Abstract
Background
Accurate identification of sentinel lymph nodes (SLNs) in patients with cancer improves detection of metastatic disease and decreases surgical morbidity. We sought to determine whether indocyanine green (ICG) fluorescent dye is superior to isosulfan blue (IB) dye in detecting SLNs in women with cervical and uterine cancers.
Methods
Patients with clinical stage I endometrial or cervical cancer undergoing curative surgery were randomly assigned 1:1 to lymphatic mapping with IB (visualized by white light) followed by ICG (visualized by near infrared imaging) or ICG followed by IB. Permuted block randomization with stratification by study site was performed using a computerized random number generator. All subjects were blinded to their randomization assignment until after the procedure. Laparoscopic surgery with the PINPOINT near infrared fluorescence imaging system (Stryker, Kalamazoo, MI) was used in all cases. The primary outcome was effectiveness of intraoperative ICG with near infrared fluorescence imaging and blue dye in the identification of lymph nodes, defined as the number of lymph nodes identified by ICG and blue dye, respectively (confirmed as lymphoid tissue by histology), divided by the number of lymph nodes identified intraoperatively and excised. Analyses were performed in both per protocol and modified intent to treat populations. The trial was registered with ClinicalTrials.gov, number NCT02209532 and is completed and closed.
Findings
In the per protocol population (n=163), a total of 517 sentinel nodes were identified. Of these, 478 (93%) were confirmed to be lymph nodes on pathologic processing: 92% (219/238) of nodes that were both blue and green, 100% (7/7) of nodes that were blue only, and 95% (252/265) of nodes that were green only (p=0·33). In the modified intent to treat population (n=176), a total of 545 nodes were identified. Of these, 513 (94%) were confirmed to be lymph nodes on pathologic processing: 92% (229/248) of nodes that were both blue and green, 100% (9/9) of nodes that were blue only, and 95% (266/279) of nodes that were green only (p=0.30).
Interpretation
ICG identifies more sentinel nodes than IB in women with cervical and uterine cancers with no difference in the pathologic confirmation of nodal tissue between the two mapping substances.
Keywords: lymphatic mapping, sentinel node, indocyanine green, cervical cancer, uterine cancer, near infrared imaging
Introduction
The disease status of the regional lymph nodes is one of the most important prognostic factors in all solid tumors and frequently drives recommendations for adjuvant chemotherapy and/or radiation therapy following surgical resection. The technique of lymphatic mapping and sentinel lymph node biopsy has improved surgeons’ ability to detect small-volume disease in lymph nodes while greatly reducing intraoperative and postoperative morbidity. This technique has been validated and is widely accepted as part of the surgical treatment of vulvar and breast carcinomas and cutaneous melanoma. Gynecologic oncologists are also starting to adopt lymphatic mapping and sentinel node biopsy for women with cervical and uterine cancers. (1–3)
In lymphatic mapping for cervical and uterine cancers, mapping agents are most commonly injected into the cervix, a practice that should result in drainage to bilateral sentinel nodes in the pelvis. If lymphatic mapping does not identify bilateral sentinel nodes, well-accepted algorithms recommend complete pelvic lymphadenectomy on the side with no sentinel nodes detected. (4, 5) As complete pelvic lymphadenectomy increases surgical time and the risks of intraoperative vascular injury and postoperative lower extremity lymphedema and lymphocyst formation, it is critically important that lymphatic mapping identify bilateral sentinel nodes.
Historically, patent blue dye with or without radiocolloid has been the most commonly used mapping agent for lymphatic mapping in women with uterine cancers. However, blue dye alone identifies at least one sentinel node in only 80% of patients and bilateral sentinel nodes in as few as 50% of patients. (6) Combining blue dye with radiotracer increases the rate of detection of at least sentinel node to 88% but the rate of detection of bilateral sentinel nodes to only 51%. (6) With either technique, therefore, almost half of the women with uterine cancer undergoing lymphatic mapping may require unilateral or bilateral pelvic lymphadenectomy, which increases the risk of surgical complications and long-term morbidity.
The fluorescent dye indocyanine green (ICG) has been explored as an off label, alternative to blue dye for lymphatic mapping in cervical and uterine cancers. Small series have shown the potential of interstitial ICG injection to improved sentinel node detection rates over those observed with blue dye. (7–9) However, there were no prospective, randomized studies comparing interstitial blue dye and ICG injections for lymphatic mapping in cervical and uterine cancers. We therefore conducted the FILM (Fluorescence Imaging for Lymphatic Mapping) Trial, which was designed to compare the detection of lymph nodes after interstitial ICG injection and interstitial isosulfan blue dye injection, the standard of care, in women with cervical and uterine cancers.
