Pulmonary arterial hypertension (PAH) is a progressive, incurable disease of the pulmonary vasculature (1). Morphological manifestations of the disease within the pulmonary vasculature include the distal extension of muscularization, concentric lesions, multicellular plexiform lesions, and infiltration with immune cells. Inflammation has been on the radar for nearly 40 years as a causal mechanism in PAH (2), but no immunomodulatory therapies are in clinical use for PAH. Growing evidence further supports a role for immune cells in PAH pathogenesis, including macrophages, mast cells, and T and B lymphocytes (1). Proinflammatory mediators also contribute to PAH pathogenesis, including IL-6 and leukotriene B4 (1). In the wake of this expanding knowledge, there are multiple clinical investigations underway that target key inflammatory pathways and immune cells in PAH (3). Such immunomodulatory therapies are much needed, as PAH remains a fatal condition.
In this issue of the Journal, Goldenberg and colleagues (pp. 1566–1569) present preclinical evidence in support of a new, more specific approach to target HMGB1 (high-mobility group box 1) in PAH (4). HMGB1 is a ubiquitous nuclear protein found in all human tissues (5). It serves as a DNA-binding protein, facilitates gene transcription, and stabilizes nucleosomes under physiological conditions (5). Yet extracellular HMGB1 occurs as a consequence of cellular injury under various pathophysiological conditions, and extracellular HMGB1 promotes inflammation, tumor growth, and, as recently shown, pulmonary hypertension induced by chronic hypoxia (5–7). HMGB1 is a ligand for TLR4 (toll-like receptor 4), and TLR4 signaling mediates some of the potent proinflammatory effects of HMGB1, such as activation of macrophages and lymphocytes and induction of proinflammatory cytokines, but also direct effects on pulmonary vascular cells (6, 7). In their study, Goldenberg and colleagues show upregulation of HMGB1 and TLR4 in the lungs from patients with PAH and localize their expression to the perivascular adventitia and to the vascular tunica intima (4). In preclinical experiments, Goldenberg and coworkers first show that an anti-HMGB1 antibody attenuates severe pulmonary hypertension induced by monocrotaline or chronic hypoxia combined with SU5416 in rats (4). The use of these two rat models further represents an advancement toward models of severe pulmonary hypertension and, in the case of chronic hypoxia/SU5416 rats, toward a model of occlusive pulmonary arteriopathy. Yet the authors also recognize that inhibition of HMGB1 needs to occur in an appropriate cellular context. The different potential HMGB1 activation states lead to context-dependent variations of HMGB1 function, and hence targeting of HMGB1 could result in significant off-target effects (8). This is well illustrated by the fact that HMGB1 knockout mice fall prey to deadly hypoglycemia during the perinatal period (9). To overcome the issue of off-target effects, the authors used P5779, a new peptide that specifically binds to the TLR4 adaptor protein MD-2 (10). By binding to MD-2, P5779 prevents the interaction between MD-2 and the cytokine-inducing, disulfide-bond form of extracellular HMGB1, thus blocking HMGB1-induced TLR4 signaling. In this way, it adds significant specificity to the therapeutic approach, leaving many of the physiological functions of both intracellular HMGB1 and non–HMGB1-dependent TLR4 signaling unaffected (10). The encouraging results in the study by Goldenberg and colleagues show that P5779 attenuates the development and progression of pulmonary hypertension in the two rat models, chronic hypoxia combined with SU5416 and monocrotaline (4). A summary of the mechanism and effects of P5779 in the context of pulmonary hypertension models is provided in Figure 1. Goldenberg and colleagues further describe that no adverse effects were noted on cardiac function in these rat models (4). Moreover, in vitro, P5779 attenuates the effects of HMGB1 on migration of pulmonary artery smooth muscle cells and pulmonary artery endothelial cells, two important structural cell types with pathophysiological relevance in PAH. Goldenberg and colleagues not only further our understanding of the role of HMGB1/TLR4-associated inflammation in PAH but also provide a more specific way to target this pathway (4).
