Abstract
Maternal obesity is correlated with cardiovascular disease in offspring, with a 1.3-fold increase in events observed in offspring of obese women. We have observed that obesity-exposed oocytes demonstrate impaired mitophagy and transmit damaged mitochondria to the offspring. Accordingly, we hypothesized that maternal obesity induces cardiac mitochondrial dysfunction in the offspring via transgenerational inheritance of abnormal oocyte mitochondria. We mated female mice fed a high-fat/high-sucrose (HFS) diet (or chow) with chow-fed males and assessed cardiac structure and function in their descendants that were chow fed in each generation. All F1 to F3 descendants bred via the female in each generation were nonobese and demonstrated cardiac mitochondrial abnormalities with crystal rarefaction and reduced oxygen consumption pointing to a transgenerational effect, while obese F0 dams’ hearts were unaffected. Furthermore, male offspring from F1 to F3 generations and female F1 and F2 offspring developed increased left ventricular (LV) mass (vs. chow-fed controls). Increased LV mass was also observed in offspring generated by in vitro fertilization of obesity-exposed oocytes and gestation in nonobese surrogates, ruling out a gestational environment effect. Contrary to our hypothesis, male F1 also transmitted these effects to their offspring, ruling out maternal mitochondria as the primary mode of transmission. We conclude that transmission of obesity-induced effects in the oocyte nucleus rather than abnormal mitochondria underlie transgenerational inheritance of cardiac mitochondrial defects in descendants of obese females. These findings will spur exploration of epigenetic alterations in the oocyte genome as potential mechanisms whereby a family history of maternal obesity predisposes to cardiovascular disease in humans.
Listen to this article's corresponding podcast at https://ajpheart.podbean.com/e/maternal-obesity-induces-transgenerational-cardiac-mitochondrial-dysfunction/.
Keywords: developmental origins of health and disease, maternal obesity, mitochondria dysfunction, transgenerational inheritance
INTRODUCTION
Heart disease is the leading cause of death in the United States, accounting for one in every four deaths (22). A major risk factor for heart disease is obesity. Several studies in humans, nonhuman primates, and rodents demonstrate an association between maternal obesity and cardiovascular disease risk in offspring (3, 18, 21, 25). For example, recent epidemiological studies found that offspring of overweight and obese women were at 1.15- and 1.30-fold increased risk of cardiovascular events, respectively (25). Given that ~35% of reproductive-age women are obese (14), it is important to determine the extent of cardiovascular dysfunction in offspring and define the mechanisms by which it occurs.
To assess the effects of maternal obesity on offspring health, we have previously described a model in which female mice [designated filial (F) 0] are fed a high-fat/high-sucrose (HFS) diet for 6 wk leading to obesity and mated to males fed a chow (Con) diet while continuing on the HFS diet during pregnancy and lactation (5, 7, 26). The progeny of HFS-fed F0 mice had mitochondrial dysfunction in their skeletal muscle, despite being chow fed and nonobese (26). Specifically, we noted abnormal mitochondrial morphology, depressed respiration, and decreased concentration of electron transport system proteins (26). Additionally, the oocytes of both HFS-fed F0 mice and their F1 progeny had increased mitochondrial size, decreased mitochondrial DNA, and altered accumulation of mitochondrial dynamics-regulating proteins. Given that obesity-exposed oocytes are incapable of removing damaged mitochondria by autophagy (7), these findings suggest that obesity leads to accumulation of defective mitochondria in the oocyte that are passed on via maternal inheritance to offspring. In this study, we tested the hypothesis that maternal obesity induces cardiac mitochondrial dysfunction in the offspring via transgenerational inheritance of abnormal oocyte mitochondria.
METHODS
All experiments were approved by the Institutional Animal Care and Use Committee at the Washington University School of Medicine (St. Louis, MO).
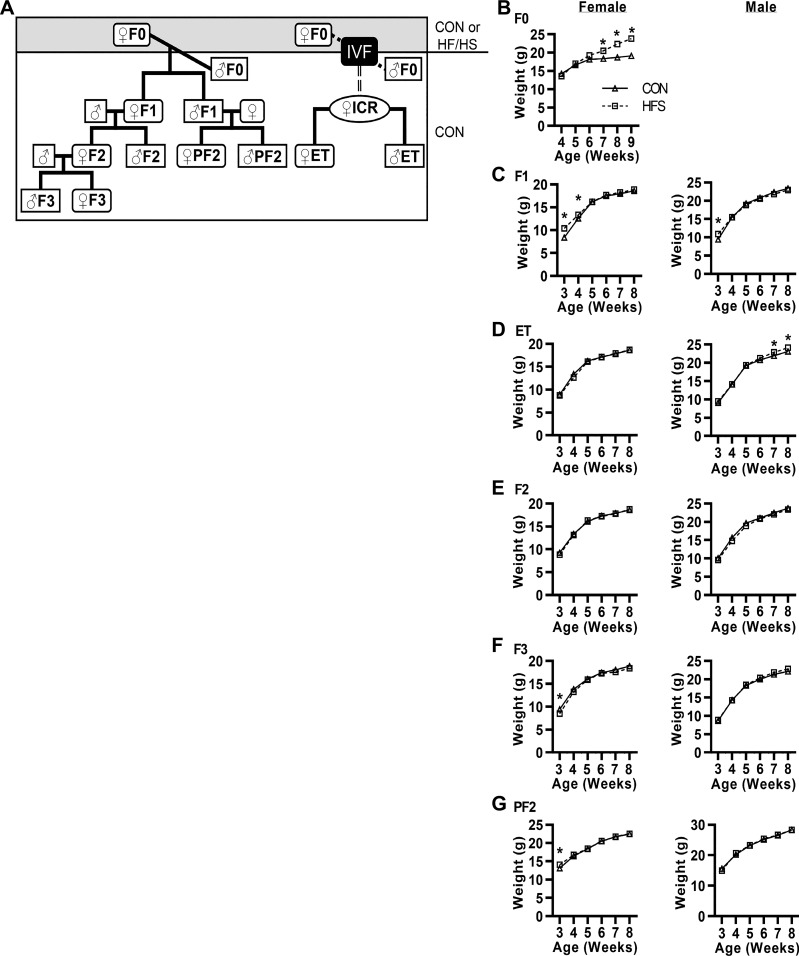
Mouse breeding and feeding scheme.
