Abstract
The endoplasmic reticulum/sarcoplasmic reticulum Ca2+ sensor stromal interaction molecule 1 (STIM1), a key mediator of store-operated Ca2+ entry, is expressed in cardiomyocytes and has been implicated in regulating multiple cardiac processes, including hypertrophic signaling. Interestingly, cardiomyocyte-restricted deletion of STIM1 (crSTIM1-KO) results in age-dependent endoplasmic reticulum stress, altered mitochondrial morphology, and dilated cardiomyopathy in mice. Here, we tested the hypothesis that STIM1 deficiency may also impact cardiac metabolism. Hearts isolated from 20-wk-old crSTIM1-KO mice exhibited a significant reduction in both oxidative and nonoxidative glucose utilization. Consistent with the reduction in glucose utilization, expression of glucose transporter 4 and AMP-activated protein kinase phosphorylation were all reduced, whereas pyruvate dehydrogenase kinase 4 and pyruvate dehydrogenase phosphorylation were increased, in crSTIM1-KO hearts. Despite similar rates of fatty acid oxidation in control and crSTIM1-KO hearts ex vivo, crSTIM1-KO hearts contained increased lipid/triglyceride content as well as increased fatty acid-binding protein 4, fatty acid synthase, acyl-CoA thioesterase 1, hormone-sensitive lipase, and adipose triglyceride lipase expression compared with control hearts, suggestive of a possible imbalance between fatty acid uptake and oxidation. Insulin-mediated alterations in AKT phosphorylation were observed in crSTIM1-KO hearts, consistent with cardiac insulin resistance. Interestingly, we observed abnormal mitochondria and increased lipid accumulation in 12-wk crSTIM1-KO hearts, suggesting that these changes may initiate the subsequent metabolic dysfunction. These results demonstrate, for the first time, that cardiomyocyte STIM1 may play a key role in regulating cardiac metabolism.
NEW & NOTEWORTHY Little is known of the physiological role of stromal interaction molecule 1 (STIM1) in the heart. Here, we demonstrate, for the first time, that hearts lacking cardiomyocyte STIM1 exhibit dysregulation of both cardiac glucose and lipid metabolism. Consequently, these results suggest a potentially novel role for STIM1 in regulating cardiac metabolism.
Keywords: cardiomyocytes, metabolism, mitochondria, stromal interaction molecule 1, store-operated calcium entry
INTRODUCTION
In cardiomyocytes, the most widely studied Ca2+ entry pathway is voltage-dependent Ca2+ entry across the sarcolemma followed by Ca2+-induced Ca2+ release from the endoplasmic reticulum (ER)/sarcoplasmic reticulum (SR) (6); thus, in the heart, the widely accepted view is that the ER/SR is the predominant source of Ca2+ for both excitation-contraction coupling as well as Ca2+ signaling. However, in many nonexcitable cell types, store-operated Ca2+ entry (SOCE) is the primary mechanism of Ca2+ signaling. SOCE occurs in response to a decrease in ER/SR Ca2+, which triggers a subsequent influx of extracellular Ca2+. In 2005, stromal interaction molecule 1 (STIM1), an ER/SR Ca2+ sensor, along with plasma membrane Orai1 channels were identified as essential mediators of the SOCE pathway (48), with STIM1 coupling to Orai1 being required to facilitate Ca2+ entry (46). STIM1 expression was first detected in the heart in 2001 (64); however, its function in cardiomyocytes has been understudied. Apart from its potential role in cardiomyocyte hypertrophy (26, 35, 61), little is known about the physiological role of STIM1-mediated SOCE in adult cardiomyocytes. Despite this, we have recently shown that cardiomyocyte-restricted deletion of STIM1 (crSTIM1-KO; i.e., >90% deletion in isolated cardiomyocytes) results in a dilated cardiomyopathy by 36 wk of age, demonstrating that STIM1 is essential for cardiomyocyte homeostasis (10). Of note, we found that STIM1 deletion was not accompanied by any compensatory changes in either STIM2 or Orai1 (10), supporting the fact that the observed changes are STIM1 specific.
Ca2+ signaling is widely recognized as playing a key role in the regulation of cardiac metabolism through its direct activation of Ca2+-dependent proteins, kinases, and phosphatases as well as via activation of several metabolic transcription factors and genes. Increases in intracellular Ca2+ have been shown to stimulate glycolysis and glucose oxidation (51). Cytoplasmic Ca2+ is also known to positively regulate the activities of several mitochondrial dehydrogenases, including pyruvate dehydrogenase (PDH), NAD-isocitrate dehydrogenase (IDH), and oxoglutarate dehydrogenase (OGDH) and the activity of the key regulators of PDH, including pyruvate dehydrogenase kinases (PDKs) (24) and pyruvate dehydrogenase phosphatases (PDPs) (3, 11). In addition, Ca2+ regulates a number of protein kinases that are known to play a key role in regulating cardiac energy metabolism, including 5′-AMP-activated protein kinase (AMPK) (31), protein kinase B (PKB/AKT) (20, 67), PKC isoforms (53), Ca2+/calmodulin-dependent protein kinase II (CAMKII) (16), phosphoinositide-dependent protein kinase 1 (PDPK1), and glycogen synthase kinase-3β (GSK-3β) (34), as well as protein phosphatases (PPs) such as PP2A, PP2B, PP2C, and PP1 in the heart (56, 68).
The relationship between fatty acid oxidation (FAO) and Ca2+ signaling is also well established, as increased Ca2+ increases FAO (40) and, interestingly, long-chain fatty acids can directly activate Ca2+ channels in cardiomyocytes (25). Ca2+ has also been shown to regulate the expression of genes encoding FAO enzymes via transcriptional regulators including the members of the peroxisome proliferator-activated receptor (PPAR) family (21, 27, 32, 66) and PPAR-γ coactivator-1α (PGC-1α) (30). In addition, the activation of both hormone-sensitive lipase (HSL) (62) and lipoprotein lipase (LPL) (63, 70) is Ca2+ dependent. Reduced HSL activity has been associated with increased Ca2+ levels, lipid formation, and storage (62). Furthermore, Ca2+ signaling has been shown to play an essential role in the membrane trafficking of receptors to the plasma membrane either directly or through modulation by AMPK; these include glucose transporters 1 and 4 (GLUT1/4) and fatty acid translocase (CD36/FAT) (2), both of which play key roles in glucose uptake and fatty acid metabolism, respectively.
Despite the known roles of Ca2+ in regulating cardiac energy metabolism, the specific Ca2+-handling pathways involved are not well characterized. Interestingly, recent studies using fibroblasts from patients with loss-of-function mutations in STIM1 have indicated that SOCE may play a role in regulating lipid metabolism and lipolysis (36), although whether this is the case in the heart remains unknown. Therefore, we used our established crSTIM1-KO mouse model to determine the effect of STIM1 deletion on cardiac metabolism. In the present study, we show, for the first time, that lack of cardiomyocyte STIM1 alters substrate utilization in the heart, resulting in reduced glucose oxidation and upregulation of lipogenic proteins associated with lipid accumulation.
MATERIALS AND METHODS
Materials.
Unless otherwise stated, all reagents and chemicals were obtained from Fisher Scientific.
Cardiomyocyte-restricted STIM1-KO mice.
All experimental protocols used in this study were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee and adhered to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996). All animals received standard chow and water on an ad libitum basis (with the exception of the insulin experiments, where animals were fasted for 6 h before experimentation), and lighting was maintained on a 12:12-h light-dark cycle. crSTIM1-KO mice were generated and their respective genotypes were determined as previously described (10). Littermate STIM1 floxed mice without Cre recombinase were used as controls. Male control and crSTIM1-KO mice (20 wk old) were used in the following experiments (with the exception of the 12-wk data shown in Fig. 8), a time point that we have shown to occur before any decline in cardiac function and development of dilated cardiomyopathy (10). All experiments (with the exception of the isolated working perfused heart experiments; data in Fig. 1) were performed in freshly isolated hearts from control and crSTIM1-KO mice. Consistent with our previous study (10), there were no significant differences between genotypes in the weight of the mice. Given the known impact of time of day on the regulation of metabolism in the heart (9, 58), all experimental data end points were collected at the same time of day to minimize any impact of circadian regulation (i.e., 10 AM).
