Abstract
Background
Malaria is considered as a major threat to health systems. It is still considered as one of the most important infectious diseases in Iran, but with an elimination goal in 2025. This study aimed to review the malaria situation in Iran over the 16 years.
Methods
The data was collected from epidemiological registration forms that had been completed by physicians and malaria focal points in the National Centers for Disease Control and Prevention.
Results
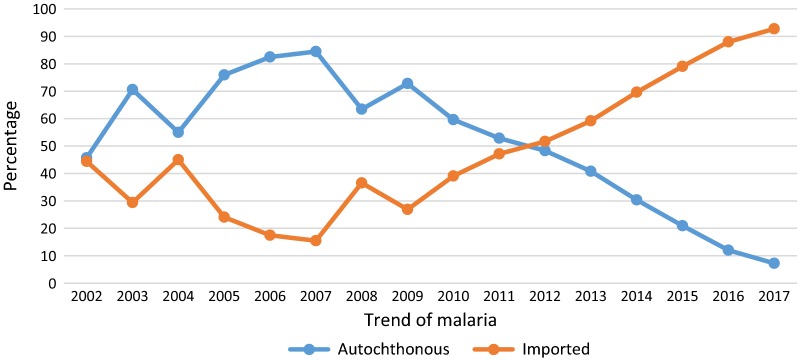
During the study period, 134,273 malaria cases were reported. The malaria incidence decreased from 0.24/1000 cases in 2002 to 0.01/1000 in 2017. From 2009 onward, the number of imported cases increased in comparison with the autochthonous and indigenous cases. Most cases were seen in males and people over 15 years of age. Moreover, the dominant registered reports were from rural areas. Most malaria cases were reported from the south and southeastern of Iran. Plasmodium vivax was the dominant species.
Conclusion
The dramatic drop in the incidence of autochthonous cases can hopefully support malaria elimination as a major goal in the near future.
Keywords: Autochthonous, Epidemiology, Malaria, Iran
Background
It is estimated that globally 219 million cases of malaria lead to 435,000 deaths in 2017. Most of the global malaria burden (80%) is carried by 15 countries in sub-Saharan Africa and India [1]. It is one of the most important communicable diseases transmitted by anopheline mosquitoes [2]. Currently, there are proven and effective tools to fight malaria, including vector control measures. As these tools are scaled up, malaria-endemic countries need to continually update the skills and competence of the health workers engaged in malaria control and elimination [3].
Iran is one of the malaria-endemic countries in the world, especially in the Sistan-Baluchestan and Hormozgan Provinces, in its southern and south-eastern areas, from where more than the four-fifths of cases are reported. Iran has a population of over 81 million, with a land area of 1,648,195 km2. Malaria is still one of the most important infectious diseases, with about 15,000 cases annually, but the total number recorded cases has dropped to less than 200, 90 and 89 locally-transmitted cases in 2015, 2016, and 2017, respectively [4–7]. The spectacular progress can be ascribed to the effective implementation of appropriate curative and preventive control interventions through a strong health care infrastructure [8]. In 2009, Iran started a malaria elimination programme with a goal to achieve this target by 2025. There has been excellent progress since, but the continued risk of importation of malaria cases from Pakistan and Afghanistan poses a huge challenge, politically, socially, operationally and technically to malaria elimination in Iran [9, 10]. The aim of this study was to analyse the malaria situation in Iran over a 16 years period.
Methods
In this study, the data on human malaria cases were provided by the national malaria surveillance system of Iran. It included coverage of all urban and rural health centres of the country during 2002–2017. The data relevant to malaria patients including gender (male and female), nationality (Iranian, Pakistani, Afghan and other nationalities), age group (0–4, 5–14, 15 years and older) and residence place (urban, rural and nomadic population), type of human malaria parasite (Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale), epidemiological classification (indigenous, introduced, and relapse as autochthonous cases and also, imported and unknown) and the outcome of treatment (recovery, hospitalization, and death) were collected and analysed by SPSS version 15 (Chicago, SPSS Inc.). The mentioned terms were used based on World Health Organization (WHO) malaria terminology [11].
