Abstract
Arsenic is a common contaminant in drinking water throughout the world, and recent studies support a link between inorganic arsenic (iAS) exposure and ischemic heart disease in men and women. Female hearts exhibit an estrogen-dependent reduction in susceptibility to myocardial ischemic injury compared with males, and as such, female hearts may be more susceptible to the endocrine-disrupting effects of iAS exposure. However, iAS exposure and susceptibility to ischemic heart injury have not been examined in mechanistic studies. Male and female mice (8 wk) were exposed to environmentally relevant concentrations of sodium arsenite (0, 10, 100, and 1,000 parts/billion) via drinking water for 4 wk. Pre- and postexposure echocardiography was performed, and postexposure plasma was collected for 17β-estradiol measurement. Hearts were excised and subjected to ischemia-reperfusion (I/R) injury via Langendorff perfusion. Exposure to 1,000 parts/billion iAS led to sex-disparate effects, such that I/R injury was exacerbated in female hearts but unexpectedly attenuated in males. Assessment of echocardiographic parameters revealed statistically significant structural remodeling in iAS-treated female hearts with no change in function; males showed no change. Plasma 17β-estradiol levels were not significantly altered by iAS in male or female mice versus nontreated controls. Although total eNOS protein levels did not change in whole heart homogenates from iAS-treated male or female mice, eNOS phosphorylation (Ser1177) was significantly elevated in iAS-treated male hearts. These results suggest that iAS exposure can induce sex-disparate effects and modulate susceptibility to ischemic heart injury by targeting distinct sex-dependent pathways.
NEW & NOTEWORTHY This is the first mechanistic study examining iAS exposure on myocardial ischemia-reperfusion injury in male and female mice. Following iAS exposure, ischemia-reperfusion injury was exacerbated in female hearts but attenuated in males. iAS treatment induced statistically significant cardiac remodeling in females, with no change in males. iAS treatment also enhanced phosphorylated eNOS levels at Ser1177, but only in male hearts. These results suggest that iAS alters susceptibility to myocardial I/R injury through distinct sex-dependent pathways.
Keywords: cardioprotection, endothelial nitric oxide synthase, environmental exposure, estrogen, sex differences
INTRODUCTION
Ischemic heart disease is a leading cause of death among both men and women across the developed world, and more importantly in the United States (7, 49). In comparison with age-matched men, premenopausal women exhibit a reduced risk for the development of cardiovascular disease (5, 23, 39). This protection has also been observed in preclinical models of ischemia-reperfusion (I/R) injury, with females typically exhibiting enhanced functional recovery and smaller infarcts compared with males (4, 9, 27, 52, 53, 55). Prior work has suggested that this cardioprotection is lost in females following ovariectomy (29, 33), suggesting a potential role for estrogen. Indeed, the administration of estrogen and/or the activation of estrogen receptors have been shown to reduce the detrimental impact of I/R injury (8, 22, 29, 33, 62). Recent work from our group and others suggests that nitric oxide also plays a critical role in sex-dependent cardioprotective signaling (9, 52, 53, 55). According to the American Heart Association, the divide in ischemic heart disease mortality rates between males and females is narrowing due to a decline in age-adjusted mortality after myocardial infarction among males from 2001 to 2003 and from 2007 to 2009, but this does not appear to be the case in females (7). Environmental factors may be influencing this increase in cardiovascular disease development in males and females.
Epidemiological studies suggest that exposure to environmental pollutants like particulate matter, gases, and metals may promote the development of cardiovascular disease (14, 18), but mechanistic studies are lacking for many of these pollutants. Inorganic arsenic (iAS) is a naturally occurring metalloid that is a common contaminant in drinking water throughout the world, resulting from both natural and industrial processes such as mining, agriculture, and fuel combustion (17, 30). The United States Environmental Protection Agency and the World Health Organization recommend that municipal water concentrations of iAS not exceed 10 parts/billion, but many private wells in the United States and groundwater sources throughout the world are far in excess of this standard (>2,000–5,000 parts/billion) (43, 54). Chronic exposure to iAS has been linked to the manifestation of a vast array of disease states, including type 2 diabetes (41) and cardiovascular disease (11–13, 24, 25, 61). Epidemiological studies associate iAS exposure with the development of peripheral vascular disease, hypertension, carotid atherosclerosis, and cardiomyopathy, as well as ischemic heart disease (11–13, 24, 25, 61). In addition to promoting cardiovascular disease, iAS is considered to be an endocrine disruptor, altering sex steroid signaling and receptor-mediated gene expression (10, 16, 63). Female hearts typically exhibit an estrogen-dependent reduction in susceptibility to ischemic injury (29, 33), and as such, female hearts may be more susceptible to the endocrine-disrupting effects of iAS exposure. However, the impact of iAS exposure on susceptibility to ischemic heart injury has not been examined in mechanistic studies.
In the current study, we exposed male and female mice to environmentally relevant doses of iAS for 4 wk and examined myocardial ischemic susceptibility using an ex vivo model of I/R injury. We found that iAS exposure led to sex-disparate effects, exacerbating injury in female hearts but unexpectedly attenuating injury in male hearts. Significant cardiac remodeling was also observed in iAS-treated females but not in iAS-treated males. Endothelial nitric oxide synthase (eNOS) phosphorylation at Ser1177 was also elevated in iAS-treated male hearts. These results suggest that iAS exposure can induce sex-dependent effects on myocardial ischemic injury.
METHODS
Animals and iAS exposure protocol.
Male and female C57BL/6J wild-type mice were purchased from Jackson Laboratory (Bar, Harbor, ME). Mice (4–5 mice/cage) were housed under specific pathogen-free conditions and were maintained on AIN-93G chow (Research Diets, New Brunswick, NJ) and Pure Life water (Nestlé Waters North America, Stamford, CT) for 1 wk before sodium arsenite exposure. Research Diets has reported that the levels of arsenic in AIN-93G chow are below the limit of detection (47). In addition, a more recent study examined arsenic levels in a number of different rodent chow formulations, and AIN-93G chow had considerably lower levels of arsenic compared with the 11 other diets that were tested (38). Nestlé also reported that the levels of arsenic in Pure Life water were below the limit of detection (0.002 parts/million) (1). As such, AIN-93G chow and Pure Life water are the preferred choices for this study. Following the 1-wk pre-exposure period, 8-wk-old mice (n = 10/group) were then given Nestlé Pure Life water containing 0 (control), 10, 100, or 1,000 parts/billion iAS in the form of sodium arsenite (Sigma-Aldrich, St. Louis, MO) for a total of 4 wk. Water containing sodium arsenite was refreshed every 2-3days to maintain effective concentrations of arsenite and minimize oxidation. For all subsequent procedures, mice were anesthetized with a mixture of ketamine (Hofspira, Lake Forest, IL) and xylazine (Sigma, St. Louis, MO) via intraperitoneal injection and anticoagulated with heparin (Fresenvis Kabi, Lake Zurich, IL). This investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH; Publication No. 85-23, Revised 2011) and was approved by the Institutional Animal Care and Use Committee of Johns Hopkins University.
Assessment of cardiac morphology and function with transthoracic echocardiography.
Transthoracic echocardiography was performed in conscious mice using a Vevo 2100 system with a 40-MHz linear transducer (FUJIFILM; VisualSonics, Toronto, ON, Canada) as described (6, 9, 20, 57, 64). The M-mode echocardiogram was acquired from the short-axis view of the left ventricle (LV) at the level of the mid-papillary muscles and at a sweep speed of 200 mm/s. From this axis view of the left ventricle, the following cardiac parameters were measured: interventricular septal thickness at end diastole (IVSd), left ventricle internal diameter at end diastole (LVIDd), left ventricle posterior wall thickness at end diastole (LVPWd), and left ventricle internal diameter at end systole (LVIDs). These parameters were used to estimate the percent fractional shortening (FS), percent ejection fraction (EF), left ventricle mass (LV mass), and relative wall thickness (RWT) as measures of cardiac contractility and left ventricle morphology. These indices were derived from the following equations: fractional shortening (%) = [(LVIDd – LVIDs)/LVIDd] × 100, ejection fraction (%) = [(LVId2 – LVIDs2)/LVIDd2] × 100, left ventricular mass (mg): 1.055 [(IVSd + LVIDd + LVPWd)3 – (LVIDd)3], where 1.055 is the specific gravity of the myocardium, and relative wall thickness = (LVPWd/LVIDd) × 2.
The end-diastolic (EDV) and end-systolic ventricular volumes (ESV), stroke volume (SV), and the percent EF were estimated using the Simpson’s method and the two-chamber view of the heart on long axis.