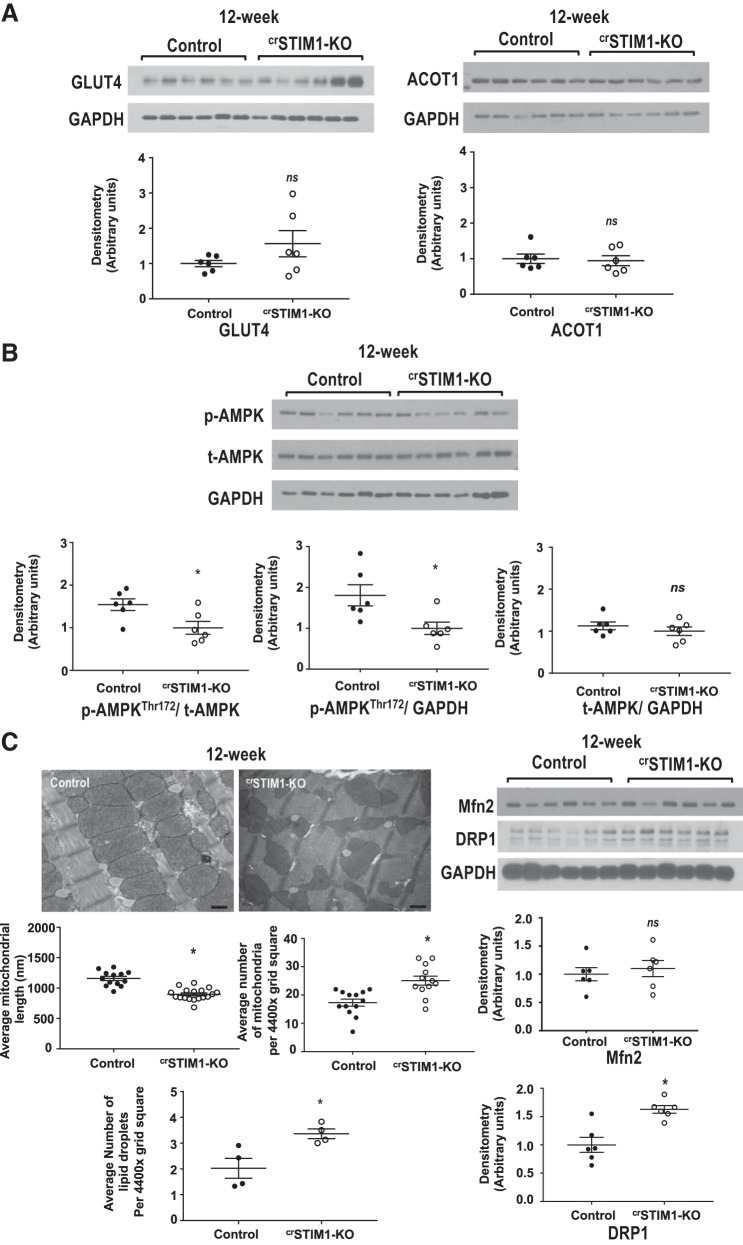
Fig. 8.
Effect of cardiomyocyte-restricted deletion of stromal interaction molecule 1 (crSTIM1-KO) on glucose, lipid, and mitochondrial metabolism at 12 wk. A, top: immunoblots illustrating glucose transporter 4 (GLUT4; left) and acyl-CoA thioesterase 1 (ACOT1; right) expression in freshly isolated hearts from 12-wk control and crSTIM1-KO mice. A, bottom: densitometric analysis of the immunoblots shown at the top. n = 6 mice/genotype. B, top: immunoblots illustrating phosphorylated (p-)AMP-activated protein kinase (AMPK)/total (t-)AMPK expression in freshly isolated hearts from 12-wk control and crSTIM1-KO mice. B, bottom: densitometric analysis of the immunoblots shown at the top. n = 6 mice/genotype. C, top left: representative transmission electron micrographs taken from left ventricular tissue isolated from 12-wk control (left) and crSTIM1-KO (right) mice. C, bottom left: quantification of average mitochondrial length, average number of mitochondria, and number of lipids per 4,400× grid square (average number of measurements per biological sample). Analysis was performed using ImageJ. n = 4 mice/genotype. C, top right: immunoblots illustrating mitofusin 2 (Mfn2) and dynamin-related protein 1 (DRP-1) expression in freshly isolated hearts from 12-wk control and crSTIM1-KO mice. C, bottom right: densitometric analysis of the immunoblots shown at the top. n = 6 mice/genotype. *P < 0.05 vs. age-matched control mice (Student’s t-test).
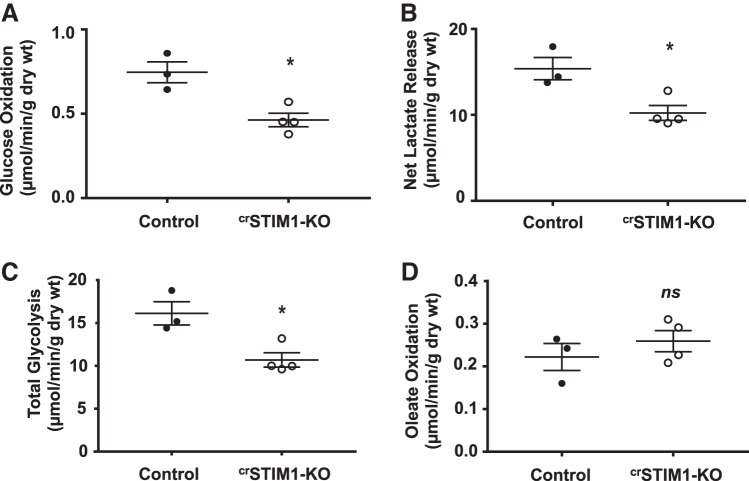
Fig. 1.
Glucose and fatty acid metabolism in ex vivo isolated perfused hearts with cardiomyocyte-restricted deletion of stromal interaction molecule 1 (crSTIM1-KO hearts). Ex vivo isolated working heart perfusion experiments performed on hearts from control and crSTIM1-KO mice. A−D: mean measurements of glucose oxidation (A), net lactate release (B), total glycolysis (glucose oxidation and net lactate release combined; C), and oleate oxidation (D). n = 3–4 mice/genotype. *P < 0.05 versus age-matched control mice (Student’s t-test).
Metabolic analysis of mouse blood plasma.
Blood (500–600 µl) obtained from the vena cavae of unfasted control and crSTIM1-KO mice was collected using EDTA-coated blood collection tubes. Blood samples were kept on ice for no longer than 30 min after collection until centrifugation to extract plasma. Collected blood plasma was immediately snap frozen and kept at −80°C until subsequent analysis. Blood plasma was examined for levels of the following circulating humoral factors: glucose, triglyceride, glycerol, free fatty acid, and insulin. Glucose was measured using a Sirrus Stanbio automated analyzer; triglyceride and glycerol were measured using commercially available colorimetric assay kits from Cayman Chemicals, free fatty acid was measured using a colorimetric kit from Cell Biolabs, and insulin was measured using a sensitive rat insulin kit from Millipore.
Isolated working heart perfusion.
Myocardial substrate utilization and contractile function were measured ex vivo using isolated working perfused mouse hearts, as previously described (9, 13, 58, 59). All hearts were perfused in the working mode (nonrecirculating manner) for 30 min with a preload of 12.5 mmHg and an afterload of 50 mmHg. Standard Krebs-Henseleit buffer was supplemented with 8 mM glucose, 1.2 mM oleate conjugated to 3% BSA (fraction V, fatty acid free, dialyzed), 10 μU/ml insulin (basal/fasting concentration), 0.05 mM l-carnitine, and 0.13 mM glycerol. Metabolic flux was assessed through the use of the following distinct radiolabeled tracers: 1) [U-14C]glucose (0.12 mCi/l) and 2) [9,10-3H]oleate (0.067 mCi/l). Measures of cardiac metabolism (e.g., glucose and oleate utilization) and function (i.e., heart rate, cardiac power, and rate-pressure product) were determined as previously described (9, 13, 58, 59). Data are presented as steady-state values (i.e., values during the last 10 min of the perfusion protocol).
Transmission electron microscopy.
As previously described (10), left ventricular tissue from control and crSTIM1-KO hearts was isolated, cut into longitudinal sections, and placed in 0.1 mol/l cacodylate buffer containing 2% glutaraldehyde-paraformaldehyde and then heat fixed for 30 min to cross link proteins and aldehydes. Lipid fixation was performed using 2% osmium tetroxide in 0.1 mol/l cacodylate buffer and 1% aqueous uranyl acetate, and tissue was embedded in Epon. Transmission electron microscopy was performed at the University of Alabama at Birmingham High Resolution Imaging Facility, and longitudinal sections of tissue were assessed at the level of the ER/SR membrane, mitochondria, and contractile filaments. Mitochondrial length measurements were taken as an index of mitochondrial size, as previously described (10).
In vivo insulin administration.
Control and crSTIM1-KO mice were fasted for 6 h (from 4 to 10 AM), after which mice received an in vivo injection (via the vena cava) of either saline or insulin (0.167 U/kg, Humulin R U-100, Eli Lilly) as previously described (37). Hearts were excised 5 min after insulin treatment, rinsed in ice-cold PBS, snap frozen, and stored for subsequent analysis.
Immunoblot analysis.
Whole heart tissue was homogenized/sonicated in lysis buffer, as previously described (10). Protein (25 µg) was separated on 7.5%, 10%, or 12% SDS-PAGE gels and subsequently transferred to polyvinylidenedifluoride (PVDF) membranes. PVDF membranes were blocked for 1 h at room temperature with 5% nonfat milk/Tris-buffered saline with Tween 20 (TBST) or 5% BSA/TBST and incubated overnight at 4°C with primary antibodies specific for the following phospho-specific proteins: phospho-AKT (Ser473, no. 9271, Cell Signaling), phospho-AKT (Thr308, no. 9275, Cell Signaling), phospho-AS160 (Thr642, no. 8881, Cell Signaling), phospho-acetyl-CoA-carboxylase (ACC; Ser79, no. 3661, Cell Signaling), phospho-GSK-3β (Ser9, no. 9336, Cell Signaling), phospho-AMPKα (Thr172, no. 2535, Cell Signaling), and phospho-PDH (Ser293, AP1062, Millipore). In addition, membranes were incubated with primary antibodies specific for GLUT4 (no. 07-1404, Millipore), GLUT1 (no. 07-1401, Millipore), PDK4 (ab38242, Abcam), PDK2 (sc100534, Santa Cruz Biotechnology), PDH (no. 2784, Cell Signaling), CD36/FAT (no. 5525, Cascade Biosciences), AMPKα (no. 2532, Cell Signaling), liver kinase B1 (LKB1; sc32245, Santa Cruz Biotechnology), GSK-3α/β (sc7291, Santa Cruz Biotechnology), hexokinase II (HK2; AB3279, Millipore), phosphofructokinase 1 (PFK1; sc67028, Santa Cruz Biotechnology), PGC-1α (sc13067, Santa Cruz Biotechnology), PPAR-β/δ (sc7197, Santa Cruz Biotechnology), PPAR-α (ab8934, Abcam), AKT (no. 9272, Cell Signaling), AS160 (no. 07-741, Millipore), ACC (no. 3662, Cell Signaling), fatty acid transporter 1 (FATP1; ab167099, Abcam), fatty acid synthase (FAS; no. 3180, Cell Signaling), fatty acid-binding protein 4 (FABP4; ab66682, Abcam), adipose triglyceride lipase (ATGL; no. 2439, Cell Signaling), HSL (no. 4107, Cell Signaling), acyl-CoA thioesterase 1 (ACOT1; ab133948, Abcam), acyl-coenzyme A:diacylglycerol acyltransferase (DGAT2; sc293211, Santa Cruz Biotechnology), mitofusin 2 (Mfn2; 1:500, Abcam), and dynamin-related protein 1 (DRP-1; 1:1,000, Thermo Fisher Scientific), after which membranes were washed in TBST. Membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies for 2 h at 4°C. Blots were exposed to ECL chemiluminescence detection, and images were obtained using autoradiograph film. Expression was normalized to the loading controls of GAPDH (1:5,000, ab8245, Abcam), β-actin (1:1,000, ab8227, Abcam), or calsequestrin (1:5,000, ab3516, Abcam), where necessary.