Results
During the period of study, 2002–2017, 134,273 malaria cases were reported by the Iranian health system. A downward trend was seen except for some years, 2003 and 2005. In addition, malaria incidence decreased from 0.24/1000 cases in 2002 to 0.01/1000 in 2017 (Table 1).
Table 1.
The incidence of malaria in Iran during 2002–2017
| Year | Population | Cases of malaria (N) | Incidence/1000 |
|---|---|---|---|
| 2002 | 64,638,790 | 15,378 | 0.24 |
| 2003 | 66,480,366 | 25,027 | 0.38 |
| 2004 | 67,278,122 | 12,007 | 0.18 |
| 2005 | 68,429,945 | 19,285 | 0.28 |
| 2006 | 69,650,436 | 15,896 | 0.23 |
| 2007 | 70,924,928 | 16,489 | 0.23 |
| 2008 | 72,583,586 | 11,333 | 0.16 |
| 2009 | 73,630,366 | 5921 | 0.08 |
| 2010 | 73,938,131 | 2963 | 0.04 |
| 2011 | 75,149,669 | 3271 | 0.04 |
| 2012 | 76,124,600 | 1623 | 0.02 |
| 2013 | 76,941,000 | 1388 | 0.02 |
| 2014 | 77,857,000 | 1251 | 0.016 |
| 2015 | 78,773,000 | 777 | 0.01 |
| 2016 | 79,996,270 | 704 | 0.01 |
| 2017 | 80,000,000 | 960 | 0.012 |
While 18,102 autochthonous cases were reported in 2003, only 89 cases were registered in 2017 as the least number of autochthonous cases. Compared with autochthonous and indigenous cases, the number of imported cases increased from 2009 on (Table 2, Fig. 1). Most cases were observed in males and people with 15 or more years of age. Furthermore, the dominant registered reports belonged to rural areas and labourers. Most malaria cases (82.20%) were reported from three south and southeastern Iranian provinces, Sistan and Baluchestan, Hormozgan and Kerman provinces (Table 3). From a parasitical point of view, P. vivax was the dominant species followed by P. falciparum.
Table 2.
The epidemiological classification of malaria in Iran
| Year | Epidemiologic classification | ||||||
|---|---|---|---|---|---|---|---|
| Autochthonousa | Imported N (%) |
Unknown N (%) |
Total | ||||
| Indigenous N (%) |
Introduced N (%) |
Relapse N (%) |
Total N (%) |
||||
| 2002 | 6274 (40.80) | 557 (3.62) | 197 (1.28) | 7028 (45.70) | 6829 (44.41) | 1521 (9.89) | 15,378 (11.45) |
| 2003 | 17,240 (68.88) | 662 (2.65) | 200 (0.80) | 18,102 (72.33) | 6925 (27.67) | 0 (0.00) | 25,027 (18.64) |
| 2004 | 5245 (43.68) | 332 (2.77) | 206 (1.71) | 5783 (48.16) | 6221 (51.82) | 3 (0.02) | 12,007 (8.94) |
| 2005 | 14,009 (72.64) | 515 (2.67) | 191 (0.99) | 14,715 (76.30) | 4570 (23.70) | 0 (0.00) | 19,285 (14.36) |
| 2006 | 12,476 (78.48) | 501 (3.16) | 131 (0.82) | 13,108 (82.46) | 2782 (17.50) | 6 (0.04) | 15,896 (11.84) |
| 2007 | 13,687 (83.00) | 197 (1.20) | 162 (0.98) | 14,046 (85.18) | 2434 (14.76) | 9 (0.06) | 16,489 (12.28) |
| 2008 | 6896 (60.85) | 118 (1.04) | 130 (1.15) | 7144 (63.04) | 4189 (36.96) | 0 (0.00) | 11,333 (8.44) |