C57BL/6J mice were obtained from The Jackson Laboratory (stock 000664; Bar Harbor, ME), and ICR mice were obtained from Envigo [Hsd: ICR (CD-1)]. Four-week-old female C57BL/6J mice were randomized to a HFS diet [Test Diet 58R3; by weight 36% fat, 35% carbohydrates (17% sucrose), 20% protein] or a Con diet [PicoLab Rodent Diet 20; by weight 11% fat, 53% carbohydrates (3% sucrose), 21% protein] (26). After 6 wk, a Con-fed 8-wk-old male was mated with the HFS-fed female in proestrus or estrus for 24 h to generate F1 offspring. After weaning, the F1 offspring were fed a Con diet. Some F1 female mice were mated with Con-fed males (both at 8 wk of age) to obtain F2 offspring. Similarly, F2 offspring were fed a Con diet, and some F2 females were mated with Con-fed male mice to produce F3 offspring. Some F1 male mice were mated with Con-fed females to produce paternal F2 (PF2) progeny. In all cases, breeders were selected such that each mouse was from a unique litter. All mice were housed in a pathogen-free barrier facility with a 12-h:12-h light-dark cycle. Food and water were available ad libitum. Weekly body weights were obtained in the mouse facility, and mice were weighed every second day of the week.
In vitro fertilization and embryo transfer.
Ten-week-old female mice were superovulated, and oocytes were collected and added to capacitated spermatozoa to perform in vitro fertilization as previously described (5). Two-cell embryos were transferred into recipient females, resulting in embryo transfer (ET) offspring (2). Briefly, pseudo-pregnant ICR mice were anesthetized, the ovary and infundibulum were gently isolated through a small incision, and ~12 embryos were injected into the ampulla. The body wall was sutured, and the skin was closed with clips.
Echocardiography.
Echocardiographic studies were conducted as previously described (11). Mice were anesthetized with Avertin until semiconscious, and the chest wall was depilated. Two-dimensional and Doppler images were obtained with a Vevo 770 Ultrasound System (VisualSonics) to assess the heart rate, left ventricular (LV) internal diameter in systole and diastole, relative wall thickness, fractional shortening, and myocardial performance index. Individuals conducting the image acquisition and analysis were blinded to the groups.
Tissue collection.
Mice were euthanized after a 4-h fast. Hearts were excised, and the atria and right ventricle removed. Isolated left ventricles were weighed for measurement of LV mass (mg), which was normalized to body weight (g; obtained immediately before euthanasia) or tibia length (tibias were isolated from both legs, air-dried overnight, measured by calipers, and averaged).
Transmission electron microscopy.
A portion of the LV apex was fixed in 2% paraformaldehyde+2.5% glutaraldehyde in 100 mM cacodylate buffer and processed for transmission electron microscopy analysis with a JEOL 1200 EX transmission electron microscope (JEOL USA, Peabody, MA) equipped with an AMT 8-megapixel digital camera (Advanced Microscopy Techniques, Woburn, MA) as previously described (11, 26). Mitochondrial abnormalities were assessed in sections from offspring of three different dams in each group, in a blinded fashion.
Preparation of permeabilized cardiac fibers and high-resolution respirometry.
High-resolution respirometry was performed as previously described (26). A section of the LV lateral wall was freshly excised; the fibers were separated, permeabilized, washed, blotted dry, and then weighed. One to two milligrams of tissue were placed in an Oxygraph 2K (OROBOROS Instruments, Innsbruck, Austria) chamber containing 2 ml of MirO5 with 20 mM creatinine monohydrate and 10 μM blebbistatin. To measure O2 flux, the following substrates were added sequentially and measurements were performed at steady state with DatLab Software (OROBOROS Instruments; final concentrations indicated): leak: 0.5 mM malate, 5 mM pyruvate, 10 mM glutamate; complex I: 5 mM ADP; complex I+II: 10 mM succinate; and complex II: 0.5 μM rotenone. Samples were assayed in random order.
Protein extraction and immunoblot analysis.
Frozen LV tissue was homogenized in ice-cold RIPA buffer with protease and phosphatase inhibitors, consisting of 50 mM Tris·HCl (pH 7.4), 5 mM EDTA (pH 8.0), 150 mM NaCl, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 1% Triton X-100, 100 mM NaF, 2 mM Na3VO4, 10 mM Na4O7P2·10H2O, 40 mM β-glycerophosphate, 0.02 mM E-64, 3 mM benzamidine, 0.01 mg/ml leupeptin, and 0.01 mg/ml aprotinin, followed by centrifugation at 14,000 g for 20 min. Immunoblotting was performed following standard methods, as previously described (10). Twenty to eighty micrograms protein were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membranes. Primary antibodies recognizing the following proteins were used: Total OXPHOS Rodent cocktail (includes ATP5A, UQCR2, MTCO1, SDHB, and NDUFB8; 1:10,000, ab110413), Mfn1 (1:2,000, ab57602), Parkin (1:2,000, ab77924), and PINK1 (1:2,000, ab23707; Abcam (Cambridge, MA); Mfn2 (1:1,000, 9482), phospho-p44/42 MAPK (1:1,000, Thr202/Tyr204, 9101), phospho-MFF (1:1,000, Ser146, 49281), and SIRT3 (1:1,000, 5490; Cell Signaling (Danvers, MA); FIS1 (1:1,000, 10956–1-AP; Proteintech); and OPA1 (1:1,000, 612606) from BD Biosciences (San Jose, CA). Image Lab software (Bio-Rad Laboratories, Hercules, CA) was used for densitometric analysis of immunoblots.
Statistical analysis.
Data are presented as means ± SE. Two-tailed Student’s t-tests were employed to detect differences between two groups, and two-way repeated measures ANOVA followed by Tukey’s post hoc testing was employed to assess differences between Con and HFS groups by age. P < 0.05 was considered statistically significant.