Langendorff heart perfusion and I/R treatment protocol.
Hearts from male and female mice were excised, cannulated, and Langendorff perfused retrogradely at a constant pressure of 100 cmH2O and a temperature of 37°C with Krebs-Henseleit buffer and allowed to beat spontaneously, as previously described (9, 52, 53). Krebs-Henseleit buffer consisted of (in mmol/l) NaCl (120), KCl (4.7), KH2PO4 (1.2), NaHCO3 (25), MgSO4 (1.2), d-glucose (11), and CaCl2 (1.75), pH 7.4. Krebs-Henseleit buffer was bubbled with 95%O2/5%CO2. Hearts were perfused in the dark as a safeguard to preserve S-nitrosothiol signaling, which is critical component for cardioprotection in female hearts (9, 53). After cannulation, a saran wrap balloon was immediately inserted into the left ventricle before ischemia to measure left ventricular developed pressure (LVDP). After 20 min of equilibration, the flow of oxygenated Krebs-Henseleit buffer through hearts was halted to induce global, normothermic ischemia for 20 min. After ischemia, the flow of oxygenated Krebs-Henseleit buffer was re-established for 120 min of reperfusion (Fig. 1A). LVDP and heart rate were recorded and digitized through a PowerLab system (AD Instruments, Dunedin, New Zealand). The rate pressure product was calculated and used as a measure of cardiac contractile function. Postischemic functional recovery was expressed as a percentage of the pre-ischemic rate pressure product. At the conclusion of the 120-min reperfusion period, hearts were perfused with 10 ml of 1% tetrazolium tetrachloride (TTC) solution over the course of 2 min. Hearts were then incubated in TTC solution for an additional 20 min at 37°C to stain for infarct, followed by fixation in 10% formalin solution. Hearts were subsequently sectioned and imaged using a dissecting scope (Nikon) and analyzed for infarct size using ImageJ software (NIH, Bethesda, MD) by an investigator blinded to the treatment groups.
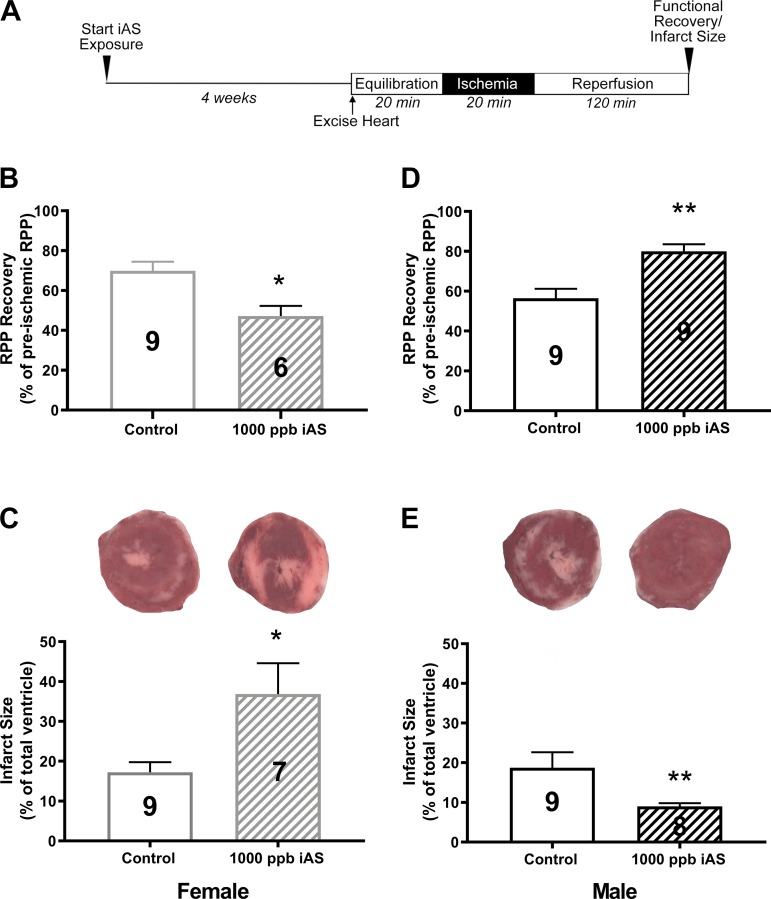
Fig. 1.
Four weeks of exposure to 1,000 parts/billion inorganic arsenic (iAS) results in sex-disparate effects on susceptibility to ischemic heart injury. A: perfusion protocol. B–E: 1,000 parts/billion exposure [B and D show postischemic functional recovery at 90 min of reperfusion; C and E show post-ischemia-reperfusion (I/R) myocardial infarct size with representative heart slices]. Numbers inside each bar represent the no. of hearts used for each group (n = 6–9 hearts/group). Data sets were analyzed using an unpaired Student’s t-test. *P < 0.05 vs. control female; **P < 0.05 vs. control male.
Water and urine arsenic, iron, and manganese measurement.
Urine samples were collected from individual mice at the end of the 4-wk exposure period using Labsand (Braintree Scientific, Braintree, MA) and stored at −80°C until analysis. Water samples were collected at the time of sodium arsenite water generation and stored at −20°C until analysis. Water and urine samples were then blinded and analyzed by an independent laboratory (Dr. Ana Rule). In brief, samples (50 μl) were diluted into 2% HNO3 and 0.5% HCl solution. The calibration curve for arsenic was built using a standard solution (Multi-element Aqueous CRM, QC Standard 21; VHG Laboratories, Manchester, NH). Ten parts/billion (vol/vol) of internal standard (CPI International, Santa Rosa, CA) was added to samples and calibration curves to control potential drifts in the signal. Metal concentrations were then measured using inductively coupled plasma mass spectrometry (Agilent 7500ce Octopole inductively coupled plasma mass spectrometer; Agilent Technologies, Santa Clara, CA). The limit of detection was 0.04 μg/l (parts/billion) for arsenic. Samples with metal concentrations below the limit of detection were substituted by the limit of detection divided by the square root of 2. For quality control and assurance, 10% duplicates, 10% blanks, Seronorm Trace Elements Urine (Accurate, Westbury, NY), and replicate sample analysis at predetermined intervals were analyzed.
Liver alanine transaminase activity.
Alanine transaminase (ALT) activity was assessed in whole liver homogenates from control and iAS-exposed male and female mice using a commercially available kit (Abcam, Cambridge, MA) per the manufacturer’s instruction. Approximately 15 mg of liver tissue was homogenized in 200 μl of assay buffer for each sample using a Dounce glass homogenizer on ice. Samples were then centrifuged at 21,130 g for 10 min at 4°C to pellet debris, and the supernatant was recovered as total crude homogenate. Sample protein concentration was determined using the Bradford protein assay. Exactly 5 μl of sample was added to 15 μl of assay buffer in an individual well of a 96-well plate. To initiate the reaction, 100 μl of a mixture containing the ALT substrates alanine and α-ketoglutarate, alanine transaminase (ALT) enzyme cofactors, and an OxiRed Probe was added to each individual well. ALT catalyzes the transfer of an amino group from alanine to α-ketoglutarate to produce pyruvate and glutamate. Pyruvate production was indirectly monitored via absorbance at 570 nm for 10 min at 37°C. ALT activity was calculated based on the amount of pyruvate that was produced between minutes 2 and 10 of the reaction. ALT activity was expressed in units where 1 unit (U) of ALT activity is the amount of ALT that generates 1.0 μmol/min pyruvate at 37°C. Samples were run in duplicate.
Plasma 17β-estradiol levels.
Blood samples were extracted from the inferior vena cava at the end of the 4-wk exposure period and collected in tubes treated with heparin. Cells were removed by centrifugation at 21,130 g for 10 min at 4°C, and the supernatant was recovered as plasma and stored at −80°C. Plasma samples were then blinded and analyzed for 17β-estradiol concentrations at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core using a commercially available estradiol ELISA kit (Calbiotech, Spring Valley, CA) per the manufacturer’s instructions. This core has extensive experience in the measurement of 17β-estradiol by ELISA (21).
Whole heart homogenate preparation.
A separate set of hearts were Langendorff-perfused for 20 min in the dark and immediately snap-frozen in liquid nitrogen. All subsequent procedures were completed in the dark. Hearts were powdered on liquid nitrogen with a mortar and pestle and resuspended in 1.0 ml of cell lysis buffer (Cell Signaling Technology, Danvers, MA) containing a protease-inhibitor cocktail (Cell Signaling Technology). Samples were then homogenized using a Dounce glass homogenizer on ice and centrifuged at 21,130 g for 10 min to pellet debris. The supernatant was recovered as total crude homogenate. Protein concentration was determined using the Bradford protein assay. Total homogenates were then aliquoted and stored at −80°C.