| 2009 | 4072 (68.77) | 95 (1.61) | 89 (1.50) | 4256 (71.88) | 1645 (27.78) | 20 (0.34) | 5921 (4.41) |
| 2010 | 1573 (53.09) | 69 (2.33) | 97 (3.27) | 1739 (58.69) | 1184 (39.96) | 40 (1.35) | 2963 (2.21) |
| 2011 | 1612 (49.28) | 78 (2.39) | 54 (1.65) | 1744 (53.32) | 1527 (46.68) | 0 (0.00) | 3271 (2.44) |
| 2012 | 733 (45.16) | 30 (1.85) | 18 (1.11) | 781 (48.12) | 842 (51.88) | 0 (0.00) | 1623 (1.21) |
| 2013 | 534 (38.47) | 26 (1.88) | 15 (1.08) | 575 (41.43) | 813 (58.57) | 0 (0.00) | 1388 (1.03) |
| 2014 | 355 (28.38) | 12 (0.96) | 22 (1.76) | 389 (31.09) | 862 (68.91) | 0 (0.00) | 1251 (0.93) |
| 2015 | 115 (14.80) | 24 (3.09) | 8 (1.03) | 147 (18.92) | 630 (81.08) | 0 (0.00) | 777 (0.58) |
| 2016 | 75 (10.65) | 10 (1.42) | 4 (0.57) | 89 (12.64) | 615 (87.36) | 0 (0.00) | 704 (0.52) |
| 2017 | 69 (7.19) | 17 (1.77) | 3 (0.31) | 89 (9.27) | 871 (90.73) | 0 (0.00) | 960 (0.72) |
| Total | 84,965 (63.28) | 3243 (2.41) | 1527 (1.14) | 89,735 (66.83) | 42,939 (31.98) | 1599 (1.19) | 134,273 (100) |
aAutochthonous indigenous + introduced + relapse
Fig. 1.
Trend of autochthonous and imported malaria cases in Iran, 2002–2017
Table 3.
Demographic and clinical characteristics of malaria patients during the period of study; 2002–2017
| Variables | Number (%) |
|---|---|
| Gender | |
| Male | 88,190 (65.68) |
| Female | 46,083 (34.32) |
| Age group | |
| 0–4 | 9614 (7.16) |
| 5–14 | 37,099 (27.63) |
| More than 15 | 87,560 (65.21) |
| Residency area | |
| Urban | 40,564 (30.21) |
| Rural | 92,165 (68.64) |
| Nomadic | 1544 (1.15) |
| Nationality | |
| Iranian | 85,344 (63.56) |
| Pakistan | 22,209 (16.54) |
| Afghan | 26,170 (19.49) |
| Others | 550 (0.41) |
| Location of patients | |
| Sistan and Baluchestan, Hormozgan, Kerman | 110,343 (82.20) |
| Other provinces | 23,930 (17.82) |
| Occupation | |
| Labourer | 53,937 (40.17) |
| Housewife | 15,347 (11.43) |
| Student | 11,172 (8.32) |
| Farmer | 6244 (4.65) |
| Driver | 10,232 (7.62) |
| Others | 37,341 (27.81) |
| Parasite species | |
| P. vivax | 116,012 (86.40) |
| P. falciparum | 15,361 (11.44) |
| P. malariae | 282 (0.21) |
| P. vivax + P. falciparum | 2618 (1.95) |
Discussion
Although a high morbidity and mortality had been reported in the past decades, the recent malaria situation shows a successful elimination in most parts of the country. In 1921 and 1924, the surveys conducted by Latychev and Gilmour showed high spleen and parasite rate in the north and centre of Iran [12]. Nowadays, malaria transmission is restricted to the south and southeast of Iran, in Sistan and Baluchestan, Hormozgan and Kerman provinces, with very low incidence [13].
