Abstract
Recent studies have identified a beneficial role for peptide tyrosine tyrosine (PYY) on pancreatic beta-cell function and survival. These effects are linked to the activation of neuropeptide Y1 receptors (NPYR1s) by PYY(1-36). However, PYY(1-36) is subject to rapid degradation by dipeptidyl peptidase-4 (DPP-4), resulting is the loss of NPYR1 activity. Therefore, the aim of this study was to develop 2 enzymatically stable PYY(1-36) analogues, namely, (P3L31P34)PYY(1-36) and PYY(1-36)(Lys12PAL), with further structural modifications to enhance NPYR1 specificity. As expected, (P3L31P34)PYY(1-36) was fully resistant to DPP-4-mediated degradation in vitro, whereas PYY(1-36) and PYY(1-36)(Lys12PAL) were both liable to DPP-4 breakdown. PYY(1-36) and (P3L31P34)PYY(1-36) induced significant reductions in glucose-stimulated insulin secretion (GSIS) from BRIN BD11 cells, but only PYY(1-36) diminished alanine-stimulated insulin secretion. In contrast, PYY(1-36)(Lys12PAL) had no impact on GSIS or alanine-induced insulin release. All 3 PYY peptides significantly enhanced proliferation in BRIN BD11 and 1.1B4 beta-cell lines, albeit only at the highest concentration examined, 10-6 M, for (P3L31P34)PYY(1-36) and PYY(1-36)(Lys12PAL) in BRIN BD11 cells. Regarding the protection of beta-cells against cytokine-induced apoptosis, PYY(1-36) induced clear protective effects. Both (P3L31P34)PYY(1-36) and PYY(1-36)(Lys12PAL) offered some protection against apoptosis in BRIN BD11 cells, but were significantly less efficacious than PYY(1-36). Similarly, in 1.1B4 cells, both PYY analogues (10-6 M) protected against cytokine-induced apoptosis, but (P3L31P34)PYY(1-36) was significantly less effective than PYY(1-36). All 3 PYY peptides had no impact on refeeding in overnight fasted mice. These data underline the beta-cell benefits of PYY(1-36) and highlight the challenges of synthesising stable, bioactive, NPYR1-specific, PYY(1-36) analogues.
Keywords: peptide YY (PYY), DPP-4, acylation, amino acid substitution, insulin secretion, beta-cell
Introduction
The native form of peptide tyrosine tyrosine (PYY) consists of 36 amino acids; however, the dipeptidyl peptidase-4 (DPP-4) degradation product, PYY(3-36), is believed to be the principal circulating PYY entity.1 This N-terminal enzymatic cleavage results in a major change in receptor specificity for PYY. As such, PYY(1-36) is an established agonist for each subtype of the target neuropeptide Y receptor (NPYR) family, namely, NPYR1, NPYR2, NPYR4, and NPYR5,2 whereas PYY(3-36) is a highly selective NPYR2 agonist.3 Indeed, much of the early work with PYY has focused predominantly on PYY(3-36) and its role in appetite regulation through the activation of hypothalamic NPYR2s.4-7 However, PYY has also been shown to be expressed and synthesised in pancreatic islet cells,8 highlighting a role for the peptide in pancreatic endocrine function.9
In this regard, recent studies have confirmed a positive effect of PYY on beta-cell survival and overall function, linked to the activation of NPYR1s.10,11 Thus, PYY(1-36) has been shown to enhance the growth and survival of beta-cells,8 ultimately leading to enhanced glycaemic control.12 In agreement with this, the ablation of PYY-expressing cells in mice results in beta-cell destruction and overt hyperglycaemia, which was partially rescued by NPYR1 activation.13 Furthermore, streptozotocin-induced beta-cell loss and insulin deficiency have been shown to decrease islet PYY expression, whereas hydrocortisone-induced beta-cell expansion was linked to an increased expression of islet PYY.8 Taken together, it is clear that the activation of islet NPYR1s by PYY offers potential as a future treatment option for diabetes, as disease pathophysiology closely linked to the loss of beta-cell mass and function.14
Therefore, the aim of this study was to synthesise and characterise 2 PYY(1-36) analogues, using current structure/function knowledge to enhance enzymatic stability and specificity towards NPYR1. Initially, Ile3 was substituted with Pro3, as this has been shown to confer DPP-4 resistance in related peptide hormones.15-17 In addition, previous studies revealed that the substitution of Gln34 for Pro34 in PYY(1-36) imparted increased NPYR1 selectivity.18 Moreover, replacing amino acids 31 of both PYY and the structurally related neuropeptide Y (NPY) peptide with leucine also inferred increased selectivity towards NPY1R.19-21 Using this structure/function information, we generated the novel peptide, (P3L31P34)PYY(1-36). Further to this, acylation of numerous regulatory peptide hormones, including gastric inhibitory polypeptide (GIP), glucagon-like peptide-1 (GLP-1), xenin, and apelin, has been shown to yield complete enzymatic resistance and significantly protract circulating biological half-life.22-30 In agreement, PYY(3-36) acylated at Lys12 is an established stable and long-acting PYY(3-36) analogue, where Ala12 is substituted for Lys12 to facilitate acylation.31 Thus, we also generated and tested PYY(1-36)(Lys12PAL) as a second potentially stable PYY-based NPYR1 agonist.
