Key Points
Question
How do levels of neurofilament light in cerebrospinal fluid (cNfL) compare between neurological conditions and with healthy controls?
Findings
Among 10 059 individuals in this systematic review and meta-analysis, cNfL was elevated in most neurological conditions compared with healthy controls, and the magnitude of the increase varies extensively. Although cNfL overlaps between most clinically similar conditions, its distribution did not overlap in frontotemporal dementia and other dementias or in Parkinson disease and atypical parkinsonian syndromes.
Meaning
The cNfL is a marker of neuronal damage and may be useful to differentiate some clinically similar conditions, such as frontotemporal dementia from Alzheimer disease and Parkinson disease from atypical parkinsonian syndromes.
This systematic review and meta-analysis assesses the associations of age, sex, and diagnosis with neurofilament light in cerebrospinal fluid and evaluates its potential in discriminating clinically similar conditions.
Abstract
Importance
Neurofilament light protein (NfL) is elevated in cerebrospinal fluid (CSF) of a number of neurological conditions compared with healthy controls (HC) and is a candidate biomarker for neuroaxonal damage. The influence of age and sex is largely unknown, and levels across neurological disorders have not been compared systematically to date.
Objectives
To assess the associations of age, sex, and diagnosis with NfL in CSF (cNfL) and to evaluate its potential in discriminating clinically similar conditions.
Data Sources
PubMed was searched for studies published between January 1, 2006, and January 1, 2016, reporting cNfL levels (using the search terms neurofilament light and cerebrospinal fluid) in neurological or psychiatric conditions and/or in HC.
Study Selection
Studies reporting NfL levels measured in lumbar CSF using a commercially available immunoassay, as well as age and sex.
Data Extraction and Synthesis
Individual-level data were requested from study authors. Generalized linear mixed-effects models were used to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels, with cohort of origin modeled as a random intercept.
Main Outcome and Measure
The cNfL levels adjusted for age and sex across diagnoses.
Results
Data were collected for 10 059 individuals (mean [SD] age, 59.7 [18.8] years; 54.1% female). Thirty-five diagnoses were identified, including inflammatory diseases of the central nervous system (n = 2795), dementias and predementia stages (n = 4284), parkinsonian disorders (n = 984), and HC (n = 1332). The cNfL was elevated compared with HC in a majority of neurological conditions studied. Highest levels were observed in cognitively impaired HIV-positive individuals (iHIV), amyotrophic lateral sclerosis, frontotemporal dementia (FTD), and Huntington disease. In 33.3% of diagnoses, including HC, multiple sclerosis, Alzheimer disease (AD), and Parkinson disease (PD), cNfL was higher in men than women. The cNfL increased with age in HC and a majority of neurological conditions, although the association was strongest in HC. The cNfL overlapped in most clinically similar diagnoses except for FTD and iHIV, which segregated from other dementias, and PD, which segregated from atypical parkinsonian syndromes.
Conclusions and Relevance
These data support the use of cNfL as a biomarker of neuroaxonal damage and indicate that age-specific and sex-specific (and in some cases disease-specific) reference values may be needed. The cNfL has potential to assist the differentiation of FTD from AD and PD from atypical parkinsonian syndromes.
Introduction
Identifying neuroaxonal damage and quantifying the intensity of this process is a critical step in patient care because it may support diagnosis and help estimate the prognosis of neurological conditions. In addition, it is essential for the evaluation of drug candidates with disease-modifying potential. Neurofilament light protein (NfL) is an abundant cytoskeletal protein exclusively expressed by central and peripheral neurons. Elevated levels of NfL in cerebrospinal fluid (CSF) were first reported in neurodegenerative conditions more than 20 years ago,1 sparking interest in the potential of this neuron-specific protein as a biomarker. Since then, elevated levels of NfL in CSF (cNfL) have been described in a number of neurological and psychiatric conditions. The magnitude of the increase in inflammatory, degenerative, infectious, ischemic, and traumatic neurological conditions, as well as in psychiatric disorders, varies between conditions and studies. To date, cNfL levels have not been compared systematically between neurological disorders, and patient numbers in individual studies are often low. A positive association between cNfL and age has been reported in healthy controls (HC)2 but was not systematically investigated in neurological conditions and may alter the performance of this biomarker across age categories. Together, these questions limit clinical implementation of cNfL. To compare cNfL levels between diagnoses, assess the association of age and sex with these variables, and evaluate the potential of cNfL level as a diagnostic biomarker, we performed a systematic review and meta-analysis on individual data collected from studies reporting cNfL levels in diseases and controls.
Methods
Search Strategy
This systematic review and meta-analysis followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines.3 We searched PubMed for articles published in English between January 1, 2006, and January 1, 2016, reporting cNfL levels (using the search terms neurofilament light and cerebrospinal fluid) in neurological or psychiatric conditions and/or in HC. Titles and abstracts were reviewed, and relevant studies were selected. The quality of primary articles was assessed using relevant criteria from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines4 and the QUADAS-2 guidelines.5 All studies were approved by local ethics committees.
Inclusion Criteria
Studies were included if lumbar cNfL was reported for neurological patients and/or HC and/or individuals with subjective neurological or cognitive complaints and/or a psychiatric condition and/or a systemic disease that may affect the central nervous system (CNS). A reference method for the measurement of cNfL is lacking to date. To limit between-cohort heterogeneity due to the measurement tool, we included only those studies that used the same commercially available immunoassay (NF-light ELISA [enzyme-linked immunosorbent assay]; UmanDiagnostics) on the market since 2006. This assay was selected because it was used in a majority of publications (71 of 112) since 2006 and was reported to be sensitive and robust.6
Data Collection
We contacted the corresponding authors to request access to individual-level cNfL, age at CSF sampling, sex, and diagnosis. An individual’s data were included only if all of those variables were available. For patients with multiple sclerosis (MS) and HIV-positive individuals, treatment status was also collected.7,8 Information on study procedures was extracted from the publication or requested from the corresponding author.
Diagnostic Categories
Diagnosis was established by the original study authors according to published criteria when applicable (Table 1). Information about the clinical subtype of neurodegenerative conditions was not retained, and all clinical subtypes of a condition were pooled in a single diagnostic group. Stroke, cardiac arrest, HIV infection, chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), Guillain-Barré syndrome (GBS), Cushing disease in remission, and optic neuritis (ON) were diagnosed according to clinical guidelines. Presymptomatic genetic frontotemporal dementia (pgFTD), Huntington disease (HD), and premanifest HD (pHD) were diagnosed by genetic testing. The HIV-infected individuals with cognitive impairment (iHIV) included individuals with mild neurocognitive impairment and individuals with HIV-associated dementia.
Table 1. Diagnostic Criteria Used by the Original Study Authors.
| Diagnosis | Abbreviation | Diagnostic Criteria |
|---|---|---|
| Multiple sclerosis and clinically isolated syndrome | MS and CIS | McDonald criteria,9 2005 revisions,10 and 2010 revisions11 |
| Alzheimer disease and mild cognitive impairment | AD and MCI | Criteria by McKhann et al12 and IWG-2 criteria13 |
| Parkinson disease | PD | United Kingdom Parkinson Disease Society Brain Bank criteria14 and National Institute of Neurological Disorders and Stroke criteria15 |
| Parkinson disease dementia | PDD | Movement Disorder Task Force16 |
| Progressive supranuclear palsy | PSP | Criteria by Litvan et al17 |
| Multiple system atrophy | MSA | Criteria by Gilman et al18 |
| Corticobasal syndrome | CBS | Criteria by Lee et al,19 criteria by Litvan et al,17 and criteria by Mathew et al20 |
| Dementia with Lewy bodies | DLB | Criteria by McKeith et al21 |
| Frontotemporal dementia (including all clinical subtypes) | FTD | Criteria by Neary et al22 and The Lund and Manchester Groups23 |
| Amyotrophic lateral sclerosis | ALS | Revised El Escorial criteria24 |
| Combined frontotemporal dementia and amyotrophic lateral sclerosis | FTD/ALS | |
| Vascular dementia | VaD | Criteria by Erkinjuntti et al25 and National Institute of Neurological Disorders and Stroke |
| Idiopathic normal-pressure hydrocephalus | iNPH | Criteria by Relkin et al26 |
| Bipolar disorder | BD | Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) |
| HIV positive with cognitive impairment (including entire spectrum of cognitive impairment) | iHIV | Global Deficit Score27 |
Abbreviation: IWG-2, International Working Group 2.