Quantification of cardiac triglyceride content.
Cardiac triglyceride content was measured from homogenate extracts from control and crSTIM1-KO hearts using the Triglyceride Colorimetric Assay kit (Cayman Chemicals) as per the manufacturer’s protocol.
Quantification of cardiac glycogen content.
Cardiac glycogen content was measured spectrophotometrically in homogenate extracts from control and crSTIM1-KO hearts, as previously described (12). The hearts used for these experiments were collected rapidly after euthanasia to ensure minimal postmortem glycogen release.
Statistical analyses.
Statistical significance was calculated using ANOVA followed by a Tukey post hoc test or Student’s t-test using GraphPad Prism 4 and 7, as appropriate, where P values of < 0.05 were deemed statistically significant. All data are presented as means ± SE. ROUT analysis was used to determine and remove any significant outliers. The present experiments were performed using a minimum of 3 mice/experiment, typically between 3 and 8 mice/manipulation. Densitometric analysis of Western blot data and quantification of lipid droplet images were performed using NIH ImageJ software.
RESULTS
Cardiomyocyte-restricted STIM1-KO hearts exhibit impaired glucose metabolism.
To determine the impact of STIM1 deletion on cardiac metabolism, hearts from control and crSTIM1-KO mice were isolated and perfused in working mode in the presence of radiolabelled glucose and oleate. We found that hearts from crSTIM1-KO mice exhibited a significant reduction in glucose oxidation, net lactate release, and total glycolysis compared with littermate control mice (Fig. 1, A−C). Myocardial glycogen levels did not exhibit differences between genotypes (0.30 ± 0.05 vs. 0.27 ± 0.04 mg/ml for control vs. crSTIM1-KO mice). There was also no significant genotype effect for oleate oxidation (Fig. 1D). There were no differences in rate-pressure products (5,492.72 ± 627.28 vs. 4,772.80 ± 465.18 beats·min−1·mmHg for control vs. crSTIM1-KO mice) or cardiac power (1.06 ± 0.17 vs. 1.02 ± 0.11 mW for control vs. crSTIM1-KO mice) between control and crSTIM1-KO hearts, suggesting that alterations in metabolism were not secondary to functional perturbations. In addition, there were no significant differences in either oxygen consumption (45.79 ± 5.29 vs. 50.34 ± 5.61 μmol·min−1·g dry wt−1 for control vs. crSTIM1-KO mice) or heart rate (315.0 ± 16.26 vs. 297.1 ± 22.13 beats/min for control vs. crSTIM1-KO mice). These heart rates are lower than those measured in vivo in mice; however, they are consistent with other studies of the ex vivo isolated working mouse heart (9, 58).
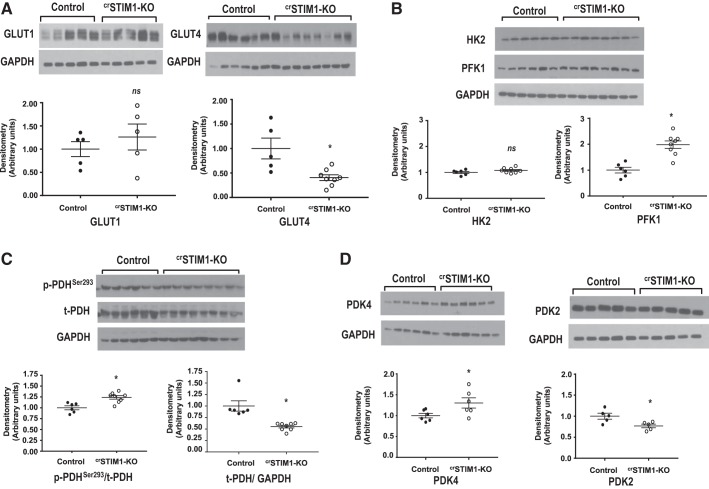
To identify potential factors contributing to decreased glucose utilization in crSTIM1-KO hearts, we examined protein levels of several regulators of glucose uptake, glycolysis, and pyruvate oxidation. Analysis of glucose transporters revealed a marked reduction in whole cell GLUT4 protein expression in crSTIM1-KO hearts; however, there were no differences in GLUT1 levels between groups (Fig. 2A). There was no significant difference in the expression of HK2 between control and crSTIM1-KO hearts; however, PFK1 expression was significantly increased (Fig. 2B). In addition, we observed a significant increase in the ratio of phospho-PDH (Ser293) to total PDH (Fig. 2C), concomitant with an increase in PDK4 expression levels in the crSTIM1-KO group (Fig. 2D). The increase in phospho-PDH/total PDH was due, in part, to the ~50% decrease in total PDH levels (P < 0.05) in crSTIM1-KO hearts. There was also a modest decrease in PDK2 expression in crSTIM1-KO versus control hearts (Fig. 2D).
Fig. 2.
Expression of key proteins involved in regulating cardiac glucose metabolism. A, top: immunoblots illustrating glucose transporter (GLUT)1 (left) and GLUT4 (right) expression in freshly isolated hearts from control and cardiomyocyte-restricted deletion of stromal interaction molecule 1 (crSTIM1-KO). A, bottom: densitometric analysis of the immunoblots shown at the top. B, top: immunoblots illustrating hexokinase 2 (HK2) and phosphofructokinase 1 (PFK1) expression in freshly isolated hearts from control and crSTIM1-KO mice. B, bottom: densitometric analysis of the immunoblots shown at the top. Protein expression was normalized to GAPDH expression. C, top: immunoblots illustrating phosphorylated (p-)pyruvate dehydrogenase (PDH)/total (t-)PDH and t-PDH/GAPDH expression in freshly isolated hearts from control and crSTIM1-KO mice. C, bottom: densitometric analysis of the immunoblots shown at the top. Protein expression was normalized to GAPDH expression. D, top: immunoblots illustrating pyruvate dehydrogenase kinase (PDK)4 and PDK2 expression in freshly isolated hearts from control and crSTIM1-KO mice. D, bottom: densitometric analysis of the immunoblots shown at the top. Protein expression was normalized to GAPDH expression. n = 5/6 control mice and n = 8 crSTIM1-KO mice (with the exception of the GLUT1 blot, where n = 5 control mice and n = 5 crSTIM1-KO mice). *P < 0.05 vs. age-matched control mice (Student’s t-test).
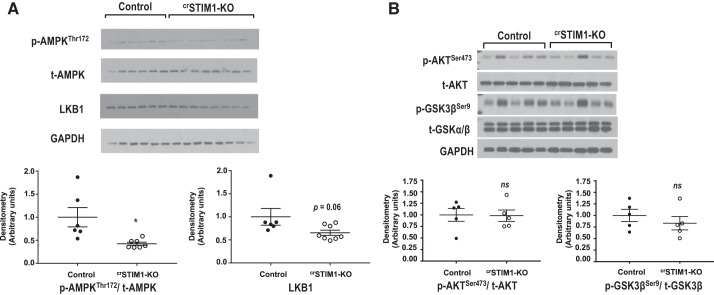
AMPK is an important regulator of cardiac metabolism, and phosphorylation of AMPK at Thr172 was markedly reduced in crSTIM1-KO hearts (Fig. 3A). AMPK phosphorylation can be regulated via LKB1, which showed a trend to being lower in crSTIM1-KO hearts (Fig. 3A). In the random fed state, there were no significant differences in the phosphorylation of AKT at Ser473 or GSK-3β at Ser9 between control and crSTIM1-KO hearts (Fig. 3B).
Fig. 3.
Effect of cardiomyocyte-restricted deletion of stromal interaction molecule 1 (crSTIM1-KO) on key kinases that regulate cardiac metabolism. A, top: Immunoblots illustrating phosphorylated (p-)AMP-activated protein kinase (AMPK)/total (t-)AMPK and liver kinase B1 (LKB1) expression in freshly isolated hearts from control and crSTIM1-KO mice. A, bottom: densitometric analysis of the immunoblots at the top. n = 5–8 mice/genotype. B, top: immunoblots illustrating p-AKT/t-AKT and p-glycogen synthase kinase-3β (GSK-3β)/t-GSK3β expression in freshly isolated hearts from control and crSTIM1-KO mice. B, bottom: densitometric analysis of immunoblots shown at the top. Protein expression was normalized to GAPDH expression. n = 5 control mice and n = 5 crSTIM1-KO mice. *P < 0.05 vs. age-matched control mice (Student’s t-test).
Impact of STIM1 deletion on cardiac lipid metabolism.
