Abstract
Cardiac dysfunction is the most frequent cause of morbidity and mortality in amyloid light chain (AL) amyloidosis caused by a clonal immunoglobulin light chain (LC). Previously published transgenic animal models of AL amyloidosis have not recapitulated the key phenotype of cardiac dysfunction seen in AL amyloidosis, which has limited our understanding of the disease mechanisms in vivo, as well as the development of targeted AL therapeutics. We have developed a transgenic zebrafish model in which a λ LC derived from a patient with AL amyloidosis is conditionally expressed in the liver under the control of the Gal4 upstream activation sequence enhancer system. Circulating LC levels of 125 µg/ml in these transgenic zebrafish are comparable to median pathological serum LC levels. Functional analysis links abnormal contractile function with evidence of cellular and molecular proteotoxicity in the heart, including increased cell death and autophagy. However, despite pathological and functional phenotypes analogous to human AL, the lifespan of the transgenic fish is comparable to control fish without the expressed AL-LC transgene. Nuclear labeling experiments suggest increased cardiac proliferation in the transgenic fish, which can be counteracted by treatment with a small molecule proliferation inhibitor leading to increased zebrafish mortality because of cardiac apoptosis and functional deterioration. This transgenic zebrafish model provides a platform to study underlying AL disease mechanisms in vivo further.
NEW & NOTEWORTHY Heart failure is a major cause of mortality in amyloid light (AL) amyloidosis, yet it has been difficult to model in animals. We report the generation of a transgenic zebrafish model for AL amyloidosis with pathological concentration of circulating human light chain protein that results in cardiac dysfunction. The light chain toxicity triggers regeneration in the zebrafish heart resulting in functional compensation early in life, but with age develops into cardiac dysfunction.
Keywords: amyloidosis, in vivo model cardiovascular disease, proteotoxicity, transgenic zebrafish
INTRODUCTION
Amyloid light chain (AL) amyloidosis is a plasma cell dyscrasia resulting from the monoclonal expansion of immunoglobulin (Ig) light chain (LC)-secreting plasma cells. Partly because of inherent structural features, these aberrant LC precursor proteins can misfold, aggregate, and form amyloid fibrils that subsequently deposit in vital organs such as the heart, kidney, and liver (7). Cardiac AL amyloidosis is particularly lethal. Amyloid cardiomyopathy is responsible for ~75% of the mortality associated with AL (6, 21). Prognosis is poor, with median survival of 6 mo (17), as patients develop progressive heart failure if the underlying neoplasia cannot be reduced using chemotherapy or novel antiplasma cell agents (such as proteasome inhibitors or immunomodulatory drugs) (22, 27).
Multiple studies have shown that precursor LC proteins initiate cardiotoxicity directly. Our previous work elucidated intrinsic signaling pathways involved in this toxicity, including cell death, mitochondrial dysfunction, and altered autophagic processes (8, 10, 19, 23, 28). Acute pathological signaling pathways involved in LC-induced cardiac toxicity have been identified; however, the absence of a chronic animal model of systemic AL amyloidosis has precluded a systematic evaluation of the key elements associated with this disorder.
Zebrafish is a well-characterized model to study mechanisms of cardiovascular disease in vivo, and facilitates scalable modeling with substantial native context. Our laboratory has previously used zebrafish to decipher signaling mechanisms associated with LC cardiotoxicity by an acute model of LC injection (23). Herein, we describe a novel chronic transgenic zebrafish model of AL amyloidosis. The amyloidogenic LC was selected from a patient with severe cardiac involvement. This transgenic zebrafish model recapitulates multiple features consistent with the human disease, including high circulating light chain concentration, LC proteotoxicity, and cardiac dysfunction and can be used to understand the molecular pathology of AL, as well as in the development of AL therapeutics.
MATERIALS AND METHODS
Animal care.
All zebrafish procedures and experiments were reviewed and approved by the Institutional Animal Care and Use Committee at Harvard Medical School and Brigham and Women’s Hospital and performed in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Wild-type zebrafish were purchased from Ekkwill Waterlife Resources (Ruskin, FL). Care and breeding of zebrafish were performed as described previously (23).
Generation of transgenic fish.
We used the upstream activation sequence (UAS)-Gal4 conditional expression system to secrete the human λ LC selectively in the liver under the control of the liver fatty acid binding protein (l-fabp) promoter. To ensure that the overexpressed protein would be secreted into circulation and not remain within the hepatocyte, a secretory sequence peptide (SSP) was appended to the chosen human AL-LC. L-fabp activation peaks at 48 h postfertilization (hpf) and remains activated through adulthood, ensuring unimpeded production of the exogenous human LC (11).
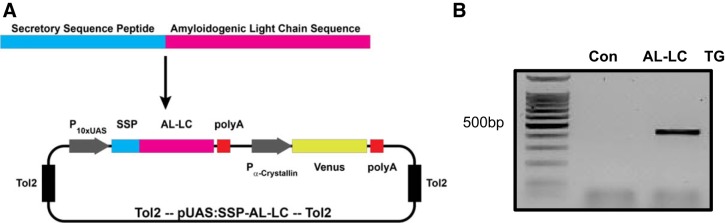
The Ig LC sequence derived from the λ 1b (IGLV1-51) germline gene was characterized from a patient with cardiac amyloidosis (identified as H7) at the Center for Research and Treatment of Systemic Amyloidosis in Pavia, Italy (25). The LC sequence spanning the variable, joining, and constant domains was synthesized synthetically. An attB1 gateway site, zebrafish SSP, and a kozak sequence (GGACC) were prepended to the H7 LC sequence, and an attB2 gateway site was appended to the H7 LC sequence by PCR. This was followed by subsequent Gateway BP cloning into pDONR221 (Thermo Scientific) and subsequently LR cloning into pDestTol2-alphacrystallin-YFP (24) with the p5E-UAS (10×UAS GAL4 promoter) and p3E-polyA (polyAdenylation sequence) from the Tol2kit v1.2 to create pEXPRTol2-UAS-SSP-H7 (Fig. 1A). Gateway recombination reactions were performed according to the manufacturer’s instructions (ThermoFisher Scientific).
Fig. 1.