Individuals with subjective neurological complaint (SNC) or subjective cognitive decline (SCD) had complaints but no objectifiable neurological condition after extensive workup. Inflammatory neurological diseases (IND) were inflammatory diseases of the CNS, excluding MS, clinically isolated syndrome (CIS), and ON. Noninflammatory neurological diseases (NID) were any CNS disease that was not of inflammatory nature. Mixed dementia (MD) was dementia of assumed mixed pathology, and dementia not specified (DNS) was dementia of uninvestigated origin. Healthy controls were individuals who did not have neurological complaints or signs of a neurological condition.
Diagnostic Groups
We clustered a subset of frequent neurological conditions into 3 groups of clinically similar disorders. These included the following: (1) untreated relapsing-remitting MS (uRRMS), individuals with relapsing-remitting MS treated with disease-modifying therapy (tRRMS), CIS, ON, primary progressive MS (PPMS), secondary progressive MS (SPMS), and IND; (2) Alzheimer disease (AD), FTD, combined FTD and amyotrophic lateral sclerosis (FTD/ALS), vascular dementia (VaD), dementia with Lewy bodies (DLB), idiopathic normal-pressure hydrocephalus (iNPH), mild cognitive impairment of suspected AD pathology (MCI), SCD, and iHIV; and (3) Parkinson disease (PD), PD dementia (PDD), DLB, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome of suspected tau underlying pathology (CBS).
cNfL Measurement
The cNfL was measured at 17 different centers using the commercially available kit (NF-light ELISA assay). The cNfL values were reported in picograms per milliliter or nanograms per liter. A systematic error in the reported concentration of cNfL was identified at 8 centers due to a misinterpretation of the assay’s protocol. The protocol indicated to perform a 1:1 dilution of CSF before performing the assay. However, because this dilution is included a priori in the value assignment of the standard curve, this initial dilution should not be corrected for at calculation of the concentration. Raw NfL values obtained from the 8 implicated centers were corrected for the systematic error (divided by 2).
Statistical Analysis
We performed an individual-level meta-analysis based on cNfL measurements provided by the corresponding authors. Linear mixed-effects models were used to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels, with cohort of origin modeled as a random intercept, using the R packages “lme4” and “lmerTest” (R Project for Statistical Computing). Age was centered according to the mean. First, we tested all 2-way and 3-way interaction terms between all fixed effects, which were retained in the model when statistically significant. No 2-way interaction of age and sex or 3-way interaction of age, sex, and diagnosis on cNfL was observed, and the best-fitting model included all fixed effects and interaction terms for diagnosis by age and diagnosis by sex. Next, we used the R package “emmeans” to obtain marginalized change folds and 95% CI cNfL and cNfL-age slope estimates for all diagnoses and to perform post hoc pairwise comparisons between diagnoses in the mean cNfL levels and in the strength of the associations between cNfL age, adjusting P values for multiple testing with the Tukey procedure. Finally, we calculated point estimates of fold-change increases for each diagnostic group compared with controls for specific ages. The consequences of study variability on the results was assessed using the intraclass correlation coefficient, which reflects the proportion of variance that can be attributed to between-study variation, for the total sample and per diagnostic group (analyses for the latter were performed on models the included the fixed effects of age and sex). Values higher than 0.60 were considered to be indicative of substantial heterogeneity. The results were considered statistically significant when they had an adjusted 2-sided P value below .05. All analyses were performed in R version 3.4.2.
Results
Data Set Characteristics, Population, and Demographics
The literature search resulted in 153 records. On the basis of title and abstract, 112 publications were selected for full-text review, and 44 data sets met our selection criteria and were included in the meta-analysis. In addition, 3 data sets unpublished at the time of data collection were provided by study authors, resulting in a total of 47 data sets (Table 2 and eFigure 1 in the Supplement). Data were obtained for 10 059 individuals (mean [SD] age, 59.7 [18.8] years; 54.1% female), and 35 diagnoses were identified, including control groups (HC [n = 1332], SNC [n = 45], and SCD [n = 24] [eTable 1 in the Supplement]), inflammatory diseases of the CNS (CIS, ON, RRMS, SPMS, PPMS, and IND [n = 2795]) (eTable 1 in the Supplement), dementias and predementia stages (MCI, AD, pgFTD, FTD, VaD, DLB, iNPH, DNS, MD, pHD, HD, iHIV, and FTD/ALS [n = 4339]) (eTable 1 in the Supplement), and parkinsonian syndromes (PD, PDD, MSA, PSP, CBS, and DLB [n = 984]) (eTable 1 in the Supplement). Three diagnostic categories were excluded from the statistical models because they had fewer than 5 observations per sex (Cushing disease, cardiac arrest, and HIV), resulting in 32 diagnostic categories and 10 012 individuals included in the analysis.
Table 2. Data Sets Included in the Meta-analysis.
| Source | Contributed Diagnostic Categories (No. of Individuals) | Diagnostic Criteria | Healthy Controls Contributed, No. |
|---|---|---|---|
| Anckarsäter et al,28 2014 | None | NA | 34 |
| Axelsson et al,29 2014 | SPMS (n = 30), PPMS (n = 5) | McDonald criteria 2010 revisions11 | 14 |
| Bäckström et al,30 2015 | PD (n = 99), MSA (n = 11), PSP (n = 12) | PD: United Kingdom Parkinson Disease Society Brain Bank criteria14 | 30 |
| MSA: Criteria by Gilman et al18 | |||
| PSP: Criteria by Litvan et al17 | |||
| Bjerke et al,31 2011 | AD (n = 30), VaD (n = 26) | AD: Criteria by McKhann et al12 | 30 |
| VaD: Criteria by Erkinjuntti et al25 | |||
| Bjerke et al,32 2014 and Jonsson et al,33 2012 | MCI (n = 31) | Criteria by McKhann et al12 | 15 |
| Bruno et al,34 2012 | None | NA | 19 |
| Burman et al,35 2014 | RRMS (n = 43), SPMS (n = 20), National Institute of Neurological Disorders and Stroke (n = 7), SNC (n = 6) | McDonald criteria 2010 revisions11 | 2 |
| Fialová et al,36 2013 | CIS (n = 32), RRMS (n = 18) | McDonald criteria 2005 revisions10 | 24 |
| Fialová et al,37 2017 | AD (n = 25), DNS (n = 13), IND (n = 17) | AD: Criteria by McKhann et al12 | 25 |
| Gunnarsson et al,38 2011 | RRMS (n = 92) | McDonald criteria 2010 revisions11 | 0 |
| Hall et al,39 2012 and Hall et al,40 2015 | AD (n = 48), PD (n = 196), PDD (n = 56), PSP (n = 53), MSA (n = 67), CBS (n = 15), DLB (n = 69) | AD: Criteria by McKhann et al12 | 150 |