Initially, DPP-4 stability of all peptides was assessed, followed by the examination of peptide effects on in vitro insulin secretion. In addition, the impact of PYY(1-36) and related analogues on pancreatic beta-cell growth and protection against apoptosis, as well as food intake in mice, was studied. The results demonstrate the positive beta-cell survival effects of PYY(1-36), suggesting possible antidiabetic utility for enzymatically stable and more potent PYY forms. However, although (P3L31P34)PYY(1-36) and PYY(1-36)(Lys12PAL) did confer some beta-cell benefits, their effectiveness was compromised when compared with the parent peptide.
Materials and Methods
Peptides
All peptides, including GLP-1 positive control, were supplied by EZBiolab Ltd (Carmel, IN, USA) at 95% purity, with peptide purity and mass confirmed in-house by high-performance liquid chromatography (HPLC) and matrix-assisted laser desorption ionisation time-of-flight mass spectrometry (MALDI-TOF MS), respectively (Table 1).
Table 1.
PYY(1-36), (P3L31P34)PYY(1-36), and PYY(Lys12PAL) amino acid sequences and characterisation.
| Peptide name | Amino acid sequence | Purity (%) | Expected mass (Da) | Calculated mass (Da) |
|---|---|---|---|---|
| PYY(1-36) | YPIKPEAPGEDASPEELNRYYASLRHYLNLVTRQRY | 99.9 | 4309.8 | 4309.7 |
| (P3L31P34)PYY(1-36) | YPPKPEAPGEDASPEELNRYYASLRHYLNLLTRPRY | 99.2 | 4277.9 | 4274.7 |
| PYY(1-36)(Lys12PAL) | YPIKPEAPGEDK(PAL)SPEELNRYYASLRHYLNLVTRQRY | 100 | 4606.4 | 4606.4 |
Abbreviations: MALDI-TOF MS, matrix-assisted laser desorption ionisation time-of-flight mass spectrometry; PYY, peptide tyrosine tyrosine; RP-HPLC, reverse phase high-performance liquid chromatography.
Peptides’ purity was confirmed using RP-HPLC on a ThermoQuest SpectraSYSTEM UV2000 chromatography system using a Phenomenex C-18 analytical column with absorbance at 214 nm. Identity of peptides was confirmed using Voyager-DE Biospectrometry MALDI-TOF MS (PerSeptive Biosystems, Framingham, MA, USA), as described previously.32
PYY degradation
PYY(1-36), (P3L31P34)PYY(1-36), and PYY(1-36)(Lys12PAL) (50 µg of each peptide) were incubated at 37°C on a plate shaker in 50 mM triethanolamine/HCl (pH 7.8) with 5 µL of pure DPP-4 enzyme (0.01 U/µL, Sigma-Aldrich, Gillingham, UK) for 0 and 8 hours. Reactions were terminated, as appropriate, via the addition of 10 µL of 10% (v/v) trifluoroacetic acid/water. Reaction mixes were separated by reverse phase high-performance liquid chromatography (RP-HPLC) using a Phenomenex C-18 analytical column (250 × 4.6 mm2), with absorbance monitored at 214 nm using a ThermoQuest SpectraSYSTEM UV2000 detector. High-performance liquid chromatography peaks were collected and identified via MALDI-TOF MS on a PerSeptive Biosystems Voyager-DE Biospectrometry (Hertfordshire, UK).