Western blots for eNOS, p-eNOS, nNOS, iNOS, Akt, p-Akt, p-AMPK, AMPK, p-PLB, PLB, and GAPDH.
Samples (30 μg) were separated on a 4–12% Bis-Tris SDS-PAGE gel and transferred to a PVDF or nitrocellulose membrane (Life Technologies, Carlsbad, CA). A molecular weight marker was included with every gel to delineate molecular weight regions of interest (high range color-coded prestained protein marker; Cell Signaling Technology; and Novex Prestained Protein Standard, Thermo Fisher Scientific, Rockford, IL). Both membrane types were blocked for 1 h with Tris-buffered saline with 0.1% Tween-20 and either 5% (wt/vol) nonfat dried milk for nonphosphorylated proteins or 5% (wt/vol) bovine serum albumin (Sigma-Aldrich) for phosphorylated proteins. Membranes were subsequently incubated with primary antibodies against phospho-eNOS Ser1177 (1:500, rabbit, 9570S; Cell Signaling Technology), phospho-eNOS Thr495 (1:500, rabbit, 9574S; Cell Signaling Technology), total eNOS (1:250, mouse, sc-376751;Santa Cruz Biotechnology, Dallas, TX), total neuronal nitric oxide synthase (nNOS; rabbit, sc-648, 1:250; Santa Cruz Biotechnology), total inducible nitric oxide synthase (iNOS; 1:250, rabbit, sc-650; Santa Cruz Biotechnology), phospho-Akt Ser473 (1:1,000, rabbit, 4060S; Cell Signaling Technology), total Akt (1:1,000, rabbit, 4691S; Cell Signaling Technology), phospho-AMPKα Thr172 (1:1,000, rabbit, 50081S; Cell Signaling Technology), total AMPKα (1:1,000, rabbit, 5832S; Cell Signaling Technology), phosphorylated phospholamban (PLB) Thr17 (1:1,000, rabbit, A010-13AP; Badrilla, Leeds, UK), total PLB (1:1,000, rabbit, PA5-26004; Thermo Fisher Scientific), or GAPDH (1:1,000, rabbit, sc-25778; Santa Cruz Biotechnology). Membranes were then probed with the corresponding secondary antibody, either anti-rabbit (7074S; Cell Signaling Technology) or anti-mouse (sc-2005; Santa Cruz Biotechnology), for 1 h and visualized by electrogenerated chemiluminescence (Life Technologies). In the case of phosphoblots, membranes were stripped with Re-blot Plus Mild Solution (EMD Millipore, Temecula, CA) and reprobed for total protein (i.e., total eNOS, Akt, AMPK, and PLB). Densitometry was assessed using ImageJ software (NIH) and normalized to GAPDH as a loading control and to a designated sample that was run in all gels to control for gel-to-gel variability.
Statistical analysis.
Results are expressed as means ± SE and were analyzed using GraphPad Prism (version 7.02; GraphPad, La Jolla, CA). Statistical outliers were identified using Grubbs’ test for the detection of outliers. Statistical significance (P < 0.05) was determined between groups using a Student’s t-test for two groups, a nonlinear regression, or a two-way ANOVA with Tukey’s multiple comparisons test.
RESULTS
iAS has sex-disparate effects on susceptibility to ischemic heart injury.
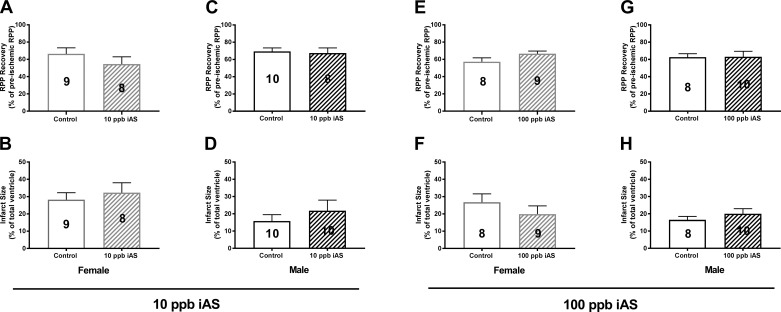
To determine the effect of iAS exposure on sex-dependent susceptibility to ischemic heart injury, male and female hearts were excised and subjected to I/R injury via Langendorff perfusion (20 min of equilibration, 20 min of ischemia, and 120 min of reperfusion; Fig. 1A). Initial hemodynamic measurements indicated no differences in baseline LVDP, heart rate, or RPP between control and iAS-treated male or female hearts at all iAS exposure doses (Table 1). However, after 20 min of ischemia and 90 min of reperfusion, we identified substantial sex-dependent changes in the response to I/R injury in our 1,000 parts/billion iAS exposure group compared with nontreated controls (Table 1). iAS-treated female hearts showed a significant decrease in postischemic recovery of function and a significant increase in infarct size compared with control (Fig. 1, B and C, and Table 1), whereas iAS-treated male hearts showed a significant increase in postischemic functional recovery and a significant reduction in infarct size (Fig. 1, D and E, and Table 1). Exposure to 10 parts/billion or 100 parts/billion iAS had no effect on I/R injury in male or female mice (Fig. 2).
Table 1.
Pre- and postischemic contractile parameters in control (0 parts/billion) and 10, 100, and 1,000 parts/billion iAS-treated male and female hearts
| Preischemia |
Postischemia |
|||||||
|---|---|---|---|---|---|---|---|---|
| Male Control | Male iAS | Female Control | Female iAS | Male Control | Male iAS | Female Control | Female iAS | |
| 10 parts/billion | ||||||||
| LVDP, cmH2O | 83.4 ± 11.3 | 76.6 ± 5.6 | 63.2 ± 6.5 | 75.8 ± 11.0 | 58.8 ± 6.5 | 62.2 ± 7.6 | 45.3 ± 7.6 | 48.9 ± 9.7 |
| HR, beats/min | 367.1 ± 15.3 | 358.3 ± 13.1 | 332.3 ± 15.2 | 378.9 ± 12.8 | 346.0 ± 10.8 | 338.7 ± 15.3 | 315.1 ± 13.5* | 305.7 ± 14.1* |
| Rate pressure product | 29968.4 ± 3743.9 | 27301.5 ± 2050.5 | 21285.0 ± 2550.3 | 28654.9 ± 4126.0 | 20420.6 ± 2523.2 | 20862.0 ± 2462.6 | 13966.6 ± 2378.3* | 15162.1 ± 2980.4* |
| 100 parts/billion | ||||||||
| LVDP, cmH2O | 84.2 ± 10.5 | 88.9 ± 14.6 | 74.7 ± 6.35 | 74.9 ± 3.7 | 57.1 ± 5.1 | 57.6 ± 8.4 | 46.9 ± 4.4†* | 56.7 ± 3.6†* |
| HR, beats/min | 335.5 ± 9.9 | 322.4 ± 17.4 | 333.8 ± 28.4 | 361.3 ± 8.7 | 297.4 ± 10.8 | 295.5 ± 14.2 | 294.8 ± 19.2 | 317.4 ± 8.7 |
| Rate pressure product | 27971.5 ± 3277.8 | 28506.9 ± 5145.5 | 25971.5 ± 3392.4 | 27044.9 ± 1446.0 | 17152.7 ± 1783.4 | 16427.0 ± 2095.5 | 14092.3 ± 1750.4†* | 17980.6 ± 1252.8†* |
| 1,000 parts/billion | ||||||||
| LVDP, cmH2O | 90.8 ± 13.3 | 81.8 ± 6.1 | 83.7 ± 6.6 | 92.4 ± 9.4 | 56.0 ± 8.5 | 72.6 ± 5.9 | 69.5 ± 6.5 | 55.5 ± 7.6* |
| HR, beats/min | 333.5 ± 28.9 | 347.0 ± 9.3 | 342.4 ± 15.7 | 359.7 ± 13.3 | 294.6 ± 24.0 | 313.2 ± 13.7 | 287.6 ± 12.4†* | 279.8 ± 15.2†* |
| Rate pressure product | 30566.9 ± 4606.1 | 28458.2 ± 2352.0 | 29147.7 ± 3361.7 | 33338.1 ± 3680.8 | 16955.9 ± 3181.8† | 22727.5 ± 2127.9 | 19885.0 ± 1809.0* | 15118.2 ± 1608.9†* |
Values are means ± SE (rate pressure product; n = 6–10 hearts/group). iAS, inorganic arsenic; LVDP, preischemic left ventricular developed pressure (cmH2O); HR, heart rate. Within-sex comparisons of data sets (male or female) were performed using a 2-way ANOVA with Tukey’s multiple comparisons test.