Materials and Methods
Study Design and Participants
The FILM Trial was an international multicenter, randomized, open-label, phase III study designed to assess the safety and effectiveness of ICG and PINPOINT near infrared fluorescence imaging (Stryker, Kalamazoo, MI) in identification of lymph nodes in women with cervical and uterine malignancies undergoing lymphatic mapping following interstitial cervical injection of colored dye (ClinicalTrials.gov identifier: NCT02209532). The protocol is available in the Appendix. The study accrued patients at eight centers in North America. The protocol was approved by institutional review boards at each center, and all patients enrolled gave written informed consent. Participating surgeons were required to have completed at least 10 lymphatic mapping procedures, including at least three with the PINPOINT platform, before the initiation of enrollment. Patients were eligible if they were 18 years of age or older, diagnosed with International Federation of Gynecology and Obstetrics clinical stage I cervical or uterine cancer, any histology, and were scheduled for curative surgery that included lymph node assessment. Any patient felt to be a safe surgical candidate was considered eligible regardless of performance status or life expectancy. Other eligibility criteria included negative nodes and absence of metastatic disease by clinical evaluation and radiologic imaging. Patients were ineligible if they had prior pelvic dissection and/or irradiation, advanced cervical or uterine cancer, T3/T4 lesions, cervical tumor size greater than 2 cm, hepatic dysfunction defined as a MELD score ≥ 10, renal dysfunction defined as serum creatinine ≥ 2·0 mg/dl, or a known allergy to ICG, iodine, iodine dyes, isosulfan blue, or triphenylmethane.
Randomization and Masking
Subjects were prospectively randomized into the FILM Clinical Trial on the day of surgery Randomization was performed as closely as possible to the mapping procedure to minimize the incidence of dropout. Subjects were randomly assigned on a one to one (1:1) basis to either the B-P Arm (LN mapping with Blue dye followed by LN mapping with PINPOINT) or the P-B Arm (LN mapping with PINPOINT followed by LN mapping with Blue dye). Randomization was stratified by study site. Permuted block randomization was performed within strata. To minimize the opportunity for the sequence to be predicted, the block size was variable and randomly chosen from small multiples of 2 (i.e. 2, 4 or 6). The randomization schedules were generated in advance using a computerized random number generator. Investigational sites did not have access to the randomization schedules. Randomization was accomplished using a secure web-based software (REDCap). As this was a within-subject comparison study, the investigator could not be blinded to the use of Blue dye or PINPOINT device. All subjects were blinded to their randomization assignment until after the procedure.
Procedures
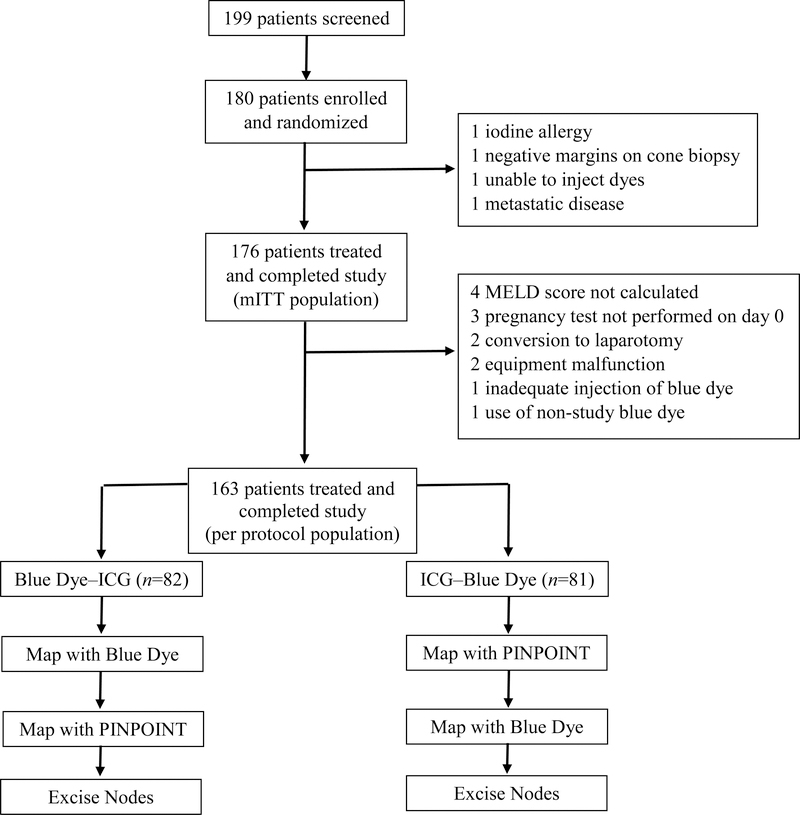
Patients were randomized 1:1 to mapping with blue dye followed by mapping with ICG with the PINPOINT platform (blue dye–ICG) or mapping with ICG with PINPOINT followed by mapping with blue dye (ICG–blue dye) (Figure 1). Patients were removed from the study if found to not meet eligibility criteria or if they requested removal at any time prior to surgery. All patients underwent laparoscopic surgery performed according to the surgeon’s standard practice. After general anesthesia was achieved, blue dye was injected in the cervix deeply and superficially at 3:00 and deeply and superficially at 9:00, and ICG was injected in the cervix deeply and superficially at 3:00 and deeply and superficially at 9:00, for a total of eight injections. For the blue dye–ICG arm, the blue dye injections were performed first; for the ICG–blue dye arm, the ICG injections were performed first. Each blue dye injection consisted of 1 ml of a 10-mg/ml blue dye solution (1% Isosulfan blue), for a total dose of 40 mg; each ICG injection consisted of 1 ml of a 1·25-mg/ml ICG solution (Novadaq Technologies, Inc, Mississagua, Canda) for a total dose of 5 mg.