Generation of a transgenic zebrafish line expressing an immunoglobulin λ 1b (IGLV1-51) light chain (LC) from a patient with AL amyloidosis. The λ LC nucleotide sequence [H7 (25)] from the clonal plasma cells of a patient with cardiac AL amyloidosis was synthesized with a secretory sequence peptide (SSP) and kozak sequence (GGACC), and cloned using Gateway BP into pDONR221 (Thermo Scientific) and subsequently subcloned with the p5E-UAS (10×UAS GAL4 promoter) and p3E-polyA (polyA sequence) from the Tol2kit v1.2 via LR cloning into pDestTol2-alphaCrystallin-YFP to create pEXPRTol2-UAS-SSP-H7 (A). Transgene incorporation was confirmed by PCR on DNA isolated from 48 hpf embryos using primers described in materials and methods (B). Con, control; hpf, hours postfertilization; TG, transgenic; UAS, upstream activation sequence; YFP, yellow fluorescent protein.
The transgenic AL-LC zebrafish were generated using the Tol2 method as described previously, by coinjection of our Tol2 site flanked pEXPRTol2-UAS-SSP-H7 plasmid (15 ng/μl) and Tol2 transposase mRNA (10 ng/μl) directly into the one cell-stage embryo (16). Following injection, 48 hpf embryos (F0) were screened based on the eye yellow fluorescent protein (YFP) marker (alphacrystallin-YFP) indicating transgene expression. At the age of 8 wk, transgenic fish were crossed with wild-type fish to confirm germline transmission (F1). F1 fish were crossed with fish with constitutive liver expression of GAL4 (l-fabp:Gal4) and an identifiable cardiac-specific green fluorescent protein (GFP) marker (cardiac myosin light chain 2, cmlc2-GFP). At 48 hpf, transgenic fish expressing H7 AL-LC-SSP were identified as those with dual markers, yellow eye/green heart, and expression was confirmed by PCR using primers H7 LC-forward (5′-GGTCACCATCTCCTGCTCCAACGTTG) and H7 LC-reverse (5′-GGTCTCCACTCCCGCCTTGACG). The resulting transgenic zebrafish are described as UAS-GAL4 (L-FABP)-H7 AL-LC. For controls, fish from the same clutch that screened positive for AL-LC transgene (+YFP eye) but were negative for liver-GAL4 expression (negative cardiac GFP signal) were used as controls for the embryonic experiments (control).
Circulating LC measurement.
Blood was collected from anesthetized adult zebrafish at 3, 12, and 24 mo of age through an incision directly below the dorsal fin, severing the main artery. Using a heparinized micropipette tip, blood was collected and centrifuged at room temperature for 20 min at 15,000 g. Clarified supernatant was analyzed by immunoblot to determine the presence of the human λ LC. Circulating LC concentrations were determined by quantification and comparison to recombinant H7 LC protein linear standard within the same immunoblot.
Immunoblot analysis.
Proteins were harvested from cellular lysates of homogenized isolated zebrafish hearts or zebrafish embryos (Cell Signaling). Total protein (20–30 μg) was loaded onto Criterion XT bis-tris precast gels (4–12%) (Invitrogen) or PAGEr Gold precast gels (4–20%) (Lonza) for electrophoresis under denaturing conditions. Separated proteins were electrotransferred to a PVDF membrane (Immobilon-FL Millipore) at 30 V for 16–18 h at 4°C. After blocking in 5% bovine serum albumin (BSA) in phosphate buffered saline (PBS) or Odyssey blocking buffer (Li-Cor), proteins of interest were incubated with appropriate primary antibodies overnight at 4°C (polyclonal rabbit anti-human λ LC, Dako cat. no. A-0193 at 1:2,000; polyclonal rabbit anti-cleaved Caspase-3, Abcam cat. no. 13847 at 1:1,000; polyclonal rabbit anti-LC3, MBL cat. no. PD-014 at 1:1,000; monoclonal mouse anti-β-actin, Sigma cat. no. 1978 clone AC-15 at 1:5,000; polyclonal rabbit anti-GAPDH, R&D cat. no. 2275-PC at 1:5,000; monoclonalmouse anti-β-tubulin at 1:5,000, Millipore cat. no. 05-661) (8). After washing, blots were incubated with corresponding near-infrared labeled secondary antibodies (Li-Cor Donkey anti-rabbit 680RD, cat. no. 926–68073 and Donkey anti-rabbit 800CW, cat. no. 926-32214 at 1:5,000). An Odyssey infrared scanner (Li-Cor) was used to determine the infrared fluorescent signal and GAPDH or β-tubulin or β-actin was used as a reference protein for normalization. The blots were quantified using Image Studio Lite (Li-Cor, version 5.2).
Immunohistochemistry.
Intact adult zebrafish were fixed in 10% neutral buffered formalin at room temperature for 48 h and isolated hearts were fixed in 4% paraformaldehyde at room temperature for 24 h, embedded in paraffin, and sectioned (5 µm). Tissues were deparaffinized in xylene, rehydrated in ethanol and nonspecific binding blocked by incubation in 3% BSA (Sigma-Aldrich cat. no. A-7908) in neutral PBS solution for 1 h. Heat-induced antigen retrieval was performed in citrate buffer pH 6.8 (Vector Laboratories cat. no. H-3300) for 1 min at 125°C under pressure in the Decloaking Chamber Plus (Biocare Medical). Slides were subsequently incubated with rabbit anti-λ light chain polyclonal antibodies (Dako, cat. no. A-0193, 1:100) at 4°C overnight, washed with PBS, and incubated with secondary antibody (Alexa Fluor 555 Goat Anti-Rabbit IgG, Molecular Probes cat. no. A-31572) at 37°C for 1 h, washed in PBS, and mounted with VECTASHIELD mounting media containing DAPI nuclear stain (Vector Laboratories). The specificity of the Dako anti-human λ antibody was confirmed by immunoblotting with purified patient λ LC and recombinant human λ LC as positive controls, transgenic zebrafish expressing the human λ LC, and control zebrafish that do not express the human λ LC, and transgenic mouse sera that express the human λ LC and wild-type controls. The specificity was validated for immunohistochemistry by staining human patient and transgenic mouse tissues expressing the human λ transgene and wild-type negative controls (29). The presence of fluorescently labeled human λ LC was visualized with a Zeiss LSM700 fluorescence confocal microscope or an Echo Revolve fluorescence microscope. Negative control staining was performed using only the secondary antibody. All staining reported was performed at the same time in the same batch.
To detect apoptotic cell death in adult heart tissues, following rehydration, sections were incubated in TUNEL reaction mixture (Roche) for 1 h at 37°C. Slides were washed with PBS and mounted with VECTASHIELD containing DAPI nuclear stain (Vector Laboratories). The presence of fluorescently labeled DNA fragments was visualized with a Zeiss LSM700 fluorescence confocal microscope.
