Key Points
Question
Is use of echocardiography associated with outcomes in acute myocardial infarction?
Findings
In this cohort study of 98 999 admissions from 397 US hospitals, higher hospital rates of echocardiography use were associated with longer length of stay and greater costs but not with differences in rates of mortality or 3-month readmission.
Meaning
Greater use of echocardiography did not appear to be associated with better patient outcomes in patients with acute myocardial infarction.
Abstract
Importance
Guidelines recommend that patients with acute myocardial infarction (AMI) undergo echocardiography for assessment of cardiac structure and ejection fraction, but little is known about the association between echocardiography as used in routine clinical management of AMI and patient outcomes.
Objective
To examine the association between risk-standardized hospital rates of transthoracic echocardiography and outcomes.
Design, Setting, and Participants
This retrospective cohort study of data from 397 US hospitals that contributed to the Premier Healthcare Informatics inpatient database from January 1, 2014, to December 31, 2014, used International Classification of Diseases, Ninth Revision (ICD-9) codes to identify 98 999 hospital admissions for patients with AMI. Data were analyzed between October 2017 and January 2019.
Exposures
Rates of transthoracic echocardiography.
Main Outcomes and Measures
Inpatient mortality, length of stay, total inpatient costs, and 3-month readmission rate.
Results
Among the 397 hospitals with more than 25 admissions for AMI in 2014, a total of 98 999 hospital admissions for AMI were identified for analysis (38.2% women; mean [SD] age, 66.5 [13.6] years), of which 69 652 (70.4%) had at least 1 transthoracic echocardiogram performed. The median (IQR) hospital risk-standardized rate of echocardiography was 72.5% (62.6%-79.1%). In models that adjusted for hospital and patient characteristics, no difference was found in inpatient mortality (odds ratio [OR], 1.02; 95% CI, 0.88-1.19) or 3-month readmission (OR, 1.01; 95% CI, 0.93-1.10) between the highest and lowest quartiles of echocardiography use (median risk-standardized echocardiography use rates of 83% vs 54%, respectively). However, hospitals with the highest rates of echocardiography had modestly longer mean lengths of stay (0.23 days; 95% CI, 0.04-0.41; P = .01) and higher mean costs ($3164; 95% CI, $1843-$4485; P < .001) per admission compared with hospitals in the lowest quartile of use. Multiple sensitivity analyses yielded similar results.
Conclusions and Relevance
In patients with AMI, hospitals in the quartile with the highest rates of echocardiography showed greater hospital costs and length of stay but few differences in clinical outcomes compared with hospitals in the quartile with the lowest rates of echocardiography. These findings suggest that more selective use of echocardiography might be used without adversely affecting clinical outcomes, particularly in hospitals with high rates of echocardiography use.
This cohort study uses a large multihospital database to evaluate the association between hospital rates of echocardiogram use and outcomes in adult patients with acute myocardial infarction.
Introduction
Echocardiography provides important prognostic information and is the primary imaging modality used to assess left ventricular ejection fraction (LVEF) in acute myocardial infarction (AMI).1,2,3 Echocardiography can also be helpful across a range of conditions including cardiogenic shock, coexisting valve disease, pericardial effusions, left ventricular thrombus, and mechanical complications of AMI.3,4 However, while echocardiography reports can inform clinical decisions and guide use of medications and procedures, only 32% of echocardiography examinations are associated with an active change in management and more than 20% of echocardiography reports are never subsequently acknowledged in the medical record.5 The yield of repeated echocardiography is even lower: new findings become apparent in only 11% of cases.6 Despite this modest diagnostic yield, clinical practice guidelines and performance measures recommend essentially universal assessment of LVEF for patients admitted with AMI.7,8,9 Yet these guidelines were written based on little evidence as to which patients are most likely to benefit from echocardiography and minimal data regarding the association between the use of echocardiography and clinical outcomes. To investigate this issue, we used a large multihospital database to evaluate the association between hospital rates of echocardiography use and patient outcomes.
Methods
Design and Setting
We used data from a geographically and structurally diverse group of hospitals (Premier Healthcare Informatics, Charlotte, NC) that represents approximately 15%-20% of all inpatient US hospitalization10,11,12; data were from 397 US hospitals that contributed to the Premier inpatient database from January 1, 2014, to December 31, 2014. Data were analyzed between October 2017 and January 2019. Unlike traditional administrative databases limited to demographic data and International Classification Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) procedure and diagnostic codes, the Premier database also draws from a hospital’s internal cost-accounting system to record date-stamped hospital service codes for medications, procedures, diagnostic tests, and therapeutic services, including echocardiography procedures. Because the data are deidentified as required by Section 164.514(b)(1) of the Health Insurance Portability and Accountability Act of 1996 Privacy Rule, the institutional review board at Baystate Health in Springfield, Massachusetts, determined this study was exempt (not human patient research) and did not require patient consent.
Patient and Hospital Factors
We used ICD-9 codes to identify all patients discharged with a principal diagnosis of AMI (410.x) in the year 2014, consistent with methods used by the Center for Medicare & Medicaid Services to define AMI.13 We recorded patients’ age, sex, race, insurance, and computed Elixhauser comorbidities and the Gagne combined comorbidity score.14,15 To control for potential confounding because of differences in disease severity, we used ICD-9 codes to identify instances of acute organ dysfunction and assessed receipt of critical care therapies (eg, inotropes, vasopressors, invasive and noninvasive ventilation, intra-aortic balloon pump, and/or arterial lines).16,17,18 Additionally, we characterized hospitals according to size, teaching status, urban or rural population served, and census region. We identified whether a hospital performed cardiac catheterization, percutaneous coronary intervention, or coronary artery bypass surgery and created indicator variables for each of these characteristics. To ensure stability in our estimates of hospital echocardiography use rates, we limited the study to patients cared for at hospitals with at least 25 AMI admissions during the study period.
