Abstract
Rationale: Higher indoor particulate matter (PM) concentrations are linked with increased asthma morbidity. Dietary intake of fatty acids, also linked with asthma outcomes, may influence this relationship.
Objectives: To determine the relationship between omega-3 and omega-6 fatty acid intake and pediatric asthma morbidity, and the association between fatty acid intake and strength of indoor, PM-related asthma symptoms, albuterol use, and systemic inflammation.
Methods: Analyses included 135 children with asthma enrolled in the AsthmaDIET Study. At baseline, 3 months, and 6 months, data included: week-long average home indoor concentration of PM ≤2.5 μm in aerodynamic diameter and PM ≤10 μm in aerodynamic diameter, dietary intake of omega-3 and omega-6 fatty acids, daily symptoms, and peripheral blood leukocytes. Asthma severity and lung function were assessed at baseline. Multivariable regression models, adjusted for known confounders, were used to determine associations between each fatty acid and outcomes of interest, with interaction terms (fatty acids × PM) in longitudinal analyses.
Measurements and Main Results: Higher omega-6 intake associated with increased odds of increased asthma severity (P = 0.02), and lower FEV1/FVC ratio (P = 0.01). Higher omega-3 intake associated with reduced effect of indoor PM ≤2.5 μm in aerodynamic diameter on symptoms (P < 0.01), whereas higher omega-6 intake associated with amplified effect of indoor PM ≤2.5 μm in aerodynamic diameter on symptoms and circulating neutrophil percentage (P < 0.01).
Conclusions: Omega-3 and omega-6 intake are associated with pediatric asthma morbidity and may modify the asthmatic response to indoor PM.
Keywords: diet, particulate matter, inflammation
At a Glance Commentary
Scientific Knowledge in the Subject
Dietary intake of fatty acids, specifically omega-3 and omega-6, may modify pediatric asthma morbidity.
What This Study Adds to the Field
We present associations between higher omega-6 intake and worse asthma severity and lung function in an urban cohort of children with asthma. Furthermore, higher omega-3 intake was associated with diminished harmful effect of indoor particulate matter (PM) exposure on respiratory symptoms, whereas higher omega-6 intake was associated with an amplified effect. Higher omega-6 intake also associated with an amplified effect of indoor PM on circulating neutrophil percentage, reflecting a modification of PM effects on systemic inflammation by fatty acid intake level. To our knowledge, this investigation represents the first evidence of a protective association between omega-3 and detrimental association between omega-6 and PM-induced asthma symptoms and systemic inflammation among school-age children with asthma. If confirmed in similar populations, alterations in omega-3 and omega-6 intake may provide opportunity for intervention to improve asthma health and reduce the harmful effects of indoor PM exposure.
Dietary intake among minority, inner city children and adolescents diverges sharply from national nutritional guidelines (1–3). This population is also disproportionately impacted by asthma (4), an inflammatory disease driven by environmental exposures, with dietary exposures also representing a potential driver of asthma morbidity (5). Dietary intake among the children largely conforms to a “Western” diet, characterized by higher intakes of unhealthy/processed foods and omega-6 fatty acids, and lower intake of healthy foods, such as fruits, vegetables, and items including omega-3 fatty acids (3, 6). These fatty acids are a source of biologically active molecules found in the lung, known as lipid-derived inflammatory mediators, providing biologic rationale for respiratory effects (7). Despite these connections, few investigations examine effects of omega-3 and omega-6 on asthma-related morbidity within pediatric populations (8).
Furthermore, children in the inner city often inhabit environments with indoor pollutants levels, specifically particulate matter (PM), that far exceed standards for acceptable ambient levels set by the Environmental Protection Agency or acceptable indoor levels set by the World Health Organization (9–13). Children with asthma spend a striking amount of the day (roughly 70% on average) indoors (14), and indoor home PM levels are independently associated with increased asthma morbidity (9). Dietary exposure to excess omega-6 fatty acids (generally proinflammatory), and reduced omega-3 fatty acids (antiinflammatory), may plausibly prime the asthmatic response to these additional environmental exposures, placing inner city children with asthma at undue risk of poor asthma outcomes.
The AsthmaDIET Study, an urban pediatric asthma cohort with detailed dietary and indoor environmental exposure measurement, provides an opportunity to simultaneously investigate the contributions of indoor PM and diet to pediatric asthma morbidity. We hypothesized that dietary intake of fatty acids would associate with measures of pediatric asthma health; specifically that higher omega-6 fatty acid intake would associate with increased asthma morbidity and that higher omega-3 fatty acid intake would associate with reduced asthma morbidity. We further hypothesized that omega-6 and omega-3 fatty acid intake would associate with amplified or diminished strength, respectively, of the asthmatic response to indoor PM. Some of the results of these studies have been previously reported in the form of an abstract (15, 16).
Methods
Study Design
The AsthmaDIET Study was an environmental cohort study of 149 children with asthma in Baltimore City, Maryland. The study was approved by the Johns Hopkins Institutional Review Board; parental consent was obtained. Participants were: 1) aged 5–12 years, 2) diagnosed by a physician with asthma with symptoms and/or reliever medication use in the past 6 months, 3) free of food allergies, and 4) not taking antioxidant supplements at enrollment.
At baseline, demographics, medication use, body mass index, asthma severity, and lung function were assessed. Each participant underwent the following 1-week assessments at baseline, 3 months, and 6 months: indoor PM concentrations, dietary intake, daytime and nocturnal asthma symptoms and albuterol use via daily diary, and phlebotomy.