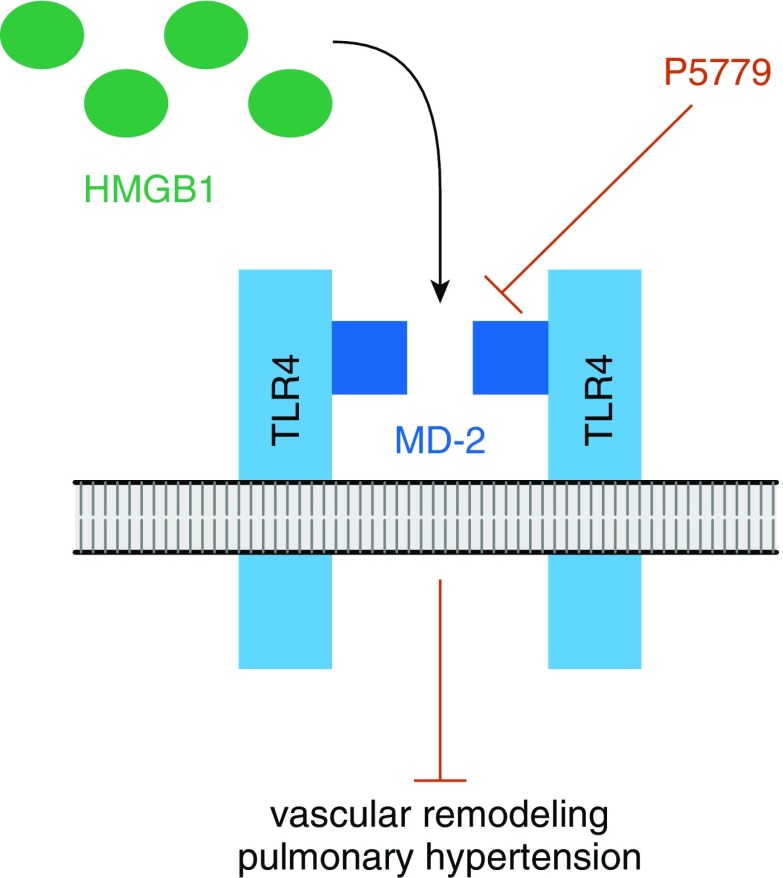
Figure 1.
Summary of the inhibitory action of P5779. P5779 inhibits the TLR4 (toll-like receptor 4)-adapter molecule MD-2 and hence blocks the interaction between extracellular HMGB1 (high-mobility group box 1) in its disulfide form and MD-2. In consequence, P5779 specifically inhibits the signaling induced by extracellular HMGB1 via TLR4. P5779 does not impact intracellular HMGB1 or TLR4 signaling that is independent of HMGB1. The in vivo experiments by Goldenberg and colleagues (4) show that P5779 reduces vascular remodeling and pulmonary hypertension during development and progression of pulmonary hypertension in two disease models.
Although the study by Goldenberg and colleagues is an important and interesting step toward disease-specific immunomodulation, some limitations are present (4). The authors do not investigate the effect of P5779 on immune cells, and their study does not contribute to a better understanding of whether P5779 modulates other essential pathways in pulmonary vascular cells. This is of particular interest, as impaired bone morphogenic protein signaling is a hallmark of endothelial PAH pathobiology, and HMGB1/TLR4 signaling can suppress bone morphogenic protein signaling (11, 12). Furthermore, as with any promising preclinical study, the path forward to clinical drug development may be difficult. The safety of P5779 in humans is unknown, and therefore it is unclear if its specificity will translate to acceptable side effects in human patients. The short half-life of P5779 could further complicate future use in clinical studies. Goldenberg and colleagues discuss that this short half-life of P5779 may be an advantage, as it will make it easier to limit treatment duration (4). However, the short half-life also raises the possibility of complicated drug delivery in humans, which could lead to an expensive therapy in a disease that needs more-affordable and less-cumbersome treatments.
We commend Goldenberg and colleagues for their findings, which contribute new information to the field of pulmonary vascular disease and offer a promising avenue for immunomodulatory therapy in patients with PAH (4).
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201902-0388ED on April 11, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herget J, Palecek F, Preclík P, Cermáková M, Vízek M, Petrovická M. Pulmonary hypertension induced by repeated pulmonary inflammation in the rat. J Appl Physiol. 1981;51:755–761. doi: 10.1152/jappl.1981.51.3.755. [DOI] [PubMed] [Google Scholar]
- 3.Spiekerkoetter E, Kawut SM, de Jesus Perez VA. New and emerging therapies for pulmonary arterial hypertension. Annu Rev Med. 2019;70:45–59. doi: 10.1146/annurev-med-041717-085955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldenberg NM, Hu Y, Hu X, Volchuk A, Zhao YD, Kucherenko MM, et al. Therapeutic targeting of high-mobility group box-1 in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2019;199:1566–1569. doi: 10.1164/rccm.201808-1597LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 6.Bauer EM, Shapiro R, Zheng H, Ahmad F, Ishizawar D, Comhair SA, et al. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med. 2013;18:1509–1518. doi: 10.2119/molmed.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer EM, Shapiro R, Billiar TR, Bauer PM. High mobility group Box 1 inhibits human pulmonary artery endothelial cell migration via a Toll-like receptor 4- and interferon response factor 3-dependent mechanism(s) J Biol Chem. 2013;288:1365–1373. doi: 10.1074/jbc.M112.434142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 9.Calogero S, Grassi F, Aguzzi A, Voigtländer T, Ferrier P, Ferrari S, et al. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet. 1999;22:276–280. doi: 10.1038/10338. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, Wang H, Ju Z, Ragab AA, Lundbäck P, Long W, et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J Exp Med. 2015;212:5–14. doi: 10.1084/jem.20141318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Tian XT, Peng Z, Li WQ, Cao YY, Li Y, et al. HMGB1/TLR4 promotes hypoxic pulmonary hypertension via suppressing BMPR2 signaling. Vascul Pharmacol. doi: 10.1016/j.vph.2018.12.006. [online ahead of print] 3 Jan 2019; DOI: 10.1016/j.vph.2018.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.