Figure 1:
Trial Profile
The surgeon identified lymph nodes and lymphatic vessels with white light for blue dye (“blue”) or near infrared imaging with the PINPOINT for ICG (“green”) depending on randomization cohort. Visibly abnormal lymph nodes were excised regardless of whether blue dye or green fluorescence was visible. To minimize the chance that leakage of blue dye or ICG would interfere with mapping, mapping was performed first on one side of the pelvis and then on the other side. In both patient groups, mapping with the first dye was completed before mapping with the second dye was started, and mapping with both dyes was completed before any lymph nodes were excised. Mapping with blue dye was considered complete when the surgeon identified all blue nodes or determined that blue nodes could not be identified. Mapping with ICG was considered complete when the surgeon identified all green nodes or determined that green nodes could not be identified and the surgeon had scanned the full 360-degree area within the abdominal cavity. Once mapping with both dyes was complete and documented, all lymph nodes identified with either dye were excised. Fluorescent and blue channels were followed in both directions to identify lymph nodes to be excised. Sentinel and non-sentinel nodes with appearance suggestive of disease were sent for intraoperative frozen section analysis at the surgeon’s discretion; normal-appearing nodes were not. Bilateral lymphatic mapping was performed according to published NCCN guidelines. (4, 5)
All SLNs resected had confirmation of nodal tissue performed by haematoxylin and eosin (H&E). Evaluation for presence of metastatic disease was performed with ultrastaging and immunohistochemistry per institutional protoco. There was no central review of pathologic specimens.
No postoperative patient follow-up was required for any primary or secondary endpoint except for adverse events/toxicities. All participants had standard of care assessments throughout the study according to the hospital/institution’s standard procedures as well as study specific visits to monitor occurrence of any adverse events/adverse device effects on postoperative Day 1, the date of discharge and Day 30 (±7 days). All subjects were followed to monitor occurrence of adverse events up to Day 30 (±7 days) post-surgery. Adverse events were characterized as mild, moderate, or severe and assigned relationship (suspected or not suspected) to either dyes or device.
Outcomes
The FILM Trial was a non-inferiority within-patient comparison study. We chose this design as it would meet our primary objective and give us reliable results with fewer patients than if we had two separate randomized arms. The primary outcome was effectiveness of intraoperative ICG with near infrared fluorescence imaging and blue dye in the identification of lymph nodes, defined as the number of lymph nodes identified by ICG and blue dye, respectively (confirmed as lymphoid tissue by histology), divided by the number of lymph nodes identified intraoperatively and excised. Secondary outcomes were the rate of intraoperative detection of at least one sentinel node and the rate of detection of bilateral sentinel nodes with ICG and with blue dye; and the incidence of adverse events with each detection method.
Sample Size and Statistical Analysis
We hypothesized that the use of ICG and near infrared imaging would be non-inferior to blue dye in the detection of sentinel nodes. A sample size of 525 nodes (approximately 175 evaluable subjects) was required to show non-inferiority of the lymph node detection rate with ICG to the lymph node detection rate with blue dye with 80% power at a 5% two-sided significance level and a 5% non-inferiority margin.
Both a modified intent-to-treat (mITT) population and a per-protocol population were used for the primary analysis. All randomized and evaluable patients were included in the mITT population. Patients were excluded from the per-protocol population if they had major deviations from the protocol. Analysis of the primary objective was conducted using a two-sided 95% confidence interval for the difference in proportions using the approach described by Nam and Kwon. (10) The clustering in question was lymph nodes nested within patient. Formal testing for heterogeneity was not completed. A non-inferiority test was conducted using the per-protocol population as a conservative approach, since this population was considered the most apt to show inferiorities. As non-inferiority was claimed, a superiority test was conducted using the mITT population, since this population was considered the most likely to show no difference. The secondary objectives of number of nodes identified per patient, detection of at least one lymph node, and detection of bilateral lymph nodes were tested using a two-sided 5% significance level with the step-down multiplicity adjustment of Benjamini and Liu. (11) Study data were collected and managed using REDCap. (12) All data were analyzed with SAS version 9.4 (SAS Institute, Cary, NC) by biostatisticians in the Department of Biostatistics at The University of Texas MD Anderson Cancer Center. The study was registered at ClinicalTrials.gov, NCT02209532.
Role of Funding Source
The sponsor (Novadaq) did participate in the study design but did not participate in the collection, analysis, or interpretation of the data or in the writing of this report. MF and DU were the only authors with access to all of the raw data. The corresponding author had full access to all of the data and the final responsibility to submit for publication. MF and DU are also supported by the NIH/NCI under award number P30CA016672.
Results
From December 21, 2015 to June 19, 2017, one hundred eighty patients were randomized, 90 to each arm, and 176 were evaluable (Figure 1). The two arms did not differ in terms of demographic factors, tumor factors, or number enrolled at each site (Table 1). One hundred sixty-nine of the patients (96%) had uterine cancer.