RESULTS
F1 progeny of obese dams have sexually dimorphic cardiac abnormalities.
Four-week-old female F0 mice were fed a HFS or Con diet for 6 wk to induce obesity (Fig. 1, A and B) followed by mating with Con-fed males while continuing on the HFS or Con diet, respectively, during pregnancy and lactation. F1 progeny were weaned on a Con diet (Fig. 1A) and studied at 8 wk of age. Although body weights did not differ between Con and HFS progeny (Fig. 1B), F1 progeny of HFS-fed dams had shorter tibias than progeny of Con-fed dams (Tables 1 and 2), suggesting altered growth in offspring. LV weight was ~10% higher in both female and male F1 progeny of HFS-fed F0 dams than in the progeny of Con-fed F0 dams (Tables 1 and 2). Echocardiographic characterization of female F1 offspring of HFS dams revealed smaller LV relative wall thickness, lower fractional shortening, and higher myocardial performance index than those of Con dams (Table 1). In contrast, the hearts of male F1 offspring of HFS dams (Table 2) had higher LV relative wall thickness vs. those from Con dams and no differences in fractional shortening or myocardial performance index. These results indicate that maternal obesity leads to sexually dimorphic heart abnormalities in F1 offspring.
Fig. 1.
Body weight in descendants of high-fat/high-sucrose (HFS)- and chow (Con)-fed dams. A: female mice (♀F0) were fed a Con or HFS diet starting at 4 wk of age. These mice were mated with Con-fed males (♂F0) to produce F1 mice or euthanized for in vitro fertilization (IVF) to generate embryo transfer (ET) mice (using pseudo-pregnant ICR strain females). F1 females were mated to generate the F2 and, subsequently, F3 mice, and F1 males were mated to generate the paternal F2 (PF2) mice. All progeny were fed a Con diet. B–G: body weights were obtained at the same time and same day of the week, once per week starting at weaning (n = 25/group). All data are expressed as means ± SE. *P < 0.05 by post hoc test after 2-way repeated measures ANOVA between Con and HFS groups, and between age within sex and generation.
Table 1.
Female physiology and echocardiography
| F0 |
F1 |
ET |
F2 |
F3 |
PF2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Con | HFS | Con | HFS | Con | HFS | Con | HFS | Con | HFS | Con | HFS |
| LV mass, mg | 63.1 ± 0.9 | 67.2 ± 2.4 | 62.4 ± 0.8 | 66.5 ± 1.4* | 70.7 ± 2.4 | 83.1 ± 3.6* | 62.2 ± 1.2 | 67.3 ± 1.4* | 65.1 ± 2.1 | 62.9 ± 1.7 | 62.2 ± 0.7 | 67.1 ± 1.2* |
| Body wt, g | 19.1 ± 0.2 | 23.8 ± 0.3* | 18.8 ± 0.6 | 18.7 ± 0.6 | 22.1 ± 0.3 | 22.2 ± 0.6 | 19.0 ± 0.6 | 18.4 ± 0.2 | 19.3 ± 0.5 | 19.7 ± 0.9 | 19.0 ± 0.1 | 19.6 ± 0.3 |
| LV mass/ body mass, mg/g | 3.30 ± 0.08 | 2.91 ± 0.14* | 3.33 ± 0.03 | 3.54 ± 0.04* | 3.25 ± 0.06 | 3.76 ± 0.18* | 3.31 ± 0.05 | 3.66 ± 00.08* | 3.37 ± 0.12 | 3.33 ± 0.14 | 3.29 ± 0.05 | 3.60 ± 0.10* |
| Tibia length, mm | 17.3 ± 0.2 | 17.3 ± 0.1 | 16.5 ± 0.1 | 15.8 ± 0.1* | 17.5 ± 0.1 | 16.8 ± 0.2* | 16.5 ± 0.1 | 16.8 ± 0.1* | 16.9 ± 0.2 | 16.6 ± 0.2* | 16.6 ± 0.1 | 16.9 ± 0.1* |
| LV mass/tibia length, mg/mm | 3.63 ± 0.05 | 3.87 ± 0.11 | 3.72 ± 0.08 | 4.20 ± 0.12* | 4.22 ± 0.15 | 4.92 ± 0.25* | 3.61 ± 0.07 | 3.98 ± 0.09* | 3.78 ± 0.10 | 3.75 ± 0.12 | 3.69 ± 0.05 | 4.03 ± 0.08* |
| LV internal diameter (diastole), mm | 3.10 ± 0.09 | 3.20 ± 0.10 | 2.93 ± 0.04 | 3.22 ± 0.09* | 3.49 ± 0.15 | 3.19 ± 0.01* | 3.53 ± 0.14 | 3.17 ± 0.07* | 3.31 ± 0.18 | 3.22 ± 0.08 | 3.12 ± 0.20 | 3.60 ± 0.05* |
| LV internal diameter (systole), mm | 1.36 ± 0.05 | 1.39 ± 0.05 | 1.28 ± 0.08 | 1.52 ± 0.07* | 1.69 ± 0.07 | 1.47 ± 0.03* | 1.75 ± 0.11 | 1.45 ± 0.03* | 1.55 ± 0.12 | 1.53 ± 0.05 | 1.43 ± 0.11 | 1.76 ± 0.03* |
| Relative wall thickness | 0.57 ± 0.04 | 0.56 ± 00.02 | 0.57 ± 0.02 | 0.49 ± 00.03* | 0.47 ± 0.03 | 0.53 ± 0.01 | 0.46 ± 0.02 | 0.54 ± 0.02* | 0.49 ± 0.05 | 0.49 ± 0.03 | 0.45 ± 0.03 | 0.49 ± 0.04 |
| Heart rate, beats/min | 631 ± 7 | 626 ± 8 | 610 ± 10 | 632 ± 12 | 586 ± 12 | 605 ± 10 | 616 ± 16 | 641.7 ± 8 | 589 ± 24 | 612 ± 5 | 614 ± 5 | 611 ± 14 |
| Fractional shortening, % | 56.3 ± 1.0 | 56.7 ± 0.1 | 58.3 ± 1.2 | 53.4 ± 0.9* | 50.2 ± 0.3 | 53.0 ± 0.6* | 52.1 ± 1.2 | 54.0 ± 1.1 | 53.2 ± 1.4 | 52.3 ± 0.7 | 53.2 ± 0.9 | 52.0 ± 1.0 |
| Myocardial performance index | 0.57 ± 0.08 | 0.48 ± 0.04 | 0.49 ± 0.01 | 0.56 ± 0.02* | 0.56 ± 0.05 | 0.52 ± 0.04 | 0.53 ± 0.04 | 0.48 ± 0.02 | 0.59 ± 0.04 | 0.57 ± 0.02 | 0.43 ± 0.03 | 0.51 ± 0.02* |
Values are means ± SE; n = 20/group for body weight, LV weight, and tibia length; n = 4–7/group for echocardiographs.