In vitro insulin secretion
The in vitro effects of PYY(1-36), (P3L31P34)PYY(1-36), and PYY(1-36)(Lys12PAL) on insulin secretion were determined using pancreatic clonal BRIN-BD11 beta-cells. The characteristics of this cell line, including glucose sensitivity and insulin secretory function, have been described in detail previously.33 Cells were cultured in RPMI 1640 medium (Gibco Life Technologies Ltd), supplemented with 10% v/v foetal bovine serum (Gibco, Thermo Fisher Scientific, Dublin, ROI) and 1% v/v antibiotics (0.1 mg/mL streptomycin and 100 U/mL penicillin) at 37°C in 5% atmospheric CO2. For experimentation, cells were seeded into 24-well plates (Falcon Ltd, Thermo Fisher Scientific, Dublin, ROI) at a density of 150 000 cells per well. Following overnight attachment, the medium was aspirated and cells were pre-incubated in 1.1 mM glucose Krebs-Ringer Buffer (KRB) for 40 minutes. Following pre-incubation, the 1.1 mM glucose solution was removed and 1 mL of KRB test solution, containing either 5.6 or 16.7 mM glucose with PYY test peptides (10-12-10-6 M) was added. PYY peptides were then incubated in the presence of alanine (10 mM) at 16.7 mM glucose to further investigate the effects on insulin secretion. For all experiments, following a 20-minute incubation period, the supernatant was collected and stored at ‒20°C until insulin concentration determination using a dextran-coated charcoal insulin radioimmunoassay.34
Beta-cell proliferation and apoptosis
To assess the effects of PYY(1-36) and related analogues (10-8 and 10-6 M) on beta-cell proliferation and apoptosis, rodent BRIN-BD1133 and human 1.1B435,36 beta-cells were seeded onto sterilised, clear-glass coverslips (16 mm diameter) and placed in 12-well plates (Falcon Ltd) at a density of 40 000 cells per well and cultured for 18 hours. Medium control, GLP-1 (10-8 and 10-6 M), and a human cytokine cocktail mix (interleukin-1β [IL-1β]: 100 U/mL; interferon-γ [IFNγ]: 20 U/mL; tumour necrosis factor-α [TNFα]: 200 U/mL) (Sigma-Aldrich) were employed as controls in both cell lines, as appropriate. Cells were then rinsed with phosphate-buffered saline (PBS) and fixed using 4% paraformaldehyde. After antigen retrieval with sodium citrate buffer at 95°C for 20 minutes, blocking was performed using 2% bis(trimethylsilyl)acetamide (BSA) for 45 minutes. For proliferation studies, the slips were then incubated at 37°C with rabbit anti-Ki-67 primary antibody (ab15580; Abcam, Cambridge, UK) and subsequently with Alexa Fluor 488 secondary antibody. Coverslips were mounted onto polysine-coated microscopic slides using a 50:50 glycerol:PBS solution and stored at 4°C until required for analysis. To assess the ability of PYY peptides to protect against cytokine-induced apoptosis, cells were seeded, washed, and fixed as above, with the exception that the medium was supplemented with the cytokine mix. The slips were then incubated at 37°C with TUNEL reaction mix for 60 minutes (Roche Diagnostics, Mannheim, Germany) and mounted onto microscopic slides, as above. All slides were viewed using a fluorescent microscope (model BX51; Olympus, Southend-on-Sea, UK) and photographed by a DP70 camera adapter system. Proliferation/TUNEL-positive frequency was determined using the cell-counter function on ImageJ software and expressed as the percentage of total cells analysed.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (version 5.0). Values are expressed as mean ± SEM. Comparative analyses between groups were performed using a one-way analysis of variance (ANOVA) with Bonferroni post hoc test or Student unpaired t-test, as appropriate. The difference between groups was considered significant if P < .05.