| PD: National Institute of Neurological Disorders and Stroke criteria15 | |||
| PDD: Movement Disorder Task Force16 | |||
| MSA: Criteria by Gilman et al18 | |||
| PSP and CBS: Criteria by Litvan et al17 | |||
| DLB: Criteria by McKeith et al21 | |||
| CBS: Criteria by Mathew et al20 | |||
| Herbert et al,41 2015 | PD (n = 64), MSA (n = 50) | PD: United Kingdom Parkinson Disease Society Brain Bank criteria14 | 70 |
| MSA: Criteria by Gilman et al18 | |||
| Hjalmarsson et al,42 2014 | Stroke (n = 20) | Clinical | 20 |
| Jakobsson et al,43 2014 and Rolstad et al,44 2015 | BD (n = 133) | Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) | 38 |
| Jeppsson et al,45 2013 | iNPH (n = 27) | Criteria by Relkin et al26 | 20 |
| Jessen Krut et al,46 2014 | iHIV (n = 13) | Global Deficit Score27 | 152 |
| Khademi et al,2 2013 Aeinehband et al,47 2015, and unpublished data | CIS (n = 203), RRMS (n = 682), IND (n = 387), National Institute of Neurological Disorders and Stroke (n = 370) | McDonald criteria9 | 30 |
| Khalil et al,48 2013 | CIS (n = 47), NID (n = 15) | McDonald criteria 2010 revisions11 | 0 |
| Kuhle et al,49 2013 | CIS (n = 62), RRMS (n = 38), SPMS (n = 25), PPMS (n = 23) | McDonald criteria 2005 revisions10 | 72 |
| Kuhle et al,50 2013 | RRMS (n = 30) | McDonald criteria 2005 revisions10 | 0 |
| Kuhle et al,51 2015 | RRMS (n = 36) | McDonald criteria 2005 revisions10 | 0 |
| Magdalinou et al,52 2015 and unpublished data | AD (n = 26), CBS (n = 16), FTD (n = 16), MSA (n = 30), PD (n = 10), PSP (n = 29) | AD: Criteria by McKhann et al12 | 28 |
| CBS: Criteria by Mathew et al20 | |||
| FTD: The Lund and Manchester Groups23 | |||
| MSA: Criteria by Gilman et al18 | |||
| PD: United Kingdom Parkinson Disease Society Brain Bank criteria14 | |||
| PSP: Criteria by Litvan et al17 | |||
| Martínez et al,53 2015 and unpublished data | PPMS (n = 17), SPMS (n = 6), RRMS (n = 192), CIS (n = 109) | McDonald criteria9 | 0 |
| Martínez et al unpublished data | CIS (n = 51), RRMS (n = 46) | McDonald criteria9 | 0 |
| Martínez et al unpublished data | NID (n = 6), IND (n = 2), stroke (n = 4), GBS (n = 1), ON (n = 1) | Clinical | 0 |
| Meeter et al,54 2016 | pgFTD (n = 42), FTD (n = 90) | Not specified | 49 |
| Menke et al,55 2015 and Lu et al,56 2015 | ALS (n = 38) | Revised El Escorial criteria24 | 20 |
| Modvig et al,57 2013 and Modvig et al,58 2016 | ON (n = 56) | Clinical | 27 |
| Modvig et al,59 2015 | ON (n = 85) | Clinical | 0 |
| Paterson et al,60 2015 | AD (n = 94) | IWG-2 criteria13 | 30 |
| Pérez-Santiago et al,61 2016 | iHIV (n = 14), HIV (n = 14) | Global Deficit Score27 | 0 |
| Pijnenburg et al,62 2015 | FTD/ALS (n = 26), FTD (n = 4), AD (n = 25), SCD (n = 24) | ALS: Revised El Escorial criteria24 | 0 |
| AD: Criteria by McKhann et al12 | |||
| Neuropathological confirmation (11 of 25 for AD, 15 of 23 for FTD) | |||
| Genetic confirmation (12 of 23 for FTD) | |||
| Pyykkö et al,63 2014 | iNPH (n = 29), MD (n = 3), AD (n = 8) | AD: Criteria by McKhann et al12 | 0 |
| iNPH: Clinical | |||
| Ragnarsson et al,64 2013 | Cushing disease (n = 12) | Clinical | 6 |
| Romme Christensen et al,65 2014 | PPMS (n = 12), SPMS (n = 12) | McDonald criteria 2005 revisions10 | 0 |
| Rosén et al,66 2014 | Cardiac arrest (n = 21) | Clinical | 20 |
| Sandberg et al,67 2016 | RRMS (n = 97), SPMS (n = 44), PPMS (n = 12) | McDonald criteria 2010 revisions11 | 0 |
| Scherling et al,68 2014 | FTD (n = 83), PSP (n = 23), CBS (n = 16), PD (n = 6), AD (n = 45) | FTD: Criteria by Neary et al22 | 54 |
| PSP: Criteria by Litvan et al17 | |||
| AD: Criteria by McKhann et al12 | |||
| CBS: Criteria by Lee et al19 | |||
| Skillbäck et al,69 2014 | AD (n = 1417), PDD (n = 45), FTD (n = 146), LBD (n = 114), MD (n = 517), VaD (n = 465), DNS (n = 545) | AD: IWG-2 criteria13 | 107 |
| DNS: International Statistical Classification of Diseases, 10th Revision | |||
| PDD: Movement Disorder Task Force16 | |||
| FTD: The Lund and Manchester Groups23 | |||
| DLB: Criteria by McKeith et al21 | |||
| VaD: National Institute of Neurological Disorders and Stroke | |||
| Stilund et al,70 2015 | RRMS (n = 44), PPMS (n = 15), CIS (n = 27), SNC (n = 39) | McDonald criteria 2010 revisions11 | 0 |
| Tortelli et al,71 2015 and Tortelli et al,72 2012 | CIDP (n = 25), ALS (n = 37), MCI (n = 3), AD (n = 15), MSA (n = 1), CBS (n = 2), NID (n = 5) | ALS: Revised El Escorial criteria24 | 0 |
| CIDP: Clinical | |||
| AD and MCI: Criteria by McKhann et al12 | |||
| CBS: Criteria by Lee et al19 | |||
| MSA: Criteria by Gilman et al18 | |||
| Tortorella et al,73 2015 | CIS (n = 21) | McDonald criteria 2005 revisions10 | 0 |
| Trentini et al,74 2014 | PPMS (n = 21), SPMS (n = 10), National Institute of Neurological Disorders and Stroke (n = 15) | McDonald criteria9 | 0 |
| Vågberg et al,75 2015 | None | NA | 53 |
| Villar et al,76 2015 | RRMS (n = 98), CIS (n = 29) | McDonald criteria 2010 revisions11 | 37 |
| Wild et al,77 2015 | HD (n = 30), pHD (n = 13) | Genetic testing | 14 |
| Zetterberg et al,78 2016 | MCI (n = 193), AD (n = 95) | Criteria by McKhann et al12 | 111 |
Abbreviations: AD, Alzheimer disease; ALS, amyotrophic lateral sclerosis; BD, bipolar disorder; CBS, corticobasal syndrome; CIDP, chronic inflammatory demyelinating polyradiculopathy; CIS, clinically isolated syndrome; DLB, dementia with Lewy bodies; DNS, dementia not specified; FTD, frontotemporal dementia; FTD/ALS, combined frontotemporal dementia and amyotrophic lateral sclerosis; GBS, Guillain-Barré syndrome; HD, Huntington disease; iHIV, HIV positive with cognitive impairment; IND, inflammatory neurological disorders other than multiple sclerosis; iNPH, idiopathic normal-pressure hydrocephalus; IWG-2, International Working Group 2; MCI, mild cognitive impairment; MD, mixed dementia; MSA, multiple system atrophy; NA, not applicable; NID, noninflammatory neurological disorders; ON, optic neuritis; PD, Parkinson disease; PDD, Parkinson disease dementia; pgFTD, presymptomatic genetic frontotemporal dementia; pHD, premanifest Huntington disease; PPMS, primary progressive multiple sclerosis; PSP, progressive supranuclear palsy; SCD, subjective cognitive decline; SNC, subjective neurological complaint; SPMS, secondary progressive multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; VaD, vascular dementia.
cNfL Distribution Across Diagnoses
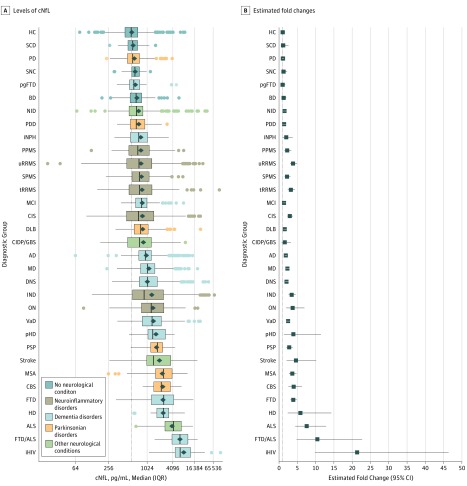
We first examined the distribution of cNfL across diagnostic categories (Figure 1). The cNfL was increased compared with HC in most neurological conditions (Figure 1A). The fold changes compared with HC varied extensively between individual conditions, with the largest effect sizes observed in iHIV (21.36; 95% CI, 9.86-46.30), FTD/ALS (10.48; 95% CI, 4.85-22.67), ALS (7.58; 95% CI, 4.49-12.81), and HD (5.88; 95% CI, 2.43-14.27) (Figure 1B; eTable 2 in the Supplement).