Exposure Assessments
Impactors designed to collect particles with an aerodynamic diameter ≤2.5 μm (PM2.5) and ≤10 μm (PM10) were placed in the home, recording average week-long PM concentrations. Dietary intake was assessed at the end of each monitoring week via a Baltimore-specific quantitative food frequency questionnaire with 7-day recall (6), from which omega-3 and omega-6 intake were derived. Further exposure assessment details are found in the online supplement.
Respiratory and Inflammatory Outcomes
Baseline asthma severity was defined via National Asthma Education and Prevention Program guidelines (17) using symptoms, medication use, activity interference, and spirometry (obtained via KoKo spirometer in accordance with American Thoracic Society guidelines) (18), as detailed in the online supplement. Predicted values for FEV1 and FVC were calculated by formulae of Hankinson and colleagues (19). Daily diary responses recorded symptoms and albuterol use at baseline, 3 months, and 6 months. Daytime asthma symptoms (trouble breathing, bother, activity limitation) were presented as none (0) or any symptom reported (1). Nocturnal awakenings and albuterol use were dichotomized to none versus any (use).
Whole blood leukocyte counts, and percentage neutrophils and percentage eosinophils, were measured at the end of each home monitoring week in serum samples.
Statistical Analyses
Variable distribution was examined and log transformation completed when appropriate to meet model assumptions. Within subject, intraclass correlation for exposure variables (PM, omega-6, and omega-3) was calculated via linear mixed model with random intercept to evaluate between- and within-individual variability. All analyses were performed using the individual fatty acid levels and a ratio (omega-6/omega-3) as predictors; because use of the ratio resulted in null effect, results are not presented.
Baseline associations between usual, or within-person average (average across visits by individual), fatty acid intake, and asthma severity were tested via Kruskall-Wallis. Ordered logistic regression was used to model the association between fatty acid intake and odds ratio (OR) of worse asthma severity, adjusted for the opposing fatty acid, age, gender, body mass index, caloric intake, and caregiver education. Relationships between usual (within-person average) fatty acid intake and lung function were modeled via linear regression, adjusted for aforementioned covariates in addition to inhaled corticosteroid use.
In repeated measure analyses, the modifying effects of fatty acid intake by visit on the association between weekly indoor PM exposure and daily outcomes (e.g., daytime symptoms, nocturnal awakenings, albuterol use) or weekly outcomes (e.g., peripheral blood leukocytes) were tested via two-way (omega-3 and PM or omega-6 and PM) interaction terms using generalized estimating equations (binomial family/logit link/exchangeable correlation/robust SE for binary outcomes or Gaussian family/identity link/exchangeable correlation/robust SE for continuous outcomes), adjusted for the aforementioned covariates and season. A sensitivity analysis adding air nicotine (see online supplement) levels to account for secondhand smoke exposure, and an additional exploration for modification of omega and PM associations by atopy (see online supplement) were performed to discern unique exposures or phenotypes that may influence observed relationships. Final models were chosen based on Wald tests of the interested regression terms, and satisfaction of appropriate modeling assumptions.
Analyses were completed using STATA version 15. Statistical significance was defined as P less than 0.05.
Results
Study Population
Participants with complete, corresponding diet and PM data for one or more of the monitoring periods (n = 135) (see Figure E1 in the online supplement) were included (Table 1). Mean age was 9.5 years (SD, 2.2 yr), and roughly half of the cohort was female (47%) and/or overweight/obese (50%). Participants were predominantly African American (96%) with public insurance (91%), and most caregivers (71%) reported at least a high school education. One-third of participants had mild asthma (34%), one-third moderate asthma (33%), and one-third severe asthma (33%). Most reported albuterol use (66%) within the last 2 weeks, and 47% reported inhaled corticosteroid use. Mean FEV1 percent predicted was 93.5%, but with noted variability between participants (SD, 16.8%). Similarly, mean FEV1/FVC ratio was 0.83, but also with substantial noted variability (SD, 0.10). Mean average daily caloric intake was 3,448 kcal. No data from analysis were excluded based on caloric estimates given that caloric intake values above relevant estimated energy requirements (20) did not correspond to outliers of omega-3 and omega-6 intake in this population sample (see Figure E2).
Table 1.
Baseline Characteristics of Children Ages 5–12 Years with Asthma Enrolled in the ASTHMA-DIET Study*
| Characteristics | n (%) or Mean ± SD |
|---|---|
| Children | |
| Age | 9.5 ± 2.2 |
| Female | 64 (47.4) |
| Black/African American | 130 (96.3) |
| Body mass index, percentile† | 71.3 ± 30.4 |
| Underweight (<5%) | 7 (5.3) |
| Normal weight (5–85%) | 61 (45.9) |
| Overweight (85–95%) | 26 (19.5) |
| Obese (≥95%) | 39 (29.3) |
| Asthma medication use | |
| Albuterol‡ | 85 (65.9) |
| Inhaled corticosteroid§ | 61 (46.9) |
| Other (cromolyn, leukotriene modifier, theophylline, oral corticosteroids)‡ | 35 (27.1) |
| Asthma severity | |
| Mild intermittent | 34 (25.2) |
| Mild persistent | 12 (8.9) |
| Moderate persistent | 44 (32.6) |
| Severe persistent | 45 (33.3) |
| Health insurance | |
| Private insurance | 11 (8.1) |
| Public insurance | 123 (91.1) |
| Other | 1 (0.7) |
| Lung function | |
| Prebronchodilator FEV1% predicted|| | 93.5 ± 16.8 |
| Prebronchodilator FVC % predicted¶ | 97.5 ± 16.8 |
| FEV1/FVC§ | 0.83 ± 0.10 |
| Total caloric intake, kcal | 3,448 (1,306) |
| Caregivers | |
| Education | |
| Not high school graduate | 39 (28.9) |
| High school graduate | 66 (48.9) |
| At least some college | 30 (22.2) |
| Household income (annual) | |
| <$25,000 | 56 (41.5) |
| $25,000–$50,000 | 18 (13.3) |
| >$50,000 | 3 (2.2) |
| Not reported | 58 (43.0) |
N = 135.
