Abstract
Polyneuropathy is a common complication to diabetes. Neuropathies within the enteric nervous system are associated with gastroenteropathy and marked symptoms that severely reduce quality of life. Symptoms are pleomorphic but include nausea, vomiting, dysphagia, dyspepsia, pain, bloating, diarrhoea, constipation and faecal incontinence. The aims of this review are fourfold. First, to provide a summary of the pathophysiology underlying diabetic gastroenteropathy. Secondly to give an overview of the diagnostic methods. Thirdly, to provide clinicians with a focussed overview of current and future methods for pharmacological and nonpharmacological treatment modalities. Pharmacological management is categorised according to symptoms arising from the upper or lower gut as well as sensory dysfunctions. Dietary management is central to improvement of symptoms and is discussed in detail, and neuromodulatory treatment modalities and other emerging management strategies for diabetic gastroenteropathy are discussed. Finally, we propose a diagnostic/investigation algorithm that can be used to support multidisciplinary management.
Keywords: diabetes mellitus, complications, diabetic neuropathies, enteric nervous system, gastrointestinal motility, gastrointestinal transit, pharmacology, enteropathy
Introduction
The development of diabetic polyneuropathy can potentially alter neuronal structure and function anywhere in the peripheral, central and enteric nervous systems. Where small and large fibres (unmyelinated C and myelinated Aβ and Aδ fibres) in the somatic sensory nervous system are affected, the typical manifestation is a distal symmetrical polyneuropathy.1 Importantly, these symptoms can also be found in people with prediabetes.2 The somatic polyneuropathy is characterised by alterations in the sensory system, and classically presents with marked changes in a number of sensations (including temperature and fine touch, balance, etc.) and can be painful or painless, although the former predominates.2 In community samples with diabetes, the prevalence of clinical symptoms is in the order of 30%, although this is likely to represent an underestimate as many people are not formally diagnosed.3
Although often overlooked, effects in the autonomic nervous system most likely occur concurrently with those observed in the somatic nervous system. For example it is plausible that early damage to small fibres, preceding large fibre neuropathy, of the somatic nerves, which can be observed in skin biopsies4 also take place in other small fibres such as in the enteric nervous system. Autonomic neuropathy can be clinically silent and is often present when polyneuropathy of the somatic nerves is present. The diagnosis is typically based on cardiovascular abnormalities. In particular, studies of RR-complexes in electrocardiograms have been used to describe heart rate variability, leading to the diagnosis of cardiac autonomic neuropathy. However, autonomic neuropathy also leads to gastrointestinal (GI) complications such as diabetic gastroenteropathy, which may affect the entire length of the GI tract. The underlying pathophysiology, which is driven by a multitude of factors, including microenvironmental factors such as hyperglycaemia, have a negative impact on the enteric motor and sensory functions and manifest as symptoms related to, e.g., motility and secretory dysfunctions.5–7 Hence, people with diabetic gastroenteropathy may experience a variety of burdensome symptoms, including dysphagia, dyspepsia, pain, bloating, diarrhoea, constipation and faecal incontinence, all of which adversely influences quality of life.
Although the focus of this review is diabetic gastroenteropathy, it should be emphasized that functional GI disorders are prevalent in the community. Hence, the presentation of diabetic gastroenteropathy and functional disorders such as functional dyspepsia and irritable bowel syndrome overlap and cannot be distinguished on the basis of symptoms or medical history (i.e. diabetes) alone. In fact, examinations of small bowel biopsies from patients with diabetes revealed neuropathy and myopathy only in a minority of patients.8 It is also clear that individuals with chronic disease have a higher prevalence of psychiatric disorders and are exposed to more stress than healthy subjects. These factors play an important role in the presentation and perceived severity of functional GI diseases.
The aims of this review are, however, to describe the pathophysiology of GI complications to diabetic neuropathy (gastroenteropathy) and to outline diagnostics and recent pharmacological and nonpharmacological treatment modalities for this burdensome condition.
Pathophysiology of diabetes-induced gastrointestinal complications
The term diabetic gastroenteropathy encompasses the cumulative impact that diabetes exerts on the GI tract. The pathophysiology is multifactorial and to date remains incompletely understood. However, changes in the neuronal microenvironment are believed to be a major driver in the pathogenesis. Microvascular complications to diabetes lead to alterations of blood flow in the GI wall, and hence also to alterations in the microenvironment. Smooth muscle myopathy is also thought to be a contributing factor to diabetic gastroenteropathy. However, smooth muscle myopathy may not be a primary disturbance, but is more likely a result of smooth muscle cell atrophy induced through reduced trophic cues from interstitial cells of Cajal (which are also reduced in number).9 This link between reduced numbers of interstitial cells of Cajal and smooth muscle myopathy has been observed in animal models of diabetic gastroparesis and likely contributes to abnormal motility.
The autonomic nervous system comprises (a) the sympathetic nervous system, (b) the parasympathetic nervous system (whose main neural substrate is the vagus nerve) and, according to the early and recently re-established definition, (c) the enteric nervous system.10,11 Alterations in either of these systems are involved in the underlying mechanism of burdensome GI complications in diabetes. As both the enteric nervous system and central nervous system (CNS) are involved in the bidirectional regulation and control of the GI homeostasis, any changes in either of these interconnected systems may result in altered GI function.
Changes at the level of the enteric nervous system
In diabetes, the microenvironment within the enteric nervous system is significantly altered due to the effect of e.g. long-term hyperglycaemia; oxidative stress; inflammation; reduced levels of neuro transmitters, local hormones and nerve growth factors; and increased levels of fatty acids.12,13 Recently, altered gut luminal microbiota has also been proposed to exert an influence. For example, lowered numbers of bacteria involved in production of short chain fatty acids have been observed in diabetes. Short chain fatty acids have anti-inflammatory effects in the GI wall and promote the secretion of the incretin hormone glucagon-like peptide-1 (GLP-1). Lower levels of GLP-1 influence glucose metabolism and can increase low-grade inflammation.14–18
Various components of the enteric nervous system, (including enteric neurons, interstitial cells of Cajal, enteric glial cells) and smooth muscle cells are affected by the changes described above. Enteric neurons and interstitial cells of Cajal are particularly vulnerable to hyperglycaemia.19 When hyperglycaemic episodes are frequent, or when hyperglycaemia is persistent, shifts in the intracellular glucose metabolism of neurons occur, consequently leading to the formation of advanced glycation end-products, osmotic and oxidative stress as well as inflammation. Collectively, this leads to cellular damage and ultimately to cell death, a process often referred to as glucose neurotoxicity. These mechanisms are primarily described in the peripheral nervous system, but similar mechanisms are present in the enteric nervous system.20
The pathophysiological changes described above may lead to various degrees of diabetes-induced damage to enteric neurones. Preferential loss of large fibre neurons in the dorsal root ganglion and inhibitory motor neurons in the gut wall have been observed. In particular, selective loss of nitric oxide synthase and neuropeptide Y expressing inhibitory motor neurons has been shown in the human diabetic colon.21 This obviously has consequences for the contractile activity of the smooth muscle layers in the GI tract and hence the motility pattern. Furthermore, loss of interstitial cells of Cajal throughout the GI tract has been reported in both animal models and in humans,22,23 causing reduced frequency of spontaneous muscular contractions. In addition, decreased activity of gastric enteric glial cells has been observed in animal models of diabetes.24 This may contribute to the development of gastroenteropathy in diabetes mainly because enteric glial cell functions such as neurotrophic support, immunosuppression and anti-inflammation is diminished with decreased activity.25,26
In contrast to the loss of motor neurons, it has recently been shown that levels of neurones containing substance P and calcitonin gene-related peptide are increased in the stomach of porcine models of diabetes.27 Both these molecules are primarily involved in the afferent transmission of GI sensory and nociceptive information. Hence, such alterations may be related to the pathophysiological mechanisms of sensory symptom generation. Like in the somatic system, when sensory nerves are affected by enteropathy, pain from the gut can likely be spontaneous or evoked by external or internal stimuli such as disturbances in motility and glandular functions. On the other hand, previous experimental studies where the gut was stimulated with electrical, mechanical and thermal stimuli, hypoalgesia to peripheral stimulation of both the upper and lower gut were shown, likely reflecting abnormal central processing of the afferent activity.28 Moreover, rectal pain thresholds are correlated to cutaneous and autonomic dysfunction.29,30 The autonomic components of visceral hyperalgesia have only been investigated in detail in healthy subjects, but similar mechanisms are likely involved in diabetes.31
Changes at the level of the autonomic nervous system
Autonomic afferent and efferent signalling through the vagus nerve is directly involved in the extensive communication with the brain, forming the so-called gut-brain-axis. In both people with diabetes and in animal models, the number of neurons in the sympathetic and parasympathetic ganglions of the vagus nerve connected to the GI tract is reduced32–34 as well as structural changes in the axons have been reported.35,36 In consequence, autonomic neuropathy, influencing the vagus nerve, contributes to reduced GI function.
