Abstract
Rationale: Patients managed at a long-term acute-care hospital (LTACH) for weaning from prolonged mechanical ventilation are at risk for profound muscle weakness and disability.
Objectives: To investigate effects of prolonged ventilation on survival, muscle function, and its impact on quality of life at 6 and 12 months after LTACH discharge.
Methods: This was a prospective, longitudinal study conducted in 315 patients being weaned from prolonged ventilation at an LTACH.
Measurements and Main Results: At discharge, 53.7% of patients were detached from the ventilator and 1-year survival was 66.9%. On enrollment, maximum inspiratory pressure (Pimax) was 41.3 (95% confidence interval, 39.4–43.2) cm H2O (53.1% predicted), whereas handgrip strength was 16.4 (95% confidence interval, 14.4–18.7) kPa (21.5% predicted). At discharge, Pimax did not change, whereas handgrip strength increased by 34.8% (P < 0.001). Between discharge and 6 months, handgrip strength increased 6.2 times more than did Pimax. Between discharge and 6 months, Katz activities-of-daily-living summary score improved by 64.4%; improvement in Katz summary score was related to improvement in handgrip strength (r = −0.51; P < 0.001). By 12 months, physical summary score and mental summary score of 36-item Short-Form Survey returned to preillness values. When asked, 84.7% of survivors indicated willingness to undergo mechanical ventilation again.
Conclusions: Among patients receiving prolonged mechanical ventilation at an LTACH, 53.7% were detached from the ventilator at discharge and 1-year survival was 66.9%. Respiratory strength was well maintained, whereas peripheral strength was severely impaired throughout hospitalization. Six months after discharge, improvement in muscle function enabled patients to perform daily activities, and 84.7% indicated willingness to undergo mechanical ventilation again.
Keywords: prolonged mechanical ventilation, survival, respiratory muscles, handgrip strength
At a Glance Commentary
Scientific Knowledge on the Subject
Patients managed at a long-term acute-care hospital for weaning from prolonged mechanical ventilation are at risk for profound muscle weakness and disability, but its impact on functional recovery and quality of life has not been investigated.
What This Study Adds to the Field
This is the first study to provide longitudinal measurements of survival, muscle function, and quality-of-life evaluation in ventilated patients during a long-term acute-care hospital stay and 6 and 12 months after discharge. Half (53.7%) of patients were detached from the ventilator at discharge and their 1-year survival was 66.9%. Respiratory strength was well maintained, whereas peripheral strength was severely impaired. Improvement in muscle function was associated with an increased ability to perform daily activities independently, and 84.7% of survivors indicated willingness to undergo ventilation again if deemed necessary.
Patients requiring prolonged mechanical ventilation are commonly transferred to a long-term acute-care hospital (LTACH) for weaning and rehabilitation (1–5). In 2015, Medicare spent $5.3 billion on care in 426 LTACHs (6). Despite the increasing role of LTACHs in ventilated patients, long-term outcome (survival and quality of life [QOL]) data in such patients are scarce (7, 8).
In a prospective trial conducted at an LTACH comparing methods of weaning from prolonged ventilation, we found that 45% of 500 enrolled patients were alive 1 year after discharge; factors contributing to their survival are poorly understood (1, 2, 9–11). Survivors are expected to have profound muscle weakness (12–15), but its impact on functional recovery has not been investigated. Because long-term outcome may be poor and the weaning process daunting to patients (16, 17), some wonder whether it is worthwhile to provide prolonged ventilation (18). Although this negative perception seems to be widespread, direct data on patient QOL and a patient’s own assessment of the ordeal are limited.
Accordingly, we prospectively followed patients between arrival at an LTACH to 1 year after discharge (as part of our original clinical trial) to assess the effects of prolonged ventilation on survival, muscle function, and its impact on QOL. We also assessed patient willingness to undergo another episode of prolonged ventilation. To minimize dropouts, postdischarge assessments were performed predominantly at patient residences.
Methods
Setting
The study was conducted between 2003 and 2010 at RML Specialty Hospital, a free-standing LTACH, and the patient’s place of residence 6 and 12 months after discharge (see the online supplement).
Patients
Of the 500 patients who were enrolled in our clinical trial comparing weaning methods in LTACH patients (see online supplement) (1), the last 315 consecutive patients were invited to participate in a 1-year outcome study (see Figure E1 in the online supplement). Inclusion/exclusion criteria have been published (1). Patients were considered ventilator-detached if they could breathe without ventilator assistance at LTACH discharge. Patients were considered ventilator-attached if they required ventilator support at LTACH discharge. The study was approved by the institutional review board and informed consent obtained from patients/surrogates.
Measurements
Respiratory muscle strength was assessed by measuring maximum inspiratory pressure (Pimax) generated against an occluded airway at residual volume (19). In nontracheotomized patients, Pimax was measured through the mouth using a nose-clip and flanged mouthpiece (20). In tracheotomized patients, Pimax was measured through either the tracheostomy stoma or mouth (see online supplement) (19, 21–23).
Handgrip strength was assessed using a dynamometer while patients were seated upright (see online supplement) (24). Values are expressed as kilopascals.
Health-related QOL was assessed using the Medical-Outcomes Study 36-item Short-Form General-Health Survey (SF-36) (25). SF-36 measures QOL in eight domains; scores in each domain range from 0 (worst) to 100 (best) (see online supplement) (26). Domain scores were combined into two summary (physical/mental) scores, and compared with normative data, standardized to a mean of 50 ± 10 (SD).
Functional status was assessed by patient ability to perform six daily tasks using Katz Activities-of-Daily-Living questionnaire (27). Scores for each task are aggregated to generate a sum score (range, 0 to 18); sum score less than or equal to six indicates independence (see online supplement).
Preference about mechanical ventilation
Patients were asked if they remembered having shortness of breath, nightmares, or problems communicating during weaning. Patients were then asked: “If necessary, would you go through the process of being put on a ventilator and everything that happened afterward again, if it was necessary to save your life? In other words, was it all worth it”?
Protocol
Enrollment
Pimax and handgrip strength were measured. Using Katz and SF-36 questionnaires, patients were asked to estimate functional status and QOL 2 weeks before hospitalization for critical illness (28, 29); this is considered preillness function (see online supplement). When patients were unable to answer, questionnaires were answered by surrogates (44.8% of assessments) (30).
Discharge
Pimax and handgrip strength were measured, and patients were asked to complete Katz and SF-36 questionnaires. When patients were unable to answer, questionnaires were answered by surrogates (see online supplement).