A malaria pre-elimination programme with WHO technical support started Iran in 2009 [14]. It was set to interrupt P. falciparum transmission and make Iran a malaria-free country by the end of 2015 and 2025, respectively [15]. All areas were classified into four categories based on new autochthonous malaria cases per 1000 population: (1) intensified control areas (Annual Parasite index/1000 > 5), (2) pre-elimination (5 > API/1000 > 1), (3) elimination (API/1000 < 1), (4) preventing reintroduction (without autochthonous malaria cases within 36 months). Actually, vector control and other interventions were programmed and implemented based on the class of each area [16]. “Improved access to early diagnosis and prompt treatment, expanded coverage of integrated vector management (IVM) and enhanced surveillance” were the most important strategic aspects of this programme [15]. The Iranian Ministry of Health received a Global Fund grants in 2008 and 2011 to reduce the local transmission by 80%. Despite six malaria deaths from 2012 to 2015 [17], malaria cases declined from 16,489 in 2007 to 704 in 2016. After 2017, the government contribution was the only source of malaria financing [1]. The development of a malaria early warning system, training of microscopists, rural malaria mobile teams, community volunteers, surveillance increase, operational research and building capacity of human resources have been considered as the main activities affecting this reduction [18]. Current malaria surveillance in Iran includes passive and active case finding among suspected individuals with relevant symptoms like fever. Active case finding is done by health workers (Behvarz) or volunteers. The examination of microscopic slides is used routinely for malaria diagnosis. Standard operating procedures for quality assurance of malaria diagnostic tests was performed for all positive and 10% of negative slides [19, 20]. The rapid diagnostic test (RDT), introduced in 2009, is usually used in remote areas (> 50 km from malaria diagnostic centres) among migrants, outbreaks and when microscopic detection is not possible [18]. Using this method by trained volunteers in rural communities can play an important role in early case finding [20]. Based on the national malaria treatment guidelines, chloroquine, primaquine and artesunate together with sulfadoxine/pyrimethamine are recommended as the first-line treatment for P. vivax and P. falciparum, respectively [18].
Based on the results, malaria incidence has been reduced from 333/1000 cases in 1921 to 0.01/1000 in 2017. Several studies have been conducted with the aim of finding the reasons for this decline. The improvement of socio-economic indicators, including access to electricity and water pipe network, as well as climate changes have been proposed as explanatory reasons for these changes [21]. Nevertheless, the measures taken to malaria elimination including entomological studies and interventions, such as periodic indoor residual spraying (IRS) [22, 23], insecticide resistance monitoring [24], using various larvicides [25, 26], distribution of free insecticide-treated nets (ITN) [27], and improvement tools for early detection especially using RDTs [28] can be considered as having the strongest impact on this reduction. In some studies, several factors “including increased funding, effective vector control, strengthening of health systems, improved case management with more effective treatment regimens and improved case reporting and surveillance” have been documented as the reasons of malaria elimination [29].
In the current study, malaria cases were mostly reported from three south and southeastern Iranian provinces. Most of these areas have humid and suitable weather for vectors development. Sistan and Baluchestan with an oriental climate is a vast land and has a maritime border with Pakistan in the east and Oman Sea in the south. A suitable climate for breeding various Anopheles species, including most malaria vectors, exist in this part of the country: Anopheles stephensi, Anopheles culicifacies, Anopheles dthali, Anopheles fluviatilis, Anopheles superpictus, Anopheles maculipennis and Anopheles sacharovi [30–33]. The first five of these vectors can be found in the southeast of the country, together with the majority of malaria cases [32]. In addition, Anopheles pulcherrimus has been considered as a potential malaria vector in this area based on immunological parasite detection [two-site immunoradiometric assay (IRMA)] [34]. Although all eight species may be collected in indoor places, some of them such as An. stephensi are considered as domestic species with endophilic behaviour. The distribution of long-lasting impregnated nets (LLINs), IRS and larviciding are applied as important strategies by Iran national malaria control programme. These free interventions are done along with other free services such as malaria diagnosis-treatment provided by the health centres [21]. Over the past years, it was proven that the high coverage of IRS application in the south of Iran has caused resistance to pyrethroid and carbamate insecticides in adult stage An. stephensi. Also, the resistance monitoring on the larval populations of this main malaria vector showed susceptibility to temephos [24, 35].
The results of this study showed that the imported cases increased from 2009 onward, compared to indigenous cases. Population movements, especially from the eastern neighbouring countries endemic for malaria, have been noted as an important factor. A study conducted in the south of Iran showed that the presence of foreign immigrants could cause malaria outbreaks and change the classification of cleared up and potential foci [10]. Thus, it seems that the increase in imported malaria cases is related to foreign immigrants, such as Pakistani and Afghan refugees [36]. Similarly to Iran, in Saudi Arabia, despite a decreasing trend in malaria cases between 1999 and 2010, the proportion of imported increased from 23 to 99% [29].
