Abstract
Due to its expression in various malignant tumors, the neurotensin receptor 1 (NTS1R) has been suggested and explored as a target for tumor diagnosis and therapy. Animal model-based investigations of various radiolabeled NTS1R ligands derived from the hexapeptide neurotensin(8–13) (NT(8–13)), e.g. 68Ga- and 18F-labeled compounds for PET diagnostics, give rise to optimize such radiotracers for clinical use. As NT(8–13) is rapidly degraded in vivo; structural modifications are required in terms of increased metabolic stability. In this study, the stabilization of the peptide backbone of NT(8–13) against enzymatic degradation was systematically explored by performing an N-methyl scan, replacing Ile12 by tert-butylglycine12 (Tle12) and N-terminal acylation. N-Methylation of either arginine, Arg8, or Arg9, combined with the Ile12/Tle12 exchange, proved to be most favorable with respect to NTS1R affinity (Ki < 2 nM) and stability in human plasma (t1/2 > 48 h), a valuable result regarding the development of radiopharmaceuticals derived from NT(8–13).
Keywords: Peptide, neurotensin, NT(8−13), neurotensin receptor, plasma stability
The neuromodulator neurotensin (NT), a 13 amino acid peptide (Figure 1), is found in the central nervous system (CNS), mediating e.g. analgesic effects, as well as in the periphery (primarily in the gastrointestinal tract).1,2 The carboxy-terminal hexapeptide of NT (NT(8–13) (1), Figure 1), is biologically equi-active to NT.3 The physiological effects of NT are mediated by three cell-surface receptors: the NT receptors 1 and 2 (NTS1R, NTS2R), both G-protein coupled receptors,4 and the NTS3R, which belongs to the Vps10p-domain receptor family.2,5 The NTS1R has increasingly gained interest as a target for tumor diagnosis and therapy, as it was reported to be (over)expressed in a variety of malignancies, among them the prognostically poor pancreatic adenocarcinoma, Ewing’s sarcoma, breast cancer, and colorectal carcinoma.6−9 Thus, radiolabeled NTS1R ligands harbor the potential of being used as radiopharmaceuticals. The majority of such compounds (e.g., 68Ga- and 18F-labeled for PET diagnostics, 177Lu-labeled for radioendotherapy) has been derived from the agonist 1.10−19 Noteworthily, also NTS1R ligands derived from nonpeptidic antagonists have been explored as radiodiagnostics and radiotherapeutics.20,21 Recently reported data of a clinical trial on the treatment of pancreatic adenocarcinoma in men by 177Lu-labeled NTS1R antagonists give reason to develop clinical trial candidates with improved properties.22 Therefore, peptidic NTS1R ligands, such as radiolabeled derivatives of 1, should be considered for clinical trials.
Figure 1.
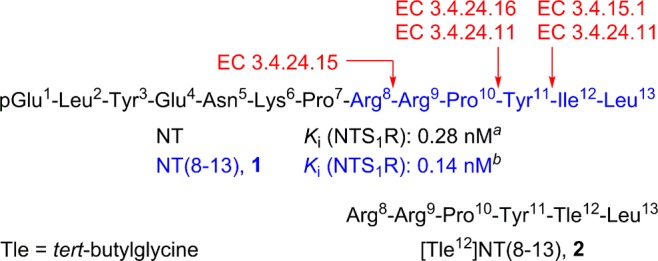
Amino acid sequences of neurotensin, 1 (NT(8–13), in blue) and 2, as well as major enzymatic cleavage sites (in red) of 1.3,24,25 EC 3.4.24.15: metalloendopeptidase 24.15, EC 3.4.24.16: metalloendopeptidase 24.16, EC 3.4.24.11: neutral endopeptidase 24.11, EC 3.4.15.1: angiotensin converting enzyme (ACE).24,25aBarroso et al.41bKeller et al.42
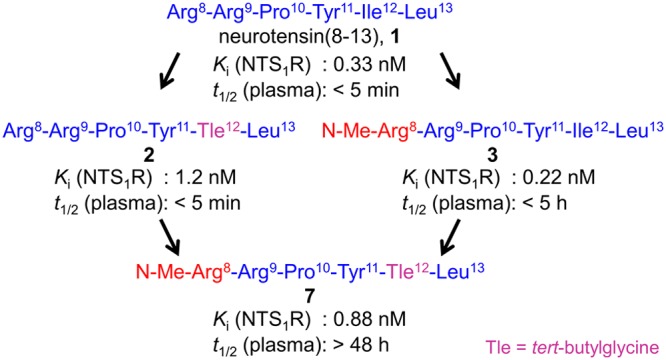
A major drawback of peptide 1 is its rapid degradation in vivo by peptidases (see Figure 1).23,24 Enzymatic degradation of 1 occurs at three major sites: the Arg8–Arg9 bond, the Pro10–Tyr11 bond, and the bond between Tyr11 and Ile12 (cf. Figure 1).24,25 The predominant approaches to stabilize the backbone of 1 are N-methylation of Arg8 or Arg9, N-terminal acylation, and the exchange of Ile12 by tert-butylglycine (Tle).10−15,17,26−38 However, for some interesting analogs of 1, such as N-methylated derivatives, investigations on the stability are lacking.33,39 It is worth mentioning that described derivatives of 1, containing Tle12 instead of Ile12, include additional structural modifications throughout;10−12,29,31,32,38,40 that is, [Tle12]NT(8–13) (2, cf. Figure 1) has not been reported to date to the best of the authors’ knowledge. Therefore, it is difficult to estimate the impact of the Ile12/Tle12 exchange on the stability of Tle12-containing derivatives of 1.