Follow-up procedure
Patients were contacted at 6 and 12 months after LTACH discharge to determine their status (survival and location). For patients living at home, a face-to-face visit was scheduled. During the visit, Pimax and handgrip strength were measured; SF-36, Katz, and preference-about-mechanical-ventilation questionnaires were completed by the patient. For patients who refused a home visit, a telephone interview was conducted and the previously mentioned questionnaires were administered. If successful contact was not achieved after three telephone attempts, questionnaires were mailed (see online supplement). For patients at an institution, a family member was contacted and asked if he/she was willing to fill out the questionnaires (see online supplement).
Statistical Analysis
Cox proportional hazards regression model adjusted for baseline characteristics was used to obtain hazard ratio (HR) of survival up to 12 months after discharge. Variables included in the model were tested for collinearity and no serious collinearity was identified among the variables (see online supplement) (31). The proportional hazards assumption was verified using Schoenfeld residuals. Kaplan-Meier curves were used to assess survival up to 12 months after discharge in ventilator-attached and ventilator-detached patients.
Primary analysis
Change in five primary outcomes (handgrip strength, Pimax, Katz summary score, SF-36 physical summary score, SF-36 mental summary score) over time were assessed separately using a mixed-effects model for repeated measures and first-order autoregressive-covariance structure (see online supplement) (32). In each model, patients were assigned as random effects; one of the five outcome variables were assigned as the dependent variable, and time was coded as an ordinal independent variable: this constitutes our primary analysis. Further details of analysis are included in the online supplement. Results are presented as estimated-marginal means with 95% confidence interval (CI). P values were two-sided: P less than 0.05 was considered statistically significant.
Missing data management
Table E2 shows frequency of missing outcomes. The missing-completely-at-random test (33) was significant, indicating that missing data did not occur completely at random (see online supplement).
To determine whether missing data compromised interpretation of our results, two sensitivity analyses were performed post hoc. First, the relationship between missingness of a covariate and patient characteristics at enrollment was investigated using logistic-regression (see online supplement). Then, the primary analysis was repeated with additional variables associated with missingness (see Table E3) added as adjusting independent variables. Second, primary analysis was repeated with imputed values to replace missing values (see online supplement).
Results
Of 315 patients included in the study (original cohort), 52 (16.5%) patients died between enrollment and discharge; 253 of 260 eligible patients (97.3%) were evaluated at discharge (see Figure E1). Between discharge and 6 months, an additional 115 patients died, and 118 of 139 eligible patients were evaluated at 6-month follow-up. Another 13 patients died before 12-month follow-up (cumulative mortality, 57.1%), and 108 of 126 eligible patients (34.3% of original cohort) were evaluated at 12 months (see Figure E1). Of 315 patients, 169 (53.7%) were detached from the ventilator and 94 (29.8%) were still attached to the ventilator at LTACH discharge (see Table E4); the remaining 16.5% died during the LTACH stay.
Long-Term Survival
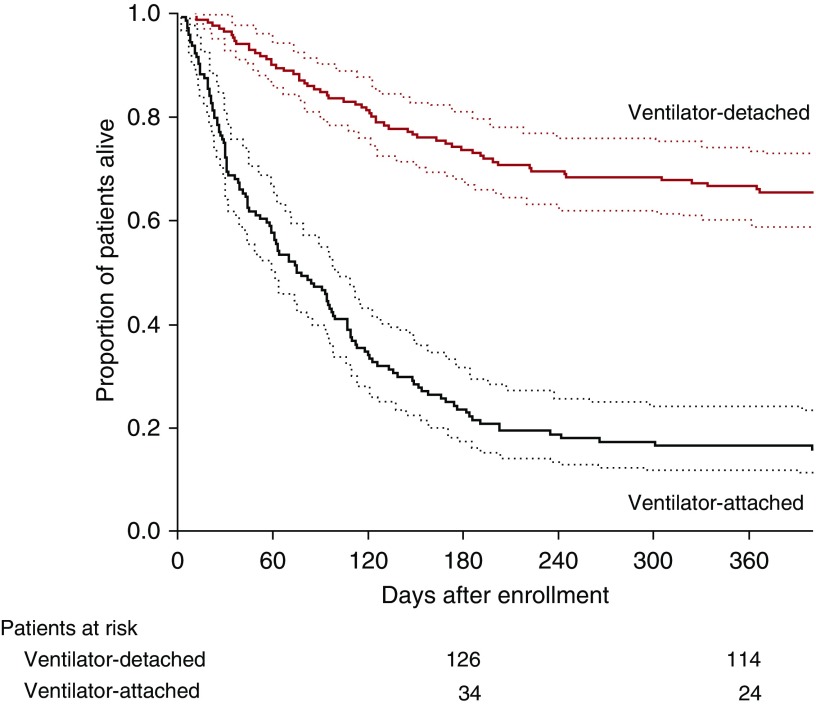
Table 1 shows the baseline characteristics of 12-month survivors versus nonsurvivors. Using a Cox proportional hazards model to adjust for baseline covariates, four variables were associated with 1-year survival: Simplified Acute Physiology Score II (HR, 1.03; 95% CI, 1.01–1.05; P = 0.0002), body mass index (HR, 0.97; 95% CI, 0.96–0.99; P = 0.004), Acute Physiology and Chronic Health Evaluation II (HR, 1.04; 95% CI, 1.00–1.09; P = 0.03), and being detached from the ventilator at discharge (HR, 0.31; 95% CI, 0.22–0.44; P < 0.0001) (see Table E5). Figure 1 shows a Kaplan-Meier plot of proportion alive in ventilator-detached patients and ventilator-attached patients up to 1 year after discharge. The 1-year survival was 66.9% for ventilator-detached patients and 16.4% for ventilator-attached patients.
Table 1.