Figure 1. Neurofilament Light in Cerebrospinal Fluid (cNfL) Levels Across Diagnostic Categories.
A, Levels of cNfL are shown corrected for age and sex. B, Estimated fold changes are compared with healthy controls (HC). AD indicates Alzheimer disease; ALS, amyotrophic lateral sclerosis; BD, bipolar disorder; CBS, corticobasal syndrome; CIDP/GBS, chronic inflammatory demyelinating polyradiculopathy and Guillain-Barré syndrome; CIS, clinically isolated syndrome; DLB, dementia with Lewy bodies; DNS, dementia not specified; FTD, frontotemporal dementia; FTD/ALS, combined frontotemporal dementia and amyotrophic lateral sclerosis; HD, Huntington disease; iHIV, HIV positive with cognitive impairment; IND, inflammatory neurological disorders other than multiple sclerosis; iNPH, idiopathic normal-pressure hydrocephalus; MCI, mild cognitive impairment; MD, mixed dementia; MSA, multiple system atrophy; NID, noninflammatory neurological disorders; ON, optic neuritis; PD, Parkinson disease; PDD, Parkinson disease dementia; pgFTD, presymptomatic genetic frontotemporal dementia; pHD, premanifest Huntington disease; PPMS, primary progressive multiple sclerosis; PSP, progressive supranuclear palsy; SCD, subjective cognitive decline; SNC, subjective neurological complaint; SPMS, secondary progressive multiple sclerosis; tRRMS, treated relapsing-remitting multiple sclerosis; uRRMS, untreated relapsing-remitting multiple sclerosis; and VaD, vascular dementia.
Association of cNfL With Age and Sex
In HC, we observed a yearly increase of 3.30% (95% CI, 2.98%-3.62%) in cNfL levels (eTable 2 in the Supplement). A positive association between cNfL and age was also observed in individuals with subjective complaints, BD, and in most neurodegenerative conditions (eTable 2 in the Supplement). In MS, iHIV, and rapidly progressive neurodegenerative conditions (FTD, ALS, FTD/ALS, MSA, PSP, CBS, and HD), no such association was observed (eTable 2 in the Supplement). In HC, cNfL was higher in men (26.0%, 95% CI, 16.0%-37.0%) (eTable 3 in the Supplement). This was also the case in a minority of neurological conditions, including MS, AD, VaD, and PD (eTable 3 in the Supplement).
cNfL Levels Within 3 Groups of Clinically Similar Disorders
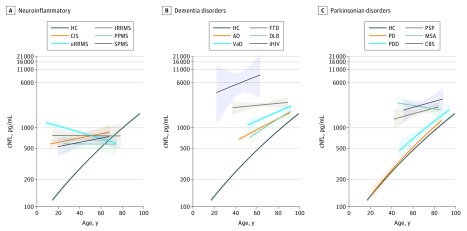
We next compared cNfL between neurological conditions within 3 groups of clinically similar disorders. In inflammatory conditions of the CNS, the mean cNfL levels were similar in ON, CIS, and MS subtypes (eTable 4A in the Supplement). The association between cNfL and age was positive in ON, CIS, and IND but was negative in uRRMS (Figure 2A; eFigure 2 and eTable 2 in the Supplement). The ratio of cNfL between ON and CIS, ON and IND, and CIS and IND remained stable across the age range of the study, while the ratio between uRRMS and CIS decreased with increasing age (eTable 5A in the Supplement). No association between cNfL and age was observed in tRRMS and PPMS (Figure 2A and eTable 4 in the Supplement). The ratio of cNfL between uRRMS and tRRMS and between uRRMS and PPMS remained stable across the age range of the study (eTable 5B in the Supplement). No association between cNfL and age was observed in SPMS (Figure 2A; and eTable 2 in the Supplement). Although cNfL levels tended to be higher in young uRRMS compared with age-corresponding SPMS, this did not reach statistical significance (eTable 5C in the Supplement). In dementias and related disorders, the mean cNfL levels were statistically significantly higher in FTD compared with other causes of dementia, such as AD (2.08; 95% CI, 1.72-2.56 [eTable 4B in the Supplement]), VaD (1.56; 95% CI, 1.25-1.96 [eTable 4B in the Supplement]), and DLB (2.50; 95% CI, 1.89-3.33 [eTable 4B in the Supplement]). An association of cNfL with age was positive in AD, VaD, and DLB but was absent in FTD (Figure 2B; eFigure 2B and eTable 4B in the Supplement). The ratio of cNfL between AD and FTD increased with age; in individuals 90 years and older, the distribution of cNfL in both conditions overlapped (eTable 5D in the Supplement). An association between cNfL and age was absent in FTD and FTD/ALS, while it was present in pgFTD (eFigure 2 and eTable 2 in the Supplement). A positive association with age was observed in AD, MCI, and SCD (Figure 2B; eTable 2 in the Supplement), and the ratio of cNfL between AD and MCI remained stable across the age range (eTable 5E in the Supplement). In parkinsonian syndromes, the mean cNfL levels did not differ between PD and PDD and between PDD and DLB, while they were higher in MSA, PSP, and CBS compared with PD (eTable 4C in the Supplement). In MSA, PSP, and CBS, no association with age was observed, while a positive association was found in PD, PDD, and DLB (Figure 2C and eTable 2 in the Supplement). The ratio of cNfL between MSA and PD, PSP and PD, and CBS and PD decreased with age but remained high across the age range of the study (Figure 2C and eTable 5G in the Supplement).
Figure 2. Neurofilament Light in Cerebrospinal Fluid (cNfL) in Neurological Conditions According to Age.
A-C, Log cNfL values are shown according to age across diagnoses. Shading around regression lines represents standard errors. AD indicates Alzheimer disease; CBS, corticobasal syndrome; CIS, clinically isolated syndrome; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; HC, healthy controls; iHIV, HIV positive with cognitive impairment; MSA, multiple system atrophy; PD, Parkinson disease; PDD, Parkinson disease dementia; PPMS, primary progressive multiple sclerosis; PSP, progressive supranuclear palsy; SPMS, secondary progressive multiple sclerosis; tRRMS, treated relapsing-remitting multiple sclerosis; uRRMS, untreated relapsing-remitting multiple sclerosis; and VaD, vascular dementia.
Assessment of Cohort Heterogeneity
In this meta-analysis, we pooled individual patient data originating from 42 different data sets. To estimate the proportion of the total variance of cNfL accounted for by the data set (cohort) of origin, we calculated the intraclass coefficient for cohort-related random intercepts. Across the total sample (n = 10 012), the intraclass coefficient was low at 0.15. Likewise, in a majority of diagnostic categories, the intraclass coefficient was low to moderate (<0.60). However, in 7 of the 32 diagnostic categories (MD, DNS, PDD, DLB, NID, iHIV, and stroke), the intraclass coefficients were high (>0.60), indicating that a large proportion of the variance in cNfL was due to the data set of origin (eTable 6 in the Supplement).
