Abstract
The Notch ligand delta-like ligand 4 (Dll4), upregulated by VEGF, is a key regulator of vessel morphogenesis and function, controlling tip and stalk cell selection during sprouting angiogenesis. Inhibition of Dll4 results in hypersprouting, nonfunctional, poorly perfused vessels, suggesting a role for Dll4 in the formation of mature, reactive, functional vessels, with low permeability and able to restrict fluid and solute exchange. We tested the hypothesis that Dll4 controls transvascular fluid exchange. A recombinant protein expressing only the extracellular portion of Dll4 [soluble Dll4 (sDll4)] induced Notch signaling in endothelial cells (ECs), resulting in increased expression of vascular-endothelial cadherin, but not the tight junctional protein zonula occludens 1, at intercellular junctions. sDll4 decreased the permeability of FITC-labeled albumin across EC monolayers, and this effect was abrogated by coculture with the γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester. One of the known molecular effectors responsible for strengthening EC-EC contacts is PKA, so we tested the effect of modulation of PKA on the sDll4-mediated reduction of permeability. Inhibition of PKA reversed the sDll4-mediated reduction in permeability and reduced expression of the Notch target gene Hey1. Knockdown of PKA reduced sDLL4-mediated vascular-endothelial cadherin junctional expression. sDll4 also caused a significant decrease in the hydraulic conductivity of rat mesenteric microvessels in vivo. This reduction was abolished upon coperfusion with the PKA inhibitor H89 dihydrochloride. These results indicate that Dll4 signaling through Notch activation acts through a cAMP/PKA pathway upon intercellular adherens junctions, but not tight junctions, to regulate endothelial barrier function.
NEW & NOTEWORTHY Notch signaling reduces vascular permeability through stimulation of cAMP-dependent protein kinase A.
Keywords: delta-like ligand 4, endothelial cells, Notch, vascular permeability
INTRODUCTION
In mature and quiescent functional vascular networks, the vessel wall forms a semipermeable barrier, which regulates the exchange of fluid and solutes between the blood and tissues. Vascular permeability is also tightly controlled during physiological angiogenesis, in development, in the female reproductive cycle, and during wound healing (6). Dysregulated vessel permeability is a symptom of pathologies such as cancer, diabetes, and cardiovascular disease, where it results in edema, facilitation of metastatic spread, vision loss, proteinuria, and kidney failure (15, 18, 30).
VEGF is the principal mediator of the angiogenic switch and a potent inducer of vessel permeability (5, 7, 25, 40), and, as such, therapies targeting VEGF were developed to reduce permeability back to prepathological levels (38) (44). Administration of anti-VEGF antibodies succeeded in reducing permeability in preclinical trials in cancer and retinal disease (26, 28), and clinical trials have demonstrated that anti-VEGF antibodies reduce edema in the retina in a substantial proportion of patients, but not all (12). This suggests that regulation of permeability during angiogenesis has multiple components (22). As a result, the therapeutic potential of other molecules, and especially those that act through lateral inhibition such as delta-like ligand 4 (Dll4), has started to be explored (31).
Dll4 is overexpressed in the tumor vasculature (32) and in tumor-bearing animals. The use of a neutralizing antibody has led to an inhibition of tumor growth (37), which was shown to be a consequence of nonproductive angiogenesis (31). Tumors treated with Dll4 inhibitors exhibited reduced pericyte coverage, increased vascular leakage, and impaired vascular integrity (13, 24), which supported the rationale behind Dll4-targeted therapies for cancer treatment. It also hinted at a role for Dll4 in the maintenance of endothelial barrier integrity. More recently, targeting of a delta-like 1 homolog, a tumor pericyte-associated antigen and Notch antagonist, has led to the development of combined vaccination approaches that lead to reduced vascular permeability and vascular normalization (11) (16). We therefore tested the hypothesis that Dll4 could control the permeability of endothelial barriers in vitro and in vivo.
METHODS
Cells, recombinant proteins, and inhibitors.
Human dermal blood endothelial cells (HDBECs) were purchased from PromoCell (Heidelberg, Germany), cultured in EBM-2 complete media (Lonza), and maintained at 37°C in a humidified chamber with 5% CO2. Human umbilical vein endothelial cells (HUVECS) were cultured and expanded in endothelial cell (EC) basal medium (PromoCell).
Recombinant human VEGF-A165a (40 ng/ml), recombinant human soluble Dll4 (sDll4; aa 27–524, 1 μg/ml), the γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT; Tocris BioScience), and the PKA inhibitor N-(2-{[3-(4-bromophenyl)-2-propenyl]amino}ethyl)-5-isoquinolinesulfonamide dihydrochloride (H89; Tocris BioScience, 1 μM) were used to treat HDBECs or to perfuse mesenteric vessels in vivo. For knockdown experiments, 24 h before transfection, HUVECs were seeded at 20,000 cells/cm2. For immunofluorescence, cells were cultured on coverslips coated with 0.2% gelatin in PBS (Sigma-Aldrich). Four different PKA siRNA (Sigma-Aldrich) (Table 1) or scrambled siRNA were transfected using Oligofectamine (Invitrogen) at a final concentration of 200 nM in 100 µl OptiMEM (GIBCO) as per the manufacturer’s instructions.
Table 1.
siRNA sequences
| siRNA | Sequence 5′-3′ |
|---|---|
| PKA1F | 5′-GAACACACCCUGAAUGAAAUU-3′ |
| PKA1F AS | 5′-UUUCAUUCAGGGUGUGUUCUU-3′ |
| PKA2F | 5′-GAACACAGCCCACUUGGAUUU-3′ |
| PKA2FAS | 5′-AUCCAAGUGGGCUGUGUUCUU-3′ |
| PKFA3F | 5′-CAAGGACAACUCAAACUUAUU-3′ |
| PKFA3FAS | 5′-UAAGUUUGAGUUGUCCUUGUU-3′ |
| PKFA4 | 5′-GCUAAGGGCAAAUGAACGAUU-3′ |
| PKA4FAS | 5′-UCGUUCAUUUGCCCUUAGCUU-3′ |
Quantitative RT-PCR.
RNA extraction was performed with TRI reagent (Sigma), and cDNA was generated using the Takara Prime script RT kit. Prevalidated primers for the human genes HES1 and HEY1 as well as GAPDH were purchased from Qiagen. Human β-actin primers and human PKA primers are shown in Table 2. PCRs were performed in triplicate with 10 μl Lightcycler 480 SYBR Green 1 mastermix (Roche), 2 μl primer sets, 2 μl cDNA, and 8 μl water. Expression relative to control was calculated from the threshold cycle (CT) and calculated as the difference between the test CT and the housekeeping gene (ΔCT) subtracted from the mean control sample ΔCT (ΔΔCT) and assuming a doubling efficiency of 1 ().
Table 2.