Characteristics of Study Population at Enrollment according to Whether Patients Survived to 1 Year after Discharge from an LTACH
| Variable | 12-mo Survivors (n = 132) | Nonsurvivors (n = 183)* | P Value† |
|---|---|---|---|
| Age, yr, median (IQR) | 64 (56–71) | 74 (67–79) | <0.001 |
| Sex, F/M (% F) | 52/80 (39) | 69/114 (38) | 0.76 |
| Postoperative, n (%) | 62 (47) | 73 (40) | 0.21 |
| Acute lung injury,‡n (%) | 47 (36) | 64 (35) | 0.91 |
| Chronic obstructive pulmonary disease, n (%) | 7 (5) | 19 (10) | 0.11 |
| Neuromuscular, n (%) | 16 (12) | 27 (15) | 0.50 |
| SAPS II, median (IQR) | 23 (18–31) | 35 (27–42) | <0.001 |
| APACHE II, median (IQR) | 14 (10–16) | 17 (14–20) | <0.001 |
| Body mass index, kg/m2, median (IQR) | 30.3 (24.5–36.9) | 27.4 (23.1–32.8) | 0.007 |
| Albumin, mg/dl | 2.3 (2.0–2.5) | 2.1 (1.8–2.4) | 0.001 |
| Variables measured at enrollment | |||
| Pimax, cm H2O, median (IQR) | 42 (36–54) | 38 (24–44) | 0.003 |
| Handgrip strength, kPa, median (IQR) | 18 (0–30) | 14 (0–25) | 0.01 |
| Preillness status | |||
| Katz summary score, mean (95% CI) | 2.2 (1.5–3.0) | 3.2 (2.5–4.0) | 0.07 |
| SF-36 physical summary score, median (IQR) | 39 (31–47) | 34 (26–41) | <0.001 |
| SF-36 mental summary score, median (IQR) | 50 (45–57) | 50.5 (41–57) | 0.56 |
| Duration of mechanical ventilation | |||
| At randomization,§ d, median (IQR) | 26 (21–35) | 29 (21–40) | 0.15 |
| At LTACH, || d, median (IQR) | 12 (5–27) | 32 (12–51) | <0.001 |
| Length of stay at LTACH,¶ d, median (IQR) | 33 (23.8–47) | 40 (27–55) | 0.006 |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; CI = confidence interval; IQR = interquartile range; LTACH = long-term acute-care hospital; Pimax = maximum inspiratory pressure; SAPS = Simplified Acute Physiology Score; SF-36 = Medical-Outcomes Study 36-item Short-Form General-Health Survey.
Three of the patients who withdrew at 6 months died before 12 months.
The Wilcoxon rank sum test was used for continuous variables, and the chi-square test was used for categorical variables.
Patients were categorized as having acute lung injury if they had pneumonia or pulmonary edema as the precipitating cause of respiratory failure.
Duration of ventilation at randomization was calculated from the day of intubation at the ICU to the day the patient was randomized at the LTACH.
Duration of ventilation at the LTACH was calculated from the day of admission to the LTACH to the last day that the patient was attached to the ventilator at the LTACH.
Length of stay at LTACH was calculated from the day of admission to the LTACH to the day the patient was discharged from the LTACH.
Figure 1.
Proportion of patients alive in the ventilator-detached group and ventilator-attached group. Dotted lines represent 95% confidence intervals.
Muscle Strength
Respiratory muscle strength
Between admission and discharge, Pimax did not change, 41.3 (95% CI, 39.4–43.2; 53.1% predicted [34]) versus 42.9 (95% CI, 40.9–44.9) cm H2O (55.9% predicted). Pimax increased to 54.4 (95% CI, 51.2–57.6) cm H2O (67.5% predicted) at 6 months (P < 0.001) and did not change at 12 months, 58.0 (95% CI, 54.5–61.6) cm H2O (70.1% predicted) (Table 2).
Table 2.
Mixed-Effects Model (Primary Analysis)
| Estimated Marginal Means (95% Confidence Interval)* |
|||||||
|---|---|---|---|---|---|---|---|
| Outcome Variables | Enrollment | Discharge | P Value† | 6 mo | P Value‡ | 12 mo | P Value§ |
| Pimax, cm H2O | 41.3 (39.4–43.2) (n = 306) | 42.9 (40.9–44.9) (n = 249) | 0.61 | 54.4 (51.2–57.6) (n = 80) | <0.001 | 58.0 (54.5–61.6) (n = 77) | <0.001 |
| Handgrip strength, kPa | 16.4 (14.1–18.7) (n = 308) | 22.1 (19.7–24.5) (n = 251) | <0.001 | 58.6 (55.0–62.1) (n = 84) | <0.001 | 62.5 (58.4–66.5) (n = 79) | <0.001 |
| Katz summary score | 2.8 (2.2–3.3)|| (n = 301) | 14.6 (13.9–15.2) (n = 227) | <0.001 | 5.2 (4.3–6.0) (n = 118) | <0.001 | 4.7 (3.8–5.6) (n = 108) | 0.002 |
| SF-36 physical summary score | 36.1 (35.0–37.3)|| (n = 299) | 24.0 (22.7–25.3) (n = 225) | <0.001 | 33.2 (31.4–35.0) (n = 117) | <0.001 | 36.5 (34.6–38.5) (n = 108) | 0.99 |
| SF-36 mental summary score | 49.1 (47.9–50.3)|| (n = 299) | 46.8 (45.5–48.2) (n = 225) | 0.02 | 52.5 (50.6–54.3) (n = 117) | <0.001 | 51.9 (49.9–53.9) (n = 108) | 0.11 |
Definition of abbreviations: Pimax = maximum inspiratory pressure; SF-36 = Medical-Outcomes Study 36-item Short-Form General-Health Survey.
Estimated marginal means derived from the mixed-effects model for repeated measures.
Comparison between enrollment and discharge.
Comparison between discharge and 6 months.
Comparison between enrollment and 12 months.
Preillness value.
Handgrip strength
Between admission and discharge, handgrip strength increased from 16.4 (95% CI, 14.1–18.7) kPa (21.5% predicted [35]) to 22.1 (95% CI, 19.7–24.5) kPa (29.1% predicted) (P < 0.001). Handgrip strength increased to 58.6 (95% CI, 55.0–62.1) kPa (81.0% predicted) at 6 months (P < 0.001) and did not change at 12 months, 62.5 (95% CI, 58.4–66.5) kPa (85.9% predicted) (Table 2).
Functional Status and QOL
Functional status
Before critical illness, Katz summary score for entire cohort was 2.8 (95% CI, 2.3–3.3). At discharge, the score was 14.6 (95% CI, 13.9–15.2) (requiring full assistance). Katz summary score at 6 months decreased to 5.2 (95% CI, 4.3–6.0; independent or requiring special equipment; P < 0.001) and remained unchanged at 12-months, 4.7 (95% CI, 3.8–5.6) (Table 2). Katz summary score correlated with handgrip strength at 6 (Spearman r = −0.51; P < 0.001) and 12 months (r = −0.52; P < 0.001), indicating that patients with objective muscle weakness were less capable of performing daily activities. A weaker correlation was observed between Katz summary score and Pimax at 6 (r = −0.27; P = 0.02) and 12 months (r = −0.22; P = 0.06). Percentage of patients needing assistance in performing daily activities (Katz summary score >6) was 16.7% at preillness, 95.6% at discharge, 22.0% at 6 months, and 25.9% at 6 months (Table 3).