Changes at the level of the CNS
Although the blood–brain barrier offers some protection to the brain against hyperglycaemia, it has been observed that the microstructure in specific brain regions (diabetic encephalopathy) involved in visceral sensory processing is changed in diabetes.37
Manifestation of sensory symptoms from the abdomen, such as nausea, vomiting and pain, as well as unspecific fullness, shooting sensations, etc., may relate to abnormal function of the sensory visceral nerves in combination with the encephalopathy (Figure 1).
Figure 1.
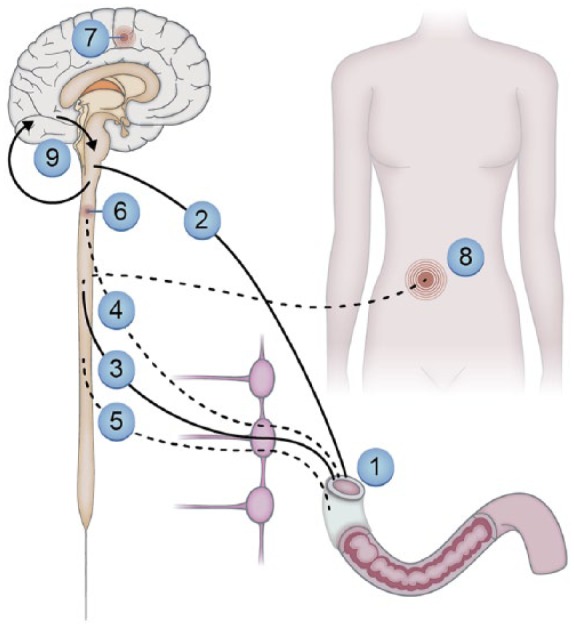
Neural pathways and mechanisms that may lead to pain and other sensory symptoms from the gastrointestinal system in people with diabetic neuropathy.
Neuropathic changes in the enteric nervous system where e.g., motility dysfunction can lead to sensory symptoms: (1) autonomic neuropathy influencing the parasympathetic (2) and (3) sympathetic pathways. Due to cross-talk between the nerve pathways and involvement of inhibitory pathways, this may indirectly modulate sensations from the gut; negative impact on visceral (4) (and somatic (5) if the peritoneum is involved) afferents lead to spontaneous and evoked pain. Structural and functional changes in the spinal cord (6) and brain (7) may lead to spontaneous and evoked pain, amplify afferent barrage and give abnormal referred pain to somatic structures (8). Spino-bulbo-spinal loops (9) that normally control pain intensity often malfunction in people with diabetes.
We have previously shown that central reorganisation of brain responses to visceral stimuli is associated to the burden of GI symptoms as well as heart rate variability as a proxy for diabetic autonomic neuropathy.38,39 This was supported by another study where visceral hyposensitivity was correlated to an increase in somatic referred pain areas, indicating central neuropathic-like changes.28 Although controversies exist regarding the pathogenesis of these changes, the findings in the CNS may be secondary to the peripheral neuropathy, because the reduced afferent activity may cause adaptive shrinking.37,40 Finally, there is evidence from both neurophysiological and imaging studies that descending pathways from the brainstem that normally ‘gates’ the incoming nociceptive barrage is malfunctioning in people with diabetes.30,41 Hence, as in somatic peripheral neuropathy, abnormal central sensory processing and hyper-excitability may explain the visceral symptoms despite the peripheral hypoalgesia (as described above).42 Such neuroplastic mechanisms were also seen e.g. in people with chronic pancreatitis, which is thought to have a strong neuropathic component, and this validated the findings.43
In summary, long-term diabetes induces marked structural and functional changes of the GI wall, parasympathetic, sympathetic and CNS alterations. Especially, intrinsic and extrinsic neuronal communication of the GI tract is altered. Taken together, this leads to burdensome panenteric alteration of GI sensation and function, including the biomechanics that drive GI motility.44,45
Diagnosis
As outlined above, diabetic gastroenteropathy can affect the entire GI tract, and consequently symptoms are not only very heterogeneous, they are also unspecific. The cardinal symptoms are nausea, vomiting, bloating and early satiety, however, symptoms range from dysphagia and heartburn to faecal incontinence. Thus, based on the patient reported symptoms, it is hardly possible to distinguish sequela of diabetes-associated GI dysfunction from organic diseases. This diagnostic dilemma is further aggravated by the fact that people with type 2 diabetes have an increased risk for a multitude of organic GI diseases, including reflux oesophagitis, gallstones and GI malignancies.46 An increased cancer risk has also been observed in type 1 diabetes.47 Moreover, increased prevalence of other diseases with an autoimmune component affecting the GI tract such as coeliac disease48 are frequent and need to be taken into consideration.
Accordingly, it is important to first exclude organic disease using appropriate laboratory tests, endoscopy and imaging techniques in people presenting with symptoms that could be attributed to diabetic gastroenteropathy (Figure 2). If these tests are unrevealing and symptoms do not respond to simple therapeutic measures (e.g. laxatives in chronic constipation), GI function tests can be used to diagnose disturbances associated with diabetic gastroenteropathy. Again, most clinically available function tests identify and quantify dysfunction of various GI segments and organs without being able to specifically prove the causation by or the relative importance of diabetic gastroenteropathy. For instance, severe oesophageal hypomotility diagnosed by high resolution manometry can explain nonobstructive dysphagia in a person with diabetes, but could also be due to other aetiologies. Therefore, clinical plausibility and affection of other organ systems, e.g. cardiac autonomic neuropathy, should be taken into account. Impaired pancreatic polypeptide release has been suggested for specific diagnosis of gastroenteropathy,49 but measurements are not widely available. Likewise, antroduodenojenunal manometry with increased frequency of phase III-motility and hypomotility during phase II as well as postprandially is typical of autonomic neuropathy, but available only at highly specialized centres and reserved for people with very severe symptoms.50
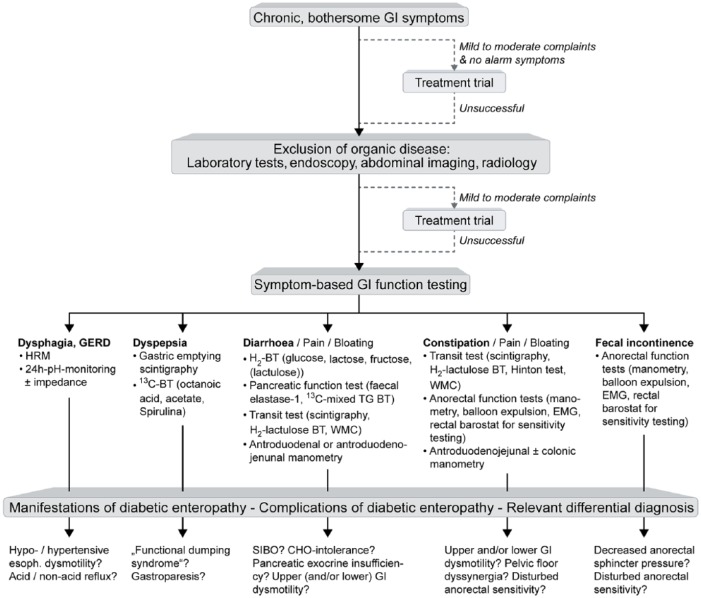
Figure 2.