To detect apoptotic cell death in embryonic zebrafish hearts, hearts were dissected from zebrafish embryos in Tyrode’s solution (137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 0.2 mM Na2HPO4, 12 mM NaHCO3, 5.5 mM d-glucose; Sigma-Aldrich) containing 3% BSA. Hearts were transferred to a microwell plate and fixed in 4% paraformaldehyde for 20 min, in PBS and permeabilized overnight in PBS containing 0.1% Tween. The following day, hearts were washed 3 times in PBS and incubated with the TUNEL reaction mixture (Roche, In Situ Cell Death Detection Kit cat. no. 12156792910) in a moisture chamber for 1 h at 37°C. Hearts were washed with PBS and incubated in Hoescht Pentahydrate (Invitrogen, cat. no. H-21491) at 5 µg/ml for 30 min and subsequently placed directly into VECTASHIELD mounting media (Vector Laboratories). Confocal images were taken using excitation wavelengths of 555 nm, and 5–7 pictures were taken from each slide using an LSM700 confocal microscope (Zeiss). The proportion of apoptotic cell death was calculated as TUNEL-positive nuclei divided by total nuclei. TUNEL-positive nuclei and the total nuclei were manually counted using thresholded images and ImageJ software (NIH). All counting was performed in a blinded fashion.
For proliferating cells staining, embryos were incubated in 250 μM of 5-ethynyl-2′-deoxyuridine (EdU) in E3 medium, containing (in mM) 5 NaCl, 0.17 KCl, 0.33 CaCl2, 0.33 MgSO4, and 10 HEPES (pH 7.1) for 3 h at 28°C. Similarly, the embryonic hearts were excised and processed as above. Labeling was performed as per the manufacturer’s instructions (Click-iT, Invitrogen). Hearts were washed with PBS and incubated in Hoescht Pentahydrate (Invitrogen, cat. no. H-21491) at 5 µg/ml for 30 min and subsequently placed directly into VECTASHIELD mounting media (Vector Laboratories). Confocal images were taken using excitation wavelengths of 485 and 555 nm, and 5–7 pictures were taken from each slide using an LSM700 confocal microscope (Zeiss). The proportion of proliferating cell was calculated as EdU-positive nuclei divided by total nuclei.
Congo red staining.
Formalin-fixed, paraffin-embedded 5-µm sections of adult zebrafish were stained with alkaline Congo red (Sigma-Aldrich) for 20 min and lightly counterstained with Mayer’s hematoxylin as previously described (29).
Electron microscopy.
Isolated zebrafish hearts were fixed for 24 h in 2.5% glutaraldehyde, 1.25% paraformaldehyde, and 0.03% picric acid in 0.1 M sodium cacodylate buffer (pH 7.4). Samples were then washed in water three times and osmicated in 1% osmium tetroxide/1.5% potassium ferrocyanide for 3 h. Following several washes, samples were incubated 1% aqueous uranyl acetate for 1 h followed by another two washes in water and then subsequently dehydrated in grades of alcohol (10 min each: 50, 70, 90, 2 × 10 min 100%). The samples were incubated in propyleneoxide for 1 h and infiltrated overnight in a 1:1 mixture of propyleneoxide and TAAB Epon mix (Marivac Canada, Inc., St. Laurent, Canada). The following day the samples were embedded in TAAB Epon mix and polymerized at 60°C for 48 h. Ultrathin sections (~80 nm) were cut with a Reichert Ultracut-S microtome, placed on copper grids stained with 0.2% lead citrate, and examined in the Philips TecnaiG2 Spirit BioTWIN. Images were recorded using an AMT 2k charge-coupled device camera.
Echocardiography.
For echocardiographic assessment of cardiac function, 4- and 20-mo-old control and transgenic zebrafish were immobilized in a sponge submersed in water. Under anesthesia (0.02% Tricane), long axis B-mode image acquisition and Color Doppler echocardiography were performed using the MS700 probe (Vevo3100, Visual Sonics). Peak aortic flow velocity was measured at 50 MHz. Heart rates were measured as the interval between five consecutive aortic flow peaks. For fractional area change (FAC), measurements of the ventricular inner wall at systole and diastole were manually traced to obtain respective ventricular area (VA) in systole (VAs) and diastole (VAd) using Visual Sonics software. Subsequently, FAC was obtained using the formula (VAd − VAs)/VAd. Six to thirteen fish were assessed per experimental group.
SB431542 treatment.
SB431542 (10 µM; Sigma cat. no. S4317) was administered to embryos in E3 medium in 35-mm dishes with 12–16 control or transgenic 48 hpf embryos per dish. Equal volume of DMSO was added as vehicle control with the final DMSO concentration not exceeding 1% in the well.
Survival.
For baseline survival study, time 0 was established as 24 hpf following bleaching and sterilization of embryos. Survival was assessed weekly for zebrafish over the course of 24 mo. For survival following SB431542 treatment, time 0 was established at 48 hpf when inhibitor treatment began. Survival was assessed daily for treated zebrafish over the course of 14 days. Deceased animals were removed. Zebrafish were housed in a normal circulating flow environment during the survival assessment; n ≥ 40 per group.
Statistical analysis.
The methodology used and data representation are consistent with the reporting guidelines published by American Journal of Physiology (20). All data are reported as means ± SE. Statistical differences between experimental groups were determined using a student’s t-test on Graphpad Prism software and Microsoft Excel. Individual P values are denoted within the figure legends. P values of <0.05 were considered significant.
RESULTS
Generation of a transgenic zebrafish line expressing human AL-LC.
