Abstract
Underperfusion of active skeletal muscle causes metabolites to accumulate and stimulate group III and IV skeletal muscle afferents, which triggers a powerful pressor response termed the muscle metaboreflex. Muscle metaboreflex activation (MMA) during submaximal dynamic exercise in healthy individuals increases arterial pressure mainly via substantial increases in cardiac output (CO). The increases in CO occur via the combination of tachycardia and increased ventricular contractility. Importantly, MMA also elicits substantial central blood volume mobilization, which allows the ventricular responses to sustain the increases in CO. Otherwise preload would fall and the increases in CO could not be maintained. In subjects with systolic heart failure (HF), the ability to increase CO during exercise and MMA is markedly reduced, which has been attributed to impaired ventricular contractility. Whether the ability to maintain preload during MMA in HF is preserved is unknown. Using a conscious chronically instrumented canine model, we observed that MMA in HF is able to raise central blood volume similarly as in normal subjects. Therefore, the loss of the ability to raise CO during MMA in HF is not because of the loss of the ability to mobilize blood volume centrally.
NEW & NOTEWORTHY In normal subjects during dynamic exercise muscle metaboreflex activation elicits large increases in cardiac output that occur via increases in heart rate, ventricular contractility, and, importantly, marked central blood volume mobilization that acts to maintain ventricular preload, thereby allowing the changes in cardiac function to maintain the increases in cardiac output. In subjects with heart failure, the ability to raise cardiac output during muscle metaboreflex activation is impaired. We investigated whether this is because of the inability to maintain ventricular preload. We found that this reflex is still able to elicit large increases in central blood volume, and therefore the limited ability to raise cardiac output likely stems from ventricular dysfunction and not the ability to maintain preload.
Keywords: exercise pressor reflex; ischemic skeletal muscle; muscle blood flow; sympathetically mediated vasoconstriction, venous volume
INTRODUCTION
When oxygen demand within active skeletal muscle exceeds oxygen delivery, metabolites can accumulate and stimulate skeletal muscle afferents that elicit a reflex pressor response termed the muscle metaboreflex (3, 7, 11–13, 18, 28, 32). The muscle metaboreflex can be thought of as a “flow sensitive-flow raising” reflex in that, under normal circumstances during submaximal dynamic exercise, this reflex is triggered by relative hypoperfusion (e.g., “flow sensitive”), and the major mechanism used by the reflex to correct the flow deficit is to raise the total systemic blood flow (e.g., cardiac output-“flow raising”) (21, 24). Muscle metaboreflex activation causes a reflex tachycardia and substantial increases in ventricular contractility (7, 11, 19, 20, 22, 27, 28). However, these alone would be ineffectual in sustaining increases in cardiac output because of the inverse relationship between cardiac output and ventricular filling pressure (29–31); as cardiac output rises there is a translocation of blood volume from the central venous side to the peripheral arterial side of the circulation, and cardiac filling pressure falls (10, 17, 29, 31). Thus, without substantial central blood volume mobilization, the ability to sustain increases in cardiac output are limited. Translocation of blood volume centrally will sustain ventricular filling pressure in the face of increased cardiac output or will raise central venous pressure if cardiac output is unchanged. Previously, we demonstrated that, in normal canines, the muscle metaboreflex is capable of eliciting profound central blood volume mobilization (29) with a strength of over three times that of the carotid arterial baroreflex (5, 29). These studies (5, 29) were performed with cardiac output held constant, and increases in central venous pressure were used to gauge increases in central blood volume mobilization. Thus, during metaboreflex activation, the combination of increases in ventricular contractility and substantial central blood volume mobilization acts to sustain or even slightly increase stroke volume, thereby causing marked increases in cardiac output with the reflex tachycardia (7, 20).
In subjects with systolic heart failure, the ability to raise cardiac output is often markedly limited. With metaboreflex engagement, a reflex tachycardia occurs; however, stroke volume often falls, which thereby attenuates the ability to raise cardiac output (2, 8, 9, 11, 14, 22, 26). The inherent ventricular dysfunction that occurs in systolic heart failure limits the ability to raise ventricular contractility, which is also exacerbated by enhanced vasoconstriction of the coronary vasculature limiting myocardial perfusion and oxygen delivery (1, 6). However, whether impaired ability to elicit central blood volume mobilization during exercise also occurs in these subjects is unclear. If so, this could contribute to the reduced ability to raise cardiac output, especially so since preload sensitivity, the ability to increase stroke volume with increases in ventricular filling (Frank-Starling mechanism), is likely reduced (15). Therefore, our aim was to investigate whether metaboreflex-induced increases in central blood volume mobilization are reduced in subjects with heart failure with reduced ejection fraction.