Receipt of Echocardiography and Outcome Measures
We considered a Premier service code for transthoracic echocardiography as the primary variable of interest, although we also recorded the use of transesophageal and stress echo.19 We measured all echocardiography examinations performed during each admission and the hospital day of service on which the test was performed. We also noted use of cardiac magnetic resonance imaging, contrast ventriculography (at the time of cardiac catheterization), and nuclear cardiac imaging, including multigated radionuclide angiography and single-photon emission computed tomography to assess other tests that may have measured LVEF. However, we ultimately focused our analysis on transthoracic echocardiography because all other tests were used infrequently.
We evaluated 4 main outcomes: inpatient mortality, hospital length of stay, total inpatient costs, and 3-month readmission among survivors. Because length of stay and total cost values were highly skewed, we winsorized them at the first and 99th percentiles. We also assessed only total costs because hospitals account differently for room and board, tests, procedures, laboratory results, and pharmacy costs.20 For hospital readmission, we included admissions to the same hospital within 3 months.21
Statistical Analysis
Descriptive statistics on patient and hospital characteristics were summarized as frequency and percentage for categorical variables, means and SDs, or as quartiles (median, 25th, and 75th percentiles) for continuous variables. We used generalized estimating equation models to examine associations between echocardiography use and unadjusted outcomes and to account for patient clustering within hospitals.
For our primary analysis, we focused on hospital rates of echocardiography use because this approach minimizes confounding by indication, more directly addresses policy questions,22 and has been used in previous studies of echocardiography and cardiac testing.10,12,23,24 We first calculated hospital rates of echocardiography use and then developed a multivariable hierarchical generalized linear model for echocardiography use with a random effect for the hospital. This model included patient demographics, comorbidities, and the presence of acute organ failure. It did not include hospital characteristics or critical care therapies, which allowed comparison between hospitals and avoided overadjustment for hospital-specific processes and patterns of care. We computed the median odds ratio (MOR) to quantify the strength of hospital-level variations in echocardiography use relative to patient-level covariates.25
We then computed a risk-standardized echocardiography rate for each hospital as the ratio of the expected echocardiography rate (based on patient characteristics at a hospital and the average hospital effect) to the predicted echocardiography use rate (based on patient characteristics at a hospital and the hospital-specific effect) multiplied by the overall unadjusted echocardiography rate. Hospitals were grouped by quartiles of risk-standardized echocardiography rate. We then assessed balance in patient characteristics, comparing highest use (quartile 4) to lowest use (quartile 1) via absolute standardized differences, in which a value >10% suggests a clinically meaningful difference in baseline characteristics.26,27 Hospital characteristics were compared using χ2 statistics. A significance level of P < .05 was set, and P values were 2-tailed.
To assess a potential association of echocardiography with medication use, we evaluated hospital prescription rates of anticoagulants (warfarin, rivaroxaban, apixaban, or dabigatran) and angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs). Spearman correlation coefficients and scatterplots were used to assess the association between risk-standardized echocardiography rates and hospital rates of nuclear imaging and ACEi or ARB prescriptions.
Using the quartile of risk-standardized echocardiography rate as the primary predictor, we modeled patient-level outcomes, adjusting for patient demographics, comorbidities, acute organ failures, hospital characteristics, and hospital interventional capabilities via hierarchical generalized linear models with a random intercept for each hospital. We used identity link models for winsorized length of stay and inpatient cost and logit link models for inpatient mortality and 3-month readmission. We compared high and low quartiles and overall differences across all 4 quartiles in these models. In our mortality model, for patients with more than 1 admission, we randomly chose 1 admission for analysis. Finally, we performed several sensitivity analyses by (1) excluding patients with a length of stay of 2 days or fewer, (2) excluding patients transferred in or out of the hospital, and (3) stratifying analyses based on receipt of cardiac catheterization and/or revascularization. A complete description of sensitivity analyses is available in the eMethods in the Supplement. All analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Among the 397 hospitals with 25 or more admissions for AMI in 2014, we included 98 999 admissions (38.2% women; mean [SD] age, 66.5 [13.6] years), of which 69 652 (70.4%) had 1 or more transthoracic echocardiograms performed. Repeated echocardiography tests were uncommon; only 4107 patients (4.1%) had more than 1 echocardiogram, and most of these (3903, 95%) were transesophageal echocardiography examinations completed after a transthoracic echocardiogram. Stress echocardiography was rare with only 776 patients (0.8%) undergoing this test. Most echocardiography was performed on hospital day 1 or 2 (81.4% [56 715 of 69 652] of studies). Nuclear cardiac imaging (4743 [4.8%]), ventriculography (1678 [1.7%]), and cardiac magnetic resonance imaging (295 [0.3%]) were also uncommon. Because these tests overlapped with echocardiography, overall assessment of LVEF increased by 3.2% to 73.6%.
Patients who underwent an echocardiogram (n = 69 652) compared with patients without an echocardiogram (n = 29 347) were more likely to have heart failure (24 214 [34.8%] vs 7287 [24.8%]; absolute standardized difference, 21.85) and pulmonary disease (4829 [6.9%] vs 1197 [4.1%]; absolute standardized difference, 12.54); be cared for in an intensive care unit (41 107 [59.0%] vs 12 859 [43.8%]; absolute standardized difference, 30.77); and receive noninvasive ventilation (6618 [9.5%] vs 1487 [5.1%]; absolute standardized difference, 17.13), invasive ventilation (12 099 [17.4%] vs 3384 [11.5%]; absolute standardized difference, 16.67), inotropes (8395 [12.1%] vs 2050 [7.0%]; absolute standardized difference, 17.33), vasopressors (15 223 [21.9%] vs 4368 [14.9%]; absolute standardized difference, 18.08), balloon pumps (3569 [5.1%] vs 830 [2.8%]; absolute standardized difference, 11.77), and nuclear imaging studies (3869 [5.6%] vs 874 [3.0%]; absolute standardized difference, 12.80) (eTable 1 in the Supplement). In unadjusted analyses, patients who underwent an echocardiogram compared with patients who did not receive an echocardiogram had longer length of stay (mean [SD] days, 5.1 [4.5] vs 3.3 [3.1]; P < .001) and higher costs (mean total cost [SD], $19 646 [$17 602] vs $13 455 [$11 507]; P < .001), but a lower proportion of inpatient mortality (mortality mean [SD], 3089 [4.5] vs 1524 [5.4]; P < .001) and lower proportion of 3-month readmission (readmission mean [SD], 12 130 [18.2] vs 5266 [18.9]; P = .01).