Table 1:
Patient Demographics
| Blue Dye–ICG Arm (n = 87) |
ICG–Blue Dye Arm (n = 89) |
|
|---|---|---|
| Age, median (range), years | 63 (31–88) | 63 (32–83) |
| BMI, median (range), kg/m2 | 32·7 (19·8–48·4) | 31·7 (19·3–58·6) |
| Race, n (%) | ||
| White | 66 (76) | 73 (82) |
| Hispanic | 10 (11) | 12 (14) |
| African American | 5 (6) | 2 (2) |
| Asian | 4 (5) | 2 (2) |
| Other | 2 (2) | 0 (0) |
| ASA Classification, n (%) | ||
| I | 10 (11) | 11 (12) |
| II | 38 (44) | 39 (44) |
| III | 34 (39) | 32 (36) |
| Unknown | 5 (6) | 7 (8) |
| Type of cancer, n (%) | ||
| Uterine | 84 (97) | 85 (96) |
| Cervical | 3 (3) | 4 (4) |
| Enrollment site, n (%) | ||
| MD Anderson | 19 (22) | 19 (21) |
| CHU de Quebec | 17 (19) | 17 (19) |
| Duke Cancer Institute | 12 (14) | 12 (14) |
| Lee Memorial | 12 (14) | 12 (14) |
| Memorial Sloan-Kettering | 11 (13) | 11 (12) |
| O’Connor Hospital | 7 (8) | 7 (8) |
| HIMA San Pablo | 5 (6) | 7 (8) |
| Sunnybrook HSC | 4 (4) | 4 (4) |
ASA, American Society of Anesthesiology.
Results in the Per-protocol Population
One hundred sixty-three patients had no major protocol deviations and constituted the per-protocol population and primary analytical cohort. In this population, 517 nodes were identified. Of the 517 sentinel nodes, 478 (93%) were confirmed to be lymph nodes on pathologic processing: 92% (219/238) of nodes that were both blue and green, 100% (7/7) of nodes that were blue only, and 95% (252/265) of nodes that were green only (p=0·33). (Table 2)
Table 2:
Sentinel lymph node (SLN) identification rates by indocyanine green and blue dye
| Per Protocol Population | Modified Intent to Treat Population | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | Type of Dye | % Detected | Proportion | p-value | % Detected | Proportion | p-value |
| Confirmation of Nodal Tissue in SLN | Blue + ICG | 92% | 219/238 | 0·33 | 92% | 229/248 | 0·30 |
| ICG Only | 95% | 252/265 | 95% | 266/279 | |||
| Blue Only | 100% | 7/7 | 100% | 9/9 | |||
| Identification of ≥ 1 SLN | ICG | 98% | 159/163 | < 0·0001 | 96% | 168/176 | <0·0001 |
| Blue Dye | 76% | 124/163 | 74% | 131/176 | |||
| Identification of Bilateral SLNs |
ICG | 81% | 132/163 | < 0·0001 | 78% | 138/176 | <0·0001 |
| Blue Dye | 32% | 54/163 | 31% | 54/176 | |||
At least one node was identified in 159 patients (98%) with ICG and in 124 patients (76%) with blue dye. Thus, we were able to conclude that ICG is not inferior to blue dye in the identification of nodes (p<0·0001). Bilateral sentinel nodes were identified in 134 patients (82%). Bilateral sentinel nodes were identified in 54 patients (32%) with blue dye and 132 patients (81%) with ICG (p<0·0001).
Results in the Modified Intent to Treat (mITT) Population
One hundred seventy-six evaluable patients had no major protocol deviations and constituted the mITT population. In this cohort, at least one sentinel node was identified in 172 women (98%) and a total of 545 nodes were identified. Of the 545 sentinel nodes, 513 (94%) were confirmed to be lymph nodes on pathologic processing: 92% (229/248) of nodes that were both blue and green, 100% (9/9) of nodes that were blue only, and 95% (266/279) of nodes that were green only (p=0.30). (Table 2)
At least one node was identified in 168 patients (96%) with ICG and 131 patients (74%) with blue dye (p< 0·0001). Thus, we were able to conclude that ICG is superior to blue dye in the identification of nodes. Difference in node identification was also conducted by site to examine site effect. Difference estimates and 95% confidence intervals indicated that estimates of superiority were consistent throughout all sites. Bilateral sentinel nodes were identified in 141 patients (80%). Bilateral sentinel nodes were identified in 54 patients (31 %) with blue dye and 138 patients (78%) with ICG (p<0·0001).
In the 545 nodes (mean 3·1 nodes/patient) identified among the mITT population, blue dye identified 257 sentinel nodes (mean 1·46/patient), and ICG identified 527 sentinel nodes (mean 2·99/patient) (p<0·0001). Nine nodes (2%) were blue only, 279 (51%) were green only, 248 (46%) were both blue and green, and nine were neither blue nor green but suspicious by visualization and/or palpation.
Randomization group did not affect the ability of blue dye or ICG to detect any or bilateral sentinel nodes. Among the 87 patients in the blue dye–ICG arm, at least one sentinel node was identified in 63 patients (72%) with blue dye and in 83 patients (95%) with ICG. Among the 89 patients in the ICG–blue dye arm, at least one sentinel node was identified in 68 patients (76%) with blue dye and in 85 patients (96%) with ICG. In the blue dye–ICG arm, ICG detected a sentinel node in 22 patients (25%) without a sentinel node detected by blue dye. In the ICG–blue dye arm, blue dye detected a sentinel node in two patients (2%) without a sentinel node detected by ICG.
