Abstract
The further optimization of a recently disclosed series of inverse agonists of the nuclear receptor RORC2 is described. Investigations into the left-hand side of compound 1, guided by X-ray crystal structures, led to the substitution of the 4-aryl-thiophenyl residue with the hexafluoro-2-phenyl-propan-2-ol moiety. This change resulted in to compound 28, which combined improved drug-like properties with good cell potency and a significantly lower dose, using an early dose to man prediction. Target engagement in vivo was demonstrated in the thymus of mice by a reduction in the number of double positive T cells after oral dosing.
Keywords: Retinoic acid-related orphan receptor C, autoimmune diseases, nuclear receptor, IL-17, dose to man prediction
Inverse agonists for the retinoic acid-related orphan receptor gamma C2 (RORC2) have been intensely pursued as potential drugs for the treatment of autoimmune disorders.1−7 RORC2 is expressed exclusively in the thymus and in cells of the immune system, where it acts as the key transcription factor for the differentiation and development of TH17 and other IL-17 producing cells.8 Blocking TH17 maturation and cytokine expression with an inverse agonist of RORC2 presents an attractive alternative to antibody treatment.9 Several antibodies targeting diverse parts of the IL-17 pathway have achieved clinical proof-of-concept in autoimmune diseases such as psoriasis, psoriatic arthritis, and ankylosing spondylitis.10−12
Previously we have described a class of inverse agonists of RORC2, exemplified by 1 (Table 1).13 Compound 1 and analogues bind inside the ligand-binding domain (LBD) of RORC2 and modulate receptor function through the ether substituent of the 4-arylthienyl group, which interferes with the cofactor recruitment site on helix 12 (AF2 domain).13,14 Large, lipophilic ether groups were necessary to achieve inverse agonism in compounds devoid of the acetamide function shown in 1. Its introduction allowed us to reduce the size of the left-hand side to a methoxy-substituted aryl and to improve physicochemical properties.
Table 1. Profile of Compound 1a.
| IL-17 cell pIC50 total/freeb | 7.2/7.9 |
| hPPB (% free)c | 1.3 |
| Clint RH/HH (μL/min/106 cells)d | 5/5.5 |
| Rat PKe: Cl (mL/min/kg), t1/2 (h), Vss (L/kg), F (%) | 2.5, 2.7, 0.34, 19 |
| eDtM (mg, QD)f | 2400 |
See Supporting Information for assay details.
Inhibition of IL-17 production from human primary TH17 cells; free potency calculated based on protein content in the assay medium.
Binding to human plasma protein determined by equilibrium dialysis.
Rat and human hepatocyte intrinsic clearance.
Pharmacokinetic profile in rat.
Early dose to man prediction.
Although 1 combined good inhibition of IL-17A production in the cell assay with oral bioavailability in rodents, prediction of the clinically efficacious dose, used within AstraZeneca to evaluate compound quality, revealed that gram amounts of 1 would be required for once-daily treatment.15
The calculation is based on a conservative approach, where we assumed that 75% of the dose of 1 would be absorbed from the gut and that the minimum concentration required for effective depletion of IL-17 over 24 h would be equal to three times the unbound potency in the IL-17 cell assay. Clearance was predicted from human hepatocytes and the volume of distribution using the Oie–Tozer equation.15
Parallel with the SAR leading to 1, we had investigated the possibility to simplify the 4-arylthienyl motif of the nonacetamide series, exemplified by 3 (Table 2), to improve its physicochemical properties.
Table 2. Modification of the LHSa.
![]()
pIC50 data are the mean of at least two independent measurements unless otherwise stated; see Table S1.
Recruitment of SRC1-derived coactivator peptide; pIC50 SEM is <0.37.
Inhibition of IL-17A production from human primary TH17 cells; % efficacy (% eff.) relative to DMSO control; pIC50 SEM is <0.31.
Distribution coefficient between 1-octanol and aqueous phosphate buffer at pH 7.4.
Determined from DMSO stock solutions in aqueous buffer at pH 7.4. ND, not determined.