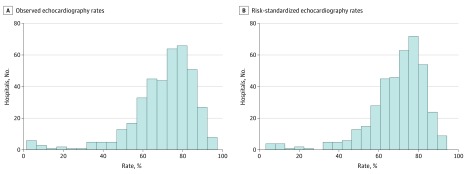
Observed hospital echocardiography rates ranged from 2% to 95.3%, with a median (interquartile range [IQR] of 73.9% (62.8% to 81.1%). The risk-standardized echocardiography model (eTable 2 in the Supplement) had modest discrimination (area under the curve, 0.72). The MOR for hospital effect was 2.26 (95% CI, 1.99-2.30), which was greater than any individual patient-level factor (odds ratio [OR] range, 0.6-1.6). The mean (SD) and median (IQR) risk-standardized hospital echocardiography rates were 68.8% (15.6%) and 72.5% (62.6%-79.1%), with a median use rate of 54%, 67%, 76%, and 83% for quartiles 1, 2, 3, and 4, respectively. Figure 1 highlights the significant between-hospital variations in echocardiography use rates and the changes seen after risk standardization.
Figure 1. Distribution of Hospital Echocardiography Rates in Patients Hospitalized With Acute Myocardial Infarction.
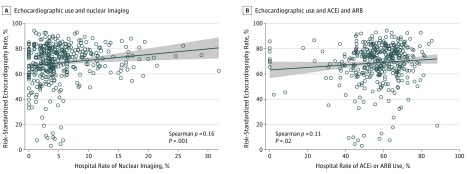
Compared with hospitals in the lowest quartile of risk-standardized echocardiography rates, hospitals in quartile 4 had patients who were more often insured by Medicaid (quartile 4 proportion of patients, 2442 [9.5%] vs quartile 1 proportion of patients, 1321 [6.4%]; absolute standardized difference, 13.91), cared for in intensive or intermediate care services (intensive or intermediate care, quartile 4, 15 447 [59.8%] vs quartile 1, 10 593 [51.1%]; absolute standardized difference, 17.58) or contained a proportion of patients who underwent invasive ventriculography (quartile 4, 1414 [5.5%] vs quartile 1, 23 [0.1%]; absolute standardized difference, 32.99) (Table 1). Other patient characteristics, such as age, sex, race, comorbidities, and organ failure, were not associated with risk-standardized echocardiography rates. Higher hospital risk-standardized echocardiography rates were associated with higher rates of nuclear imaging (Spearman ρ = 0.16, P = .001; Figure 2A) and ACEi or ARB use (Spearman ρ = 0.11, P = .02; Figure 2B) but not anticoagulants. Compared with lower risk-standardized echocardiography rates (quartile 1), hospitals with higher risk-standardized echocardiography rates (quartile 4) had a higher proportion of hospitals capable of performing cardiac catheterization (quartile 4 rate, 94 [94.9%] vs quartile 1 rate, 81 [81.8%]; P = .004) or percutaneous coronary intervention (quartile 4 rate, 86 [86.9%] vs quartile 1, 67 [67.7%]; P = .001) (Table 2), but this proportion did not translate to different rates of use of either procedure (Table 1). Other hospital characteristics, including number of beds and hospital capability to perform coronary artery bypass graft surgery, were not different between quartiles.
Table 1. Patient Characteristics by Quartiles of Hospital Risk-Standardized Echocardiography Ratesa.
| Variable | No. | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Absolute Standardized Difference (Quartile 4 vs Quartile 1) | |
| Risk-standardized echocardiography rate | NA | ||||
| Median (range) | 54.0 (3.2-62.5) | 66.8 (62.6-72.4) | 75.7 (72.5-79.1) | 83.2 (79.1-94.3) | |
| Mean (SD) | 48.3 (15.8) | 67.3 (3.0) | 75.7 (1.9) | 83.9 (3.7) | |
| Patients | 20 728 (20.9) | 30 342 (30.6) | 22 102 (22.3) | 25 827 (26.1) | |
| Age | |||||
| Mean (SD), y | 66.5 (13.6) | 66.7 (13.7) | 67.1 (13.7) | 65.7 (13.5) | 5.77 |
| Median (IQR), y | 66 (57-77) | 67 (57-78) | 67 (57-78) | 66 (56-76) | |
| Female sex | 8192 (39.5) | 11 605 (38.2) | 8600 (38.9) | 9454 (36.6) | 6.01 |
| Race/ethnicity | |||||
| White | 14 868 (71.7) | 22 202 (73.2) | 13 081 (59.2) | 19 415 (75.2) | 9.96 |
| Black | 2770 (13.4) | 2999 (9.9) | 1807 (8.2) | 2691 (10.4) | |
| Hispanic | 867 (4.2) | 2182 (7.2) | 2158 (9.8) | 1171 (4.5) | |
| Other | 2223 (10.7) | 2959 (9.8) | 5056 (22.9) | 2550 (9.9) | |
| Insurance payer | |||||
| Medicare | 12 288 (59.3) | 17 510 (57.7) | 12 586 (56.9) | 14 606 (56.6) | 13.91 |