Aiming at a systematic study on the stabilization of the NT(8–13) core structure, we synthesized compound 2, performed an N-methyl scan of 1, combined N-methylation with the Ile12/Tle12 exchange, and, additionally, prepared N-terminally acylated derivatives of 1. All compounds were studied with respect to NTS1R binding and plasma stability.
2
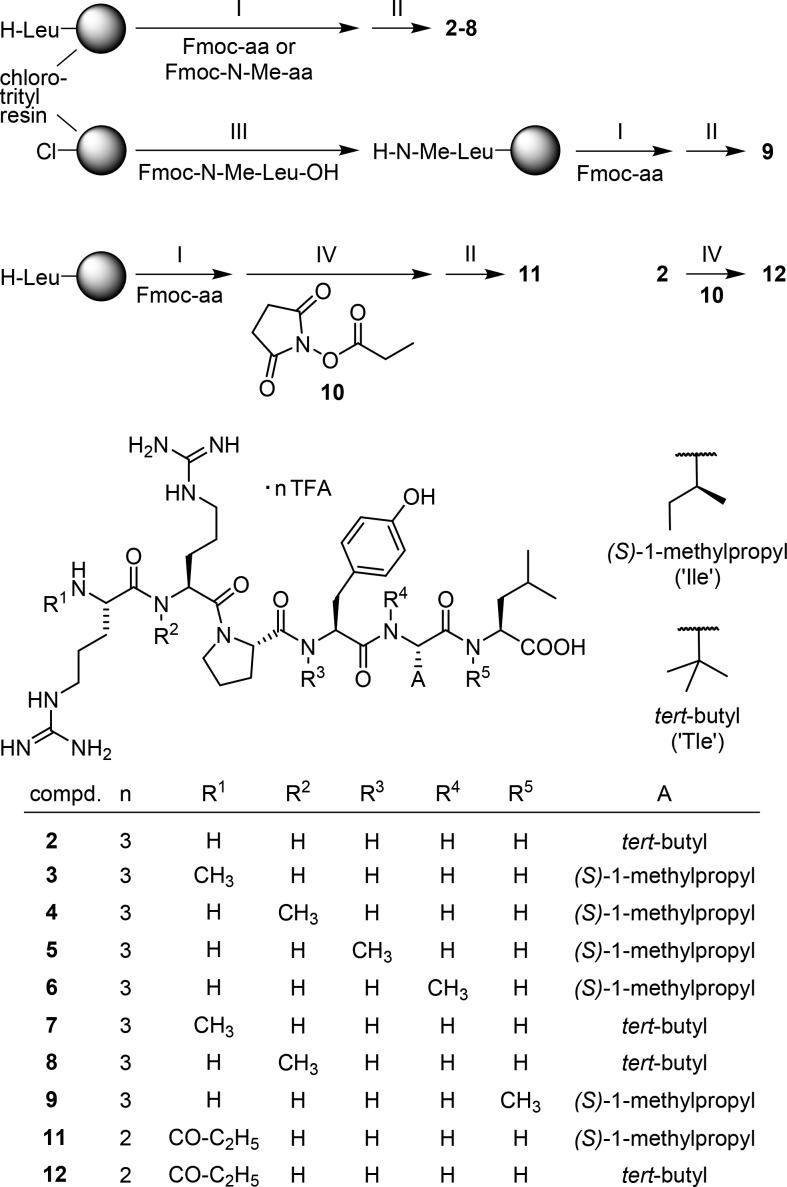
Peptides , 3,344,335,33,396,337, 8, and 9(33) were prepared by solid-phase peptide synthesis (SPPS) according to the 9-fluorenylmethoxycarbonyl (Fmoc) protecting group strategy using 1-hydroxybenzotriazole (HOBt)/O-(1H-benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) and diisopropylethylamine (DIPEA) for amide bond formation (Scheme 1). Coupling of Fmoc-protected amino acids to the secondary amino group of N-methylated amino acids turned out to be the yield limiting factor in the cases of 5, 6, and 9 (overall yields 18%, 15%, and 20%, respectively). The N-terminally propionylated derivative 11 was obtained by treatment of the respective resin-bound, side chain-protected, but N-terminally deprotected precursor peptide with succinimidyl propionate (10) followed by cleavage off the resin and side chain deprotection. By contrast, the N-terminally propionylated peptide 12 was prepared by solution phase treatment of 2 with compound 10 (Scheme 1).
Scheme 1. Syntheses of the NT(8–13) Derivatives 2–9, 11, and 12.
