Abstract
A wide array of different factors and processes have been linked to the biochemical underpinnings of glioblastoma multiforme (GBM) and glioblastoma stem cells (GSC), with no clear framework in which these may be integrated. Consequently, treatment of GBM/GSC is generally regarded as very poor. This article provides a framework that is based on alterations in the regulation of the melatonergic pathways within mitochondria of GBM/GSC. It is proposed that the presence of high levels of mitochondria-synthesized melatonin is toxic to GBM/GSC, with a number of processes in GBM/GSC acting to limit melatonin’s synthesis in mitochondria. One such factor is the aryl hydrocarbon receptor, which increases cytochrome P450 (CYP)1b1 in mitochondria, leading to the ‘backward’ conversion of melatonin to N-acetylserotonin (NAS). N-acetylserotonin has some similar, but some important differential effects compared with melatonin, including its activation of the tyrosine receptor kinase B (TrkB) receptor. TrkB activation is important to GBM/GSC survival and proliferation. A plethora of significant, but previously disparate, data on GBM/GSC can then be integrated within this framework, including miR-451, AMP-activated protein kinase (AMPK)/mTOR, 14-3-3 proteins, sirtuins, tryptophan 2,3-dioxygenase, and the kynurenine pathways. Such a conceptualization provides a framework for the development of more effective treatment for this poorly managed condition.
Keywords: glioblastoma; glioblastoma stem-like cell; melatonin; kynurenine; N-acetylserotonin; mitochondria; aryl hydrocarbon receptor; tryptophan 2,3-dioxygenase; sirtuins; treatment
Introduction
A plethora of intracellular processes, receptors, and epigenetic processes have been proposed to regulate glioblastoma multiforme (GBM)/glioblastoma stem cell (GSC) survival, proliferation, and migration. Consequently, there is a wide array of disparate data for this poorly managed condition, including alterations in microRNAs (miRNAs), 14-3-3 proteins, AMP-activated protein kinase (AMPK), mammalian target of rapamycin (mTOR), sirtuins, aryl hydrocarbon receptor (AhR), endoplasmic reticulum (ER), mTOR, sphingosine-1-phosphate (S1P) receptors and levels, small GTPases, kynurenine pathway products, tyrosine receptor kinase B (TrkB), chromosome 4q35, purinergic signalling, shifting between glycolysis and oxidative phosphorylation, and alterations in the melatonergic pathway regulation.1 All of these changes have been linked to alterations in mitochondria functioning and the interaction of GBM/GSC with other cells in the tumour microenvironment.1
The microenvironments, both within and surrounding GBM/GSC, are important determinants of GBM/GSC gene expressions, which have been proposed to form ‘go or grow’ phenotypes. This suggests a shifting between proliferation and motility, an idea that has maintained popularity over a couple of decades.2 Microenvironments are clearly important, with unfavourable environments, such as lower oxygen/nutrients, inducing migration in search of better conditions, while limiting proliferation capacity. Attempts at treatment can interact with such microenvironment-driven changes in GBM/GSC phenotypes, with bevacizumab increasing the more migratory phenotype.3 As such, GBM/GSC expression patterns are in intimate interaction with the cells and fluxes of the local microenvironment.
This article reviews these commonly described changes that underpin the pathophysiological complexity of GBM/GSC, before integrating these factors and processes in a model that highlights the underexplored role of the melatonergic pathway in modulating, and mediating, the mitochondria-driven changes in GBM/GSC and the tumour microenvironment.
Common Biochemical Changes in GBM/GSC
miR-451
A plethora of data show a role for alterations in the miRNA, miR-451, in the pathophysiology of GBM/GSC.4,5 Interestingly, the tumour microenvironment seems to modulate miR-451 levels in GBM/GSC.6 Zhao and colleagues6 found miR-451 levels to be relatively low in central regions and high in peripheral regions of GBM, with higher miR-451 levels facilitating proliferation, while repressing migration.6 Such data indicate the importance of miR-451 in determining GBM/GSC responses, as well as highlighting how putative phenotypes may exemplify microenvironment-driven heterogeneity and plasticity of responses. Recent work suggests that the 14-3-3 protein, AMPK-mTOR pathway, and the small GTPase, Rac1, may be intimately associated with such alterations in miR-451 levels,6–8 highlighting the role that miR-451 can play in the heterogeneous biological underpinnings of GBM/GSC. It is important to note that miR-451 represses levels of 14-3-3ζ protein, which is necessary for the stabilization of aralkylamine N-acetyltransferase (AANAT) and therefore for N-acetylserotonin (NAS) synthesis and the activation of the melatonergic pathways.9 Variations in miR-451 may therefore be associated with the differential regulation of the melatonergic pathways.
miR-7
Another miRNA, miR-7, can inhibit both cell migration and proliferation in GBM/GSC,10 as well as increasing sensitivity to treatment in drug-resistant GBM cells.11 Such effects seem mediated by the regulation of the transcription factor, Yin Yang (YY)1.11 Interestingly, miR-7 inhibits NAS and melatonin synthesis in the porcine pineal gland,12 while YY1 is a significant regulator of retinal NAS and melatonin synthesis.13 Importantly, miR-7 also downregulates 14-3-3ζ in glioma cell lines,14 suggesting that some of the impacts of miR-7 may be via a decrease in the stability of AANAT and therefore of NAS synthesis.
14-3-3
Increased levels of various isoforms of 14-3-3 protein are associated with apoptosis resistance and poorer survival rates in GBM/GSC patients.15,16 Downregulation of 14-3-3ζ sensitizes human glioblastoma cells to apoptosis induction, with higher levels of 14-3-3ζ being associated with a higher grade of glioma.17 Higher levels of 14-3-3 are also evident in GSC.18
As indicated above, both miR-7 and miR-451 can repress 14-3-3ζ in both tumour and nonneoplastic cells.8,14,19 The loss of 14-3-3 sensitizes GBM/GSC to apoptosis as well as preventing NAS synthesis, thereby preventing any trophic effects of NAS via the TrkB. N-acetylserotonin is a brain-derived neurotrophic factor (BDNF) mimic and activates the BDNF receptor, TrkB, which is significant driver of GSC proliferation. As such, the miRNAs regulation of 14-3-3, which stabilizes AANAT and therefore NAS synthesis, is a significant modulator of TrkB-mediated GSC proliferation. It should also be noted that the conversion of NAS to melatonin may be prevented by a number of processes, allowing variations in the NAS/melatonin ratio to be regulated by GBM/GSC processes, as detailed in the following.
Kynurenine and AhR
The AhR is also a known modulator of GBM/GSC, including contributing to genotoxic damage.20 These authors show that the activation of tryptophan-2,3-dioxygenase (TDO), leading to the production of AhR ligands, kynurenine, and kynurenic acid, is a major AhR activator in GBM/GSC.20 The AhR modulates, and can be modulated by, a number of miRNAs.21 However, it requires investigation as to whether the AhR regulates, or is regulated by, miRNAS that can increase the NAS/melatonin ratio and therefore TrkB activation. It is also of note that the activation of the kynurenine pathways via TDO could decrease the levels of tryptophan availability for the synthesis of serotonin, NAS, and melatonin.