Discussion
In this meta-analysis that included 10 012 individuals, we found that cNfL was increased compared with HC in most neurological conditions studied. The largest effect sizes were observed in iHIV, FTD/ALS, ALS, and HD, while the effect sizes in inflammatory conditions of the CNS were low. Other neurological disorders showed much subtler increases that failed to reach statistical significance (PD and CIDP/GBS). However, the effect sizes in these conditions were positive, and larger sample sizes may allow for more robust estimates. In HC, we observed a positive association between cNfL and age. A positive association, albeit weaker, was also present in a majority of neurological conditions. An association with sex was absent in most diagnostic categories except for HC, PPMS, AD, VaD, and PD, where levels were higher in men. In clinically similar disorders, the distribution of cNfL relative to age mostly overlapped, suggesting limited use for differential diagnosis. Exceptions were FTD, which segregated from other common causes of dementia (including AD and VaD), and PD, which segregated from atypical parkinsonian syndromes. These data indicate that cNfL may contribute to the differentiation of these conditions, particularly in younger individuals.
cNfL and Age
In about two-thirds of the diagnoses, including HC, we observed a positive association between cNfL and age. In the control groups (HC, SNC, and SCD), as well as in pgFTD and BD, the association of cNfL with age was strongest. This positive association in diagnostic categories without an overt neurological condition may reflect a decrease in CSF clearance with age, the presence of a preclinical age-related neurological condition, or age-related neuronal loss.79 The association of cNfL with age in HC implies that age-specific reference values may be needed and that the diagnostic potential of cNfL may decrease with age. In neurological conditions with substantially elevated levels of cNfL, such as FTD, ALS, FTD/ALS, HD, and iHIV, as well as in atypical parkinsonian syndromes, no association with age was observed, suggesting that neuropathological processes may cause plateau levels or mask age associations. In MS, an association with age was absent or negative, which may reflect the observation that younger patients with MS have more active diseases.2
cNfL and Sex
In a minority of diagnoses, including HC, cNfL was higher in men than women. The clinical relevance of these findings is uncertain, but the results suggest that sex-specific reference values may be needed.
Other Determinants of cNfL Levels
Age, sex, and the random (cohort) association explained 46% of the variance of cNfL in the best-fitting model, indicating that many determinants of cNfL remain to be identified. Disease duration and severity could influence cNfL levels. However, these data were not available in the data sets that were included in this meta-analysis, and studies designed specifically to evaluate the association of these variables and others (eg, smoking, physical activity, and body size) are ongoing.
cNfL in Inflammatory Conditions of the CNS, Including MS
The cNfL was increased in all inflammatory conditions of the CNS examined in this meta-analysis, but the effect sizes were small. The distribution of cNfL in CIS, ON, and RRMS overlapped, which may be expected because CIS and a proportion of ON are initial manifestations of RRMS. Neurodegeneration has a central role in MS, contributing to disease progression and long-term disability.80 Poor understanding of the processes driving neurodegeneration, together with the lack of biomarkers allowing dynamic measurement of its rate, hampers the development of specific treatments.81 The cNfL has been reported to correlate with brain atrophy,50,82 which is considered a marker of neurodegeneration.83,84 We found that levels of cNfL did not differ statistically significantly between RRMS, PPMS, and SPMS, indicating that on a population level cNfL may not differentiate acute inflammation-induced neuronal damage in the context of relapses from progressive neurodegeneration if the consequences of recent relapses or novel lesion formation are not considered. In individual patients, cNfL has been reported to reflect acute neuronal and axonal damage in MS, with levels transiently increasing during relapse.85,86,87 We found that cNfL levels in uRRMS and tRRMS did not differ statistically significantly. However, patients with the most active RRMS with potentially highest cNfL levels are also those who are most likely to be treated, and cNfL has been reported to decrease after treatment initiation in individual patients.38,50,51
cNfL in Dementia and ALS
The higher levels of cNfL observed in FTD compared with other frequent causes of dementias, including AD, VaD and DLB, may be related to the anatomical location of neurodegeneration or the rate of neuronal death. This finding suggests that cNfL may support the differentiation of FTD from other dementias, in line with a recent study88 not included in this meta-analysis, which reported that in combination with YKL40 and Aβ42 cNfL assists in the differentiation between FTD and AD with high accuracy. In iHIV, which included both mild cognitive impairment due to HIV and HIV-associated dementia, we observed highest levels of cNfL, setting it apart from neurodegenerative and vascular causes of dementia. This may reflect a high rate of neuroaxonal damage due to the presence of HIV and the inflammatory response to it in the CNS, or it may indicate additional peripheral nervous system damage contributing to the elevation of cNfL. In predementia stages, such as MCI and pgFTD, cNfL values were similar to levels in HC, suggesting that CNS damage must reach a certain extent before it is reflected by increased cNfL. However, the pgFTD cohort was small (n = 42); therefore, a small effect size could have been missed. The cNfL levels were highly elevated in ALS and FTD/ALS compared with HC. These results are in line with single-center studies not included in this meta-analysis that used different assays to measure NfL in CSF.89 Together with the high levels of cNfL observed in stroke, these findings indicate that the rate of neuroaxonal damage may be an important determinant of the magnitude of NfL increase in CSF, possibly by overriding CSF clearance mechanisms.
cNfL in Degenerative Parkinsonian Syndromes
In degenerative parkinsonian syndromes, cNfL clustered into 2 groups. The first group consisted of PD, PDD, and DLB, in which cNfL levels were similar to those in HC, and the second group consisted of atypical parkinsonian syndromes MSA, PSP, and CBS, with elevated levels of cNfL compared with HC and the absence of association with age. This finding is in line with the results of another meta-analysis90 that focused on parkinsonian disorders, examining data sets not included in the present meta-analysis, further underscoring the robustness of our findings. These data have important clinical implications because they suggest a potential for cNfL in supporting the differentiation of PD from atypical parkinsonian syndromes. Accurate and early differential diagnosis of these conditions is crucial because their prognosis and management differ substantially.
Serum NfL
A few years ago, an ultrasensitive assay was developed that allows measurement of NfL in serum (sNfL). This assay uses the same antibody pair as the immunoassay used in the studies included in this meta-analysis, and studies91,92 have reported high correlations between serum and CSF levels. These findings indicate that sNfL may replace cNfL. In addition, it may likely be that the findings of the present meta-analysis, which collected data over 10 years, can be readily translated to sNfL.
Limitations of the Study
Our systematic review and meta-analysis has some limitations. In all studies included in the meta-analysis except one,93 diagnosis was based on clinical criteria. This limitation is mostly a concern for dementias and parkinsonian syndromes, for which definitive diagnosis requires postmortem examination. However, the agreement between clinical and pathological diagnoses was reported to be high when diagnoses were established in specialized centers using consensus criteria.94,95 For AD and MCI, 2 consensus criteria were applied (criteria by McKhann et al12 and the International Working Group 2 [IWG-2] criteria13), for which a high concordance rate was reported.96 For VaD, the 2 consensus diagnostic criteria used (criteria by Erkinjuntti et al25 and the National Institute of Neurological Disorders and Stroke criteria) were also reported to have a high agreement.97 For PD, 2 consensus criteria were applied, for which concordance evaluation is not available. For ALS, FTD, PSP, MSA, PDD, DLB, and iHIV, the same consensus criteria were applied in all studies. In MS, the McDonald criteria were revised over time, and this may have influenced classification of RRMS and CIS. A further limitation is the inability to capture dementia of multifactorial origin, which may have increased heterogeneity in the dementia diagnostic categories and blurred the difference in cNfL distributions between dementia subtypes. Further classification of neurodegenerative conditions into clinical phenotypes could not be performed because this information was absent in a majority of studies. Therefore, the specific value of cNfL in subphenotypes could have been missed in this meta-analysis. In addition, for some conditions, data and age ranges were limited, resulting in large standard errors and low statistical power, and conclusions for these conditions should be interpreted with caution. Finally, we included only those studies that used a specific immunoassay for cNfL in an attempt to reduce heterogeneity due to the analytical procedure. However, the range of conditions that were explored in the studies not included in the meta-analysis for the same reason did not differ from those included.