Table 3.
Independence in Daily Activities before Illness, at Discharge, and 6 and 12 Months after Discharge from an LTACH
| Preillness (n = 301) | Discharge (n = 227) | 6 mo (n = 118) | 12 mo (n =108) | |
|---|---|---|---|---|
| Bathing, n (%) | ||||
| No help | 208 (69) | 6 (3) | 64 (54) | 60 (55) |
| Special equipment | 35 (12) | 4 (2) | 22 (19) | 17 (16) |
| Partly assisted | 24 (8) | 19 (8) | 6 (5) | 12 (11) |
| Assisted | 34 (11) | 198 (87) | 26 (22) | 19 (18) |
| Dressing, n (%) | ||||
| No help | 231 (77) | 9 (4) | 82 (70) | 75 (69) |
| Special clothes | 5 (2) | 6 (3) | 5 (4) | 3 (3) |
| Assistance in tying shoes | 24 (8) | 11 (5) | 5 (4) | 3 (3) |
| Other assistance | 41 (13) | 201 (88) | 26 (22) | 27 (25) |
| Toileting, n (%) | ||||
| Normal | 217 (72) | 5 (2) | 68 (58) | 61 (57) |
| Special equipment | 45 (15) | 17 (8) | 25 (21) | 26 (24) |
| Assistance | 27 (9) | 35 (15) | 5 (4) | 9 (8) |
| Not possible to go to bathroom | 12 (4) | 170 (75) | 20 (17) | 12 (11) |
| Transfer, n (%) | ||||
| Normal | 225 (75) | 8 (4) | 71 (60) | 69 (64) |
| Special equipment | 37 (12) | 17 (7) | 20 (17) | 13 (12) |
| Help | 29 (10) | 146 (65) | 14 (12) | 19 (18) |
| Does not get out of bed | 10 (3) | 55 (24) | 13 (11) | 7 (6) |
| Continence, n (%) | ||||
| Normal | 251 (83) | 30 (13) | 83 (70) | 79 (73) |
| Special medications | 5 (2) | 7 (3) | 4 (4) | 2 (2) |
| Occasional accidents | 28 (9) | 10 (5) | 11 (9) | 15 (14) |
| Help | 17 (6) | 180 (79) | 20 (17) | 12 (11) |
| Feeding, n (%) | ||||
| Normal | 258 (86) | 44 (19) | 95 (80) | 89 (82) |
| Special equipment | 7 (2) | 19 (8) | 5 (4) | 2 (2) |
| Little help with cutting and buttering | 14 (5) | 88 (39) | 4 (4) | 5 (5) |
| Help | 22 (7) | 76 (34) | 14 (12) | 12 (11) |
Definition of abbreviation: LTACH = long-term acute-care hospital.
Quality-of-life
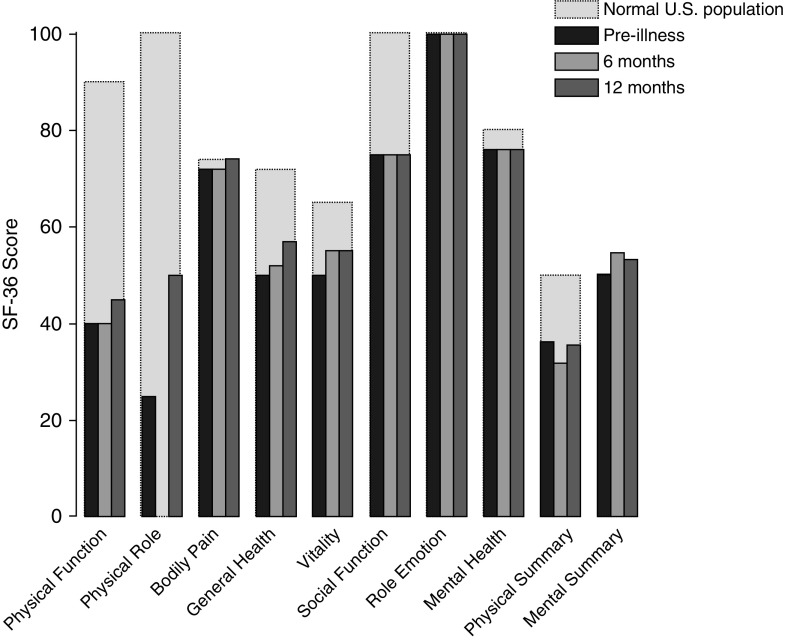
Figure 2 shows scores of domains of SF-36 questionnaire at preillness and 6 and 12 months after discharge. Before critical illness, physical summary score was 36.1 (95% CI, 35.0–37.3; normal, 50). At discharge, score was 24.0 (95% CI, 22.7–25.3). Between discharge and 6 months, score increased to 33.2 (95% CI, 31.4–35.0; P < 0.001) and remained unchanged at 12 months (36.5; 95% CI, 34.6–38.5) (Table 2). Physical summary score correlated with handgrip strength at 6 (r = 0.46; P < 0.001) and 12 months (r = 0.47; P < 0.001). Physical summary score correlated with Pimax at 6 (r = 0.45; P < 0.001) and 12 months (r = 0.34; P = 0.002).
Figure 2.
Medical-Outcomes Study 36-item Short-Form General-Health Survey (SF-36) at preillness (n = 299), 6 months (n = 117), and 12 months (n = 108) after discharge in long-term acute-care hospital patients compared with U.S. general population. Preillness score for all domains of the SF-36 questionnaire were below those of age- and sex-matched U.S. population except for emotional role, which was similar to published norms (25). At 6 months, all domains returned to preillness values except for physical role, which was zero at 6 months. Bars represent median value.
Before critical illness, mental summary score was 49.1 (95% CI, 47.9–50.3; normal, 50). At discharge, score was 46.8 (95% CI, 45.5–48.2). Between discharge and 6 months, score increased to 52.5 (95% CI, 50.6–54.3; P < 0.001) and remained unchanged at 12 months (51.9; 95% CI, 49.9–53.9) (Table 2).
Preference about Mechanical Ventilation
Patients were asked about their weaning experience 6 and 12 months after discharge. Because responses were equivalent at both times, only 6-month data are presented. Of 118 patients (79.7% of survivors; 37% of original cohort) interviewed, 34.5% recalled shortness of breath, 38.8% reported problems communicating with staff/family, and 8.6% recalled nightmares.