Reagents and conditions: (I) Fmoc strategy SPPS using HBTU/HOBt and DIPEA, solvent: DMF/NMP (80:20 v/v), 35 °C, 2 × 1 h or 2 × 2 h, Fmoc-deprotection: 20% piperidine in DMF/NMP (80:20 v/v), rt, 2 × 8–10 min; (II) (1) hexafluoro-2-propanol (HFIP)/CH2Cl2 (1:3 v/v), rt, 2 × 20 min, (2) TFA/H2O (95:5 v/v), rt, 3 h; (III) DIPEA, solvent: CH2Cl2, 35 °C, 14 h; (IV) DIPEA, solvent: DMF/NMP (80:20 v/v), rt, 1 h; overall yields 77% (2), 67% (3), 56% (4), 18% (5), 15% (6), 42% (7), 38% (8), 20% (9), 56% (11), 85% (12).
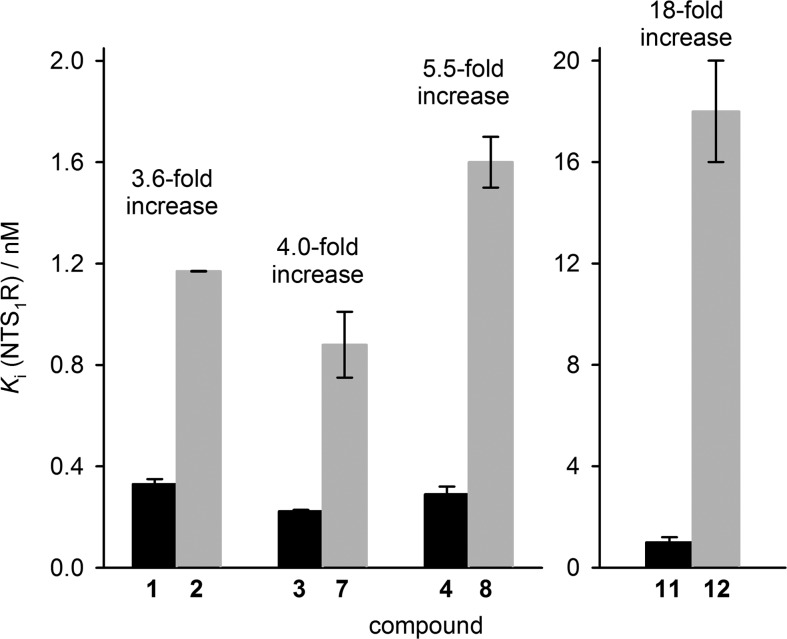
NTS1R binding data (Ki values) were determined for 1–9, 11, and 12 by competition binding with [3H]UR-MK30042 ([3H]13, for structure see Figure S1, Supporting Information) at intact hNTS1R expressing HT-29 colon carcinoma cells (Table 1). The replacement of Ile12 by Tle12 in 1 (compound 2) resulted in a minor decrease in NTS1R affinity (Ki values of 1 and 2: 0.33 vs 1.17 nM, cf. Table 1). Regarding the N-methyl scan of 1 (peptides 3–6 and 9), methylation at Arg8 or Arg9 (3, 4) did not affect NTS1R affinity (Ki < 0.5 nM, Table 1). By contrast, N-methylation of Tyr11, Ile12, or Leu13 (5, 6, 9) led to a considerable decrease in NTS1R affinity (Ki values: > 1,000 nM, 60 nM and 880 nM, respectively, cf. Table 1). As expected, the combination of the N-methylation at Arg8 or Arg9 with the replacement of Ile12 by Tle12 (peptides 7 and 8) resulted in NTS1R affinities comparable to that of 2 (Table 1). The N-terminally propionylated analogs of 1 and 2 (compounds 11 and 12) exhibited Ki values (NTS1R) of 1.0 and 18 nM, respectively.
Table 1. Peptide Sequences and NTS1R Affinities of 1–9, 11, and 12, as Well as Stabilities of 1–9, 11, and 12 in Human Plasma/PBS (1:2 v/v) (37 °C).