Conclusions
Our study was designed to compare cNfL levels across neurological conditions and controls, assess the association of age and sex with these variables, and evaluate the potential of cNfL to differentiate clinically similar conditions. Our meta-analysis found that cNfL was elevated in a majority of the neurological conditions included in this study. Although cNfL overlapped between most clinically similar conditions, its distribution did not overlap in FTD compared with other dementia subtypes or in PD compared with atypical parkinsonian syndromes, indicating clinical potential in differentiating these conditions.
eFigure 1. Flow Diagram of Literature Search and Study Selection
eFigure 2. cNfL in Neurological Conditions According to Age
eTable 1. Diagnostic Categories and Their Demographics
eTable 2. Cerebrospinal Fluid Neurofilament Light Percent Fold-Change Compared to Healthy Controls and Percent Yearly Decrease
eTable 3. Ratio Between Male and Female Mean Cerebrospinal Fluid Neurofilament Light Values
eTable 4. Mean Cerebrospinal Fluid Neurofilament Light Ratios Across Clinically Similar Disorders
eTable 5. Mean Cerebrospinal Fluid Neurofilament Light Ratios per 10-Years Age Ranges
eTable 6. Intercohort Heterogeneity Coefficient for Diagnostic Categories With More Than One Contributing Cohort
References
- 1.Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelsø C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem. 1996;67(5):2013-2018. doi: 10.1046/j.1471-4159.1996.67052013.x [DOI] [PubMed] [Google Scholar]
- 2.Khademi M, Dring AM, Gilthorpe JD, et al. Intense inflammation and nerve damage in early multiple sclerosis subsides at older age: a reflection by cerebrospinal fluid biomarkers. PLoS One. 2013;8(5):e63172. doi: 10.1371/journal.pone.0063172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 4.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 2007;18(6):805-835. Medline:18049195 doi: 10.1097/EDE.0b013e3181577511 [DOI] [PubMed] [Google Scholar]
- 5.Whiting PF, Rutjes AW, Westwood ME, et al. ; QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529-536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 6.Petzold A, Altintas A, Andreoni L, et al. Neurofilament ELISA validation. J Immunol Methods. 2010;352(1-2):23-31. doi: 10.1016/j.jim.2009.09.014 [DOI] [PubMed] [Google Scholar]
- 7.Teunissen CE, Khalil M. Neurofilaments as biomarkers in multiple sclerosis. Mult Scler. 2012;18(5):552-556. doi: 10.1177/1352458512443092 [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz A, Blennow K, Hagberg L, et al. Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert Rev Mol Diagn. 2017;17(8):761-770. doi: 10.1080/14737159.2017.1341313 [DOI] [PubMed] [Google Scholar]
- 9.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127. doi: 10.1002/ana.1032 [DOI] [PubMed] [Google Scholar]
- 10.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840-846. doi: 10.1002/ana.20703 [DOI] [PubMed] [Google Scholar]
- 11.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald Criteria. Ann Neurol. 2011;69(2):292-302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria [published correction appears in Lancet Neurol. 2014;13(8):757]. Lancet Neurol. 2014;13(6):614-629. doi: 10.1016/S1474-4422(14)70090-0 [DOI] [PubMed] [Google Scholar]
- 14.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181-184. doi: 10.1136/jnnp.55.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33-39. doi: 10.1001/archneur.56.1.33 [DOI] [PubMed] [Google Scholar]
- 16.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689-1707. doi: 10.1002/mds.21507 [DOI] [PubMed] [Google Scholar]
- 17.Litvan I, Agid Y, Jankovic J, et al. Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology. 1996;46(4):922-930. doi: 10.1212/WNL.46.4.922 [DOI] [PubMed] [Google Scholar]
- 18.Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163(1):94-98. doi: 10.1016/S0022-510X(98)00304-9 [DOI] [PubMed] [Google Scholar]
- 19.Lee SE, Rabinovici GD, Mayo MC, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol. 2011;70(2):327-340. doi: 10.1002/ana.22424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathew R, Bak TH, Hodges JR. Diagnostic criteria for corticobasal syndrome: a comparative study. J Neurol Neurosurg Psychiatry. 2012;83(4):405-410. doi: 10.1136/jnnp-2011-300875 [DOI] [PubMed] [Google Scholar]
- 21.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. doi: 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546-1554. doi: 10.1212/WNL.51.6.1546 [DOI] [PubMed] [Google Scholar]
- 23.The Lund and Manchester Groups Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1994;57(4):416-418. doi: 10.1136/jnnp.57.4.416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludolph A, Drory V, Hardiman O, et al. ; WFN Research Group on ALS/MND . A revision of the El Escorial criteria: 2015. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(5-6):291-292. doi: 10.3109/21678421.2015.1049183 [DOI] [PubMed] [Google Scholar]
- 25.Erkinjuntti T, Haltia M, Palo J, Sulkava R, Paetau A. Accuracy of the clinical diagnosis of vascular dementia: a prospective clinical and post-mortem neuropathological study. J Neurol Neurosurg Psychiatry. 1988;51(8):1037-1044. doi: 10.1136/jnnp.51.8.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(3)(suppl):S4-S16. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez R, Heaton RK, Moore DJ, et al. ; HIV Neurobehavioral Research Center Group . Computerized reaction time battery versus a traditional neuropsychological battery: detecting HIV-related impairments. J Int Neuropsychol Soc. 2003;9(1):64-71. doi: 10.1017/S1355617703910071 [DOI] [PubMed] [Google Scholar]
- 28.Anckarsäter R, Anckarsäter H, Bromander S, Blennow K, Wass C, Zetterberg H. Non-neurological surgery and cerebrospinal fluid biomarkers for neuronal and astroglial integrity. J Neural Transm (Vienna). 2014;121(6):649-653. doi: 10.1007/s00702-013-1156-0 [DOI] [PubMed] [Google Scholar]
- 29.Axelsson M, Malmeström C, Gunnarsson M, et al. Immunosuppressive therapy reduces axonal damage in progressive multiple sclerosis. Mult Scler. 2014;20(1):43-50. doi: 10.1177/1352458513490544 [DOI] [PubMed] [Google Scholar]
- 30.Bäckström DC, Eriksson Domellöf M, Linder J, et al. Cerebrospinal fluid patterns and the risk of future dementia in early, incident Parkinson disease. JAMA Neurol. 2015;72(10):1175-1182. doi: 10.1001/jamaneurol.2015.1449 [DOI] [PubMed] [Google Scholar]
- 31.Bjerke M, Zetterberg H, Edman Å, Blennow K, Wallin A, Andreasson U. Cerebrospinal fluid matrix metalloproteinases and tissue inhibitor of metalloproteinases in combination with subcortical and cortical biomarkers in vascular dementia and Alzheimer’s disease. J Alzheimers Dis. 2011;27(3):665-676. doi: 10.3233/JAD-2011-110566 [DOI] [PubMed] [Google Scholar]
- 32.Bjerke M, Jonsson M, Nordlund A, et al. Cerebrovascular biomarker profile is related to white matter disease and ventricular dilation in a LADIS substudy. Dement Geriatr Cogn Dis Extra. 2014;4(3):385-394. doi: 10.1159/000366119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonsson M, Zetterberg H, Rolstad S, et al. Low cerebrospinal fluid sulfatide predicts progression of white matter lesions: the LADIS study. Dement Geriatr Cogn Disord. 2012;34(1):61-67. doi: 10.1159/000341576 [DOI] [PubMed] [Google Scholar]
- 34.Bruno D, Pomara N, Nierenberg J, et al. Levels of cerebrospinal fluid neurofilament light protein in healthy elderly vary as a function of TOMM40 variants. Exp Gerontol. 2012;47(5):347-352. doi: 10.1016/j.exger.2011.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burman J, Zetterberg H, Fransson M, Loskog AS, Raininko R, Fagius J. Assessing tissue damage in multiple sclerosis: a biomarker approach. Acta Neurol Scand. 2014;130(2):81-89. doi: 10.1111/ane.12239 [DOI] [PubMed] [Google Scholar]