Interestingly, GBM-derived kynurenine can significantly modulate the immune responses in the tumour microenvironment.22 These authors showed kynurenine to activate the AhR in tumour-associated macrophages, a process involving the suppression of macrophage NF-κB, in turn modulating macrophage responses and the influence of macrophages on the activity of other immune cells in the tumour environment.22 It may be of note that NF-κB activation in macrophages is necessary for the induction and release of melatonin by these cells, suggesting that TDO-induced kynurenine, via macrophage NF-κB inhibition, may act to inhibit the release and effects of melatonin in the tumour microenvironment.23 Melatonin may also act to inhibit the AhR.24
It should also be noted that the AhR-activating effects of kynurenine may be distinct from those of kynurenic acid in the tumour microenvironment. Although both TDO products can activate the AhR, kynurenic acid may significantly inhibit the effects of the high levels of glutamate released by GBM/GSC,25 given that kynurenic acid inhibits N-methyl-d-aspartate (NMDA) receptor activation.26 The high levels of NMDA receptor–mediated excitotoxic damage induced by GBM glutamate release is important in neighbour cell death and often thought to contribute to pathway clearance for proliferating and migrating GBM/GSC. As such, kynurenine may be more likely to benefit GBM/GSC survival and migration.
AMP-activated protein kinase
AMP-activated protein kinase has an important role in cellular energy homeostasis, being generally associated with driving the uptake of glucose and fatty acids and increasing levels of oxidation when cellular energy/mitochondria functioning levels are low. AMP-activated protein kinase has classically been seen as a tumour suppressor, with a large body of data showing AMPK to inhibit cell growth. However, such inhibition can be transient, allowing AMPK to provide cancer cells pro-survival flexibility under different challenging conditions. Glioblastoma multiforme can hijack the AMPK-regulated stress response pathway, which is highly conserved in nonneoplastic cells, given the high levels of AMPK isoforms expressed in GBM.27 These authors also show that AMPK inhibition reduces the viability of patient-derived GSCs.27
AMPK is also intimately linked to the regulation of miR-451, which has been proposed to be a mechanism whereby GBM may adapt their cellular phenotype under different stress conditions4,28. AMPK/miR-451 are proposed to form a negative reciprocal feedback loop, suggesting that miR-451 may be finely regulated under different conditions.4 This may be complicated by the effects of miR-451 on AMPK regulation and other processes in different cell types, with miR-451 antagonism increasing AMPK activity in intestinal epithelial cells,29 while in GBM it is proposed that miR-451 mediates the shifting of GBM from proliferation to migration in response to different environmental conditions.6 This indicates that AMPK and the synthesis of NAS may be dynamically regulated in association with miR-451 levels.
mTOR
mTOR regulates cell metabolism and proliferation and is increased in aggressive tumours, including GBM/GSC, contributing to heightened levels of glucose uptake and utilization.30 It is proposed that a decrease in miR-451 in low-glucose environments contributes to an increase in AMPK and decrease in mTOR, which drives higher levels of migration, while relatively increased miR-451 in higher glucose environments will lead to a decrease in AMPK and increase in mTOR leading to higher levels of proliferation.31 As such, alterations in the availability of glucose, via changes in miR-451, may be associated with the proliferation/migration balance via changes in the AMPK-mTOR pathway. Such a schema has readily lent itself to a perspective of 2 GBM/GSC phenotypes.4 As indicated above, miR-451 would also concurrently differentially regulate the melatonergic pathways in mitochondria, again indicating that the NAS synthesis and its activation of TrkB may be dynamically regulated in association with AMPK, mTOR, and miR-451, in response to different microenvironments.
Purinergic signalling
A number of studies have investigated the role of extracellular ATP, acting via the P2 receptors, in the pathophysiology of GBM/GSC. Interestingly, 10-150 μM ATP at the P2Y1 receptor, or P2Y1 receptor agonists, increases GSC proliferation.32 These authors, and other research labs, have shown that significantly higher extracellular levels of ATP lead to a decrease in GSC proliferation and at dramatically higher levels, via the P2X7 receptor, may lead to apoptosis.33 As such, extracellular ATP can have variable concentration-dependent effects via distinct purinergic receptors. It should be noted that the P2Y1 receptor can also act to significantly increase the NAS/melatonin ratio, as shown in pinealocytes,34 indicating that P2Y1 receptor activation will also increase NAS effects at the TrkB, as detailed in the following.
S1P and small GTPases
It would seem likely that GBM/GSC proliferation and migration arises from an increase in S1P receptor-induced small GTPase35 and that variations in migration may be linked to alterations in the levels of S1P receptor subtypes and sphingosine kinase–induced S1P36 in rearranged rafts, paralleling such changes in neurogenesis.37 As to whether the P2Y1 receptor modulates the S1P receptors and small GTPases as in platelets requires investigation in GBM/GSC,38 including as to whether any concurrent changes in the NAS/melatonin ratio are evident. Rearranged lipid rafts, and associated receptor clusters, are an integral aspect of the plasticity of GBM/GSC, especially in response to tumour environment changes. Recent work showing that dysregulating cholesterol synthesis and uptake in the tumour microenvironment is damaging to GBM/GSC likely reflects such alterations in raft-associated receptors and the changes in intracellular pathways that occur, including in S1P receptor subtype–driven small GTPases.39 As such, alterations in S1P receptor subtypes in rearranged rafts will be coordinated with alterations in AMPK/mTOR/miR-451 and the regulation of NAS levels and effects at TrkB.
TrkB
TrkB is a neurotrophin receptor, which mediates many of the trophic effects of BDNF. TrkB is expressed on mitochondria membranes, suggesting that TrkB is well placed to be activated by mitochondria TrkB ligands, such as NAS.40 TrkB increases the proliferation of GSC.41 GBM/GSC exosomes containing TrkB are also important in transferring aggressiveness to neighbouring GBM,42 suggesting that trophic activation of TrkB is important to the pathophysiology of GBM/GSC. As noted, the melatonin precursor, NAS, has BDNF mimicking effects via the TrkB,43 suggesting that NAS synthesis and release could be an important factor in the initiation of aggressive processes in GBM/GSC. It may also be of note that NAS can increase BDNF synthesis and release, as shown in the murine dentate gyrus.44 The induction of BDNF seems of some importance in GBM, and maybe especially GSC, with a decrease in miR-210 contributing to increased BDNF expression in GSC.45 Anti-TrkB pharmaceuticals show some promise in tumour management in rodent models.46
4q35
Chromosome region 4q35 is a not an uncommon deletion in GBM and some other cancers, often in association with the tumour suppressor gene, FAT1,47 which is sited at 4q35.48 It is also of note that the melatonin MT1 receptor, which is frequently silenced in many different tumours,49 is also sited at 4q35.50 As well as the inhibition of melatonin synthesis, melatonin’s effects via the MT1 receptor may also be prevented in some GBM/GSC.
Sirtuins
Most sirtuins are NAD+-dependent deacetylases and linked to cell survival, longevity, and optimized mitochondria functioning in nonneoplastic cells. Being NAD+ dependent links sirtuin activity to the availability of cellular NAD+, including its induction at the end of the kynurenine pathway. A number of different sirtuins have been associated with the regulation of GBM/GSC. Sirtuin-3, which is predominantly mitochondria-located, interacts with the mitochondrial chaperone, TRAP1, to modulate the mitochondria response to stress and maintain the ‘stemness’ of GSC.51 Factors that act to inhibit sirtuin-3 lead to a decrease in the viability of GBM,52 with factors acting to increase sirtuin-3 leading to an increased radioresistance in GBM cell lines.53 Melatonin and sirtuin-3 are proposed not only to interact but also to have coevolved in mitochondria, suggesting that the putative loss of melatonin synthesis in mitochondria may modulate the functions and effects of sirtuin-3. As such, an increase in the NAS/melatonin ratio leads to the loss of melatonin’s mitochondria effects, as well as the trophic effects of NAS.