In the blue dye–ICG arm, blue dye identified bilateral sentinel nodes in 28 patients (32%), ICG identified bilateral sentinel nodes in 67 patients (77%), and ICG identified bilateral sentinel nodes in 40 patients (46%) without bilateral sentinel nodes identified by blue dye. In the ICG–blue dye arm, blue dye identified bilateral sentinel nodes in 26 patients (29%), ICG identified bilateral sentinel nodes in 71 patients (80%), and blue dye identified bilateral sentinel nodes in two patients (2%) without bilateral sentinel nodes identified by ICG.
Sixteen patients (9%) had metastatic disease in 21 sentinel nodes—13 (62%) both blue and green, none blue only, and 8 (38%) green only. No metastatic sentinel nodes would have been missed with use of ICG alone. Macrometastatic disease was found in eight sentinel nodes (38%), micrometastatic disease in 5 (24%), and isolated tumor cells in 8 (38%).
There were no allergic reactions or adverse events attributable to either blue dye or ICG.
Discussion
Although this study was designed as a non-inferiority trial, we were able to conclude that ICG was also superior to blue dye in identifying sentinel lymph nodes in women with cervical and uterine cancer because ICG identified sentinel lymph nodes in a much larger proportion of patients than blue dye did. ICG was also significantly superior to blue dye in detecting at least one sentinel node and in detecting bilateral sentinel nodes. The use of ICG and blue dye together does not seem necessary as the addition of blue dye to ICG identified few sentinel nodes beyond those identified with ICG alone. All metastatic sentinel nodes were detected with ICG, but over one-third of them would have been missed had blue dye alone been utilized. Finally, interstitial injection of ICG appears to be safe as there were no adverse events related to the compound. Breast cancer, like uterine cancer, has highly predictable lymphatic drainage. In uterine cancer, more than 95% of sentinel nodes are found in the pelvis, and in breast cancer, virtually all sentinel nodes are found in the axilla. Because of this, preoperative lymphatic mapping with radiocolloid and dynamic imaging (e.g. lymphoscintigraphy or SPECT/CT) to determine site of incision is unnecessary for lymphatic mapping in breast cancer. Improving the detection of sentinel nodes intraoperatively, therefore, is a major focus of research and innovation for breast cancer surgeons. The use of ICG for detecting sentinel nodes in women with breast cancer may be an improvement over current methods. For example, a direct comparison showed that ICG detected a sentinel node in 97% to 100% of patients with breast cancer whereas blue dyes detected a sentinel node in 88% to 89%. (13, 14) In addition, ICG detects more sentinel nodes per patient than blue dye. When compared to radiocolloid, ICG also identifies a sentinel node is significantly more patients (OR = 1·29) and may find more metastatic disease in sentinel nodes. (15)
For melanoma, interest in ICG as an alternate to radiocolloid for lymphatic mapping and sentinel node biopsy has been somewhat more tempered. Lymphatic drainage of melanoma can often be ambiguous, particularly for lesions located on the trunk or head and neck. (16) Preoperative lymphatic mapping with dynamic imaging (lymphoscintigraphy or SPECT/CT) is often necessary to identify draining nodal basins and to determine sites for surgical incision and exploration. The penetrance of near infrared cameras to detect sentinel nodes through the skin is only 2 to 3 mm, so utilizing ICG as a substitute for radiocolloid has proven largely ineffective, especially for lesions located in anatomic areas with ambiguous drainage. (17)
Lymphatic mapping and sentinel node biopsy is an image-guided precision surgical procedure that is increasingly being adopted for the surgical staging of apparent cervical- and uterine-confined malignancies. As use of ICG for lymphatic mapping in cervical and uterine malignancies becomes more routine, we must remain aware of the potential pitfalls of this new exciting technology. One major potential pitfall is removal of a presumed mapped lymph node that on final pathology is determined to be only lymphatic trunks or adipose tissue without nodal tissue. This phenomenon seems to be more associated with increased ICG utilization. In our study, approximately 5% to 6% of presumed “nodes” with bright signal on near infrared imaging were not confirmed to be nodes on pathology. This is likely due to the bright green effect of the imaging with a laser-based near infrared system. Occasionally, dilated lymphatic channels can lead to a very bright signal that leads to the channels being mistaken for nodes and excised. Mistaken excision of lymphatic trunks or adipose tissue instead of a node is potentially a major pitfall as we move more and more toward performing sentinel node biopsy without complete lymphadenectomy: failure to excise a true node could leave the disease status of an entire hemipelvis unknown. Attention to the details of mapping and the use of color-segmented fluorescence technology and the SPY mode to ensure the highest precision and discrimination between nodes, channels, and stained adipose tissue cannot be emphasized enough. If the surgeon has any doubt, it is reasonable to send the presumed nodal tissue for an intraoperative pathologic consultation to confirm nodal content.
A second major potential pitfall of lymphatic mapping and sentinel node biopsy for cervical and uterine malignancies is the removal of multiple bright green “sentinel” nodes, many of which are not true sentinel nodes but rather second- and third-echelon nodes. This pitfall also relates to the bright signal from a laser-based near infrared imaging platform. Unlike blue dye, which rarely can be seen beyond the first or true sentinel node to secondary-echelon nodes, ICG often produces bright green signal in not only the true sentinel node but also secondary, tertiary, and more distant nodal basins. The surgeon should be aware of this potential pitfall and may choose to remove secondary and tertiary nodes but should not call them “sentinel.” Further, the enhanced pathology protocol evaluation should be limited to the true sentinel nodes, which in most studies average about three per patient with uterine malignancy.