Asthma severity based on symptoms, medication use, activity interference, and spirometry.
n = 133.
n = 129.
n = 130.
n = 119.
n = 118.
Within-person median (interquartile range) omega-6 and omega-3 levels were 4.64 g (3.37–6.35 g) and 0.32 g (0.21–0.45 g), respectively, with corresponding intraclass correlations of 0.16 (95% confidence interval [CI], 0.07–0.31) and 0.20 (95% CI, 0.11–0.35). Within-person median (interquartile range) PM concentrations revealed PM2.5 and PM10 levels of 26.8 μg/m3 (19.0–40.4 μg/m3) and 39.0 μg/m3 (26.6–54.7 μg/m3), respectively, with corresponding intraclass correlations of 0.54 (95% CI, 0.44–0.64) and 0.53 (95% CI, 0.43–0.63).
Association between Average Fatty Acid Intake and Baseline Asthma Severity and Lung Function
Univariate associations between fatty acid intake and categories of asthma severity revealed a positive trend toward higher omega-6 intake and worse asthma severity (P = 0.06) (Figure 1A). After adjustment for confounders, each additional gram of omega-6 intake was associated with increased likelihood of higher asthma severity category (OR, 1.29; P = 0.02) (Figure 1C). There was no statistically significant association between omega-3 intake and asthma severity in univariate or adjusted models (Figures 1B and 1D).
Figure 1.
Fatty acid intake and associations with asthma severity. (A and B) Univariate associations between reported omega-3 (A, odds ratio [OR] per 0.1-g increase) and omega-6 (B, OR per 1-g increase) intake and asthma severity shown in Tukey plots. P values were determined via Kruskall-Wallis equality of populations rank test. (C and D) Predicted probability of severe persistent asthma (based on the ordered logistic regression using four categories of asthma severity) by intake of omega-3 (C) and omega-6 (D); point estimates and 95% confidence intervals are represented with each fatty acid adjusted for the other. int. = intermittent; Mod. = moderate; per. = persistent; Pr = probability.
Regarding lung function, adjusted models similarly revealed an association between each additional gram of omega-6 intake and lower FEV1/FVC (β −0.012; P = 0.01) driven by reductions in FEV1 (β −1.41; P = 0.12), whereas omega-3 intake did not demonstrate statistically significant associations with lung function parameters in any models (data not shown).
Fatty Acid Intake Modified Relationships between Repeated PM Measures, Symptoms, and Albuterol Use
Daytime symptoms were reported on 508 of 2,068 days (19.8%), with individual symptom reports of trouble breathing on 18.8% of days, bother caused by asthma on 16.5% of days, and limitation in activity caused by asthma on 13.3% of days. Any albuterol use was reported on 468 of 2,135 days (18.0%), and nocturnal symptoms were noted on 176 of 2,412 days (6.8%). Total number of days varied by outcome measure because of missing daily diary responses from some of the participants.
Fully adjusted models including both omega-6 and omega-3 did not reveal a primary effect of PM2.5 or PM10 exposure on daytime symptoms (PM2.5: OR, 1.00; P = 0.90; PM10: OR, 0.99; P = 0.75), albuterol use (PM2.5: OR, 0.97; P = 0.51; PM10: OR, 0.96; P = 0.44), or nocturnal symptoms (PM2.5: OR, 0.96; P = 0.19; PM10: OR, 0.97; P = 0.26). Likewise, omega-3 and omega-6 intake did not demonstrate primary associations with symptoms or albuterol use in adjusted models (data not shown). However, significant two-way interactions between each fatty acid and both indoor PM2.5 and PM10 were noted for all outcomes.
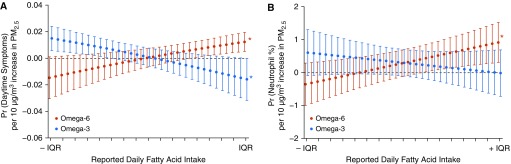
Specifically, the association between PM2.5 and daytime symptoms was augmented at higher levels of omega-6 intake (OR for interaction [ORintx], 1.02 per 1-g increase in omega-6; [P for interaction (Pintx)] < 0.01) and mitigated at higher levels of omega-3 intake (ORintx, 0.96 per 0.1-g increase in omega-3; Pintx < 0.01) (Figure 2). Intake of omega-3 and omega-6 similarly modified the association between PM10 and daytime symptoms, with augmentation at higher omega-6 (ORintx, 1.02 per 1-g increase in omega-6; Pintx = 0.03), and mitigation at higher omega-3 (ORintx, 0.97 per 0.1-g increase in omega-3; Pintx = 0.01) (see Figure E3). The harmful effects of omega-6 on the relationship between PM2.5 or PM10 and symptoms were most pronounced at lower levels of omega-3; conversely the beneficial effects of omega-3 were most pronounced at higher levels of omega-6. Adjustment for air nicotine did not alter results, nor were relationships influenced by atopic status of the participants (data not shown).