Changing the left-hand side of 3 to a para-substituted biphenyl as in 5 was superior with respect to potency to 3 or the meta-substituted analogue 4, but did not improve physicochemical properties.16 The X-ray crystal structure of compound 2, a cell active (pIC50 = 5.81) inverse agonist, identified from a high-throughput screening campaign, helped us in addressing this issue.17 Soaking 2 in the agonist form of the RORC2 LBD returned the (R)-enantiomer as the bound species (Figure 1). An overlay with 1 showed that both compounds established the same hydrogen bond interactions with their amide moieties to Glu379 and Phe377, respectively. The isopropyl group on the phenyl substituent of 2 partially filled the volume occupied by the methoxy residue of the 4-aryl group on the thiophene, indicating that the 4-chlorophenyl group in 5 could be replaced with a simpler residue.
Figure 1.

Overlay of 1 (1.84 Å, PDB: 6ESN, carbons in cyan) and HTS hit 2 (2.1 Å, PDB: 6R7A carbons in orange) in the RORC2 LBD.
Replacement of the 4-chlorophenyl group in 5 with isopropyl resulted in inactive compound 6. Speculating that either a polar group, mimicking the methoxy group in 1, or enhanced steric bulk could lead to active compounds led us to the preparation of analogues containing a diverse set of para-substituents. While introduction of an isopropyl cyano (7) or methoxy (8) substituent improved activity in the FRET and cell assay and also physicochemical properties, the bis-trifluoromethyl-isopropanol moiety in 9 proved to be the optimal choice, leading to efficacious inhibition of IL-17A production. The introduction of the two trifluoromethyl groups led to a relatively high logD, but overall solubility with respect to 3 was improved, due to the slight acidic nature of the hydroxy group (measured pKa = 8.85). Methoxy and nitrile analogues 10 and 11 exhibited marginally improved potency in the cell assay but were 8-fold less soluble. Elimination of the hydroxy group from 9 reduced activity by about 10-fold (12), whereas elimination of one trifluoromethyl group resulted in inactive compound 13.
The hexafluoro-2-phenyl-propan-2-ol moiety is part of T0901317, a known agonist of the liver X receptor, from which diverse groups developed structurally diverse inverse agonists of RORC2.18−20 Compound 9 did not display any agonistic activity against LXRα and β up to a concentration of 10 μM in a reporter gene assay. It was also found to be inactive against other ROR isoforms and related retinoic acid receptors α, β, and γ.
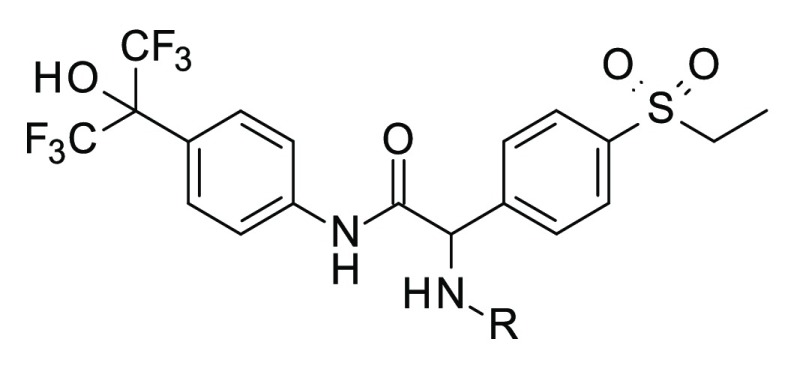
Combining the hexafluoro-2-phenyl-propan-2-ol moiety with the phenylglycine residue found in 1 resulted in 14, which combined enhanced cell potency and full efficacy in suppressing IL-17 production with superior metabolic stability and solubility compared to 1 (Table 3). The unsubstituted amine 15 is about 6-fold less potent in the cell assay. This potency difference is not reflected in the FRET assay, which is also observed for other compounds in Table 3. The result is due to the assay conditions, placing a limit on the highest pIC50 that can be determined at around 7.5. Compounds 14 and 15 displayed agonistic activity on RORA in the lower micromolar range, as determined by a cofactor recruitment assay, but were inactive as inverse agonists, as all compounds in Table 3. Compound 14 was also inactive against RORB or other nuclear receptors such as retinoic acid receptors α, β, and γ.