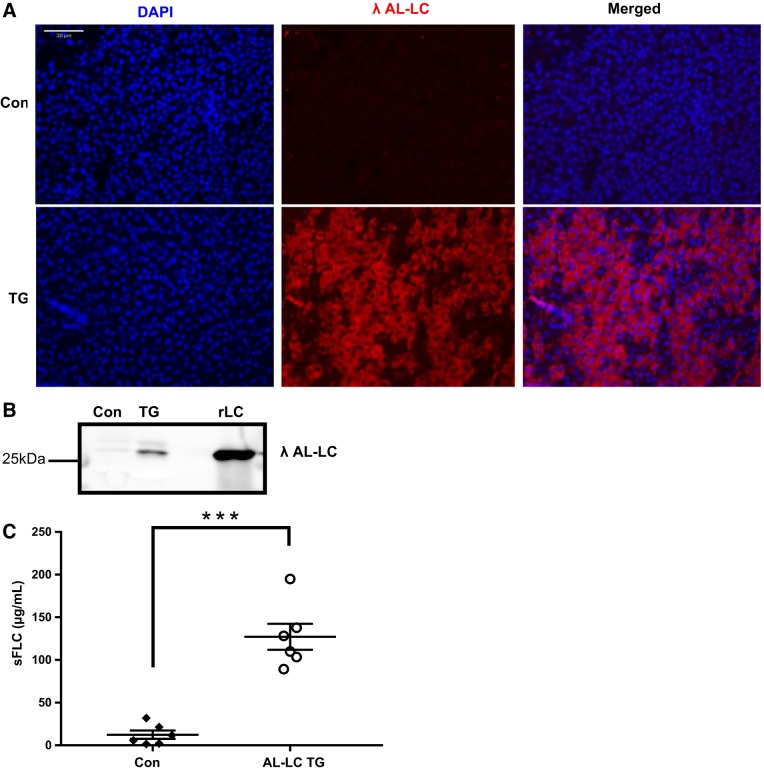
AL-LC transgene incorporation was confirmed by PCR genotype analysis using primers designed to amplify the human LC transgene insert (Fig. 1B). Expression of LC protein in the liver was verified by immunohistochemistry (Fig. 2A). The secreted LC was detectable in the adult fish circulation throughout development and adulthood to 2 yr of age (Fig. 2B) with a serum concentration of ~125 mg/l (Fig. 2C). Patient serum LC concentrations vary widely, but this is comparable to the median LC concentration measured in newly diagnosed patients (4).
Fig. 2.
Human LC protein is expressed in the liver and secreted into circulation. The human λ LC protein was detected in the liver of transgenic fish by immunohistochemistry (red staining). Scale bar represents 30 µm (A). Human λ LC protein is detectable in the serum from transgenic fish at 2 yr of age (B). Quantification of serum-free LC (sFLC) levels using recombinant H7 protein (rLC) as a standard for quantification (***P < 0.001) (C). A concentration (125 µg/ml) comparable to patients with AL amyloidosis was detectable in the serum. Con, control; TG, transgenic.
AL-LC expression induced cardiac proteotoxicity and functional compensation in early adult transgenic zebrafish that diminishes with age.
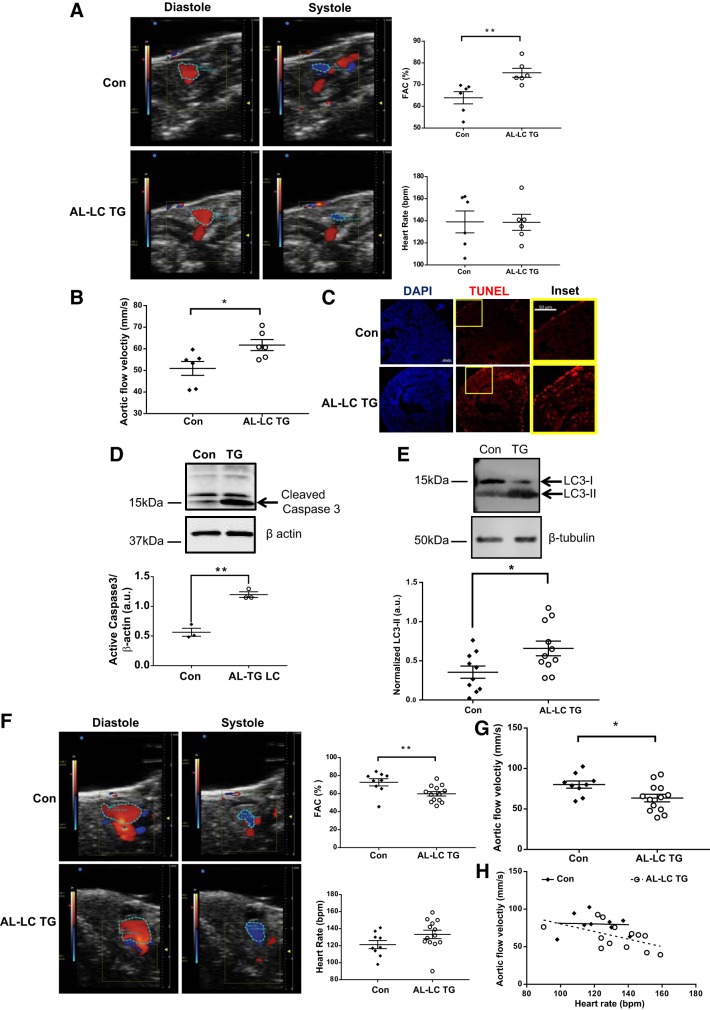
Patients with AL amyloidosis often present with heart failure where systolic functional parameters such as ejection fraction are preserved (15). Cardiac function was analyzed by color Doppler echocardiography in adult zebrafish at 4 mo of age to measure a similar systolic functional parameter of FAC. Notably, FAC was elevated in transgenic zebrafish hearts (Fig. 3A, top right). Heart rate was comparable between the two groups (Fig. 3A, bottom right). In addition, analysis of cardiac blood flow showed increased flow velocity in the transgenic hearts compared with controls (Fig. 3B).
Fig. 3.
Human AL-LC induced functional, biochemical, and ultrastructural alterations in the hearts of the transgenic fish. Representative images of ventricular area (VA) in diastole (VAd) and systole (VAs) obtained from color Doppler at 4 mo of age in control and transgenic fish (left). Quantification of fractional area change (FAC; top right). Heart rate of control and transgenic fish during acquisition (bottom right) (A). Peak aortic blood flow velocity at 4 mo in control and transgenic fish, n = 6 fish per experimental group (*P = 0.02; **P = 0.008) (B). Increased apoptosis was observed in the transgenic zebrafish at 3 mo old compared with control fish, as evidenced by the presence of TUNEL-positive nuclei in representative myocardial sections and an increase in cleaved caspase-3 detected by immunoblot of isolated heart protein lysates (P = 0.001) (C and D). Representative immunoblot showing increased LC3-II (P = 0.02) expression in transgenic myocardium compared with controls at 1 yr of age is indicative of abnormal authophagic flux (E). Representative images of VA in VAd and VAs obtained from color Doppler at 20 mo of age in control and transgenic fish (left). Quantification of FAC (top right). Heart rate of control and transgenic fish during acquisition (bottom right) (F). Peak aortic blood flow velocity at 20 mo in control and transgenic fish, n = 9 (control), n = 13 (transgenic) (*P = 0.02; **P = 0.009) (G). Scatter plot showing negative correlation between heart rate and aortic flow rate in transgenic fish (P = 0.06) (H). bpm, beats/min; Con, control; TG, transgenic.