In contrast, the induction of sirtuin-1 leads to apoptosis in some GBM cell lines,54 while other data indicate that sirtuin-1 inhibition is important for apoptosis via its regulation of p53.55 Such superficially conflicting data highlight the importance of other concurrent changes in tumour cells. Data indicate that some of the effects of sirtuin-1 may be mediated by its deacetylation of sirtuin-3,56 which, as noted above, may be at least partly dependent on the synthesis of melatonin within mitochondria. It requires investigation as to how the regulation of the melatonergic pathways in GBM/GSC interacts with the levels and effects of different sirtuins, including as to how increased NAS synthesis and TrkB activation interact with sirtuin-1 effects in GBM/GSC.
Overall, the above highlights the complex sets of data that have been collected on the pathophysiological processes evident in GBM/GSC. These factors and processes may be determined by core changes involving the AhR, IDO, and kynurenine metabolites in the upregulation of the NAS/melatonin ratio and consequent TrkB activation by NAS. The integration of the wider bodies of data of GBM/GSC in the following is mediated via their association with these key processes.
Integrative Model
There are many conflicting results as to the pathophysiological changes occurring in GBM/GSC, with a plethora of intracellular processes, receptors, and epigenetic processes shown to occur and have relevance to GBM/GSC survival, proliferation, and migration. This arises to some degree due to differences in cell lines and in primary samples used, as well as dynamic interactions of GBM/GSC with an ever-changing microenvironment. As to whether the orchestrated changes in response to local microenvironmental factors can be explained by oscillations between 2 phenotypes, migratory and proliferative, is probably simplistic. Intracellular heterogeneity is long appreciated as an important factor in GBM, as in most other tumours.57 GSC can show considerable variability in energy production and metabolism, with some showing a predominant utilization of glycolysis, while others use oxidative phosphorylation.58 It would seem more likely that such a gradation of reactive responses reflects plasticity responses to microenvironment changes. It would also seem that alterations in mitochondria functioning are at the core of such plasticity responses.
Recent work suggests that many, if not all, mitochondria-containing cells produce NAS and melatonin, quite likely within mitochondria, including within glia, neurons, and immune cells.59–61 This has still to be determined within GBM/GSC. However, the presence of melatonin within GBM/GSC is likely to prove problematic, given that melatonin in these cells, as in most tumours, inhibits proliferation and migration as well as inducing apoptosis.62 This would suggest that GBM/GSC, as with most tumours, would have their behaviour and survival threatened unless they are able to suppress melatonin synthesis and/or melatonin’s effects. The synthesis and presence of melatonin within mitochondria would be expected to modulate many of the key mitochondria processes that underpin the changes in transcription and patterned gene expressions as well as the variable proliferation and migratory responses that can occur in GBM/GSC.
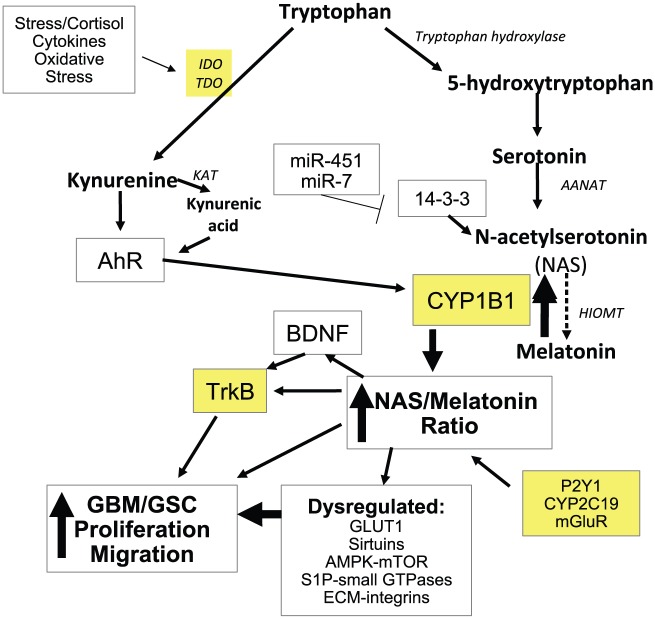
It should be emphasized that NAS, as well as being the immediate precursor of melatonin synthesis, is usually also co-released with melatonin and has many similar attributes to melatonin, including being a powerful antioxidant and anti-inflammatory. However, NAS has some distinct effects, including the activation of the neurotrophin receptor, TrkB.43 Given that TrkB is an important regulator of GBM/GSC survival and of the spread of aggressiveness, at least in part via GBM/GSC exosomes,42 variations in the regulation of the NAS/melatonin ratio may be of some importance. A wide array of previously disparate data in GBM/GSC may be intimately linked to the regulation of NAS and melatonin synthesis and uptake in GBM/GSC, including data on miRNAs, PEPT1/2, 14-3-3 proteins, AMPK, mTOR, sirtuins, AhR, and S1P as well as the small GTPases, Rac1, and RhoA (see Figure 1).
Figure 1.
The activation of TDO, and possibly IDO, leads to an increase in kynurenine and kynurenic acid, which can activate the AhR, leading to a number of important changes in GBM/GSC, including the dramatic increase in CYP1B1 in mitochondria. Mitochondria CYP1B1 leads to the conversion of melatonin to NAS, which can then activate TrkB directly as well as via BDNF induction. This can increase the proliferation and migration of GBM/GSC. Such an increase in the NAS/melatonin ratio may also be mediated by CYP2C19, P2Y1 receptor, and mGluR. A number of microRNAs, including miR-451 and miR-7, via 14-3-3 regulation, will also modulate NAS and melatonin synthesis, given 14-3-3 is necessary for the stabilization of AANAT. The loss of melatonin and increase in NAS will have impacts on sirtuins, AMPK-mTOR, GLUT1, S1P-small GTPases, and ECM-integrin interactions. Such changes have significant impacts on mitochondria functioning and therefore in responses to the dynamic alterations evident in the tumour microenvironment, including ECM interactions with the integrins and the regulation of cholesterol uptake and membrane lipid rafts. Sites for treatment targets are highlighted in yellow. AANAT indicates aralkylamine N-acetyltransferase; AhR, aryl hydrocarbon receptor; AMPK, AMP-activated protein kinase; BDNF, brain-derived neurotrophic factor; CYP, cytochrome P450; ECM, extracellular matrix; GBM, glioblastoma multiforme; GSC, glioblastoma stem-like cells; GLUT1, glucose transporter 1; HIOMT, N-Acetylserotonin O-methyltransferase; IDO, indoleamine 2,3-dioxygenase; KAT, kynurenine aminotransferase; mGluR, metabotropic glutamate receptor; NAS, N-acetylserotonin; P2Y1, purinergic receptor; S1P, sphingosine-1-phosphate; TDO, tryptophan 2,3-dioxygenase; TrkB, tyrosine receptor kinase B.