Table 3. Impact of the Amine Substituent on Activity and Metabolic Stabilitya.
| Cmpd | R | FRET pIC50 (% eff.)b | IL-17 Cell pIC50 (% eff.)c | logDd | Sol. (μM)e | RH Clintf | RORA pIC50g |
|---|---|---|---|---|---|---|---|
| 1 | 7.2 (−72) | 7.2 (−72) | 3.3 | 205 | 5 | 4.5 | |
| 14 | C(O)Me | 7.4 (−99) | 7.3 (−91) | 3.1 | 621 | <1 | 5.6 |
| 15 | H | 7.4 (−94) | 6.6 (−91) | 2.8 | 150 | 2.5 | 5.8 |
| 16 | C(O)OMe | 7.3 (−97) | 7.2 (−88) | 3.6 | 150 | 2.5 | <4.5 |
| 17 | C(O)NHMe | 7.4 (−98) | 6.6 (−89) | 3.2 | 155 | 5.4 | ND |
| 18 | SO2Me | 7.1 (−97) | 6.9 (−91) | 2.7 | 447 | <1 | ND |
| 19 | C(O)CH2–iPr | 7.1 (−94) | 7.8 (−96) | >4 | 28 | 9 | <4.5 |
| 20 | C(O)CH2C(OH)Me2 | 7.1 (−97) | 7.3 (−91) | 3.3 | 241 | 1.3 | <4.5 |
| 21 | C(O)Ph | 7.0 (−97) | 7.4 (−95) | >4 | 1.4 | 3.6 | ND |
| 22 | C(O)CH2Ph | 7.3 (−92) | 8.5 (−95) | 4 | 11 | 23 | 5.7 |
| 23 | C(O)(CH2)2Ph | 7.1 (−100) | 7.4 (−97) | >4 | 0.03 | 54 | ND |
| 24 | C(O)CH2–2-pyridyl | 7.3 (−97) | 7.9 (−97) | 3.3 | 2.7 | 17 | <4.5 |
| 25 | C(O)CH2–2-pyridazyl | 7.2 (−95) | 7.0 (−89) | 2.5 | 110 | 4.3 | ND |
| 26 | C(O)CH2–3-pyridazyl | 7.2 (−95) | 7.6 (−89) | 2.9 | 3.4 | 24 | <4.5 |
| 27 | C(O)CH2–3-pyrimidyl | 7.3 (−97) | 7.4 (−90) | 2.8 | 177 | 6 | ND |
| 28 | C(O)Me (R) | 7.5 (−95) | 7.5 (−95) | 3.0 | 381 | <1 | 6.3 |
| 29 | C(O)Me (S) | 5.6 (−98) | 7.1 (−91) | 3.1 | 326 | <1 | <4.5 |
pIC50 data are the mean of at least two independent measurements unless otherwise stated; see Table S1.
Recruitment of SRC1-derived coactivator peptide; pIC50 SEM is <0.37.
Inhibition of IL-17A production from human primary TH17 cells; % efficacy (% eff.) relative to DMSO control; pIC50 SEM is <0.31.
Distribution coefficient between 1-octanol and aqueous phosphate buffer at pH 7.4.
Determined from DMSO stock solutions in aqueous buffer at pH 7.4. ND, not determined.
Rat hepatocyte intrinsic clearance (μL/min1/106 cells).
Time-resolved (TR)-FRET assay in agonist mode using a peptide derived from PGC1α 130–154; ND, not determined.
We briefly investigated the SAR around the acetamide residue to see if we could further improve cell potency, without compromising on the physicochemical properties. Carbamate 16 was comparable in potency to amide 14, whereas urea 17 and sulphonamide 18 were inferior. Increasing the size of the amide substituent to isobutyl, as in 19, improved cell potency by about 3-fold, but at the expense of metabolic stability and solubility. Introduction of a hydroxy group into the isobutyl residue as in 20 improved overall properties, but not cell potency.
Aromatic residues were tolerated, and benzoate 21 or phenylpropionate 23 showed cell potencies similar to 14. Phenylacetamide 22 was an exception, being 10-fold more active in the cell assay. As observed for 19, the higher logD resulted in higher turnover in rat hepatocytes and a loss in solubility. Incorporation of nitrogen atoms as in compounds 24–27 caused generally a loss in cell potency with respect to 22. This was less pronounced for the 2-pyridyl and the 3-pyridazinyl analogues 24 and 26, but despite their lower logD, turnover in rat hepatocytes and solubility did not improve. Interestingly, the carbamate linkage in 16 and the bulkier amide residues in 19 or 20 as well as introduction of nitrogen atoms into 22 abolished activity on RORA.