| % intact peptide
in plasmab after the specified incubation
times: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| compd | sequence | Ki [nM] NTS1Ra | 10 min | 30 min | 1 h | 2 h | 6 h | 24 h | 48 h |
| 1 | Arg-Arg-Pro-Tyr-Ile-Leu | 0.33 [0.35, 0.31] (lit. 0.14c) | 23.1 ± 0.2 | n.d. | <1 | n.d. | n.d. | <1 | <1 |
| 2 | Arg-Arg-Pro-Tyr-Tle-Leu | 1.17 [1.17, 1.17] | 10.8 ± 0.5 | n.d. | <1 | n.d. | n.d. | <1 | <1 |
| 3 | N(Me)-Arg-Arg-Pro-Tyr-Ile-Leu | 0.223 ± 0.005(lit. 0.29d) | 92.1 ± 0.1 | 88.2 ± 0.2 | 79.7 ± 0.1 | 70.8 ± 0.1 | n.d. | n.d. | n.d. |
| 4 | Arg-N(Me)-Arg-Pro-Tyr-Ile-Leu | 0.29 ± 0.03(lit. 0.51e) | >99 | 93.6 ± 0.1 | 83.7 ± 0.3 | 66.4 ± 0.1 | n.d. | n.d. | n.d. |
| 5 | Arg-Arg-Pro-N(Me)-Tyr-Ile-Leu | >1,000(lit. 5,100e) | 22.9 ± 0.2 | <1 | <1 | <1 | n.d. | n.d. | n.d. |
| 6 | Arg-Arg-Pro-Tyr-N(Me)-Ile-Leu | 60 ± 5(lit. 160e) | 2.6 ± 0.5 | <1 | <1 | <1 | n.d. | n.d. | n.d. |
| 7 | N(Me)-Arg-Arg-Pro-Tyr-Tle-Leu | 0.88 ± 0.13 | n.d. | n.d. | >99 | n.d. | >99 | 98.3 ± 0.8 | 86.8 ± 0.3 |
| 8 | Arg-N(Me)-Arg-Pro-Tyr-Tle-Leu | 1.6 ± 0.1 | n.d. | n.d. | >99 | n.d. | >99 | >99 | >99 |
| 9 | Arg-Arg-Pro-Tyr-Ile-N(Me)-Leu | 880 ± 260(lit. 190e) | 39.9 ± 0.9 | <1 | <1 | <1 | n.d. | n.d. | n.d. |
| 11 | Propionyl-Arg-Arg-Pro-Tyr-Ile-Leu | 1.0 ± 0.2 | >99 | 84.0 ± 0.1 | 71.8 ± 0.2 | 32.4 ± 0.1 | n.d. | n.d. | n.d. |
| 12 | Propionyl-Arg-Arg-Pro-Tyr-Tle-Leu | 18 ± 2 | n.d. | n.d. | >99 | n.d. | >99 | >99 | 92.5 ± 0.9 |
Determined by radioligand competition binding with [3H]13 at HT-29 cells; mean values from two (1, 2), three (3, 4) or four (6–9, 11, 12) independent experiments, each performed in triplicate (for n > 2 Ki values are given ± SEM; in the case of n = 2 individual Ki values are given in square brackets).
The initial concentration of the peptides in plasma/PBS (1:2 v/v) was 100 μM; presented are mean values ± SEM from three independent experiments (SEM not given if no decomposition was observed).
Keller et al.42
Orwig et al.34
Härterich et al.33 n.d. = not determined.
Figure 2 illustrates a general decrease in NTS1R affinity caused by the replacement of Ile12 by Tle12 in 1, 3, 4, and 11, giving 2, 7, 8, and 12, respectively, and a dependency of the extent of the decrease in affinity on the primary structure of the peptides. This is in agreement with reported NTS1R binding data of derivatives of 1 containing Tle12.10,11,27,31,38,40
Figure 2.
Decrease in NTS1R affinity (increase in Ki) resulting from the exchange of Ile12 by Tle12 in 1, 3, 4, and 11 (black bars) giving 2, 7, 8, and 12 (gray bars), respectively. Note: the scales of the Y-axes are different.
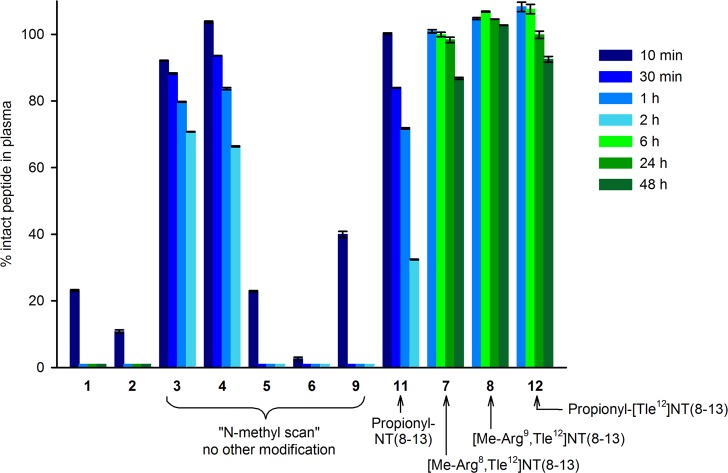
In order to investigate the effect of N-methylation (3–9), Ile12/Tle12 exchange (2, 7, 8, 12), and N-terminal acylation (11, 12) on the stability of the peptides against enzymatic cleavage, the stability of all compounds was investigated in human plasma at 37 °C for up to 48 h (Figure 3, Table 1). Whereas N-methylation of Arg8 or Arg9 in 1 (compounds 3 and 4) significantly enhanced the peptide stability in plasma compared to 1, methylation of Tyr11, Ile12, and Leu13 (5, 6, 9) did not lead to higher plasma stabilities. Strikingly, peptide 2, which differed from 1 only with respect to the replacement of Ile12 by Tle12, proved to be as unstable as 1 (Figure 3, Table 1). However, the combination of the Ile12/Tle12 exchange with N-methylation of Arg8 or Arg9 (7, 8) resulted in significantly higher plasma stabilities (t1/2 > 48 h) compared to 3 and 4. These results confirmed that both N-terminal (cleavage between Arg8 and Arg9) and C-terminal (cleavage between Tyr11 and Ile12) degradation are highly relevant, and they revealed that the former occurs faster than the latter. As in the case of N-terminal methylation of 1 (peptide 3), N-terminal propionylation of 1 (peptide 11) resulted in a moderate increase in enzymatic stability compared to 1 (t1/2 of 11 between 1 and 2 h, cf. Table 1). The combination of N-terminal propionylation with an Ile12/Tle12 exchange (compound 12) led to an excellent plasma stability as also observed in the case of combining N-terminal methylation with an Ile12/Tle12 exchange (peptide 7) (Figure 3, Table 1).