Primer sequences and sources
| Primer | Source | Sequence | Catalogue number |
|---|---|---|---|
| Hes1 | Qiagen | Not disclosed by supplier | QT00039648 |
| Hey1 | Qiagen | Not disclosed by supplier | QT00035644 |
| GAPDH | Qiagen | Not disclosed by supplier | QT00079247 |
| Actin | |||
| Foward | Qiagen | 5′-CCCAGCACAATGAAGATCAA-3′ | |
| Reverse | Qiagen | 5′-CGATCCACACGGAGTACTTG-3′ | |
| PKA | |||
| Forward | Qiagen | 5′-GAAGATCGTCTCTGGGAAGT-3′ | |
| Reverse | Qiagen | 5′-TGACCCCATTCTTGAGGTTC-3′ |
Transwell assay.
Corning transwell inserts (6.5-mm diameter, 0.4-μm pore size) coated with 1% gelatin solution were used to seed HDBECs at a density of 5 × 104 cells/insert. Relative media volumes were 100 μl in the insert and 600 μl in the lower compartment to avoid changes in hydrostatic pressure. Once cells had become confluent, FITC-BSA solution (1 mg/ml) was added to both compartments, and treatments started after 30 min of equilibration. Samples of phenol-free media were taken from both compartments at 30-min intervals, and their absorbance was read at 492/520-nm absorption/emission.
Immunofluorescence, staining, and imaging.
Sterile 13-mm2 coverslips coated with 0.2 mg/cm2 fibronectin were used to plate 3 × 104 HDBECs or 0.2% gelatin for HUVECs. Cells were incubated until a monolayer was formed and then treated for 4 h with vehicle, VEGF, or sDll4. The media were then discarded, and cells were fixed in ice-cold ethanol at −20°C for 30 min, washed in PBS, and incubated at 4°C overnight with rabbit polyclonal anti-activated Notch1 antibody (ab8925, Abcam, 1:200), polyclonal rabbit anti-vascular-endothelial (VE-)cadherin antibody (ab33168, Abcam, 5 μg/ml), or polyclonal rabbit anti-zonula occludens (ZO)-1 antibody (ab59720, Abcam, 10 μg/ml). The following day, cells were washed and stained with donkey anti-rabbit Alexa Fluor 555-conjugated antibody (Life Technologies, 1 μg/ml) and phalloidin Alexa Fluor 488-conjugated antibody (Life Technologies, 1:500). Nuclei were stained with DAPI, and coverslips were mounted onto microscope slides with anti-fade Vectashield. z-Stack images of five regions from each coverslip were acquired with a confocal microscope at ×40 magnification. Analysis was undertaken blinded using ImageJ analysis software.
All in vitro experiments were performed in triplicate and repeated three times unless otherwise stated. Data are presented as means ± SE with the number of experimental independent replicates being the n number given and used for statistical analysis. Post hoc power analysis indicates that all significant differences were achieved with a power greater than 80%. Power for nonsignificant differences is given where stated. Power was calculated using G Power. The percentage of thick and thin junctions and junctional gaps was statistically analyzed using two-way ANOVA with a Bonferroni posttest; fluorescence intensity for both VE-cadherin and ZO-1 staining was statistically analyzed using one-way ANOVA with a Bonferroni posttest. Both analyses used confidence intervals of 95%.
Measurement of hydraulic conductivity.
All animal experiments were conducted according to the Animal (Scientific Procedures) Act of 1986, according to United Kingdom legislation, and conducted in the named establishments under the authority of the Home Office.
Male Han Wistar rats (n = 5 rats/group) were anaesthetized with 2% isoflurane vaporized in 100% O2, and a laparotomy was performed under sterile conditions. The mesentery was draped over a quartz pillar bathed in warmed mammalian Ringer solution and the animal moved to the imaging rig. A refillable glass micropipette was then used to cannulate a postcapillary venule, and the vessel was continuously perfused with a solution of 1% BSA (Sigma) in Ringer solution (pH 7.40). Washed red blood cells (RBCs) were used as flow markers, and the vessel was occluded at 15- to 20-s intervals. After ~8 min, the pipette was refilled again with either control BSA solution, sDll4, or the PKA inhibitor H89, and repeated occlusion of the vessel was continued. At the end of the experiment, the animal was killed by cervical dislocation while still under anesthesia. Video recordings of each vessel were analyzed to calculate hydraulic conductivity (Lp). During each vessel occlusion, we measured the vessel radius and the distance between the occlusion site and a single RBC and calculated its velocity; with this information, we calculated the transcapillary water flow per unit area (Jv/S), as previously described (39). Lp was then calculated as the slope of the relation between Jv/S and pressure. An unpaired t-test was performed using the fold change compared with baseline maximal responses for BSA versus sDll4. One-way ANOVA (with a Bonferroni posttest) was performed for comparison of maximal responses for sDll4 versus H89 and H89 + sDll4. Confidence levels were set at 95%, and all data are presented as means ± SE.
RESULTS
sDll4 activates Notch signaling in ECs.
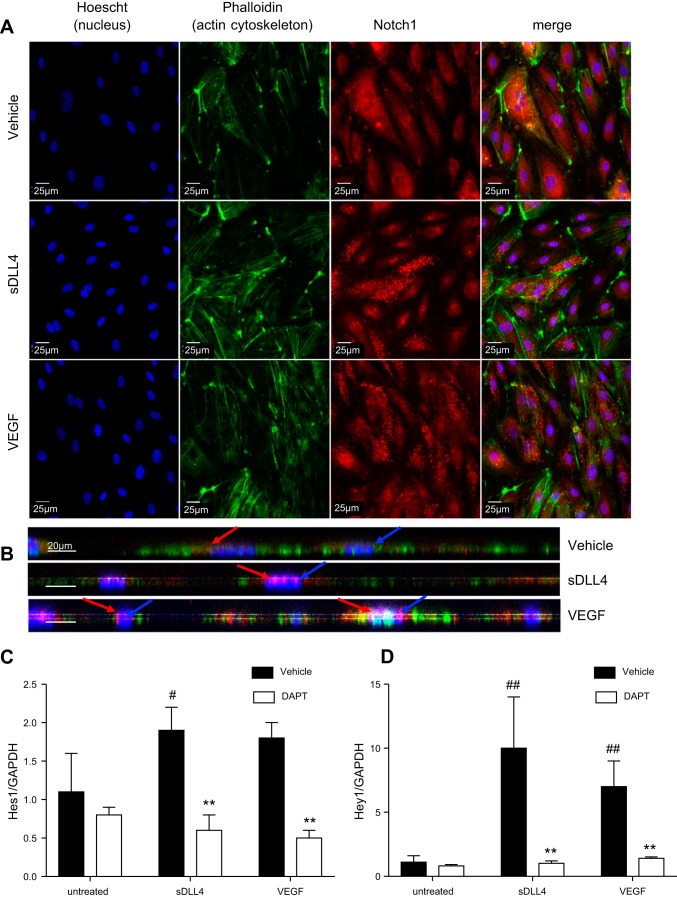
To determine whether we could experimentally induce Dll4 forward signaling we used recombinant soluble Dll4 protein. In confluent cultured ECs, Notch staining was diffuse and more abundant in the cytoplasm (Fig. 1A), whereas treatment with either 1 µg/ml sDll4 or 40 ng/ml VEGF-A led to the appearance of punctate staining of Notch, indicating proteolytic cleavage of Notch, resulting in the release of the Notch intracellular domain (NICD; Fig. 1A). Moreover, in cells treated with 1 µg/ml sDll4, Notch staining tended to be localized inside the nucleus (see side projection in Fig. 1B).