Diagnostic algorithm in persons with suspected diabetic gastroenteropathy.
People with bothersome gastrointestinal symptoms should first undergo laboratory and imaging tests for exclusion of organic disease. If these are not helpful and symptoms are severe, or do not respond to simple therapeutic measures, GI function tests should be performed based on individual symptom pattern. These may reveal manifestations of diabetic gastroenteropathy (e.g. typical small bowel dysmotility in a person with diarrhoea), complications of diabetic gastroenteropathy (e.g. small bowel bacterial overgrowth) or differential diagnoses (e.g. lactose intolerance). BT: breath test; CHO: carbohydrate; EMG: Electromyography; GERD: gastro-oesophageal reflux disease; GI: gastrointestinal; HRM: high resolution manometry; SIBO: small bowel bacterial overgrowth; TG: triglyceride; WMC: wireless motility capsule.
Gastric emptying tests are recommended at an early stage in people with diabetes and dyspeptic symptoms. One reason for this is that gastric emptying is of paramount importance for blood glucose control.51 Another reason is that approximately 20% of people with diabetes and disturbed gastric emptying have functional dumping syndrome, i.e. accelerated gastric emptying without prior upper GI surgery, in contrast to the more frequently reported complication gastroparesis. Both disturbances cannot be differentiated reliably based on symptoms, but obviously require different treatment strategies.50,52 Recommendations regarding optimal use of gastric emptying tests are reviewed in detail elsewhere.50,53 Even in the absence of dyspeptic symptoms, gastric emptying testing can play a role in exclusion of diabetic gastropathy as a cause of impaired blood glucose control not responding to antidiabetic medication.
Once settled on the diagnosis diabetic gastroenteropathy, treatment options are not overwhelming, but the cornerstone is tight glycaemic control.
New diagnostic modalities
The following modalities are selected because they are directly applicable in clinical practice and therefore can be used to assess diabetic neuropathy.
13C-breath test
This test reflects the conversion of 13C isotope to 13C-CO by hepatic metabolism after absorption from the small intestine, thereby serving as a proxy for gastric emptying. This modality may not be as sophisticated as, for instance, magnetic resonance imaging or scintigraphy. However, this very accessible technique correlates well with scintigraphic data, can be used repeatedly in the same person, and may be a marked improvement in assessing gastric emptying in clinical situations.
The wireless motility capsule
This system compromises an indigestible capsule that continuously measures pressure, temperature and pH as it passes through the GI tract.54 Although the modality has been available for some time, the optimal yield from this technology is still to be determined. The pH measurements are used mainly to establish the GI segment. However, with the growing evidence of the influence of gut microbiota on gastroenteropathy and metabolism in general, one could speculate that associations between the pH of the different segments and composition of the gut microbiome could be of interest. For example, more acidic caecal pH has been demonstrated in type 1 diabetes, which may represent increased caecal fermentation.55
3D-transit system
Finally, a number of emerging modalities are currently being developed for research use. The ambulatory Motilis 3D-transit system tracks electromagnetic capsules as they traverse the GI tract and measures changes in position, velocity of movements and orientation of the capsules. This reflects gut contractile activity and progression dynamics. Anatomical information allows for detailed description of colonic motility, including regional transit times and motor patterns.56
Management
Management of blood glucose fluctuations
There is no cure for diabetic gastroenteropathy. Hence, the treatment aims are to delay progression, ease symptoms, manage complications and if possible restore function. The primary strategy to achieve this remains tight glycaemic control. Glycaemic management should be guided by age, disease duration and overall health and, if successful, symptoms may improve. Dietary and lifestyle advice can provide persons with diabetes with tools for better long-term glycaemic control. In people with diabetes and gastroparesis, it can often be helpful to administer pre-prandial insulin after the meal or in reduced amount. Employment of an insulin pump may further contribute to tight glycaemic control in persons with insulin-dependent diabetes. Devices that allow for continuous glucose monitoring in interstitial fluids in real time have become available and enable monitoring of time spent in target glucose range (‘time in range’) as well as warning trends toward hypoglycaemia or hyperglycaemia in real time.57 This modality has already proven its potential in modelling intestinal glucose absorption,58 and thus it can be expected to be a future important tool in validating glucose metabolism of the enteric system in physiological and pathophysiological setups. Continuous glucose monitoring is recommended by national and international medical organisations and expert clinician consensus guidelines, both in combination with pumps and in persons on multiple daily insulin injections.57,59–61 As the number of hyper- and hypoglycaemic events are reduced, the concept is believed to have a neuroprotective effect. Besides optimisation of glycaemic control, no available treatments address the underlying polyneuropathy. Hence available nonpharmacological and pharmacological treatment options targets symptoms of gastroenteropathy, the latter of which may be complicated by altered drug absorption in the diabetic gut.
Pharmacological management: absorption of medications
Widespread disease of the GI tract will have consequences for the absorption of orally administered drugs.62 However, only few studies on small populations have addressed the effects of diabetes on net absorption of drugs.63 As diabetes-induced structural and functional alterations are observed throughout the GI tract, diabetic gastroenteropathy may alter drug absorption after oral administration.
Two important factors may be affected: (1) The release of drug substance from the controlled release formulations, which are designed to release drug with a predefined rate throughout the GI-tract. Changes in the intraluminal environment due to gastroenteropathy may alter drug release. (2) Drug absorption following oral administration, which is possible throughout the GI-tract, with the upper small intestine as the main site for absorption.64 Several changes of GI physiology and function related to diabetic gastroenteropathy may therefore influence drug absorption (Table 1). This can ultimately result in therapeutic failure due to altered plasma levels.
Table 1.
| GI factors influencing drug absorption | Consequences |
|---|---|
| Dysmotility (gastroparesis, functional dumping syndrome, diarrhoea and constipation inclusive) |
Altered transit time and/or luminal water content |
| Small intestinal bacterial overgrowth | Altered pH and transit time |
| Altered secretory function | Reduced or increased luminal water content |
| Altered enteric microbiota | Altered pH and luminal drug metabolism |
| Structural remodelling of the wall of the GI tract | Altered intestinal transporters and gut wall metabolism |
| Reduced microvascular blood flow | Altered absorption to systemic circulation |
GI: Gastrointestinal.
One study in diabetes demonstrated that alterations in gut transit time impacts mainly the pharmacokinetics of drugs with poor intestinal absorption and controlled release formulations.63 Further studies on disease–drug interactions are needed as the study on the influence of GI dysfunction on drug absorption from oral formulations is still in its beginning.62,67
In conclusion, potential unpredictable drug absorption and the likelihood of treatment failure should be considered in people where diabetic gastroenteropathy may be present.68
Pharmacological management of motility dysfunction in the upper GI tract
Prokinetics
A number of prokinetic drugs have been studied for the management of motility disturbances in the upper GI tract in people with diabetes, and have generally proven to be effective for symptom improvement in placebo-controlled trials.69–72 Until now, no absolute association between symptom improvement and changes in upper GI motility before or after treatment have been shown.70 This may be due to heterogeneity of study groups and use of sub-optimal methods for measuring gastric emptying time. Hence, two new metanalysis showed that there may in fact be an association between upper GI motility and symptoms when optimal methods are used.53,73 On the other hand it cannot be ruled out that central effects of prokinetics may explain the observed improvement of symptoms. The classical prokinetic drugs fall into two categories based on their primary molecular target. The D2-receptor antagonists metoclopramide and domperidone have been used to treat gastroparesis for many years and have proven effective for this indication in randomised placebo-controlled trials.69 A multicentre study comparing the effectiveness of metoclopramide and domperidone found the drugs to be equally effective against symptoms of gastroparesis, but with more adverse effects in the CNS reported in persons treated with metoclopramide.71 This finding can be explained by the ability of metoclopramide to cross the blood-brain barrier to a higher degree, thereby having an increased potential for mediating central adverse effects. In February 2009, the U.S. Food and Drug Administration (FDA) and European Medicines Agency appointed black box warnings for long-term use (more than 12 weeks) of metoclopramide due to the risk of irreversible tardive dyskinesia, which has limited its use. The FDA has approved only metoclopramide for gastroparesis, although the risks for cardiovascular side effects seems to be higher than for domperidone.74 In Europe, domperidone is most often used, but caution should be taken due to risk of cardiac arrhythmias in the presence of prolonged QT-syndrome. Risk factors such as female sex, age above 65 years, electrolyte disturbances, polypharmacy, known heart disease, etc. should be taken into consideration when D2-receptor antagonists are used.75 Erythromycin is a motilin and cholinergic receptor agonist that has also been used for decades to treat upper motility dysfunction in people with diabetes.70 However, its clinical efficacy often diminishes after 2–4 weeks due to tachyphylaxis, and many patients experience adverse effect during long-term use.70 A survey among people with gastroparesis observed a correlation between willingness to accept potentially lethal side effects and symptom severity.76 This emphasizes how burdensome GI complications are.