P < 0.05 by Student’s t-test between Con and HFS groups within sex and generation.
Table 2.
Male physiology and echocardiography
| F1 |
ET |
F2 |
F3 |
PF2 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Con | HFS | Con | HFS | Con | HFS | Con | HFS | Con | HFS |
| LV mass, mg | 76.6 ± 0.9 | 80.2 ± 1.5* | 88.8 ± 1.9 | 98.4 ± 4.1* | 77.6 ± 1.4 | 82.8 ± 1.8* | 75.1 ± 1.8 | 83.0 ± 2.6* | 76.3 ± 1.8 | 83.6 ± 1.2* |
| Body wt, g | 23.3 ± 0.2 | 22.7 ± 0.32 | 26.5 ± 0.4 | 25.8 ± 0.5 | 23.2 ± 0.3 | 22.4 ± 0.3 | 21.3 ± 0.5 | 22.7 ± 0.5 | 22.1 ± 0.4 | 23.4 ± 0.3* |
| LV mass/body mass, mg/g | 3.39 ± 0.03 | 3.63 ± 0.03* | 3.40 ± 0.04 | 3.70 ± 0.06* | 3.37 ± 0.07 | 3.74 ± 0.03* | 3.44 ± 0.15 | 3.57 ± 0.19* | 3.28 ± 0.04 | 3.53 ± 0.05* |
| Tibia length mm | 17.0 ± 0.1 | 16.5 ± 0.2* | 17.5 ± 0.1 | 17.1 ± 0.1* | 16.5 ± 0.1 | 17.0 ± 0.1* | 16.8 ± 0.1 | 17.2 ± 0.1* | 16.8 ± 0.1 | 17.4 ± 0.1* |
| LV Mass/tibia length, mg/mm | 4.49 ± 0.06 | 4.97 ± 0.13* | 4.84 ± 0.09 | 5.75 ± 0.23* | 4.51 ± 0.08 | 4.86 ± 0.09* | 4.35 ± 0.09 | 4.92 ± 0.17* | 4.22 ± 0.10 | 4.84 ± 0.05* |
| LV internal diameter (diastole), mm | 3.87 ± 0.10 | 3.39 ± 0.11* | 3.35 ± 0.05 | 3.55 ± 0.08* | 3.38 ± 0.07 | 3.52 ± 0.02* | 3.51 ± 0.24 | 3.54 ± 0.12 | 3.45 ± 0.08 | 3.06 ± 0.06* |
| LV internal diameter (systole), mm | 1.93 ± 0.04 | 1.60 ± 0.04* | 1.60 ± 0.02 | 1.80 ± 0.07* | 1.60 ± 0.05 | 1.78 ± 0.01* | 1.63 ± 0.10 | 1.60 ± 0.05 | 1.65 ± 0.07 | 1.47 ± 0.04* |
| Relative wall thickness | 0.44 ± 0.01 | 0.56 ± 0.06* | 0.52 ± 0.01 | 0.46 ± 0.01* | 0.55 ± 0.01 | 0.50 ± 0.01* | 0.53 ± 0.06 | 0.46 ± 0.02 | 0.50 ± 0.02 | 0.62 ± 0.02* |
| Heart rate, beats/min | 617 ± 8 | 621 ± 9 | 638 ± 15 | 627 ± 18 | 624 ± 8 | 626 ± 14 | 611 ± 13 | 592 ± 7 | 590 ± 12 | 602 ± 4 |
| Fractional shortening, % | 50.2 ± 0.1 | 51.2 ± 0.9 | 50.8 ± 0.4 | 51.8 ± 1.2 | 53.0 ± 0.8 | 49.5 ± 0.4 | 53.5 ± 1.2 | 54.7 ± 0.5 | 52.3 ± 1.1 | 53.1 ± 1.4 |
| Myocardial performance index | 0.49 ± 0.06 | 0.48 ± 0.03 | 0.40 ± 0.04 | 0.54 ± 0.04* | 0.53 ± 0.04 | 0.55 ± 0.05 | 0.55 ± 0.03 | 0.53 ± 0.02 | 0.46 ± 0.02 | 0.59 ± 0.03* |
Values are means ± SE; n = 20/group for body weight, LV weight, and tibia length; n = 5/group for echocardiographs except, and n = 7 for F2 males and n = 4 for PF2 mice.
P < 0.05 by Student’s t-test between Con and HFS groups within sex and generation.
F1 male and female progeny of obese dams have cardiac mitochondrial abnormalities.