When asked (6 mo after discharge) whether they would go through the process of mechanical ventilation again, 100 (84.7%) answered yes, 10 answered no, and eight were unsure. Compared with patients who would be willing to undergo ventilation again, patients not willing had lower SF-36 physical (28.2 ± 8.3 vs. 34.4 ± 11.2; P = 0.04) and mental (47.8 ± 13.7 vs. 53.3 ± 10.1; P = 0.06) summary scores at 6-month follow-up.
Sensitivity and Subgroup Analysis
Reanalyzing data to include variables associated with missingness in mixed-effects models did not change main findings (see Table E6). Reanalyzing data with imputed values substituted for missing values yielded results similar to the primary analysis (see Table E7). To ensure that data loss consequent to death did not influence the results, pairwise comparison of the raw data was performed using paired Student’s t tests. Differences obtained with Student’s t tests (which include only alive patients with paired data) and differences obtained with the mixed-model analysis at the specified time-point for the five outcomes were similar (see Table E8).
Potential confounders that could have influenced the results were randomization status and weaning method (see online supplement). Reanalyzing data to include potential confounders in mixed-effects models showed that interpretation of the main findings remained the same regardless of randomization status and weaning method (see online supplement).
Discussion
Studies on impact of short-term (median, 9-d) ventilation on outcome in ICU survivors have been conducted (15, 36–42), but one cannot extrapolate from short-term ventilated patients to LTACH patients who require prolonged ventilation (median, 53-d): the clinical picture and experience of the two groups differ substantially (13). This is the first study to provide longitudinal measurements of survival, muscle function, and QOL evaluation in LTACH patients. At discharge, 53.7% of patients were detached from the ventilator and their 1-year survival was 66.9%. Between admission and discharge from LTACH, Pimax did not change, whereas handgrip strength increased. Between discharge and 6-month follow-up, handgrip strength increased by 165.2% and Pimax increased by 26.8%. At 6-month follow-up, 78.0% of patients were able to perform daily activities without assistance (Katz summary score ≤6); improvement in Katz summary score was related to improvement in handgrip strength. When LTACH survivors were asked if they would be willing to undergo prolonged ventilation again if deemed necessary, 84.7% said they would be willing.
Some clinicians perceive that prognosis of patients requiring prolonged mechanical ventilation is poor. That perception is accurate regarding patients who remain attached to a ventilator after repeated weaning attempts in an LTACH: only 16.4% of ventilator-attached patients were alive 1 year after study enrollment. Conversely, survival of patients detached from the ventilator during their LTACH stay was 66.9%, similar to survival of patients who receive short-term ventilation in an ICU (64.6%) (36, 38, 40, 43). Particularly striking was the high proportion of patients admitted to our LTACH who were successfully detached from the ventilator: 53.7%. The better-than-expected outcome among patients (albeit patients enrolled in a clinical trial) underscores the need for clinicians to alter their mindset about the management of patients receiving prolonged ventilation. Instead of limiting (or abandoning) weaning efforts based on perceived poor prognosis, clinicians should adopt a more aggressive approach and evaluate patient performance in the complete absence of ventilator assistance (trach-collar trials or T-tube trials), which facilitates earlier ventilator discontinuation (1). Such an approach minimizes the risk of failing to identify patients who can be detached from the ventilator (44).
The possibility that mechanical ventilation induces respiratory muscle atrophy and injury is arousing much interest (45–48). If any group of patients is at risk for this disorder, it should be patients requiring prolonged ventilation (47). Our ventilator-attached patients received mechanical ventilation for 46.2 days at the LTACH, yet Pimax did not decrease between admission and discharge (36.2 ± 1.0 vs. 35.2 ± 1.3 cm H2O). These novel data signify that ventilator-induced diaphragmatic dysfunction did not ensue during the LTACH stay. Moreover, if respiratory muscle strength was an important factor for ventilator detachment, one would have expected Pimax to increase over time in ventilator-detached patients. In reality, Pimax did not change between admission and discharge (45.4 ± 1.3 vs. 48.1 ± 1.3 cm H2O), indicating that an increase in respiratory muscle strength was not an important determinant of ventilator detachment (47). The latter observation is in accordance with our findings in ventilated patients in an ICU setting; Pimax was not higher in weaning-success versus weaning-failure patients (46.3 ± 3.1 vs. 41.6 ± 5.3 cm H2O) (19). In another experimental study, we showed that direct neurophysiologic measurement of diaphragmatic contractility (achieved by invasive measurements of transdiaphragmatic pressure in response to phrenic-nerve stimulation) was equivalent in weaning-failure and weaning-success patients: 8.9 ± 2.2 and 10.3 ± 1.5 cm H2O (49). That Pimax was lower in the ventilator-attached patients than in the ventilator-detached patients suggests that respiratory muscle weakness may have been a factor in why some patients remained attached to the ventilator.
When patients arrived at the LTACH, Pimax was considerably higher than handgrip strength: 53.1% versus 21.5% predicted. The better preservation of respiratory versus limb-muscle strength probably arose because respiratory muscles were contracting around-the-clock (for ventilator triggering) from the time ventilation had been instituted in the home ICU (50). In contrast, limb muscles were almost completely inactive throughout this period and predisposed to development of atrophy and weakness (51).
In contrast to Pimax, limb-muscle strength increased during LTACH stay. One explanation for the increase was very low handgrip strength on arrival, which afforded greater opportunity for improvement as compared with Pimax. Another contributor was the nature of rehabilitation. Professional therapists performed whole-body exercises (3–5 times/wk) specifically designed toward strengthening limb muscles. In contrast, rehabilitation specifically targeting inspiratory muscles was not provided (20).
At 6 months, handgrip strength increased by 165.2%, reaching near-normal values (81.0% predicted). The increase in muscle strength may reflect the action of rehabilitation therapy received in acute or subacute rehabilitation facilities following discharge. In addition to increase in size of muscle fibers and enhanced contractility, increases in recruitment of muscle units and motor neuron firing rate may also have contributed to the increase in handgrip strength (52, 53). Psychological factors modulate muscle recruitment during voluntary contractions (54, 55). Patient mental well-being (SF-36 mental summary score) increased significantly between discharge and 6-month follow-up, suggesting a link between enhanced voluntary activation and increase in handgrip strength.