P < 0.05 vs. control, preischemia;
P < 0.05 vs. iAS, preischemia.
Fig. 2.
Four weeks of exposure to 10 or 100 parts/billion inorganic arsenic (iAS) does not alter susceptibility to ischemic heart injury. A–D: 10 parts/billion exposure [A and C show postischemic functional recovery at 90 min of reperfusion; B and D: post-ischemia-reperfusion (I/R) myocardial infarct size]. E–H: 100 parts/billion exposure (E and G show postischemic functional recovery at 90 min of reperfusion; F and H show post-I/R myocardial infarct size. Numbers inside each bar represent the no. of hearts used for each group (n = 8–10 hearts/group). Data sets were analyzed using an unpaired Student’s t-test.
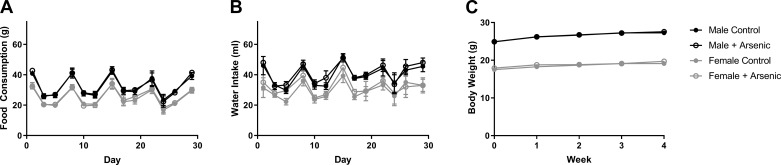
Because dietary intake and dehydration can modulate susceptibility to I/R injury (35), feed and water intake were monitored during acclimation and throughout the duration of the exposure, but no significant differences were observed between the 0 and 1,000 parts/billion iAS treatment groups (Fig. 3, A and B). Body weight was also not different between 0 and 1,000 parts/billion iAS treatment groups (Fig. 3C). No significant differences were noted in food intake, water intake, or body weight in the 10 or 100 parts/billion iAS treatment groups (data not shown). In addition, no mice died during the 4-wk exposure period at any of the iAS exposure doses.
Fig. 3.
Feed consumption, water intake, and body weight are not altered with 1,000 parts/billion inorganic arsenic (iAS) exposure. A: feed consumption for male and female mice during exposure protocol. B: water intake for male and female mice during exposure protocol. C: weekly body weight for male and female mice during exposure protocol (n = 15–20 mice/group). Data sets were analyzed using a nonlinear regression.
To confirm iAS exposure, we used inductively coupled plasma mass spectrometry to quantify urine arsenic. Because differences in myocardial injury were observed only with 1,000 parts/billion iAS exposure, we examined total arsenic levels in the urine of male and female mice exposed to 0 and 1,000 parts/billion of iAS and detected significantly higher levels of arsenic in 1,000 parts/billion iAS-treated male and female mice compared with 0 parts/billion control mice (control male: 24.7 ± 0.9 parts/billion versus arsenic male: 643.6 ± 164.5 parts/billion, P < 0.05 vs. control male; control female: 12.04 ± 2.0 parts/billion vs. arsenic female: 685.2 ± 10.1 parts/billion, P < 0.05 vs. control female). Urine iron and manganese levels were also assessed, but no differences were detected in the excretion of these metals in male or female mice treated with or without iAS (data not shown). We also analyzed water samples from the water that was used for dosing the mice with 1,000 parts/billion iAS and found that the iAS level was 735.12 ± 8.38 parts/billion. As such, the arsenic exposure dose was actually lower than anticipated. This discrepancy can likely be attributed to the presence of impurities in the sodium arsenite salt used to generate the water for dosing the mice and the possible oxidation of iAS. To maintain iAS levels and minimize iAS oxidation during the 4-wk exposure period, water was replaced every 2–3 days.
iAS exposure induces cardiac remodeling in females.
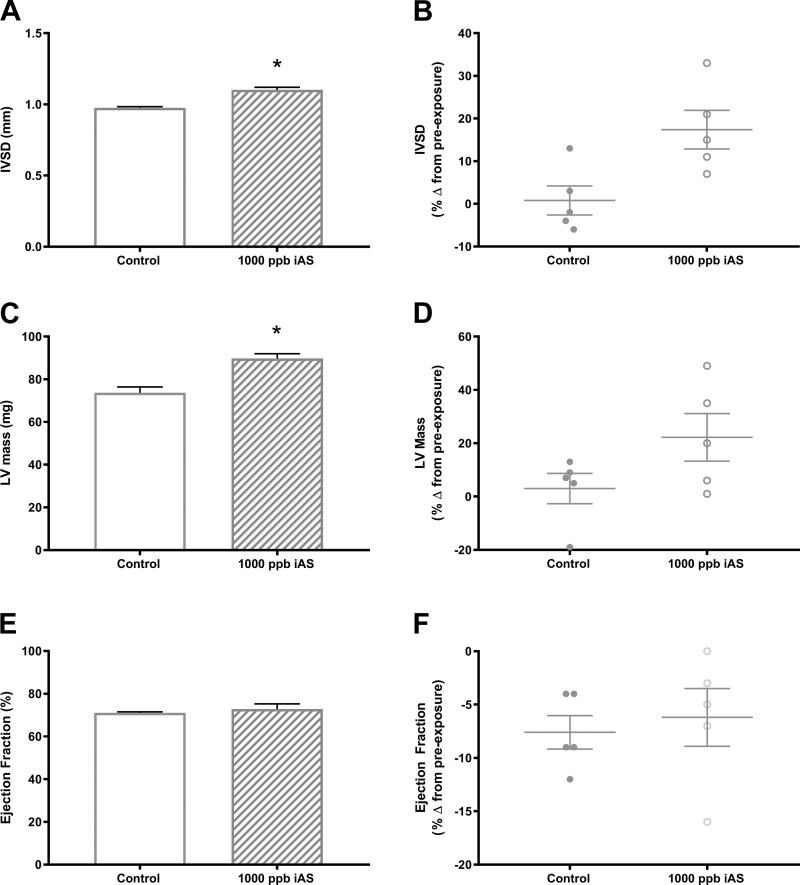
Because iAS exposure is associated with major risk factors for the development of pathological cardiac hypertrophy, which can also alter susceptibility to ischemic heart injury (48), we next assessed cardiac morphology and function. Pre- and postexposure transthoracic echocardiography was conducted for iAS-treated and nontreated male and female mice (Supplemental Tables S1–S3; Supplemental Material for this article can be found at https://doi.org/10.5281/zenodo.2573356). We observed significant increases in interventricular septal thickness at end-diastole and left ventricle mass in 1,000 parts/billion iAS-treated females compared with nontreated controls (Fig. 4, A–D). However, myocardial function, measured as ejection fraction, was not altered in 1,000 parts/billion iAS-treated females (Fig. 4, E and F). No significant changes were observed with regard to myocardial morphology or function in 1,000 parts/billion iAS-exposed male hearts (Supplemental Table S1; https://doi.org/10.5281/zenodo.2573356). In addition, we did not detect significant changes in cardiac morphology or function at the 10 or 100 parts/billion iAS exposure doses in either male or female mice (Supplemental Tables S2 and S3; https://doi.org/10.5281/zenodo.2573356). Because the 10 and 100 parts/billion iAS exposure doses were without effect on myocardial ischemic injury, morphology, or function, the remaining experiments were conducted using only 1,000 parts/billion iAS-treated and nontreated male and female mice.
Fig. 4.
Four week of exposure to 1,000 parts/billion inorganic arsenic (iAS) induces significant changes in cardiac morphology in females. Cardiac morphology and function were assessed by transthoracic echocardiography following exposure to 0 (control) or 1,000 parts/billion iAS for 4 wk. A and B: interventricular septal thickness at end diastole (IVSd) with control and iAS treatment. C and D: left ventricle (LV) mass with control and iAS treatment. E and F: ejection fraction with control and iAS treatment (n = 5 mice/group). Data sets were analyzed using an unpaired Student’s t-test. *P < 0.05 vs. control female.
Liver toxicity was not observed with iAS exposure.
The liver is a major target of iAS toxicity, and noncirrhotic fibrosis is common in individuals chronically exposed to a high dose of iAS (15, 51). Therefore, we next examined liver toxicity as a potential mechanism for the exacerbation of myocardial ischemic injury in females. ALT activity was assessed in livers from 1,000 parts/billion iAS-treated and nontreated mice as measure of hepatotoxicity (3), but no differences were observed in either male or female mice (control male: 156.3 ± 11.6 mU/μg vs. arsenic male: 150.1 ± 7.1 mU/μg; control female: 168.0 ± 11.3 mU/μg vs. arsenic female: 159.0 ± 7.8 mU/μg).
iAS exposure does not alter circulating 17β-estradiol levels in males or females.