A number of factors are known to increase the NAS/melatonin ratio. Melatonin can be converted back to NAS by a process of O-demethylation, which may be driven by CYP2C19.63 Perhaps more important in GBM/GSC, and many other tumours, melatonin can be directly converted back to NAS by CYP1B1, with CYP1B1 being predominantly present in mitochondria.64,65 As noted above, the AhR is an important regulator of GBM/GSC. In nonneoplastic cells, AhR-induced CYP1B1 in mitochondria leads to increased oxidative stress and suboptimal mitochondria functioning,65 highlighting the clear impact that CYP1B1 can have on core mitochondria processes. Importantly, CYP1B1 is highly overexpressed in a wide array of different tumours, including lung, breast, hepatic, kidney, skin, gastrointestinal, prostate, bladder, and ovarian cancers, while CYP1B1 is absent or shows very low expression in healthy tissue.66–73 Clearly, the dramatic increase in mitochondria CYP1B1 in GBM/GSC and most other cancers suggests a significant role for CYP1B1 in mitochondria functioning in tumour cells.
Interestingly, CYP1B1 is powerfully induced by the AhR in inflammatory breast cancer stem cells,67 suggesting that AhR activation in cancer stem cells may be significant regulators of CYP1B1 and therefore of the NAS/melatonin ratio. This would suggest that TDO induction and consequent kynurenine and kynurenic acid activation of the AhR may dramatically increase the NAS/melatonin ratio, via CYP1B1, while also decreasing the levels of tryptophan available for NAS and melatonin synthesis. This could suggest lower levels of NAS and melatonin, but a dramatic increase in the NAS/melatonin ratio. CYP1B1 is inhibited by miR-187-5p in non-small-cell lung cancers,69 and it requires investigation as to whether miR-187-5p, or other miRNAs, modulate GBM/GSC, including via the regulation of CYP1B1.
AhR induction of mitochondria CYP1B1 suggests that many of the other processes regulated by the AhR and altered in GBM may be coordinated with an increase in CYP1B1 and NAS/melatonin ratio. Transforming growth factor (TGF)-β levels are raised in GBM/GSC, being associated with increased proliferation and migration.74 TGF-β may also be intimately associated with cholesterol regulation in GBM/GSC, with the putative efficacy of statins in GBM/GSC treatment being mediated via a decrease in TGF-β.75 These authors found statins to reduce TGF-β activity, cell viability and invasiveness, small GTPase activity, and Smad3 phosphorylation and activity, as well as the expression of TGF-β targets ZYX and SERPINE1 in GBM and GSC cells. The AhR is a major determinant of TGF-β levels and effects in GBM, at least in part via the regulation of integrins and their interaction with the extracellular matrix.76 As such, AhR induction of mitochondria CYP1B1 may be linked to not only changes in the melatonergic pathways and other GBM/GSC factors but also to alterations in the intercellular interactions that occur in the tumour microenvironment.
It is also of note that AANAT, which generates NAS, has been found in different mitochondria compartments, namely, the matrix and intermembrane space, as reviewed in Tan and Reiter.59 It is unclear as to why this should be the case, although these authors propose that AANAT is active when within the matrix, primarily as there is a ready availability of N-acetyl-coenzyme A substrate within the matrix, with localization in the mitochondria intramembrane space indicating AANAT that is either in the process of being transported to the matrix or stored there for when required and/or is being silenced by its reverse transport from the matrix. The putative transporters, and factors that could act to regulate such transport, between mitochondria compartments have still to be investigated, which may be important to determine, given the relevance of the melatonergic pathways to the regulation of GBM/GSC mitochondria functioning.
As well as CYP1B1 and CYP2C19 impacts on melatonin conversion to NAS, other factors and processes may also modulate the NAS/melatonin ratio. In pinealocytes, ATP via the P2Y1 receptor potentiates NE-induced NAS, which is also associated with an inhibition of NAS conversion to melatonin.34 Interestingly, 10-150 μM ATP at the P2Y1 receptor, or P2Y1 receptor agonists, increases GSC proliferation.32 These authors, and other researcher labs, have shown that significantly higher extracellular levels of ATP lead to a decrease in GSC proliferation and at dramatically higher levels via the P2X7 receptor may lead to apoptosis.33 It requires investigation of GSC as to whether such differential effects of different concentrations of ATP have similar impacts on the NAS/melatonin ratio. It will be important to determine the intracellular processes involved in ATP’s concentration-dependent regulation of the NAS/melatonin ratio, including as to whether this involves the upregulation of CYP1B1 or as to whether the P2Y1 receptor is another route whereby GBM/GSC may act to regulate the NAS/melatonin ratio.
There is little data on the role of the P2Y1 receptor in the regulation of GBM/GSC, although in some cell types P2Y1 receptor activation induces protein kinase C (PKC)δ, with PKCδ being an important regulator of many GBM/GSC processes.77,78 The P2Y1 receptor is also associated with the regulation of astrocyte AMPK,79 mTOR-associated migratory effects,80 and astrocyte excitability,81 suggesting impacts on processes that are important to the pathophysiology of GBM/GSC, as well as indicating a role for P2Y1 receptors in the wider tumour microenvironment. The high levels of glutamate efflux by GBM/GSC would also increase CYP1B1 in astrocytes, via the metabotropic glutamate receptor (mGluR)5.82 There is increasing interest in the roles of mGluRs in GBM/GSC,83 which may include paracrine and autocrine effects on CYP1B1 and an increased NAS/melatonin ratio, as well as the classical role of glutamate in excitotoxicity-driven ‘pathway clearance’.25
Although the synthesis and uptake of melatonin would seem dangerous to the survival of GBM/GSC, both GBM and GSC have high levels of proton-driven oligopeptide transporter (PEPT1/2),84 which can uptake melatonin into mitochondria in cancer cells.85 This could suggest that mitochondria in GSC, as with other cancer cells, may be capable of high melatonin uptake.85 It is also of note that other factors are also taken up by PEPT1/2, including 5-aminolevulinic acid (5-ALA)-induced protoporphyrin IX, which is highly expressed in GSC.84 The presence of high levels of PEPT1/2 on the mitochondria of GSC could suggest that these cells may be importantly regulated by melatonin, including that released by neighbouring cells. The heightened expression of PEPT1/2 in GBM/GSC has driven the search for peptides that may be useful in the treatment of GBM/GSC and most other cancers.86
Another means by which melatonin production in the tumour microenvironment may act to regulate GBM/GSC is via the regulation of glucose uptake. Melatonin can be taken up by the glucose transporter (GLUT1), with melatonin uptake being upregulated by the presence of glucose.87 Melatonin also lowers levels of glucose uptake and modifies GLUT1 transporter expression levels, as shown in prostate cancer cells.88 It is important to note that in these cells, glucose supplementation promotes prostate cancer progression, with melatonin attenuating this glucose-induced tumour progression.88 This may have some relevance to the survival rates in GBM patients, given that hyperglycaemia is associated with a lower survival duration.89 Melatonin reduced lactate labelling in prostate cancer cells as well as decreasing tricarboxylic acid cycle metabolites and ATP production.88
Such alterations in energy availability are likely to have significant impacts on mitochondria functioning in GBM/GSC, with melatonin’s inhibition of glucose uptake via the GLUT1 likely to contribute to the suppression of melatonin synthesis in these cells. It is also likely that this will impact on the contents of vesicles and exosomes produced by GBM/GSC, such as miRNAs, with consequences for regulation of glia and immune cells in the tumour microenvironment.90
As such, alterations in the NAS/melatonin ratio have links to longstanding ideas regarding the nature of the tumour microenvironment and its influence on tumour survival, indicating that GBM/GSC may have a number of means in shaping this tumour microenvironment, including variations in the NAS/melatonin ratio. This suggests a dynamic 2-way interaction of tumour and tumour microenvironment, which would be modulated by levels of melatonin synthesis and uptake.