Results
DPP-4 stability
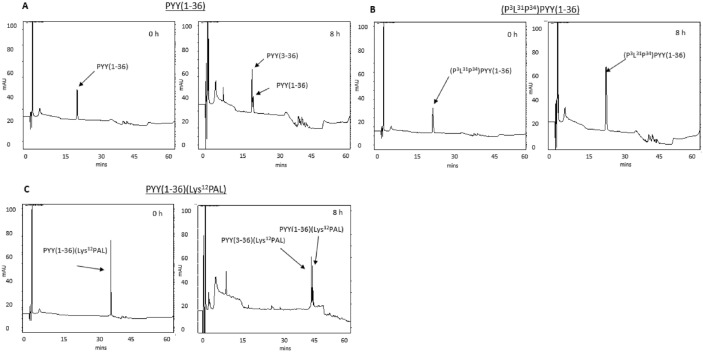
Incubation of PYY(1-36) with DPP-4 resulted in the generation of PYY(3-36) (Figure 1A and Supplementary Figure 1). Similarly, PYY(1-36)(Lys12PAL) was also N-terminally degraded by DPP-4 (Figure 1B and Supplementary Figure 1). In contrast, (P3L31P34)PYY(1-36) was completely resistant to DPP-4 degradation over the 8-hour incubation period (Figure 1C).
Figure 1.
HPLC profiles obtained following the incubation of (A) PYY(1-36), (B) (P3L31P34)PYY(1-36), and (C) PYY(1-36)(Lys12PAL) with purified DPP-4. Peptides (50 µg; n = 3) were incubated at 37°C with 5 µL DPP-4 enzyme (0.01 U/µL) in 50 mM triethanolamine-HCl. Reactions were stopped using 10% (v/v) trifluoroacetic acid/water and reaction mixes separated by HPLC. Peptide or peptide fragment masses were determined by MALDI-TOF MS (see Supplementary Data). DPP-4 indicates dipeptidyl peptidase-4; HPLC, high-performance liquid chromatography; MALDI-TOF MS, matrix-assisted laser desorption ionisation time-of-flight mass spectrometry; PYY, peptide tyrosine tyrosine.
Effects of PYY(1-36), (P3L31P34)PYY(1-36), and PYY(1-36)(Lys12PAL) on insulin secretion from rodent BRIN BD11 beta-cells
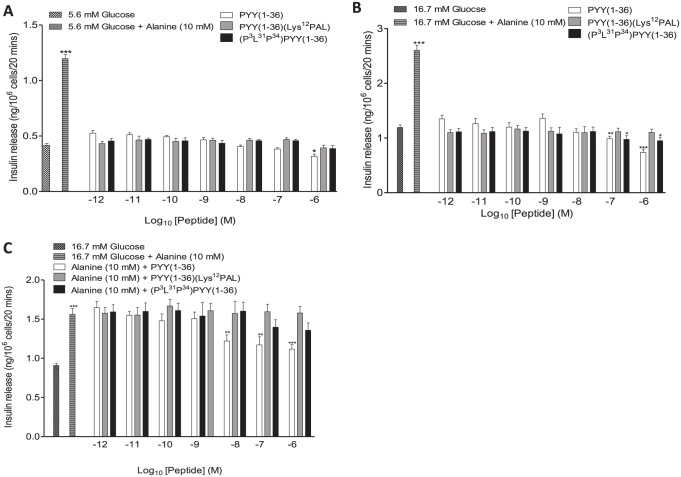
PYY(1-36) significantly (P < .05) inhibited insulin secretion from BRIN BD11 cells at 5.6 mM glucose, albeit only at the highest concentration (10-6 M) examined (Figure 2A). Similarly, at 16.7 mM glucose PYY(1-36) also significantly (at 10-7 and 10-6 M, P < .01 to P < .001, respectively) decreased the insulin secretory response (Figure 2A). (P3L31P34)PYY(1-36) and PYY(1-36)(Lys12PAL) did not modulate insulin secretion at 5.6 mM glucose (Figure 2A). However, at 16.7 mM glucose (P3L31P34)PYY(1-36) decreased (P < .05) glucose-stimulated insulin secretion from BRIN BD11 cells (Figure 2B). When incubated at 16.7 mM glucose supplemented with 10 mM alanine, PYY(1-36), but not (P3L31P34)PYY(1-36) or PYY(1-36)(Lys12PAL), reduced (P < .01 to P < .001) alanine-induced augmentations of insulin release (Figure 2C).