Fig. 1.
Soluble delta-like ligand 4 (sDll4) promotes Notch intracellular signaling in endothelial cells. A: representative confocal images of human dermal blood endothelial cells (HDBECs) treated with VEGF, sDll4, or vehicle control and then stained for Notch1 (red) and phalloidin (green) with nuclear counterstain for Hoechst (blue) to reveal nuclear localization and punctate staining of Notch1 upon VEGF or sDll4 treatment. B: z-projection of cells demonstrating nuclear (blue arrow) expression of Notch1 (red arrow) in sDll4- and VEGF-treated cells but not in vehicle-treated cells. Scale bars = 25 μm. C and D: VEGF and sDll4 treatment resulted in an indistinguishable increase in the expression of Notch1 target genes Hes1 (C) and Hey1 (D) relative to vehicle, as assessed by droplet digtal PCR; this upregulation in RNA transcription was impaired by cotreatment of HDBECs with N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT). **P < 0.01 compared with vehicle; #P < 0.05 and ##P < 0.01 compared with untreated.
To ascertain whether increased NICD translocation translates to induction of translation of target genes, we compared levels of expression of the Notch target genes Hes1 and Hey1. HDBECs were incubated until 80% confluent and then treated for 4 h with vehicle (negative control), VEGF (positive control), or sDll4. Treatment of HDBECs with 1 µg/ml sDll4 recombinant protein upregulated both Hes1 (Fig. 1B) and Hey1 transcripts (Fig. 1C) compared with untreated cells and at a level similar to that elicited by VEGF stimulation. Although this upregulation was more pronounced for Hey1 (around 10-fold increase compared with untreated cells; Fig. 1C) than for Hes1 (around 2-fold increase compared with untreated cells; Fig. 1B), it was completely abrogated by treatment with the γ-secretase inhibitor DAPT, both for Hes1 and Hey1 (Fig. 1, B and C), indicating that sDll4-mediated signaling acts in a manner similar to the canonical understanding of Dll4-Notch1 signaling.
sDll4 promotes endothelial junctional protein expression.
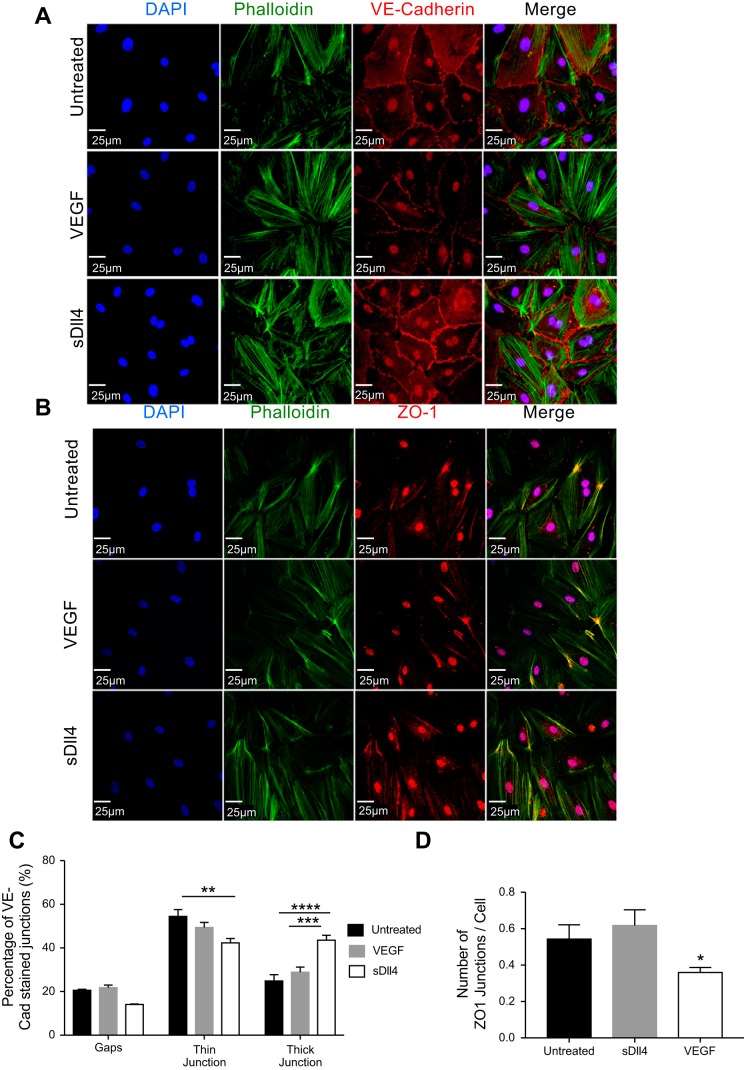
To investigate the effects of Dll4-Notch signaling on vascular permeability, we first investigated changes in junctional proteins. We treated EC monolayers with VEGF or sDll4 and stained cells with phalloidin for cytoskeletal protein F-actin and VE-cadherin for adherens junctions (Fig. 2A) or ZO-1 for tight junctions (Fig. 2B). sDll4 treatment led to a rearrangement of actin fibers in cultured ECs, which were located around the periphery of the cells compared with the parallel arrangement seen in untreated and VEGF-treated cells (Fig. 2A). VE-cadherin staining tended to be localized at EC-EC junctions in untreated cells (Fig. 2A). Similarly, ZO-1 expression was enhanced by sDll4 but reduced by VEGF-A (Fig. 2B). The VE-cadherin-positive junctions could be divided into a thick and thin morphology (Fig. 2C). In untreated cells, the VE-cadherin-positive thin junctions formed the majority (54.5%) and the thick junctions accounted for only 20.9% of the total junctional length. Whereas VEGF caused no significant difference in the distributions of the types of adherens junctions compared with untreated cells, sDll4 significantly decreased the percentage of thin junctions and significantly increased the percentage of thick junctions (Fig. 2C). VEGF treatment also resulted in the lowest number of ZO-1-positive junctions per cell (Fig. 2D). Together, these results indicate that sDll4 leads to a rearrangement of the proteins comprising adherens junctions that could result in an improvement in the barrier function of EC monolayers.
Fig. 2.