Because female hearts normally exhibit an estrogen-dependent reduction in susceptibility to ischemic injury and we observed significantly greater I/R injury in females with iAS exposure, we next examined plasma 17β-estradiol levels from 1,000 parts/billion iAS-treated male and female mice. 17β-Estradiol levels showed a trending decrease in plasma from iAS-treated females compared nontreated female controls, but this decrease was ultimately not statistically significant (control female: 10.33 ± 1.88 pg/ml vs. arsenic female: 6.71 ± 0.89 pg/ml, P = 0.0954 vs. control female). No change in 17β-estradiol levels was observed in iAS-treated males (control male: 7.19 ± 0.96 pg/ml vs. arsenic male: 7.10 ± 1.08 pg/ml).
iAS alters myocardial nitric oxide signaling.
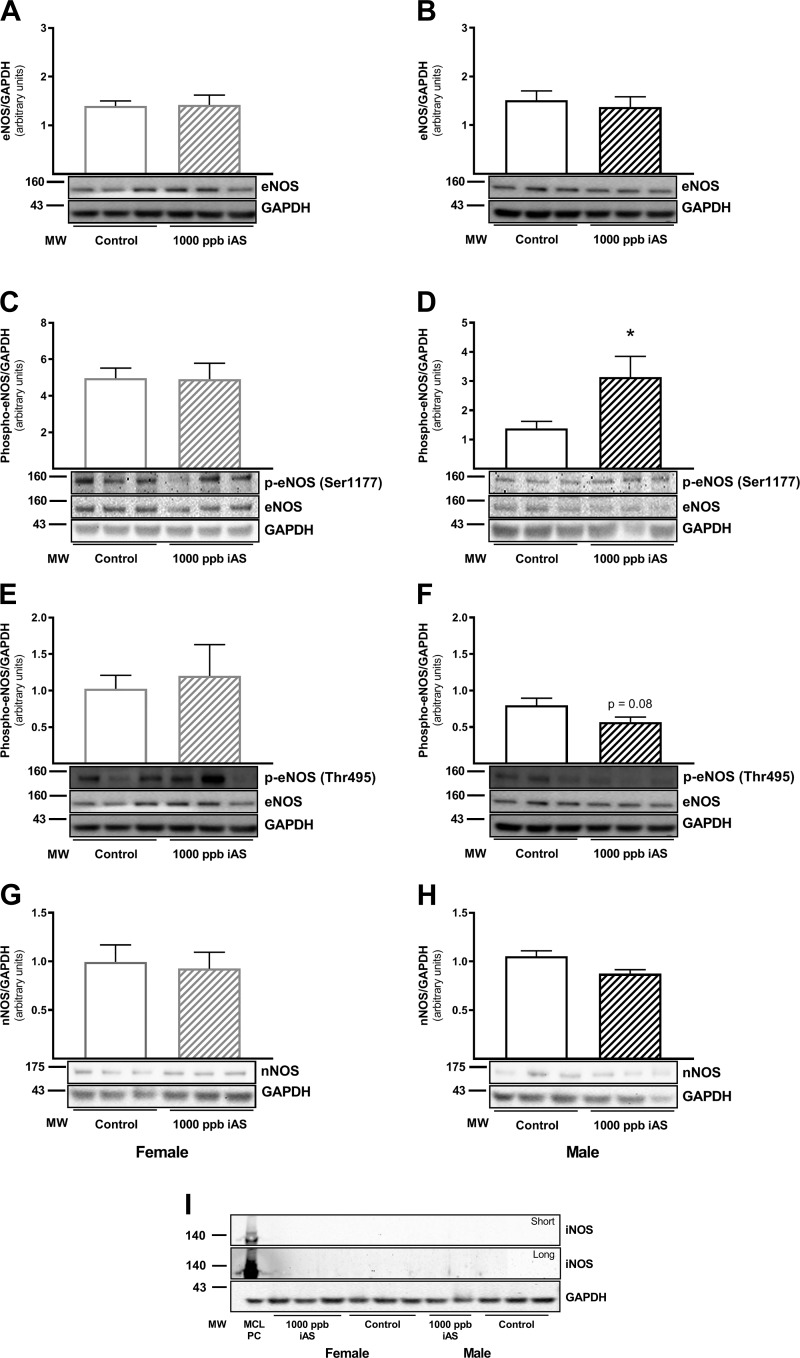
NO signaling is a key component of sex-dependent cardioprotection (39, 52, 53), so we next examined eNOS expression and phosphorylation (Fig. 5, A–F). We found that whereas total eNOS protein levels remained unchanged in hearts from iAS-treated females and males compared with nontreated controls (Fig. 5, A and B), eNOS phosphorylation was significantly elevated at the activation site (Ser1177) in iAS-treated male hearts (Fig. 5D). Ser1177 phosphorylation remained unchanged in iAS-treated female hearts (Fig. 5C). Conversely, eNOS phosphorylation at the inhibitory site (Thr495) was unchanged in hearts from iAS-treated females compared with nontreated controls (Fig. 5E), but Thr495 phosphorylation showed a trending decrease in hearts from iAS-treated male mice, although this change was ultimately not statistically significant (Fig. 5F). Additionally, we examined total nNOS and iNOS protein expression levels with iAS exposure. No significant changes in nNOS protein levels were noted with iAS exposure in either female or male hearts (Fig. 5, G and H), and iNOS expression was not detected (Fig. 5I). Macrophage cell lysate was used as a positive control for iNOS.
Fig. 5.
Four weeks of exposure to 1,000 parts/billion inorganic arsenic (iAS) modulates myocardial endothelial nitric oxide synthase (eNOS) phosphorylation in males. A and B: Western blot depicting total eNOS levels in control and 1,000 parts/billion iAS-treated male and female hearts. C and D: Western blot depicting phosphorylated (Ser1177) eNOS levels in control and 1,000 parts/billion iAS-treated male and female hearts. E and F: Western blot depicting phosphorylated (Thr495) eNOS levels in control and 1,000 parts/billion iAS-treated male and female hearts. G and H: Western blot depicting total neuronal nitric oxide synthase (nNOS) levels in control and 1,000 parts/billion iAS-treated male and female hearts. I: Western blot depicting total inducible nitric oxide synthase (iNOS) levels in control and 1,000 parts/billion iAS-treated male and female hearts with a short and long chemiluminescent exposure. Macrophage cell lysate (MCL) was used as a positive control for iNOS (n = 5–10 hearts/group). Data sets were analyzed using an unpaired Student’s t-test; *P < 0.05 vs. control male.
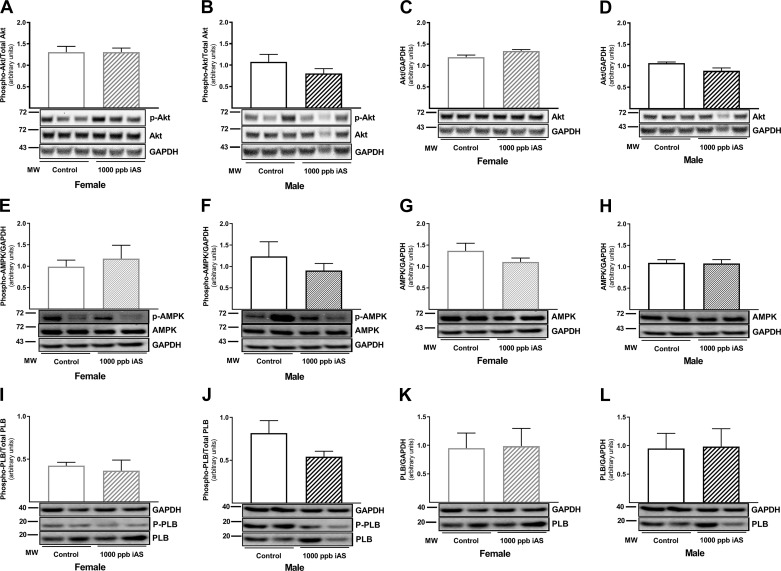
To determine a potential mechanism for the increase in eNOS phosphorylation observed at Ser1177 in iAS-treated male mice, we initially measured phospho-Akt and phospho-AMPK levels since both kinases have been shown to phosphorylate Ser1177 of eNOS (37). However, no changes were observed with regard to phospho-Akt (Fig. 6, A and B) or total Akt levels (Fig. 6, C and D) in iAS-treated male or female hearts. Phospho-AMPK and total AMPK levels also remained unchanged in iAS-treated male and female hearts (Fig. 6, E–H). CaMKII has also been shown to phosphorylate eNOS at Ser1177 (37), so we next examined phospholamban (PLB) phosphorylation levels at Thr17 as a surrogate marker for CaMKII activation. At baseline, we found significantly higher PLB phosphorylation levels at Thr17 in male hearts versus females (Fig. 6, I–L), which is consistent with the estrogen-dependent suppression of CaMKII activity demonstrated in previous work (34). In iAS-treated male mice, PLB Thr17 phosphorylation was decreased versus non-iAS treated controls, but this decrease was not significant (Fig. 6j). PLB Thr17 phosphorylation did not change with iAS exposure in female hearts (Fig. 6I). Total PLB levels also remained unchanged in iAS-treated male and female hearts (Fig. 6, K and L).