The prokinetic action of erythromycin is likely a drug class effect, and other macrolides with less toxicity may be used, but evidence from controlled trials is not available. Novel molecular targets, including highly selective serotonin agonists, are currently under investigation for debilitating symptoms associated with diabetic gastroparesis, but have not yet been approved for this indication.72 Prucalopride is currently available for other GI dysmotility disorders (see next section). It has a safe cardiovascular profile and may be used off-label for treatment in selected cases.77 The synthetic ghrelin analogue, relamorelin, with prokinetic properties, also appears to be safe and has shown promising results for the treatment of gastroparesis in phase IIA studies.78 Hence, relamorelin may be a potential drug to use in the future.
Tricyclic antidepressants
Low-dose nortriptyline, amitriptyline, and desipramine can diminish symptoms in people with diabetes and chronic vomiting, who had an inadequate response to prokinetics.79 A multicentre trial has reported that amitriptyline relieve symptoms, although gastric emptying time was not lowered, in subgroups of patients with painful functional dyspepsia. This indicates a mechanism of action of tricyclic antidepressants on visceral hypersensitivity and not gastric emptying.80 However, in a large multicentre randomised controlled trial in adults with idiopathic gastroparesis, the use of nortriptyline (up to 75 mg per day) compared with placebo for 15 weeks did not improve the overall symptom score.81 Thus, further research is warranted in order to make any conclusive recommendations.
Taken together, the serotonin and ghrelin receptor agonists have generally been well tolerated and safe in humans without the cardiac or neurologic adverse effects associated with ‘classic prokinetics’. Therefore, these agents (and potentially tricyclic antidepressants) comprise promising therapeutics for future treatment of upper motility dysfunction in diabetes and other upper GI dysmotility disorders.
Pharmacological management of motility dysfunction in the lower GI tract
Natural bulking, osmotic and stimulant laxatives
Lower GI symptoms in people with diabetes type 1 are associated with poor glycaemic control and quality of life.82 In randomised, placebo controlled trials, the intake of the natural bulking psyllium (10 g bid) or flaxseed (10 g bid) reduced symptoms of constipation and improved glycaemic control in people with type 2 diabetes.83,84
No studies have specifically investigated the effects of laxatives in persons with constipation as a complication due to diabetic gastroenteropathy. It is commonly suggested, but not evidence based, to start with osmotic laxatives such as magnesia or polyethylene glycol. If this is insufficient, stimulant laxatives such as bisacodyle, senna or picosulfate can be added. Lubiprostone, a chloride channel activator, increases secretion from the colon, thereby reducing colonic transit time and increasing the number of spontaneous bowel movements in persons with diabetes-related constipation.85 Prucalopride, a 5-HT4 agonist, reduces transit time throughout the GI tract, and especially through the colon. Though not evaluated in DM, the pharmacological profile of prucalopride indicates that it is useful against constipation as part of the panenteric disorder often found in diabetes. Linaclotide, although registered for irritated bowel syndrome, may also be used in selected cases.86 Finally, the enzyme transglucosidase (used as a dietary supplement) changes the gut microbiome and increases the weekly number of bowel movements in people with type 2 diabetes and constipation.87
Antibiotics, enzyme supplementation and dietary intervention
In addition, people with diabetes have an increased prevalence of diarrhoea as sequela to small intestinal bacterial overgrowth, bile acid diarrhoea, pancreatic insufficiency and coeliac disease. These should be treated specifically with antibiotics, bile acid sequestrants, enzyme supplement or gluten-free diet whenever appropriate.
Antidiarrhoeal products
Compared to the general population, people with diabetes have a two-fold risk of having diarrhoea (11 vs 6 %).88 Very often, diarrhoea is induced by glucose-stabilizing treatment such as metformin or other medications commonly taken by people with diabetes. If no underlying condition is found for development of diarrhoea, pharmacological treatment will usually include dietary assessment and intervention in combination with loperamide, an opioid receptor agonist. Uncontrolled observational studies have shown that the α2 adrenergic agonist clonidine may reduce diarrhoea in people with diabetes. The use of clonidine is, however, restricted by its cardiovascular side effects.89
Furthermore, diabetes significantly increases the risk of having faecal incontinence, which in frequently is aggravated by diarrhoea. If an underlying cause of diarrhoea can be identified, it must be addressed. If not, loperamide or dietary intervention may be indicated. In a number of persons, faecal incontinence is associated with neuropathy and reduced sensibility of the anal canal. In these cases, loperamide, suppositories or enemas should be considered. Treatment of refractory cases is very complex and may even require a stoma.
Neuromodulation
Neuromodulatory electrical stimulation of the sacral nerve, which is described in detail later, is an emerging technique for the treatment of faecal incontinence and potentially sensitivity in the anal canal.90 However, the role of sacral nerve stimulation in people with diabetic gastroenteropathy has not been investigated specifically.
Pharmacological management of sensory symptoms
In the management of abdominal pain in diabetes it is often impossible to distinguish between the different organ manifestations. This is due to the vague presentation of visceral symptoms with changing presentation of the pain and referral to somatic structures, and this should be taken into consideration.91 It can also be difficult to distinguish between symptoms such as nausea and pain evoked by dysmotility and those that are related to the sensory (and central) neuropathy per se. In such cases the primary reason for pain such as constipation should be treated primarily. If all such reasons for the pain can be excluded, management should be directed against the neuropathy. Although only few studies have addressed treatment of visceral sensory symptoms in people with diabetes, the pharmaceutical options most often applied are reviewed here. On the other hand, the individual variability in phenotypical presentation of pain in diseases is greater between people than between pain syndromes. This indicates that mechanistic aetiologies and subsequent successful treatment should be based at the level of the individual rather than the disease per se.92 Hence, the recommendation for pharmacological management of visceral neuropathic pain follows the guidelines used for somatic neuropathies.93
Tricyclic antidepressants
As stated above tricyclic antidepressive medications can be used to manage symptoms in gastroparesis, likely because many of the clinical presentations are consequences to sensory neuropathy. A detailed description is outside the scope of this paper, but for disorders with peripheral and central neuropathy, these adjuvant analgesics are often used.
Selective serotonin-noradrenaline reuptake inhibitors and gabapentinoids
Depending on the clinical situation, other pharmacological compounds for the use of treating diabetes induced somatic and neuropathic pain also include selective serotonin-noradrenaline reuptake inhibitors as well as the gabapentinoids (gabapentin and pregabalin), and the drugs can be used in combination.
Opioids
In difficult and severe cases, more potent analgesics such as opioids may be needed, but, if possible, long-term treatment should be avoided due to adverse effects, e.g. high risk of masking hypoglycaemic events.94,95 Although most drugs have a certain potency, all have GI (or CNS) side effects and safety is often the major limitation in pain management, especially for opioids.75,96 Hence, the balance between effect and side-effects is more relevant than the potency of the analgesics. This balance is however highly individual, and as no valid predictors for individualised treatment is available, a period with ‘trial and error’ is often necessary. There should also be awareness on opioid-induced bowel dysfunction that by itself may lead to gastroparesis and constipation and lead to a vicious circle that can intensify the pain.97,98
Modern pain management
There is always more to analgesia than analgesics, and pharmacological management can seldom stand alone. Therefore, modern pain management of diabetic gastroenteropathy also includes e.g., invasive procedures, supportive care and nursing (multimodal analgesia). Finally, it should not be forgotten that individual experiences and manifestations of pain are influenced by complex interactions between sensory, pathophysiological, affective, socio-cultural, behavioural and cognitive elements. An active screening for psychiatric comorbidity, including anxiety and depression, should be done as up to 40% of chronic pain patients are depressed, and identification of mood disorders may select persons where adjuvant therapy with antidepressants are particular beneficial.99 It should be stressed that treatment of abdominal pain secondary to diabetic gastroenteropathy is complex and involves a multidisciplinary approach including diabetologists, gastroenterologists, pain specialists, dietitians and psychologists.