The X-ray cocrystal structures of 14 and 22, obtained by soaking the compounds into the apo crystals of RORC2-LBD in the presence of bound SRC2 peptide, returned their respective R-isomers as the bound species (Figure 2). We have shown previously that the ligand interactions in such agonist conformation would render the same binding mode as in the cocrystals without the coactivator peptide.21 Their right-hand side moieties overlapped well and established the same hydrogen bonds inside the pocket and outside the LBD, namely, to Arg364, Phe377, and Glu379. The enhanced cell activity of 22 can be explained by the interaction of the phenylacetamide with a hydrophobic stretch outside the LBD.
Figure 2.

Overlay of 1 (1.84 Å, PDB: 6ESN, carbons in cyan), 14 (1.8 Å, PDB: 6R7J, carbons in green), and 22 (1.5 Å, PDB: 6R7K, carbons in pink) with key interactions to the RORC2 LBD.
On the left-hand side, the bis-trifluoromethyl groups occupied a space in front of the 4-aryl residue of 1, extending above and below the plane of the phenyl residues, which overlap with the thiophene of 1. The interaction with Ser404 is lost, and this might be the reason for the activity observed on RORA. The Phe-C(CF3)2OH moiety is located in a hydrophobic environment, at about 7–8 Å away from the cofactor recruitment site, but not exactly in the same place as in previously reported inverse agonists (see Figure S3).20 Its local conformation, with the hydroxy group pointing towards helix 12, suggests that it modulates receptor function through indirect interactions with this region (Figure S1). Although a more thorough analysis is needed, an overlay of the LBD’s of RORA and RORC suggested that the observed selectivity against RORA with either carbamate, branched, or polar acetamide residues could be due to a narrower and more hydrophobic channel in RORA (Figure S2).
Compound 14 was separated into its enantiomers, and the more active enantiomer 28 was assigned the (R)-configuration, based on the X-ray structure. The (S)-isomer 29 was about 100-fold less active in the FRET assay but showed higher than expected activity in the cell assay. We believe this to be due to racemization of the compounds in the medium of the cell assay, as we have previously demonstrated for a close derivative of 1.13 Isomer 28 was also more active on RORA. Further profiling of 22, 28, and 29 revealed that they showed good crossover to mouse, as evaluated by radioligand binding assays (Table 4). Compound 22 showed moderate stability in human hepatocytes, similar to 1, whereas 28 and 29 were stable. Single dose rat PK of 22 was characterized by high plasma clearance and low bioavailability, in line with clearance data from rat hepatocytes. The rat PK of 28 was comparable to 1, revealing moderate plasma clearance and reasonable oral bioavailability. Compared to 1, permeability and efflux of 28, determined in Caco2-cells, were not significantly improved (0.3/0.49 × 10–6 cm/s and 50/17, respectively). We calculated the early dose to man as described above for compound 1. A clear improvement for compound 28, with respect to 1 and 22, is evident, due mainly to its enhanced metabolic stability, resulting in a significant reduction in predicted human clearance.
Table 4. Profile of Selected Compounds.
| Cmpd | 22 | 28 | 29 |
|---|---|---|---|
| hRORC SPAa | 7.4 | 7.3 | 6.1 |
| mRORC SPAa | 7.6 | 6.9 | 5.4 |
| hPPB (% free)b | 0.72 | 12 | 10 |
| IL-17 cell pIC50 freec | 9.4 | 7.6 | 7.3 |
| Clint HHd (μL/min/10–6 cells) | 4.3 | <1 | <1 |
| eDTM (mg, QD)e | 900 | 250 | |
| Rat PKf | |||
| Cl (mL/min/kg), t1/2 (h) | 53, 4.9 | 18.2, 3.7 | ND |
| Vss (L/kg), F (%) | 10.2, 5 | 2.2, 20 | ND |
| Mouse PKf | |||
| Cl (mL/min/kg), t1/2 (h) | ND | 14, 3.3 | 16, 2.9 |
| Vss(L/kg), F (%) | ND | 2.4, 27 | 2.7, 28 |
Scintillation proximity competition binding assay (SPA).
Binding to human plasma protein determined by equilibrium dialysis.
Inhibition of IL-17A production from human primary TH17 cells; % efficacy (% eff.) relative to DMSO control; pIC50 SEM is <0.31.
Distribution coefficient between 1-octanol and aqueous phosphate buffer at pH 7.4.
Determined from DMSO stock solutions in aqueous buffer at pH 7.4.
Pharmacokinetic profile in rat and mouse. For experimental conditions, see Supporting Information. ND, not determined.