| Medicaid | 1321 (6.4) | 2543 (8.4) | 2288 (10.4) | 2442 (9.5) | |
| Managed care | 3983 (19.2) | 5990 (19.7) | 4468 (20.2) | 4622 (17.9) | |
| Commercial indemnity | 999 (4.8) | 1748 (5.8) | 1027 (4.6) | 1529 (5.9) | |
| Self-pay | 1343 (6.5) | 1489 (4.9) | 908 (4.1) | 1439 (5.6) | |
| Other | 794 (3.8) | 1062 (3.5) | 825 (3.7) | 1189 (4.6) | |
| Gagne Combined Comorbidity score | |||||
| Mean (SD) | 2.1 (2.7) | 2.0 (2.7) | 2.0 (2.7) | 2.0 (2.7) | 3.25 |
| Median (IQR) | 1 (0-4) | 1 (0-4) | 1 (0-4) | 1 (0-4) | |
| Comorbiditiesb | |||||
| Hypertension | 16 504 (79.6) | 24 122 (79.5) | 17 541 (79.4) | 20 423 (79.1) | 1.35 |
| Hypothyroidism | 8284 (40.0) | 11 961 (39.4) | 92 35 (41.8) | 10 345 (40.1) | 0.18 |
| Congestive heart failure | 6638 (32.0) | 9517 (31.4) | 7249 (32.8) | 8097 (31.4) | 1.45 |
| Chronic obstructive pulmonary disease | 4931 (23.8) | 6789 (22.4) | 4844 (21.9) | 6069 (23.5) | 0.68 |
| Renal failure | 4393 (21.2) | 6386 (21.0) | 4948 (22.4) | 5311 (20.6) | 1.55 |
| Obesity | 3872 (18.7) | 5768 (19.0) | 3885 (17.6) | 4941 (19.1) | 1.15 |
| Deficiency anemia | 3657 (17.6) | 5423 (17.9) | 4168 (18.9) | 4461 (17.3) | 0.98 |
| Valvular disease | 3053 (14.7) | 4971 (16.4) | 3325 (15.0) | 3894 (15.1) | 0.98 |
| Diabetes | 2574 (12.4) | 3541 (11.7) | 2670 (12.1) | 3254 (12.6) | 0.55 |
| Peripheral vascular disease | 2814 (13.6) | 4043 (13.3) | 2623 (11.9) | 3379 (13.1) | 1.45 |
| Depression | 1970 (9.5) | 2882 (9.5) | 1754 (7.9) | 2478 (9.6) | 0.31 |
| Other neurological disorders | 1452 (7.0) | 2060 (6.8) | 1353 (6.1) | 1622 (6.3) | 2.91 |
| Pulmonary circulation disease | 1212 (5.8) | 1950 (6.4) | 1329 (6.0) | 1535 (5.9) | 0.41 |
| Critical care therapies | |||||
| Intensive/Intermediate care unit, d | 10 593 (51.1) | 15 542 (51.2) | 12 384 (56.0) | 15 447 (59.8) | 17.58 |
| Median (IQR) | 2 (1-4) | 2 (1-4) | 2 (1-4) | 2 (1-4) | |
| Mean (SD) | 3.2 (3.7) | 3.2 (4.1) | 3.4 (4.3) | 3.5 (4.5) | 8.80 |
| Vasodilators | 7622 (36.8) | 12 031 (39.7) | 7466 (33.8) | 10 620 (41.1) | 8.93 |
| Noninvasive ventilation | 1528 (7.4) | 2548 (8.4) | 1787 (8.1) | 2242 (8.7) | 4.82 |
| Invasive mechanical ventilation | 2957 (14.3) | 5141 (16.9) | 3133 (14.2) | 4252 (16.5) | 6.10 |
| Vasopressors | 3799 (18.3) | 5997 (19.8) | 4066 (18.4) | 5729 (22.2) | 9.60 |
| Arterial line | 858 (4.1) | 1712 (5.6) | 800 (3.6) | 739 (2.9) | 6.96 |
| Intra-aortic balloon pump | 861 (4.2) | 1330 (4.4) | 1088 (4.9) | 1120 (4.3) | 0.91 |
| Inotropes | 2199 (10.6) | 3135 (10.3) | 2130 (9.6) | 2981 (11.5) | 2.97 |
| Other cardiac tests/therapies | |||||
| Nuclear imaging | 755 (3.6) | 1420 (4.7) | 1223 (5.5) | 1345 (5.2) | 7.62 |
| Ventriculography | 23 (0.1) | 73 (0.2) | 177 (0.8) | 1414 (5.5) | 32.99 |
| Cardiac magnetic resonance imaging | 65 (0.3) | 110 (0.4) | 54 (0.2) | 66 (0.3) | 1.09 |
| ACEis or ARBs | 12 398 (59.8) | 18 270 (60.2) | 13 249 (59.9) | 16 094 (62.3) | 5.10 |
| Anticoagulants | 1632 (7.9) | 2902 (9.6) | 2069 (9.4) | 2487 (9.6) | 6.20 |
| Procedures | |||||
| Coronary artery bypass | 1798 (8.7) | 3200 (10.5) | 1753 (7.9) | 2651 (10.3) | 5.43 |
| Percutaneous coronary intervention | 9385 (45.3) | 14 414 (47.5) | 10 481 (47.4) | 12 544 (48.6) | 6.60 |
| Cardiac catheterization | 14 982 (72.3) | 22 970 (75.7) | 16 404 (74.2) | 19 756 (76.5) | 9.67 |
| Organ failure | |||||
| Respiratory | 2205 (10.6) | 3228 (10.6) | 2195 (9.9) | 2750 (10.6) | 0.03 |
| Cardiac | 2812 (13.6) | 4223 (13.9) | 2793 (12.6) | 3416 (13.2) | 1.00 |
| Acute renal | 3540 (17.1) | 5308 (17.5) | 3840 (17.4) | 4457 (17.3) | 0.47 |
| Neurological | 1085 (5.2) | 1576 (5.2) | 1062 (4.8) | 1325 (5.1) | 0.47 |
| Hematological | 1143 (5.5) | 1914 (6.3) | 1267 (5.7) | 1446 (5.6) | 0.37 |
| Hepatic | 302 (1.5) | 470 (1.5) | 312 (1.4) | 417 (1.6) | 1.28 |
| Acidosis | 991 (4.8) | 1555 (5.1) | 1057 (4.8) | 1232 (4.8) | 0.05 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; IQR, interquartile range; NA, not applicable.