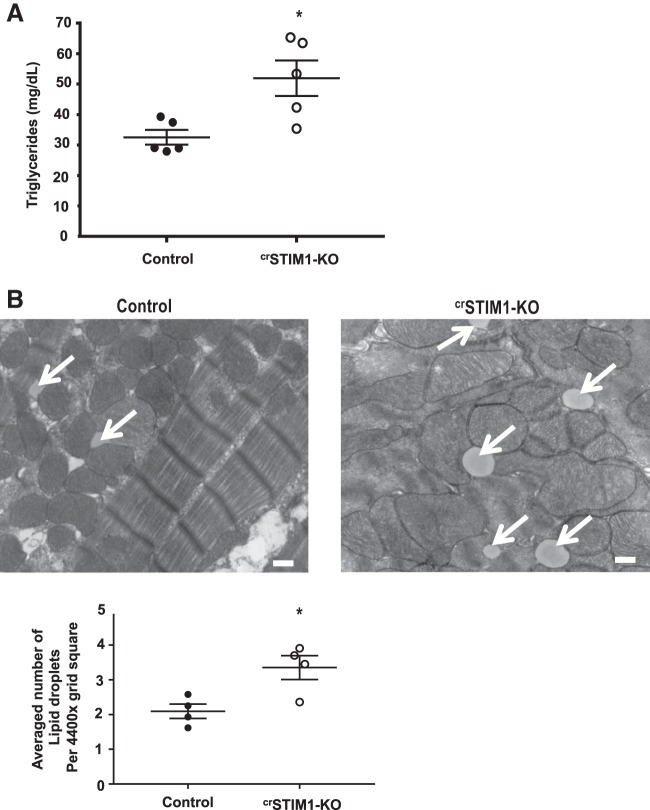
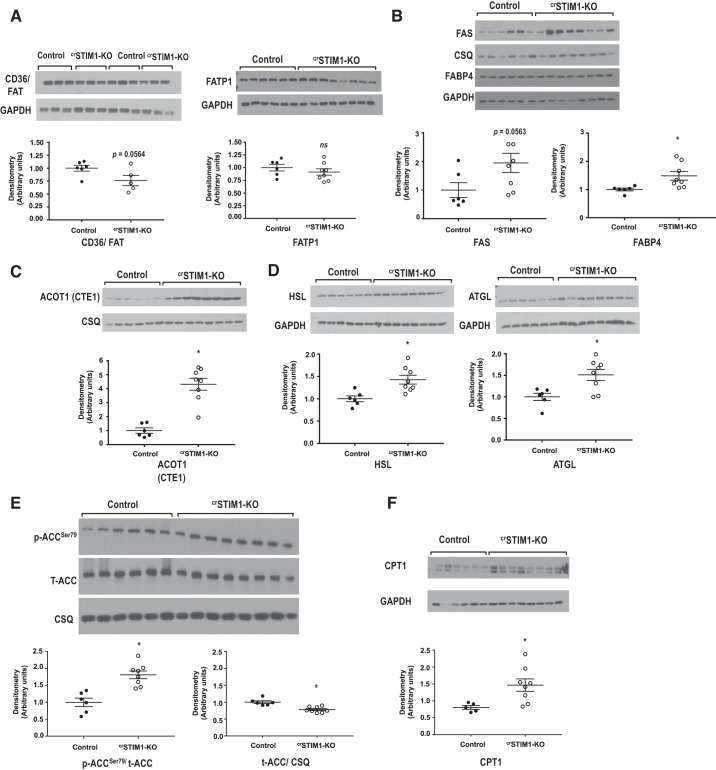
There were no differences in fatty acid oxidation between groups (Fig. 1D); however, myocardial triglyceride content was significantly increased in crSTIM1-KO hearts (Fig. 4A). There was also a significant increase in lipid droplet accumulation in crSTIM1-KO hearts (Fig. 4B). Accumulation of lipids can occur due to an imbalance between fatty acid uptake and oxidation and/or altered triglyceride turnover. We therefore examined expression of a number of proteins involved in fatty acid metabolism. Immunoblot analysis revealed no alterations in CD36/FAT or FATP1 levels in crSTIM1-KO hearts (Fig. 5A), both of which contribute to fatty acid transport across the plasma membrane. In contrast, however, we observed increases in FAS and FABP4 (Fig. 5B) and a greater than fourfold increase in ACOT1 in crSTIM1-KO hearts (Fig. 5C). Both HSL and ATGL levels were also significantly increased in response to loss of STIM1 (Fig. 5D). ACC is another key regulator of fatty acid metabolism. We found that the ratio of phospho-ACC (Ser79) to total ACC was increased in crSTIM1-KO hearts, which was associated with a small but significant decrease in total ACC (Fig. 5E). Carnitine palmitoyltransferase I (CPT1), a key regulator of fatty acid oxidation, was significantly increased in the hearts of crSTIM1-KO mice (Fig. 5F).
Fig. 4.
Effect of cardiomyocyte-restricted deletion of stromal interaction molecule 1 (crSTIM1-KO) on lipid droplets and triglyceride levels. A: triglyceride content of whole heart lysates from control and crSTIM1-KO mice. n = 5 mice/genotype. B, top: representative transmission electron micrographs taken from left ventricular tissue isolated from control (left) and crSTIM1-KO mice (right). Arrows depict the lipid droplets. Scale bar = 500 nm. B, bottom: quantification of the average number of lipid droplets located in a 4,400× grid square (average number of measurements per biological sample). n = 3–4 mice/genotype. Analysis was performed using ImageJ. * P < 0.05 vs. age-matched control mice (Student’s t-test).
Fig. 5.
Effect of cardiomyocyte-restricted deletion of stromal interaction molecule 1 (crSTIM1-KO) on proteins that regulate lipid metabolism. A, top: immunoblots illustrating fatty acid translocase (CD36/FAT) and fatty acid transporter 1 (FATP1) whole cell expression levels in freshly isolated control and crSTIM1-KO hearts. A, bottom: densitometric analysis of the immunoblots shown at the top. B, top: Immunoblots illustrating fatty acid synthase (FAS) and fatty acid-binding protein 4 (FABP4) whole cell expression levels in freshly isolated control and crSTIM1-KO hearts. B, bottom: densitometric analysis of the immunoblots shown at the top. C, top: immunoblots illustrating acyl-CoA thioesterase 1 (ACOT1) whole cell expression levels in freshly isolated control and crSTIM1-KO hearts. C, bottom: densitometric analysis of the immunoblots shown at the top. D, top: immunoblots illustrating hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) whole cell expression levels in freshly isolated control and crSTIM1-KO hearts. D, bottom: densitometric analysis of the immunoblots shown at the top. E, top: immunoblots illustrating phosphorylated (p-)acetyl-CoA-carboxylase (ACC)/total (t-)ACC and t-ACC/calsequestrin (CSQ) in freshly isolated hearts from control and crSTIM1-KO mice. E, bottom: densitometric analysis of the immunoblots shown at the top. F, top: immunoblots illustrating whole cell carnitine palmitoyltransferase I (CPT1) expression in freshly isolated hearts from control and crSTIM1-KO mice. F, bottom: densitometric analysis of the immunoblots shown at the top. Protein expression was normalized to GAPDH or CSQ expression, where necessary. For FATP1, FAS, FABP4, and ACOT1 blots, n = 6 control mice and n = 7/8 crSTIM1-KO mice; for CD36/FAT, ACC, and CPT1 blots, n = 5/6 control mice and n = 5/6 crSTIM1-KO mice. *P < 0.05 vs. age-matched control mice (Student’s t-test).
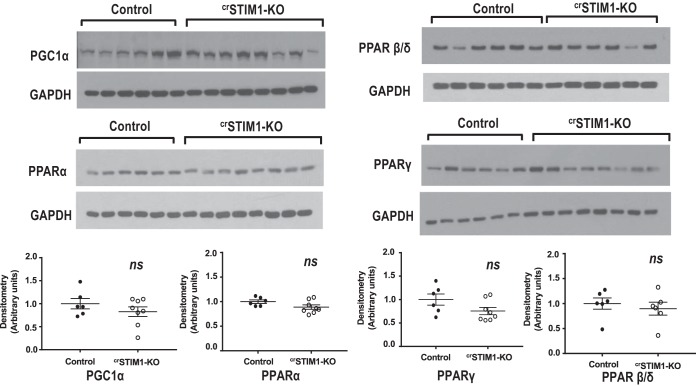
Since the transcription of lipid metabolism genes is regulated by the Ca2+-sensitive PPAR family of receptors, we also examined whole cell expression of PPAR-α, PPAR-β/δ, and PGC-1α in control and crSTIM1-KO hearts. There were no significant differences in the expression of these proteins between the hearts of both genotypes (Fig. 6).
Fig. 6.
Effects of cardiomyocyte-restricted deletion of stromal interaction molecule 1 (crSTIM1-KO) on the expression of peroxisome proliferator-activated receptor (PPAR) and PPAR-related proteins. Top: immunoblots illustrating PPAR-γ coactivator-1α (PGC-1α), PPAR-α, PPAR-β/δ and PPAR-γ in freshly isolated hearts from control and crSTIM1-KO mice. Bottom: densitometric analysis of the immunoblots shown at the top. Protein expression was normalized to GADPH expression. Data were analyzed by a Student’s t-test. For PGC-1α, PPAR-α, and PPAR-γ blots, n = 6 control mice and n = 8 crSTIM1-KO; for the PPAR-β/δ blot, n = 6 control mice and n = 6 crSTIM1-KO mice.
Table 1 shows that plasma glucose, free fatty acids, and triglycerides as well as glycerol and insulin were not different between crSTIM1-KO and littermate control mice. Thus, the observed changes in cardiac metabolism cannot be attributed to differences in available substrates between genotypes.