Fig. 6.
Four weeks of exposure to 1,000 parts/billion inorganic arsenic (iAS) does not alter Akt, AMPK, or CaMKII activation. A and B: Western blot depicting phosphorylated (Ser473) Akt levels in control and 1,000 parts/billion iAS-treated male and female hearts. C and D: Western blot depicting total Akt levels in control and 1,000 parts/billion iAS-treated male and female hearts. E and F: Western blot depicting phosphorylated (Thr172) AMPK levels in control and 1,000 parts/billion iAS-treated male and female hearts. G and H: Western blot depicting total AMPK levels in control and 1,000 parts/billion iAS-treated male and female hearts. I and J: Western blot depicting phosphorylated (Thr17) phospholamban (PLB) levels in control and 1,000 parts/billion iAS-treated male and female hearts. K and L: Western blot depicting total PLB levels in control and 1,000 parts/billion iAS-treated male and female hearts (n = 5 hearts/group). Data sets were analyzed using an unpaired Student’s t-test.
DISCUSSION
We report for the first time that exposure to an environmentally relevant dose of iAS induces sex-disparate effects and alters susceptibility to myocardial I/R injury in part by targeting distinct, sex-dependent pathways in the heart. iAS exposure exacerbated myocardial I/R injury in females at the highest dose (measured to be 735 parts/billion) but surprisingly reduced injury in male hearts exposed to the same dose of iAS (Fig. 1). Although the Environmental Protection Agency and World Health Organization drinking water standard is 10 parts/billion, many private wells and groundwater sources throughout the world greatly exceed this standard (>2,000 to 5,000 parts/billion) (43, 54). Furthermore, a number of epidemiological studies suggest a link between iAS exposure and the development of ischemic heart disease in both men and women (25, 36, 40, 58), so our findings of iAS-induced cardioprotection in males were quite unexpected and may result from the short exposure window used in our model. Nonetheless, we further examined the mechanism underlying the sex-disparate effects of iAS on myocardial I/R injury.
iAS has been shown to induce hypertensive effects (11–13, 26, 32), which could lead to pathologic cardiac hypertrophy. An epidemiological study of children in Mexico also linked early life iAS exposure to elevated blood pressure, higher left ventricle mass, and reduced ejection fraction (44). Thus, we initially examined cardiac morphology and function using echocardiography and noted significant changes in interventricular septal thickness at end diastole and left ventricle mass in female hearts exposed to 1000 parts/billion iAS with no change in cardiac function (Fig. 4). Male hearts were unaffected at the same dose of iAS (Supplemental Table S1 (https://doi.org/10.5281/zenodo.2573356). These results are consistent with prior work demonstrating an increase in interventricular septal thickness at end-diastole and left ventricle mass in female mice exposed to 100 parts/billion iAS for 22 wk (50). This iAS-induced increase in left ventricle mass likely makes the female heart more susceptible to ischemic injury, as previous work suggests (48). Future studies will examine the effect of 1,000 parts/billion iAS on blood pressure and cardiac morphology over longer periods of exposure.
We also considered hepatotoxicity as a potential mechanism, since the liver is a major target of iAS-induced toxicity (15, 51). Although ALT activity has been shown to be elevated in livers from mice exposed to 3,200 parts/billion iAS for 12 mo and 4,900 parts/billion iAS for 10 mo (51, 56), we did not detect a change ALT activity in liver extracts from either male or female mice treated with 1,000 parts/billion iAS for 4 wk. These results are consistent with prior studies utilizing similar iAS exposure models in mice (2, 31) and suggest that susceptibility to ischemic injury may be altered in the heart at subhepatotoxic doses of iAS.
iAS has also been shown to disrupt estrogen signaling (10, 16, 63), which is a key component of sex-dependent cardioprotection (39). Therefore, we examined circulating 17β-estradiol levels in plasma from iAS-treated and nontreated mice, but 17β-estradiol levels were not significantly altered in either iAS-treated males or females compared with nontreated controls. However, it is important to note that endocrine disruption can occur independently from direct changes in hormone levels, for example, by interruption of steroid receptor interactions with DNA response elements (19).
Additionally, the genetic deletion of eNOS has been shown to abrogate protection in female hearts (55), suggesting a key role for eNOS in cardioprotection, as we and others have shown (52, 53, 55). Therefore, we next examined eNOS expression and phosphorylation levels using Western blot. Although iAS has been shown to decrease eNOS expression in vascular tissue (28, 59), total eNOS expression was not altered in whole heart homogenates from either male or female mice exposed to 1,000 parts/billion iAS (Fig. 5). However, we did find eNOS phosphorylation levels to be increased at Ser1177, but only in male hearts (Fig. 5). Phosphorylation of eNOS at Ser1177 has been shown to increase the activity of the enzyme (37). Conversely, phosphorylation of the inhibitory site for eNOS (Thr495) was not altered with iAS exposure in either male or female hearts (Fig. 5). These results suggest that eNOS phosphorylation may be partly responsible for the protection observed in iAS-treated male hearts, but additional experiments are required to confirm a role for eNOS.
To determine the kinase responsible for our observed increase in the phosphorylation of eNOS at Ser1177 in iAS-treated male hearts (Fig. 6), we next examined Akt, AMPK, and CaMKII, but none of these kinases appeared to be responsible for the observed increase. However, it is interesting to speculate that perhaps CaMKII activation may be suppressed in iAS-treated male hearts, and this inhibition could contribute to cardioprotection, as previous studies suggest (34, 60). Taken together, our results suggest that iAS exposure exacerbates myocardial I/R injury in female mice by inducing cardiac remodeling independently from eNOS expression or phosphorylation. iAS-treated male mice, on the other hand, showed a reduction in myocardial I/R injury, which may result from an increase in phospho-eNOS levels and a decrease in CaMKII activation in the heart.
Study limitations.
There are a number of limitations that require acknowledgment. Regarding our iAS exposure model, the iAS exposure window was narrow and limited to 4 wk. Exposure to longer durations of iAS is likely to yield a more dramatic phenotype in both male and female mice (i.e., overt hypertrophy) and may yield different phenotypes with regard to I/R injury. As such, although we observed protection in male hearts with a 4-wk exposure to 1,000 parts/billion iAS, we expect that exposure to iAS for longer time periods would eventually exacerbate I/R injury in males based on the available epidemiological evidence (25, 36, 40, 58). Although adult mice were also exposed in the current study, prenatal or early life exposure to iAS is likely to yield a more dramatic phenotype. Additionally, we did not directly measure blood pressure or pulse wave velocity in iAS-treated and nontreated mice, so we cannot completely exclude a potential vascular effect in our iAS exposure model. There are also key differences between mice and humans with regard to arsenic metabolism that ultimately make mice less susceptible to arsenic-induced toxicity compared with humans (45, 46). Furthermore, circulating 17β-estradiol levels are low in mice compared with humans and other species (42), so the measurement of 17β-estradiol levels in plasma by immunoassay can be difficult. Future experiments will use a more sensitive measure to determine whether 17β-estradiol is affected in iAS-treated female mice.
Conclusions.
In summary, we have shown that an environmental exposure can modulate susceptibility to ischemic injury in the mouse heart. More specifically, we have demonstrated for the first time that iAS exposure induces distinct changes in male and female hearts, thereby driving sex-dependent differences in the response to myocardial I/R injury. Our results further suggest that exposure to environmentally relevant concentrations of iAS may be of greater detriment to females.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants R00-HL-114721 and R01-HL-136496 (to M. J. Kohr), the American Heart Association Grant 16GRNT30420019 (to M. J. Kohr), and the Institute for Gender Specific Medicine (to M. J. Kohr).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.V. and M.J.K. conceived and designed research; R.V., K.M.C., P.S., R.K., N.M., N.T., D.B., and R.C. performed experiments; R.V., K.M.C., P.S., and D.B. analyzed data; R.V., K.M.C., P.S., A.R., and M.J.K. interpreted results of experiments; R.V., K.M.C., P.S., and M.J.K. prepared figures; R.V. and M.J.K. drafted manuscript; R.V., K.M.C., P.S., R.K., N.T., D.B., A.R., and M.J.K. edited and revised manuscript; R.V., K.M.C., P.S., R.K., N.M., N.T., D.B., R.C., A.R., and M.J.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the technical assistance of the Cardiovascular Physiology and Surgery Core at The Johns Hopkins University School of Medicine.