We established an in vivo model that allowed us to evaluate the effect of RORC2 inhibition in the thymus of mice. Maturation and survival of double positive (CD4+CD8+) T cells in the thymus depends on RORC2. It has been shown that germline knockout of the receptor in the mouse leads to a reduction of the number of double positive cells.22
The mouse PK for 28 and 29 displayed almost identical profiles and was similar to the rat PK of 28 (Table 4). Only a small extent of racemization (<5%) was observed in plasma at 24 h.
Compounds were then dosed orally at 50 mg/kg twice a day for 2 days to six-week-old C57BL/6 female mice. Afterwards, a single cell suspension from each thymus was prepared and cells stained for flow cytometric analysis to assess the absolute numbers of double positive (CD4+CD8+) thymocytes (Figure 3). Compound 28 significantly reduced their number by about 40% (p = 0.008), whereas enantiomer 29 had no effect. Exposure levels for compounds 28 and 29 at 48 h were similar, with 240 ± 150 and 145 ± 45 nM, respectively. Free terminal plasma exposure of compound 28 was calculated to be 28 nM, which corresponds to the unbound IC50 value in the cell assay. The lack of effect for 29 is in line with its weak binding activity on mouse RORC.
Figure 3.
Effect of compounds 28 and 29 on the number of CD4+CD8+ T cells in the thymus of mice.
In summary we have further improved our series of phenylglycine based RORC2 inverse agonist. Investigations into the left-hand side of compound 1, inspired by the X-ray cocrystal structure of 2, led to the substitution of the aryl-thiophenyl residue with a hexafluoro-2-phenyl-propan-2-ol moiety, reducing the number of aromatic rings from three to two and led to compound 28, which showed good cellular potency with full suppression of IL-17 production. It also resulted in an overall improvement in drug-like properties. Using early dose to man prediction as a benchmark, compound 28 requires 6-fold lower dosing compared to 1. Oral dosing to mice showed engagement of RORC2 in vivo, as demonstrated by a reduction in the number of double positive T cells in the thymus.
For the synthesis of phenylglycine derivatives, amine 15 was prepared by amide coupling of 30 to acid 32(13) and subsequent deprotection of the Boc group. Reaction with the appropriate acylating reagent furnished the desired products. Acetate 14 was further separated into the enantiomers 28 and 29 (Scheme 1).
Scheme 1. Synthesis of Analogues 14, 16, 22, 28, and 29.
Reagents and conditions: (a) HATU, i-PrNEt2, CH2Cl2 85%; HCl, 1,4-dioxane, RT or TFA, CH2Cl2, quant.; (b) for 14: MeCN, AcCl, NEt3, 89%; for 16: MeCN, MeOCOCl, i-PrNEt2, 64%; for 22: PhCH2CO2H, T3P, TEA, CH2Cl2, RT, 85%; (c) SFC, 30% i-PrOH in CO2, 120 bar: 28 (41%), 29 (41%).
Acknowledgments
The authors would like to thank the analytical group, the SSL group, and Anna Malmberg for the purification and characterization of compounds. We acknowledge Catarina Johansson for SPR measurements of compound 2, Agnes Leffler and Elisabeth Bäck for providing the cell data, and the IMED RIA in vivo team for support of the mouse study.
Glossary
ABBREVIATIONS
- FRET
fluorescence resonance energy transfer
- HATU
O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium-hexafluor-phosphate
- LBD
ligand binding domain
- PK
pharmacokinetics
- ROR
retinoic acid receptor-related orphan receptor
- SAR
structure–activity relationship
- SPA
scintillation proximity assay
- SRC
steroid receptor coactivator
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.9b00158.
Experimental procedures and characterization data for intermediates and all final compounds, conditions for biological assays, and crystal structure information (PDF)
Accession Codes
PDB accession codes 6R7A (2), 6R7K (22), and 6R7J (14/28). The authors will release the atomic coordinates and experimental data upon publication.
Author Contributions
F.N., A.L., J.J., J.M., and T.G.H. conceived and designed the experiments; F.N., S.v.B., R.I.O., T.H., M.L., J.M., M.Lep., R.C., and Y.Xi. designed and/or synthesized compounds; A.A. and Y.X. provided X-ray structure data; A.L., M.C., J.J., N.K., M.R., M.B., and A.L. designed and/or performed and analyzed the in vivo studies; J.M. and E.L.H. provided in vitro assay data;. F.N. wrote the manuscript with contributions from Y.X., A.L., J.M., S.v.B., and M.C.