Soluble delta-like ligand 4 (sDll4) promotes the rearrangement of adherens junctions. A and B: representative confocal images of human dermal blood endothelial cells (HDBECs) treated with VEGF, sDll4, or vehicle control and then stained for phalloidin (green) and vascular-endothelial (VE-)cadherin for adherens junctions (A; red) or zonula occludens (ZO)-1 for tight junctions (red; B) with nuclear counterstain for DAPI (blue). Scale bars = 25 μm. C and D: image analysis was performed using FIJI to quantify the percentage of intercellular space occupied by gaps or VE-cadherin-positive intercellular junctions between cells (C) and the number of ZO-1-positive junctions per cell (D). VEGF treatment led to an increase in the number of gaps and a decrease in the number of junctions per cell, whereas sDll4 promoted the formation of thicker adherens junctions. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001 (two-way ANOVA with 95% confidence intervals and a Bonferroni posttest).
sDll4 improves endothelial barrier function in vitro.
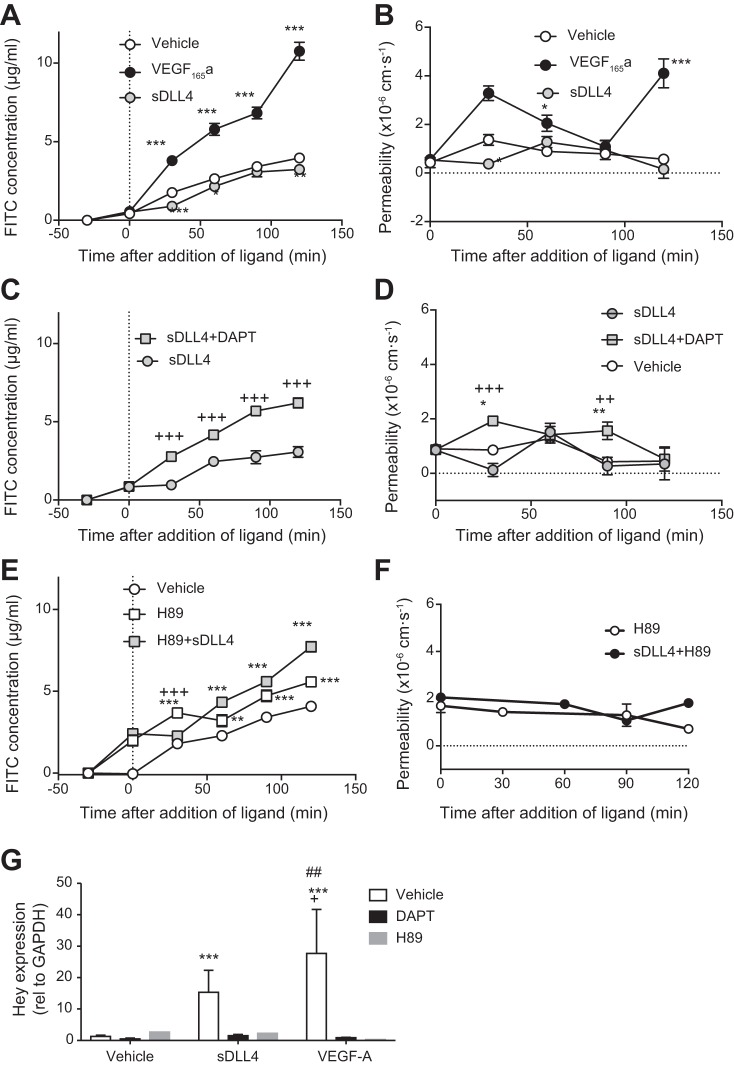
Having established that sDll4 induced Notch signaling in a manner similar to VEGF and that it could affect barrier protein expression in a manner opposite to VEGF, we assessed the effect of sDll4 on permeability of confluent cells. We used a transwell assay to measure the movement of FITC-labeled albumin across a monolayer of ECs (Fig. 3). Treatment of cells with 40 ng/ml VEGF-A resulted in an increase in FITC-BSA in the lower well (Fig. 3A). In contrast, 1 µg/ml sDll4 reduced FITC-BSA in the lower wells compared with control. We calculated the permeability of the monolayer, assuming negligible active transport or convection, based on the solute flux (mass of FITC-BSA that crossed the transwell per 30-min time period), the area of the membrane, and the concentration gradient at that time point. Figure 3B shows that sDll4 resulted in a transient decrease in permeability at 30 min compared with control. VEGF-A resulted in the characteristic biphasic increase in permeability. To determine whether the reduction in permeability by Dll4 was induced through Notch, we treated cells with the γ-secretase inhibitor DAPT for 30 min before addition of the recombinant proteins to the media. This reversed the decrease in FITC-BSA transport (Fig. 3C) and the decrease in permeability (Fig. 3D).
Fig. 3.
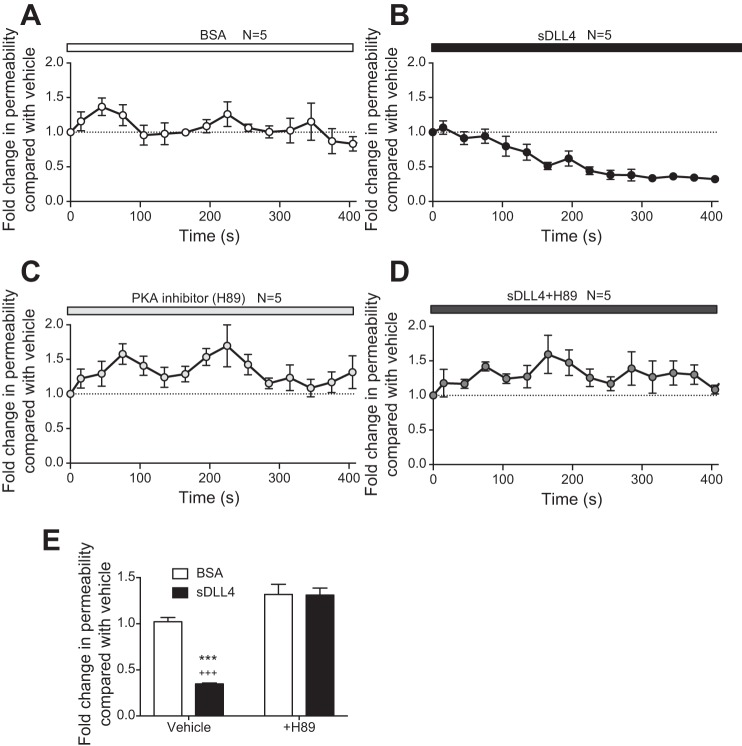
Soluble delta-like ligand 4 (sDll4) decreases endothelial monolayer permeability via cAMP. A: in endothelial cell monolayers, the flux of FITC-labeled BSA reached 4 μg/ml after 2 h (n = 6), whereas in the presence of VEGF-165A, it increased to over 10 μg/ml (n = 8). sDll4 decreased the FITC concentration to below that of vehicle and significantly below that of VEGF (n = 8). B: this translated in a typical biphasic response for VEGF permeability, which was counteracted in a mirroring behavior by the effect of sDll4 on the endothelial cell monolayer. C and D: inhibition of sDll4 (N = 5) with N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT; n = 5) increased FITC concentration (C) and permeability above vehicle (D). E: PKA inhibition with H89 resulted in increased permeability above vehicle (n = 3), which was not impaired by sDll4 (n = 3). F: in effect, permeability was the same for H89 and sDll4 + H89. G: both DAPT and H89 were able to abolish both VEGF- and sDll4-mediated increases in Hey1 gene expression (n = 3 per group). P < 0.001 (ANOVA with a Bonferroni posttest); *P < 0.05, **P < 0.01, and ***P < 0.001 compared with vehicle; #P < 0.05 and ##P < 0.01 compared with DAPT; +P < 0.05, ++P < 0.01, and +++P < 0.001 compared with H89 (ANOVA with a Bonferroni posttest).