METHODS
Experiments were conducted on conscious adult mongrel dogs (n = 5, weight 21–26 kg, 2 female, 3 male) selected for their willingness to run on a treadmill. We have previously shown that gender has little impact on the strength and mechanisms of the muscle metaboreflex in canines (16). All procedures were reviewed and approved by the Wayne State University Animal Care Committee and in accordance to the National Institutes of Health guidelines.
Surgical Preparation
The animals were prepared in a series of three sterile surgical procedures performed with a 10- to 14-day interval between surgeries. Preoperative care included acepromazine (0.2 mg/kg im) 30 min before anesthetic induction for preoperative sedation. Anesthesia was induced with intravenous thiopental sodium (25 mg/kg) and maintained with isoflurane gas (1–3%). Postoperative analgesia was delivered via transdermal fentanyl patch (Duragesic, 125–150 μg/h for 72 h; Janssen Pharmaceutica). Prophylactic antibiotic therapy included cefazolin (500 mg iv) was given immediately before and after each surgery and cephalexin (30 mg/kg by oral administration, two times/day). During recovery from surgery, buprenorphine (0.015 mg/kg iv), maleate acepromazine (0.1 mg/kg im), and oral etorolac (10–15 mg·kg−1·day−1, oral administration) were administered, as needed, for supplemental analgesia and sedation. Experiments were conducted at least 1 wk after the last surgery.
First surgery.
The thoracic cavity was opened via a left thoracotomy (4th intercostal space). The pericardium was opened, and a ultrasonic perivascular flow probe (20PAU; Transonic Systems) was placed around the ascending aorta to measure cardiac output (CO). Three stainless steel ventricular pacing electrodes were sutured to the apex of the left ventricle for subsequent ventricular pacing. The pericardium was reapproximated loosely over the heart, the flow probe cable and electrodes were tunneled subcutaneously and exteriorized between the scapulas, and the chest was closed in layers.
Second surgery.
Via retroperitoneal approach, an incision was made in the left flank cranial to the iliac crest, to expose the abdominal aorta and implant an ultrasonic perivascular flow probe (10PAU; Transonic Systems) around the terminal aorta for measuring hind limb blood flow (HLBF). All arterial side branches between the common iliac arteries and the flow probe were ligated and severed. In addition, two perivascular hydraulic occluders (In Vivo Metrics) were positioned around the terminal aorta (distal to the flow probe) for HLBF reductions. A polyvinyl catheter (19 gauge, S54-HL, Tygon; Norton) was inserted through a ligated lumbar artery and secured in the terminal aorta, cranial to the probe and occluders to measure mean arterial pressure (MAP). The flow probe cable, vascular occluder tubing, and catheter were tunneled subcutaneously and exteriorized between the scapulas.
Third surgery.
In the final surgical session, a catheter was introduced in the jugular vein and advanced to the arterial-caval junction to measure central venous pressure (CVP). This catheter was also tunneled subcutaneously and exteriorized between the scapulas.
Experimental Procedures
All experiments were performed after the animals had fully recovered from instrumentation (i.e., active, afebrile, and of good appetite). Before the experimental sessions, each animal was transported to the laboratory, allowed to roam freely for 15–30 min, and then led to the treadmill. The blood flow probes were connected to the flowmeters (Transonic System). Heart rate (HR) was computed by a cardiotachometer triggered by the CO signal. The arterial and venous catheters were connected to pressure transducers (Transpac IV; Abbott Laboratories).
All data were recorded on a computerized analog-to-digital recording system for subsequent off-line analyses. For a given experimental session, steady-state data were collected during rest, during mild exercise (3.2 km/h) with unrestricted blood flow to the hind limbs, and during muscle metaboreflex activation (MMA). MMA was elicited by progressive graded reductions in HLBF via partial inflations of the vascular occluders placed on the terminal aorta as described in previous studies (2, 3, 11). Data were acquired with the animal standing quietly on the treadmill until the measured variables achieved steady-state values. The treadmill was then started at 3.2 km/h. Once steady state was achieved, hind limb blood flow was decreased by partially inflating the vascular occluders. The partial occlusion was sustained until the measured variables achieved steady values. Further reductions in hind limb perfusion were then imposed after each steady-state level was achieved until a large rise in systemic arterial pressure was elicited. Steady state was generally reached within 3–5 min.
Mild exercise with normal cardiac function.
Steady-state data were collected at rest, mild exercise (3.2 km/h) with unrestricted blood flow to the hind limbs, and MMA.
Mild exercise with constant HR.
On another day, the animals were connected to a fixed-rate pacemaker built in our laboratory and paced at 225 beats/min to keep HR constant during MMA. Steady-state data were recorded at rest before and after pacing, and during exercise and MMA with pacing.
Mild exercise with constant HR plus limited contractility.