- 36.Fialová L, Bartos A, Švarcová J, Zimova D, Kotoucova J. Serum and cerebrospinal fluid heavy neurofilaments and antibodies against them in early multiple sclerosis. J Neuroimmunol. 2013;259(1-2):81-87. doi: 10.1016/j.jneuroim.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 37.Fialová L, Bartos A, Švarcová J. Neurofilaments and tau proteins in cerebrospinal fluid and serum in dementias and neuroinflammation. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161(3):286-295. doi: 10.5507/bp.2017.038 [DOI] [PubMed] [Google Scholar]
- 38.Gunnarsson M, Malmeström C, Axelsson M, et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol. 2011;69(1):83-89. doi: 10.1002/ana.22247 [DOI] [PubMed] [Google Scholar]
- 39.Hall S, Öhrfelt A, Constantinescu R, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol. 2012;69(11):1445-1452. doi: 10.1001/archneurol.2012.1654 [DOI] [PubMed] [Google Scholar]
- 40.Hall S, Surova Y, Öhrfelt A, Zetterberg H, Lindqvist D, Hansson O. CSF biomarkers and clinical progression of Parkinson disease. Neurology. 2015;84(1):57-63. doi: 10.1212/WNL.0000000000001098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herbert MK, Aerts MB, Beenes M, et al. CSF neurofilament light chain but not FLT3 ligand discriminates parkinsonian disorders. Front Neurol. 2015;6(May):91. doi: 10.3389/fneur.2015.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hjalmarsson C, Bjerke M, Andersson B, et al. Neuronal and glia-related biomarkers in cerebrospinal fluid of patients with acute ischemic stroke. J Cent Nerv Syst Dis. 2014;6:51-58. doi: 10.4137/JCNSD.S13821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jakobsson J, Bjerke M, Ekman CJ, et al. Elevated concentrations of neurofilament light chain in the cerebrospinal fluid of bipolar disorder patients. Neuropsychopharmacology. 2014;39(10):2349-2356. doi: 10.1038/npp.2014.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rolstad S, Jakobsson J, Sellgren C, et al. Cognitive performance and cerebrospinal fluid biomarkers of neurodegeneration: a study of patients with bipolar disorder and healthy controls. PLoS One. 2015;10(5):e0127100. doi: 10.1371/journal.pone.0127100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeppsson A, Zetterberg H, Blennow K, Wikkelsø C. Idiopathic normal-pressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology. 2013;80(15):1385-1392. doi: 10.1212/WNL.0b013e31828c2fda [DOI] [PubMed] [Google Scholar]
- 46.Jessen Krut J, Mellberg T, Price RW, et al. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One. 2014;9(2):e88591. doi: 10.1371/journal.pone.0088591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aeinehband S, Lindblom RPF, Al Nimer F, et al. Complement component C3 and butyrylcholinesterase activity are associated with neurodegeneration and clinical disability in multiple sclerosis. PLoS One. 2015;10(4):e0122048. doi: 10.1371/journal.pone.0122048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khalil M, Enzinger C, Langkammer C, et al. CSF neurofilament and N-acetylaspartate related brain changes in clinically isolated syndrome. Mult Scler. 2013;19(4):436-442. doi: 10.1177/1352458512458010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuhle J, Plattner K, Bestwick JP, et al. A comparative study of CSF neurofilament light and heavy chain protein in MS. Mult Scler. 2013;19(12):1597-1603. doi: 10.1177/1352458513482374 [DOI] [PubMed] [Google Scholar]
- 50.Kuhle J, Malmeström C, Axelsson M, et al. Neurofilament light and heavy subunits compared as therapeutic biomarkers in multiple sclerosis. Acta Neurol Scand. 2013;128(6):e33-e36. doi: 10.1111/ane.12151 [DOI] [PubMed] [Google Scholar]
- 51.Kuhle J, Disanto G, Lorscheider J, et al. Fingolimod and CSF neurofilament light chain levels in relapsing-remitting multiple sclerosis. Neurology. 2015;84(16):1639-1643. doi: 10.1212/WNL.0000000000001491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magdalinou NK, Paterson RW, Schott JM, et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 2015;86(11):1240-1247. doi: 10.1136/jnnp-2014-309562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martínez MAM, Olsson B, Bau L, et al. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult Scler. 2015;21(5):550-561. doi: 10.1177/1352458514549397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meeter LH, Dopper EG, Jiskoot LC, et al. Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Ann Clin Transl Neurol. 2016;3(8):623-636. doi: 10.1002/acn3.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menke RA, Gray E, Lu CH, et al. CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann Clin Transl Neurol. 2015;2(7):748-755. doi: 10.1002/acn3.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu CH, Macdonald-Wallis C, Gray E, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis [published correction appears in Neurology. 2015;85(10):921]. Neurology. 2015;84(22):2247-2257. doi: 10.1212/WNL.0000000000001642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Modvig S, Degn M, Horwitz H, et al. Relationship between cerebrospinal fluid biomarkers for inflammation, demyelination and neurodegeneration in acute optic neuritis. PLoS One. 2013;8(10):e77163. doi: 10.1371/journal.pone.0077163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Modvig S, Degn M, Sander B, et al. Cerebrospinal fluid neurofilament light chain levels predict visual outcome after optic neuritis. Mult Scler. 2016;22(5):590-598. doi: 10.1177/1352458515599074 [DOI] [PubMed] [Google Scholar]
- 59.Modvig S, Degn M, Roed H, et al. Cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain predict multiple sclerosis development and disability after optic neuritis. Mult Scler. 2015;21(14):1761-1770. doi: 10.1177/1352458515574148 [DOI] [PubMed] [Google Scholar]
- 60.Paterson RW, Toombs J, Slattery CF, et al. Dissecting IWG-2 typical and atypical Alzheimer’s disease: insights from cerebrospinal fluid analysis. J Neurol. 2015;262(12):2722-2730. doi: 10.1007/s00415-015-7904-3 [DOI] [PubMed] [Google Scholar]
- 61.Pérez-Santiago J, Schrier RD, de Oliveira MF, et al. Cell-free mitochondrial DNA in CSF is associated with early viral rebound, inflammation, and severity of neurocognitive deficits in HIV infection. J Neurovirol. 2016;22(2):191-200. doi: 10.1007/s13365-015-0384-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pijnenburg YA, Verwey NA, van der Flier WM, Scheltens P, Teunissen CE. Discriminative and prognostic potential of cerebrospinal fluid phosphoTau/tau ratio and neurofilaments for frontotemporal dementia subtypes. Alzheimers Dement (Amst). 2015;1(4):505-512. doi: 10.1016/j.dadm.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pyykkö OT, Lumela M, Rummukainen J, et al. Cerebrospinal fluid biomarker and brain biopsy findings in idiopathic normal pressure hydrocephalus. PLoS One. 2014;9(3):e91974. doi: 10.1371/journal.pone.0091974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ragnarsson O, Berglund P, Eder DN, et al. Neurodegenerative and inflammatory biomarkers in cerebrospinal fluid in patients with Cushing’s syndrome in remission. Eur J Endocrinol. 2013;169(2):211-215. doi: 10.1530/EJE-13-0205 [DOI] [PubMed] [Google Scholar]
- 65.Romme Christensen J, Ratzer R, Börnsen L, et al. Natalizumab in progressive MS: results of an open-label, phase 2A, proof-of-concept trial. Neurology. 2014;82(17):1499-1507. doi: 10.1212/WNL.0000000000000361 [DOI] [PubMed] [Google Scholar]
- 66.Rosén C, Rosén H, Andreasson U, et al. Cerebrospinal fluid biomarkers in cardiac arrest survivors. Resuscitation. 2014;85(2):227-232. doi: 10.1016/j.resuscitation.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 67.Sandberg L, Biström M, Salzer J, Vågberg M, Svenningsson A, Sundström P. Vitamin D and axonal injury in multiple sclerosis. Mult Scler. 2016;22(8):1027-1031. doi: 10.1177/1352458515606986 [DOI] [PubMed] [Google Scholar]