Mechanisms underlying decreased permeability are not well described. The most extensive literature on reduced permeability concerns the involvement of PKA signaling through cAMP (1). We therefore investigated whether the PKA inhibitor H89 could reverse the decrease in permeability in HDBEC monolayers. Treatment of cells with 10 µM H89 resulted in a significant increase in FITC-BSA in the lower well above vehicle control (Fig. 3E). Treatment with sDll4 did not reduce the BSA-FITC concentration below control in the presence of H89, and after 1 h there was more, not less, FITC-BSA in sDll4-treated wells than in the vehicle control. Calculation of permeability (Fig. 3F) showed that the H89 baseline value was higher and that sDll4 did not reduce permeability in the presence of H89.
PKA inhibition impairs Notch signaling.
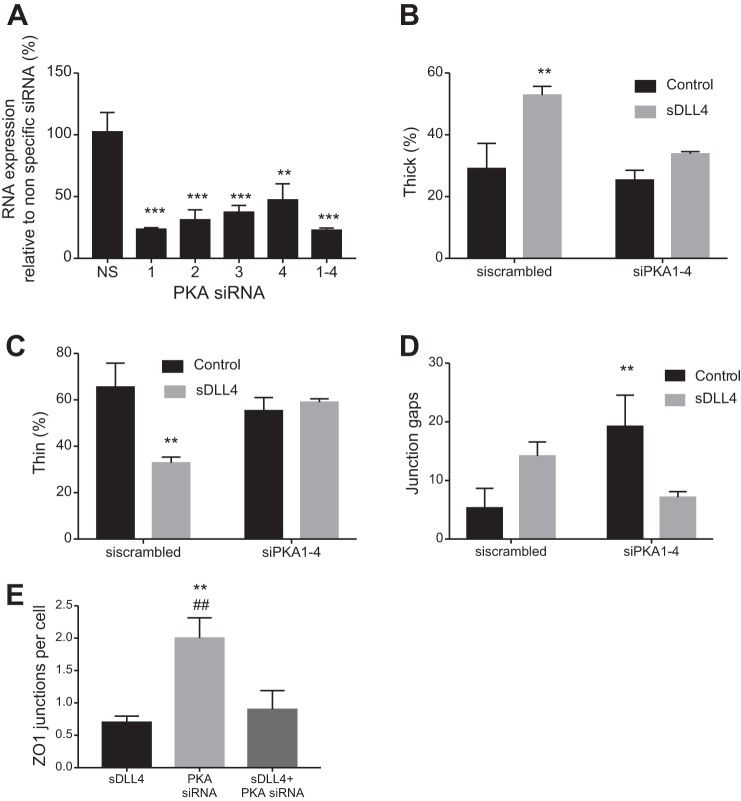
Dll4-Notch has not previously been shown to signal through PKA. Therefore, to determine whether downstream transcriptional activation of Notch target genes could be abrogated by treatment with H89, we measured Hey expression. We cotreated HDBECs with VEGF-A and DAPT or sDll4 and DAPT and obtained results similar to those reported earlier (Fig. 1). Figure 3G also shows that when HDBECs were cotreated with DAPT or H89 (concomitantly with VEGF-A or sDll4), they showed reduced Hey1 expression compared with vehicle. To confirm that PKA inhibition was behind the sDLL4-mediated alteration in barrier function, HUVECs were transfected with four different PKA siRNA or a combination of all four siRNAs, and cells were stained for junctional proteins. All four siRNAs resulted in reduced expression of PKA, and combining all four resulted in a highly significant 77.1 ± 1.6% reduction in RNA expression (Fig. 4A). The DLL4-mediated increase in thick (Fig. 4B) and decrease in thin (Fig. 4C) junctions was blocked by PKA knockdown. PKA knockdown by itself also increased the number of gaps in VE-cadherin stained junctions, but DLL4 again had no effect (Fig. 4D). Interestingly, ZO-1 staining was increased by PKA knockdown (Fig. 4E). These results confirm that activation of Notch signaling, both for its target genes and adherens junctional integrity, requires intact cAMP/PKA signaling.
Fig. 4.
Formation of adherens junctions by delta-like ligand 4 (DLL4) is PKA dependent. A: knockdown of PKA using four different PKA siRNAs. Cells were treated with the indicated siRNAs, and RNA was extracted and amplified by quantitative PCR after 48 h. n = 3 human umbilical vein endothelial cells were stained for vascular-endothelial (VE-)cadherin and zonula occludens (ZO)-1, and junctional type was calculated as described in Fig. 2. The increase in thick junctions (B) and decrease in thin junctions (C) was blocked by PKA knockdown. D: PKA knockdown increased the percentage of gaps in the absence but not the presence of soluble DLL4 (sDLL4). E: ZO-1 junctional staining was increased by PKA knockdown in the absence but not the presence of sDLL4. **P < 0.01 and ***P < 0.001 compared with the scrambled control; ##P < 0.01 compared with sDLL4. In B−E, n = 5 (ANOVA with a Bonferroni posttest).
sDll4 reduces permeability to water in vivo.
To ascertain whether Dll4-Notch signaling resulted in a decrease in vascular permeability in vivo, we measured the effect of sDll4 on individually perfused microvessels of the intact rat mesentery. We used an intravital perfusion and microscopy system to cannulate and perfuse postcapillary venules in the mesentery of anesthetized rats. This enabled control of the oncotic and hydrostatic pressure gradients, expose cells to shear stress, and rely on intact cell signaling. We used the Landis-Michel technique to measure Lp, where RBCs are used as flow markers (Fig. 5). Control experiments showed that refilling of the cannulation pipette with 1% BSA solution did not change Lp (Fig. 5A). In contrast, within 2 min of perfusion with sDll4, Lp consistently began to fall below baseline, plateauing at a minimal value after 5 min, where it remained until the end of the experiment (~10 min; Fig. 5B).
Fig. 5.