Data are given as number (%) of patients unless otherwise specified.
Elixhauser comorbidities with greater than 5% prevalence.
Figure 2. Association Between Hospital Risk-Standardized Echocardiography Rates and Nuclear Imaging and Angiotensin-Converting Enzyme Inhibitor (ACEi) or Angiotensin Receptor Blocker (ARB) Use.
A, Hospital rates of echocardiography use and hospital rates of nuclear imaging. B, Hospital use rate of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.
Table 2. Hospital Characteristics by Quartiles of Risk-Standardized Echocardiography Rates.
| Hospital Characteristic | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Valuea |
|---|---|---|---|---|---|
| Risk-standardized echocardiography rate, % median (range) | 54 (3.2-62.5) | 67 (62.6-72.4) | 76 (72.5-79.1) | 83 (79.1-94.3) | NA |
| No. of hospitals | 99 | 99 | 100 | 99 | |
| Hospital size, beds | |||||
| ≤200 | 34 (34.3) | 32 (32.3) | 36 (36.0) | 25 (25.2) | .10 |
| 201-400 | 48 (48.5) | 30 (30.3) | 37 (37.0) | 45 (45.4) | |
| ≥401 | 17 (17.2) | 37 (37.4) | 27 (27.0) | 29 (29.3) | |
| Urban location | 77 (77.8) | 75 (75.8) | 83 (83.0) | 80 (80.8) | .60 |
| Teaching hospital | 28 (28.3) | 34 (34.3) | 31 (31.0) | 28 (28.3) | .99 |
| Hospital region | |||||
| Northeast | 9 (9.1) | 18 (18.2) | 16 (16.0) | 13 (13.1) | .19 |
| Midwest | 21 (21.2) | 26 (26.3) | 23 (23.0) | 26 (26.3) | |
| West | 11 (11.1) | 7 (7.1) | 20 (20.0) | 17 (17.2) | |
| South | 58 (58.6) | 48 (48.5) | 41 (41.0) | 43 (43.4) | |
| Services available at hospital | |||||
| Cardiac surgery | 50 (50.5) | 57 (57.6) | 54 (54.0) | 59 (59.6) | .20 |
| Percutaneous coronary intervention | 67 (67.7) | 78 (78.8) | 86 (86.0) | 86 (86.9) | .001 |
| Cardiac catheterization | 81 (81.8) | 89 (89.9) | 94 (94.0) | 94 (94.9) | .004 |
Abbreviation: NA, not applicable.
P Value was determined with χ2 test comparing quartile 4 vs quartile 1.
In adjusted analyses, no difference was found in inpatient mortality (OR, 1.02; 95% CI, 0.88-1.19) or 3-month readmission (OR, 1.01; 95% CI, 0.93-1.10) between the highest and lowest quartiles of echocardiography use. However, patients treated at hospitals in the highest quartile of echocardiography use had a modestly longer mean length of stay (0.23 days; 95% CI, 0.04-0.41; P = .01) and higher mean costs per admission ($3164; 95% CI, $1843-$4485; P < .001) compared with those at the lowest quartile of use (Table 3). Multiple sensitivity analyses yielded similar results (eResults; eTables 3-8 in the Supplement).
Table 3. Outcomes by Risk-Standardized Quartiles of Echocardiography Use.
| Variable | Unadjusted Outcomes | Fully Adjusted Outcome Effect Size Estimate, OR (95% CI)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Value (Quartile 4 vs Quartile 1) | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Value (Quartile 4 vs Quartile 1) | Overall P Value | ||
| Length of stay, db,c | 4.3 (4.0) | 4.6 (4.3) | 4.6 (4.3) | 4.7 (4.3) | .03 | 1 [Reference] | 0.12 (−0.06 to 0.30) | 0.17 (−0.01 to 0.36) | 0.23 (0.04 to 0.41) | .01 | .09 | |
| Total cost, $c,d | 15 747 (14 282) | 17 105 (15 817) | 19 633 (17 807) | 18 737 (16 734) | <.001 | 1 [Reference] | 842 (−467 to 2150) | 3232 (1912 to 4551) | 3164 (1843 to 4485) | <.001 | <.001 | |
| In-hospital mortalitye,f | 919 (4.6) | 1513 (5.1) | 1019 (4.8) | 1162 (4.6) | .79 | 1 [Reference] | 1.15 (0.99 to 1.34) | 1.09 (0.94 to 1.27) | 1.02 (0.88 to 1.19) | .80 | .22 | |
| 3-mo Readmission among survivorsf | 3599 (18.2) | 5191 (18.0) | 4201 (19.9) | 4405 (17.9) | .40 | 1 [Reference] | 1.01 (0.93 to 1.10) | 1.16 (1.06 to 1.27) | 1.00 (0.92 to 1.09) | .80 | .001 | |
Abbreviation: OR, odds ratio.
Hierarchical generalized linear models accounted for patient clustering within hospitals and adjusted for patient demographics, comorbidities, acute organ failures, hospital characteristics, and hospital interventional capabilities.
Winsorized at the 99th percentile.
Expressed as mean (SD) in unadjusted outcomes.
Winsorized at the first and 99th percentiles.
One admission was randomly selected.
Expressed as No. (%) of patients in unadjusted outcomes.
Discussion
In this large sample of US hospitals, more than 70% of patients hospitalized for AMI underwent an echocardiogram and 74% had an evaluation of LVEF. Risk-standardized echocardiography rates varied significantly across hospitals, with median echocardiography use rates of 54% in the lowest quartile vs 83% in the highest quartile. Our analyses suggest that the strongest predictor of receipt of echocardiogram was the hospital to which the patient was admitted, which trumped any individual patient characteristic. When comparing outcomes at hospitals in the lowest vs highest quartiles, we observed no differences in mortality or 3-month readmission; however, hospitals with high echocardiography rates had a modestly longer length of stay and significantly higher total costs. Thus, our analyses suggest that, within the context of current clinical practice in the United States, more selective ordering of echocardiography has the potential to reduce costs without adversely affecting clinical outcomes, particularly at high-use hospitals.