Previous studies demonstrated evidence of LC cellular and molecular toxicity, including increased apoptosis and enhanced autophagy (8). We examined if these molecular phenotypes were present in our zebrafish AL model. TUNEL staining revealed an increase in cardiac cell death in the transgenic fish hearts compared with controls (Fig. 3C) with a concomitant increase in cleaved Caspase-3 expression by immunoblot (Fig. 3D). Concomitantly, LC3-II levels were also increased in the transgenic fish hearts (Fig. 3E). Congo red staining revealed no evidence of amyloid fibrils in major organs. Cardiac AL amyloidosis is typically observed in patients who are older. To address age-dependent phenotypes in our transgenic model, cardiac function was analyzed by color Doppler echocardiography in adult zebrafish at 20 mo. Consistent with late onset of cardiac dysfunction, FAC was reduced in the transgenic hearts (Fig. 3F, top right). In addition, analysis of cardiac blood flow showed decreased flow velocity in the transgenic hearts compared with controls (Fig. 3G). Although heart rate was statistically comparable between the two groups (Fig. 3F, bottom right), we observed a higher heart rate in the transgenic fish that correlated negatively with aortic flow velocity (Fig. 3H, P = 0.06) suggesting that functional deterioration was not because of heart rate. Together these data show that our AL-LC transgenic zebrafish model recapitulates many clinical and molecular phenotypes of AL disease.
Functional cardiac compensation and cardiac regeneration is evident in embryonic transgenic zebrafish.
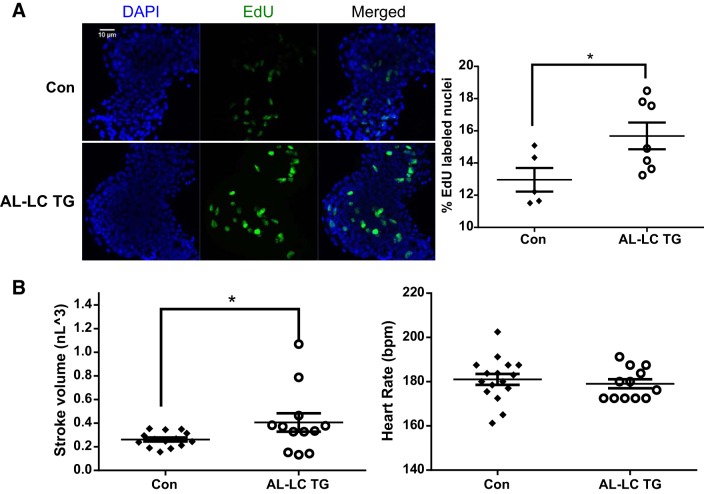
In patients with AL amyloidosis, cardiac involvement is associated with a high rate of mortality (6, 21). Although cardiac phenotype was evident in our transgenic zebrafish model, the AL-LC transgenic fish did not progress to an overt heart failure phenotype and had normal life spans. We suspected that the lack of heart failure and increased mortality in our model fish was a result of the capacity for cardiac regeneration and repair that zebrafish possess. We measured cellular proliferation in zebrafish embryos. EdU cell staining revealed an increase in proliferating cells in transgenic hearts compared with control hearts (12.96 vs. 15.68%, P = 0.0418, Fig. 4A). Cardiac functional analysis demonstrated an elevated cardiac stroke volume in the transgenic embryos at 48 hpf (0.262 vs. 0.406 nl3, P = 0.0328, Fig. 4B left). Together, these data are suggestive of functional compensation that begins early in AL-LC disease progression.
Fig. 4.
Early signs of functional as well as cellular compensation begin in AL-LC transgenic fish embryos. Increased proliferation as indicated by increased EdU-labeled nuclei (green) were detectable in the 48 hpf transgenic zebrafish hearts compared with control hearts (*P = 0.04) (A). Dimensional analysis to measure cardiac function was performed at 48 hpf as previously described in (23) revealing an increase in stroke volume (*P = 0.03, left) while maintaining a comparable heart rate (B). bpm, beats/min; Con, control; EdU, 5-ethynyl-2′-deoxyuridine; hpf, hours postfertilization; TG, transgenic.
Inhibition of regenerative proliferation compromises cardiac function and survival.
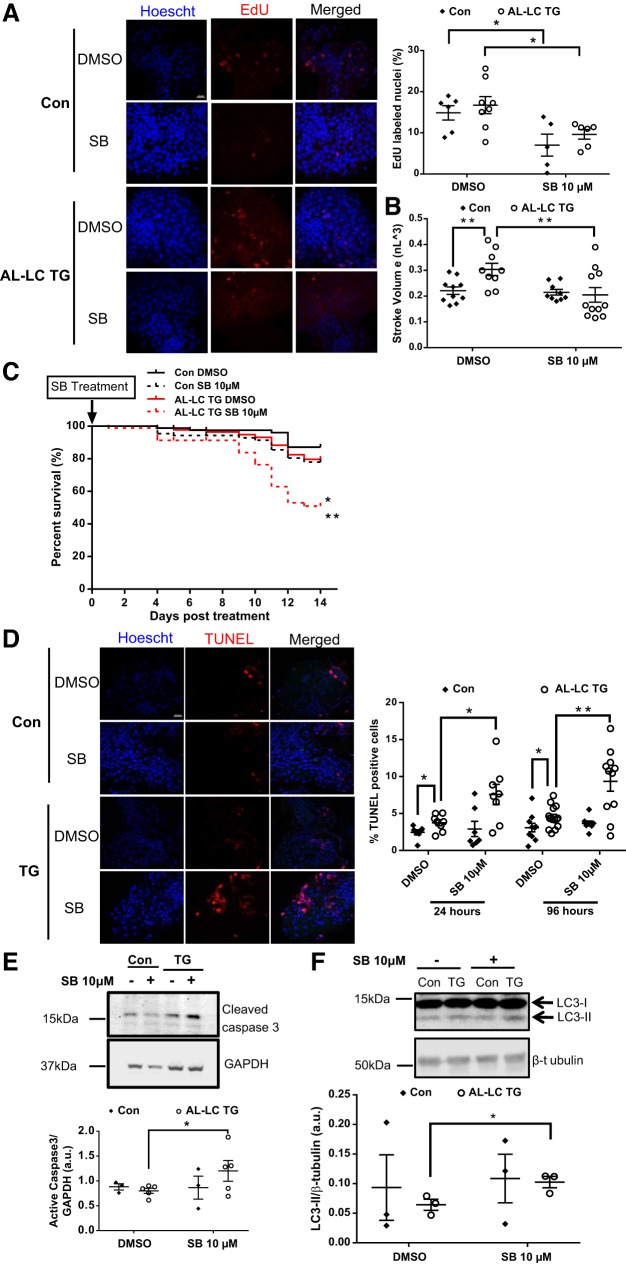
Previous studies have shown that transforming growth factor-β is required for myocardial regeneration in zebrafish (2). Given that we hypothesize that AL-LC zebrafish did not show appreciable heart failure and mortality because of increased cardiac cell proliferation, we tested whether inhibition of cell proliferation would lead to heart failure and mortality. Chemical inhibition of transforming growth factor-β signaling in 48 hpf embryos using 10 µM SB431542 for 24 h inhibited proliferation in both the control (14.85 DMSO vs. 7.0% SB431542, P = 0.03) and transgenic (16.74 DMSO vs. 9.62% SB431542, P = 0.02, Fig. 5A ) fish embryo hearts. Treatment with SB431542 for 24 h also ameliorated the compensatory increase in cardiac function shown by increased stroke volume in the transgenic fish (0.02 nl3 vs. 0.03 nl3, P = 0.007) to the level of control hearts (0.03 nl3 DMSO vs. 0.02 nl3 SB431542, P = 0.02, Fig. 5B). Because the percentage of proliferating cardiac myocytes increases through days 2–5 postfertilization (2), 10 µM SB431542 treatment was continued to 96 h beginning at 48 hpf. The extended inhibitor treatment resulted in further decreased transgenic fish survival. At day 14, the transgenic fish survival in the inhibitor-treated group was 50 compared with 80% in the DMSO control (P = 0.0003, Fig. 5C).