Dietary management
People with diabetic gastroenteropathy are at risk of developing dehydration and poor nutritional status.100 Most studies on dietary treatment in diabetes have included people with gastroparesis who may have insufficient dietary intake due to early satiety, postprandial fullness, nausea and vomiting. However, the literature regarding the evidence for the nutritional intervention is scarce. Among people with gastroparesis, 64% had insufficient daily intake of energy, vitamins and minerals.100 In contrast, another study demonstrated that people with diabetes were capable of maintaining a sufficient daily caloric intake.101 The normal dietetic recommendation for diabetics includes a high-fibre content, which is not appropriate for people with gastroparesis. A small particle diet cause less upper GI symptoms than a conventional diet and represents food items that are easily processed into small particle size (maximum 2 mm in diameter). In a study by Olausson et al., the fibre and fat content of the diet was normal, but it excluded husks/peels (e.g. corn), membranes (e.g. orange), stringy foods (e.g. rhubarb), seeds and grains (e.g. whole grain), and poorly digestible particles (e.g. salad).102
It has been observed that gastric emptying is significantly delayed in healthy volunteers after a high-fat meal compared with a low-fat meal.103 However, the majority of well-controlled studies indicate that gastric emptying upon high-fat meals is similar to other nutrient compositions matched for calorie and volume.104 One explanation may be that high-fat meals increase visceral sensitivity. This may explain, why correspondingly, a high-fat solid meal causes more symptoms than a low-fat liquid meal in people with diabetic or idiopathic gastroparesis.105,106 Also, high-fat liquid meals were better tolerated than high-fat solid meals.107 High-osmolality liquids with more than 350 mOsm.kg delay gastric emptying in healthy persons108 but the effect in people with diabetes is unknown.
Since fat, fibre, meal-size and consistency of foods all seem to influence gastric emptying and symptoms, the dietetic intervention in people with diabetes and gastroparesis should include several small meals (five to six) with a small particle size, a moderate-to-low content of fat and fibre, and a high content of liquids both in and alongside the meal. In case of weight loss, high-fat liquid meals might be an option. Oral intake is preferred, but in people with severe symptoms and weight loss, enteral (particular jejunal) or parenteral nutrition can be indicated.109
Neuromodulatory treatment
Although not neuromodulatory per se, a novel nonpharmacological vibrating capsule is assumed to induce a normal peristaltic wave in the large intestine to alleviate constipation. Although it has been shown to improve transit times in some people with functional constipation by a conveying by vibration approach,110 further evidence is needed.
Electrical stimulation of the GI tract was first used over 50 years ago to improve motility in postoperative ileus.111 Subsequent studies demonstrated that gastric electrical stimulation could improve gastric emptying and gastric dysrhythmias,112 both important pathophysiological features of gastroparesis.113 Gastric electrical stimulation is currently used for the treatment of people with gastroparesis whose symptoms are refractory to medical interventions, particularly in the context of weight loss, however, it has not affected the transit time. The method is invasive and involves laparoscopic surgical placement of electrodes on the externa muscular wall of the gastric antrum (Figure 3a). These electrodes are then subsequently attached to a programmable signal generator box, which is implanted in a subcutaneous pouch in the left flank. The complication rates are in the order of 10%, the most common being subcutaneous pocket infection.114 The mechanism of action of gastric electrical stimulation is incompletely understood, but it is thought that it may either modulate vagus nerve function to improve gastric accommodation or influences vagal afferent signalling to the CNS. Outcome and efficacy data on gastroparesis in people with diabetes are largely derived from open-label single-centre studies in highly selected persons. Meta-analytic evidence, however, suggests that gastric electrical stimulation improves the cardinal symptoms of gastroparesis, such as nausea and vomiting, improves generic quality of life and reduces the need for enteral or parenteral nutritional support.115
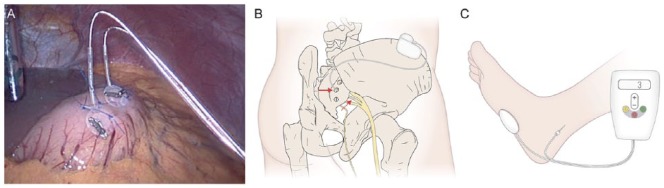
Figure 3.
Neuromodulatory modalities in gastroenteropathy.
(a) A laparoscopic view of gastric electrical stimulation electrodes sutured to the wall of the gastric antrum. Photograph courtesy of Sri Kardirkamanthan, Broomfield Hospital, Essex, UK. (b) A schematic representation of sacral nerve stimulation demonstrating the electrodes and signal generator. (c) Percutaneous tibial nerve stimulation. In this photograph, the tibial nerve is being stimulated using a 34-gauge needle inserted in/around the tibial nerve with a cutaneous electrode on the sole of the foot.
It has been shown that acute hyperglycaemia inhibits anal sphincter function, leading to a reduction in rectal compliance, which can lead to faecal incontinence.116 Although the mainstay of management remains medical, a number of neuromodulatory therapies are emerging, such as sacral nerve stimulation or percutaneous tibial nerve stimulation. Typically, a test or trial stimulation period is undertaken where the signal generator is external, and, if successful, this can be internalised in a subcutaneous pouch (Figure 3b). The majority of the studies to date have used cross-over designs, which have demonstrated a degree of efficacy in reducing the number of episodes of faecal incontinence, although these have not been specifically designed for people with diabetes.117 Albeit not tested in diabetes, percutaneous tibial nerve stimulation is a novel ambulatory therapy for faecal incontinence (Figure 3c). However, a large multi-centre randomised controlled trial comparing 12 weeks of percutaneous tibial nerve stimulation with sham stimulation did not demonstrate any benefit over 12 weeks of treatment.118
Non-invasive electrical stimulation is a promising novel approach for the treatment of abdominal symptoms that appears to increase gastric emptying and colon transit time. If proven, this would constitute a very applicable treatment approach.119
Conclusion
The understanding of diabetic neuropathy has improved rapidly over the last decade. Increased understanding of the different symptoms, and how they interact with motor and secretory functions of the gut may be a major breakthrough the treatment of the, often very diffuse, symptoms and complaints that have a major impact on quality of life. The management of diabetic gastroenteropathy is still difficult and should involve a multidisciplinary team including pharmacologists, nurses, dieticians, diabetologists, gastroenterologists and surgeons as well as health professionals from other specialities. On the other hand, new techniques to unravel the gut function as well as better treatment modalities are emerging. Together with increased awareness on the symptoms and better glycaemic control, diabetic gastroenteropathy and its different manifestations will undoubtedly be less burdensome in the near future.
Acknowledgments
Simon Lykkemark is acknowledged for his work with the illustrations.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Asbjørn Mohr Drewes  https://orcid.org/0000-0002-8833-1984
https://orcid.org/0000-0002-8833-1984
Contributor Information
Theresa Meldgaard, Mech-Sense, Department of Gastroenterology and Hepatology, Aalborg University Hospital, Denmark; Department of Clinical Medicine, Aalborg University, Denmark.
Jutta Keller, Israelitic Hospital in Hamburg, Academic Hospital University of Hamburg, Germany.
Anne Estrup Olesen, Mech-Sense, Department of Gastroenterology and Hepatology and Department of Clinical Medicine, Aalborg University Hospital, Denmark; Department of Clinical Medicine, Aalborg University, Denmark.
Søren Schou Olesen, Mech-Sense, Department of Gastroenterology and Hepatology and Department of Clinical Medicine, Aalborg University Hospital, Denmark; Department of Clinical Medicine, Aalborg University, Denmark.