All animal experiments were approved by the Pharmaron’s Institutional Animal Care and Use Committee (IACUC) in Pharmaron and by the Gothenburg Ethics Committee for Experimental Animals Sweden; both conform to Directive 2010/63/EU. Animal handling conformed to standards established by the Council of Europe ETS123 AppA, NIH guidelines on animal welfare, Chinese legislation and Pharmaron’s IACUC policies and procedures, the Helsinki Convention for the Use and Care of Animals, Swedish legislation, and AstraZeneca Global Internal Standards.
The authors declare no competing financial interest.
Supplementary Material
References
- Cyr P.; Bronner S. M.; Crawford J. J. Recent progress on nuclear receptor RORγ modulators. Bioorg. Med. Chem. Lett. 2016, 26, 4387–4393. 10.1016/j.bmcl.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Pandya V. B.; Kumar S.; Sachchidanand; Sharma R.; Desai R. C. Combating Autoimmune Diseases With Retinoic Acid Receptor-Related Orphan Receptor-γ (RORγ or RORc) Inhibitors: Hits and Misses. J. Med. Chem. 2018, 61, 10976–10995. 10.1021/acs.jmedchem.8b00588. [DOI] [PubMed] [Google Scholar]
- Schnute M. E.; Wennerstål M.; Alley J.; Bengtsson M.; Blinn J. R.; Bolten C. W.; Braden T.; Bonn T.; Carlsson B.; Caspers N.; Chen M.; Choi C.; Collis L. P.; Crouse K.; Färnegårdh M.; Fennell K. F.; Fish S.; Flick A. C.; Goos-Nilsson A.; Gullberg H.; Harris P. K.; Heasley S. E.; Hegen M.; Hromockyj A. E.; Hu X.; Husman B.; Janosik T.; Jones P.; Kaila N.; Kallin E.; Kauppi B.; Kiefer J. R.; Knafels J.; Koehler K.; Kruger L.; Kurumbail R. G.; Kyne R. E.; Li W.; Löfstedt J.; Long S. A.; Menard C. A.; Mente S.; Messing D.; Meyers M. J.; Napierata L.; Nöteberg D.; Nuhant P.; Pelc M. J.; Prinsen M. J.; Rhönnstad P.; Backström-Rydin E.; Sandberg J.; Sandström M.; Shah F.; Sjöberg M.; Sundell A.; Taylor A. P.; Thorarensen A.; Trujillo J. I.; Trzupek J. D.; Unwalla R.; Vajdos F. F.; Weinberg R. A.; Wood D. C.; Xing L.; Zamaratski E.; Zapf C. W.; Zhao Y.; Wilhelmsson A.; Berstein G. Discovery of 3-Cyano-N-(3-(1-isobutyrylpiperidin-4-yl)-1-methyl-4-(trifluoromethyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)benzamide: A Potent, Selective, and Orally Bioavailable Retinoic Acid Receptor-Related Orphan Receptor C2 Inverse Agonist. J. Med. Chem. 2018, 61, 10415–10439. 10.1021/acs.jmedchem.8b00392. [DOI] [PubMed] [Google Scholar]
- Sasaki Y.; Odan M.; Yamamoto S.; Kida S.; Ueyama A.; Shimizu M.; Haruna T.; Watanabe A.; Okuno T. Discovery of a potent orally bioavailable retinoic acid receptor-related orphan receptor-gamma-t (RORγt) inhibitor, S18–000003. Bioorg. Med. Chem. Lett. 2018, 28, 3549–3553. 10.1016/j.bmcl.2018.09.032. [DOI] [PubMed] [Google Scholar]
- Ouvry G.; Bihl F.; Bouix-Peter C.; Christin O.; Defoin-Platel C.; Deret S.; Feret C.; Froude D.; Hacini-Rachinel F.; Harris C. S.; Hervouet C.; Lafitte G.; Luzy A.-P.; Musicki B.; Orfila D.; Parnet V.; Pascau C.; Pascau J.; Pierre R.; Raffin C.; Rossio P.; Spiesse D.; Taquet N.; Thoreau E.; Vatinel R.; Vial E.; Hennequin L. F. Sulfoximines as potent RORγ inverse agonists. Bioorg. Med. Chem. Lett. 2018, 28, 1269–1273. 10.1016/j.bmcl.2018.03.041. [DOI] [PubMed] [Google Scholar]