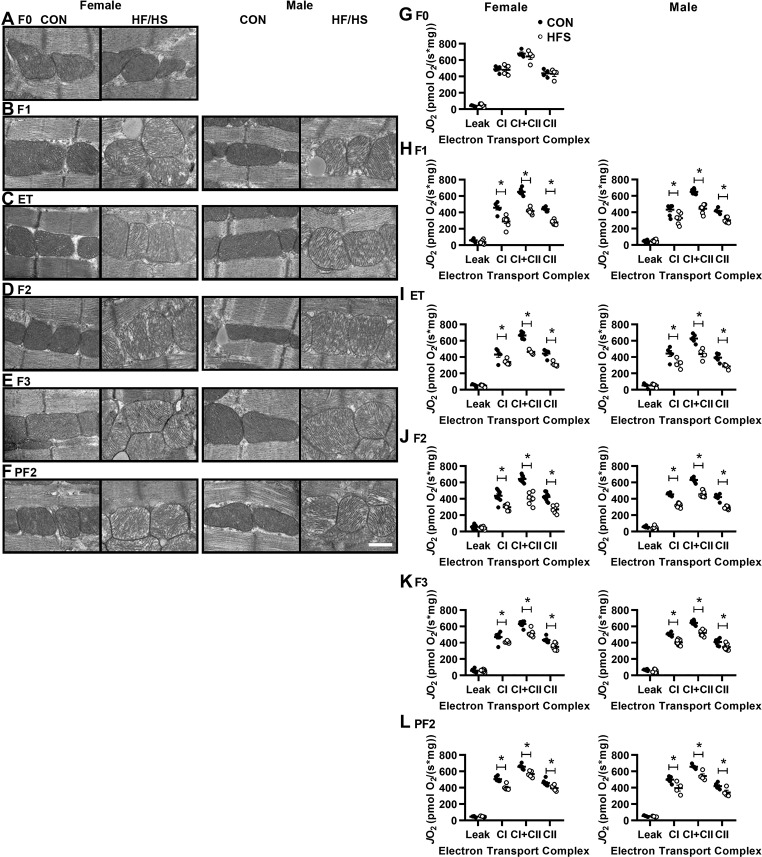
Given our previous report that maternal obesity led to mitochondrial defects in skeletal muscle of F1 progeny (26), we wondered whether maternal obesity similarly affected cardiac mitochondria. We addressed this question in three ways. First, we examined cardiac mitochondrial morphology and integrity by transmission electron microscopy. The mitochondria of F1 progeny from Con-fed dams were distinctly darker than the myofibrils, predominantly elongated and/or tubular in shape, and packed linearly between sarcomeres. In contrast, the mitochondria of both male and female F1 progeny of HFS-fed dams were less electron dense, mostly exhibited circular morphology, were disorganized with respect to the sarcomere, and displayed crystal rarefaction (Fig. 2B).
Fig. 2.
Altered mitochondrial morphology and function in descendants of HFS-fed dams. A–F: representative transmission electron microscopy images at ×25,000 magnification of myocardial sections from mice of the indicated generations as in Fig. 1; n = 3/group. Scale bar = 500 nm. G–L: high-resolution respirometry was performed to measure oxygen consumption [volume-specific oxygen flux (JO2)] in isolated, permeabilized cardiac fiber bundles in the presence of malate/pyruvate/glutamate (Leak), ADP (complex I), succinate (complex I+II), and rotenone (complex II); n = 8/group. All data are expressed as means ± SE. *P < 0.05 by Student’s t-test between Con and HFS groups, within sex and generation.
Second, we performed high-resolution respirometry in permeabilized, isolated cardiac fibers. Mitochondrial respiration in the presence of malate, glutamate, and pyruvate (a condition that measures electron leak) was identical between F1 progeny of Con- and HFS-fed dams. However, complex I respiration (measured by adding ADP) was lower in the cardiac tissue from male and female F1 progeny of HFS-fed dams than in tissue from progeny of Con-fed dams. Similarly, complex I + II respiration (measured by further addition of succinate) and complex II respiration (measured by subsequent addition of rotenone) was lower in tissue from male and female F1 progeny of HFS-fed dams than in tissue from progeny of Con-fed dams (Fig. 2H). Importantly, the ultrastructural and functional mitochondrial abnormalities were not observed in hearts of F0 dams fed a HFS diet vs. Con-fed F0 dams (Fig. 2, A and G).
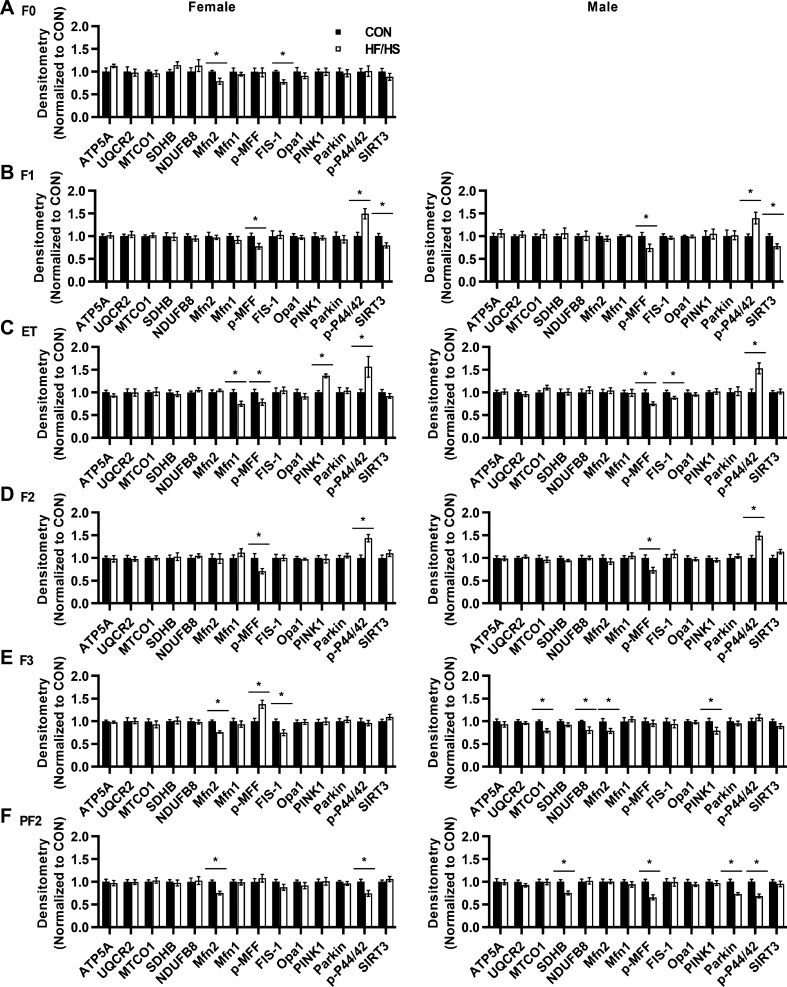
Third, we examined expression of several mitochondrial dynamics proteins (Mfn2, Mfn1, p-MFF, FIS-1, Opa1, PINK1, Parkin), electron transport chain proteins (ATP5A, UQCR2, MTCO1, SDHB, and NDUFB8), and the mitochondria-targeted sirtuin SIRT3 in cardiac tissue from F1 progeny of Con- and HFS-fed dams. We found that the concentrations of p-MFF and SIRT3 were lower in the tissue from male and female F1 offspring of HFS-fed dams vs. offspring of Con-fed dams (Fig. 3B).