Soluble delta-like ligand 4 (sDll4) decreases hydraulic conductivity (Lp) in vivo via a cAMP/PKA-dependent pathway. A: calculation of Lp in postcapillary venules of the mesentery of rats revealed that reperfusion with BSA did not alter vascular permeability (n = 5). B: perfusion with sDll4 showed a clear decrease in permeability compared with BSA within 120 s until it plateaued at 300 s (n = 5). C and D: while H89 perfusion had no effect in water permeability relative to BSA baseline (n = 5; C), perfusion with the combination of H89 and sDll4 did not result in a change in Lp despite constant fluctuations around baseline (n = 5; D). E: relative to BSA vehicle, the peak response in sDll4 gave rise to around 60% decrease in water permeability, which was completely abrogated by H89. P < 0.001 (ANOVA with a Bonferroni posttest); ***P < 0.001 compared with BSA; +++P < 0.001 compared with sDll4 + H89.
sDll4-mediated decrease in Lp acts via cAMP/PKA.
To understand whether the mechanism underlying the reduction in permeability affected by sDll4 also depended on PKA, we perfused mesenteric postcapillary venules with H89. This led to a slight but not statistically significant increase in Lp of these vessels (Fig. 5C). When sDll4 and H89 were perfused together, Lp changed in a manner similar to H89 perfusion (Fig. 5D), not sDll4, making the difference in Lp a very significant increase compared with sDll4 alone (Fig. 5E). These results clearly demonstrate that, in vivo, sDll4 is acting through a cAMP/PKA-dependent pathway to regulate microvessel permeability.
DISCUSSION
The upregulation of the Notch ligand, Dll4 (27), is a downstream consequence of VEGF signaling through VEGF receptor 2 (VEGFR2). The subsequent paracrine reciprocal interaction of Dll4 with Notch has been recognized as the principal molecular mechanism giving rise to the tip or stalk cell phenotypes in sprouting angiogenesis (20, 41). Dll4 will bind to Notch on an adjacent EC membrane, leading to the γ-secretase-mediated proteolytic cleavage of Notch and the subsequent release of the NICD (10). The NICD translocates to the nucleus, where it interacts with recombination signal binding protein for the Ig κ J region (RBPJ-κ). Upon binding, allosteric changes occur in RBPJ-κ that enable the displacement of transcriptional repressors and the subsequent transcription of Notch target genes such as Hes1 and Hey1 (19). Dll4/Notch signaling then negatively regulates VEGFR2 expression (41) with Notch playing an essential part in vascular plexus remodelling and maturation (14). To validate our experimental approach, we compared the effect of VEGF with the extracellular portion of Dll4 (sDll4) and found that sDll4 is sufficient to activate Notch signaling (Fig. 1).
During sprouting angiogenesis, VE-cadherin, the principal component of endothelial adherens junctions, is expressed on the anterior plasma membrane and in filopodia protrusions of tip cells (3). However, the continual flux in Notch levels in individual EC results in differential VE-cadherin turnover and junctional-cortex protrusions (8), which powers differential cell movement when EC compete for the tip cell position, first described in embryoid bodies (21). Thus, Dll4/Notch signaling at EC-EC junctions seems to play an essential role in maintaining endothelial barrier integrity, especially during angiogenesis. Inhibition of γ-secretase with DAPT leads to inhibition of Dll4/Notch signaling (Fig. 1), but it also has effects on other transmembrane proteins. In breast cancer cells, DAPT blocks E-cadherin cleavage (46). In cultured hippocampal neurons, endoplasmic reticulum loss was inhibited by DAPT, and this correlates with proteolytic activity affecting adherens junctions (29). In a rat model of permanent middle cerebral artery occlusion, DAPT reduced the permeability of the blood-brain barrier by decreasing the ubiquitination and degradation of occludin (47). However, when we tested the effect of sDll4 in confluent cultured ECs, we found that sDll4-treated ECs undergo a change in expression and distribution of proteins that constitute adherens junctions, but not those involved in tight junctions (Fig. 2), and reduces permeability in intact quiescent blood vessels. This suggests that the previously described reduction in permeability by inhibition of the Notch pathway may be context dependent (active or confluent ECs) and tissue dependent (blood-brain barrier compared with systemic capillaries).
cAMP has been shown to stabilize the endothelial barrier by reducing myosin light chain phosphorylation (9, 45), inhibiting the GTPase RhoA (34), preventing Rac1 inhibition (43) and acting through Epac/Rap1 to stabilize cortical actin (2), leading to an increase in cytoskeletal-associated VE-cadherin. Here, we add to this knowledge by showing that sDll4 decreases solute flux across an endothelial cell monolayer, that this is reversed by H89, an inhibitor of PKA (Fig. 3), and that this PKA mediated rearrangement controls adherens junctional formation (but not tight junctional components such as ZO-1). This indicates that PKA-dependent cAMP signaling mediates DLL4 mediated VE-cadherin assembly at adherens junctions and promotes endothelial barrier integrity.
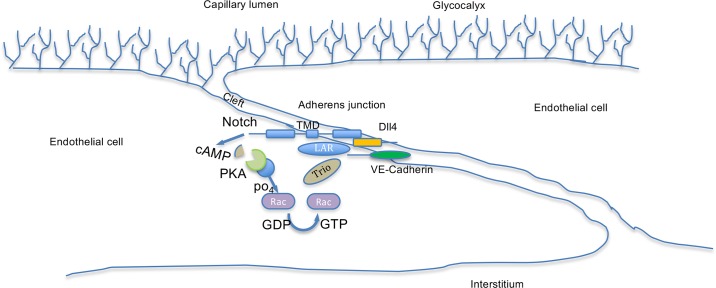
Recently, the link between VE-cadherin and Dll4/Notch signaling has been further explored (33). Using an engineered blood vessel model, the authors found that a novel leukocyte common antigen-related/Trio/Rac1 complex is formed due to Notch “noncanonical” signaling to drive assembly of adherens junctions in response to shear stress. Rac1 has been shown to be downstream of cAMP in thrombin-induced permeability enhancement in ECs (4). In the present study, we further add to mechanistic insights into Dll4/Notch mediated reduction in permeability, finding that this noncanonical signaling is through cAMP-mediated activation of PKA, which causes a reduction in barrier function, presumably through phosphorylation of Rac1, allowing it to facilitate the interaction between the Lar-Notch1 transmembrane domain-VE-cadherin complex through the guanine exchange factor-Trio axis (Fig. 6). Furthermore, we show here that this results in an actual decrease in permeability of the barrier wall in vivo (Fig. 5) rather than just a reduction in solute flux, which could be explained by hemodynamic changes.
Fig. 6.
Schematic for the junctional regulation of vascular-endothelial (VE-)cadherin by delta-like ligand 4 (Dll4)-Notch signaling. The endothelial cells, covered by a layer of glycocalyx, form an intercellular cleft where two endothelial cells meet. The cleft contains adherens junctions and tight junctions (not shown). The adherens junctions consist of VE-cadherin bound to multiple signaling molecules (not shown). Upon activation of Notch by Dll4, cAMP is generated, either directly or indirectly, which results in strengthening of the VE-cadherin junctions. Recent work by Polacheck et al. has shown that the transmembrane domain of Notch can bind to the Lar-Trio-Rac1 complex, which is known to be activated by phosphorylation by PKA.