Figure 2.
Effects of PYY(1-36), (P3L31P34)PYY(1-36), and PYY(1-36)(Lys12PAL) on insulin release from BRIN-BD11 beta-cells. BRIN BD11 cells were incubated with (A) 5.6 mM glucose, (B) 16.7 mM glucose, or (C) 16.7 mM glucose supplemented with alanine (10 mM) and the effects of PYY peptides (10-6-10-12 M) on insulin secretion determined. Values are mean ± SEM (n = 8). PYY indicates peptide tyrosine tyrosine.
*P < .05, **P < .01, and ***P < .001 compared with (A) 5.6 mM glucose, (B) 16.7 mM glucose, or (C) 16.7 mM glucose supplemented with alanine.
Effects of PYY(1-36), (P3L31P34)PYY(1-36), and PYY(1-36)(Lys12PAL) on beta-cell proliferation and protection against cytokine-induced apoptosis
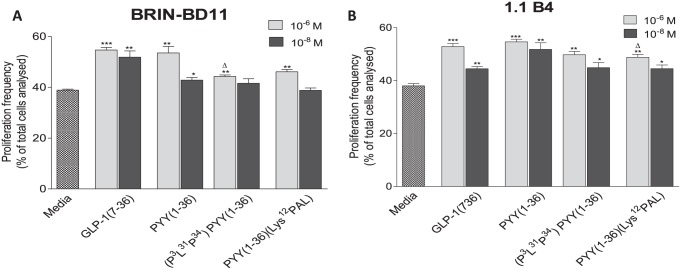
Both GLP-1 and PYY(1-36) (10-8 and 10-6 M) significantly (P < .05 to P < .001) increased BRIN BD11 and 1.1B4 beta-cell proliferation when compared with control cultures (Figure 3A and B). In addition, (P3L31P34)PYY(1-36) and PYY(1-36)(Lys12PAL) also significantly increased (P < .05 to P < .01) the proliferation of both beta-cell lines, but only at the highest concentration tested, 10-6 M, in BRIN BD11 cells (Figure 3A and B). In addition, at 10-6 M PYY(1-36) induced significantly (P < .05) increased beta-cell proliferation when compared with (P3L31P34)PYY(1-36) in BRIN BD11 cells and PYY(1-36)(Lys12PAL) in 1.1B4 cells, at the same concentration (Figure 3A and B). Representative images of Ki-67-stained beta-cells are shown in Supplementary Figures 2 and 3. Regarding protection against cytokine-induced beta-cell apoptosis, all peptides at both concentrations examined (10-8 and 10-6 M), barring PYY(1-36)(Lys12PAL) at 10-8 M, significantly (P < .05 to P < .001) reduced apoptosis in BRIN BD11 cells when compared with cytokine cocktail control (Figure 4A). However, PYY(1-36)(Lys12PAL) was significantly (P < .05 to P < .01) less efficacious in this regard than PYY(1-36), as was (P3L31P34)PYY(1-36) at 10-6 M (Figure 4A). In 1.1B4 beta-cells, all peptides, except (P3L31P34)PYY(1-36) at 10-8 M, decreased apoptosis to levels significantly (P < .05 to P < .001) lower than those of the cytokine cocktail treatment alone (Figure 4B). (P3L31P34)PYY(1-36) was significantly (P < .01) less effective at preventing apoptosis than PYY(1-36) in 1.1B4 beta-cells (Figure 4B). Representative images of TUNEL-stained beta-cells under each culture condition are shown in Supplementary Figures 4 and 5.