Fig. 3.
Analysis of proteins associated with mitochondrial function and dynamics (A–F). Quantitative analyses of immunoblots to assess the indicated proteins in groups are shown; n = 7/group. All data are expressed as means ± SE. *P < 0.05 by Student’s t-test between Con and HFS groups, within sex and generation.
Effects of maternal obesity can be transmitted without in utero exposure during gestation.
In utero exposure to maternal obesity can negatively affect offspring health by exposing the gestating offspring to abnormal circulating hormone or metabolite levels (17). Furthermore, before weaning, offspring are exposed to potential effects of maternal diet via breast milk (12, 17). Because we previously observed mitochondrial dysfunction in the oocytes of obese mice (7), we hypothesized that in utero and lactation exposure were not necessary to result in cardiac mitochondrial defects in offspring. To test this idea, we isolated oocytes from HFS- and Con-fed F0 dams, fertilized them with sperm from Con-fed males, and transferred the resulting embryos into Con-fed females to generate embryo transfer (ET) offspring (Fig. 1A). LV mass was higher in both male and female ET offspring generated using oocytes from HFS-exposed F0 dams than in the ET offspring from oocytes of Con-exposed F0 dams (Tables 1 and 2). Similarly, we noted similar cardiac mitochondrial defects in ET offspring of HFS-exposed dams as in F1 offspring (Fig. 2, C and I).
Maternal obesity leads to cardiac and mitochondrial defects in F2 and F3 progeny.
We next bred some Con-fed F1 female offspring from both HFS-fed and Con-fed F0 dams to Con-fed males to generate F2 male and female progeny, and bred some Con-fed F2 females from each group to Con-fed males to generate F3 progeny (Fig. 1A) while on a Con diet. We performed the same analyses of these offspring as for the F1 progeny. As expected, F2 and F3 offspring of HFS-fed and Con-fed F0 dams displayed similar body weights while on a Con diet (Fig. 1, E and F). F2 females and both F2 and F3 males showed higher LV mass in the HFS group than in the Con group (Tables 1 and 2), despite variable differences in echocardiographic parameters in various generations. Moreover, all F2 and F3 male and female offspring of HFS-fed F0 dams showed cardiac mitochondrial defects as assessed by electron microscopy (Fig. 2, D and E) and respirometry (Fig. 2, J and K). Additionally, we noted some differences in expression of mitochondrial proteins between F2 and F3 offspring of HFS-fed and Con-fed F0 dams (Fig. 3, D and E). Together, these results indicate that at least some cardiac and mitochondrial effects of maternal obesity are transgenerational.
Effects of maternal obesity can be transmitted through the male germline.
Thus far, our data were consistent with a model in which maternal obesity caused mitochondrial defects in oocytes that were maternally inherited in subsequent generations. To test this hypothesis, we asked whether the defects could be transmitted through the male germline, as sperm mitochondria are largely eliminated through mitophagy (28). Male F1 offspring of HFS-fed and Con-fed F0 dams were mated to Con-fed females to generate paternal F2 (PF2) offspring, while on a Con diet (Fig. 1A). PF2 offspring of HFS-exposed F0 dams also demonstrated increased LV mass vs. PF2 offspring of Con F0 dams (Tables 1 and 2). Additionally, PF2 offspring of HFS-fed F0 dams showed similar mitochondrial defects as F1, F2, F3, and ET offspring (Figs. 2, F and L). Thus we conclude that the effects of maternal obesity are not transmitted via inheritance of defective maternal mitochondria.
DISCUSSION
In this study, we tested the hypothesis that maternal obesity induces cardiac mitochondrial dysfunction in the offspring via transgenerational inheritance of abnormal oocyte mitochondria. Our results demonstrate transgenerational inheritance of mitochondrial abnormalities but disprove the hypothesized mechanism, given transmission of the abnormalities via the male germline. Specifically, we show that maternal obesity caused by exposure to a HFS diet induces cardiac mitochondrial dysfunction in three subsequent generations of mice. In utero and nursing exposure are not required for this effect. To our knowledge, this is the first study to show transgenerational inheritance of changes in mitochondrial structure and function in the heart.
Transgenerational inheritance of mitochondrial dysfunction suggests heritable epigenetic changes as the likely etiology (13). This is further supported by our previous work revealing mitochondrial abnormalities in the oocytes and skeletal muscle of F1 and F2 female progeny of HFS-fed female mice (26). We did not observe differences in expression of electron transport chain proteins between progeny of HFS-fed and Con-fed dams, suggesting that maternal obesity did not affect mitochondrial content. In contrast, expression of proteins involved in mitochondrial dynamics and mitophagy differed between offspring of Con- and HFS-fed dams, suggesting altered mitochondrial dynamics. However, these effects were not consistent across generations and could reflect variability in epigenome modifications. Given that 99% of mitochondrial proteins are encoded in the nuclear genome, the inherited epigenetic modifications likely occur in the nucleus (4, 13). A limitation of our study is that the abundance of individual mitochondrial proteins was assessed as normalized to total cardiac protein content, whereby we cannot rule out differences in mitochondrial density as a potential contributor.