Over the years, a number of papers have linked the formation of mechanosensing complexes with endothelial barrier function (17, 42). What was once thought of as arterial specification during development may be mechanistically linked to sensing of shear stress (23). Indeed, low shear stress-induced atherosclerotic plaque formation was inhibited by DAPT, with the subsequent downregulation of NICD and ICAM-1 (35). This may not be limited to ECs, since pericyte-derived Dll4 may control involution of infantile hemangioma in a VEGF-independent manner (23). Of interest was the distinguishment of tight junctional protein rearrangement from adherens junctions (Figs. 2 and 4). Whereas sDLL4 did not affect ZO-1 expression, PKA knockdown increased it, which was blocked by sDLL4. This indicates that there are two different mechanisms for regulating permeability through the two different pathways. This could be due to localization of PKA to the different junctional compartments, a mechanism proposed by Radeva et al. (36) to be mediated by A-kinase anchoring protein 12.
In summary, we show here, for the first time, that Dll4-mediated activation of Notch can reduce Lp, and hence the permeability of the capillary wall, through activation of cAMP. This supports the concept that fluid and solute exchange is limited in the normal vasculature by Notch signaling through the regulation of adherens junctions.
GRANTS
This work was supported by Medical Research Council Grants MR/K013157/1 and 1361718, British Heart Foundation Grants PG/13/85/30536 and PG/18/31/33759, and the National Eye Research Centre.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.B., V.P., N.M., L.L., A.V.B., D.O.B., and M.J.M. conceived and designed research; R.B., V.P., N.M., A.P.L., A.V.B., D.O.B., and M.J.M. performed experiments; R.B., V.P., N.M., A.P.L., A.V.B., D.O.B., and M.J.M. analyzed data; R.B., V.P., N.M., L.L., A.V.B., D.O.B., and M.J.M. interpreted results of experiments; R.B., V.P., A.V.B., D.O.B., and M.J.M. prepared figures; R.B., V.P., A.P.L., L.L., A.V.B., D.O.B., and M.J.M. drafted manuscript; R.B., V.P., N.M., A.P.L., L.L., A.V.B., D.O.B., and M.J.M. edited and revised manuscript; R.B., V.P., N.M., A.P.L., L.L., A.V.B., D.O.B., and M.J.M. approved final version of manuscript.
REFERENCES
- 1.Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. Am J Physiol Heart Circ Physiol 274: H1885–H1894, 1998. doi: 10.1152/ajpheart.1998.274.6.H1885. [DOI] [PubMed] [Google Scholar]
- 2.Adamson RH, Ly JC, Sarai RK, Lenz JF, Altangerel A, Drenckhahn D, Curry FE. Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am J Physiol Heart Circ Physiol 294: H1188–H1196, 2008. doi: 10.1152/ajpheart.00937.2007. [DOI] [PubMed] [Google Scholar]
- 3.Almagro S, Durmort C, Chervin-Pétinot A, Heyraud S, Dubois M, Lambert O, Maillefaud C, Hewat E, Schaal JP, Huber P, Gulino-Debrac D. The motor protein myosin-X transports VE-cadherin along filopodia to allow the formation of early endothelial cell-cell contacts. Mol Cell Biol 30: 1703–1717, 2010. doi: 10.1128/MCB.01226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslam M, Tanislav C, Troidl C, Schulz R, Hamm C, Gündüz D. cAMP controls the restoration of endothelial barrier function after thrombin-induced hyperpermeability via Rac1 activation. Physiol Rep 2: e12175, 2014. 10.14814/phy2.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates DO. Chronically increased hydraulic conductivity (Lp) by vascular endothelial growth factor (VEGF) is attenuated by inhibition of calcium influx. J Physiol 513: 225–233, 1998. doi: 10.1111/j.1469-7793.1998.225by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res 87: 262–271, 2010. doi: 10.1093/cvr/cvq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates DO, Curry FE. Vascular endothelial growth factor increases hydraulic conductivity of isolated perfused microvessels. Am J Physiol Heart Circ Physiol 271: H2520–H2528, 1996. doi: 10.1152/ajpheart.1996.271.6.H2520. [DOI] [PubMed] [Google Scholar]
- 8.Bentley K, Franco CA, Philippides A, Blanco R, Dierkes M, Gebala V, Stanchi F, Jones M, Aspalter IM, Cagna G, Weström S, Claesson-Welsh L, Vestweber D, Gerhardt H. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat Cell Biol 16: 309–321, 2014. doi: 10.1038/ncb2926. [DOI] [PubMed] [Google Scholar]
- 9.Bindewald K, Gündüz D, Härtel F, Peters SC, Rodewald C, Nau S, Schäfer M, Neumann J, Piper HM, Noll T. Opposite effect of cAMP signaling in endothelial barriers of different origin. Am J Physiol Cell Physiol 287: C1246–C1255, 2004. doi: 10.1152/ajpcell.00132.2004. [DOI] [PubMed] [Google Scholar]
- 10.Caolo V, van den Akker NM, Verbruggen S, Donners MM, Swennen G, Schulten H, Waltenberger J, Post MJ, Molin DG. Feed-forward signaling by membrane-bound ligand receptor circuit: the case of NOTCH delta-like 4 ligand in endothelial cells. J Biol Chem 285: 40681–40689, 2010. doi: 10.1074/jbc.M110.176065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi Sabins N, Taylor JL, Fabian KP, Appleman LJ, Maranchie JK, Stolz DB, Storkus WJ. DLK1: a novel target for immunotherapeutic remodeling of the tumor blood vasculature. Mol Ther 21: 1958–1968, 2013. doi: 10.1038/mt.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabetic Retinopathy Clinical Research Network; Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ, Elman MJ, Ferris FL, Friedman SM, Melia M, Pieramici DJ, Sun JK, Beck RW. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 372: 1193–1203, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djokovic D, Trindade A, Gigante J, Badenes M, Silva L, Liu R, Li X, Gong M, Krasnoperov V, Gill PS, Duarte A. Combination of Dll4/Notch and Ephrin-B2/EphB4 targeted therapy is highly effective in disrupting tumor angiogenesis. BMC Cancer 10: 641, 2010. doi: 10.1186/1471-2407-10-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehling M, Adams S, Benedito R, Adams RH. Notch controls retinal blood vessel maturation and quiescence. Development 140: 3051–3061, 2013. doi: 10.1242/dev.093351. [DOI] [PubMed] [Google Scholar]
- 15.Erickson KK, Sundstrom JM, Antonetti DA. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis 10: 103–117, 2007. doi: 10.1007/s10456-007-9067-z. [DOI] [PubMed] [Google Scholar]