It should be noted that CYP1B1 modulates oestrogen effects, suggesting that some of its effects in hormone-modulated cancers, such as breast cancer, may confound its putative effects via the NAS/melatonin ratio, especially as melatonin has some negative regulatory effects on the oestrogen receptor-alpha.91 It is also of note that the conversion of melatonin to NAS by CYP1B1 has been proposed to induce apoptosis in neural cancer cells.64 Clearly, this is an area needing extensive investigation across different cancer cell types, including as to confounding by oestrogen regulation and TrkB expression. It should also be noted that CYP1B1, like CYP1A1 and CYP1A2, can be localized to the ERs, as well as mitochondria, as shown in the mouse.92 Mitochondria reticulae and ER are in a close and dynamic spatial and functional relationship, especially around the nucleus, with these interfaces, referred to as mitochondria-associated membranes (MAM). Mitochondria-associated membranes regulate a plethora of functions, including lipid metabolism, Ca2+ signalling, mitochondrial maintenance regulation, and programmed cell death/survival,93 with the ER also proposed to have a role in the cause and progression of GBM/GSC.94 The relevance of the putative shift in the NAS/melatonin ratio and increased expressions of CYP1 to alterations in the spatiotemporal and functional interactions of mitochondria and ERs requires investigation.
Alterations in ER functioning are relevant to GSC survival, with stearoyl CoA desaturase (SCD1) being essential for ER homeostasis and GSC survival and proliferation.94 The pharmacological inhibition of SCD1 is toxic to GSC due to saturated fatty acids accumulation, which exacerbates ER stress, impairs DNA repair, and increases apoptosis. These authors found SCD1 inhibition to provide a 25%-100% survival rate in xenograft mouse models. The ER sensor inositol-requiring enzyme 1 (IRE1) drives the shift to apoptosis in these GSC cells when SCD1 induction is prevented.94 Interestingly, melatonin may act to suppress the induction of SCD1.95
It is also notable that sodium butyrate induces senescence and inhibits GBM invasiveness.96 Other studies show sodium butyrate to increase the differentiation of U87 glioblastoma cell lines into cholinergic neurons97 and to increase the radiosensitivity of another GBM cell line.98 Such effects have been attributed to the histone deacetylase (HDAC) inhibitory effects of sodium butyrate.99 However, it should be noted that sodium butyrate can also induce an increase in AANAT, N-acetylserotonin O-methyltransferase (HIOMT), and therefore NAS and melatonin synthesis, as shown in intestinal epithelial cells.100 As to how relevant such effects are in GBM/GSC will be important to determine. Sodium butyrate can also increase the sensitivity of tumour cells to natural killer cells,101 while also regulating autophagy via AMPK signalling.102 Sodium butyrate can increase levels of CYP1A1 in colon cancers.103 It requires investigation as to whether sodium butyrate would have any impact on the levels of mitochondria-associated CYP1B1 in GBM/GSC. Sodium butyrate is the commercially available salt version of the short-chain fatty acid produced by the intestinal microbiome, butyrate. This could suggest a role for alterations in the gut microbiome to the cause and/or course of GBM/GSC, given that butyrate readily crosses into the general circulation, where it acts on mitochondria, including immune cells, mimicking many of the effects of melatonin.
Conclusions
As indicated above, there are a plethora of disparate bodies of data on the biochemical alterations occurring in GBM/GSC, without any clear framework whereby such data could be integrated. Consequently, pharmaceutical treatment targets have been inadequately guided by research to date. This article provides a framework that links a wide array of previously disparate data and provides a rationale for the distinct cellular organizations and intracellular processes that are evident in GBM/GSC and other cancers. It is proposed that the normally highly beneficial effects of melatonin in mitochondria are toxic in GBM/GSC and other cancers, with many of the cellular organization and inductions driven by the need to shift melatonin to NAS synthesis, leading to trophic effects of NAS on TrkB, which increases GBM/GSC survival and proliferation. A core aspect of this framework is the TDO induction of kynurenine and kynurenic acid, which activates the AhR, leading to a dramatic increase in mitochondria CYP1B1, in turn dramatically increasing the NAS/melatonin ratio. Many of the risk factors and alterations in expression can be linked to such processes, including miR-451, AMPK/mTOR, 14-3-3 proteins, purinergic signalling, sirtuins, tryptophan 2,3-dioxygenase, and the kynurenine pathways. Such a conceptualization provides a framework for the development of more effective treatment for this poorly managed condition.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Both authors contributed to this article.
Disclosure and Ethics:This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material
References
- 1. Beischlag TV, Anderson G, Mazzoccoli G. Glioma: tryptophan catabolite and melatoninergic pathways link microRNA, 14-3-3, chromosome 4q35, epigenetic processes and other glioma biochemical changes. Curr Pharm Des. 2016;22:1033–1048. [DOI] [PubMed] [Google Scholar]
- 2. Giese A, Loo MA, Tran N, Haskett D, Coons SW, Berens ME. Dichotomy of astrocytoma migration and proliferation. Int J Cancer. 1996;67:275–282. [DOI] [PubMed] [Google Scholar]
- 3. de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogawa D, Ansari K, Nowicki MO, Salinska E, Bronisz A, Godlewski J. MicroRNA-451 inhibits migration of glioblastoma while making it more susceptible to conventional therapy. Noncoding RNA. 2019;5:E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gal H, Pandi G, Kanner AA, et al. MIR-451 and imatinib mesylate inhibit tumor growth of glioblastoma stem cells. Biochem Biophys Res Commun. 2008;376:86–90. [DOI] [PubMed] [Google Scholar]
- 6. Zhao K, Wang L, Li T, et al. The role of miR-451 in the switching between proliferation and migration in malignant glioma cells: AMPK signaling, mTOR modulation and Rac1 activation required. Int J Oncol. 2017;50:1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ansari KI, Ogawa D, Rooj AK, et al. Glucose-based regulation of miR-451/AMPK signaling depends on the OCT1 transcription factor. Cell Rep. 2015;11:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu D, dos Santos CO, Zhao G, et al. miR-451 protects against erythroid oxidant stress by repressing 14-3-3zeta. Genes Dev. 2010;24:1620–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Obsil T, Ghirlando R, Klein DC, Ganguly S, Dyda F. Crystal structure of the 14-3-3 zeta: serotonin N-acetyltransferase complex. Cell. 2001;105:257–267. [DOI] [PubMed] [Google Scholar]