Our results indicate that while maternal exposure to an obesogenic diet leads to cardiac mitochondrial defects in both male and female progeny, the effects on the heart muscle are somewhat sexually dimorphic, whereby the signaling pathways involved will require careful elucidation in future studies. Interestingly, we observed significant downregulation of SIRT3 protein in both male and female F1 offspring only (and not in other generations) as has been seen in livers of rat offspring of obese dams (6). We speculate that the altered expression of mitochondrial proteins is not the cause of the mitochondrial dysfunction, since the phenotype (altered mitochondrial morphology and depressed oxidative phosphorylation) persists in all generations despite conflicting expression profiles of these proteins. Also, while increased LV mass was observed in all groups (except female F3 offspring), only the hearts from male and female offspring of HFS-fed dams in F1, F2, and ET groups had a higher ratio of MAPK p-P44/42 (vs. offspring of Con-fed dams), reflecting activation of a major positive regulator of increased LV mass (23). The abundance of p-p44/42 was unchanged in F3 and significantly reduced in HFS progeny in the PF2 generation. Therefore, we speculate that the observed proteomic changes and echocardiographic alterations are influenced not only by the hypothesized nuclear epigenetic changes but also by differences in environmental exposures within each generation. For example, the F1 generation is directly exposed to maternal obesity in F0 dams; whereas the F2 generation is indirectly exposed as developing gametes within the F1 generation, while being subsequently exposed to a different in utero environment in Con-fed F1 dams. Furthermore, the epigenetic changes are inherited from one parent only and presumably “diluted” over subsequent generations.
Several studies have suggested that in utero exposures affect cardiac health in the next generation. For example, prepregnancy obesity in humans is associated with higher myocardial performance indices in fetuses between 26 and 38 wk of gestation (8). Also, human maternal obesity was associated with detrimental effects on fetal cardiac function and morphology between 14 and 32 wk of gestation, suggesting that cardiac abnormalities develop early during development (15, 16). Additionally, prepregnancy and pregnancy weight gain in humans (24) and maternal consumption of a high-fat diet during lactation in rats (19) can detrimentally affect offspring metabolic health. Our data indicate that gestational environment and lactation were not required for the effects. Conceivably, minor differences between the ET and the F1 offspring in diastolic and systolic LV diameters and protein expression could reflect lack of exposure to the diet during gestation and lactation in ET offspring or in vitro fertilization-induced alterations in DNA methylation (9).
Mitochondria are largely inherited via the maternal germline [sperm mitochondria are degraded immediately upon fertilization (28), and the male nuclear genome is transmitted (1)]. Accordingly, the transmission of cardiac mitochondrial abnormalities through the male lineage suggests inheritance of nuclear epigenetic modifications rather than transmission of abnormal mitochondria. However, given reports of rare contributions from paternal mitochondrial DNA detected in humans (20), as well as the observation of sperm mitochondria in postfertilization embryos (27), mitochondrial tracing and pronuclear transfer experiments will be required to conclusively address the underlying mechanism.
In conclusion, this study shows that maternal obesity induced by exposure to a HFS diet can program transgenerational mitochondrial defects and cardiac structural and functional alterations. This does not require exposure to an obesogenic diet during gestation or nursing and can be transmitted through the male lineage. If similar effects occur in humans, then maternal, grandmaternal, and great-grandmaternal obesity may be risk factors for development of cardiovascular disease, independently of a patient’s own weight.
GRANTS
This work is supported by National Institutes of Health Grants P30-DK-056341 (Nutrition Obesity Research Center), F32-HL-140848 (to J. L. A. Ferey), R01-HD-083895 (to K. H. Moley), and R01-HL-143431 (to K. H. Moley and A. Diwan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.L.F., A.L.B., A. Drury, A.K., T.P., B.J.D., M.D.T., A. Diwan, and K.H.M. conceived and designed research; J.L.F., A.L.B., M.R., A. Drury, S.S., Z.M., and A.K. performed experiments; J.L.F., A.L.B., A.K., T.P., and A. Diwan analyzed data; J.L.F., A.K., T.P., B.J.D., M.D.T., A. Diwan, and K.H.M. interpreted results of experiments; J.L.F. and A. Diwan prepared figures; J.L.F. drafted manuscript; J.L.F. and A. Diwan edited and revised manuscript; J.L.F., A.L.B., M.R., A. Drury, S.S., Z.M., A.K., T.P., B.J.D., M.D.T., A. Diwan, and K.H.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Wandy Beatty (Molecular Microbiology) and Carla Weinheimer (Center for Cardiovascular Research) at Washington University for technical and scientific assistance. We thank Deborah J. Frank (Obstetrics and Gynecology) for critical reading and editing of this manuscript.
REFERENCES
- 1.Al Rawi S, Louvet-Vallée S, Djeddi A, Sachse M, Culetto E, Hajjar C, Boyd L, Legouis R, Galy V. Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science 334: 1144–1147, 2011. doi: 10.1126/science.1211878. [DOI] [PubMed] [Google Scholar]
- 2.Behringer R, Gertsenstein M, Nagy K, Nagy A. Manipulating the Mouse Embryo: A Laboratory Manual (4th ed.). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2014. [Google Scholar]