- 16.Fabian KP, Chi-Sabins N, Taylor JL, Fecek R, Weinstein A, Storkus WJ. Therapeutic efficacy of combined vaccination against tumor pericyte-associated antigens DLK1 and DLK2 in mice. OncoImmunology 6: e1290035, 2017. doi: 10.1080/2162402X.2017.1290035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frye M, Dierkes M, Küppers V, Vockel M, Tomm J, Zeuschner D, Rossaint J, Zarbock A, Koh GY, Peters K, Nottebaum AF, Vestweber D. Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J Exp Med 212: 2267–2287, 2015. doi: 10.1084/jem.20150718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation 17: 206–225, 2010. doi: 10.1111/j.1549-8719.2010.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrington LS, Sainson RC, Williams CK, Taylor JM, Shi W, Li JL, Harris AL. Regulation of multiple angiogenic pathways by Dll4 and Notch in human umbilical vein endothelial cells. Microvasc Res 75: 144–154, 2008. doi: 10.1016/j.mvr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445: 776–780, 2007. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 21.Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol 12: 943–953, 2010. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 22.Jayson GC, Zweit J, Jackson A, Mulatero C, Julyan P, Ranson M, Broughton L, Wagstaff J, Hakannson L, Groenewegen G, Bailey J, Smith N, Hastings D, Lawrance J, Haroon H, Ward T, McGown AT, Tang M, Levitt D, Marreaud S, Lehmann FF, Herold M, Zwierzina H; European Organisation for Research and Treatment of Cancer Biological Therapeutic Development Group . Molecular imaging and biological evaluation of HuMV833 anti-VEGF antibody: implications for trial design of antiangiogenic antibodies. J Natl Cancer Inst 94: 1484–1493, 2002. doi: 10.1093/jnci/94.19.1484. [DOI] [PubMed] [Google Scholar]
- 23.Jones EA, Yuan L, Breant C, Watts RJ, Eichmann A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development 135: 2479–2488, 2008. doi: 10.1242/dev.014902. [DOI] [PubMed] [Google Scholar]
- 24.Kalén M, Heikura T, Karvinen H, Nitzsche A, Weber H, Esser N, Ylä-Herttuala S, Hellström M. Gamma-secretase inhibitor treatment promotes VEGF-A-driven blood vessel growth and vascular leakage but disrupts neovascular perfusion. PLoS One 6: e18709, 2011. doi: 10.1371/journal.pone.0018709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 246: 1309–1312, 1989. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenbeld HC, Ferarra N, Jain RK, Munn LL. Effect of local anti-VEGF antibody treatment on tumor microvessel permeability. Microvasc Res 57: 357–362, 1999. doi: 10.1006/mvre.1998.2140. [DOI] [PubMed] [Google Scholar]
- 27.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol 23: 14–25, 2003. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murata T, Ishibashi T, Khalil A, Hata Y, Yoshikawa H, Inomata H. Vascular endothelial growth factor plays a role in hyperpermeability of diabetic retinal vessels. Ophthalmic Res 27: 48–52, 1995. doi: 10.1159/000267567. [DOI] [PubMed] [Google Scholar]
- 29.Ng AN, Toresson H. Gamma-secretase and metalloproteinase activity regulate the distribution of endoplasmic reticulum to hippocampal neuron dendritic spines. FASEB J 22: 2832–2842, 2008. doi: 10.1096/fj.07-103903. [DOI] [PubMed] [Google Scholar]
- 30.Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, Vink H, Kastelein JJ, Stroes ES. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 55: 480–486, 2006. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- 31.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444: 1032–1037, 2006. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 32.Patel NS, Li JL, Generali D, Poulsom R, Cranston DW, Harris AL. Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Res 65: 8690–8697, 2005. doi: 10.1158/0008-5472.CAN-05-1208. [DOI] [PubMed] [Google Scholar]
- 33.Polacheck WJ, Kutys ML, Yang J, Eyckmans J, Wu Y, Vasavada H, Hirschi KK, Chen CS. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature 552: 258–262, 2017. doi: 10.1038/nature24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 284: L972–L980, 2003. doi: 10.1152/ajplung.00429.2002. [DOI] [PubMed] [Google Scholar]
- 35.Qin WD, Zhang F, Qin XJ, Wang J, Meng X, Wang H, Guo HP, Wu QZ, Wu DW, Zhang MX. Notch1 inhibition reduces low shear stress-induced plaque formation. Int J Biochem Cell Biol 72: 63–72, 2016. doi: 10.1016/j.biocel.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Radeva MY, Kugelmann D, Spindler V, Waschke J. PKA compartmentalization via AKAP220 and AKAP12 contributes to endothelial barrier regulation. PLoS One 9: e106733, 2014. doi: 10.1371/journal.pone.0106733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, Tan C, Hongo JA, de Sauvage F, Plowman G, Yan M. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444: 1083–1087, 2006. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 38.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY; MARINA Study Group . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355: 1419–1431, 2006. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 39.Salmon AH, Neal CR, Sage LM, Glass CA, Harper SJ, Bates DO. Angiopoietin-1 alters microvascular permeability coefficients in vivo via modification of endothelial glycocalyx. Cardiovasc Res 83: 24–33, 2009. doi: 10.1093/cvr/cvp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219: 983–985, 1983. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 41.Suchting S, Freitas C, le Noble F, Benedito R, Bréant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA 104: 3225–3230, 2007. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431, 2005. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 43.Waschke J, Drenckhahn D, Adamson RH, Barth H, Curry FE. cAMP protects endothelial barrier functions by preventing Rac-1 inhibition. Am J Physiol Heart Circ Physiol 287: H2427–H2433, 2004. doi: 10.1152/ajpheart.00556.2004. [DOI] [PubMed] [Google Scholar]
- 44.Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O’Reilly T, Persohn E, Rösel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch KH, Schneider MR, Drevs J, Martiny-Baron G, Totzke F. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res 60: 2178–2189, 2000. [PubMed] [Google Scholar]
- 45.Wysolmerski RB, Lagunoff D. Regulation of permeabilized endothelial cell retraction by myosin phosphorylation. Am J Physiol 261: C32–C40, 1991. doi: 10.1152/ajpcell.1991.261.1.C32. [DOI] [PubMed] [Google Scholar]
- 46.Yoo CB, Yun SM, Jo C, Koh YH. γ-Secretase-dependent cleavage of E-cadherin by staurosporine in breast cancer cells. Cell Commun Adhes 19: 11–16, 2012. doi: 10.3109/15419061.2012.665969. [DOI] [PubMed] [Google Scholar]
- 47.Zhang GS, Tian Y, Huang JY, Tao RR, Liao MH, Lu YM, Ye WF, Wang R, Fukunaga K, Lou YJ, Han F. The γ-secretase blocker DAPT reduces the permeability of the blood-brain barrier by decreasing the ubiquitination and degradation of occludin during permanent brain ischemia. CNS Neurosci Ther 19: 53–60, 2013. doi: 10.1111/cns.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]