Table 1.
Analysis of circulating humoral factors in control and crSTIM1-KO plasma samples
| Control | crSTIM1-KO | P Value | |
|---|---|---|---|
| Glucose, mg/dl | 278 ± 10 | 279 ± 7 | NS |
| Free fatty acids, meq/l | 374.0 ± 25.2 | 396.2 ± 33.4 | NS |
| Insulin, ng/ml | 0.795 ± 0.249 | 0.748 ± 0.138 | NS |
| Triglyceride, mg/dl | 87.63 ± 12.52 | 121.85 ± 15.24 | NS |
| Glycerol, mg/l | 20.04 ± 1.64 | 21.69 ± 1.78 | NS |
Values are means ± SE.
STIM1-KO, cardiomyocyte-restricted deletion of stromal interaction molecule 1; NS, not significant.
Impact of STIM1 deletion on cardiac insulin signaling.
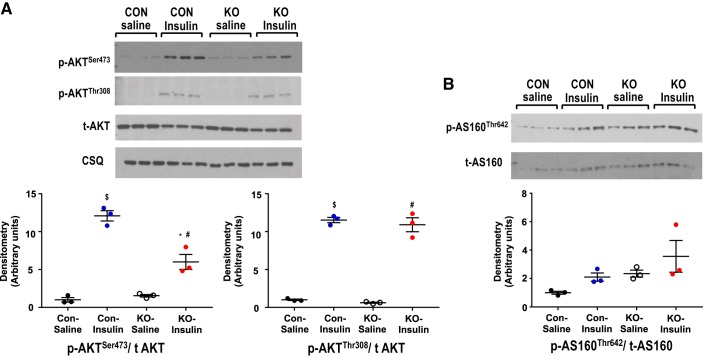
Given that insulin signaling is known to directly impact glucose metabolism, the observed changes in crSTIM1-KO hearts could be the consequence of cardiac insulin resistance; therefore, control and crSTIM1-KO mice were treated with insulin or saline, and hearts were isolated as described above in methods. As anticipated, insulin significantly increased Akt phosphorylation at both Ser473 and Thr308 in control and crSTIM1-KO hearts; however, the insulin-mediated increase in Ser473 was significantly blunted in the crSTIM1-KO group (Fig. 7A). The apparent increase in AS160 phosphorylation at Thr642 with insulin in control and crSTIM1-KO hearts was not statistically significant using ANOVA (Fig. 7B); however, a Students t-test comparing saline-treated control and saline-treated crSTIM-1-KO groups indicated a significant increase in AS160 phosphorylation in the crSTIM-1-KO group.
Fig. 7.
Effects of cardiomyocyte-restricted deletion of stromal interaction molecule 1 (crSTIM1-KO) on insulin-induced activation of Akt and AS160. A, top: immunoblots illustrating Akt phosphorylation in hearts isolated from control and crSTIM1-KO mice in response to either 5-min saline or insulin treatment (0.167 U/kg). A, bottom: densitometric analysis of the immunoblots shown at the top. Protein expression was normalized to calsequestrin (CSQ) expression. B, top: immunoblots illustrating AS160 phosphorylation in hearts isolated from control and crSTIM1-KO mice in response to either 5-min saline or insulin treatment (0.167 U/kg). B, bottom: densitometric analysis of the immunoblots shown at the top. n = 3–6 hearts/experimental group. $P < 0.05, saline-treated vs. insulin-treated control mice; *P < 0.05 insulin-treated control mice vs. insulin-treated crSTIM1-KO mice; #P < 0.05, saline-treated vs. insulin-treated crSTIM1-KO mice; &P < 0.05, saline-treated control mice vs. saline-treated crSTIM1-KO mice (ANOVA/Tukey post hoc test).
Mitochondrial alterations precede changes in the expression of GLUT4 and ACOT1.
To examine whether the changes in metabolism in crSTIM1-KO hearts are a consequence of mitochondrial dysfunction, we examined the expression of metabolic proteins that exhibited the largest changes at 20 wk in addition to mitochondrial parameters and lipid accumulation in 12-wk control and crSTIM1-KO hearts. We found that there were no significant differences in the expression of GLUT4 and ACOT1 between the hearts of both genotypes (Fig. 8A). However, we found that phosphorylation of AMPK at Thr172 was significantly reduced in 12-wk crSTIM1-KO versus control hearts (Fig. 8B). Interestingly, we observed significant changes in mitochondrial size and number at 12 wk in crSTIM1-KO hearts, which was also associated with lipid accumulation (Fig. 8C) and similar to previously reported findings at 20 wk (10). At 20 wk, we also previously observed significant differences in DRP-1 and Mfn2 expression in crSTIM1-KO hearts (10); however, in 12-wk crSTIM1-KO hearts, only DRP-1 was significantly increased without changes in Mfn2 expression (Fig. 8C).
DISCUSSION
It is well established that Ca2+ plays an important role in regulating cardiac metabolism; however, the specific Ca2+ signaling pathways that contribute have been poorly defined. Although STIM1-mediated SOCE is a highly conserved process (48), its role in the heart remains controversial. Consequently, we postulated that STIM1 may contribute to the regulation of cardiac metabolism. We found that lack of cardiomyocyte STIM1 resulted in decreased cardiac glucose metabolism and lipid accumulation. We also observed changes in the expression and phosphorylation of proteins involved in regulating carbohydrate and lipid metabolism and demonstrated that crSTIM1-KO hearts exhibit signs of insulin resistance.
Although the decrease in glucose metabolism in crSTIM1-KO hearts could lead to an energy deficit, it is unlikely that this is the case under these perfusion conditions since contractile function was similar between control and crSTIM1-KO hearts. The decrease in glucose metabolism is likely compensated by an increase in endogenous substrate utilization, most likely triglycerides; however, this was not evaluated in these studies. Changes in the relative contributions of glucose and fatty acids to oxidative metabolism can, in principle, lead to alterations in oxygen consumption; however, in these experiments, hearts were perfused with 1.2 mM oleate, and, consequently, fatty acids provided the primary substrate for oxidative energy metabolism. Thus, the reduction in glucose oxidation in crSTIM1-KO hearts would have minimal effect on overall oxygen consumption, accounting for the similarities in oxygen consumption between the groups. Myocardial substrate utilization is highly dependent on the specific carbon substrates available, the presence of hormones such as insulin, and workload. Therefore, future studies will be needed to assess the consequences of STIM1 deletion on the utilization of other substrates such as lactate, ketone bodies, and amino acids, in addition to evaluating the metabolic responses to physiological stimuli such as increased workload.
The decrease in glucose metabolism in crSTIM1-KO hearts was associated with an ~75% reduction in GLUT4 expression with no change in GLUT1 expression (Fig. 2A). GLUT4 has a higher affinity for glucose than GLUT1 and is responsible for the majority of glucose uptake in the heart (52); therefore, it is likely that the lower GLUT4 protein levels in crSTIM1-KO hearts contributes, at least in part, to the reduction in glycolysis. Since GLUT4 and GLUT1 are regulated by different transcription factors (18, 49), the selective decrease in GLUT4 expression suggests that GLUT4 but not GLUT1 transcription is STIM1 dependent.
As PFK1 is the first rate limiting step in glycolysis, the twofold increase in PFK1 could be an adaptive response to decreased glucose availability. The activity of PFK1 is regulated allosterically by a number of factors; however, its transcriptional regulation is less well understood. The heart also expresses PFK2, a bifunctional enzyme that controls glycolytic flux through regulation of fructose-2,6-bisphosphate levels and 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3, both of which could contribute to the regulation of glycolysis (8). The effects of STIM1 deletion on these regulatory proteins were not determined. We also found a decrease in PDH phosphorylation concomitant with an increase in PDK4 expression, which would also contribute to lower glucose oxidation. Since PDK4 expression is known to be regulated in part by lipid availability, it is possible that the increase in PDK4 is secondary to the increase in lipid accumulation (Fig. 4b) rather than a direct response to the loss of STIM1.
Similar to reports in other systems (7, 31, 38, 54), lack of STIM1 resulted in a marked decrease in AMPK phosphorylation and also a trend toward educed LKB1 expression. While LKB1 is the primary upstream kinase for AMPK, AMPK can also be phosphorylated via Ca2+/calmodulin-dependent kinase kinase-β, which is activated by an increase in cytosolic Ca2+. It should also be noted that AMPK phosphorylation is also regulated by PP2A, which is also activated by Ca2+; thus, an increase in PP2A activity could also reduce AMPK phosphorylation (41). However, owing to the trend in decreased LKB1 levels, PP2A-mediated regulation seems unlikely to be a factor here. Consequently, the reduction in AMPK phosphorylation may be a direct result of an absence of STIM1 and decreased AMPK activation, which could contribute to the reduction in glucose metabolism. In contrast to other reports (5, 33, 50), loss of STIM1 in the heart had no significant changes in basal AKT or GSK-3β phosphorylation.
The accumulation of triglycerides and lipid droplets (Fig. 4) in crSTIM1-KO hearts is consistent with reports showing that STIM1 regulates lipogenesis and lipid storage in Drosophila (4) and that SOCE controls transcriptional regulation of lipid metabolism through modulation of lipolysis and FAO in fibroblasts (36). The fatty acid transporters CD36/FAT and FATP1 were not significantly different between groups, suggesting that increased fatty acid transport was not a factor contributing to increased lipid accumulation (Fig. 5a). However, since translocation of CD36/FAT is both insulin (60) and Ca2+ dependent (2) and since CD36 has been implicated in SOCE (29), it is possible this could be altered in crSTIM1-KO hearts and warrants further investigation. The lipases HSL and ATGL were both increased in crSTIM1-KO groups (Fig. 5D), which is in contrast with the report by Maus et al. (36), who found that both were lower in SOCE-deficient cells. Increased ATGL expression has also been observed in hearts from diabetic mice, where triglycerides are also elevated and cardiac-specific ATGL overexpression was found to ameliorate the effects of diabetes (44, 45, 55). It was concluded that in diabetes, the increase in cardiac ATGL expression was an adaptive yet insufficient response to the increase in triglycerides. It is likely that this is also the reason for increased HSL and ATGL in crSTIM1-KO hearts.