On another day, the animals were administered atenolol (2 mg/kg iv), connected to a fixed-rate pacemaker, and paced at 225 beats/min to keep HR and ventricular contractility constant during MMA, which thereby maintains CO essentially constant (29). Steady-state data were recorded at rest before and after pacing plus atenolol, and during exercise and MMA with pacing plus atenolol.
Heart failure induction.
Heart failure (HF) was induced by rapid left ventricular pacing at 225–250 beats/min for ∼30 days. Threshold values for pacing voltage and pulse width that were necessary for ventricular capture were determined by means of a pacing system analyzer (model 5311b; Medtronic). The pacemakers were adjusted to 1.5 times the threshold values. The presence of HF was determined from hemodynamic values, such as the increase in CVP, tachycardia, and decreases in MAP and CO.
Each dog completed the same series of experiments described previously, before and after induction of HF; thus, each animal served as its own control.
Data Analysis
MAP, HR, CVP, and CO were continuously recorded during experiments. The vascular conductance of the nonischemic regions [nonischemic vascular conductance (NIVC)] was calculated as NIVC = (CO − HLBF)/(MAP − CVP). Stroke volume (SV) was calculated as CO/HR. Signals were averaged during steady-state levels over 1- to 2-min intervals. Several experiments were performed for each animal in each condition and treatment. For each experiment, the values at rest, during free-flow exercise, and maximal metaboreflex activation were determined. These values within a given permutation of condition and treatment were averaged across experiments for each animal. These average values were then averaged across all animals to determine the mean hemodynamic values for the population studied in each combination. Therefore, each animal only contributed one time to the population mean and served as its own control. Averaged responses for each animal were analyzed via three-way repeated-measures ANOVA to compare hemodynamic data for settings [rest, exercise (Ex), and MMA], and/or treatment (normal cardiac function, constant HR, constant HR plus limited contractility), and/or condition (control, HF) effects. In the event of a significant setting/treatment/condition interaction, a C-matrix test for simple effects was performed. A linear regression analysis was performed on the average values of the changes in CO versus the changes in CVP that occurred in response to metaboreflex activation (see Fig. 3) in each setting before and after HF induction. Data are reported as means ± SE, and statistical significance was ascribed as P < 0.05.
Fig. 3.
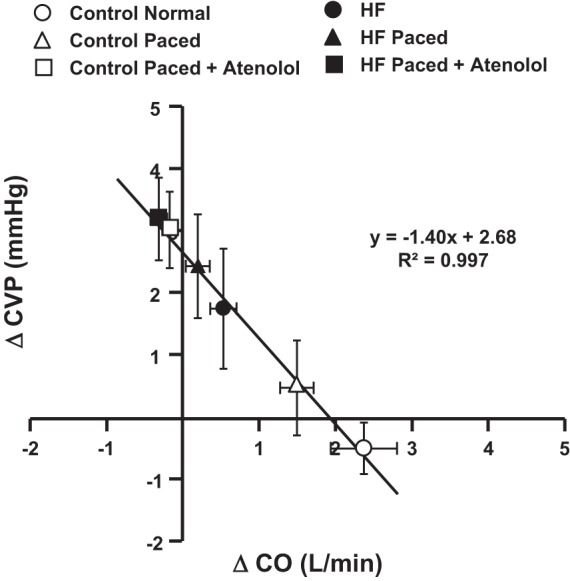
Relationship between changes in central venous pressure (ΔCVP) and cardiac output (ΔCO) in response to muscle metaboreflex activation under intrinsic heart rate (HR) and contractility responses (circles), constant HR by rapid ventricular pacing (triangles), and pacing plus β1-blockade (squares); in control (white symbols) and heart failure (black symbols) conditions. Line and equation are the results of linear regression analysis, N = 5.
RESULTS
ANOVA of HLBF data showed main effects of setting (rest, Ex, and MMA), treatment (normal, paced, and paced plus atenolol), and condition (control and HF), with no significant interaction effect between setting, treatment, and/or condition (Table 1).
Table 1.
Hind limb blood flow
| Rest | Ex | MMA | |
|---|---|---|---|
| Control | |||
| Normal | 0.63 ± 0.07 | 1.23 ± 0.08 | 0.39 ± 0.08 |
| Paced | 0.67 ± 0.06 | 1.28 ± 0.11 | 0.35 ± 0.02 |
| Paced + atenolol | 0.55 ± 0.05 | 1.07 ± 0.07 | 0.27 ± 0.02 |
| Heart Failure | |||
| Normal | 0.50 ± 0.06 | 0.98 ± 0.07 | 0.44 ± 0.03 |
| Paced | 0.45 ± 0.07 | 0.98 ± 0.13 | 0.46 ± 0.04 |
| Paced + atenolol | 0.30 ± 0.03 | 0.62 ± 0.06 | 0.34 ± 0.02 |
Hind limb blood flow (HLBF; l/min ± SE) during rest, mild exercise (Ex), and muscle metaboreflex activation (MMA) in control and heart failure conditions under normal heart rate (HR) and contractility responses, constant HR by rapid ventricular pacing (paced), and constant HR in association with limited contractility by pacing plus β1-blockade (paced plus atenolol). Significant main effect of setting (rest, Ex, MMA) and significant main effect of treatment (no treatment, paced, paced + atenolol). P < 0.05, N = 5.