Figure 3.
Stabilities of 1–9, 11, and 12 in human plasma/PBS (1:2 v/v) at 37 °C investigated for up to 48 h. Data represent mean values ± SEM from three independent experiments.
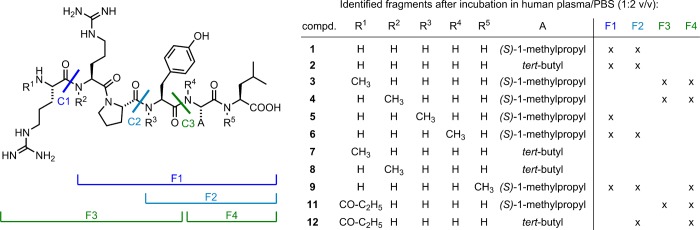
Figure 4 provides an overview of the major degradation fragments identified by LC-HRMS. The Arg8–Arg9, Pro10–Tyr11, and Tyr11–Ile12 bonds were identified as the major cleavage sites (Figure 4), being in agreement with reported data on the metabolic stability of 1.24,25 As outlined above, the present study suggests that cleavage of Arg8 in 1 occurs faster than its C-terminal degradation. This is, on the one hand, in agreement with reports in the literature;24 on the other hand, it is in disagreement with other reports, which suggest an Ile12/Tle12 exchange as the most crucial structural modification with respect to metabolic stabilization.27,28
Figure 4.
Major enzymatic cleavage sites (C1–C3) of compounds 1–9, 11, and 12 as well as corresponding fragments F1–F4, identified by LC-HRMS analysis after incubation in human plasma at 37 °C for up to 48 h.
In conclusion, the synthesis and investigation of N-methylated derivatives of NT(8–13) (1), N-terminally acylated derivatives of 1, and analogs containing Tle12 instead of Ile12 revealed that only the combination of appropriate N-terminal (e.g., N-methylation of Arg8) and C-terminal (replacement of Ile12 by Tle12) structural modifications in 1 affords highly stable (plasma half-live >48 h) congeners of 1 (compounds 7, 8, and 12). Fortunately, two of the most stable compounds (7, 8) exhibited the highest NTS1R affinities of the investigated analogs of 1. This work answers open questions concerning the controversially discussed impact of various structural modifications of 1 on the enzymatic stability, thus supporting the development of stable radiolabeled derivatives of 1, which harbor the potential of being used as radiopharmaceuticals.
Acknowledgments
The authors thank Susanne Bollwein, Brigitte Wenzl, and Maria Beer-Krön for excellent technical assistance, Christoph Müller for the synthesis of compound 10, and Armin Buschauer for providing laboratory equipment.
Glossary
Abbreviations
- 2-ClTrt
2-chlorotrityl
- 2-ClTrt-Cl
2-chlorotrityl-chloride
- DIPEA
diisopropylethylamine
- FCS
fetal calf serum
- Fmoc
9-fluorenylmethoxycarbonyl
- HBTU
O-(1H-benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate
- HFIP
1,1,1,3,3,3-hexafluoro-2-propanol
- HOBt
1-hydroxybenzotriazole
- HT-29
human colorectal adenocarcinoma cell line
- IC50
inhibitor/antagonist concentration which suppresses 50% of an agonist induced effect, or displaces 50% of a labeled ligand from the binding site
- k
retention (or capacity) factor (HPLC)
- Kd
dissociation constant obtained from a saturation binding experiment
- Ki
dissociation constant obtained from a competition binding experiment
- NT
neurotensin
- NT(8–13)
neurotensin(8–13)
- NTS1R
neurotensin receptor 1
- NTS2R
neurotensin receptor 2
- RP
reversed phase
- SEM
standard error of the mean
- SPPS
solid-phase peptide synthesis
- Tle
tert-butylglycine
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.9b00122.
General experimental conditions; experimental synthetic protocols and analytical data of compounds 2–9, 11, and 12; radioligand competition binding assay; experimental protocol for the investigation of the stability of 2–9, 11, and 12 in human plasma; Figures S1 and S2; RP-HPLC chromatograms of compounds 2–9, 11, and 12; 1H NMR spectra of compounds 2–9, 11, and 12 in DMSO-d6 and DMSO-d6/D2O (PDF)
Molecular formula strings (XLSX)
Author Contributions
L.S. performed any syntheses, radioligand competition binding experiments, and plasma stability studies. M.K. initiated and planned the project. M.K. and G.B. supervised the research. L.S., M.K., and G.B. wrote the manuscript. All authors have given approval to the final version of the manuscript.
This work was funded by the Deutsche Forschungsgemeinschaft (research grant KE 1857/1-1 and 1-2, Graduate Training Program (Graduiertenkolleg) GRK1910)
The authors declare no competing financial interest.