Pimax increased by 26.8% at 6 months, reaching 67.5% predicted, well above the threshold (35% predicted) shown to induce dyspnea or impede physical performance (56). When patients were asked at 6 months whether health impeded walking, climbing stairs, or carrying groceries, more than 70% said they were not limited (or only a little). Near maximum recovery of global muscle function by 6 months left little opportunity for further increase over ensuing follow-up, as reflected by lack of change in Pimax and handgrip strength between 6 and 12 months.
Functional status improved considerably after discharge. At 6 months, 78% of patients were functionally independent (Katz summary score ≤6) and SF-36 physical summary score was 92.0% of score before illness. Improvement in functional status likely resulted from improvements in skeletal-muscle function, as reflected by corresponding increases in handgrip strength. Support that muscle strength mediated functional recovery is the close correlations between handgrip strength and Katz summary score (r = −0.51) and SF-36 physical summary score (r = 0.46) at 6 months. Functional recovery in our patients was better than that reported in patients requiring prolonged ventilation (duration, 27 d) who were managed in an ICU (57); whole-body rehabilitation and the focus on weaning at the LTACH most likely attributed to the more favorable outcome.
The physical summary score at 1-year follow-up was similar to the score in acute-lung-injury survivors (37, 58). Given longer duration of ventilation and bed rest in our patients than in ICU-ventilated patients, one might expect our patients to exhibit lesser recovery at 1 year (15, 59, 60). Our patients were physically limited before acute illness (physical summary score, 36.1 at baseline) consequent to age and comorbidities (61). Investigators have assumed normal physical function at baseline in ICU-survivor studies (score, 50) (62). Accordingly, return to baseline required smaller improvement in functional status in our patients than in ICU survivors.
An early criticism of LTACHs was the existential objection that patient misery related to prolonged ventilation (16, 43, 63) (repeated experiences of severe dyspnea consequent to unremitting failed-weaning attempts) (64) entailed that patients would not volunteer for the ordeal if they fully understood what it involved. In reality, 84.7% of our patients said they would be willing to undergo the experience again if deemed necessary.
Nobelist Kahneman (65) notes that humans have two types of self: an experiencing-self, which addresses “Is it uncomfortable now?” and a remembering-self, which addresses “How was the overall experience?” Kahneman (65) concludes that “Memories are all we get to keep from our experience of living, and the only perspective that we can adopt as we think about our lives.” It is the remembering-self that makes decisions. Contrary to presupposition of caregivers, patients are poor at remembering duration of an unpleasant experience. Indeed, two-thirds of patients did not have unpleasant memories of time on the ventilator, explaining why 84.7% would be willing to undergo a further episode of prolonged ventilation.
This study has limitations. Assessment of muscle strength before illness was impossible because critical illness is unpredictable. Almost half (45%) of preillness QOL was estimated from surrogates because patients were too ill to fill out the questionnaires themselves. To determine if using surrogates influenced study outcome, the mixed-effect model was recomputed to include the source of the responder (patient or surrogate) as an additional independent variable (see online supplement). The use of surrogates had a significant effect on Katz summary score: preillness score was higher (lower function) in the surrogate-responder group than in the patient-responder group (see Table E9). The surrogate-responder group were sicker than the patient-responder group, which could account for higher Katz scores; an overestimation of patient functional dependence by the surrogate, however, cannot be excluded (see online supplement) (66). Muscle-strength assessments after discharge were completed in 60% of survivors. The main reason for missing measurements was inability to achieve face-to-face encounters. Sensitivity analyses revealed that missing data did not bias our results (see Tables E6 and E7). The study took 10 years to complete, which may have influenced the results. The study was conducted in a single LTACH as part of a clinical trial; thus, it is possible that our findings may not be generalizable to patients requiring prolonged ventilation at other settings. A prerequisite for generalizability (external validity) is sound internal validity (67). The major obstacle to internal validity is systematic error, which can be more carefully controlled in a single center where selection and patient care is uniform. That the ventilator-detachment rate at our LTACH was virtually identical to that reported in a study conducted in 23 LTACHs (54.1%) (7) supports the likelihood that our results are generalizable to other LTACHs.
In conclusion, two-thirds of patients who were detached from the ventilator at discharge from the LTACH were alive 1 year later. Respiratory muscle strength was well maintained in patients being weaned from prolonged ventilation at a LTACH, whereas peripheral strength was severely impaired. Six months after discharge, improvement in peripheral muscle function was associated with an increased ability to perform daily activities independently, and 84.7% indicated willingness to undergo ventilation again if deemed necessary.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the Pulmonary and Critical Care Medicine fellows of Loyola University Stritch School of Medicine and the medical staff at RML Hospital for their assistance with recruitment; Jay Shannon, M.D. (CEO, Cook County Health, Chicago, IL), for serving as the off-site safety monitor and the Institutional Review Board of RML Hospital for serving as the on-site safety monitors. None received compensation beyond their normal salaries.
Footnotes
Supported by the National Institute of Nursing Research (R01-NR008782).