- 68.Scherling CS, Hall T, Berisha F, et al. Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Ann Neurol. 2014;75(1):116-126. doi: 10.1002/ana.24052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skillbäck T, Farahmand B, Bartlett JW, et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. 2014;83(21):1945-1953. doi: 10.1212/WNL.0000000000001015 [DOI] [PubMed] [Google Scholar]
- 70.Stilund M, Gjelstrup MC, Petersen T, Møller HJ, Rasmussen PV, Christensen T. Biomarkers of inflammation and axonal degeneration/damage in patients with newly diagnosed multiple sclerosis: contributions of the soluble CD163 CSF/serum ratio to a biomarker panel. PLoS One. 2015;10(4):e0119681. doi: 10.1371/journal.pone.0119681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tortelli R, Copetti M, Ruggieri M, et al. Cerebrospinal fluid neurofilament light chain levels: marker of progression to generalized amyotrophic lateral sclerosis. Eur J Neurol. 2015;22(1):215-218. doi: 10.1111/ene.12421 [DOI] [PubMed] [Google Scholar]
- 72.Tortelli R, Ruggieri M, Cortese R, et al. Elevated cerebrospinal fluid neurofilament light levels in patients with amyotrophic lateral sclerosis: a possible marker of disease severity and progression. Eur J Neurol. 2012;19(12):1561-1567. doi: 10.1111/j.1468-1331.2012.03777.x [DOI] [PubMed] [Google Scholar]
- 73.Tortorella C, Direnzo V, Taurisano P, et al. Cerebrospinal fluid neurofilament tracks fMRI correlates of attention at the first attack of multiple sclerosis. Mult Scler. 2015;21(4):396-401. doi: 10.1177/1352458514546789 [DOI] [PubMed] [Google Scholar]
- 74.Trentini A, Comabella M, Tintoré M, et al. N-acetylaspartate and neurofilaments as biomarkers of axonal damage in patients with progressive forms of multiple sclerosis. J Neurol. 2014;261(12):2338-2343. doi: 10.1007/s00415-014-7507-4 [DOI] [PubMed] [Google Scholar]
- 75.Vågberg M, Norgren N, Dring A, et al. Levels and age dependency of neurofilament light and glial fibrillary acidic protein in healthy individuals and their relation to the brain parenchymal fraction. PLoS One. 2015;10(8):e0135886. doi: 10.1371/journal.pone.0135886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Villar LM, Picón C, Costa-Frossard L, et al. Cerebrospinal fluid immunological biomarkers associated with axonal damage in multiple sclerosis. Eur J Neurol. 2015;22(8):1169-1175. doi: 10.1111/ene.12579 [DOI] [PubMed] [Google Scholar]
- 77.Wild EJ, Boggio R, Langbehn D, et al. Quantification of mutant huntingtin protein in cerebrospinal fluid from Huntington’s disease patients. J Clin Invest. 2015;125(5):1979-1986. doi: 10.1172/JCI80743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zetterberg H, Skillbäck T, Mattsson N, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 2016;73(1):60-67. doi: 10.1001/jamaneurol.2015.3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384(2):312-320. doi: [DOI] [PubMed] [Google Scholar]
- 80.Cohen JA, Reingold SC, Polman CH, Wolinsky JS; International Advisory Committee on Clinical Trials in Multiple Sclerosis . Disability outcome measures in multiple sclerosis clinical trials: current status and future prospects. Lancet Neurol. 2012;11(5):467-476. doi: 10.1016/S1474-4422(12)70059-5 [DOI] [PubMed] [Google Scholar]
- 81.Stadelmann C, Wegner C, Brück W. Inflammation, demyelination, and degeneration - recent insights from MS pathology. Biochim Biophys Acta. 2011;1812(2):275-282. doi: 10.1016/j.bbadis.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 82.Gaiottino J, Norgren N, Dobson R, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One. 2013;8(9):e75091. doi: 10.1371/journal.pone.0075091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tortorella C, Direnzo V, Ruggieri M, et al. Cerebrospinal fluid neurofilament light levels mark grey matter volume in clinically isolated syndrome suggestive of multiple sclerosis [published correction appears in Mult Scler. 2019;25(2):302]. Mult Scler J. 2018;24(8):1039-1045. [DOI] [PubMed] [Google Scholar]
- 84.De Stefano N, Matthews PM, Filippi M, et al. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology. 2003;60(7):1157-1162. doi: 10.1212/01.WNL.0000055926.69643.03 [DOI] [PubMed] [Google Scholar]
- 85.Malmeström C, Haghighi S, Rosengren L, Andersen O, Lycke J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology. 2003;61(12):1720-1725. doi: 10.1212/01.WNL.0000098880.19793.B6 [DOI] [PubMed] [Google Scholar]
- 86.Norgren N, Sundström P, Svenningsson A, Rosengren L, Stigbrand T, Gunnarsson M. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology. 2004;63(9):1586-1590. doi: 10.1212/01.WNL.0000142988.49341.D1 [DOI] [PubMed] [Google Scholar]
- 87.Giovannoni G, Nath A. After the storm: neurofilament levels as a surrogate endpoint for neuroaxonal damage. Neurology. 2011;76(14):1200-1201. doi: 10.1212/WNL.0b013e3182143345 [DOI] [PubMed] [Google Scholar]
- 88.Alcolea D, Vilaplana E, Suárez-Calvet M, et al. CSF sAPPβ, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology. 2017;89(2):178-188. doi: 10.1212/WNL.0000000000004088 [DOI] [PubMed] [Google Scholar]
- 89.Reijn TS, Abdo WF, Schelhaas HJ, Verbeek MM. CSF neurofilament protein analysis in the differential diagnosis of ALS. J Neurol. 2009;256(4):615-619. doi: 10.1007/s00415-009-0131-z [DOI] [PubMed] [Google Scholar]
- 90.Hu X, Yang Y, Gong D. Cerebrospinal fluid levels of neurofilament light chain in multiple system atrophy relative to Parkinson’s disease: a meta-analysis. Neurol Sci. 2017;38(3):407-414. doi: 10.1007/s10072-016-2783-7 [DOI] [PubMed] [Google Scholar]
- 91.Soylu-Kucharz R, Sandelius Å, Sjögren M, et al. Neurofilament light protein in CSF and blood is associated with neurodegeneration and disease severity in Huntington’s disease R6/2 mice. Sci Rep. 2017;7(1):14114. doi: 10.1038/s41598-017-14179-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bergman J, Dring A, Zetterberg H, et al. Neurofilament light in CSF and serum is a sensitive marker for axonal white matter injury in MS. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e271. doi: 10.1212/NXI.0000000000000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Teunissen CE, Elias N, Koel-Simmelink MJ, et al. Novel diagnostic cerebrospinal fluid biomarkers for pathologic subtypes of frontotemporal dementia identified by proteomics. Alzheimers Dement (Amst). 2016;2:86-94. doi: 10.1016/j.dadm.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125(pt 4):861-870. doi: 10.1093/brain/awf080 [DOI] [PubMed] [Google Scholar]
- 95.Plassman BL, Khachaturian AS, Townsend JJ, et al. Comparison of clinical and neuropathologic diagnoses of Alzheimer’s disease in 3 epidemiologic samples. Alzheimers Dement. 2006;2(1):2-11. doi: 10.1016/j.jalz.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 96.Visser PJ, Vos S, van Rossum I, Scheltens P. Comparison of International Working Group criteria and National Institute on Aging–Alzheimer’s Association criteria for Alzheimer’s disease. Alzheimers Dement. 2012;8(6):560-563. doi: 10.1016/j.jalz.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 97.Pohjasvaara T, Mäntylä R, Ylikoski R, Kaste M, Erkinjuntti T; National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l’Enseignement en Neurosciences. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. Stroke. 2000;31(12):2952-2957. doi: 10.1161/01.STR.31.12.2952 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow Diagram of Literature Search and Study Selection
eFigure 2. cNfL in Neurological Conditions According to Age
eTable 1. Diagnostic Categories and Their Demographics
eTable 2. Cerebrospinal Fluid Neurofilament Light Percent Fold-Change Compared to Healthy Controls and Percent Yearly Decrease
eTable 3. Ratio Between Male and Female Mean Cerebrospinal Fluid Neurofilament Light Values
eTable 4. Mean Cerebrospinal Fluid Neurofilament Light Ratios Across Clinically Similar Disorders
eTable 5. Mean Cerebrospinal Fluid Neurofilament Light Ratios per 10-Years Age Ranges
eTable 6. Intercohort Heterogeneity Coefficient for Diagnostic Categories With More Than One Contributing Cohort