Klaus Krogh, Department of Hepatology and Gastroenterology, Aarhus University Hospital, Denmark.
Mette Borre, Department of Hepatology and Gastroenterology, Aarhus University Hospital, Denmark.
Adam Farmer, Department of Gastroenterology, University Hospitals of North Midlands, Stoke on Trent, Staffordshire, UK; Centre for Digestive Diseases, Blizard Institute of Cell and Molecular Science, Wingate Institute of Neurogastroenterology, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, UK.
Birgitte Brock, Department of Clinical Research, Steno Diabetes Center Copenhagen (SDCC), Denmark.
Christina Brock, Mech-Sense, Department of Gastroenterology and Hepatology and Department of Clinical Medicine, Aalborg University Hospital, Denmark; Department of Clinical Medicine, Aalborg University, Denmark.
Asbjørn Mohr Drewes, Mech-Sense, Department of Gastroenterology and Hepatology and Department of Clinical Medicine, Aalborg University Hospital, Denmark; Department of Clinical Medicine, Aalborg University, Denmark.
References
- 1. Terkelsen AJ, Karlsson P, Lauria G, et al. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol 2017; 16: 934–944. [DOI] [PubMed] [Google Scholar]
- 2. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care 2017; 40: 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abbott CA, Malik RA, van Ross ERE, et al. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011; 34: 2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breiner A, Lovblom LE, Perkins BA, et al. Does the prevailing hypothesis that small-fiber dysfunction precedes large-fiber dysfunction apply to type 1 diabetic patients? Diabetes Care 2014; 37: 1418–1424. [DOI] [PubMed] [Google Scholar]
- 5. Spångéus A, El-Salhy M, Suhr O, et al. Prevalence of gastrointestinal symptoms in young and middle-aged diabetic patients. Scand J Gastroenterol 1999; 34: 1196–202. [DOI] [PubMed] [Google Scholar]
- 6. Ricci JA, Siddique R, Stewart WF, et al. Upper gastrointestinal symptoms in a U.S. national sample of adults with diabetes. Scand J Gastroenterol 2000; 35: 152–159. [DOI] [PubMed] [Google Scholar]
- 7. Bytzer P, Talley NJ, Hammer J, et al. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol 2002; 97: 604–611. [DOI] [PubMed] [Google Scholar]
- 8. Lindberg G, Törnblom H, Iwarzon M, et al. Full-thickness biopsy findings in chronic intestinal pseudo-obstruction and enteric dysmotility. Gut 2009; 58: 1084–1090. [DOI] [PubMed] [Google Scholar]
- 9. Horváth VJ, Vittal H, Lörincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology 2006; 130: 759–770. [DOI] [PubMed] [Google Scholar]
- 10. Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol 2016; 13: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langley JN. The autonomic nervous system part I, 1921, https://openlibrary.org/books/OL24179879M/The_autonomic_nervous_system
- 12. Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil 2014; 26: 611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bagyánszki M, Bódi N. Diabetes-related alterations in the enteric nervous system and its microenvironment. World J Diabetes 2012; 3: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature 2009; 457: 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012; 490: 55–60. [DOI] [PubMed] [Google Scholar]
- 16. Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013; 498: 99–103. [DOI] [PubMed] [Google Scholar]
- 17. Needell JC, Zipris D. The role of the intestinal microbiome in type 1 diabetes pathogenesis. Curr Diab Rep 2016; 16: 89. [DOI] [PubMed] [Google Scholar]
- 18. Aw W, Fukuda S. Understanding the role of the gut ecosystem in diabetes mellitus. J Diabetes Investig 2018; 9: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meldgaard T, Olesen SS, Farmer AD, et al. Diabetic enteropathy: from molecule to mechanism-based treatment. J Diabetes Res 2018; 2018: 3827301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meldgaard T, Olesen SS, Farmer AD, et al. Diabetic enteropathy: from molecule to mechanism-based treatment. J Diabetes Res 2018; 2018: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chandrasekharan B, Anitha M, Blatt R, et al. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil 2011; 23: 131–138.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi KM, Gibbons SJ, Nguyen T V, et al. Heme oxygenase-1 protects interstitial cells of cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology 2008; 135: 2055–2064.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iwasaki H, Kajimura M, Osawa S, et al. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol 2006; 41: 1076–1087. [DOI] [PubMed] [Google Scholar]
- 24. Qi R, Yang W, Chen J. Role of enteric glial cells in gastric motility in diabetic rats at different stages. J Huazhong Univ Sci Technol - Med Sci 2013; 33: 496–500. [DOI] [PubMed] [Google Scholar]
- 25. Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest 2006; 116: 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pereira RVF, Tronchini EA, Tashima CM, et al. L-glutamine supplementation prevents myenteric neuron loss and has gliatrophic effects in the ileum of diabetic rats. Dig Dis Sci 2011; 56: 3507–3516. [DOI] [PubMed] [Google Scholar]
- 27. Bulc M, Palus K, Całka J, et al. Changes in immunoreactivity of sensory substances within the enteric nervous system of the porcine stomach during experimentally induced diabetes. J Diabetes Res 2018; 2018: 4735659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frøkjaer JB, Andersen SD, Ejskaer N, et al. Gut sensations in diabetic autonomic neuropathy. Pain 2007; 131: 320–329. [DOI] [PubMed] [Google Scholar]
- 29. Søfteland E, Brock C, Frøkjær JB, et al. Association between visceral, cardiac and sensorimotor polyneuropathies in diabetes mellitus. J Diabetes Complications 2014; 28: 370–377. [DOI] [PubMed] [Google Scholar]
- 30. Brock C, Søfteland E, Frøkjær JB, et al. Associations between sensorimotor, autonomic and central neuropathies in diabetes mellitus. J Diabetes Metab 2014; 05: 1–6. [Google Scholar]
- 31. Botha C, Farmer AD, Nilsson M, et al. Preliminary report: modulation of parasympathetic nervous system tone influences oesophageal pain hypersensitivity. Gut 2015; 64: 611–617. [DOI] [PubMed] [Google Scholar]
- 32. Carroll SL, Byer SJ, Dorsey DA, et al. Ganglion-specific patterns of diabetes-modulated gene expression are established in prevertebral and paravertebral sympathetic ganglia prior to the development of neuroaxonal dystrophy. J Neuropathol Exp Neurol 2004; 63: 1144–1154. [DOI] [PubMed] [Google Scholar]
- 33. Guo C, Quobatari A, Shangguan Y, et al. Diabetic autonomic neuropathy: evidence for apoptosis in situ in the rat. Neurogastroenterol Motil 2004; 16: 335–345. [DOI] [PubMed] [Google Scholar]
- 34. Tay SS, Wong WC. Short- and long-term effects of streptozotocin-induced diabetes on the dorsal motor nucleus of the vagus nerve in the rat. Acta Anat (Basel) 1994; 150: 274–281. [DOI] [PubMed] [Google Scholar]
- 35. Guy RJ, Dawson JL, Garrett JR, et al. Diabetic gastroparesis from autonomic neuropathy: surgical considerations and changes in vagus nerve morphology. J Neurol Neurosurg Psychiatry 1984; 47: 686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith B. Neuropathology of the oesophagus in diabetes mellitus. J Neurol Neurosurg Psychiatry 1974; 37: 1151–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Drewes AM, Søfteland E, Dimcevski G, et al. Brain changes in diabetes mellitus patients with gastrointestinal symptoms. World J Diabetes 2016; 7: 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brock C, Graversen C, Frøkjaer JB, et al. Peripheral and central nervous contribution to gastrointestinal symptoms in diabetic patients with autonomic neuropathy. Eur J Pain 2013; 17: 820–831. [DOI] [PubMed] [Google Scholar]