Supplementary Material
References
- Myers R. M.; Shearman J. W.; Kitching M. O.; Ramos-Montoya A.; Neal D. E.; Ley S. V. Cancer, chemistry, and the cell: molecules that interact with the neurotensin receptors. ACS Chem. Biol. 2009, 4, 503–525. 10.1021/cb900038e. [DOI] [PubMed] [Google Scholar]
- Vincent J.; Mazella J.; Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol. Sci. 1999, 20, 302–309. 10.1016/S0165-6147(99)01357-7. [DOI] [PubMed] [Google Scholar]
- Carraway R.; Leeman S. E. The amino acid sequence of a hypothalamic peptide, neurotensin. J. Biol. Chem. 1975, 250, 1907–1911. [PubMed] [Google Scholar]
- Tanaka K.; Masu M.; Nakanishi S. Structure and functional expression of the cloned rat neurotensin receptor. Neuron 1990, 4, 847–854. 10.1016/0896-6273(90)90137-5. [DOI] [PubMed] [Google Scholar]
- Mazella J.; Zsürger N.; Navarro V.; Chabry J.; Kaghad M.; Caput D.; Ferrara P.; Vita N.; Gully D.; Maffrand J. P.; Vincent J. P. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J. Biol. Chem. 1998, 273, 26273–26276. 10.1074/jbc.273.41.26273. [DOI] [PubMed] [Google Scholar]
- Maoret J.-J.; Pospai D.; Rouyer-Fessard C.; Couvineau A.; Laboisse C.; Voisin T.; Laburthe M. Neurotensin receptor and its mRNA are expressed in many human colon cancer cell lines but not in normal colonic epithelium: binding studies and RT-PCR experiments. Biochem. Biophys. Res. Commun. 1994, 203, 465–471. 10.1006/bbrc.1994.2205. [DOI] [PubMed] [Google Scholar]
- Reubi J. C.; Waser B.; Friess H.; Büchler M.; Laissue J. Neurotensin receptors: a new marker for human ductal pancreatic adenocarcinoma. Gut 1998, 42, 546–550. 10.1136/gut.42.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi J. C.; Waser B.; Schaer J.; Laissue J. A. Neurotensin receptors in human neoplasms: high incidence in Ewing’s sarcoma. Int. J. Cancer 1999, 82, 213–218. . [DOI] [PubMed] [Google Scholar]
- Souazé F.; Dupouy S.; Viardot-Foucault V.; Bruyneel E.; Attoub S.; Gespach C.; Gompel A.; Forgez P. Expression of neurotensin and NT1 receptor in human breast cancer: a potential role in tumor progression. Cancer Res. 2006, 66, 6243–6249. 10.1158/0008-5472.CAN-06-0450. [DOI] [PubMed] [Google Scholar]
- Bergmann R.; Scheunemann M.; Heichert C.; Mäding P.; Wittrisch H.; Kretzschmar M.; Rodig H.; Tourwé D.; Iterbeke K.; Chavatte K.; Zips D.; Reubi J. C.; Johannsen B. Biodistribution and catabolism of 18F-labeled neurotensin(8–13) analogs. Nucl. Med. Biol. 2002, 29, 61–72. 10.1016/S0969-8051(01)00284-0. [DOI] [PubMed] [Google Scholar]
- Alshoukr F.; Rosant C.; Maes V.; Abdelhak J.; Raguin O.; Burg S.; Sarda L.; Barbet J.; Tourwé D.; Pelaprat D.; Gruaz-Guyon A. Novel neurotensin analogues for radioisotope targeting to neurotensin receptor-positive tumors. Bioconjugate Chem. 2009, 20, 1602–1610. 10.1021/bc900151z. [DOI] [PubMed] [Google Scholar]
- García-Garayoa E.; Bläuenstein P.; Blanc A.; Maes V.; Tourwé D.; Schubiger P. A. A stable neurotensin-based radiopharmaceutical for targeted imaging and therapy of neurotensin receptor-positive tumours. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 37–47. 10.1007/s00259-008-0894-y. [DOI] [PubMed] [Google Scholar]
- Maschauer S.; Einsiedel J.; Hocke C.; Hübner H.; Kuwert T.; Gmeiner P.; Prante O. Synthesis of a 68Ga-labeled peptoid-peptide hybrid for imaging of neurotensin receptor expression in vivo. ACS Med. Chem. Lett. 2010, 1, 224–228. 10.1021/ml1000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschauer S.; Einsiedel J.; Haubner R.; Hocke C.; Ocker M.; Hübner H.; Kuwert T.; Gmeiner P.; Prante O. Labeling and Glycosylation of Peptides Using Click Chemistry: A general approach to 18F-glycopeptides as effective imaging probes for positron emission tomography. Angew. Chem., Int. Ed. 2010, 49, 976–979. 10.1002/anie.200904137. [DOI] [PubMed] [Google Scholar]
- Alshoukr F.; Prignon A.; Brans L.; Jallane A.; Mendes S.; Talbot J.-N.; Tourwé D.; Barbet J.; Gruaz-Guyon A. Novel DOTA-neurotensin analogues for 111In scintigraphy and 68Ga PET imaging of neurotensin receptor-positive tumors. Bioconjugate Chem. 2011, 22, 1374–1385. 10.1021/bc200078p. [DOI] [PubMed] [Google Scholar]