Plasmodium vivax was found to be the dominant malaria parasite species in Iran. It is reported globally and can develop in both temperate and tropical climates. Early appearance of its gametocytes, efficient transmission by Anopheles vectors at lower parasite densities, faster development of sporozoites within the mosquito and wider viable temperature ranges than P. falciparum, have been suggested as important reasons for the wider geographical distribution of P. vivax. In addition, it was documented that the vector control methods, such as ITNs were more effective in reducing P. falciparum transmission than P. vivax. In 2010, it was estimated that 2.49 billion people live in areas with the risk of P. vivax infection [37]. Plasmodium vivax is endemic in a third of the Earth’s land surface, 44 million square kilometres. Fifty-one percent of this area is located in Africa, 22% in the Americas and 27% in Asia [38]. It is believed that vivax malaria is predominant along Iran’s entire borders and in other neighbouring countries, including Afghanistan, Iraq, Oman, Pakistan, Saudi Arabia, Syria, Yemen, Armenia, Azerbaijan, Tajikistan and Turkey [39].
In the present study, most cases were seen in males and in people above 15 years of age mostly from rural areas. It has been noted that an increased proportion of malaria cases among adult men is a notable epidemiological aspect in malaria-eliminating countries. This high proportion is related to occupational and behavioural factors that cause more contact with infective vectors than women. Because of the prevalence of asymptomatic cases with low parasite densities among adult men, these so-called “hot-pops” (hot populations) can play an important role in causing outbreaks [29]. A piece of research carried out in Thailand showed that malaria can be considered as a rural disease associated with agricultural labourers [40]. A study conducted in China reported that socio-economic development is very relevant to malaria reduction. It was concluded that labourers may not have been paid enough to be able to afford malaria treatment [41]. Another study also showed that malaria in overseas Chinese labourers who returned to China represented an increasing trend in the 2001–2011 period. The researchers explained that due to global economic integration, a large number of labourers may emigrate to malaria-endemic countries [42]. However, it seems that because of free health care services, especially malaria treatment in Iran, the economic conditions of rural residents have no effect on malaria outbreaks, but their social situation can affect knowledge, attitude and practice [13].
Generally, the epidemiological classification is calculated based on a compilation of criteria [43]. The classification of malaria cases as autochthonous, indigenous, induced, imported or relapsing are very important and needs an improved surveillance health system [11]. The recognition and assessment of imported malaria cases are very important especially in the clear up foci. In addition, in malaria‐endemic areas, a misclassification of autochthonous cases instead of imported ones can affect the control programmes [44, 45]. In malaria-eliminating countries, imported malaria is as important as indigenous cases and can be considered as the main threat to achieving elimination [46]. In Italy, during 2009–2011, two autochthonous cases of malaria were considered as a threat for public health. Nevertheless, they were not proven and had been reported as probable cases [47]. These findings can show the importance of probable autochthonous cases in a non-endemic malaria setting. In fact, they resulted from an improved epidemiological survey. It seems that the improvement of the surveillance system was one of the most important activities done by the Iranian health system to eliminate malaria.
Conclusion
The overall results of this study show that although Iran has taken great steps to eliminate malaria, it is not yet ready for certification. The dramatic drop in the incidence of autochthonous cases can be promising in supporting this major goal in the near future. Thus, high political commitment and efficient inter-sectoral collaboration are the two main factors for successful elimination.
Acknowledgements
The authors are grateful to the National Program for Malaria Control Office, Center of Disease Control & Prevention, Ministry of Health and Medical Education, Tehran, Iran.
Abbreviations
- ITN
insecticide-treated nets
- ACT
artemisinin-based combination therapy
- IRS
indoor residual spraying
- RDT
rapid malaria diagnostic test
Authors’ contributions
HV and AS developed the study concept and design. AR, AS, FN, and JN collected the data. AS, HV and AR analyzed and interpreted the data. AS, JN, and AR wrote the manuscript. AS and HV and AR revised and edited the manuscript. All authors read and approved the final manuscript.
Funding
This study received financial support from the National Program for Malaria Control, Center of Disease Control & Prevention, Ministry of Health and Medical Education, Tehran, Iran
This study was supported “Tehran University of Medical Sciences and Health Services (Grant Number 2131)”
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Ethical clearance was earned from the National Program for Malaria Control, Center of Disease Control & Prevention, Ministry of Health and Medical Education, Tehran, Iran.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. World malaria report 2018. Geneva: World Health Organization; 2018. https://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf. Accessed 16 Jan 2019.