Figure 2.
Marginal effects of indoor particulate matter ≤2.5 μm in aerodynamic diameter (PM2.5) on daytime symptoms and peripheral neutrophil % is modified by fatty acid intake. (A) Predicted probability of daytime symptoms (n = 2,467 person-days) and (B) peripheral neutrophil percentage (n = 264 serum samples) per 10 μg/m3 increase in PM2.5 at levels from −1 to +1 interquartile range of reported intake in the AsthmaDIET Study. Omega-6 and omega-3 included simultaneously in generalized estimating equation model above, adjusted for age, gender, body mass index, caloric intake (quadratic term for symptoms outcome only), caregiver education, inhaled corticosteroid use, and season. To display relationships, each model is held constant at the median level of the opposing fatty acid. *Statistical significance. IQR = interquartile range; Pr = probability.
The components of the composite daytime symptom variable (trouble breathing, bother, activity limitation) were examined separately, with fatty acid intake modifying the effects of PM2.5 individually on trouble breathing, bother caused by asthma, and activity limitation consistent with the composite score (Table 2). Results were similar for PM10 (see Table E1). Intake of omega-6 additionally augmented the association between PM2.5 and albuterol use (ORintx, 1.03 per 1-g increase in omega-6; Pintx = 0.02) and nocturnal symptoms (ORintx, 1.02 per 1-g increase in omega-6; Pintx = 0.04); no significant interaction was noted between omega-3 and PM2.5 for these outcomes (Table 2). Intake of omega-6 additionally augmented the association between PM10 and nocturnal symptoms (ORintx, 1.02 per 1-g increase in omega-6; Pintx = 0.03); no significant interaction was noted between omega-6 and PM10 for albuterol use or for omega-3 and PM10 for albuterol use or nocturnal symptoms (see Table E1).
Table 2.
Marginal Effects of Indoor PM on Daily Symptoms Modified by Omega-3 and Omega-6 Fatty Acid Intake
| PM2.5 |
PM2.5 × Omega-3 |
PM2.5 × Omega-6 |
n* | ||||
|---|---|---|---|---|---|---|---|
| OR (SE) | P Value | OR (SE) | P Value | OR (SE) | P Value | ||
| Trouble breathing | 1.00 (0.02) | 0.98 | 0.97 (0.01) | <0.01 | 1.02 (0.01) | <0.01 | 2,463 |
| Bother caused by asthma | 1.01 (0.03) | 0.83 | 0.97 (0.01) | <0.01 | 1.02 (0.01) | <0.001 | 2,467 |
| Limitation in activity because of asthma | 1.00 (0.03) | 0.87 | 0.97 (0.01) | 0.02 | 1.02 (0.01) | <0.01 | 2,465 |
| Albuterol use | 0.98 (0.04) | 0.55 | 0.98 (0.02) | 0.23 | 1.03 (0.01) | 0.02 | 2,500 |
| Nocturnal symptoms | 0.98 (0.02) | 0.29 | 0.99 (0.02) | 0.60 | 1.02 (0.01) | 0.04 | 2,486 |
Definition of abbreviations: OR = odds ratio; PM = particulate matter; PM2.5 = PM ≤2.5 μm in aerodynamic diameter.
Omega-6 and omega-3 were included simultaneously in a generalized estimating equation model with a robust SE estimator, adjusted for age, gender, body mass index, caloric intake, caregiver education, inhaled corticosteroid use, and season. Effect estimates are shown per 10-μg/m3 change in PM, 0.1-g change in omega-3, and 1.0-g change in omega-6, centered at median levels. Interaction terms are bold if significant at Pintx < 0.1.
n for analyses represents person-days.
Fatty Acid Intake Modified Relationships between Repeated PM Measures and Blood Neutrophils
Fully adjusted models including both omega-6 and omega-3 likewise did not reveal a primary association between PM2.5 or PM10 exposure on total blood leukocyte counts (leukocyte counts log transformed to meet model assumptions; PM2.5: β 0.0001; P = 0.97; PM10: β 0.002; P = 0.65), percentage eosinophils (PM2.5: β −0.08; P = 0.07; PM10: β −0.05; P = 0.39), or percentage neutrophils (PM2.5: β 0.21; P = 0.34; PM10: β 0.20; P = 0.42). Likewise, omega-3 and omega-6 intake did not demonstrate primary associations with these outcomes (data not shown).
With regard to two-way interactions, the association between PM2.5 and percentage neutrophils was augmented by increasing omega-6 intake (βintx = 0.17 per 1-g increase in omega-6; Pintx < 0.01) with no significant effects of omega-3 noted (Figure 2; see Figure E3 and Table E2). Results were similar by PM10 exposure (see Table E2). Fatty acid intake did not demonstrate consistent, significant effects on the relationship between PM2.5 or PM10 on total leukocyte counts or percentage eosinophils. Adjustment for air nicotine did not alter results, nor were relationships influenced by atopic status of the participants (data not shown).