Our findings that blue dye detected at least one sentinel node in 74% of patients and ICG detected at least one sentinel node in 96% of patients are similar to what has been reported in the literature. (6) However, blue dye alone detected bilateral lymph nodes in only 31% of patients, a rate lower than the 55% to 60% reported in previous studies. (18, 19) All surgeons participating in our study had attained proficiency in the mapping procedure, so it is difficult to attribute the low rate of detection of bilateral nodes with blue dye alone to poor technique or learning curve. Some might argue that the use of blue dye alone instead of combined blue dye and radiocolloid was responsible for the low rate of detection of bilateral sentinel nodes. Retrospective studies have compared ICG to blue dye plus radiocolloid with mixed results. Multiple single institution studies have found ICG superior to blue dye and radiocolloid in detecting bilateral sentinel nodes after a cervical injection (20, 21) while a large multi-institutional retrospective study did not show a difference between the two techniques. (22) Systematic reviews and meta-analyses also present conflicting results with some showing combination blue dye and radiocolloid equivalent to ICG in detecting bilateral sentinel nodes (23) while others show that compared to use of blue dye alone, using combined tracers may improve detection of at least one sentinel node but not detection of bilateral nodes. (6) Although some might contend that the combination of blue dye and radiocolloid is superior to blue dye alone and equivalent to ICG in detecting sentinel nodes, there are no studies, prospective or retrospective, that show the combination of blue dye and radiocolloid is superior to ICG. Finally, the relatively high median body mass index (BMI) of our patients may have affected detection of bilateral nodes. The median BMI was 32 kg/m2, and 36% of the patients were morbidly obese (BMI >35 kg/m2). In one retrospective study, increasing BMI adversely affected detection of bilateral sentinel nodes with ICG or blue dye. However, the effect was much more pronounced with blue dye alone than with ICG alone. (8)
Because of the high rate of obesity in women with uterine cancer, many gynecologic oncologists have adopted the robotic system to assist in performing hysterectomy and staging, citing as their reason that the robotic system makes it easier to perform the lymphadenectomy portion of the surgery in women with higher BMI. (24, 25) The most commonly used robotic platform, the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA), includes an option for intraoperative near infrared imaging called the Firefly (originally developed by Novadaq technologies). The FIRES trial, a recent prospective study, showed that ICG and near infrared imaging in the da Vinci system had a sensitivity of 97% in detecting metastatic disease in sentinel nodes. (26) The FILM protocol did not require completion lymphadenectomy after sentinel node detection so sensitivity and negative predictive values for the PINPOINT system cannot be calculated in this study. The PINPOINT technology and image display employed in our study differs from that of the Firefly, so the results of our study cannot be extrapolated to robotic surgery. In the FIRES study, the rate of identification of at least one sentinel node was 86%, slightly lower than the 96% rate of identification with ICG and the PINPOINT system in our present study. In addition, the ICG utilized in this study (IC2000) may differ from the ICG utilized in the FIRES trial. That being said, we do believe that the ability to perform lymphatic mapping and sentinel node detection with ICG and near infrared imaging is likely similar in the two systems, and there are possibly applications of the technique in other cancers, including breast cancer and melanoma. Some institutions may balk at the initial capital expenditures needed to incorporate the use of ICG and near infrared imaging for lymphatic mapping in patients with cervical and uterine cancers. However, prior to quickly abandoning this option as “too expensive”, hospital administrators should consider the following. First, the purchase of a near infrared imaging system is not limited in application to only lymphatic mapping for gynecologic malignancies. Once the system is in the operating suite, surgeons are likely to utilize the technology for other purposes such as evaluating perfusion of bowel or esophageal anastomoses or delineating anatomy during cholecystectomy. (27–29) Increase use of the technology for those purposes will likely lead to improved patient safety and decreased cost related to the management of surgical complications. Next, the alternative to the ICG and near infrared imaging approach is to continue with the combination of blue dye and radiocolloid in these patients for, as discussed, blue dye alone for lymphatic mapping is suboptimal. Although blue dye is relatively inexpensive, the use of radiocolloid is not. Administration of radiocolloid requires additional nuclear medicine technicians, nurses, and physicians as well as use of nuclear medicine facilities. In addition, capital expenditures for open and laparoscopic gamma probes and their maintenance are also required. Finally, incorporating sentinel lymph node biopsy with ICG and near infrared imaging may be the most cost-effective approach in treating women with gynecologic cancers when compared to complete lymphadenectomy in all patients or lymphatic mapping and sentinel node biopsy with radiocolloid and/or blue dye. (30)
This study is limited in its inability to determine the sensitivity, negative predictive value, and oncologic outcomes for lymphatic mapping and sentinel node biopsy in women with cervical and uterine cancers. The protocol was designed only to compare ICG and blue dye as mapping substances in these patients and not as a trial to determine the validity of the sentinel node concept. Some have criticized the protocol for utilizing blue dye alone as the comparator as opposed to combination blue dye and radiocolloid. Although the combination of blue dye and radiocolloid may have improved detection rates in the standard arm, based on the retrospective data in the literature, we believe ICG would still be superior in detecting sentinel nodes in women with cervical and uterine cancers. The results presented reflect the outcomes from surgeons experienced with the procedure and the technology. Surgeons who are new to lymphatic mapping and sentinel lymph node biopsy should perform the procedure followed by complete lymphadenectomy until they are assured that they have achieved proficiency in accurately detecting sentinel nodes. Finally, as mentioned, the results can only be assumed valid for the compound (IC2000) and near infrared system (PINPOINT) utilized in the study. However, we believe that studies utilizing other formulations of ICG and other near infrared imaging systems are likely to yield similar results.