- Maschauer S.; Ruckdeschel T.; Tripal P.; Haubner R.; Einsiedel J.; Hübner H.; Gmeiner P.; Kuwert T.; Prante O. In vivo monitoring of the antiangiogenic effect of neurotensin receptor-mediated radiotherapy by small-animal positron emission tomography: a pilot study. Pharmaceuticals 2014, 7, 464–481. 10.3390/ph7040464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y.; Shi W.; Zhou Z.; Wagh N. K.; Fan W.; Brusnahan S. K.; Garrison J. C. Evaluation of DOTA-chelated neurotensin analogs with spacer-enhanced biological performance for neurotensin-receptor-1-positive tumor targeting. Nucl. Med. Biol. 2015, 42, 816–823. 10.1016/j.nucmedbio.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschauer S.; Einsiedel J.; Hübner H.; Gmeiner P.; Prante O. 18F- and 68Ga-labeled neurotensin peptides for PET imaging of neurotensin receptor 1. J. Med. Chem. 2016, 59, 6480–6492. 10.1021/acs.jmedchem.6b00675. [DOI] [PubMed] [Google Scholar]
- Deng H.; Wang H.; Zhang H.; Wang M.; Giglio B.; Ma X.; Jiang G.; Yuan H.; Wu Z.; Li Z. Imaging neurotensin receptor in prostate cancer with 64Cu-labeled neurotensin analogs. Mol. Imaging 2017, 16, 1536012117711369. 10.1177/1536012117711369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C.; Maschauer S.; Hübner H.; Gmeiner P.; Prante O. Synthesis and evaluation of a 18F-labeled diarylpyrazole glycoconjugate for the imaging of NTS1-positive tumors. J. Med. Chem. 2013, 56, 9361–9365. 10.1021/jm401491e. [DOI] [PubMed] [Google Scholar]
- Schulz J.; Rohracker M.; Stiebler M.; Goldschmidt J.; Grosser O. S.; Osterkamp F.; Pethe A.; Reineke U.; Smerling C.; Amthauer H. Comparative evaluation of the biodistribution profiles of a series of nonpeptidic neurotensin receptor-1 antagonists reveals a promising candidate for theranostic applications. J. Nucl. Med. 2016, 57, 1120–1123. 10.2967/jnumed.115.170530. [DOI] [PubMed] [Google Scholar]
- Baum R. P.; Singh A.; Schuchardt C.; Kulkarni H. R.; Klette I.; Wiessalla S.; Osterkamp F.; Reineke U.; Smerling C. 177Lu-3BP-227 for neurotensin receptor 1-targeted therapy of metastatic pancreatic adenocarcinoma: first clinical results. J. Nucl. Med. 2018, 59, 809–814. 10.2967/jnumed.117.193847. [DOI] [PubMed] [Google Scholar]
- Pedersen J. H.; Beck H.; Shokouh-Amiri M.; Fischer A. Effect of neurotensin in the dumping syndrome. Scand. J. Gastroenterol. 1986, 21, 478–482. 10.3109/00365528609015165. [DOI] [PubMed] [Google Scholar]
- García-Garayoa E.; Allemann-Tannahill L.; Bläuenstein P.; Willmann M.; Carrel-Rémy N.; Tourwé D.; Iterbeke K.; Conrath P.; Schubiger P. A. In vitro and in vivo evaluation of new radiolabeled neurotensin(8–13) analogues with high affinity for NT1 receptors. Nucl. Med. Biol. 2001, 28, 75–84. 10.1016/S0969-8051(00)00190-6. [DOI] [PubMed] [Google Scholar]
- Kitabgi P.; De Nadai F.; Rovère C.; Bidard J. N. Biosynthesis, maturation, release, and degradation of neurotensin and neuromedin N. Ann. N. Y. Acad. Sci. 1992, 668, 30–42. 10.1111/j.1749-6632.1992.tb27337.x. [DOI] [PubMed] [Google Scholar]
- Tyler-McMahon B. M.; Boules M.; Richelson E. Neurotensin: peptide for the next millennium. Regul. Pept. 2000, 93, 125–136. 10.1016/S0167-0115(00)00183-X. [DOI] [PubMed] [Google Scholar]
- Bruehlmeier M.; García-Garayoa E.; Blanc A.; Holzer B.; Gergely S.; Tourwé D.; Schubiger P. A.; Bläuenstein P. Stabilization of neurotensin analogues: effect on peptide catabolism, biodistribution and tumor binding. Nucl. Med. Biol. 2002, 29, 321–327. 10.1016/S0969-8051(01)00304-3. [DOI] [PubMed] [Google Scholar]
- García-Garayoa E.; Bläuenstein P.; Bruehlmeier M.; Blanc A.; Iterbeke K.; Conrath P.; Tourwé D.; Schubiger P. A. Preclinical evaluation of a new, stabilized neurotensin(8–13) pseudopeptide radiolabeled with 99mTc. J. Nucl. Med. 2002, 43, 374–383. [PubMed] [Google Scholar]