To be clear, our findings should not be interpreted to mean that echocardiography provides no value in AMI. Because ACEis and ARBs are frequently indicated in patients with reduced LVEF, echocardiography can direct the use of these medications to improve patient outcomes.28,29 Echocardiography is also essential in determining patient eligibility for defibrillator use30 and developing a clinical suspicion for left ventricular thrombus.4 We found a small positive correlation between higher echocardiogram use and higher ACEi/ARB use; in a sensitivity analysis, we found that higher echocardiogram use was associated with somewhat lower readmission rates among patients who did not undergo cardiac catheterization. However, our overall results suggest that, at the margins, there may be clinical circumstances in which an echocardiogram can be safely deferred. Along these lines, it is known that echocardiography is associated with management changes in only 32% of cases, and repeated echocardiography yields new findings in only 11% of studies.5,6 Although our study was not designed to address this issue, our clinical experience suggests that an echocardiogram is unlikely to change management in several clinical situations, such as for patients in whom LVEF is already known to be markedly reduced; for patients with prior echocardiography in which image quality was so low as to make any future study nondiagnostic; for patients with a completely normal electrocardiogram31; or patients in whom AMI is diagnosed because of a mildly elevated troponin but who have few (if any) clinical symptoms or clinical suspicions for reduced LVEF. Future research should focus on identifying the clinical situations in which echocardiography can be safely deferred.
We carried out a series of hospital-level analyses because we found patterns in echocardiography use and patient characteristics which suggested that a patient-level analysis would be limited by confounding in which patients who are sicker would preferentially be chosen to receive echocardiography. This decision was supported by higher use of critical care therapies and greater comorbidities among patients with an echocardiogram. Further, in a sensitivity analysis when excluding patients with a short hospital length of stay (many of whom died prior to the performance of an echocardiogram or, conversely, were low acuity and thus discharged quickly), we observed higher unadjusted mortality rates among patients with an echocardiogram. We found more variation between hospitals in use of echocardiography than we did based on any individual patient characteristic, and adjustment only narrowed the distribution of risk-standardized echocardiography rates by a small amount.
Our results contrast with those of a study that reported echocardiography being associated with a 26% lower risk of mortality in AMI.32 However, that analysis relied on an ICD-9 procedure code that recorded echocardiography results in only 7% of patients, which is markedly different than our finding of greater than 70% use of echocardiography when applying billing codes.19 Moreover, that study used a patient-level analysis, did not adjust for hospital effects, and did not perform sensitivity analyses excluding early deaths prior to the performance of echocardiography. The Worcester Heart Attack Study29 noted an increase in echocardiography use from 4% in 1975 to 73% in 2003. A secondary finding of this study was that echocardiography use was associated with lower mortality, but they did not undertake a full analysis that accounted for a substantial difference in baseline characteristics. A study by Hernandez and colleagues33 showed that assessment of LVEF in AMI was associated with lower mortality, but this study involved patients enrolled in a clinical trial that began in 2001 when clinical practice patterns were significantly different and contrast ventriculography was frequently performed. As a result, the relevance of these studies is unclear regarding our current understanding of the association between contemporary echocardiography use and outcomes.
In contrast to these studies, our findings are consistent with several studies showing that higher rates of echocardiography testing are not associated with improved patient outcomes. Clough et al34 demonstrated that higher rates of outpatient echocardiography, catheterization, and myocardial perfusion imaging were not associated with differences in mortality or hospitalization in an outpatient Medicare cohort, which was similar to the findings of Kini and colleagues23 in a population with heart failure. Similarly, Safavi et al10 found that higher noninvasive imaging for suspected acute coronary syndrome was associated with higher rates of hospitalization and invasive procedures but no changes in patient-centered outcomes. Cohen et al12 found that routine echocardiography inhemodynamically stable acute pulmonary embolism was associated with higher use of thrombolysis, bleeding, and cost, but was not associated with changes in mortality.
Because an individual echocardiogram costs considerably less than $3100, the difference in costs between high- and low-rate hospitals is not explained solely by variation in the use of echocardiography. Instead, the higher costs noted at hospitals with high echocardiography usage may reflect a hospital culture that encourages more testing, procedures, and resource use overall.35 Hospitals with higher rates of echocardiography use also showed higher rates of nuclear testing and invasive ventriculography and greater use of intensive care unit services. This same general finding has also been encountered in several other studies showing that increased resource availability was associated with higher resource use without changes in outcomes.36,37,38,39
Limitations
Our study has several limitations. First, our data set did not include information about outpatient procedures (either before or after the AMI), and this protocol confines our conclusions primarily to the value of an inpatient echocardiogram performed during an admission for AMI. Second, the data set lacks long-term outcomes. It is possible that patients with an inpatient echocardiogram after AMI receive better in-hospital care,28,33 which in turn may be associated with better long-term outcomes. However, we believe this situation is less likely because we saw little association between an inpatient echocardiogram and 3-month readmission. Moreover, prior studies have shown no association between echocardiography testing intensity and long-term outcomes, including readmission.23,34 Third, although our data set captures echocardiography and cardiac imaging testing, the results of these tests (such as LVEF) are unknown. This lack of imaging results limits our ability to adjust for LVEF levels but not our ability to evaluate associations between test performance and outcomes. Fourth, hospital participation in the Premier database is voluntary, and the hospitals are not fully representative of US acute care hospitals, with overrepresentation of hospitals located in the southern United States. Given that we found lower rates of echocardiography among hospitals in the south, this finding may partially explain why our overall LVEF assessment percentage is somewhat lower than reported in the Get With the Guidelines database.28 However, this situation should not limit the validity of the comparisons between hospitals in which we had complete data capture.