The US Food and Drug Administration (FDA) indications for ICG include only intravenous injection to determine cardiac output, to evaluate perfusion of solid organs, and to perform ophthalmic angiography. Interstitial injection of ICG for lymphatic mapping is currently an off-label use. The manufacturer has submitted an application to the FDA based on the results from our study for on-label use of interstitial injection of ICG combined with near infrared imaging for lymphatic mapping. For women with cervical and uterine cancers, there is mounting evidence that lymphatic mapping and sentinel node biopsy not only improves detection of disease in regional nodes but also decreases operative morbidity. As lymphatic mapping and sentinel node biopsy become the standard of care in the surgical treatment of cervical and uterine cancers, improving sentinel node detection, particularly detection of bilateral sentinel nodes, will become the focus of research. As shown in this study, the incorporation of ICG cervical injection and near infrared imaging into the mapping procedure is a monumental improvement over current approaches (i.e. blue dye and/or radiocolloid) and likely will become standard in these and other solid tumors.
Supplementary Material
Research in Context.
Evidence before this Study
Prior to development of the concept for the FILM study and the production of the subsequent protocol, we performed a comprehensive review of the medical literature to evaluate the off label utilization of indocyanine green and near infrared imaging for lymphatic mapping and sentinel lymph node biopsy in all solid tumors. This was performed using PubMed and clinicaltrials.gov with no date or language restrictions. Search terms included “indocyanine green”, “ICG”, “near infrared imaging”, “lymphatic mapping”, and “sentinel node”. We identified multiple small, single institution retrospective reports of interstitial injection of ICG for lymphatic mapping in a wide range of solid tumors including breast, uterine, cervical, vulvar, lung, kidney, and colon cancers as well as cutaneous melanoma. All of these studies showed utilizing ICG with near infrared imaging was feasible and safe for performing lymphatic mapping in solid tumors and that the technique might potentially be an improvement over currently FDA approved mapping substances such as patent blue dyes and radiocolloids. However, there were no prospective, controlled studies comparing the use of ICG as a mapping substance to standard of care.
Added Value of this Study
Although The FILM study was designed as a non-inferiority comparison of the effectiveness of intraoperative ICG with near infrared imaging to blue dye in the identification of lymph nodes, the results actually showed superiority of the experimental arm over standard of care. To our knowledge, this is the first study to compare ICG and blue dye as mapping substances in a prospective, randomized control study. ICG with near infrared imaging identified significantly more sentinel nodes and more bilateral sentinel nodes compared to blue dye. ICG also identified all sentinel nodes with metastatic disease while blue dye missed a large proportion of them.
Implications of All the Available Evidence
The results of this study will serve as the basis for an application to the FDA for on-label use of interstitial injection of ICG combined with near infrared imaging for lymphatic mapping. ICG so dramatically improves sentinel lymph node identification rates and detection of metastatic disease that once an FDA label has been approved, it will likely become standard of care for lymphatic mapping and sentinel lymph node biopsy for women with cervical and uterine cancers and will likely be adopted as the mapping substance of choice across multiple subspecialties in surgical oncology.
Acknowledgments
Declaration of Interests
MF reports grants from Novadaq/Stryker, during the conduct of the study; personal fees from Novadaq/Stryker, grants from Navidea, personal fees from Johnson and Johnson, personal fees from Genentech, outside the submitted work.
Footnotes
The other authors declared no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holloway RW, Abu-Rustum NR, Backes FJ, et al. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecologic oncology. 2017;146(2):405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoury-Collado F, St Clair C, Abu-Rustum NR. Sentinel Lymph Node Mapping in Endometrial Cancer: An Update. The oncologist. 2016;21(4):461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salvo G, Ramirez PT, Levenback CF, et al. Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage cervical cancer. Gynecologic oncology. 2017;145(1):96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Cervical Cancer (Version 1.2018) 2017. [updated October 25, 2017 Available from: https://www.nccn.org/professionals/physician_gls/PDF/cervical.pdf. Last accessed March 1, 2018.
- 5.National Comprehensive Cancer Network. Uterine Neoplasms Cancer (Version 1.2018) 2017. [updated October 31, 2017. Available from: https://www.nccn.org/professionals/physician_gls/PDF/uterine.pdf. Last accessed March 1, 2018.