Author Contributions: A.J. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A.J., L.A.H., and M.J.T. contributed to the conception and design of the study. L.A.D. contributed to data acquisition. B.J.B.G. conducted statistical analysis. A.J., B.J.B.G., L.A.D., E.G.C., D.M.L., L.A.H., and M.J.T. interpreted the data. A.J. and M.J.T. drafted the manuscript, and all authors have provided critical revision for important intellectual content. All authors have read and approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201806-1131OC on January 9, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jubran A, Grant BJ, Duffner LA, Collins EG, Lanuza DM, Hoffman LA, et al. Effect of pressure support vs unassisted breathing through a tracheostomy collar on weaning duration in patients requiring prolonged mechanical ventilation: a randomized trial. JAMA. 2013;309:671–677. doi: 10.1001/jama.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn JM, Carson SS. Generating evidence on best practice in long-term acute care hospitals. JAMA. 2013;309:719–720. doi: 10.1001/jama.2013.848. [DOI] [PubMed] [Google Scholar]
- 3.Dres M, Mancebo J, Curley GF. Update in critical care 2015. Am J Respir Crit Care Med. 2016;194:19–25. doi: 10.1164/rccm.201602-0339UP. [DOI] [PubMed] [Google Scholar]
- 4.Carson SS, Bach PB, Brzozowski L, Leff A. Outcomes after long-term acute care: an analysis of 133 mechanically ventilated patients. Am J Respir Crit Care Med. 1999;159:1568–1573. doi: 10.1164/ajrccm.159.5.9809002. [DOI] [PubMed] [Google Scholar]
- 5.Polverino E, Nava S, Ferrer M, Ceriana P, Clini E, Spada E, et al. Patients’ characterization, hospital course and clinical outcomes in five Italian respiratory intensive care units. Intensive Care Med. 2010;36:137–142. doi: 10.1007/s00134-009-1658-2. [DOI] [PubMed] [Google Scholar]
- 6.Medicare Payment Advisory Commission. Report to the Congress: Medicare payment policy. Washington, DC: Medicare Payment Advisory Commission; 2017. [Google Scholar]
- 7.Scheinhorn DJ, Hassenpflug MS, Votto JJ, Chao DC, Epstein SK, Doig GS, et al. Ventilation Outcomes Study Group. Post-ICU mechanical ventilation at 23 long-term care hospitals: a multicenter outcomes study. Chest. 2007;131:85–93. doi: 10.1378/chest.06-1081. [DOI] [PubMed] [Google Scholar]
- 8.Bigatello LM, Stelfox HT, Berra L, Schmidt U, Gettings EM. Outcome of patients undergoing prolonged mechanical ventilation after critical illness. Crit Care Med. 2007;35:2491–2497. doi: 10.1097/01.CCM.0000287589.16724.B2. [DOI] [PubMed] [Google Scholar]
- 9.Carson SS, Kahn JM, Hough CL, Seeley EJ, White DB, Douglas IS, et al. ProVent Investigators. A multicenter mortality prediction model for patients receiving prolonged mechanical ventilation. Crit Care Med. 2012;40:1171–1176. doi: 10.1097/CCM.0b013e3182387d43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schönhofer B, Euteneuer S, Nava S, Suchi S, Köhler D. Survival of mechanically ventilated patients admitted to a specialised weaning centre. Intensive Care Med. 2002;28:908–916. doi: 10.1007/s00134-002-1287-5. [DOI] [PubMed] [Google Scholar]
- 11.Herridge MS. Fifty years of research in ARDS: long-term follow-up after acute respiratory distress syndrome. Insights for managing medical complexity after critical illness. Am J Respir Crit Care Med. 2017;196:1380–1384. doi: 10.1164/rccm.201704-0815ED. [DOI] [PubMed] [Google Scholar]
- 12.Azoulay E, Vincent JL, Angus DC, Arabi YM, Brochard L, Brett SJ, et al. Recovery after critical illness: putting the puzzle together—a consensus of 29. Crit Care. 2017;21:296. doi: 10.1186/s13054-017-1887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. 2010;182:446–454. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, et al. Groupe de Réflexion et d’Etude des Neuromyopathies en Réanimation. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 15.Herridge MS. Long-term outcomes after mechanical ventilation. In: Tobin MJ, editor. Principles and practice of mechanical ventilation. New York, NY: McGraw-Hill; 2013. pp. 1501–1516. [Google Scholar]
- 16.Jubran A, Lawm G, Duffner LA, Collins EG, Lanuza DM, Hoffman LA, et al. Post-traumatic stress disorder after weaning from prolonged mechanical ventilation. Intensive Care Med. 2010;36:2030–2037. doi: 10.1007/s00134-010-1972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotondi AJ, Chelluri L, Sirio C, Mendelsohn A, Schulz R, Belle S, et al. Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. 2002;30:746–752. doi: 10.1097/00003246-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Schneiderman LJ, Spragg RG. Ethical decisions in discontinuing mechanical ventilation. N Engl J Med. 1988;318:984–988. doi: 10.1056/NEJM198804143181509. [DOI] [PubMed] [Google Scholar]
- 19.Jubran A, Tobin MJ. Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am J Respir Crit Care Med. 1997;155:906–915. doi: 10.1164/ajrccm.155.3.9117025. [DOI] [PubMed] [Google Scholar]
- 20.Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168:10–48. doi: 10.1164/rccm.2206020. [DOI] [PubMed] [Google Scholar]
- 21.Jubran A, Van de Graaff WB, Tobin MJ. Variability of patient-ventilator interaction with pressure support ventilation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152:129–136. doi: 10.1164/ajrccm.152.1.7599811. [DOI] [PubMed] [Google Scholar]
- 22.Parthasarathy S, Jubran A, Laghi F, Tobin MJ. Sternomastoid, rib cage, and expiratory muscle activity during weaning failure. J Appl Physiol (1985) 2007;103:140–147. doi: 10.1152/japplphysiol.00904.2006. [DOI] [PubMed] [Google Scholar]
- 23.Vitacca M, Paneroni M, Bianchi L, Clini E, Vianello A, Ceriana P, et al. Maximal inspiratory and expiratory pressure measurement in tracheotomised patients. Eur Respir J. 2006;27:343–349. doi: 10.1183/09031936.06.00002705. [DOI] [PubMed] [Google Scholar]
- 24.Laghi F, Langbein WE, Antonescu-Turcu A, Jubran A, Bammert C, Tobin MJ. Respiratory and skeletal muscles in hypogonadal men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:598–605. doi: 10.1164/rccm.200412-1643OC. [DOI] [PubMed] [Google Scholar]
- 25.Needham DM, Sepulveda KA, Dinglas VD, Chessare CM, Friedman LA, Bingham CO, III, et al. Core outcome measures for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Am J Respir Crit Care Med. 2017;196:1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 27.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the index of ADL. A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 28.Montuclard L, Garrouste-Orgeas M, Timsit JF, Misset B, De Jonghe B, Carlet J. Outcome, functional autonomy, and quality of life of elderly patients with a long-term intensive care unit stay. Crit Care Med. 2000;28:3389–3395. doi: 10.1097/00003246-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Cuthbertson BH, Wunsch H. Long-term outcomes after critical illness: the best predictor of the future is the past. Am J Respir Crit Care Med. 2016;194:132–134. doi: 10.1164/rccm.201602-0257ED. [DOI] [PubMed] [Google Scholar]