- Kono M.; Ochida A.; Oda T.; Imada T.; Banno Y.; Taya N.; Masada S.; Kawamoto T.; Yonemori K.; Nara Y.; Fukase Y.; Yukawa T.; Tokuhara H.; Skene R.; Sang B. C.; Hoffman I. D.; Snell G. P.; Uga K.; Shibata A.; Igaki K.; Nakamura Y.; Nakagawa H.; Tsuchimori N.; Yamasaki M.; Shirai J.; Yamamoto S. Discovery of [ cis-3-({(5 R)-5-[(7-Fluoro-1,1-dimethyl-2,3-dihydro-1 H-inden-5-yl)carbamoyl]-2-methoxy-7,8-dihydro-1,6-naphthyridin-6(5 H)-yl}carbonyl)cyclobutyl]acetic Acid (TAK-828F) as a Potent, Selective, and Orally Available Novel Retinoic Acid Receptor-Related Orphan Receptor gammat Inverse Agonist. J. Med. Chem. 2018, 61, 2973–2988. 10.1021/acs.jmedchem.8b00061. [DOI] [PubMed] [Google Scholar]
- Kummer D. A.; Cummings M. D.; Abad M.; Barbay J.; Castro G.; Wolin R.; Kreutter K. D.; Maharoof U.; Milligan C.; Nishimura R.; Pierce J.; Schalk-Hihi C.; Spurlino J.; Urbanski M.; Venkatesan H.; Wang A.; Woods C.; Xue X.; Edwards J. P.; Fourie A. M.; Leonard K. Identification and structure activity relationships of quinoline tertiary alcohol modulators of RORγt. Bioorg. Med. Chem. Lett. 2017, 27, 2047–2057. 10.1016/j.bmcl.2017.02.044. [DOI] [PubMed] [Google Scholar]
- Cook D. N.; Kang H. S.; Jetten A. M. Retinoic Acid-Related Orphan Receptors (RORs): Regulatory Functions in Immunity, Development, Circadian Rhythm, and Metabolism. Nucl. Receptor Res. 2015, 2, 101185. 10.11131/2015/101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassolas-Molina H.; Raymond E.; Labadia M.; Wahle J.; Ferrer-Picón E.; Panzenbeck M.; Zheng J.; Harcken C.; Hughes R.; Turner M.; Smith D.; Calderón-Gómez E.; Esteller M.; Carrasco A.; Esteve M.; Dotti I.; Corraliza A. M.; Masamunt M. C.; Arajol C.; Guardiola J.; Ricart E.; Nabozny G.; Salas A. An RORγt Oral Inhibitor Modulates IL-17 Responses in Peripheral Blood and Intestinal Mucosa of Crohn’s Disease Patients. Front. Immunol. 2018, 9, 2307. 10.3389/fimmu.2018.02307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease P. J. Inhibition of interleukin-17, interleukin-23 and the TH17 cell pathway in the treatment of psoriatic arthritis and psoriasis. Curr. Opin. Rheumatol. 2015, 27, 127–133. 10.1097/BOR.0000000000000147. [DOI] [PubMed] [Google Scholar]
- Boutet M.-A.; Nerviani A.; Gallo Afflitto G.; Pitzalis C. Role of the IL-23/IL-17 Axis in Psoriasis and Psoriatic Arthritis: The Clinical Importance of Its Divergence in Skin and Joints. Int. J. Mol. Sci. 2018, 19, 530. 10.3390/ijms19020530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten D.; Sieper J.; Braun J.; Baraliakos X.; Dougados M.; Emery P.; Deodhar A.; Porter B.; Martin R.; Andersson M.; Mpofu S.; Richards H. B. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 2015, 373, 2534–2548. 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- Narjes F.; Xue Y.; von Berg S.; Malmberg J.; Llinas A.; Olsson R. I.; Jirholt J.; Grindebacke H.; Leffler A.; Hossain N.; Lepistö M.; Thunberg L.; Leek H.; Aagaard A.; McPheat J.; Hansson E. L.; Bäck E.; Tångefjord S.; Chen R.; Xiong Y.; Hongbin G.; Hansson T. G. Potent and Orally Bioavailable Inverse Agonists of RORγt Resulting from Structure-Based Design. J. Med. Chem. 2018, 61, 7796–7813. 10.1021/acs.jmedchem.8b00783. [DOI] [PubMed] [Google Scholar]