- 6.Bodurtha Smith AJ, Fader AN, Tanner EJ. Sentinel lymph node assessment in endometrial cancer: a systematic review and meta-analysis. American journal of obstetrics and gynecology. 2017;216(5):459–76 e10. [DOI] [PubMed] [Google Scholar]
- 7.How J, Gotlieb WH, Press JZ, et al. Comparing indocyanine green, technetium, and blue dye for sentinel lymph node mapping in endometrial cancer. Gynecologic oncology. 2015;137(3):436–42. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson AG, Montovano M, Beavis A, et al. Impact of Obesity on Sentinel Lymph Node Mapping in Patients with Newly Diagnosed Uterine Cancer Undergoing Robotic Surgery. Annals of surgical oncology. 2016;23(8):2522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jewell EL, Huang JJ, Abu-Rustum NR, et al. Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malignancies. Gynecologic oncology. 2014;133(2):274–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam JM, Kwon D. Non-inferiority tests for clustered matched-pair data. Stat Med. 2009;28(12):1668–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamini Y, Liu W. A step-down multiple hypotheses testing procedure that controls the false discovery rate under independence. Journal of Statistical Planning and Inference. 1999;82:163–70. [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo J, Yang H, Wang S, et al. Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: a prospective cohort study. World J Surg Oncol. 2017;15(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Huang L, Wang N, Chen P. Indocyanine green detects sentinel lymph nodes in early breast cancer. J Int Med Res. 2017;45(2):514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugie T, Ikeda T, Kawaguchi A, Shimizu A, Toi M. Sentinel lymph node biopsy using indocyanine green fluorescence in early-stage breast cancer: a meta-analysis. Int J Clin Oncol. 2017;22(1):11–7. [DOI] [PubMed] [Google Scholar]
- 16.Balch CM, Ross MI. Lymphatic Mapping and Sentinel Node Biopsy in Melanoma. JAMA Surg. 2015;150(7):623–4. [DOI] [PubMed] [Google Scholar]
- 17.Stoffels I, Dissemond J, Poppel T, Schadendorf D, Klode J. Intraoperative Fluorescence Imaging for Sentinel Lymph Node Detection: Prospective Clinical Trial to Compare the Usefulness of Indocyanine Green vs Technetium Tc 99m for Identification of Sentinel Lymph Nodes. JAMA Surg. 2015;150(7):617–23. [DOI] [PubMed] [Google Scholar]
- 18.Vidal F, Leguevaque P, Motton S, et al. Evaluation of the sentinel lymph node algorithm with blue dye labeling for early-stage endometrial cancer in a multicentric setting. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2013;23(7):1237–43. [DOI] [PubMed] [Google Scholar]
- 19.Eitan R, Sabah G, Krissi H, et al. Robotic blue-dye sentinel lymph node detection for endometrial cancer - Factors predicting successful mapping. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2015;41(12):1659–63. [DOI] [PubMed] [Google Scholar]
- 20.Buda A, Crivellaro C, Elisei F, et al. Impact of Indocyanine Green for Sentinel Lymph Node Mapping in Early Stage Endometrial and Cervical Cancer: Comparison with Conventional Radiotracer (99m)Tc and/or Blue Dye. Annals of surgical oncology. 2016;23(7):2183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imboden S, Papadia A, Nauwerk M, et al. A Comparison of Radiocolloid and Indocyanine Green Fluorescence Imaging, Sentinel Lymph Node Mapping in Patients with Cervical Cancer Undergoing Laparoscopic Surgery. Annals of surgical oncology. 2015;22(13):4198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadia A, Zapardiel I, Bussi B, et al. Sentinel lymph node mapping in patients with stage I endometrial carcinoma: a focus on bilateral mapping identification by comparing radiotracer Tc99(m) with blue dye versus indocyanine green fluorescent dye. J Cancer Res Clin Oncol. 2017;143(3):475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruscito I, Gasparri ML, Braicu EI, et al. Sentinel Node Mapping in Cervical and Endometrial Cancer: Indocyanine Green Versus Other Conventional Dyes-A Meta-Analysis. Annals of surgical oncology. 2016;23(11):3749–56. [DOI] [PubMed] [Google Scholar]
- 24.Seamon LG, Bryant SA, Rheaume PS, et al. Comprehensive surgical staging for endometrial cancer in obese patients: comparing robotics and laparotomy. Obstet Gynecol. 2009;114(1):16–21. [DOI] [PubMed] [Google Scholar]
- 25.Gehrig PA, Cantrell LA, Shafer A, Abaid LN, Mendivil A, Boggess JF. What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecologic oncology. 2008;111(1):41–5. [DOI] [PubMed] [Google Scholar]
- 26.Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. The Lancet Oncology. 2017;18(3):384–92. [DOI] [PubMed] [Google Scholar]
- 27.Boogerd LSF, Handgraaf HJM, Huurman VAL, et al. The Best Approach for Laparoscopic Fluorescence Cholangiography: Overview of the Literature and Optimization of Dose and Dosing Time. Surg Innov. 2017;24(4):386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohi M, Toiyama Y, Mohri Y, et al. Prevalence of anastomotic leak and the impact of indocyanine green fluorescein imaging for evaluating blood flow in the gastric conduit following esophageal cancer surgery. Esophagus. 2017;14(4):351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ris F, Liot E, Buchs NC, et al. Multicentre phase II trial of near-infrared imaging in elective colorectal surgery. Br J Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brar H, Hogen L, Covens A. Cost-effectiveness of sentinel node biopsy and pathological ultrastaging in patients with early-stage cervical cancer. Cancer. 2017;123(10):1751–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.