- 10. Yin CY, Kong W, Jiang J, Xu H, Zhao W. miR-7-5p inhibits cell migration and invasion in glioblastoma through targeting SATB1. Oncol Lett. 2019;17:1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jia B, Liu W, Gu J, et al. MiR-7-5p suppresses stemness and enhances temozolomide sensitivity of drug-resistant glioblastoma cells by targeting Yin Yang 1. Exp Cell Res. 2019;375:73–81. [DOI] [PubMed] [Google Scholar]
- 12. Qiu J, Zhang J, Zhou Y, et al. MicroRNA-7 inhibits melatonin synthesis by acting as a linking molecule between leptin and norepinephrine signaling pathways in pig pineal gland. J Pineal Res. 2019;66:e12552. [DOI] [PubMed] [Google Scholar]
- 13. Bernard M, Voisin P. Photoreceptor-specific expression, light-dependent localization, and transcriptional targets of the zinc-finger protein Yin Yang 1 in the chicken retina. J Neurochem. 2008;105:595–604. [DOI] [PubMed] [Google Scholar]
- 14. Lu ZJ, Liu SY, Yao YQ, et al. The effect of miR-7 on behavior and global protein expression in glioma cell lines. Electrophoresis. 2011;32:3612–3620. [DOI] [PubMed] [Google Scholar]
- 15. Yang X, Cao W, Zhou J, et al. 14-3-3ζ positive expression is associated with a poor prognosis in patients with glioblastoma. Neurosurgery. 2011;68:932–938, discussion 938. [DOI] [PubMed] [Google Scholar]
- 16. Cao WD, Zhang X, Zhang JN, et al. Immunocytochemical detection of 14-3-3 in primary nervous system tumors. J Neurooncol. 2006;77:125–130. [DOI] [PubMed] [Google Scholar]
- 17. Hashemi M, Zali A, Hashemi J, Oraee-Yazdani S, Akbari A. Down-regulation of 14-3-3 zeta sensitizes human glioblastoma cells to apoptosis induction. Apoptosis. 2018;23:616–625. [DOI] [PubMed] [Google Scholar]
- 18. Im CN. Combination treatment with PPARγ ligand and its specific inhibitor GW9662 downregulates BIS and 14-3-3 gamma, inhibiting stem-like properties in glioblastoma cells. Biomed Res Int. 2017;2017:5832824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bergamaschi A, Katzenellenbogen BS. Tamoxifen downregulation of miR-451 increases 14-3-3ζ and promotes breast cancer cell survival and endocrine resistance. Oncogene. 2012;31:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bostian AC, Maddukuri L, Reed MR, et al. Kynurenine signaling increases DNA polymerase kappa expression and promotes genomic instability in glioblastoma cells. Chem Res Toxicol. 2016;29:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ovchinnikov VY, Antonets DV, Gulyaeva LF. The search of CAR, AhR, ESRs binding sites in promoters of intronic and intergenic microRNAs. J Bioinform Comput Biol. 2018;16:1750029. [DOI] [PubMed] [Google Scholar]
- 22. Takenaka MC, Gabriely G, Rothhammer V, et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci. 2019;22:729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muxel SM, Pires-Lapa MA, Monteiro AW, et al. NF-κB drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine-N-acetyltransferase (AA-NAT) gene. PLoS ONE. 2012;7:e52010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderson G, Maes M. Interactions of tryptophan and its catabolites with melatonin and the alpha 7 nicotinic receptor in central nervous system and psychiatric disorders: role of the aryl hydrocarbon receptor and direct mitochondria regulation [published online ahead of print February 16, 2017]. Int J Tryptophan Res. doi: 10.1177/1178646917691738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59:4383–4391. [PubMed] [Google Scholar]
- 26. Walczak K, Deneka-Hannemann S, Jarosz B, et al. Kynurenic acid inhibits proliferation and migration of human glioblastoma T98G cells. Pharmacol Rep. 2014;66:130–136. [DOI] [PubMed] [Google Scholar]
- 27. Chhipa RR, Fan Q, Anderson J, et al. AMP kinase promotes glioblastoma bioenergetics and tumour growth. Nat Cell Biol. 2018;20:823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanli T, Steinberg GR, Singh G, Tsakiridis T. AMP-activated protein kinase (AMPK) beyond metabolism: a novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol Ther. 2014;15:156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu H, Zhang L, Xu J, et al. AntogomiR-451 protects human gastric epithelial cells from ethanol via activating AMPK signaling. Biochem Biophys Res Commun. 2018;497:339–346. [DOI] [PubMed] [Google Scholar]
- 30. Xu X, Xu J, Knutsson L, et al. The effect of the mTOR inhibitor rapamycin on glucoCEST signal in a preclinical model of glioblastoma. Magn Reson Med. 2019;81:3798–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim Y, Kang H, Powathil G, et al. Role of extracellular matrix and microenvironment in regulation of tumor growth and LAR-mediated invasion in glioblastoma. PLoS ONE. 2018;13:e0204865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. D’Alimonte I, Nargi E, Zuccarini M, et al. Potentiation of temozolomide antitumor effect by purine receptor ligands able to restrain the in vitro growth of human glioblastoma stem cells. Purinergic Signal. 2015;11:331–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strong AD, Indart MC, Hill NR, Daniels RL. GL261 glioma tumor cells respond to ATP with an intracellular calcium rise and glutamate release. Mol Cell Biochem. 2018;446:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Souza-Teodoro LH, Dargenio-Garcia L, Petrilli-Lapa CL, et al. Adenosine triphosphate inhibits melatonin synthesis in the rat pineal gland. J Pineal Res. 2016;60:242–249. [DOI] [PubMed] [Google Scholar]
- 35. Mahajan-Thakur S, Bien-Moller S, Marx S, Schroeder H, Rauch BH. Sphingosine 1-phosphate (S1P) signaling in glioblastoma multiforme – a systematic review. Int J Mol Sci. 2017;18:E2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quint K, Stiel N, Neureiter D, et al. The role of sphingosine kinase isoforms and receptors S1P1, S1P2, S1P3, and S1P5 in primary, secondary, and recurrent glioblastomas. Tumour Biol. 2014;35:8979–8989. [DOI] [PubMed] [Google Scholar]
- 37. Anderson G, Maes M. Reconceptualizing adult neurogenesis: role for sphingosine-1-phosphate and fibroblast growth factor-1 in co-ordinating astrocyte-neuronal precursor interactions. CNS Neurol Disord Drug Targets. 2014;13:126–136. [DOI] [PubMed] [Google Scholar]
- 38. Amison RT, Jamshidi S, Rahman KM, Page CP, Pitchford SC. Diverse signalling of the platelet P2Y1 receptor leads to a dichotomy in platelet function. Eur J Pharmacol. 2018;827:58–70. [DOI] [PubMed] [Google Scholar]
- 39. Pirmoradi L, Seyfizadeh N, Ghavami S, Zeki AA, Shojaei S. Targeting cholesterol metabolism in glioblastoma: a new therapeutic approach in cancer therapy. J Investig Med. 2019;67:715–719. [DOI] [PubMed] [Google Scholar]
- 40. Wiedemann FR, Siemen D, Mawrin C, Horn TF, Dietzmann K. The neurotrophin receptor TrkB is colocalized to mitochondrial membranes. Int J Biochem Cell Biol. 2006;38:610–620. [DOI] [PubMed] [Google Scholar]