The expression of a number of other proteins involved in lipid metabolism in the heart were also increased in response to STIM1 deletion; these included FAS, FABP4, ACOT1, phospho-ACC, and CPT1 (Fig. 5). While the heart is not a lipogenic organ, it does contain FAS, and here we also showed a twofold increase in FAS levels in crSTIM1-KO hearts (Fig. 5B). Cardiac levels of FAS are increased in heart failure and are also associated with increased lipid accumulation (1) and may be linked to Ca2+ signaling in the heart via activation of CaMKII (47). The role of FABP4 in cardiomyocytes remains unclear, but it has been reported to play a role in transporting fatty acids inside the cell, and it has also been suggested that FABP4 might scavenge reactive lipids (23). ACOT1, a cytosolic enzyme responsible for the hydrolysis of fatty acyl-CoA thioester leading to CoA and free fatty acid (57), has been shown to increase in the heart under conditions of increased availability of fatty acids and elevated triglycerides (14, 42, 69) and in response to increased dietary fat (14, 65). The increases in FAS, FABP4, and ACOT1 expression are all consistent with dysregulation of lipid metabolism in crSTIM1-KO hearts.
Consistent with an overall upregulation of fatty acid metabolism pathways, we also found that CPT1 expression was increased in crSTIM1-KO hearts. This may suggest that alterations in the malonyl-CoA decarboxylase axis may contribute to metabolic changes in crSTIM1-KO hearts; however, since FAO was unchanged between genotypes, it is unlikely that it has a major contribution. The increased ACC phosphorylation in crSTIM1-KO hearts (Fig. 5E), which is indicative of increased mitochondrial β-oxidation, is paradoxical as there was no increase in exogenous FAO in crSTIM1-KO hearts. The increase in ACC phosphorylation is also inconsistent with the decreased AMPK phosphorylation in crSTIM1-KO hearts. However, it has been reported that, in the heart, ACC may be regulated by other kinases, including PKA (15). Lipid accumulation in crSTIM1-KO hearts could also be due to defects in lipophagy, thereby contributing to lipooxidative stress and lipotoxicity, which could be a contributing factor in the cardiomyopathy observed in these mice (10). However, the role of Ca2+ in regulating autophagy/lipophagy is complex, and further studies are needed to determine whether STIM1 contributes to the regulation of these processes in cardiomyocytes. It is somewhat surprising that there were no differences between groups in PGC-1α and PPAR expression given their essential role as transcriptional regulators of lipid metabolism.
Impaired insulin signaling could also be a factor in decreased glucose metabolism in crSTIM1-KO hearts and could also occur in response to increased lipid accumulation. In support of this, we found that while insulin-stimulated phosphorylation of Thr308 on Akt was similar between control and crSTIM1-KO hearts, the increase in phosphorylation of Ser473, which is required for full activation of Akt and subsequent GLUT4 translocation, was significantly blunted in crSTIM1-KO hearts. This finding is consistent with a recent report (5) demonstrating that cardiomyocyte STIM1 knockdown significantly decreased the mammalian target of rapamycin complex 2-Akt signaling axis. However, in that study, there was no examination of insulin signaling. The selective decrease in Ser473 phosphorylation suggests a defect in phosphatidylinositol 3-kinase (PI3K)-mTORC2 signaling but not the PI3K-PDK1 pathway. Surprisingly, we found no significant changes in insulin-mediated activation of AS160 in crSTIM1-KO hearts, as determined by ANOVA (Fig. 7B). Interestingly, analysis of these groups using Student’s t-test indicated that the increases in basal AS160 phosphorylation between control and crSTIM1-KO was significant, although the implications of this are unclear. Measurements of insulin-mediated glucose uptake would provide further insights. Nevertheless, these observations raise the possibility that STIM1 contributes to insulin signaling in cardiomyocytes; however, further studies are clearly needed to better understand this relationship.
In our earlier study (10), we found that in 20-wk crSTIM1-KO hearts, there were alterations in mitochondrial morphology, suggestive of an increase in mitochondrial fission. This raises the question as to whether the metabolic changes seen could occur secondary to the mitochondrial changes; therefore, we looked at a number of parameters in 12-wk crSTIM1-KO hearts. There were no significant changes in GLUT4 or ACOT1 (Fig. 8A), both of which exhibited large differences between genotypes at 20 wk of age. Interestingly, however, AMPK phosphorylation (Fig. 8B) was already significantly lower in the crSTIM1-KO group. In addition, changes in mitochondrial morphology at 12 wk were similar to those seen at 20 wk, and there was also a significant increase in lipid droplets at 12 wk (Fig. 8C). We used mitochondrial length as an index of mitochondrial size in the present study and our previous study (10); however, quantification of mitochondrial perimeter may yield additional insights. Although additional studies are clearly required, these data suggest that alterations in mitochondrial function and resulting lipid accumulation could precede and lead to the impaired glucose metabolism and insulin signaling observed at 20 wk of age. It also seems likely that the progressive mitochondrial phenotype coupled with changes in metabolism contribute to the cardiomyopathy associated with these mice later in life.
Currently, the only accepted role of STIM1 in the heart is in the regulation of cardiac hypertrophy (5, 35, 39); however, here, we have shown that loss of STIM1 in the heart is associated with a reduction in glucose metabolism, impaired insulin signaling, and dysregulation of lipid metabolism. STIM1 mutations and variants observed in humans are associated with severe immunodeficiency (43); consequently, the role of STIM1 in the cardiovascular system is typically overlooked. Interestingly, the metabolic remodelling seen here is remarkably similar to that seen in the heart in response to diabetes, which leads to decreased STIM1 protein levels (17, 28). The specific mechanisms linking STIM1 to metabolic regulation in the heart clearly warrant further investigation. Nevertheless, our findings are consistent with recent reports of STIM1-dependent alterations in lipid metabolism in other cells (4, 19, 22, 36) and suggest, for the first time, that STIM1 could be a previously unrecognized regulator of cardiac metabolism.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-110366 (to J. C. Chatham), HL-106199, HL-074259, and HL-123574 (to M. E. Young), HL-122975 (to J. C. Chatham and M. E. Young) and HL-133011 (to A. R. Wende), a William W Featheringill Postdoctoral Fellowship (University of Alabama at Birmingham Comprehensive Cardiovascular Center; to H. E. Collins), American Heart Association Southeast Affiliate Postdoctoral Fellowship 15POST25260004 (to H. E. Collins), and American Diabetes Association Postdoctoral Fellowship 1-16-PDF-024 (to H. E. Collins). This work was also supported by a University of Alabama at Birmingham AMC21 reload multi-investigator grant (to J. C. Chatham, M. E. Young, and A. R. Wende).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.E.C., B.M.P., and L.Z. performed experiments; H.E.C. and S.H.L. analyzed data; H.E.C., S.H.L., A.R.W., M.E.Y., and J.C.C. interpreted results of experiments; H.E.C. prepared figures; H.E.C. drafted manuscript; H.E.C., S.H.L., A.R.W., M.E.Y., and J.C.C. edited and revised manuscript; H.E.C., B.M.P., L.Z., S.H.L., A.R.W., M.E.Y., and J.C.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge our animal care staff for excellent animal care and all members of the Young, Wende, and Chatham laboratories for technical assistance and valued discussions.