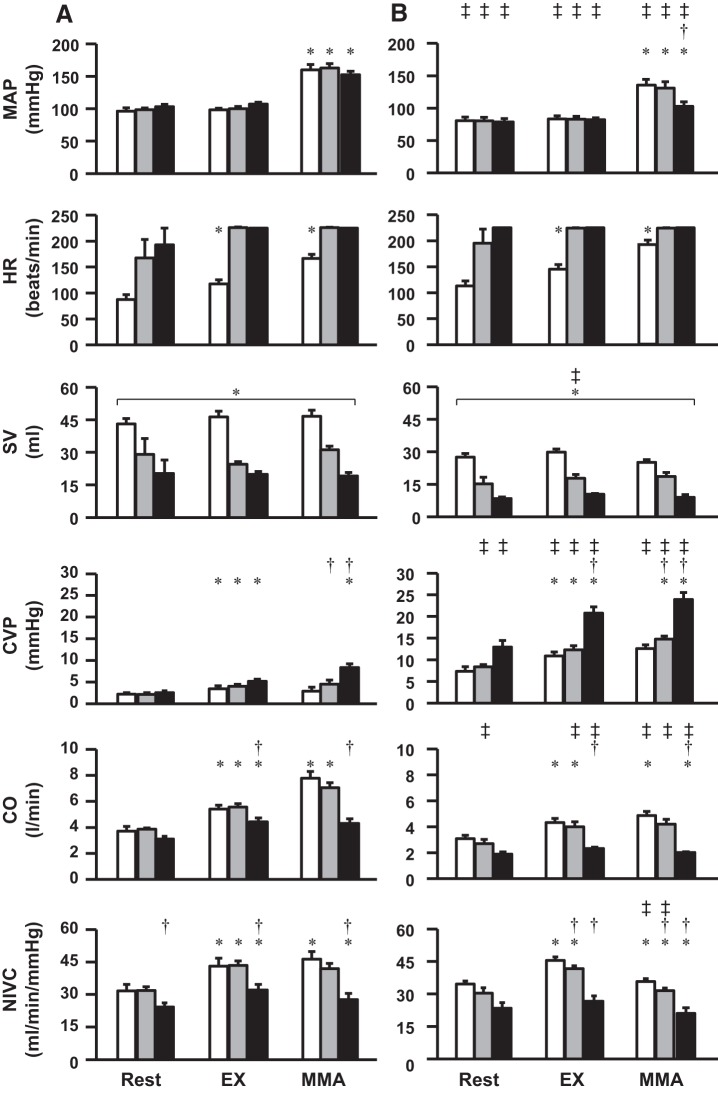
Figure 1 shows the average levels during steady state of MAP, HR, SV, CVP, CO, and NIVC during rest, Ex, and MMA, under normal circumstances (normal HR and contractility responses), during pacing (HR held constant by rapid ventricular pacing), and during pacing combined with β1-blockade (constant HR in association with limited contractility); in the same animals before (control) and after induction of HF. In control experiments under normal HR and contractility responses, MMA increased MAP, CO, and NIVC. MMA caused no significant change in CVP. ANOVA for SV showed no significant interaction effect, with only a significant main effect of settings (rest, Ex, MMA). MMA activation when HR was maintained constant caused similar increases in MAP and CO as during control experiments in normal animals. In this setting the increases in CO occurred via increases in SV at constant HR rather than increases in HR with relatively stable SV as occurred in control experiments. There were no significant changes in CVP with MMA at constant HR. Pacing plus β1-blockade virtually abolished any increase in CO during MMA activation, and a significant increase in CVP occurred. After induction of HF, hemodynamic variables, with the exception of CVP, exhibited the same pattern of responses to exercise and MMA observed in control experiments although generally smaller responses occurred. CVP was elevated at rest, during exercise, and with MMA from the values before induction of HF experiments.
Fig. 1.