- 41. Lawn S, Krishna N, Pisklakova A, et al. Neurotrophin signaling via TrkB and TrkC receptors promotes the growth of brain tumor-initiating cells. J Biol Chem. 2015;290:3814–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pinet S, Bessette B, Vedrenne N, et al. TrkB-containing exosomes promote the transfer of glioblastoma aggressiveness to YKL-40-inactivated glioblastoma cells. Oncotarget. 2016;7:50349–50364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jang SW, Liu X, Pradoldej S, et al. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc Natl Acad Sci U S A. 2010;107:3876–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoo DY, Nam SM, Kim W, et al. N-acetylserotonin increases cell proliferation and differentiating neuroblasts with tertiary dendrites through upregulation of brain-derived neurotrophic factor in the mouse dentate gyrus. J Vet Med Sci. 2011;73:1411–1416. [DOI] [PubMed] [Google Scholar]
- 45. Liu S, Jiang T, Zhong Y, Yu Y. miR-210 inhibits cell migration and invasion by targeting the brain-derived neurotrophic factor in glioblastoma [published online ahead of print February 11, 2019]. J Cell Biochem. doi: 10.1002/jcb.28414. [DOI] [PubMed] [Google Scholar]
- 46. Meldolesi J. Neurotrophin Trk receptors: new targets for cancer therapy. Rev Physiol Biochem Pharmacol. 2018;174:67–79. [DOI] [PubMed] [Google Scholar]
- 47. Morris LG, Kaufman AM, Gong Y, et al. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat Genet. 2013;45:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dunne J, Hanby AM, Poulsom R, et al. Molecular cloning and tissue expression of FAT, the human homologue of the Drosophila fat gene that is located on chromosome 4q34-q35 and encodes a putative adhesion molecule. Genomics. 1995;30:207–223. [DOI] [PubMed] [Google Scholar]
- 49. Nakamura E, Kozaki K, Tsuda H, et al. Frequent silencing of a putative tumor suppressor gene melatonin receptor 1 A (MTNR1A) in oral squamous-cell carcinoma. Cancer Sci. 2008;99:1390–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Slaugenhaupt SA, Roca AL, Liebert CB, Altherr MR, Gusella JF, Reppert SM. Mapping of the gene for the Mel1a-melatonin receptor to human chromosome 4 (MTNR1A) and mouse chromosome 8 (Mtnr1a). Genomics. 1995;27:355–357. [DOI] [PubMed] [Google Scholar]
- 51. Park HK, Hong JH, Oh YT, et al. Interplay between TRAP1 and Sirtuin-3 modulates mitochondrial respiration and oxidative stress to maintain stemness of glioma stem cells. Cancer Res. 2019;79:1369–1382. [DOI] [PubMed] [Google Scholar]
- 52. Wang G, Fu XL, Wang JJ, Guan R, Sun Y, Tony To SS. Inhibition of glycolytic metabolism in glioblastoma cells by Pt3glc combinated with PI3K inhibitor via SIRT3-mediated mitochondrial and PI3K/Akt-MAPK pathway. J Cell Physiol. 2019;234:5888–5903. [DOI] [PubMed] [Google Scholar]
- 53. Liu R, Fan M, Candas D, et al. CDK1-mediated SIRT3 activation enhances mitochondrial function and tumor radioresistance. Mol Cancer Ther. 2015;14:2090–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yao ZQ, Zhang X, Zhen Y, et al. A novel small-molecule activator of Sirtuin-1 induces autophagic cell death/mitophagy as a potential therapeutic strategy in glioblastoma. Cell Death Dis. 2018;9:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dixit D, Sharma V, Ghosh S, Mehta VS, Sen E. Inhibition of casein kinase-2 induces p53-dependent cell cycle arrest and sensitizes glioblastoma cells to tumor necrosis factor (TNFα)-induced apoptosis through SIRT1 inhibition. Cell Death Dis. 2012;3:e271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kwon S, Seok S, Yau P, Li X, Kemper B, Kemper JK. Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3. J Biol Chem. 2017;292:17312–17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li X, Thirumalai D. Share, but unequally: a plausible mechanism for emergence and maintenance of intratumour heterogeneity. J R Soc Interface. 2019;16:20180820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shibao S, Minami N, Koike N, et al. Metabolic heterogeneity and plasticity of glioma stem cells in a mouse glioblastoma model. Neuro Oncol. 2018;20:343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tan D-X, Reiter RJ. Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019;2:44–66. [Google Scholar]
- 60. Anderson G, Maes M. Local melatonin regulates inflammation resolution: a common factor in neurodegenerative, psychiatric and systemic inflammatory disorders. CNS Neurol Disord Drug Targets. 2014;13:817–827. [DOI] [PubMed] [Google Scholar]
- 61. Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J Pineal Res. 2013;54:127–138. [DOI] [PubMed] [Google Scholar]
- 62. Zheng X, Pang B, Gu G, et al. Melatonin inhibits glioblastoma stem-like cells through suppression of EZH2-NOTCH1 signaling axis. Int J Biol Sci. 2017;13:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ma X, Idle JR, Krausz KW, Gonzalez FJ. Metabolism of melatonin by human cytochromes p450. Drug Metab Dispos. 2005;33:489–494. [DOI] [PubMed] [Google Scholar]
- 64. Yu Z, Tian X, Peng Y, et al. Mitochondrial cytochrome P450 (CYP) 1B1 is responsible for melatonin-induced apoptosis in neural cancer cells. J Pineal Res. 2018;65:e12478. [DOI] [PubMed] [Google Scholar]
- 65. Bansal S, Leu AN, Gonzalez FJ, et al. Mitochondrial targeting of cytochrome P450 (CYP) 1B1 and its role in polycyclic aromatic hydrocarbon-induced mitochondrial dysfunction. J Biol Chem. 2014;289:9936–9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bulus H, Oguztuzun S, Guler Simsek G, et al. Expression of CYP and GST in human normal and colon tumor tissues. Biotech Histochem. 2019;94:1–9. [DOI] [PubMed] [Google Scholar]
- 67. Mohamed HT, Gadalla R, El-Husseiny N, et al. Inflammatory breast cancer: activation of the aryl hydrocarbon receptor and its target CYP1B1 correlates closely with Wnt5a/b-β-catenin signalling, the stem cell phenotype and disease progression. J Adv Res. 2018;16:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chang I, Mitsui Y, Kim SK, et al. Cytochrome P450 1B1 inhibition suppresses tumorigenicity of prostate cancer via caspase-1 activation. Oncotarget. 2017;8:39087–39100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mao M, Wu Z, Chen J. MicroRNA-187-5p suppresses cancer cell progression in non-small cell lung cancer (NSCLC) through down-regulation of CYP1B1. Biochem Biophys Res Commun. 2016;478:649–655. [DOI] [PubMed] [Google Scholar]
- 70. Siddens LK, Bunde KL, Harper TA, Jr, et al. Cytochrome P450 1b1 in polycyclic aromatic hydrocarbon (PAH)-induced skin carcinogenesis: tumorigenicity of individual PAHs and coal-tar extract, DNA adduction and expression of select genes in the Cyp1b1 knockout mouse. Toxicol Appl Pharmacol. 2015;287:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mu W, Hu C, Zhang H, et al. miR-27b synergizes with anticancer drugs via p53 activation and CYP1B1 suppression. Cell Res. 2015;25:477–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhu Z, Mu Y, Qi C, et al. CYP1B1 enhances the resistance of epithelial ovarian cancer cells to paclitaxel in vivo and in vitro. Int J Mol Med. 2015;35:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Androutsopoulos VP, Spyrou I, Ploumidis A, et al. Expression profile of CYP1A1 and CYP1B1 enzymes in colon and bladder tumors. PLoS ONE. 2013;8:e82487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ma C, Wei F, Xia H, et al. MicroRNA-10b mediates TGF-β1-regulated glioblastoma proliferation, migration and epithelial-mesenchymal transition. Int J Oncol. 2017;50:1739–1748. [DOI] [PubMed] [Google Scholar]