Discussion
Poor dietary habits and high air pollution exposure are two risk factors that may contribute to disproportionate asthma burden in low-income populations. We present data from a prospective cohort of children with asthma in Baltimore City, demonstrating associations between reported omega-3 and omega-6 fatty acid intake and asthma health. Specifically, higher omega-6 intake was linked to more severe asthma and reduced lung function. Furthermore, both omega-6 and omega-3 intake modified the association between indoor PM exposure, asthma symptoms, and albuterol use, with diminished strength of PM effects noted at higher levels of omega-3 and amplification of PM effects seen at higher levels of omega-6 intake. Higher omega-6 intake also associated with augmentation of PM effects on systemic inflammation (i.e., percent neutrophilia). To our knowledge, this investigation represents the first evidence of a protective association between omega-3 and a detrimental association between omega-6 and PM-induced asthma symptoms and systemic inflammation among school-age children with asthma.
Literature evaluating the association between omega-3 and omega-6 intake and pediatric asthma diagnosis has been mixed, and to our knowledge no epidemiologic evaluations have been published in cohorts limited to school-age children with asthma. Studies focused on asthma symptoms in mixed populations (pediatric asthma and nonasthma populations) demonstrate varied results (21–28), although favor a protective effect of omega-3 intake on respiratory symptoms and provide limited insight into omega-6 effects. Three small trials of fatty acid intake manipulation in children with asthma also provide mixed results (29–31), perhaps because of heterogeneity of design and population, although all demonstrated improvement in at least one outcome (lung function [29, 31] or systemic inflammation [30]) in response to omega-3 supplementation. Our data would suggest that both omega-3 intake augmentation and omega-6 intake reduction may present potent targets for future investigations aimed at reducing asthma morbidity within the present urban, pediatric population. Furthermore, the absolute levels of intake, rather than ratio of omega-6 to omega-3 intake, may be important in influencing asthma health.
In addition to demonstrating an association between fatty acid intake, asthma severity, and lung function, our results show that fatty acid intake may determine individual susceptibility to air pollution exposure. Associations between higher indoor PM concentrations and increased asthma symptoms, exacerbations, and reduced lung function in urban children are well-established (32), and short-term PM exposure is linked to increased peripheral blood neutrophilia in healthy volunteers (33). Because PM exposure is an established and potent contributor to pediatric asthma morbidity and systemic inflammation, the importance of omega-3 and omega-6 intake to the asthmatic response to PM further strengthens the relevance of our findings.
Although not yet fully understood, the health effects on PM exposure are suggested to be mediated through biologic mechanisms including airway and systemic oxidative stress and inflammation (34, 35). Omega-6 and omega-3 fatty acids are sources of lipid-derived inflammatory and proresolving mediators, metabolites that arbitrate pulmonary and systemic inflammation (7) and are plausible as biologic mediators of the PM response. The omega-3 fatty acids alpha-linoleic acid, eicosapentaenoic acid, and docosahexaenoic acid are precursors of resolvins, protectins, and maresins. These three classes of molecules regulate neutrophil infiltration, coordinate clearance of apoptosed neutrophils by macrophages, and adjust cytokine production in response to inflammatory triggers to promote resolution of inflammation (36).
In contrast, omega-6 fatty acids play a more complex role as precursors for both pro and antiinflammatory mediators. Arachadonic acid is a precursor for proresolving mediators called lipoxins (i.e., LXA4) (37–40) and prostaglandin E2 (41), which inhibit proinflammatory cytokines/leukotrienes and inflammatory cell recruitment, and increase smooth muscle relaxation in the lungs. However, the potential antiinflammatory omega-6 effects are counterbalanced and perhaps overwhelmed by proinflammatory effects. Arachadonic acid also serves as the precursor for prostaglandin D2, which likely contributes to bronchoconstriction and may increase proinflammatory cytokine production (42), and cysteinyl leukotrienes, which contribute to bronchoconstriction, increased vascular permeability and mucus secretion, airway remodeling, and the release of additional proinflammatory cytokines (43, 44). The relative concentrations of these biologically active molecules may plausibly influence individual response to PM.
Emerging data support a role for mitigation of air pollution health risks via nutrition in a range of chronic diseases (45, 46). Murine studies demonstrate protection against PM2.5-induced pulmonary and systemic inflammation and oxidative stress in mice with augmented tissue levels of omega-3 (47). Consistent with these effects, limited omega-3 supplementation trials in human populations suggest protection against the inflammatory effects of PM. For example, in a randomized double-blind controlled trial of 50 elderly adults in Mexico City, Romieu and colleagues (48) demonstrated that omega-3 supplementation was associated with increasing plasma antioxidant activity, hypothesized to act as a defense against PM. One prior study demonstrated mitigation of nasal lavage IL-8 levels in response to ambient air pollution among children with asthma who reported dietary intake consistent with a Mediterranean diet (characteristically high in omega-3) (49).
Several limitations to the current investigation are noted. Omega-3 and omega-6 intake were obtained through food frequency questionnaire assessment, with the possibility of exposure misclassification or residual confounding caused by overreport or underreport of dietary intake by the children in the study. However, caregivers were enlisted for completion of surveys and to assist with meal recall, thus improving accuracy. Total caloric intake values (Table 1) do not demonstrate the underreporting noted in other pediatric studies (50, 51). Intake assessment targeted foods demonstrated to be consumed in the local community based on previous research (6), providing content validity to the assessment. And although reported fatty acid intake levels have not been compared with red blood cell membrane fatty acid levels, a biologic measure unavailable in this cohort, reported fatty acid intake has been correlated with cell membrane levels in other studies using food frequency questionnaire methodology, providing validity to the approach (52, 53). The diet of the children in the presented Baltimore City cohort was highly skewed toward low omega-3 intake (median, 0.32 g), typical of an American-style diet. Although low omega-3 intake is consistent with prior reports of dietary intake in Baltimore City and other urban minority populations (3, 6), increasing confidence in dietary results, the generally low levels of omega-3 intake in the cohort limit accuracy of extrapolation regarding potential effect of high-dose omega-3 supplementation or dietary intake, as provided in previous trials, within this population.