Conclusions
Rates of echocardiography in the setting of AMI vary between hospitals; however, higher rates were not associated with better clinical outcomes but were associated with higher costs and longer length of stay. Although echocardiography plays an important role in the treatment of many patients with AMI, these findings suggest that a more selective approach may be safe and may reduce costs, particularly at high-use hospitals.
eMethods. Sensitivity Analyses
eResults. Sensitivity Results
eTable 1. Patient Characteristics and Unadjusted Outcomes by Use of Echocardiography at Hospitals with At Least 25 Admissions for Acute Myocardial Infarction
eTable 2. Risk Standardized Model
eTable 3. Death and Echo Rates by Patient Hospital Length of Stay
eTable 4. Sensitivity Analysis Cohorts
eTable 5. Patient Characteristics and Unadjusted Outcomes By Use of Echocardiography When Excluding Length of Stay ≤ 2 Days
eTable 6. Sensitivity Analysis Excluding Patients with Hospital Length of Stay of ≤2 days
eTable 7. Sensitivity Analysis Excluding Hospital Transfers
eTable 8. Sensitivity Analysis by Catheterization and Revascularization Subgroups
References
- 1.Krumholz HM, Chen J, Chen YT, Wang Y, Radford MJ. Predicting one-year mortality among elderly survivors of hospitalization for an acute myocardial infarction: results from the Cooperative Cardiovascular Project. J Am Coll Cardiol. 2001;38(2):453-459. doi: 10.1016/S0735-1097(01)01395-X [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee JT, Beshansky JR, Ruthazer R, et al. . In-hospital measurement of left ventricular ejection fraction and one-year outcomes in acute coronary syndromes: results from the IMMEDIATE Trial. Cardiovasc Ultrasound. 2016;14(1):29. doi: 10.1186/s12947-016-0068-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prastaro M, Pirozzi E, Gaibazzi N, et al. . Expert review on the prognostic role of echocardiography after acute myocardial infarction. J Am Soc Echocardiogr. 2017;30(5):431-443.e2. doi: 10.1016/j.echo.2017.01.020 [DOI] [PubMed] [Google Scholar]
- 4.Weinsaft JW, Kim J, Medicherla CB, et al. . Echocardiography algorithm for post-myocardial infarction LV thrombus: a gatekeeper for thrombus evaluation by delayed enhancement CMR. JACC Cardiovasc Imaging. 2016;9(5):505-515. doi: 10.1016/j.jcmg.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matulevicius SA, Rohatgi A, Das SR, Price AL, DeLuna A, Reimold SC. Appropriate use and clinical impact of transthoracic echocardiography. JAMA Intern Med. 2013;173(17):1600-1607. doi: 10.1001/jamainternmed.2013.8972 [DOI] [PubMed] [Google Scholar]
- 6.Hua A, McCaughan V, Wright M, et al. . Appropriateness, diagnostic value, and outcomes of repeat testing following index echocardiography. Echocardiography. 2018;35(1):24-29. doi: 10.1111/echo.13726 [DOI] [PubMed] [Google Scholar]
- 7.Amsterdam EA, Wenger NK, Brindis RG, et al. . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139-e228. doi: 10.1016/j.jacc.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 8.O’Gara PT, Kushner FG, Ascheim DD, et al. . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):e78-e140. doi: 10.1016/j.jacc.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 9.Jneid H, Addison D, Bhatt DL, et al. . 2017 AHA/ACC clinical performance and quality measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2017;70(16):2048-2090. doi: 10.1016/j.jacc.2017.06.032 [DOI] [PubMed] [Google Scholar]
- 10.Safavi KC, Li SX, Dharmarajan K, et al. . Hospital variation in the use of noninvasive cardiac imaging and its association with downstream testing, interventions, and outcomes. JAMA Intern Med. 2014;174(4):546-553. doi: 10.1001/jamainternmed.2013.14407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Main ML, Hibberd MG, Ryan A, Lowe TJ, Miller P, Bhat G. Acute mortality in critically ill patients undergoing echocardiography with or without an ultrasound contrast agent. JACC Cardiovasc Imaging. 2014;7(1):40-48. doi: 10.1016/j.jcmg.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 12.Cohen DM, Winter M, Lindenauer PK, Walkey AJ. Echocardiogram in the evaluation of hemodynamically stable acute pulmonary embolism: national practices and clinical outcomes. Ann Am Thorac Soc. 2018;15(5):581-588. doi: 10.1513/AnnalsATS.201707-577OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim N, Bernheim SM, Ott LS, et al. . An administrative claims measure of payments made for Medicare patients for a 30-day episode of care for acute myocardial infarction. Med Care. 2015;53(6):542-549. doi: 10.1097/MLR.0000000000000361 [DOI] [PubMed] [Google Scholar]
- 14.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749-759. doi: 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 16.Lagu T, Pekow PS, Shieh MS, et al. . Validation and comparison of seven mortality prediction models for hospitalized patients with acute decompensated heart failure. Circ Heart Fail. 2016;9(8):e002912. doi: 10.1161/CIRCHEARTFAILURE.115.002912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagu T, Lindenauer PK, Rothberg MB, et al. . Development and validation of a model that uses enhanced administrative data to predict mortality in patients with sepsis. Crit Care Med. 2011;39(11):2425-2430. doi: 10.1097/CCM.0b013e31822572e3 [DOI] [PubMed] [Google Scholar]