Fig. 5.
Proliferation inhibition in transgenic zebrafish using SB431542 sensitizes them to LC cardiac toxicity lowering survival, increasing cardiac cell death and dysfunction. Representative images (left) from isolated control and AL-LC TG embryonic zebrafish hearts following 24-h SB431542 treatment display a decrease in EdU-labeled nuclei. Quantification of EdU-positive cells in zebrafish heart following 24-h SB431542 treatment. n = 5–7 zebrafish embryos/group; P = 0.03 for control embryos with DMSO vs. SB431542; P = 0.02 for TG DMSO vs. SB431542 (A). Dimensional analysis performed as (23) at 72 hpf following a 24-h SB431542 treatment revealed a loss of the cardiac functional compensation by stroke volume (P = 0.02) (B). Kaplan-Meier curve shows that survival is significantly decreased in AL-LC transgenic zebrafish embryos treated with 10 µM SB431542 for 96 h administered starting at 2 days postfertilization (*P = 0.002 for control embryos with SB431542 vs. TG SB431542; **P = 0.0003 for TG with DMSO vs. SB431542); n ≥ 40 zebrafish embryos/group (C). Representative images of isolated control and AL-LC TG zebrafish hearts following 24-h and 96-h SB431542 treatment revealed increased TUNEL-stained nuclei (red, left). Quantification of TUNEL-positive cells in isolated zebrafish hearts following 24-h and 96-h SB431542 treatment. Treatment for both durations resulted in a significant increase in cell death in the AL-LC TG fish hearts, n = 7–14 zebrafish embryos/group; P = 0.02 at 24 h; P = 0.008 at 96 h (D). Further evidence of apoptosis was observed with an increase in cleaved caspase-3 protein by immunoblotting of the AL-LC TG embryos treated with 10 μM SB431542 (P = 0.048) (E). Indication of abnormal authophagic flux was observed via increased LC3-II protein levels as measured by immunoblotting of the AL-LC TG embryos treated with 10 μM SB431542 (P = 0.045). n = 3 separate experiments with 7–14 zebrafish embryos/group (F). Con, control; EdU, 5-ethynyl-2′-deoxyuridine; hpf, hours postfertilization; SB, SB431542; TG, transgenic.
Apoptosis was measured to assess if the decrease in cardiac function and overall survival in the transgenic fish was the result of increased cell death. One day following SB431542 treatment, there was a marked increase in TUNEL-positive cells in the AL-LC TG fish hearts (3.74 DMSO vs. 7.57% SB431542, P = 0.02). This increased cell death was consistent with extended 96-h SB431542 treatment (4.48 DMSO vs. 9.34% SB431542, P = 0.0008, Fig. 5D). Further supporting an apoptotic mechanism, we also observed increased cleaved caspase-3 activation following SB431542 treatment in AL-TG fish at 6 dpf (Fig. 5E). SB431542 did not impact cell death in control hearts. In addition, we also observed increased levels of LC3-II following SB431542 treatment in transgenic embryos compared with the controls (Fig. 5F). Together these data show that inhibition of cellular proliferation by chemical inhibition removes the compensatory effects provided by increased proliferation and reveals the underlying heart failure and mortality phenotypes associated with AL.
DISCUSSION
Although progress has been made toward identifying pathological signaling pathways contributing to AL amyloidosis, one of the major hurdles in understanding the underlying mechanisms of the disease is the lack of a systemic animal model of the disease especially with cardiac involvement. Ward et al. (29) developed a transgenic mouse ubiquitously expressing human amyloidogenic light chain. Although LC was detectable in the serum, aside from amyloid fibril detection in the low pH stomach environment, the mice did not exhibit cardiac disease (29). Herein, we present the generation and phenotypic characterization of a transgenic zebrafish model expressing a human AL-LC that recapitulates many features of the initial phases of systemic amyloidosis.
The generation of this disease model and the expression strategy of the transgene was designed to be representative of clinical presentation of the disease. Therefore, two main considerations were made: expression strategy and choice of LC sequence. The targeted expression strategy centered upon achieving high levels of circulating LC protein as opposed to modulating expression of the protein in an organ-specific manner. Analysis of plasma from the transgenic zebrafish showed elevated circulating light chain levels comparable to the mean pathological serum-free light chain concentrations (~125 mg/l) observed in patients (4). Because L-FABP remains activated through adulthood, this ensured unimpeded production of the exogenous human LC (11). As expected, this elevated plasma LC concentration was maintained in adult fish over the time period of 3 mo to 2 yr of age.