The investigations herein bear repeating in a population with a higher representation of a “healthy” and balanced diet. Furthermore, adequate daily intakes in this age group are defined as 0.9–1.6 g/d of omega-3 (alpha-linoleic acid) and 10–16 g/d omega-6 (linoleic acid), based on average intake levels in healthy populations (20). Intake of both omega-3 and omega-6 fell below adequate intake levels within this cohort. Despite overall low levels of omega-6 intake, a trend toward harm with increasing intake was noted, and may have implications on the appropriateness of adequate intake recommendations in populations with asthma. Applicability of conclusions to pediatric cohorts with notably different distributions of intake warrants further investigation.
Despite noted limitations, the study design and original results demonstrate important strengths. Prospective design with multiple visits per participant adds potency to conclusions and provides the power necessary to examine the complex relationships between diet, environmental exposures, and asthma-related morbidity. Although ambient exposures are widely characterized and often based on modeling or predicted exposures, studies examining the effect of indoor exposures on asthma health are less prevalent. This owes to the cost and time commitment necessary to directly sample individual home environments. However, given high concentrations of PM in the indoor environment in this population (54, 55), the amount of time children with asthma spend in the home environment (14), and therefore the relative contribution of indoor pollutants to daily exposures, the indoor environment is highly relevant and provides distinct but complementary evidence to studies of outdoor PM exposures, representing a further strength of the current analysis. The population of children with asthma represented here, minority, largely of low socioeconomic status, and living in an urban environment, are disproportionately affected by asthma and asthma morbidity (4), and are exposed to poor diet (3, 6) and excessive indoor air pollution (10, 11). Identification of two modifiable risk factors (dietary intake and indoor air quality) for asthma-related morbidity in this population is a major strength. Finally, although the data are observational, the presentation of consistent relationships between omega-3 and omega-6 in regards to symptoms and systemic inflammation outcomes lends consistency of effect, an additional criterion necessary for the establishment of causality.
In summary, we present results from an inner-city pediatric cohort with asthma demonstrating an association between higher omega-6 levels and increased asthma severity, and modification by reported omega-3 (protective) and omega-6 (harmful) fatty acid intake of the association between indoor PM exposure and asthma symptoms and systemic inflammation. These results highlight that this highly vulnerable and at-risk population has potentially two modifiable exposures (unfavorable fatty acid intake profiles and high indoor PM exposures) combining to effectively worsen asthma morbidity. As evidence continues to gather regarding an effect of dietary intake on pediatric asthma health, it is critical to scrutinize the contribution and role of diet in the context of environmental exposures linked to respiratory morbidity. It is possible that the collective improvement of dietary and indoor air exposures may have the greatest impact on improving asthma health.
Supplementary Material
Footnotes
Supported by grants from the National Institute for Environmental Health Sciences of the NIH (P50ES018176, N.N.H.; P01ES018176, P50ES015903, and K24ES021098, G.D.; K23ES029105, E.P.B.), the National Center for Advancing Translational Sciences of the NIH (KL2TR001077, E.P.B.), and the U.S. Environmental Protection Agency (EPA) (agreement number 83615201 and 83451001, N.N.H.). This manuscript has not been formally reviewed by the EPA or NIH, and the views expressed in this document are solely those of the authors and do not necessarily reflect those of the EPA or NIH.
Author Contributions: All authors listed on this work have provided substantial contributions to the conception or the design of the work, including acquisition, analysis, or interpretation of data; drafting the work or revising critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201808-1474OC on March 29, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Affenito SG, Thompson DR, Franko DL, Striegel-Moore RH, Daniels SR, Barton BA, et al. Longitudinal assessment of micronutrient intake among African-American and white girls: the National Heart, Lung, and Blood Institute Growth and Health Study. J Am Diet Assoc. 2007;107:1113–1123. doi: 10.1016/j.jada.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Di Noia J, Schinke SP, Contento IR. Dietary fat intake among urban, African American adolescents. Eat Behav. 2008;9:251–256. doi: 10.1016/j.eatbeh.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Jahns L, Tussing-Humphreys L, Xie B, Rockett H, Liang H, et al. Dietary intake patterns of low-income urban African-American adolescents. J Am Diet Assoc. 2010;110:1340–1345. doi: 10.1016/j.jada.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Center for Health Statistics. FASTATS 2015. 2017 [updated 2017 Jan 19; accessed 2019 Jan 5]. Available from: http://www.cdc.gov/nchs/fastats/default.htm.