REFERENCES
- 1.[No authors listed]. Nestlé Pure Life 2017 Water Analysis Report (Online) Stamford, CT: Nestlé Waters North America, 2017. https://www.nestle-watersna.com/asset-library/documents/pl_eng.pdf. [Google Scholar]
- 2.Arteel GE, Guo L, Schlierf T, Beier JI, Kaiser JP, Chen TS, Liu M, Conklin DJ, Miller HL, von Montfort C, States JC. Subhepatotoxic exposure to arsenic enhances lipopolysaccharide-induced liver injury in mice. Toxicol Appl Pharmacol 226: 128–139, 2008. doi: 10.1016/j.taap.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubrecht J, Schomaker SJ, Amacher DE. Emerging hepatotoxicity biomarkers and their potential to improve understanding and management of drug-induced liver injury. Genome Med 5: 85, 2013. doi: 10.1186/gm489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae S, Zhang L. Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol Exp Ther 315: 1125–1135, 2005. doi: 10.1124/jpet.105.090803. [DOI] [PubMed] [Google Scholar]
- 5.Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation 95: 252–264, 1997. doi: 10.1161/01.CIR.95.1.252. [DOI] [PubMed] [Google Scholar]
- 6.Benavides-Vallve C, Corbacho D, Iglesias-Garcia O, Pelacho B, Albiasu E, Castaño S, Muñoz-Barrutia A, Prosper F, Ortiz-de-Solorzano C. New strategies for echocardiographic evaluation of left ventricular function in a mouse model of long-term myocardial infarction. PLoS One 7: e41691, 2012. doi: 10.1371/journal.pone.0041691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UK, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2018 Update: A report from the American Heart Association. Circulation 137: e67–e492, 2018. [Erratum in Circulation 137: e493, 2018.] doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 8.Booth EA, Obeid NR, Lucchesi BR. Activation of estrogen receptor-alpha protects the in vivo rabbit heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 289: H2039–H2047, 2005. doi: 10.1152/ajpheart.00479.2005. [DOI] [PubMed] [Google Scholar]
- 9.Casin KM, Fallica J, Mackowski N, Veenema RJ, Chan A, St. Paul A, Zhu G, Bedja D, Biswal S, Kohr MJ. S-nitrosoglutathione reductase is essential for protecting the female heart from ischemia-reperfusion injury. Circ Res 123: 1232–1243, 2018. doi: 10.1161/CIRCRESAHA.118.313956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee A, Chatterji U. Arsenic abrogates the estrogen-signaling pathway in the rat uterus. Reprod Biol Endocrinol 8: 80, 2010. doi: 10.1186/1477-7827-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol 16: 504–510, 1996. doi: 10.1161/01.ATV.16.4.504. [DOI] [PubMed] [Google Scholar]
- 12.Chen CJ, Hsueh YM, Lai MS, Shyu MP, Chen SY, Wu MM, Kuo TL, Tai TY. Increased prevalence of hypertension and long-term arsenic exposure. Hypertension 25: 53–60, 1995. doi: 10.1161/01.HYP.25.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Factor-Litvak P, Howe GR, Graziano JH, Brandt-Rauf P, Parvez F, van Geen A, Ahsan H. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: a population-based, cross-sectional study. Am J Epidemiol 165: 541–552, 2007. doi: 10.1093/aje/kwk037. [DOI] [PubMed] [Google Scholar]
- 14.Cosselman KE, Navas-Acien A, Kaufman JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol 12: 627–642, 2015. doi: 10.1038/nrcardio.2015.152. [DOI] [PubMed] [Google Scholar]
- 15.Das N, Paul S, Chatterjee D, Banerjee N, Majumder NS, Sarma N, Sau TJ, Basu S, Banerjee S, Majumder P, Bandyopadhyay AK, States JC, Giri AK. Arsenic exposure through drinking water increases the risk of liver and cardiovascular diseases in the population of West Bengal, India. BMC Public Health 12: 639, 2012. doi: 10.1186/1471-2458-12-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davey JC, Bodwell JE, Gosse JA, Hamilton JW. Arsenic as an endocrine disruptor: effects of arsenic on estrogen receptor-mediated gene expression in vivo and in cell culture. Toxicol Sci 98: 75–86, 2007. doi: 10.1093/toxsci/kfm013. [DOI] [PubMed] [Google Scholar]
- 17.Garelick H, Jones H, Dybowska A, Valsami-Jones E. Arsenic pollution sources. Rev Environ Contam Toxicol 197: 17–60, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Gorr MW, Falvo MJ, Wold LE. Air pollution and other environmental modulators of cardiac function. Compr Physiol 7: 1479–1495, 2017. doi: 10.1002/cphy.c170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosse JA, Taylor VF, Jackson BP, Hamilton JW, Bodwell JE. Monomethylated trivalent arsenic species disrupt steroid receptor interactions with their DNA response elements at non-cytotoxic cellular concentrations. J Appl Toxicol 34: 498–505, 2014. doi: 10.1002/jat.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science 332: 361–365, 2011. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology 152: 4443–4447, 2011. doi: 10.1210/en.2011-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hale SL, Birnbaum Y, Kloner RA. beta-Estradiol, but not alpha-estradiol, reduced myocardial necrosis in rabbits after ischemia and reperfusion. Am Heart J 132: 258–262, 1996. doi: 10.1016/S0002-8703(96)90419-6. [DOI] [PubMed] [Google Scholar]
- 23.Hayward CS, Kelly RP, Collins P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res 46: 28–49, 2000. doi: 10.1016/S0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh YC, Lien LM, Chung WT, Hsieh FI, Hsieh PF, Wu MM, Tseng HP, Chiou HY, Chen CJ. Significantly increased risk of carotid atherosclerosis with arsenic exposure and polymorphisms in arsenic metabolism genes. Environ Res 111: 804–810, 2011. doi: 10.1016/j.envres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Hsueh YM, Wu WL, Huang YL, Chiou HY, Tseng CH, Chen CJ. Low serum carotene level and increased risk of ischemic heart disease related to long-term arsenic exposure. Atherosclerosis 141: 249–257, 1998. doi: 10.1016/S0021-9150(98)00178-6. [DOI] [PubMed] [Google Scholar]
- 26.Huang YK, Tseng CH, Huang YL, Yang MH, Chen CJ, Hsueh YM. Arsenic methylation capability and hypertension risk in subjects living in arseniasis-hyperendemic areas in southwestern Taiwan. Toxicol Appl Pharmacol 218: 135–142, 2007. doi: 10.1016/j.taap.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Johnson MS, Moore RL, Brown DA. Sex differences in myocardial infarct size are abolished by sarcolemmal KATP channel blockade in rat. Am J Physiol Heart Circ Physiol 290: H2644–H2647, 2006. doi: 10.1152/ajpheart.01291.2005. [DOI] [PubMed] [Google Scholar]
- 28.Kesavan M, Sarath TS, Kannan K, Suresh S, Gupta P, Vijayakaran K, Sankar P, Kurade NP, Mishra SK, Sarkar SN. Atorvastatin restores arsenic-induced vascular dysfunction in rats: modulation of nitric oxide signaling and inflammatory mediators. Toxicol Appl Pharmacol 280: 107–116, 2014. doi: 10.1016/j.taap.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res 106: 1681–1691, 2010. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larios R, Fernández-Martínez R, Álvarez R, Rucandio I. Arsenic pollution and fractionation in sediments and mine waste samples from different mine sites. Sci Total Environ 431: 426–435, 2012. doi: 10.1016/j.scitotenv.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 31.Lemaire M, Lemarié CA, Molina MF, Schiffrin EL, Lehoux S, Mann KK. Exposure to moderate arsenic concentrations increases atherosclerosis in ApoE−/− mouse model. Toxicol Sci 122: 211–221, 2011. doi: 10.1093/toxsci/kfr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Li B, Xi S, Zheng Q, Wang D, Sun G. Association of urinary monomethylated arsenic concentration and risk of hypertension: a cross-sectional study from arsenic contaminated areas in northwestern China. Environ Health 12: 37, 2013. doi: 10.1186/1476-069X-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin J, Steenbergen C, Murphy E, Sun J. Estrogen receptor-beta activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation 120: 245–254, 2009. doi: 10.1161/CIRCULATIONAHA.109.868729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Y, Cheng WT, Wu S, Wong TM. Oestrogen confers cardioprotection by suppressing Ca2+/calmodulin-dependent protein kinase II. Br J Pharmacol 157: 705–715, 2009. doi: 10.1111/j.1476-5381.2009.