We selected the sequence of amyloidogenic light chain from a patient with severe cardiac involvement, which had been previously characterized in vitro (12, 18). In a study by Diomede et al. (3), both the recombinant and Bence Jones forms of this specific H7 AL-LC induced toxicity in a Caenorhabditis elegans model system. Exposure of AL-LC caused pharyngeal “pumping” impairment, an organ considered as the ortholog of vertebrate heart (3). In our transgenic fish, LC is continuously produced and kills cells; therefore, it is fully expected that the cell death and injury remains as long as the toxic LC proteins are circulating in the system. Indeed, we observed LC-induced cell death in embryos as well as adult fish. However, we believe that the AL-LC-induced cellular toxicity was likely counteracted by cardiac proliferation in the transgenic fish myocardium as suggested by our inhibitor experimets. A modest 50% inhibition of proliferation sensitized the transgenic embryos to cell death and other consequences of LC toxicity, with subsequent overall mortality. Moreover, although it is well known that zebrafish maintain regeneration capacity (14), it was not apparent that such intricate relationship between regeneration could have served to combat a degenerative disease. Phenotypic characterization that demonstrates cardiotoxicity resulting in subsequent cardiac dysfunction in our transgenic fish supports previous data and expands further into a chronic model of AL amyloidosis.
A recent analysis of LC proteins, which included the H7 LC protein, attributed low fold stability and high protein dynamics resulting in increased proteolysis as key features contributing to amyloid formation (25). In addition to the inherent properties of LC protein, microenvironments within the cellular milieu have been implicated in providing conditions conducive for amyloid formation (1). Age-related changes are known to affect protein stability/misfolding (13). We did not observe any amyloid deposition by Congo red staining in our transgenic zebrafish up until 2 yr, which is around the midst of their approximate average lifespan. Given that AL amyloidosis is an age-onset disease, it is tempting to speculate that further aging may result in an amyloidogenic phenotype. The plethora of in vitro studies have shown the potential of LC protein for amyloid formation, and our transgenic zebrafish certainly provides a model in which specific pathways that contribute to the amyloidogenicity can be studied at an organismal level. Moreover, although infiltration of AL-LC fibrils contributes to the passive stiffness of the heart that presents as restrictive cardiomyopathy, this notion has been challenged by data suggesting that precursor protein itself may greatly influence the overall AL amyloidosis pathogenesis (5, 19, 26, 28).
The studies presented here demonstrate a novel in vivo systemic AL amyloidosis zebrafish model mimicking clinical presentation of disease. Our phenotypic characterization was consistent with studies indicating changes in cell death and mitochondrial homeostasis as a critical mechanism contributing to cardiac toxicity. Our systemic overexpression strategy links any observed phenotype as directly causal to LC, as is the case with clinical AL presentation. The sustained and pathological levels of systemic amyloidogenic LC in our transgenic fish model may contribute to understanding the outstanding fundamental questions unanswered in AL amyloidosis, which will facilitate development of improved and specific therapies for this disease.
GRANTS
This work was supported by National Heart, Lung, Blood Institute Grants R01-HL-132511 and R01-HL-128135 (to R. Liao).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M., S.J., E.P.B., C.A.M., and R.L. conceived and designed research; S.M., S.J., Deepak Mishra, and Deepa Mishra performed experiments; S.M., S.J., J.E.W., E.P.B., C.A.M., and R.L. analyzed data; S.M., S.J., J.E.W., E.P.B., S.F., C.A.M., and R.L. interpreted results of experiments; S.M., S.J., J.E.W., and R.L. prepared figures; S.M., S.J., J.E.W., and R.L. drafted manuscript; S.M., S.J., J.E.W., E.P.B., I.M., S.F., F.L., G.M., S.D., C.A.M., and R.L. edited and revised manuscript; S.M., S.J., J.E.W., G.M., C.A.M., and R.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Richard N. Mitchell for thourough examination of pathology images, Brigham and Women’s Hospital Aquatics Facilty for zebrafish maintenance and husbandry, Brigham and Women’s Hospital Cardiovascular Physiology Core for assistance in echocardiography, and Roberto Sacripanti for technical assistance.
Present addresses: S. Mishra, Thermo Fisher Scientific, 5781 Van Allen Way, Carlsbad, CA, 92008; I. Morgado and R. Liao, Cardiovascular Inst., Stanford Univ. School of Medicine, 1651 Page Mill Rd., Rm. 2330, Palo Alto, CA 94304; and Deepa Mishra, The Law Offices of T. C. Theofrastous, 11320 Hessler Rd., Cleveland, OH 44106.
REFERENCES
- 1.Buxbaum JN. Animal models of human amyloidoses: are transgenic mice worth the time and trouble? FEBS Lett 583: 2663–2673, 2009. doi: 10.1016/j.febslet.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi WY, Gemberling M, Wang J, Holdway JE, Shen MC, Karlstrom RO, Poss KD. In vivo monitoring of cardiomyocyte proliferation to identify chemical modifiers of heart regeneration. Development 140: 660–666, 2013. doi: 10.1242/dev.088526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diomede L, Romeo M, Rognoni P, Beeg M, Foray C, Ghibaudi E, Palladini G, Cherny RA, Verga L, Capello GL, Perfetti V, Fiordaliso F, Merlini G, Salmona M. Cardiac light chain amyloidosis: the role of metal ions in oxidative stress and mitochondrial damage. Antioxid Redox Signal 27: 567–582, 2017. doi: 10.1089/ars.2016.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dispenzieri A, Lacy MQ, Katzmann JA, Rajkumar SV, Abraham RS, Hayman SR, Kumar SK, Clark R, Kyle RA, Litzow MR, Inwards DJ, Ansell SM, Micallef IM, Porrata LF, Elliott MA, Johnston PB, Greipp PR, Witzig TE, Zeldenrust SR, Russell SJ, Gastineau D, Gertz MA. Absolute values of immunoglobulin free light chains are prognostic in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood 107: 3378–3383, 2006. doi: 10.1182/blood-2005-07-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubrey SW, Cha K, Skinner M, LaValley M, Falk RH. Familial and primary (AL) cardiac amyloidosis: echocardiographically similar diseases with distinctly different clinical outcomes. Heart 78: 74–82, 1997. doi: 10.1136/hrt.78.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falk RH. Cardiac amyloidosis: a treatable disease, often overlooked. Circulation 124: 1079–1085, 2011. doi: 10.1161/CIRCULATIONAHA.110.010447. [DOI] [PubMed] [Google Scholar]