- 2.Smith DL, Mckenzie F. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malar J. 2004;3:13. doi: 10.1186/1475-2875-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benelli G, Beier JC. Current vector control challenges in the fight against malaria. Acta Trop. 2017;174:91–96. doi: 10.1016/j.actatropica.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Sheikhzadeh K, Haghdoost AA, Bahrampour A, Raeisi A, Zolala F, et al. Predicting malaria transmission risk in endemic areas of Iran: a multilevel modeling using climate and socioeconomic indicators. Iran Red Crescent Med J. 2017;19:e45132. doi: 10.5812/ircmj.45132. [DOI] [Google Scholar]
- 5.Ostovar A, Raeisi A, Haghdoost AA, Ranjbar M, Rahimi A. Lessons learnt from malaria epidemics in the Islamic Republic of Iran. East Mediterr Health J. 2012;18:864–869. doi: 10.26719/2012.18.8.864. [DOI] [PubMed] [Google Scholar]
- 6.McKelvie WR, Haghdoost AA, Raeisi A. Defining and detecting malaria epidemics in south-east Iran. Malar J. 2012;11:81. doi: 10.1186/1475-2875-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raeisi A, Gouya MM, Nadim A, Ranjbar M, Hasanzehi A, Fallahnezhad M, et al. Determination of malaria epidemiological status in Iran’s malarious areas as baseline information for implementation of malaria elimination program in Iran. Iran J Public Health. 2013;42:326–333. [PMC free article] [PubMed] [Google Scholar]
- 8.Lankarani KB, Alavian SM, Peymani P. Health in the Islamic Republic of Iran, challenges and progresses. Med J Islam Repub Iran. 2013;27:42–49. [PMC free article] [PubMed] [Google Scholar]
- 9.Hemami MR, Sari AA, Raeisi A, Vatandoost H, Majdzadeh R. Malaria elimination in iran, importance and challenges. Int J Prev Med. 2013;4:88–94. [PMC free article] [PubMed] [Google Scholar]
- 10.Raeisi A, Nejati J, Ansari-moghaddam A, Sakeni M, Faraji L, Paktinat B, et al. Effects of foreign immigrants on malaria situation in cleared up and potential foci in one of the highest malaria burden district of southern Iran. Malar J. 2012;11:P81. doi: 10.1186/1475-2875-11-S1-P81. [DOI] [Google Scholar]
- 11.World Health Organization . WHO malaria terminology. Geneva: World Health Organization; 2016. [Google Scholar]
- 12.Edrissian GhH. Malaria in Iran: past and present situation. Iran J Parasitol. 2006;1:1–14. [Google Scholar]
- 13.Nejati J, Moosa-Kazemi SH, Saghafipour A, Soofi K. Knowledge, attitude and practice (KAP) on malaria, from high malaria burden rural communities, southeastern Iran. J Parasit Dis. 2018;42:62–67. doi: 10.1007/s12639-017-0965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kafil SH. Malaria in Iran: is the elimination phase a target? Ann Trop Med Public Health. 2017;10:1062. doi: 10.4103/1755-6783.196621. [DOI] [Google Scholar]
- 15.UCSF Global Health Group. Eliminating malaria in Iran; 2015. https://globalhealthsciences.ucsf.edu/sites/globalhealthsciences.ucsf.edu/files/pub/iran2015-final.pdf. Accessed 11 Mar 2015.
- 16.CDC. Ministry Health and Medical Education . Malaria elimination program in Islamic Republic of Iran (in vision of 2025) Tehran: Ministry Health and Medical Education; 2008. [Google Scholar]
- 17.Piroozi B, Moradi G, Safari H, Faraji L, Sima S, Alinia C, et al. Incidence, mortality, and burden of malaria and its geographical distribution in Iran during 2002–2015. Iran J Public Health. 2019;48(Suppl 1):53–61. [Google Scholar]
- 18.Schapira AM, Zaim M, Raeisi A, Ranjbar M, Kolifarhood G, Nikpour F, et al. History of the successful struggle against malaria in the Islamic Republic of Iran: from the earliest records to imminent elimination. Tehran: Shayan Gostar Publ Tehran; 2018. [Google Scholar]
- 19.Laboratory Diagnosis of Malaria. Operational Guidelines for Laboratory Technicians. Government of India; National Vector Borne Diseases Control Programme; Ministry of Health & Family Welfare, Editors. NVBDCP, India, 2009.