Shown are mean arterial pressure (MAP), heart rate (HR), stroke volume (SV), central venous pressure (CVP), cardiac output (CO), and nonischemic vascular conductance (NIVC); during rest, mild exercise (Ex), and muscle metaboreflex activation (MMA); under intrinsic HR and contractility responses (white bars), constant HR by rapid ventricular pacing (gray bars), and constant HR in association with limited contractility by pacing plus β1-blockade (black bars); in control (A) and heart failure (B) conditions.*P < 0.05 vs. previous setting (Ex vs. rest; MMA vs. Ex); †P < 0.05 vs. no treatment (pacing vs. no treatment; pacing plus atenolol vs. no treatment) within the same setting and condition; ‡P < 0.05 vs. previous condition (heart failure vs. control) within same setting and treatment; brackets with *, significant main effect of settings (rest, Ex, MMA); brackets with ‡significant main effect of condition (heart failure, control), N = 5.
Figure 2 shows the relationship between CO and CVP during free-flow exercise and MMA in all settings and conditions. The broken lines show the responses in CVP and CO from exercise to peak muscle metaboreflex activation. Data points represent the average for CVP and CO under normal circumstances (normal HR and contractility responses), during pacing (HR held constant by rapid ventricular pacing), and during pacing combined with β1-blockade in the same animals before (control) and after induction of HF. For example, in control experiments in normal animals (Fig. 2, bottom right), on average, MMA increased CO by >2 l/min with little change in CVP. Similar responses occurred in normal animals with constant HR. However, when pacing was combined with β1-blockade, baseline CO was reduced and CVP increased during exercise, and, with MMA, no significant increase in CO occurred and CVP rose significantly. In HF, in control experiments, the MMA-induced rise in CO was markedly attenuated, and CVP tended to rise. With constant HR, MMA induced little change in CO, and a significant rise in CVP occurred; with additional β1-blockade CO tended to fall slightly with MMA while CVP rose.
Fig. 2.
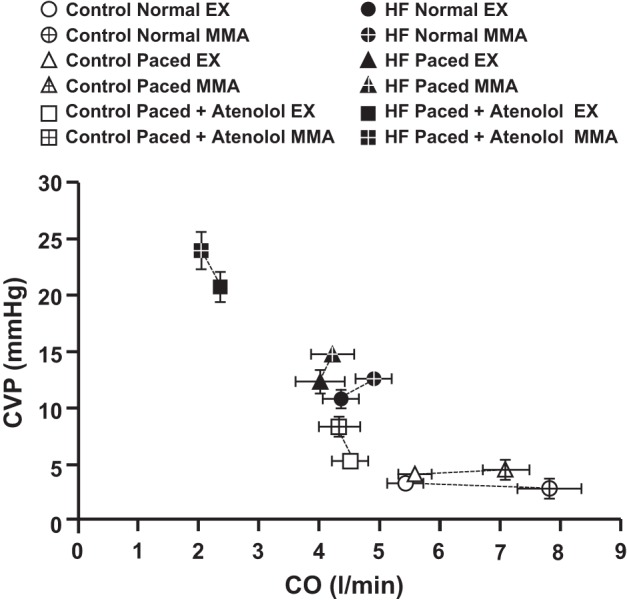
Average levels of central venous pressure (CVP) and cardiac output (CO) during steady-state exercise (solid symbols) and peak muscle metaboreflex activation (crossed symbols); under intrinsic heart rate (HR) and contractility responses (circles), constant HR by rapid ventricular pacing (triangles), and constant HR in association with limited contractility by pacing plus β1-blockade (squares); in control (white symbols) and heart failure (black symbols), N = 5.
Figure 3 shows the inverse relationship between the average change in CO and the average change in CVP observed during peak MMA in each setting and condition. A linear regression analysis was performed on these points showing a highly linear relationship with R2 > 0.99. When large increases in CO occurred with MMA, the changes in CVP were very small. Conversely, when the rise in CO was attenuated or abolished, the rise in CVP with MMA increased substantially. The y-intercept was Δ2.7 mmHg, indicating that, if there was no change in CO, then the muscle metaboreflex would increase CVP by 2.7 mmHg. This increase in CVP with MMA at constant CO was attenuated by 1.4 mmHg for every 1.0 l/min that CO rose during MMA (regression slope) such, during control experiments in normal animals, with the observed increases in CO (>2 l/min), very little change in CVP occurred.
DISCUSSION
This is the first study to investigate whether HF affects the ability of the muscle metaboreflex to increase central blood volume mobilization. We found that, after induction of HF, similar increases in central blood volume mobilization occurred during metaboreflex activation as in normal subjects when the responses are normalized to the ability to raise CO. Thus, the impaired ability to raise CO during metaboreflex activation in subjects with HF likely does not stem from a depressed ability to maintain cardiac filling pressures but likely stems from the impaired ability to raise ventricular contractility because of both the inherent ventricular dysfunction and the accentuated reflex coronary vasoconstriction, which limits myocardial blood flow and O2 delivery (1, 6, 8, 11, 26).