- 39. Brock C, Søfteland E, Gunterberg V, et al. Diabetic autonomic neuropathy affects symptom generation and brain-gut axis. Diabetes Care 2013; 36: 3698–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Apkarian A V. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004; 24: 10410–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Segerdahl AR, Themistocleous AC, Fido D, et al. A brain-based pain facilitation mechanism contributes to painful diabetic polyneuropathy. Brain 2018; 141: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frøkjær JB, Egsgaard LL, Graversen C, et al. Gastrointestinal symptoms in type-1 diabetes: is it all about brain plasticity? Eur J Pain 2011; 15: 249–257. [DOI] [PubMed] [Google Scholar]
- 43. Drewes AM, Krarup AL, Detlefsen S, et al. Pain in chronic pancreatitis: the role of neuropathic pain mechanisms. Gut 2008; 57: 1616–1627. [DOI] [PubMed] [Google Scholar]
- 44. Zhao M, Liao D, Zhao J. Diabetes-induced mechanophysiological changes in the small intestine and colon. World J Diabetes 2017; 8: 249–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao J-B, Frøkjaer JB, Drewes AM, et al. Upper gastrointestinal sensory-motor dysfunction in diabetes mellitus. World J Gastroenterol 2006; 12: 2846–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Jong RGPJ, Peeters PJHL, Burden AM, et al. Gastrointestinal cancer incidence in type 2 diabetes mellitus; results from a large population-based cohort study in the UK. Cancer Epidemiol 2018; 54: 104–111. [DOI] [PubMed] [Google Scholar]
- 47. Hsu P-C, Lin W-H, Kuo T-H, et al. A population-based cohort study of all-cause and site-specific cancer incidence among patients with type 1 diabetes mellitus in taiwan. J Epidemiol 2015; 25: 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Van den Driessche A, Eenkhoorn V, Van Gaal L, et al. Type 1 diabetes and autoimmune polyglandular syndrome: a clinical review. Neth J Med 2009; 67: 376–387. [PubMed] [Google Scholar]
- 49. Krarup T, Schwartz TW, Hilsted J, et al. Impaired response of pancreatic polypeptide to hypoglycaemia: an early sign of autonomic neuropathy in diabetics. Br Med J 1979; 2: 1544–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keller J, Bassotti G, Clarke J, et al. Expert consensus document: Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat Rev Gastroenterol Hepatol 2018; 15: 291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Olausson EA, Grundin H, Isaksson M, et al. Postprandial plasma glucose response and gastrointestinal symptom severity in patients with diabetic gastroparesis. J Diabetes Sci Technol 2014; 8: 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Balan K, Sonoda LI, Seshadri N, et al. Clinical significance of scintigraphic rapid gastric emptying. Nucl Med Commun 2011; 32: 1185–1189. [DOI] [PubMed] [Google Scholar]
- 53. Vijayvargiya P, Jameie-Oskooei S, Camilleri M, et al. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut 2019; 68: 804–813. [DOI] [PubMed] [Google Scholar]
- 54. Farmer AD, Scott SM, Hobson AR. Gastrointestinal motility revisited: The wireless motility capsule. United Eur Gastroenterol J 2013; 1: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Farmer AD, Pedersen AG, Brock B, et al. Type 1 diabetic patients with peripheral neuropathy have pan-enteric prolongation of gastrointestinal transit times and an altered caecal pH profile. Diabetologia 2017; 60: 709–718. [DOI] [PubMed] [Google Scholar]
- 56. Mark EB, Poulsen JL, Haase A-M, et al. Ambulatory assessment of colonic motility using the electromagnetic capsule tracking system. Neurogastroenterol Motil 2018: e13451. [DOI] [PubMed] [Google Scholar]
- 57. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017; 40: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Knopp JL, Signal M, Harris DL, et al. Modelling intestinal glucose absorption in premature infants using continuous glucose monitoring data. Comput Methods Programs Biomed 2018; 2: 37. [DOI] [PubMed] [Google Scholar]
- 59. Parkin CG, Homberg A, Hinzmann R. 10th annual symposium on self-monitoring of blood glucose, April 27–29, 2017, Warsaw, Poland. Diabetes Technol Ther 2018; 20: 68–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. American diabetes association. 6. Glycemic targets: standards of medical care in diabetes-2018. Diabetes Care 2018; 41: S55–S64. [DOI] [PubMed] [Google Scholar]
- 61. Borot S, Benhamou PY, Atlan C, et al. Practical implementation, education and interpretation guidelines for continuous glucose monitoring: a French position statement. Diabetes Metab 2018; 44: 61–72. [DOI] [PubMed] [Google Scholar]
- 62. Hatton GB, Madla CM, Rabbie SC, et al. All disease begins in the gut: Influence of gastrointestinal disorders and surgery on oral drug performance. Int J Pharm 2018; 548: 408–422. [DOI] [PubMed] [Google Scholar]
- 63. Li J, Guo H-F, Liu C, et al. Prediction of drug disposition in diabetic patients by means of a physiologically based pharmacokinetic model. Clin Pharmacokinet 2015; 54: 179–193. [DOI] [PubMed] [Google Scholar]
- 64. Washington N, Washington C, Wilson CG. Physiological pharmaceutics barriers to drug absorption. 2nd ed. London: Taylor and Francis, 2001. [Google Scholar]
- 65. Wegeberg A-ML, Brock C, Brock B, et al. Regional gastrointestinal pH profile is altered in patients with type 1 diabetes and peripheral neuropathy. Neurogastroenterol Motil 2018; 30: e13407. [DOI] [PubMed] [Google Scholar]
- 66. Li J, Guo H-F, Liu C, et al. Prediction of drug disposition in diabetic patients by means of a physiologically based pharmacokinetic model. Clin Pharmacokinet 2015; 54: 179–193. [DOI] [PubMed] [Google Scholar]
- 67. Dostalek M, Akhlaghi F, Puzanovova M. Effect of diabetes mellitus on pharmacokinetic and pharmacodynamic properties of drugs. Clin Pharmacokinet 2012; 51: 481–499. [DOI] [PubMed] [Google Scholar]
- 68. Ward N. The impact of intestinal failure on oral drug absorption: a review. J Gastrointest Surg 2010; 14: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 69. McCallum RW, Ricci DA, Rakatansky H, et al. A multicenter placebo-controlled clinical trial of oral metoclopramide in diabetic gastroparesis. Diabetes Care 1983; 6: 463–467. [DOI] [PubMed] [Google Scholar]
- 70. Janssens J, Peeters TL, Vantrappen G, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med 1990; 322: 1028–1031. [DOI] [PubMed] [Google Scholar]
- 71. Patterson D, Abell T, Rothstein R, et al. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol 1999; 94: 1230–1234. [DOI] [PubMed] [Google Scholar]
- 72. Camilleri M. Novel diet, drugs, and gastric interventions for gastroparesis. Clin Gastroenterol Hepatol 2016; 14: 1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vijayvargiya P, Camilleri M, Chedid V, et al. Effects of promotility agents on gastric emptying and symptoms: a systematic review and meta-analysis. Gastroenterology 2019; 156: 1650–1660. [DOI] [PubMed] [Google Scholar]
- 74. Bor S, Demir M, Ozdemir O, et al. A meta-analysis on the cardiac safety profile of domperidone compared to metoclopramide. United Eur Gastroenterol J 2018; 6: 1331–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Giudicessi JR, Ackerman MJ, Camilleri M. Cardiovascular safety of prokinetic agents: A focus on drug-induced arrhythmias. Neurogastroenterol Motil 2018; 30: e13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Navas CM, Crowell MD, Lacy BE. The willingness of patients with gastroparesis to take risks with medications. Aliment Pharmacol Ther 2019; 1–8. [DOI] [PubMed] [Google Scholar]
- 77. Tack J, Camilleri M. New developments in the treatment of gastroparesis and functional dyspepsia. Curr Opin Pharmacol 2018; 43: 111–117. [DOI] [PubMed] [Google Scholar]
- 78. Chedid V, Camilleri M. Relamorelin for the treatment of gastrointestinal motility disorders. Expert Opin Investig Drugs 2017; 26: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 79. Sawhney MS, Prakash C, Lustman PJ, et al. Tricyclic antidepressants for chronic vomiting in diabetic patients. Dig Dis Sci 2007; 52: 418–424. [DOI] [PubMed] [Google Scholar]