- 3.Blackmore HL, Niu Y, Fernandez-Twinn DS, Tarry-Adkins JL, Giussani DA, Ozanne SE. Maternal diet-induced obesity programs cardiovascular dysfunction in adult male mouse offspring independent of current body weight. Endocrinology 155: 3970–3980, 2014. doi: 10.1210/en.2014-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boengler K, Heusch G, Schulz R. Nuclear-encoded mitochondrial proteins and their role in cardioprotection. Biochim Biophys Acta 1813: 1286–1294, 2011. doi: 10.1016/j.bbamcr.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Boots CE, Boudoures A, Zhang W, Drury A, Moley KH. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum Reprod 31: 2090–2097, 2016. doi: 10.1093/humrep/dew181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borengasser SJ, Lau F, Kang P, Blackburn ML, Ronis MJJ, Badger TM, Shankar K. Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS One 6: e24068, 2011. doi: 10.1371/journal.pone.0024068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boudoures AL, Saben J, Drury A, Scheaffer S, Modi Z, Zhang W, Moley KH. Obesity-exposed oocytes accumulate and transmit damaged mitochondria due to an inability to activate mitophagy. Dev Biol 426: 126–138, 2017. doi: 10.1016/j.ydbio.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Ece I, Uner A, Balli S, Kibar AE, Oflaz MB, Kurdoglu M. The effects of pre-pregnancy obesity on fetal cardiac functions. Pediatr Cardiol 35: 838–843, 2014. doi: 10.1007/s00246-014-0863-0. [DOI] [PubMed] [Google Scholar]
- 9.Estill MS, Bolnick JM, Waterland RA, Bolnick AD, Diamond MP, Krawetz SA. Assisted reproductive technology alters deoxyribonucleic acid methylation profiles in bloodspots of newborn infants. Fertil Steril 106: 629–639.e10, 2016. doi: 10.1016/j.fertnstert.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Ferey JL, Brault JJ, Smith CA, Witczak CA. Constitutive activation of CaMKKα signaling is sufficient but not necessary for mTORC1 activation and growth in mouse skeletal muscle. Am J Physiol Endocrinol Metab 307: E686–E694, 2014. doi: 10.1152/ajpendo.00322.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godar RJ, Ma X, Liu H, Murphy JT, Weinheimer CJ, Kovacs A, Crosby SD, Saftig P, Diwan A. Repetitive stimulation of autophagy-lysosome machinery by intermittent fasting preconditions the myocardium to ischemia-reperfusion injury. Autophagy 11: 1537–1560, 2015. doi: 10.1080/15548627.2015.1063768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes RM, Bueno FG, Schamber CR, de Mello JCP, de Oliveira JC, Francisco FA, Moreira VM, Junior MDF, Pedrino GR, de Freitas Mathias PC, Miranda RA, de Moraes SMF, Natali MRM. Maternal diet-induced obesity during suckling period programs offspring obese phenotype and hypothalamic leptin/insulin resistance. J Nutr Biochem 61: 24–32, 2018. doi: 10.1016/j.jnutbio.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell 157: 95–109, 2014. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinkle SN, Sharma AJ, Kim SY, Park S, Dalenius K, Brindley PL, Grummer-Strawn LM. Prepregnancy obesity trends among low-income women, United States, 1999-2008. Matern Child Health J 16: 1339–1348, 2012. doi: 10.1007/s10995-011-0898-2. [DOI] [PubMed] [Google Scholar]
- 15.Ingul CB, Lorås L, Tegnander E, Eik-Nes SH, Brantberg A. Maternal obesity affects fetal myocardial function as early as in the first trimester. Ultrasound Obstet Gynecol 47: 433–442, 2016. doi: 10.1002/uog.14841. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni A, Li L, Craft M, Nanda M, Lorenzo JMM, Danford D, Kutty S. Fetal myocardial deformation in maternal diabetes mellitus and obesity. Ultrasound Obstet Gynecol 49: 630–636, 2017. doi: 10.1002/uog.15971. [DOI] [PubMed] [Google Scholar]
- 17.Lager S, Samulesson A-M, Taylor PD, Poston L, Powell TL, Jansson T. Diet-induced obesity in mice reduces placental efficiency and inhibits placental mTOR signaling. Physiol Rep 2: e00242, 2014. doi: 10.1002/phy2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loche E, Blackmore HL, Carpenter AA, Beeson JH, Pinnock A, Ashmore TJ, Aiken CE, de Almeida-Faria J, Schoonejans JM, Giussani DA, Fernandez-Twinn DS, Ozanne SE. Maternal diet-induced obesity programmes cardiac dysfunction in male mice independently of post-weaning diet. Cardiovasc Res 114: 1372–1384, 2018. doi: 10.1093/cvr/cvy082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Los Ríos EA, Ruiz-Herrera X, Tinoco-Pantoja V, López-Barrera F, Martínez de la Escalera G, Clapp C, Macotela Y. Impaired prolactin actions mediate altered offspring metabolism induced by maternal high-fat feeding during lactation. FASEB J 32: 3457–3470, 2018. doi: 10.1096/fj.201701154R. [DOI] [PubMed] [Google Scholar]
- 20.Luo S, Valencia CA, Zhang J, Lee N-C, Slone J, Gui B, Wang X, Li Z, Dell S, Brown J, Chen SM, Chien Y-H, Hwu W-L, Fan P-C, Wong L-J, Atwal PS, Huang T. Biparental inheritance of mitochondrial DNA in humans. Proc Natl Acad Sci USA 115: 13039–13044, 2018. doi: 10.1073/pnas.1810946115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maloyan A, Muralimanoharan S, Huffman S, Cox LA, Nathanielsz PW, Myatt L, Nijland MJ. Identification and comparative analyses of myocardial miRNAs involved in the fetal response to maternal obesity. Physiol Genomics 45: 889–900, 2013. doi: 10.1152/physiolgenomics.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131: e29–e322, 2015. [Errata in Circulation 133: e417, 2016; Circulation 131: e535, 2015] 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 23.Mutlak M, Kehat I. Extracellular signal-regulated kinases 1/2 as regulators of cardiac hypertrophy. Front Pharmacol 6: 149, 2015. doi: 10.3389/fphar.2015.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nucci D, Chiavarini M, Duca E, Pieroni L, Salmasi L, Minelli L. Pre-pregnancy body mass index, gestational weight gain and adverse birth outcomes: some evidence from Italy. Ann Ig 30: 140–152, 2018. doi: 10.7416/ai.2018.2205. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, Sarwar N, Lee AJ, Bhattacharya S, Norman JE. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ 347, aug13 1: f4539, 2013. doi: 10.1136/bmj.f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saben JL, Boudoures AL, Asghar Z, Thompson A, Drury A, Zhang W, Chi M, Cusumano A, Scheaffer S, Moley KH. Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep 16: 1–8, 2016. doi: 10.1016/j.celrep.2016.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sathananthan AH, Ratnam SS, Ng SC, Tarín JJ, Gianaroli L, Trounson A. The sperm centriole: its inheritance, replication and perpetuation in early human embryos. Hum Reprod 11: 345–356, 1996. doi: 10.1093/HUMREP/11.2.345. [DOI] [PubMed] [Google Scholar]
- 28.Sutovsky P, Song W-H. Post-fertilisation sperm mitophagy: the tale of Mitochondrial Eve and Steve. Reprod Fertil Dev 30: 56–63, 2017. doi: 10.1071/RD17364. [DOI] [PubMed] [Google Scholar]