- 5.Oland AA, Booster GD, Bender BG. Psychological and lifestyle risk factors for asthma exacerbations and morbidity in children. World Allergy Organ J. 2017;10:35. doi: 10.1186/s40413-017-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolahdooz F, Butler JL, Christiansen K, Diette GB, Breysse PN, Hansel NN, et al. Food and nutrient intake in African American children and adolescents aged 5 to 16 years in Baltimore city. J Am Coll Nutr. 2016;35:205–216. doi: 10.1080/07315724.2014.959206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wendell SG, Baffi C, Holguin F. Fatty acids, inflammation, and asthma. J Allergy Clin Immunol. 2014;133:1255–1264. doi: 10.1016/j.jaci.2013.12.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miles EA, Calder PC. Omega-6 and omega-3 polyunsaturated fatty acids and allergic diseases in infancy and childhood. Curr Pharm Des. 2014;20:946–953. doi: 10.2174/138161282006140220125732. [DOI] [PubMed] [Google Scholar]
- 9.McCormack MC, Breysse PN, Matsui EC, Hansel NN, Peng RD, Curtin-Brosnan J, et al. Center for Childhood Asthma in the Urban Environment. Indoor particulate matter increases asthma morbidity in children with non-atopic and atopic asthma. Ann Allergy Asthma Immunol. 2011;106:308–315. doi: 10.1016/j.anai.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormack MC, Breysse PN, Matsui EC, Hansel NN, Williams D, Curtin-Brosnan J, et al. Center for Childhood Asthma in the Urban Environment. In-home particle concentrations and childhood asthma morbidity. Environ Health Perspect. 2009;117:294–298. doi: 10.1289/ehp.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hulin M, Caillaud D, Annesi-Maesano I. Indoor air pollution and childhood asthma: variations between urban and rural areas. Indoor Air. 2010;20:502–514. doi: 10.1111/j.1600-0668.2010.00673.x. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Environmental Protection Agency. Indoor air quality 2016[updated 2018 Sep 19; accessed 2019 Jan 1]. Available from: https://www.epa.gov/indoor-air-quality-iaq/indoor-particulate-matter
- 13.World Health Organization. WHO air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide: global update 2005. Summary of risk assessment. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 14.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 15.Brigham E, McCormack MC, Woo H, Rice J, Koehler K, Vulcain T, et al. Omega-3 and omega-6 fatty acid intake modifies response to indoor air pollution in children with asthma [abstract] Presented at the NIEHS/EPA Children’s Environmental Health Centers Annual Meeting and Social Media Workshop. October 22, 2018, Durham, NC.
- 16.Brigham E, McCormack MC, Woo H, Rice J, Koehler K, Vulcain T, et al. Omega-3 and omega-6 fatty acid intake modifies response to indoor air pollution in children with asthma[abstract]. Am J Respir Crit Care Med 2018197A1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Heart, Lung, and Blood Institute. Expert panel report 3: guidelines for the diagnosis and management of asthma. Bethesda, MD: United States Department of Health and Human Services; 2007. [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 20.Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington, DC: National Academic Press; 2005. [DOI] [PubMed] [Google Scholar]
- 21.Guilleminault L, Williams EJ, Scott HA, Berthon BS, Jensen M, Wood LG. Diet and asthma: is it time to adapt our message? Nutrients. 2017;9:E1227. doi: 10.3390/nu9111227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27–34. doi: 10.1016/j.alit.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Saadeh D, Salameh P, Caillaud D, Charpin D, De Blay F, Kopferschmitt C, et al. Prevalence and association of asthma and allergic sensitization with dietary factors in schoolchildren: data from the French six cities study. BMC Public Health. 2015;15:993. doi: 10.1186/s12889-015-2320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Innocenzo S, Matos SM, Prado MS, Santos CA, Assis AM, Cruz AA, et al. Dietary pattern, asthma, and atopic and non-atopic wheezing in children and adolescents: SCAALA study, Salvador, Bahia State, Brazil [in Portuguese] Cad Saude Publica. 2014;30:1849–1860. doi: 10.1590/0102-311x00165513. [DOI] [PubMed] [Google Scholar]
- 25.D’Auria E, Miraglia Del Giudice M, Barberi S, Mandelli M, Verduci E, Leonardi S, et al. Omega-3 fatty acids and asthma in children. Allergy Asthma Proc. 2014;35:233–240. doi: 10.2500/aap.2014.35.3736. [DOI] [PubMed] [Google Scholar]
- 26.Nagel G, Weinmayr G, Kleiner A, Garcia-Marcos L, Strachan DP ISAAC Phase Two Study Group. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) phase two. Thorax. 2010;65:516–522. doi: 10.1136/thx.2009.128256. [DOI] [PubMed] [Google Scholar]
- 27.Tabak C, Wijga AH, de Meer G, Janssen NA, Brunekreef B, Smit HA. Diet and asthma in Dutch school children (ISAAC-2) Thorax. 2006;61:1048–1053. doi: 10.1136/thx.2005.043034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns JS, Dockery DW, Neas LM, Schwartz J, Coull BA, Raizenne M, et al. Low dietary nutrient intakes and respiratory health in adolescents. Chest. 2007;132:238–245. doi: 10.1378/chest.07-0038. [DOI] [PubMed] [Google Scholar]
- 29.Hodge L, Salome CM, Hughes JM, Liu-Brennan D, Rimmer J, Allman M, et al. Effect of dietary intake of omega-3 and omega-6 fatty acids on severity of asthma in children. Eur Respir J. 1998;11:361–365. doi: 10.1183/09031936.98.11020361. [DOI] [PubMed] [Google Scholar]
- 30.Nagakura T, Matsuda S, Shichijyo K, Sugimoto H, Hata K. Dietary supplementation with fish oil rich in omega-3 polyunsaturated fatty acids in children with bronchial asthma. Eur Respir J. 2000;16:861–865. doi: 10.1183/09031936.00.16586100. [DOI] [PubMed] [Google Scholar]