Because of the inverse relationship between CO and central blood volume, many previous studies investigating control of central blood volume mobilization were performed in acute experiments using an anesthetized animal preparation on cardiac bypass at constant pump speed (10, 17). In this setting with “CO” constant, changes in the volume of a central reservoir supplying the pump directly reflected changes in blood volume mobilization. Such an experimental preparation would be extremely challenging to maintain in a conscious subject during dynamic exercise, and this has limited our understanding of the control of central blood volume during exercise. However, if CO can be controlled, then changes in central venous pressure can be used as an index of changes in central blood volume (5). Wyss et al. (33) developed an elegant canine model to maintain CO virtually constant on a beat-by-beat basis. After induction of atrioventricular block, a computer continuously monitored stroke volume on a beat-by-beat basis and then adjusted ventricular R-R interval such that CO was held constant within a limited range (5, 23, 25). Sheriff et al. (31) used this preparation to show that the slope of the linear inverse relationship between CO and central venous pressure remains similar between rest and mild to moderate dynamic exercise but that this line is shifted to higher CO and CVP as workload increases. Because this inverse relationship between CO and CVP exists, the observation that CVP remains essentially constant during metaboreflex activation despite large increases in CO is indirect evidence that substantial blood volume mobilization must occur. Otherwise CVP should fall (11, 20, 29). If filling pressure fell, it is likely that large increases in CO could not be sustained. Figure 3 shows that the magnitude of the increases in CVP with MMA is inversely dependent on how much CO increases. During MMA in control conditions, the large reflex-induced central blood volume mobilization (which would increase CVP) is matched by the translocation of blood volume from the central veins to the peripheral circulation caused by the large increase in CO (which would decrease CVP), and thus little or no change in CVP occurs. As the rise in CO with MMA is attenuated, the reflex central blood volume mobilization exceeds the translocation of volume away from the central veins (via CO), and the increases in CVP become progressively greater such that, when no increase in CO occurs, the reflex rise in CVP is ~2.7 mmHg (Fig. 3 regression y-intercept).
In our initial experiments, we attempted a more simplified model, and, rather than induce atrioventricular block, we maintained heart rate constant via overdrive pacing of the ventricles at a rate much higher than ever achieved during metaboreflex activation at mild workloads (225 beats/min). This approach completely failed to maintain CO constant because, although heart rate remained constant, stroke volume rose markedly, and similar increases in CO occurred (20). Because CVP did not significantly change, we concluded that this increase in stroke volume at constant heart rate reflected large increases in ventricular contractility (20). We then attenuated the increase in contractility by using a β-adrenergic receptor antagonist in combination with constant ventricular pacing. This combination successfully abolished any significant increase in CO during metaboreflex activation, and substantial metaboreflex-induced increases in CVP occurred (29).
After induction of HF, the normal rise in CO with metaboreflex activation was markedly attenuated, and significant increases in CVP occurred, again indirectly indicating substantial central blood volume mobilization (29). Pacing alone virtually abolished the small increases in CO seen with metaboreflex activation in HF, and similar increases in CVP occurred as seen in control experiments with combined constant HR and β-blockade. When we added β-blockade to constant HR in the animals after induction of HF, there were small decreases in CO with metaboreflex activation, likely because of the increased afterload and poor ventricular function, and the increases in CVP were slightly larger. The increases in CVP with metaboreflex activation at constant CO were similar to those predicted to occur based on the normal increases in CO and the slope of the CO-CVP relationship previously observed (31). Thus, the inability to raise CO during metaboreflex activation in HF does not stem from a loss of the ability to raise central blood volume mobilization but likely reflects the poor ventricular performance.
Limitations
HF changes a plethora of cardiovascular parameters, including baseline levels of CO, stroke volume, regional blood flows, arterial and central venous pressures as well as sympathetic activity and plasma levels of vasoactive hormones. We used changes in central venous pressure as an index of changes in central blood volume mobilization, and it is possible that venous compliance was different after induction of HF. However, Balmain et al. concluded that, whereas venous compliance was reduced in human subjects diagnosed with HF with preserved ejection fraction, it was unchanged in patients with HF with reduced ejection fraction (4). The rapid ventricular pacing model causes marked reductions in ejection fraction and systolic failure as evident by the markedly lower stroke volume, tachycardia, and low CO (11, 26). However, we did not measure venous compliance in these studies, so it is possible that the same change in CVP could be caused by a smaller central blood volume mobilization if central venous compliance was reduced. Inasmuch as filling pressure is an important determinant of cardiac preload, our observations that muscle metaboreflex activation can still elicit increases in CVP indicate that the impaired ability to raise CO does not likely stem from a failure to adequately support ventricular preload.