- 7.Falk RH, Alexander KM, Liao R, Dorbala S. AL (light-chain) cardiac amyloidosis: a review of diagnosis and therapy. J Am Coll Cardiol 68: 1323–1341, 2016. doi: 10.1016/j.jacc.2016.06.053. [DOI] [PubMed] [Google Scholar]
- 8.Guan J, Mishra S, Qiu Y, Shi J, Trudeau K, Las G, Liesa M, Shirihai OS, Connors LH, Seldin DC, Falk RH, MacRae CA, Liao R. Lysosomal dysfunction and impaired autophagy underlie the pathogenesis of amyloidogenic light chain-mediated cardiotoxicity. EMBO Mol Med 6: 1493–1507, 2014. [Erratum in EMBO Mol Med 7: 688, 2015.] 10.15252/emmm.201404190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan J, Mishra S, Shi J, Plovie E, Qiu Y, Cao X, Gianni D, Jiang B, Del Monte F, Connors LH, Seldin DC, Lavatelli F, Rognoni P, Palladini G, Merlini G, Falk RH, Semigran MJ, Dec GW JR, Macrae CA, Liao R. Stanniocalcin1 is a key mediator of amyloidogenic light chain induced cardiotoxicity. Basic Res Cardiol 108: 378, 2013. doi: 10.1007/s00395-013-0378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Her GM, Chiang CC, Chen WY, Wu JL. In vivo studies of liver-type fatty acid binding protein (L-FABP) gene expression in liver of transgenic zebrafish (Danio rerio). FEBS Lett 538: 125–133, 2003. doi: 10.1016/S0014-5793(03)00157-1. [DOI] [PubMed] [Google Scholar]
- 12.Imperlini E, Gnecchi M, Rognoni P, Sabidò E, Ciuffreda MC, Palladini G, Espadas G, Mancuso FM, Bozzola M, Malpasso G, Valentini V, Palladini G, Orrù S, Ferraro G, Milani P, Perlini S, Salvatore F, Merlini G, Lavatelli F. Proteotoxicity in cardiac amyloidosis: amyloidogenic light chains affect the levels of intracellular proteins in human heart cells. Sci Rep 7: 15661, 2017. doi: 10.1038/s41598-017-15424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med 21: 1406–1415, 2015. doi: 10.1038/nm.4001. [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi K, Poss KD. Cardiac regenerative capacity and mechanisms. Annu Rev Cell Dev Biol 28: 719–741, 2012. doi: 10.1146/annurev-cellbio-101011-155739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation 107: 2446–2452, 2003. doi: 10.1161/01.CIR.0000068313.67758.4F. [DOI] [PubMed] [Google Scholar]
- 16.Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236: 3088–3099, 2007. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- 17.Kyle RA, Linos A, Beard CM, Linke RP, Gertz MA, O’Fallon WM, Kurland LT. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood 79: 1817–1822, 1992. [PubMed] [Google Scholar]
- 18.Lavatelli F, Imperlini E, Orrù S, Rognoni P, Sarnataro D, Palladini G, Malpasso G, Soriano ME, Di Fonzo A, Valentini V, Gnecchi M, Perlini S, Salvatore F, Merlini G. Novel mitochondrial protein interactors of immunoglobulin light chains causing heart amyloidosis. FASEB J 29: 4614–4628, 2015. doi: 10.1096/fj.15-272179. [DOI] [PubMed] [Google Scholar]
- 19.Liao R, Jain M, Teller P, Connors LH, Ngoy S, Skinner M, Falk RH, Apstein CS. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation 104: 1594–1597, 2001. doi: 10.1161/circ.104.14.1594. [DOI] [PubMed] [Google Scholar]
- 20.Lindsey ML, Gray GA, Wood SK, Curran-Everett D. Statistical considerations in reporting cardiovascular research. Am J Physiol Heart Circ Physiol 315: H303–H313, 2018. doi: 10.1152/ajpheart.00309.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madan S, Dispenzieri A, Lacy MQ, Buadi F, Hayman SR, Zeldenrust SR, Rajkumar SV, Gertz MA, Kumar SK. Clinical features and treatment response of light chain (AL) amyloidosis diagnosed in patients with previous diagnosis of multiple myeloma. Mayo Clin Proc 85: 232–238, 2010. doi: 10.4065/mcp.2009.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merlini G, Dispenzieri A, Sanchorawala V, Schönland SO, Palladini G, Hawkins PN, Gertz MA. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers 4: 38, 2018. doi: 10.1038/s41572-018-0034-3. [DOI] [PubMed] [Google Scholar]
- 23.Mishra S, Guan J, Plovie E, Seldin DC, Connors LH, Merlini G, Falk RH, MacRae CA, Liao R. Human amyloidogenic light chain proteins result in cardiac dysfunction, cell death, and early mortality in zebrafish. Am J Physiol Heart Circ Physiol 305: H95–H103, 2013. doi: 10.1152/ajpheart.00186.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosimann C, Puller AC, Lawson KL, Tschopp P, Amsterdam A, Zon LI. Site-directed zebrafish transgenesis into single landing sites with the phiC31 integrase system. Dev Dyn 242: 949–963, 2013. doi: 10.1002/dvdy.23989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oberti L, Rognoni P, Barbiroli A, Lavatelli F, Russo R, Maritan M, Palladini G, Bolognesi M, Merlini G, Ricagno S. Concurrent structural and biophysical traits link with immunoglobulin light chains amyloid propensity. Sci Rep 7: 16809, 2017. doi: 10.1038/s41598-017-16953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palladini G, Lavatelli F, Russo P, Perlini S, Perfetti V, Bosoni T, Obici L, Bradwell AR, D’Eril GM, Fogari R, Moratti R, Merlini G. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood 107: 3854–3858, 2006. doi: 10.1182/blood-2005-11-4385. [DOI] [PubMed] [Google Scholar]
- 27.Palladini G, Sachchithanantham S, Milani P, Gillmore J, Foli A, Lachmann H, Basset M, Hawkins P, Merlini G, Wechalekar AD. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood 126: 612–615, 2015. doi: 10.1182/blood-2015-01-620302. [DOI] [PubMed] [Google Scholar]
- 28.Shi J, Guan J, Jiang B, Brenner DA, Del Monte F, Ward JE, Connors LH, Sawyer DB, Semigran MJ, Macgillivray TE, Seldin DC, Falk R, Liao R. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc Natl Acad Sci USA 107: 4188–4193, 2010. doi: 10.1073/pnas.0912263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward JE, Ren R, Toraldo G, Soohoo P, Guan J, O’Hara C, Jasuja R, Trinkaus-Randall V, Liao R, Connors LH, Seldin DC. Doxycycline reduces fibril formation in a transgenic mouse model of AL amyloidosis. Blood 118: 6610–6617, 2011. doi: 10.1182/blood-2011-04-351643. [DOI] [PMC free article] [PubMed] [Google Scholar]