- 31.Farjadian S, Moghtaderi M, Kalani M, Gholami T, Hosseini Teshnizi S. Effects of omega-3 fatty acids on serum levels of T-helper cytokines in children with asthma. Cytokine. 2016;85:61–66. doi: 10.1016/j.cyto.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Breysse PN, Diette GB, Matsui EC, Butz AM, Hansel NN, McCormack MC. Indoor air pollution and asthma in children. Proc Am Thorac Soc. 2010;7:102–106. doi: 10.1513/pats.200908-083RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salvi S, Blomberg A, Rudell B, Kelly F, Sandström T, Holgate ST, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159:702–709. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- 34.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011:487074. doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz J. Air pollution and children’s health. Pediatrics. 2004;113(Suppl. 4):1037–1043. [PubMed] [Google Scholar]
- 36.Duvall MG, Levy BD. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol. 2016;785:144–155. doi: 10.1016/j.ejphar.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandrasekharan JA, Sharma-Walia N. Lipoxins: nature’s way to resolve inflammation. J Inflamm Res. 2015;8:181–192. doi: 10.2147/JIR.S90380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ono E, Dutile S, Kazani S, Wechsler ME, Yang J, Hammock BD, et al. National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am J Respir Crit Care Med. 2014;190:886–897. doi: 10.1164/rccm.201403-0544OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gagliardo R, Gras D, La Grutta S, Chanez P, Di Sano C, Albano GD, et al. Airway lipoxin A4/formyl peptide receptor 2-lipoxin receptor levels in pediatric patients with severe asthma. J Allergy Clin Immunol. 2016;137:1796–1806. doi: 10.1016/j.jaci.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 40.Bonnans C, Vachier I, Chavis C, Godard P, Bousquet J, Chanez P. Lipoxins are potential endogenous antiinflammatory mediators in asthma. Am J Respir Crit Care Med. 2002;165:1531–1535. doi: 10.1164/rccm.200201-053OC. [DOI] [PubMed] [Google Scholar]
- 41.Sastre B, del Pozo V. Role of PGE2 in asthma and nonasthmatic eosinophilic bronchitis. Mediators Inflamm. 2012;2012:645383. doi: 10.1155/2012/645383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, et al. Prostaglandin D₂ pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131:1504–1512. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 44.Misso NL, Thompson P. Prostaglandins and leukotrienes: mediators of inflammation and asthma. Enfield, NH: Science Publishers; 2012. [Google Scholar]
- 45.Romieu I, Castro-Giner F, Kunzli N, Sunyer J. Air pollution, oxidative stress and dietary supplementation: a review. Eur Respir J. 2008;31:179–197. doi: 10.1183/09031936.00128106. [DOI] [PubMed] [Google Scholar]
- 46.Tashakkor AY, Chow KS, Carlsten C. Modification by antioxidant supplementation of changes in human lung function associated with air pollutant exposure: a systematic review. BMC Public Health. 2011;11:532. doi: 10.1186/1471-2458-11-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li XY, Hao L, Liu YH, Chen CY, Pai VJ, Kang JX. Protection against fine particle-induced pulmonary and systemic inflammation by omega-3 polyunsaturated fatty acids. Biochim Biophys Acta, Gen Subj. 2017;1861:577–584. doi: 10.1016/j.bbagen.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Romieu I, Garcia-Esteban R, Sunyer J, Rios C, Alcaraz-Zubeldia M, Velasco SR, et al. The effect of supplementation with omega-3 polyunsaturated fatty acids on markers of oxidative stress in elderly exposed to PM(2.5) Environ Health Perspect. 2008;116:1237–1242. doi: 10.1289/ehp.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romieu I, Barraza-Villarreal A, Escamilla-Núñez C, Texcalac-Sangrador JL, Hernandez-Cadena L, Díaz-Sánchez D, et al. Dietary intake, lung function and airway inflammation in Mexico City school children exposed to air pollutants. Respir Res. 2009;10:122. doi: 10.1186/1465-9921-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Champagne CM, Baker NB, DeLany JP, Harsha DW, Bray GA. Assessment of energy intake underreporting by doubly labeled water and observations on reported nutrient intakes in children. J Am Diet Assoc. 1998;98:426–433. doi: 10.1016/S0002-8223(98)00097-2. [DOI] [PubMed] [Google Scholar]
- 51.Burrows TL, Martin RJ, Collins CE. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J Am Diet Assoc. 2010;110:1501–1510. doi: 10.1016/j.jada.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Burrows T, Berthon B, Garg ML, Collins CE. A comparative validation of a child food frequency questionnaire using red blood cell membrane fatty acids. Eur J Clin Nutr. 2012;66:825–829. doi: 10.1038/ejcn.2012.26. [DOI] [PubMed] [Google Scholar]
- 53.Orton HD, Szabo NJ, Clare-Salzler M, Norris JM. Comparison between omega-3 and omega-6 polyunsaturated fatty acid intakes as assessed by a food frequency questionnaire and erythrocyte membrane fatty acid composition in young children. Eur J Clin Nutr. 2008;62:733–738. doi: 10.1038/sj.ejcn.1602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simons E, Curtin-Brosnan J, Buckley T, Breysse P, Eggleston PA. Indoor environmental differences between inner city and suburban homes of children with asthma. J Urban Health. 2007;84:577–590. doi: 10.1007/s11524-007-9205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Breysse PN, Buckley TJ, Williams D, Beck CM, Jo SJ, Merriman B, et al. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. Environ Res. 2005;98:167–176. doi: 10.1016/j.envres.2004.07.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.