- Kallen J.; Izaac A.; Be C.; Arista L.; Orain D.; Kaupmann K.; Guntermann C.; Hoegenauer K.; Hintermann S. Structural States of RORγt: X-ray Elucidation of Molecular Mechanisms and Binding Interactions for Natural and Synthetic Compounds. ChemMedChem 2017, 12, 1014–1021. 10.1002/cmdc.201700278. [DOI] [PubMed] [Google Scholar]
- Page K. M. Validation of Early Human Dose Prediction: A Key Metric for Compound Progression in Drug Discovery. Mol. Pharmaceutics 2016, 13, 609–620. 10.1021/acs.molpharmaceut.5b00840. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Cai W.; Cheng Y.; Yang T.; Liu Q.; Zhang G.; Meng Q.; Han F.; Huang Y.; Zhou L.; Xiang Z.; Zhao Y.-G.; Xu Y.; Cheng Z.; Lu S.; Wu Q.; Xiang J.-N.; Elliott J. D.; Leung S.; Ren F.; Lin X. Discovery of Biaryl Amides as Potent, Orally Bioavailable, and CNS Penetrant RORγt Inhibitors. ACS Med. Chem. Lett. 2015, 6, 787–792. 10.1021/acsmedchemlett.5b00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai J.; Tomata Y.; Kono M.; Ochida A.; Fukase Y.; Sato A.; Masada S.; Kawamoto T.; Yonemori K.; Koyama R.; Nakagawa H.; Nakayama M.; Uga K.; Shibata A.; Koga K.; Okui T.; Shirasaki M.; Skene R.; Sang B.; Hoffman I.; Lane W.; Fujitani Y.; Yamasaki M.; Yamamoto S. Discovery of orally efficacious RORγt inverse agonists, part 1: Identification of novel phenylglycinamides as lead scaffolds. Bioorg. Med. Chem. 2018, 26, 483–500. 10.1016/j.bmc.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Fauber B. P.; de Leon Boenig G.; Burton B.; Eidenschenk C.; Everett C.; Gobbi A.; Hymowitz S. G.; Johnson A. R.; Liimatta M.; Lockey P.; Norman M.; Ouyang W.; Rene O.; Wong H. Structure-based design of substituted hexafluoroisopropanol-arylsulfonamides as modulators of RORc. Bioorg. Med. Chem. Lett. 2013, 23, 6604–6609. 10.1016/j.bmcl.2013.10.054. [DOI] [PubMed] [Google Scholar]
- Solt L. A.; Kumar N.; He Y.; Kamenecka T. M.; Griffin P. R.; Burris T. P. Identification of a Selective RORγ Ligand That Suppresses TH17 Cells and Stimulates T Regulatory Cells. ACS Chem. Biol. 2012, 7, 1515–1519. 10.1021/cb3002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J. J. W.; Lu Z.; Jiang B.; Stachura S.; Weigelt C. A.; Sack J. S.; Khan J.; Ruzanov M.; Galella M. A.; Wu D.-R.; Yarde M.; Shen D.-R.; Shuster D. J.; Borowski V.; Xie J. H.; Zhang L.; Vanteru S.; Gupta A. K.; Mathur A.; Zhao Q.; Foster W.; Salter-Cid L. M.; Carter P. H.; Dhar T. G. M. Structure-based Discovery of Phenyl (3-Phenylpyrrolidin-3-yl)sulfones as Selective, Orally Active RORγt Inverse Agonists. ACS Med. Chem. Lett. 2019, 10, 367–373. 10.1021/acsmedchemlett.9b00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson R. I.; Xue Y.; von Berg S.; Aagaard A.; McPheat J.; Hansson E. L.; Bernstroem J.; Hansson P.; Jirholt J.; Grindebacke H.; Leffler A.; Chen R.; Xiong Y.; Ge H.; Hansson T. G.; Narjes F. Benzoxazepines Achieve Potent Suppression of IL-17 Release in Human T-Helper 17 (TH17) Cells through an Induced-Fit Binding Mode to the Nuclear Receptor RORγ. ChemMedChem 2016, 11, 207–216. 10.1002/cmdc.201500432. [DOI] [PubMed] [Google Scholar]
- Eberl G.; Littman D. R. The role of the nuclear hormone receptor RORγt in the development of lymph nodes and Peyer’s patches. Immunol. Rev. 2003, 195, 81–90. 10.1034/j.1600-065X.2003.00074.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.