- 80. Talley NJ, Locke GR, Saito YA, et al. Effect of amitriptyline and escitalopram on functional dyspepsia: a multicenter, randomized Controlled Study. Gastroenterology 2015; 149: 340–349.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA 2013; 310: 2640–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Leeds JS, Hadjivassiliou M, Tesfaye S, et al. Lower gastrointestinal symptoms are associated with worse glycemic control and quality of life in type 1 diabetes mellitus. BMJ Open Diabetes Res Care 2018; 6: e000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Soltanian N, Janghorbani M, Adibi P. Effects of psyllium vs. placebo on constipation, weight, glycemia, and lipids: A randomized trial in patients with type 2 diabetes and chronic constipation. Complement Ther Med 2018; 40: 1–7. [DOI] [PubMed] [Google Scholar]
- 84. Soltanian N, Janghorbani M. A randomized trial of the effects of flaxseed to manage constipation, weight, glycemia, and lipids in constipated patients with type 2 diabetes. Nutr Metab. Epub ahead of print 2018. DOI: 10.1186/s12986-018-0273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Christie J, Shroff S, Shahnavaz N, et al. A randomized, double-blind, placebo-controlled trial to examine the effectiveness of lubiprostone on constipation symptoms and colon transit time in diabetic patients. Am J Gastroenterol 2017; 112: 356–364. [DOI] [PubMed] [Google Scholar]
- 86. Quigley EMM, Tack J, Chey WD, et al. Randomised clinical trials: linaclotide phase 3 studies in IBS-C - a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment Pharmacol Ther 2013; 37: 49–61. [DOI] [PubMed] [Google Scholar]
- 87. Shimozato A, Sasaki M, Ogasawara N, et al. Transglucosidase improves the bowel movements in type 2 diabetes mellitus patients: a preliminary randomized double-blind, placebo-controlled study. United Eur Gastroenterol J 2017; 5: 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sommers T, Mitsuhashi S, Singh P, et al. Prevalence of chronic constipation and chronic diarrhea in diabetic Individuals in the united States. Am J Gastroenterol Epub ahead of print 2018. DOI: 10.1038/s41395-018-0418-8. [DOI] [PubMed] [Google Scholar]
- 89. Fedorak RN, Field M, Chang EB. Treatment of diabetic diarrhea with clonidine. Ann Intern Med 1985; 102: 197–199. [DOI] [PubMed] [Google Scholar]
- 90. Haas S, Brock C, Krogh K, et al. Does sacral nerve stimulation improve continence through enhanced sensitivity of the anal canal? a pilot study. Dis Colon Rectum 2016; 59: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 91. Olesen AE, Farmer AD, Olesen SS, et al. Management of chronic visceral pain. Pain Manag 2016; 6: 469–486. [DOI] [PubMed] [Google Scholar]
- 92. Edwards RR, Dworkin RH, Turk DC, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain 2016; 157: 1851–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ang L, Cowdin N, Mizokami-Stout K, et al. Update on the management of diabetic neuropathy. Diabetes Spectr 2018; 31: 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. O’Brien T, Christrup LL, Drewes AM, et al. European pain federation position paper on appropriate opioid use in chronic pain management. Eur J Pain 2017; 21: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Drewes AM, Munkholm P, Simrén M, et al. Topical review definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction-Recommendations of the Nordic Working Group. Scand J Pain 2016; 11: 111–122. [DOI] [PubMed] [Google Scholar]
- 97. Farmer AD, Drewes AM, Chiarioni G, et al. Pathophysiology and management of opioid-induced constipation: European expert consensus statement. United Eur Gastroenterol J Epub ahead of print 14 December 2018. DOI: 10.1177/2050640618818305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Poulsen JL, Brock C, Olesen AE, et al. Evolving paradigms in the treatment of opioid-induced bowel dysfunction. Therap Adv Gastroenterol 2015; 8: 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Drewes AM, Bouwense SAW, Campbell CM, et al. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology 2017; 17: 720–731. [DOI] [PubMed] [Google Scholar]
- 100. Parkman HP, Yates KP, Hasler WL, et al. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology 2011; 141: 486–98, 498.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Koch KL, Hasler WL, Yates KP, et al. Baseline features and differences in 48 week clinical outcomes in patients with gastroparesis and type 1 vs type 2 diabetes. Neurogastroenterol Motil 2016; 28: 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Olausson EA, Störsrud S, Grundin H, et al. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol 2014; 109: 375–385. [DOI] [PubMed] [Google Scholar]
- 103. Clegg M, Shafat A. Energy and macronutrient composition of breakfast affect gastric emptying of lunch and subsequent food intake, satiety and satiation. Appetite 2010; 54: 517–523. [DOI] [PubMed] [Google Scholar]
- 104. Goetze O, Steingoetter A, Menne D, et al. The effect of macronutrients on gastric volume responses and gastric emptying in humans: A magnetic resonance imaging study. Am J Physiol Gastrointest Liver Physiol 2007; 292: G11–G17. [DOI] [PubMed] [Google Scholar]
- 105. Marciani L, Cox EF, Pritchard SE, et al. Additive effects of gastric volumes and macronutrient composition on the sensation of postprandial fullness in humans. Eur J Clin Nutr 2015; 69: 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fried M, Feinle C. The role of fat and cholecystokinin in functional dyspepsia. Gut 2002; 51 Suppl 1: i54–i57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Homko CJ, Duffy F, Friedenberg FK, et al. Effect of dietary fat and food consistency on gastroparesis symptoms in patients with gastroparesis. Neurogastroenterol Motil 2015; 27: 501–508. [DOI] [PubMed] [Google Scholar]
- 108. Shi X, Osterberg KL, Petrie H, et al. Effect of different osmolalities, CHO types, and [CHO] on gastric emptying in humans. Med Sci Sports Exerc 2017; 49: 1015–1021. [DOI] [PubMed] [Google Scholar]
- 109. Fontana RJ, Barnett JL. Jejunostomy tube placement in refractory diabetic gastroparesis: a retrospective review. Am J Gastroenterol 1996; 91: 2174–2178. [PubMed] [Google Scholar]
- 110. Nelson AD, Camilleri M, Acosta A, et al. A single-center, prospective, double-blind, sham-controlled, randomized study of the effect of a vibrating capsule on colonic transit in patients with chronic constipation. Neurogastroenterol Motil 2017; 29: 1–6. [DOI] [PubMed] [Google Scholar]
- 111. Soffer EE. Gastric electrical stimulation for gastroparesis. J Neurogastroenterol Motil 2012; 18: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Eagon JC, Kelly KA. Effects of gastric pacing on canine gastric motility and emptying. Am J Physiol 1993; 265: G767–G774. [DOI] [PubMed] [Google Scholar]
- 113. Vanormelingen C, Tack J, Andrews CN. Diabetic gastroparesis. Br Med Bull 2013; 105: 213–230. [DOI] [PubMed] [Google Scholar]
- 114. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol 2011; 9: 314–319.e1. [DOI] [PubMed] [Google Scholar]
- 115. O’Grady G, Egbuji JU, Du P, et al. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World J Surg 2009; 33: 1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Russo A, Botten R, Kong M-F, et al. Effects of acute hyperglycaemia on anorectal motor and sensory function in diabetes mellitus. Diabet Med 2004; 21: 176–182. [DOI] [PubMed] [Google Scholar]
- 117. Thaha MA, Abukar AA, Thin NN, et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane database Syst Rev 2015; 2015: CD004464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Knowles CH, Horrocks EJ, Bremner SA, et al. Percutaneous tibial nerve stimulation versus sham electrical stimulation for the treatment of faecal incontinence in adults (CONFIDeNT): a double-blind, multicentre, pragmatic, parallel-group, randomised controlled trial. Lancet (London, England) 2015; 386: 1640–1648. [DOI] [PubMed] [Google Scholar]
- 119. Yik YI, Ed M, Hutson J, et al. Home-based transabdominal interferential electrical stimulation for six months improves paediatric slow transit constipation (STC). Neuromodulation J Int Neuromodulation Soc 2018; 21: 676–681. [DOI] [PubMed] [Google Scholar]