- 20.UNDP. Intensified malaria control in high burden provinces towards falciparum elimination; 2017. http://www.ir.undp.org/content/iran/en/home/operations/projects/health_and_development/Malaria.html. Accessed Jan 2017.
- 21.Nejati J, Tabatabaei SM, Salehi M, Saghafipour A, Mozafari E. Some probable factors affecting the malaria situation before and at the beginning of a pre-elimination program in southeastern Iran. J Parasit Dis. 2017;41:503–509. doi: 10.1007/s12639-016-0838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nejati J, Mahjoob M, Kiyani M, Keyhani A, Hasanzehi A. Status of indoor residual spraying by deltamethrin in malaria elimination program, southeastern Iran. Iran J Toxicol. 2012;6:600–604. [Google Scholar]
- 23.Hanafi-Bojd AA, Vatandoost H, Oshaghi MA, Haghdoost AA, Shahi M, Sedaghat MM. Entomological and epidemiological attributes for malaria transmission and implementation of vector control in southern Iran. Acta Trop. 2012;121:85–92. doi: 10.1016/j.actatropica.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Vatandoost H, Hanafi-Bojd AA. Indication of pyrethroid resistance in the main malaria vector, Anopheles stephensi from Iran. Asian Pac J Trop Med. 2012;5:722–726. doi: 10.1016/S1995-7645(12)60114-X. [DOI] [PubMed] [Google Scholar]
- 25.Vatandoost H, Sanei Dehkordi A, Sadeghi SM, Davari B, Karimian F, Abai MR, et al. Identification of chemical constituents and larvicidal activity of Kelussia odoratissima Mozaffarian essential oil against two mosquito vectors Anopheles stephensi and Culex pipiens (Diptera: Culicidae) Exp Parasitol. 2012;132:470–474. doi: 10.1016/j.exppara.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Mozaffari E, Abai MR, Khanavi M, Vatandoost H, Sedaghat MM, Moridnia A, et al. Chemical composition, larvicidal and repellency properties of Cionura erecta (L.) Griseb. against malaria vector, Anopheles stephensi Liston (Diptera: Culicidae) J Arthropod Borne Dis. 2014;8:147–155. [PMC free article] [PubMed] [Google Scholar]
- 27.Soleimani Ahmadi M, Vatandoost H, Shaeghi M, Raeisi A, Abedi F, Eshraghian MR, et al. Effects of educational intervention on long-lasting insecticidal nets use in a malarious area, southeast Iran. Acta Med Iran. 2012;50:279–287. [PubMed] [Google Scholar]
- 28.Ehtesham R, Fazaeli A, Raeisi A, Keshavarz H, Heidari A. Detection of mixed-species infections of Plasmodium falciparum and Plasmodium vivax by nested PCR and rapid diagnostic tests in southeastern Iran. Am J Trop Med Hyg. 2015;93:181–185. doi: 10.4269/ajtmh.14-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–911. doi: 10.1016/S0140-6736(13)60310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nejati J, Saghafipour A, Vatandoost H, Moosa-Kazemi SH, Motevalli Haghi A, Sanei-Dehkordi A. Bionomics of Anopheles subpictus (Diptera: Culicidae) in a malaria endemic area, Southeastern Iran. J Med Entomol. 2018;55:1182–1187. doi: 10.1093/jme/tjy079. [DOI] [PubMed] [Google Scholar]
- 31.Nejati J, Vatandoost H, Oshghi MA, Salehi M, Mozafari E, Moosa-Kazemi SH. Some ecological attributes of malarial vector Anopheles superpictus Grassi in endemic foci in southeastern Iran. Asian Pac J Trop Biomed. 2013;3:1003–1008. doi: 10.1016/S2221-1691(13)60193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanafi-Bojd AA, Azari-Hamidian S, Vatandoost H, Charrahy Z. Spatio-temporal distribution of malaria vectors (Diptera: Culicidae) across different climatic zones of Iran. Asian Pac J Trop Med. 2011;4:498–504. doi: 10.1016/S1995-7645(11)60134-X. [DOI] [PubMed] [Google Scholar]