GRANTS
This work was supported by National Heart, Lung and, Blood Institute Grants HL-55473 and HL-126706.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S.O’L. conceived and designed research; D.S.O’L. and R.A.A. performed experiments; D.S.O’L., D.S., and R.A.A. analyzed data; D.S.O’L., D.S., and R.A.A. interpreted results of experiments; D.S.O’L. and D.S. prepared figures; D.S.O’L. drafted manuscript; D.S.O’L., D.S., and R.A.A. edited and revised manuscript; D.S.O’L., D.S., and R.A.A. approved final version of manuscript.
REFERENCES
- 1.Ansorge EJ, Augustyniak RA, Perinot ML, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, O’Leary DS. Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. Am J Physiol Heart Circ Physiol 288: H1381–H1388, 2005. doi: 10.1152/ajpheart.00985.2004. [DOI] [PubMed] [Google Scholar]
- 2.Augustyniak RA, Ansorge EJ, Kim JK, Sala-Mercado JA, Hammond RL, Rossi NF, O’Leary DS. Cardiovascular responses to exercise and muscle metaboreflex activation during the recovery from pacing-induced heart failure. J Appl Physiol (1985) 101: 14–22, 2006. doi: 10.1152/japplphysiol.00072.2006. [DOI] [PubMed] [Google Scholar]
- 3.Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O’Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001. doi: 10.1152/ajpheart.2001.280.4.H1645. [DOI] [PubMed] [Google Scholar]
- 4.Balmain S, Padmanabhan N, Ferrell WR, Morton JJ, McMurray JJ. Differences in arterial compliance, microvascular function and venous capacitance between patients with heart failure and either preserved or reduced left ventricular systolic function. Eur J Heart Fail 9: 865–871, 2007. doi: 10.1016/j.ejheart.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Bennett TD, Wyss CR, Scher AM. Changes in vascular capacity in awake dogs in response to carotid sinus occlusion and administration of catecholamines. Circ Res 55: 440–453, 1984. doi: 10.1161/01.RES.55.4.440. [DOI] [PubMed] [Google Scholar]
- 6.Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O’Leary DS. Muscle metaboreflex-induced coronary vasoconstriction limits ventricular contractility during dynamic exercise in heart failure. Am J Physiol Heart Circ Physiol 304: H1029–H1037, 2013. doi: 10.1152/ajpheart.00879.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crisafulli A, Piras F, Filippi M, Piredda C, Chiappori P, Melis F, Milia R, Tocco F, Concu A. Role of heart rate and stroke volume during muscle metaboreflex-induced cardiac output increase: differences between activation during and after exercise. J Physiol Sci 61: 385–394, 2011. doi: 10.1007/s12576-011-0163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- 9.Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJ, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc 35: 221–228, 2003. doi: 10.1249/01.MSS.0000048639.02548.24. [DOI] [PubMed] [Google Scholar]
- 10.Guyton AC, Douglas BH, Langston JB, Richardson TQ, Abernathy B. Instantaneous increase in mean circulatory pressure and cardiac output at onset of muscular activity. Circ Res 11: 431–441, 1962. doi: 10.1161/01.RES.11.3.431. [DOI] [PubMed] [Google Scholar]
- 11.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O’Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000. doi: 10.1152/ajpheart.2000.278.3.H818. [DOI] [PubMed] [Google Scholar]
- 12.Hansen J, Thomas GD, Jacobsen TN, Victor RG. Muscle metaboreflex triggers parallel sympathetic activation in exercising and resting human skeletal muscle. Am J Physiol Heart Circ Physiol 266: H2508–H2514, 1994. doi: 10.1152/ajpheart.1994.266.6.H2508. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman MP. Metaboreflex control of the heart. J Physiol 588: 1037–1038, 2010. doi: 10.1113/jphysiol.2010.189241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaur J, Senador D, Krishnan AC, Hanna HW, Alvarez A, Machado TM, O’Leary DS. Muscle metaboreflex-induced vasoconstriction in the ischemic active muscle is exaggerated in heart failure. Am J Physiol Heart Circ Physiol 314: H11–H18, 2018. doi: 10.1152/ajpheart.00375.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komamura K, Shannon RP, Ihara T, Shen YT, Mirsky I, Bishop SP, Vatner SF. Exhaustion of Frank-Starling mechanism in conscious dogs with heart failure. Am J Physiol Heart Circ Physiol 265: H1119–H1131, 1993. doi: 10.1152/ajpheart.1993.265.4.H1119. [DOI] [PubMed] [Google Scholar]