- 75. Xiao A, Brenneman B, Floyd D, et al. Statins affect human glioblastoma and other cancers through TGF-β inhibition. Oncotarget. 2019;10:1716–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Silginer M, Burghardt I, Gramatzki D, et al. The aryl hydrocarbon receptor links integrin signaling to the TGF-β pathway. Oncogene. 2016;35:3260–3271. [DOI] [PubMed] [Google Scholar]
- 77. Misuth M, Joniova J, Horvath D, et al. The flashlights on a distinct role of protein kinase C δ: phosphorylation of regulatory and catalytic domain upon oxidative stress in glioma cells. Cell Signal. 2017;34:11–22. [DOI] [PubMed] [Google Scholar]
- 78. Hwang E, Yoo KC, Kang SG, et al. PKCδ activated by c-MET enhances infiltration of human glioblastoma cells through NOTCH2 signaling. Oncotarget. 2016;7:4890–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cui J, Ou S, He WJ, Du L, Zhao YD, Ruan HZ. Prevention of extracellular ADP-induced ATP accumulation of the cultured rat spinal astrocytes via P2Y(1)-mediated inhibition of AMPK. Neurosci Lett. 2011;503:244–249. [DOI] [PubMed] [Google Scholar]
- 80. Gerasimovskaya EV, Woodward HN, Tucker DA, Stenmark KR. Extracellular ATP is a pro-angiogenic factor for pulmonary artery vasa vasorum endothelial cells. Angiogenesis. 2008;11:169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wellmann M, Alvarez-Ferradas C, Maturana CJ, Saez JC, Bonansco C. Astroglial Ca2+-dependent hyperexcitability requires P2Y1 purinergic receptors and pannexin-1 channel activation in a chronic model of epilepsy. Front Cell Neurosci. 2018;12:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yu X, Wu J, Hu M, et al. Glutamate affects the CYP1B1- and CYP2U1-mediated hydroxylation of arachidonic acid metabolism via astrocytic mGlu5 receptor. Int J Biochem Cell Biol. 2019;110:111–121. [DOI] [PubMed] [Google Scholar]
- 83. Pereira MSL, Klamt F, Thome CC, Worm PV, de Oliveira DL. Metabotropic glutamate receptors as a new therapeutic target for malignant gliomas. Oncotarget. 2017;8:22279–22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fujishiro T, Nonoguchi N, Pavliukov M, et al. 5-Aminolevulinic acid-mediated photodynamic therapy can target human glioma stem-like cells refractory to antineoplastic agents. Photodiagnosis Photodyn Ther. 2018;24:58–68. [DOI] [PubMed] [Google Scholar]
- 85. Huo X, Wang C, Yu Z, et al. Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: an implication of the therapeutic potential [published online ahead of print May 2017]. J Pineal Res. 2017. doi: 10.1111/jpi.12390. [DOI] [PubMed] [Google Scholar]
- 86. Raucher D. Tumor targeting peptides: novel therapeutic strategies in glioblastoma. Curr Opin Pharmacol. 2019;47:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hevia D, Gonzalez-Menendez P, Quiros-Gonzalez I, et al. Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J Pineal Res. 2015;58:234–250. [DOI] [PubMed] [Google Scholar]
- 88. Hevia D, Gonzalez-Menendez P, Fernandez-Fernandez M, et al. Melatonin decreases glucose metabolism in prostate cancer cells: a 13C stable isotope-resolved metabolomic study. Int J Mol Sci. 2017;18:E1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lu VM, Goyal A, Vaughan LS, McDonald KL. The impact of hyperglycemia on survival in glioblastoma: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2018;170:165–169. [DOI] [PubMed] [Google Scholar]
- 90. Yang JK, Liu HJ, Wang Y, et al. Exosomal miR-214-5p released from glioblastoma cells modulates inflammatory response of microglia after lipopolysaccharide stimulation through targeting CXCR5. CNS Neurol Disord Drug Targets. 2019;18:78–87. [DOI] [PubMed] [Google Scholar]
- 91. Lopes J, Arnosti D, Trosko JE, Tai MH, Zuccari D. Melatonin decreases estrogen receptor binding to estrogen response elements sites on the OCT4 gene in human breast cancer stem cells. Genes Cancer. 2016;7:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dong H, Shertzer HG, Genter MB, et al. Mitochondrial targeting of mouse NQO1 and CYP1B1 proteins. Biochem Biophys Res Commun. 2013;435:727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schrader M, Godinho LF, Costello JL, Islinger M. The different facets of organelle interplay-an overview of organelle interactions. Front Cell Dev Biol. 2015;3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pinkham K, Park DJ, Hashemiaghdam A, et al. Stearoyl CoA desaturase is essential for regulation of endoplasmic reticulum homeostasis and tumor growth in glioblastoma cancer stem cells. Stem Cell Reports. 2019;12:712–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mi Y, Tan D, He Y, Zhou X, Zhou Q, Ji S. Melatonin modulates lipid metabolism in HepG2 cells cultured in high concentrations of oleic acid: AMPK pathway activation may play an important role. Cell Biochem Biophys. 2018;76:463–470. [DOI] [PubMed] [Google Scholar]
- 96. Nakagawa H, Sasagawa S, Itoh K. Sodium butyrate induces senescence and inhibits the invasiveness of glioblastoma cells. Oncol Lett. 2018;15:1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liu H, Xia J, Wang T, Li W, Song Y, Tan G. Differentiation of human glioblastoma U87 cells into cholinergic neuron. Neurosci Lett. 2019;704:1–7. [DOI] [PubMed] [Google Scholar]
- 98. Li Y, Zhou H, Xing E, et al. Contribution of decreased expression of Ku70 to enhanced radiosensitivity by sodium butyrate in glioblastoma cell line (U251). J Huazhong Univ Sci Technolog Med Sci. 2011;31:359. [DOI] [PubMed] [Google Scholar]
- 99. Egler V, Korur S, Failly M, et al. Histone deacetylase inhibition and blockade of the glycolytic pathway synergistically induce glioblastoma cell death. Clin Cancer Res. 2008;14:3132–3140. [DOI] [PubMed] [Google Scholar]
- 100. Jin CJ, Engstler AJ, Sellmann C, et al. Sodium butyrate protects mice from the development of the early signs of non-alcoholic fatty liver disease: role of melatonin and lipid peroxidation [published online ahead of print November 23, 2016]. Br J Nutr. doi: 10.1017/S0007114516004025. [DOI] [PubMed] [Google Scholar]
- 101. Schmudde M, Braun A, Pende D, et al. Histone deacetylase inhibitors sensitize tumour cells for cytotoxic effects of natural killer cells. Cancer Lett. 2008;272:110–121. [DOI] [PubMed] [Google Scholar]
- 102. Luo S, Li Z, Mao L, Chen S, Sun S. Sodium butyrate induces autophagy in colorectal cancer cells through LKB1/AMPK signaling. J Physiol Biochem. 2019;75:53–63. [DOI] [PubMed] [Google Scholar]
- 103. Zapletal O, Tylichova Z, Neca J, et al. Butyrate alters expression of cytochrome P450 1A1 and metabolism of benzo[a]pyrene via its histone deacetylase activity in colon epithelial cell models. Arch Toxicol. 2017;91:2135–2150. [DOI] [PubMed] [Google Scholar]