- 33.Azari-Hamidian S, Norouzi B, Harbach RE. A detailed review of the mosquitoes (Diptera: Culicidae) of Iran and their medical and veterinary importance. Acta Trop. 2019;194:106–122. doi: 10.1016/j.actatropica.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Zaim M, Subbarao S, Manouchehri A, Cochrane A. Role of Anopheles culicifacies s.l. and An. pulcherrimus in malaria transmission in Ghassreghand (Baluchistan), Iran. J Am Mosq Control Assoc. 1993;9:23–26. [PubMed] [Google Scholar]
- 35.Abbasi M, Hanafi-Bojd AA, Yaghoobi-Ershadi MR, Vatandoost H, Oshaghi MA, Hazratian T, et al. Resistance status of main malaria vector, Anopheles stephensi Liston (Diptera: Culicidae) to insecticides in a malaria Endemic Area, Southern Iran. Asian Pac J Trop Med. 2019;12:43–48. doi: 10.4103/1995-7645.250344. [DOI] [Google Scholar]
- 36.Stark K, Schöneberg I. Increase in malaria cases imported from Pakistan to Germany in 2012. Euro Surveill. 2012;17:20320. doi: 10.2807/ese.17.47.20320-en. [DOI] [PubMed] [Google Scholar]
- 37.Battle KE, Gething PW, Elyazar IR, Moyes CL, Sinka ME, Howes RE, et al. The global public health significance of Plasmodium vivax. Adv Parasitol. 2012;80:1–111. doi: 10.1016/B978-0-12-397900-1.00001-3. [DOI] [PubMed] [Google Scholar]
- 38.Gething PW, Elyazar IR, Moyes CL, Smith DL, Battle KE, Guerra CA. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis. 2012;6:e1814. doi: 10.1371/journal.pntd.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howes RE, Battle KE, Mendis KN, Smith DL, Cibulskis RE, Baird JK. Global Epidemiology of Plasmodium vivax. Am J Trop Med Hyg. 2016;95:15–34. doi: 10.4269/ajtmh.16-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kühner A. The impact of public health programs on economic development: report of a study of malaria in Thailand. Inter J Health Serv. 1971;1:285–292. doi: 10.2190/BDFL-PVH7-EGG1-MDW7. [DOI] [PubMed] [Google Scholar]
- 41.Wang SQ, Li YC, Zhang ZM, Wang GZ, Hu XM, Qualls WA, et al. Prevention measures and socio-economic development result in a decrease in malaria in Hainan, China. Malar J. 2014;13:362. doi: 10.1186/1475-2875-13-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Hsiang MS, Zhou H, Wang W, Cao Y, Gosling RD, et al. Malaria in overseas labourers returning to China: an analysis of imported malaria in Jiangsu Province, 2001–2011. Malar J. 2014;13:29. doi: 10.1186/1475-2875-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Nam N, de Vries PJ, Van Toi L, Nagelkerke N. Malaria control in Vietnam: the Binh Thuan experience. Trop Med Int Health. 2005;10:357–365. doi: 10.1111/j.1365-3156.2005.01387.x. [DOI] [PubMed] [Google Scholar]
- 44.Choi KM, Choi YK, Kang YA, Seo SY, Lee HW, Cho SH, et al. Study of the genetic discrimination between imported and autochthonous cases of malaria in South Korea. J Travel Med. 2011;18:63–66. doi: 10.1111/j.1708-8305.2010.00473.x. [DOI] [PubMed] [Google Scholar]
- 45.Piperaki ET, Daikos GL. Malaria in Europe: emerging threat or minor nuisance? Clin Microbiol Infect. 2016;22:487–493. doi: 10.1016/j.cmi.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 46.Fullman N, Burstein R, Lim SS, Medlin C, Gakidou E. Nets, spray or both? The effectiveness of insecticide-treated nets and indoor residual spraying in reducing malaria morbidity and child mortality in sub-Saharan Africa. Malar J. 2013;12:62. doi: 10.1186/1475-2875-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romi R, Boccolini D, Menegon M, Rezza G. Probable autochthonous introduced malaria cases in Italy in 2009–2011 and the risk of local vector-borne transmission. Euro Surveill. 2012;17:20325. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.