- 16.Laprad SL, Augustyniak RA, Hammond RL, O’Leary DS. Does gender influence the strength and mechanisms of the muscle metaboreflex during dynamic exercise in dogs? Am J Physiol Regul Integr Comp Physiol 276: R1203–R1208, 1999. doi: 10.1152/ajpregu.1999.276.4.R1203. [DOI] [PubMed] [Google Scholar]
- 17.Levy MN. The cardiac and vascular factors that determine systemic blood flow. Circ Res 44: 739–747, 1979. doi: 10.1161/01.RES.44.6.739. [DOI] [PubMed] [Google Scholar]
- 18.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol 589: 6191–6204, 2011. doi: 10.1113/jphysiol.2011.214429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Leary DS. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol (1985) 74: 1748–1754, 1993. doi: 10.1152/jappl.1993.74.4.1748. [DOI] [PubMed] [Google Scholar]
- 20.O’Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol Heart Circ Physiol 275: H220–H224, 1998. doi: 10.1152/ajpheart.1998.275.1.H220. [DOI] [PubMed] [Google Scholar]
- 21.O’Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physioll 276: H1399–H1403, 1999. doi: 10.1152/ajpheart.1999.276.4.H1399. [DOI] [PubMed] [Google Scholar]
- 22.O’Leary DS, Sala-Mercado JA, Augustyniak RA, Hammond RL, Rossi NF, Ansorge EJ. Impaired muscle metaboreflex-induced increases in ventricular function in heart failure. Am J Physiol Heart Circ Physiol 287: H2612–H2618, 2004. doi: 10.1152/ajpheart.00604.2004. [DOI] [PubMed] [Google Scholar]
- 23.O’Leary DS, Scher AM. Arterial pressure control after chronic carotid sinus denervation. Am J Physiol Heart Circ Physiol 255: H910–H916, 1988. doi: 10.1152/ajpheart.1988.255.4.H910. [DOI] [PubMed] [Google Scholar]
- 24.O’Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995. doi: 10.1152/ajpheart.1995.268.3.H980. [DOI] [PubMed] [Google Scholar]
- 25.Raymundo H, Scher AM, O’Leary DS, Sampson PD. Cardiovascular control by arterial and cardiopulmonary baroreceptors in awake dogs with atrioventricular block. Am J Physiol Heart Circ Physiol 257: H2048–H2058, 1989. doi: 10.1152/ajpheart.1989.257.6.H2048. [DOI] [PubMed] [Google Scholar]
- 26.Sala-Mercado JA, Hammond RL, Kim JK, McDonald PJ, Stephenson LW, O'Leary DS. Heart failure attenuates muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 292: H2159–H2166, 2006. doi: 10.1152/ajpheart.01240.2006. [DOI] [PubMed] [Google Scholar]
- 27.Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O'Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290: H751–H757, 2006. doi: 10.1152/ajpheart.00869.2005. [DOI] [PubMed] [Google Scholar]
- 28.Sala-Mercado JA, Spranger MD, Abu-Hamdah R, Kaur J, Coutsos M, Stayer D, Augustyniak RA, O’Leary DS. Attenuated muscle metaboreflex-induced increases in cardiac function in hypertension. Am J Physiol Heart Circ Physiol 305: H1548–H1554, 2013. doi: 10.1152/ajpheart.00478.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheriff DD, Augustyniak RA, O’Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998. doi: 10.1152/ajpheart.1998.275.3.H767. [DOI] [PubMed] [Google Scholar]
- 30.Sheriff DD, Luo Z. Capacitive function of the heart: influence of acute changes in heart volume on mean right atrial pressure. Am J Physiol Heart Circ Physiol 272: H553–H558, 1997. doi: 10.1152/ajpheart.1997.272.1.H553. [DOI] [PubMed] [Google Scholar]
- 31.Sheriff DD, Zhou XP, Scher AM, Rowell LB. Dependence of cardiac filling pressure on cardiac output during rest and dynamic exercise in dogs. Am J Physiol Heart Circ Physiol 265: H316–H322, 1993. doi: 10.1152/ajpheart.1993.265.1.H316. [DOI] [PubMed] [Google Scholar]
- 32.Spranger MD, Sala-Mercado JA, Coutsos M, Kaur J, Stayer D, Augustyniak RA, O’Leary DS. Role of cardiac output versus peripheral vasoconstriction in mediating muscle metaboreflex pressor responses: dynamic exercise versus postexercise muscle ischemia. Am J Physiol Regul Integr Comp Physiol 304: R657–R663, 2013. doi: 10.1152/ajpregu.00601.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyss CR, Bennett TD, Scher AM. Beat-by-beat control of cardiac output in awake dogs with atrioventricular block. Am J Physiol Heart Circ Physiol 242: H1118–H1121, 1982. doi: 10.1152/ajpheart.1982.242.6.H1118. [DOI] [PubMed] [Google Scholar]