REFERENCES
- 1.Abdalla S, Fu X, Elzahwy SS, Klaetschke K, Streichert T, Quitterer U. Up-regulation of the cardiac lipid metabolism at the onset of heart failure. Cardiovasc Hematol Agents Med Chem 9: 190–206, 2011. doi: 10.2174/187152511797037583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angin Y, Schwenk RW, Nergiz-Unal R, Hoebers N, Heemskerk JW, Kuijpers MJ, Coumans WA, van Zandvoort MA, Bonen A, Neumann D, Glatz JF, Luiken JJ. Calcium signaling recruits substrate transporters GLUT4 and CD36 to the sarcolemma without increasing cardiac substrate uptake. Am J Physiol Endocrinol Metab 307: E225–E236, 2014. doi: 10.1152/ajpendo.00655.2013. [DOI] [PubMed] [Google Scholar]
- 3.Balaban RS. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim Biophys Acta 1787: 1334–1341, 2009. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumbach J, Hummel P, Bickmeyer I, Kowalczyk KM, Frank M, Knorr K, Hildebrandt A, Riedel D, Jäckle H, Kühnlein RP. A Drosophila in vivo screen identifies store-operated calcium entry as a key regulator of adiposity. Cell Metab 19: 331–343, 2014. doi: 10.1016/j.cmet.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Bénard L, Oh JG, Cacheux M, Lee A, Nonnenmacher M, Matasic DS, Kohlbrenner E, Kho C, Pavoine C, Hajjar RJ, Hulot JS. Cardiac Stim1 silencing impairs adaptive hypertrophy and promotes heart failure through inactivation of mTORC2/Akt signaling. Circulation 133: 1458–1471, 2016. doi: 10.1161/CIRCULATIONAHA.115.020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 7.Bhavsar SK, Schmidt S, Bobbala D, Nurbaeva MK, Hosseinzadeh Z, Merches K, Fajol A, Wilmes J, Lang F. AMPKalpha1-sensitivity of Orai1 and Ca2+ entry in T-lymphocytes. Cell Physiol Biochem 32: 687–698, 2013. doi: 10.1159/000354472. [DOI] [PubMed] [Google Scholar]
- 8.Bockus LB, Matsuzaki S, Vadvalkar SS, Young ZT, Giorgione JR, Newhardt MF, Kinter M, Humphries KM. Cardiac insulin signaling regulates glycolysis through phosphofructokinase 2 content and activity. J Am Heart Assoc 6: e007159, 2017. doi: 10.1161/JAHA.117.007159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED, Young ME. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol 294: H1036–H1047, 2008. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 10.Collins HE, He L, Zou L, Qu J, Zhou L, Litovsky SH, Yang Q, Young ME, Marchase RB, Chatham JC. Stromal interaction molecule 1 is essential for normal cardiac homeostasis through modulation of ER and mitochondrial function. Am J Physiol Heart Circ Physiol 306: H1231–H1239, 2014. doi: 10.1152/ajpheart.00075.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787: 1309–1316, 2009. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Durgan DJ, Moore MW, Ha NP, Egbejimi O, Fields A, Mbawuike U, Egbejimi A, Shaw CA, Bray MS, Nannegari V, Hickson-Bick DL, Heird WC, Dyck JR, Chandler MP, Young ME. Circadian rhythms in myocardial metabolism and contractile function: influence of workload and oleate. Am J Physiol Heart Circ Physiol 293: H2385–H2393, 2007. doi: 10.1152/ajpheart.01361.2006. [DOI] [PubMed] [Google Scholar]
- 13.Durgan DJ, Pat BM, Laczy B, Bradley JA, Tsai JY, Grenett MH, Ratcliffe WF, Brewer RA, Nagendran J, Villegas-Montoya C, Zou C, Zou L, Johnson RL Jr, Dyck JR, Bray MS, Gamble KL, Chatham JC, Young ME. O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J Biol Chem 286: 44606–44619, 2011. doi: 10.1074/jbc.M111.278903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durgan DJ, Smith JK, Hotze MA, Egbejimi O, Cuthbert KD, Zaha VG, Dyck JR, Abel ED, Young ME. Distinct transcriptional regulation of long-chain acyl-CoA synthetase isoforms and cytosolic thioesterase 1 in the rodent heart by fatty acids and insulin. Am J Physiol Heart Circ Physiol 290: H2480–H2497, 2006. doi: 10.1152/ajpheart.01344.2005. [DOI] [PubMed] [Google Scholar]
- 15.Dyck JR, Kudo N, Barr AJ, Davies SP, Hardie DG, Lopaschuk GD. Phosphorylation control of cardiac acetyl-CoA carboxylase by cAMP-dependent protein kinase and 5′-AMP activated protein kinase. Eur J Biochem 262: 184–190, 1999. doi: 10.1046/j.1432-1327.1999.00371.x. [DOI] [PubMed] [Google Scholar]
- 16.Erickson JR. Mechanisms of CaMKII Activation in the Heart. Front Pharmacol 5: 59, 2014. doi: 10.3389/fphar.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estrada IA, Donthamsetty R, Debski P, Zhou MH, Zhang SL, Yuan JX, Han W, Makino A. STIM1 restores coronary endothelial function in type 1 diabetic mice. Circ Res 111: 1166–1175, 2012. doi: 10.1161/CIRCRESAHA.112.275743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fandos C, Sánchez-Feutrie M, Santalucía T, Viñals F, Cadefau J, Gumà A, Cussó R, Kaliman P, Canicio J, Palacín M, Zorzano A. GLUT1 glucose transporter gene transcription is repressed by Sp3. Evidence for a regulatory role of Sp3 during myogenesis. J Mol Biol 294: 103–119, 1999. doi: 10.1006/jmbi.1999.3216. [DOI] [PubMed] [Google Scholar]
- 19.Graham SJ, Black MJ, Soboloff J, Gill DL, Dziadek MA, Johnstone LS. Stim1, an endoplasmic reticulum Ca2+ sensor, negatively regulates 3T3-L1 pre-adipocyte differentiation. Differentiation 77: 239–247, 2009. doi: 10.1016/j.diff.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graves BM, Simerly T, Li C, Williams DL, Wondergem R. Phosphoinositide-3-kinase/akt - dependent signaling is required for maintenance of [Ca2+]i, ICa, and Ca2+ transients in HL-1 cardiomyocytes. J Biomed Sci 19: 59, 2012. doi: 10.1186/1423-0127-19-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto R, Umemoto S, Guo F, Umeji K, Itoh S, Kishi H, Kobayashi S, Matsuzaki M. Nifedipine activates PPARgamma and exerts antioxidative action through Cu/ZnSOD independent of blood-pressure lowering in SHRSP. J Atheroscler Thromb 17: 785–795, 2010. doi: 10.5551/jat.4556. [DOI] [PubMed] [Google Scholar]
- 22.Holowka D, Korzeniowski MK, Bryant KL, Baird B. Polyunsaturated fatty acids inhibit stimulated coupling between the ER Ca2+ sensor STIM1 and the Ca2+ channel protein Orai1 in a process that correlates with inhibition of stimulated STIM1 oligomerization. Biochim Biophys Acta 1841: 1210–1216, 2014. doi: 10.1016/j.bbalip.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotamisligil GS, Bernlohr DA. Metabolic functions of FABPs−mechanisms and therapeutic implications. Nat Rev Endocrinol 11: 592–605, 2015. doi: 10.1038/nrendo.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houten SM, Chegary M, Te Brinke H, Wijnen WJ, Glatz JF, Luiken JJ, Wijburg FA, Wanders RJ. Pyruvate dehydrogenase kinase 4 expression is synergistically induced by AMP-activated protein kinase and fatty acids. Cell Mol Life Sci 66: 1283–1294, 2009. doi: 10.1007/s00018-009-9066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang JM, Xian H, Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl Acad Sci USA 89: 6452–6456, 1992. doi: 10.1073/pnas.89.14.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hulot JS, Fauconnier J, Ramanujam D, Chaanine A, Aubart F, Sassi Y, Merkle S, Cazorla O, Ouillé A, Dupuis M, Hadri L, Jeong D, Mühlstedt S, Schmitt J, Braun A, Bénard L, Saliba Y, Laggerbauer B, Nieswandt B, Lacampagne A, Hajjar RJ, Lompré AM, Engelhardt S. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation 124: 796–805, 2011. doi: 10.1161/CIRCULATIONAHA.111.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishii N, Matsumura T, Kinoshita H, Fukuda K, Motoshima H, Senokuchi T, Nakao S, Tsutsumi A, Kim-Mitsuyama S, Kawada T, Takeya M, Miyamura N, Nishikawa T, Araki E. Nifedipine induces peroxisome proliferator-activated receptor-gamma activation in macrophages and suppresses the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 30: 1598–1605, 2010. doi: 10.1161/ATVBAHA.109.202309. [DOI] [PubMed] [Google Scholar]
- 28.Jardín I, López JJ, Zbidi H, Bartegi A, Salido GM, Rosado JA. Attenuated store-operated divalent cation entry and association between STIM1, Orai1, hTRPC1 and hTRPC6 in platelets from type 2 diabetic patients. Blood Cells Mol Dis 46: 252–260, 2011. doi: 10.1016/j.bcmd.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Kuda O, Jenkins CM, Skinner JR, Moon SH, Su X, Gross RW, Abumrad NA. CD36 protein is involved in store-operated calcium flux, phospholipase A2 activation, and production of prostaglandin E2. J Biol Chem 286: 17785–17795, 2011. doi: 10.1074/jbc.M111.232975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusuhara K, Madsen K, Jensen L, Hellsten Y, Pilegaard H. Calcium signalling in the regulation of PGC-1alpha, PDK4 and HKII mRNA expression. Biol Chem 388: 481–488, 2007. doi: 10.1515/BC.2007.052. [DOI] [PubMed] [Google Scholar]
- 31.Lang F, Eylenstein A, Shumilina E. Regulation of Orai1/STIM1 by the kinases SGK1 and AMPK. Cell Calcium 52: 347–354, 2012. doi: 10.1016/j.ceca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Lee KS, Park JH, Lee S, Lim HJ, Park HY. PPARdelta activation inhibits angiotensin II induced cardiomyocyte hypertrophy by suppressing intracellular Ca2+ signaling pathway. J Cell Biochem 106: 823–834, 2009. doi: 10.1002/jcb.22038. [DOI] [PubMed] [Google Scholar]
- 33.Li T, Finch EA, Graham V, Zhang ZS, Ding JD, Burch J, Oh-hora M, Rosenberg P. STIM1-Ca2+ signaling is required for the hypertrophic growth of skeletal muscle in mice. Mol Cell Biol 32: 3009–3017, 2012. doi: 10.1128/MCB.06599-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu SY, Huang ZM, Huang WK, Liu XY, Chen YY, Shi T, Zhang J. How calcium inhibits the magnesium-dependent kinase GSK3β: a molecular simulation study. Proteins 81: 740–753, 2013. doi: 10.1002/prot.24221. [DOI] [PubMed] [Google Scholar]