- 18.Walkey AJ, Lagu T, Lindenauer PK. Trends in sepsis and infection sources in the United States: a population-based study. Ann Am Thorac Soc. 2015;12(2):216-220. doi: 10.1513/AnnalsATS.201411-498BC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pack QR, Priya A, Lagu TC, et al. . Inpatient echocardiography use for common cardiovascular conditions. Circulation. 2018;137(16):1745-1747. doi: 10.1161/CIRCULATIONAHA.117.032256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagu T, Krumholz HM, Dharmarajan K, et al. . Spending more, doing more, or both? an alternative method for quantifying utilization during hospitalizations. J Hosp Med. 2013;8(7):373-379. doi: 10.1002/jhm.2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pack QR, Priya A, Lagu T, et al. . Development and validation of a predictive model for short- and medium-term hospital readmission following heart valve surgery. J Am Heart Assoc. 2016;5(9):e003544. doi: 10.1161/JAHA.116.003544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston SC, Henneman T, McCulloch CE, van der Laan M. Modeling treatment effects on binary outcomes with grouped-treatment variables and individual covariates. Am J Epidemiol. 2002;156(8):753-760. doi: 10.1093/aje/kwf095 [DOI] [PubMed] [Google Scholar]
- 23.Kini V, McCarthy FH, Rajaei S, Epstein AJ, Heidenreich PA, Groeneveld PW. Variation in use of echocardiography among veterans who use the Veterans Health Administration vs Medicare. Am Heart J. 2015;170(4):805-811. doi: 10.1016/j.ahj.2015.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institute of Medicine Variation in Health Care Spending: Target Decision Making, Not Geography. Washington, DC: The National Academies Press; 2013. doi: 10.17226/18393. [DOI] [PubMed] [Google Scholar]
- 25.Merlo J, Chaix B, Ohlsson H, et al. . A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60(4):290-297. doi: 10.1136/jech.2004.029454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang D, Dalton JE A unified approach to measuring the effect size between two groups using SAS. SAS Global Forum 2012;Paper 335-2012. [Google Scholar]
- 28.Miller AL, Dib C, Li L, et al. . Left ventricular ejection fraction assessment among patients with acute myocardial infarction and its association with hospital quality of care and evidence-based therapy use. Circ Cardiovasc Qual Outcomes. 2012;5(5):662-671. doi: 10.1161/CIRCOUTCOMES.112.965012 [DOI] [PubMed] [Google Scholar]
- 29.Santolucito PA, Tighe DA, Lessard D, et al. . Changing trends in the evaluation of ejection fraction in patients hospitalized with acute myocardial infarction: the Worcester Heart Attack Study. Am Heart J. 2008;155(3):485-493. doi: 10.1016/j.ahj.2007.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adabag S, Patton KK, Buxton AE, et al. . Association of implantable cardioverter defibrillators with survival in patients with and without improved ejection fraction: secondary analysis of the Sudden Cardiac Death in Heart Failure Trial. JAMA Cardiol. 2017;2(7):767-774. doi: 10.1001/jamacardio.2017.1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Keefe JH Jr, Zinsmeister AR, Gibbons RJ. Value of normal electrocardiographic findings in predicting resting left ventricular function in patients with chest pain and suspected coronary artery disease. Am J Med. 1989;86(6, pt 1):658-662. doi: 10.1016/0002-9343(89)90439-7 [DOI] [PubMed] [Google Scholar]
- 32.Papolos A, Narula J, Bavishi C, Chaudhry FA, Sengupta PPUS. US hospital use of echocardiography: insights from the Nationwide Inpatient Sample. J Am Coll Cardiol. 2016;67(5):502-511. doi: 10.1016/j.jacc.2015.10.090 [DOI] [PubMed] [Google Scholar]
- 33.Hernandez AF, Velazquez EJ, Solomon SD, et al. ; VALIANT Registry . Left ventricular assessment in myocardial infarction: the VALIANT registry. Arch Intern Med. 2005;165(18):2162-2169. doi: 10.1001/archinte.165.18.2162 [DOI] [PubMed] [Google Scholar]
- 34.Clough JD, Rajkumar R, Crim MT, et al. . Practice-level variation in outpatient cardiac care and association with outcomes. J Am Heart Assoc. 2016;5(2):e002594. doi: 10.1161/JAHA.115.002594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X, Li SX, Lin H, et al. . Hospital phenotypes in the management of patients admitted for acute myocardial infarction. Med Care. 2016;54(10):929-936. doi: 10.1097/MLR.0000000000000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fanaroff AC, Peterson ED, Chen AY, et al. . Intensive care unit utilization and mortality among Medicare patients hospitalized with non–ST-segment elevation myocardial infarction. JAMA Cardiol. 2017;2(1):36-44. doi: 10.1001/jamacardio.2016.3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucas FL, Sirovich BE, Gallagher PM, Siewers AE, Wennberg DE. Variation in cardiologists’ propensity to test and treat: is it associated with regional variation in utilization? Circ Cardiovasc Qual Outcomes. 2010;3(3):253-260. doi: 10.1161/CIRCOUTCOMES.108.840009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delamater PL, Messina JP, Grady SC, WinklerPrins V, Shortridge AM. Do more hospital beds lead to higher hospitalization rates? a spatial examination of Roemer’s Law. PLoS One. 2013;8(2):e54900. doi: 10.1371/journal.pone.0054900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nallamothu BK, Rogers MA, Chernew ME, Krumholz HM, Eagle KA, Birkmeyer JD. Opening of specialty cardiac hospitals and use of coronary revascularization in Medicare beneficiaries. JAMA. 2007;297(9):962-968. doi: 10.1001/jama.297.9.962 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Sensitivity Analyses
eResults. Sensitivity Results
eTable 1. Patient Characteristics and Unadjusted Outcomes by Use of Echocardiography at Hospitals with At Least 25 Admissions for Acute Myocardial Infarction
eTable 2. Risk Standardized Model
eTable 3. Death and Echo Rates by Patient Hospital Length of Stay
eTable 4. Sensitivity Analysis Cohorts
eTable 5. Patient Characteristics and Unadjusted Outcomes By Use of Echocardiography When Excluding Length of Stay ≤ 2 Days
eTable 6. Sensitivity Analysis Excluding Patients with Hospital Length of Stay of ≤2 days
eTable 7. Sensitivity Analysis Excluding Hospital Transfers
eTable 8. Sensitivity Analysis by Catheterization and Revascularization Subgroups