- Bläuenstein P.; García-Garayoa E.; Rüegg D.; Blanc A.; Tourwé D.; Beck-Sickinger A.; Schubiger P. A. Improving the tumor uptake of 99mTc-labeled neuropeptides using stabilized peptide analogues. Cancer Biother.Radiopharm. 2004, 19, 181–188. 10.1089/108497804323071959. [DOI] [PubMed] [Google Scholar]
- Maes V.; García-Garayoa E.; Bläuenstein P.; Tourwé D. Novel 99mTc-labeled neurotensin analogues with optimized biodistribution properties. J. Med. Chem. 2006, 49, 1833–1836. 10.1021/jm051172f. [DOI] [PubMed] [Google Scholar]
- Nock B. A.; Nikolopoulou A.; Reubi J. C.; Maes V.; Conrath P.; Tourwé D.; Maina T. Toward stable N4-modified neurotensins for NTS1-receptor-targeted tumor imaging with 99mTc. J. Med. Chem. 2006, 49, 4767–4776. 10.1021/jm060415g. [DOI] [PubMed] [Google Scholar]
- Maina T.; Nikolopoulou A.; Stathopoulou E.; Galanis A. S.; Cordopatis P.; Nock B. A. [99mTc]Demotensin 5 and 6 in the NTS1-R-targeted imaging of tumours: synthesis and preclinical results. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1804–1814. 10.1007/s00259-007-0489-z. [DOI] [PubMed] [Google Scholar]
- Härterich S.; Koschatzky S.; Einsiedel J.; Gmeiner P. Novel insights into GPCR—Peptide interactions: Mutations in extracellular loop 1, ligand backbone methylations and molecular modeling of neurotensin receptor 1. Bioorg. Med. Chem. 2008, 16, 9359–9368. 10.1016/j.bmc.2008.08.051. [DOI] [PubMed] [Google Scholar]
- Orwig K. S.; Lassetter M. R.; Hadden M. K.; Dix T. A. Comparison of N-terminal modifications on neurotensin(8–13) analogues correlates peptide stability but not binding affinity with in vivo efficacy. J. Med. Chem. 2009, 52, 1803–1813. 10.1021/jm801072v. [DOI] [PubMed] [Google Scholar]
- Boules M.; Liang Y.; Briody S.; Miura T.; Fauq I.; Oliveros A.; Wilson M.; Khaniyev S.; Williams K.; Li Z.; Qi Y.; Katovich M.; Richelson E. NT79: A novel neurotensin analog with selective behavioral effects. Brain Res. 2010, 1308, 35–46. 10.1016/j.brainres.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einsiedel J.; Held C.; Hervet M.; Plomer M.; Tschammer N.; Hübner H.; Gmeiner P. Discovery of highly potent and neurotensin receptor 2 selective neurotensin mimetics. J. Med. Chem. 2011, 54, 2915–2923. 10.1021/jm200006c. [DOI] [PubMed] [Google Scholar]
- Held C.; Plomer M.; Hübner H.; Meltretter J.; Pischetsrieder M.; Gmeiner P. Development of a metabolically stable neurotensin receptor 2 (NTS2) ligand. ChemMedChem 2013, 8, 75–81. 10.1002/cmdc.201200376. [DOI] [PubMed] [Google Scholar]
- Mascarin A.; Valverde I. E.; Mindt T. L. Structure-activity relationship studies of amino acid substitutions in radiolabeled neurotensin conjugates. ChemMedChem 2016, 11, 102–107. 10.1002/cmdc.201500468. [DOI] [PubMed] [Google Scholar]
- Bittermann H.; Einsiedel J.; Hübner H.; Gmeiner P. Evaluation of lactam-bridged neurotensin analogues adjusting ψ(Pro10) close to the experimentally derived bioactive conformation of NT(8–13). J. Med. Chem. 2004, 47, 5587–5590. 10.1021/jm049644y. [DOI] [PubMed] [Google Scholar]
- Kokko K. P.; Hadden M. K.; Orwig K. S.; Mazella J.; Dix T. A. In vitro analysis of stable, receptor-selective neurotensin[8–13] analogues. J. Med. Chem. 2003, 46, 4141–4148. 10.1021/jm0300633. [DOI] [PubMed] [Google Scholar]
- Barroso S.; Richard F.; Nicolas-Ethève D.; Reversat J. L.; Bernassau J. M.; Kitabgi P.; Labbé-Jullié C. Identification of residues involved in neurotensin binding and modeling of the agonist binding site in neurotensin receptor 1. J. Biol. Chem. 2000, 275, 328–336. 10.1074/jbc.275.1.328. [DOI] [PubMed] [Google Scholar]
- Keller M.; Kuhn K. K.; Einsiedel J.; Hübner H.; Biselli S.; Mollereau C.; Wifling D.; Svobodova J.; Bernhardt G.; Cabrele C.; Vanderheyden P. M.; Gmeiner P.; Buschauer A. Mimicking of Arginine by functionalized Nω-carbamoylated arginine as a new broadly applicable approach to labeled bioactive peptides: high affinity angiotensin, neuropeptide Y, neuropeptide FF, and neurotensin receptor ligands as examples. J. Med. Chem. 2016, 59, 1925–1945. 10.1021/acs.jmedchem.5b01495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.