- 35.Luo X, Hojayev B, Jiang N, Wang ZV, Tandan S, Rakalin A, Rothermel BA, Gillette TG, Hill JA. STIM1-dependent store-operated Ca2+ entry is required for pathological cardiac hypertrophy. J Mol Cell Cardiol 52: 136–147, 2012. doi: 10.1016/j.yjmcc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maus M, Cuk M, Patel B, Lian J, Ouimet M, Kaufmann U, Yang J, Horvath R, Hornig-Do HT, Chrzanowska-Lightowlers ZM, Moore KJ, Cuervo AM, Feske S. Store-operated Ca2+ entry controls induction of lipolysis and the transcriptional reprogramming to lipid metabolism. Cell Metab 25: 698–712, 2017. doi: 10.1016/j.cmet.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGinnis GR, Tang Y, Brewer RA, Brahma MK, Stanley HL, Shanmugam G, Rajasekaran NS, Rowe GC, Frank SJ, Wende AR, Abel ED, Taegtmeyer H, Litovsky S, Darley-Usmar V, Zhang J, Chatham JC, Young ME. Genetic disruption of the cardiomyocyte circadian clock differentially influences insulin-mediated processes in the heart. J Mol Cell Cardiol 110: 80–95, 2017. doi: 10.1016/j.yjmcc.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol 31: 3531–3545, 2011. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohba T, Watanabe H, Murakami M, Sato T, Ono K, Ito H. Essential role of STIM1 in the development of cardiomyocyte hypertrophy. Biochem Biophys Res Commun 389: 172–176, 2009. doi: 10.1016/j.bbrc.2009.08.117. [DOI] [PubMed] [Google Scholar]
- 40.Otto DA, Ontko JA. Activation of mitochondrial fatty acid oxidation by calcium. Conversion to the energized state. J Biol Chem 253: 789–799, 1978. [PubMed] [Google Scholar]
- 41.Park S, Scheffler TL, Rossie SS, Gerrard DE. AMPK activity is regulated by calcium-mediated protein phosphatase 2A activity. Cell Calcium 53: 217–223, 2013. doi: 10.1016/j.ceca.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Peliciari-Garcia RA, Goel M, Aristorenas JA, Shah K, He L, Yang Q, Shalev A, Bailey SM, Prabhu SD, Chatham JC, Gamble KL, Young ME. Altered myocardial metabolic adaptation to increased fatty acid availability in cardiomyocyte-specific CLOCK mutant mice. Biochim Biophys Acta 1861: 1579–1595, 2016. doi: 10.1016/j.bbalip.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picard C, McCarl CA, Papolos A, Khalil S, Lüthy K, Hivroz C, LeDeist F, Rieux-Laucat F, Rechavi G, Rao A, Fischer A, Feske S. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med 360: 1971–1980, 2009. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pulinilkunnil T, Kienesberger PC, Nagendran J, Sharma N, Young ME, Dyck JR. Cardiac-specific adipose triglyceride lipase overexpression protects from cardiac steatosis and dilated cardiomyopathy following diet-induced obesity. Int J Obes 38: 205–215, 2014. doi: 10.1038/ijo.2013.103. [DOI] [PubMed] [Google Scholar]
- 45.Pulinilkunnil T, Kienesberger PC, Nagendran J, Waller TJ, Young ME, Kershaw EE, Korbutt G, Haemmerle G, Zechner R, Dyck JR. Myocardial adipose triglyceride lipase overexpression protects diabetic mice from the development of lipotoxic cardiomyopathy. Diabetes 62: 1464–1477, 2013. doi: 10.2337/db12-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev 231: 10–22, 2009. doi: 10.1111/j.1600-065X.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- 47.Razani B, Zhang H, Schulze PC, Schilling JD, Verbsky J, Lodhi IJ, Topkara VK, Feng C, Coleman T, Kovacs A, Kelly DP, Saffitz JE, Dorn GW II, Nichols CG, Semenkovich CF. Fatty acid synthase modulates homeostatic responses to myocardial stress. J Biol Chem 286: 30949–30961, 2011. doi: 10.1074/jbc.M111.230508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445, 2005. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santalucía T, Moreno H, Palacín M, Yacoub MH, Brand NJ, Zorzano A. A novel functional co-operation between MyoD, MEF2 and TRalpha1 is sufficient for the induction of GLUT4 gene transcription. J Mol Biol 314: 195–204, 2001. doi: 10.1006/jmbi.2001.5091. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt S, Liu G, Liu G, Yang W, Honisch S, Pantelakos S, Stournaras C, Hönig A, Lang F. Enhanced Orai1 and STIM1 expression as well as store operated Ca2+ entry in therapy resistant ovary carcinoma cells. Oncotarget 5: 4799–4810, 2014. doi: 10.18632/oncotarget.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schönekess BO, Brindley PG, Lopaschuk GD. Calcium regulation of glycolysis, glucose oxidation, and fatty acid oxidation in the aerobic and ischemic heart. Can J Physiol Pharmacol 73: 1632–1640, 1995. doi: 10.1139/y95-725. [DOI] [PubMed] [Google Scholar]
- 52.Shao D, Tian R. Glucose transporters in cardiac metabolism and hypertrophy. Compr Physiol 6: 331–351, 2015. doi: 10.1002/cphy.c150016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steinberg SF. Cardiac actions of protein kinase C isoforms. Physiology (Bethesda) 27: 130–139, 2012. doi: 10.1152/physiol.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sundivakkam PC, Natarajan V, Malik AB, Tiruppathi C. Store-operated Ca2+ entry (SOCE) induced by protease-activated receptor-1 mediates STIM1 protein phosphorylation to inhibit SOCE in endothelial cells through AMP-activated protein kinase and p38β mitogen-activated protein kinase. J Biol Chem 288: 17030–17041, 2013. doi: 10.1074/jbc.M112.411272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki J, Shen WJ, Nelson BD, Patel S, Veerkamp JH, Selwood SP, Murphy GM Jr, Reaven E, Kraemer FB. Absence of cardiac lipid accumulation in transgenic mice with heart-specific HSL overexpression. Am J Physiol Endocrinol Metab 281: E857–E866, 2001. doi: 10.1152/ajpendo.2001.281.4.E857. [DOI] [PubMed] [Google Scholar]
- 56.Terentyev D, Hamilton S. Regulation of sarcoplasmic reticulum Ca2+ release by serine-threonine phosphatases in the heart. J Mol Cell Cardiol 101: 156–164, 2016. doi: 10.1016/j.yjmcc.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tillander V, Alexson SEH, Cohen DE. Deactivating fatty acids: acyl-CoA thioesterase-mediated control of lipid metabolism. Trends Endocrinol Metab 28: 473–484, 2017. doi: 10.1016/j.tem.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, Villegas-Montoya C, Jahoor A, Gonzalez R, Garvey ME, Boland B, Blasier Z, McElfresh TA, Nannegari V, Chow CW, Heird WC, Chandler MP, Dyck JR, Bray MS, Young ME. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem 285: 2918–2929, 2010. doi: 10.1074/jbc.M109.077800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsai JY, Villegas-Montoya C, Boland BB, Blasier Z, Egbejimi O, Gonzalez R, Kueht M, McElfresh TA, Brewer RA, Chandler MP, Bray MS, Young ME. Influence of dark phase restricted high fat feeding on myocardial adaptation in mice. J Mol Cell Cardiol 55: 147–155, 2013. doi: 10.1016/j.yjmcc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Oort MM, van Doorn JM, Bonen A, Glatz JF, van der Horst DJ, Rodenburg KW, Luiken JJ. Insulin-induced translocation of CD36 to the plasma membrane is reversible and shows similarity to that of GLUT4. Biochim Biophys Acta 1781: 61–71, 2008. doi: 10.1016/j.bbalip.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 61.Voelkers M, Salz M, Herzog N, Frank D, Dolatabadi N, Frey N, Gude N, Friedrich O, Koch WJ, Katus HA, Sussman MA, Most P. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol 48: 1329–1334, 2010. doi: 10.1016/j.yjmcc.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watt MJ, Steinberg GR, Heigenhauser GJ, Spriet LL, Dyck DJ. Hormone-sensitive lipase activity and triacylglycerol hydrolysis are decreased in rat soleus muscle by cyclopiazonic acid. Am J Physiol Endocrinol Metab 285: E412–E419, 2003. doi: 10.1152/ajpendo.00023.2003. [DOI] [PubMed] [Google Scholar]
- 63.Whayne TF Jr, Felts JM. Activation of lipoprotein lipase. Evaluation of calcium, magnesium, and ammonium as cofactors. Circ Res 28: 649–654, 1971. doi: 10.1161/01.RES.28.6.649. [DOI] [PubMed] [Google Scholar]
- 64.Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, Smith PJ, Dziadek MA. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J 357: 673–685, 2001. doi: 10.1042/bj3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J 406: 457–467, 2007. doi: 10.1042/BJ20070392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie Y, Gu ZJ, Wu MX, Huang TC, Ou JS, Ni HS, Lin MH, Yuan WL, Wang JF, Chen YX. Disruption of calcium homeostasis by cardiac-specific over-expression of PPAR-γ in mice: A role in ventricular arrhythmia. Life Sci 167: 12–21, 2016. doi: 10.1016/j.lfs.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 67.Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 396: 584–587, 1998. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 68.Yu L, Xu J, Minobe E, Kameyama A, Yang L, Feng R, Hao L, Kameyama M. Role of protein phosphatases in the run down of guinea pig cardiac Cav1.2 Ca2+ channels. Am J Physiol Cell Physiol 310: C773–C779, 2016. doi: 10.1152/ajpcell.00199.2015. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Qiao C, Chang L, Guo Y, Fan Y, Villacorta L, Chen YE, Zhang J. Cardiomyocyte overexpression of FABP4 aggravates pressure overload-induced heart hypertrophy. PLoS One 11: e0157372, 2016. doi: 10.1371/journal.pone.0157372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L, Lookene A, Wu G, Olivecrona G. Calcium triggers folding of lipoprotein lipase into active dimers. J Biol Chem 280: 42580–42591, 2005. doi: 10.1074/jbc.M507252200. [DOI] [PubMed] [Google Scholar]