- 30.Pol MC, Buurman BM, de Vos R, de Rooij SE. Patient and proxy rating agreements on activities of daily living and the instrumental activities of daily living of acutely hospitalized older adults. J Am Geriatr Soc. 2011;59:1554–1556. doi: 10.1111/j.1532-5415.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 31.Glantz SA, Slinker BK, Neilands TB. Primer of applied regression & analysis of variance. New York, NY: McGraw-Hill; 2016. [Google Scholar]
- 32.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 33.Little RJ, D’Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367:1355–1360. doi: 10.1056/NEJMsr1203730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enright PL, Kronmal RA, Manolio TA, Schenker MB, Hyatt RE Cardiovascular Health Study Research Group. Respiratory muscle strength in the elderly: correlates and reference values. Am J Respir Crit Care Med. 1994;149:430–438. doi: 10.1164/ajrccm.149.2.8306041. [DOI] [PubMed] [Google Scholar]
- 35.Desrosiers J, Bravo G, Hebert R, Dutil E. Normative data for grip strength of elderly men and women. Am J Occup Ther. 1995;49:637–644. doi: 10.5014/ajot.49.7.637. [DOI] [PubMed] [Google Scholar]
- 36.Chelluri L, Im KA, Belle SH, Schulz R, Rotondi AJ, Donahoe MP, et al. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med. 2004;32:61–69. doi: 10.1097/01.CCM.0000098029.65347.F9. [DOI] [PubMed] [Google Scholar]
- 37.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 38.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 39.Needham DM, Dinglas VD, Morris PE, Jackson JC, Hough CL, Mendez-Tellez PA, et al. NIH NHLBI ARDS Network. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding: EDEN trial follow-up. Am J Respir Crit Care Med. 2013;188:567–576. doi: 10.1164/rccm.201304-0651OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Douglas SL, Daly BJ, Gordon N, Brennan PF. Survival and quality of life: short-term versus long-term ventilator patients. Crit Care Med. 2002;30:2655–2662. doi: 10.1097/00003246-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Dinglas VD, Aronson Friedman L, Colantuoni E, Mendez-Tellez PA, Shanholtz CB, Ciesla ND, et al. Muscle weakness and 5-year survival in acute respiratory distress syndrome survivors. Crit Care Med. 2017;45:446–453. doi: 10.1097/CCM.0000000000002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herridge MS, Chu LM, Matte A, Tomlinson G, Chan L, Thomas C, et al. RECOVER Program Investigators (Phase 1: towards RECOVER); Canadian Critical Care Trials Group. The RECOVER program: disability risk groups and 1-year outcome after 7 or more days of mechanical ventilation. Am J Respir Crit Care Med. 2016;194:831–844. doi: 10.1164/rccm.201512-2343OC. [DOI] [PubMed] [Google Scholar]
- 44.Tobin MJ, Jubran A. Weaning from mechanical ventilation. In: Tobin MJ, editor. Principles and practice of mechanical ventilation. 3rd ed. New York, NY: McGraw-Hill; 2013. pp. 1307–1351. [Google Scholar]
- 45.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 46.Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;170:626–632. doi: 10.1164/rccm.200401-042OC. [DOI] [PubMed] [Google Scholar]
- 47.Tobin MJ, Laghi F, Jubran A. Ventilatory failure, ventilator support, and ventilator weaning. Compr Physiol. 2012;2:2871–2921. doi: 10.1002/cphy.c110030. [DOI] [PubMed] [Google Scholar]
- 48.Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. 2018;197:204–213. doi: 10.1164/rccm.201703-0536OC. [DOI] [PubMed] [Google Scholar]
- 49.Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, et al. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med. 2003;167:120–127. doi: 10.1164/rccm.200210-1246OC. [DOI] [PubMed] [Google Scholar]
- 50.Leung P, Jubran A, Tobin MJ. Comparison of assisted ventilator modes on triggering, patient effort, and dyspnea. Am J Respir Crit Care Med. 1997;155:1940–1948. doi: 10.1164/ajrccm.155.6.9196100. [DOI] [PubMed] [Google Scholar]
- 51.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 52.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 53.Laghi F, Shaikh HS, Morales D, Sinderby C, Jubran A, Tobin MJ. Diaphragmatic neuromechanical coupling and mechanisms of hypercapnia during inspiratory loading. Respir Physiol Neurobiol. 2014;198:32–41. doi: 10.1016/j.resp.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Allen GM, Hickie I, Gandevia SC, McKenzie DK. Impaired voluntary drive to breathe: a possible link between depression and unexplained ventilatory failure in asthmatic patients. Thorax. 1994;49:881–884. doi: 10.1136/thx.49.9.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rantanen T, Penninx BW, Masaki K, Lintunen T, Foley D, Guralnik JM. Depressed mood and body mass index as predictors of muscle strength decline in old men. J Am Geriatr Soc. 2000;48:613–617. doi: 10.1111/j.1532-5415.2000.tb04717.x. [DOI] [PubMed] [Google Scholar]
- 56.Hamilton AL, Killian KJ, Summers E, Jones NL. Muscle strength, symptom intensity, and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med. 1995;152:2021–2031. doi: 10.1164/ajrccm.152.6.8520771. [DOI] [PubMed] [Google Scholar]
- 57.Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010;153:167–175. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 59.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42:849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Needham DM, Wozniak AW, Hough CL, Morris PE, Dinglas VD, Jackson JC, et al. National Institutes of Health NHLBI ARDS Network. Risk factors for physical impairment after acute lung injury in a national, multicenter study. Am J Respir Crit Care Med. 2014;189:1214–1224. doi: 10.1164/rccm.201401-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scheinhorn DJ, Hassenpflug MS, Votto JJ, Chao DC, Epstein SK, Doig GS, et al. Ventilation Outcomes Study Group. Ventilator-dependent survivors of catastrophic illness transferred to 23 long-term care hospitals for weaning from prolonged mechanical ventilation. Chest. 2007;131:76–84. doi: 10.1378/chest.06-1079. [DOI] [PubMed] [Google Scholar]
- 62.Hua M, Wunsch H. Reporting data on long-term follow-up of critical care trials. Thorax. 2016;71:395–396. doi: 10.1136/thoraxjnl-2016-208466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jubran A, Lawm G, Kelly J, Duffner LA, Gungor G, Collins EG, et al. Depressive disorders during weaning from prolonged mechanical ventilation. Intensive Care Med. 2010;36:828–835. doi: 10.1007/s00134-010-1842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banzett RB, Similowski T, Brown R. Addressing respiratory discomfort in the ventilated patient. In: Tobin MJ, editor. Principles and practice of mechanical ventilation. 3rd ed. New York, NY: McGraw-Hill; 2013. pp. 1267–1292. [Google Scholar]
- 65.Kahneman D. Thinking, fast and slow. New York, NY: Farrar, Straus and Giroux; 2011. pp. 377–385. [Google Scholar]
- 66.Gifford JM, Husain N, Dinglas VD, Colantuoni E, Needham DM. Baseline quality of life before intensive care: a comparison of patient versus proxy responses. Crit Care Med. 2010;38:855–860. doi: 10.1097/CCM.0b013e3181cd10c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rothman K. Modern epidemiology. Boston, MA: Little, Brown; 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.