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Müller C, de Jong M, van IJcken W, IJzermans JN, Hoeijmakers JH, de Bruin RW. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell 9: 40–53, 2010. doi: 10.1111/j.1474-9726.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon KA, Oberoi S, Barchowsky A, Chen Y, Guallar E, Nachman KE, Rahman M, Sohel N, D’Ippoliti D, Wade TJ, James KA, Farzan SF, Karagas MR, Ahsan H, Navas-Acien A. A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int J Epidemiol 46: 1924–1939, 2017. doi: 10.1093/ije/dyx202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42: 271–279, 2007. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 38.Murko M, Elek B, Styblo M, Thomas DJ, Francesconi KA. Dose and Diet - Sources of Arsenic Intake in Mouse in Utero Exposure Scenarios. Chem Res Toxicol 31: 156–164, 2018. doi: 10.1021/acs.chemrestox.7b00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy E, Lagranha C, Deschamps A, Kohr M, Nguyen T, Wong R, Sun J, Steenbergen C. Mechanism of cardioprotection: what can we learn from females? Pediatr Cardiol 32: 354–359, 2011. doi: 10.1007/s00246-010-9877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, Guallar E. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol 162: 1037–1049, 2005. doi: 10.1093/aje/kwi330. [DOI] [PubMed] [Google Scholar]
- 41.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 300: 814–822, 2008. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson ME, Vandenput L, Tivesten Å, Norlén AK, Lagerquist MK, Windahl SH, Börjesson AE, Farman HH, Poutanen M, Benrick A, Maliqueo M, Stener-Victorin E, Ryberg H, Ohlsson C. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology 156: 2492–2502, 2015. doi: 10.1210/en.2014-1890. [DOI] [PubMed] [Google Scholar]
- 43.Nordstrom DK. Public health. Worldwide occurrences of arsenic in ground water. Science 296: 2143–2145, 2002. doi: 10.1126/science.1072375. [DOI] [PubMed] [Google Scholar]
- 44.Osorio-Yáñez C, Ayllon-Vergara JC, Arreola-Mendoza L, Aguilar-Madrid G, Hernández-Castellanos E, Sánchez-Peña LC, Del Razo LM. Blood pressure, left ventricular geometry, and systolic function in children exposed to inorganic arsenic. Environ Health Perspect 123: 629–635, 2015. doi: 10.1289/ehp.1307327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul DS, Hernández-Zavala A, Walton FS, Adair BM, Dedina J, Matousek T, Stýblo M. Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes. Toxicol Appl Pharmacol 222: 305–314, 2007. doi: 10.1016/j.taap.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul DS, Walton FS, Saunders RJ, Stýblo M. Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. Environ Health Perspect 119: 1104–1109, 2011. doi: 10.1289/ehp.1003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pellizzon M. Focus: Arsenic in Grain-Based Laboratory Animal Diets and Effects on the Rodent Toxicological Phenotype (Online). New Brunswick, NJ: Research Diets, 2014. https://researchdiets.com/system/resources/W1siZiIsIjIwMTgvMDMvMjkvNmR5d2F3cTlqOF9XU19BcnNlbmljX2luX0xhYm9yYXRvcnlfQW5pbWFsc193ZWIucGRmIl1d/WS_Arsenic_in_Laboratory_Animals_web.pdf. [Google Scholar]
- 48.Penna C, Tullio F, Perrelli MG, Moro F, Abbadessa G, Piccione F, Carriero V, Racca S, Pagliaro P. Ischemia/reperfusion injury is increased and cardioprotection by a postconditioning protocol is lost as cardiac hypertrophy develops in nandrolone treated rats. Basic Res Cardiol 106: 409–420, 2011. doi: 10.1007/s00395-010-0143-y. [DOI] [PubMed] [Google Scholar]
- 49.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, Catalá-López F, Choi JY, Christensen H, Cirillo M, Cooper L Jr, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HM, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DA, Sobngwi E, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 70: 1–25, 2017. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez-Soria P, Broka D, Monks SL, Camenisch TD. Chronic low-level arsenite exposure through drinking water increases blood pressure and promotes concentric left ventricular hypertrophy in female mice. Toxicol Pathol 40: 504–512, 2012. doi: 10.1177/0192623311432297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santra A, Maiti A, Das S, Lahiri S, Charkaborty SK, Mazumder DN. Hepatic damage caused by chronic arsenic toxicity in experimental animals. J Toxicol Clin Toxicol 38: 395–405, 2000. doi: 10.1081/CLT-100100949. [DOI] [PubMed] [Google Scholar]
- 52.Shao Q, Casin KM, Mackowski N, Murphy E, Steenbergen C, Kohr MJ. Adenosine A1 receptor activation increases myocardial protein S-nitrosothiols and elicits protection from ischemia-reperfusion injury in male and female hearts. PLoS One 12: e0177315, 2017. doi: 10.1371/journal.pone.0177315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shao Q, Fallica J, Casin KM, Murphy E, Steenbergen C, Kohr MJ. Characterization of the sex-dependent myocardial S-nitrosothiol proteome. Am J Physiol Heart Circ Physiol 310: H505–H515, 2016. doi: 10.1152/ajpheart.00681.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17: 517–568, 2002. doi: 10.1016/S0883-2927(02)00018-5. [DOI] [Google Scholar]
- 55.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res 98: 403–411, 2006. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 56.Tan M, Schmidt RH, Beier JI, Watson WH, Zhong H, States JC, Arteel GE. Chronic subhepatotoxic exposure to arsenic enhances hepatic injury caused by high fat diet in mice. Toxicol Appl Pharmacol 257: 356–364, 2011. doi: 10.1016/j.taap.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka N, Dalton N, Mao L, Rockman HA, Peterson KL, Gottshall KR, Hunter JJ, Chien KR, Ross J Jr. Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation 94: 1109–1117, 1996. doi: 10.1161/01.CIR.94.5.1109. [DOI] [PubMed] [Google Scholar]
- 58.Tseng CH, Chong CK, Tseng CP, Hsueh YM, Chiou HY, Tseng CC, Chen CJ. Long-term arsenic exposure and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan. Toxicol Lett 137: 15–21, 2003. doi: 10.1016/S0378-4274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- 59.Tsou TC, Tsai FY, Hsieh YW, Li LA, Yeh SC, Chang LW. Arsenite induces endothelial cytotoxicity by down-regulation of vascular endothelial nitric oxide synthase. Toxicol Appl Pharmacol 208: 277–284, 2005. doi: 10.1016/j.taap.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, Hajjar RJ, Kranias EG, Mundiña-Weilenmann C, Mattiazzi A. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovasc Res 73: 689–698, 2007. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Wang CH, Jeng JS, Yip PK, Chen CL, Hsu LI, Hsueh YM, Chiou HY, Wu MM, Chen CJ. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation 105: 1804–1809, 2002. doi: 10.1161/01.CIR.0000015862.64816.B2. [DOI] [PubMed] [Google Scholar]
- 62.Wang M, Crisostomo P, Wairiuko GM, Meldrum DR. Estrogen receptor-alpha mediates acute myocardial protection in females. Am J Physiol Heart Circ Physiol 290: H2204–H2209, 2006. doi: 10.1152/ajpheart.01219.2005. [DOI] [PubMed] [Google Scholar]
- 63.Watson WH, Yager JD. Arsenic: extension of its endocrine disruption potential to interference with estrogen receptor-mediated signaling. Toxicol Sci 98: 1–4, 2007. doi: 10.1093/toxsci/kfm111. [DOI] [PubMed] [Google Scholar]
- 64.Wei H, Bedja D, Koitabashi N, Xing D, Chen J, Fox-Talbot K, Rouf R, Chen S, Steenbergen C, Harmon JW, Dietz HC, Gabrielson KL, Kass DA, Semenza GL. Endothelial expression of hypoxia-inducible factor 1 protects the murine heart and aorta from pressure overload by suppression of TGF-β signaling. Proc Natl Acad Sci USA 109: E841–E850, 2012. doi: 10.1073/pnas.1202081109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]