Figure 3.
Effects of PYY(1-36), (P3L31P34)PYY(1-36), and PYY(1-36)(Lys12PAL) on (A) rodent BRIN-BD11 and (B) human 1.1 B4 beta-cell proliferation. Cells were cultured (16 hours) with PYY peptides or GLP-1 (10-8 and 10-6 M) and proliferation assessed by Ki-67 staining. Values are mean ± SEM (n = 3). GLP-1 indicates glucagon-like peptide-1; PYY, peptide tyrosine tyrosine.
*P < .05, **P < .01, and ***P < .001 compared with control culture.
ΔP < .05 compared with PYY(1-36).
Representative images for each treatment are provided in Supplementary Data.
Figure 4.
Effects of PYY(1-36), (P3L31P34)PYY(1-36), and PYY(1-36)(Lys12PAL) on protection against cytokine-induced apoptosis in (A) rodent BRIN-BD11 and (B) human 1.1 B4 beta-cells. Cells were cultured (16 hours) with PYY peptides or GLP-1 (10-8 and 10-6 M) in the presence of a cytokine cocktail and apoptosis detected using the TUNEL assay. Values are mean ± SEM (n = 3). GLP-1 indicates glucagon-like peptide-1; PYY, peptide tyrosine tyrosine.
*P < .05, **P < .01, and ***P < .001 compared with cytokine cocktail.
++P < .01 and +++P < .001 compared with RPMI medium control.
ΔP < .05 and ΔΔP < .01 compared with PYY(1-36) at the same concentration.
Representative images for each treatment are provided in Supplementary Data.
Discussion
Since the discovery of the satiety-inducing effects of the DPP-4 degradation product of PYY(1-36), namely, PYY(3-36),37 most PYY-based research has revolved around the activation of hypothalamic NPYR2 receptors by PYY(3-36) and possible anti-obesity effects.1,31 However, more recent evidence reveals that the NPYR1 is expressed on pancreatic islet cells and that PYY(1-36) is synthesised and secreted locally within islets, with postulated beneficial local actions.8,9 This study has consequently aimed to synthesise and characterise PYY(1-36) peptide analogues with enhanced enzymatic stability and improved NPYR1 selectivity, to fully harness PYY-related pancreatic benefits. To date, the only report of a long-acting PYY analogue with NPYR1 activity is a dual NPYR1/NPYR2 agonist named X-PYY,13 which interestingly has deletion of the first 2 N-terminal amino acids that would be considered to diminish NPYR1-mediated effects.3
(P3L31P34)PYY(1-36) and PYY(1-36)(Lys12PAL) were designed based on current structure/function knowledge known to extend biological half-life and/or promote NPYR1 selectivity of peptide-based drugs.6,16,18,31,38 Following successful synthesis, the susceptibility of the PYY peptides to DPP-4 degradation was examined. In contrast to native PYY(1-36), which was efficiently degraded by DPP-4 to PYY(3-36),39 (P3L31P34)PYY(1-36) was completely resistant to DPP-4-mediated enzymatic degradation. This confirms that the substitution of Ile3 in PYY(1-36) with a proline residue renders the peptide resistant to the actions of DPP-4, as documented for other regulatory peptide hormones.15 Thus, unlike native PYY(1-36), (P3L31P34)PYY(1-36) does not undergo removal of the N-terminal Tyr1-Pro2 dipeptide, known to generate a more specific NPYR2 agonist.3,39 Indeed, the additional structural modifications at positions 31 and 34 in (P3L31P34)PYY(1-36) should render the peptide a stable, long-acting, NPYR1 agonist.18,21 Somewhat surprisingly, PYY(1-36)(Lys12PAL) was susceptible to DPP-4 degradation in the in vitro system, unlike related fatty-acid-derivatised regulatory peptide hormone analogues where acylation has been shown to mask the cleavage site for DPP-4.22-30 This difference could be related to the unique three-dimensional structure of PYY that includes an N-terminal left-handed polyproline-like helix, a typical mid-chain α-helix and β-turn that together give rise to the characteristic ‘PP-fold’ of the NPY family of peptides.40 In addition, the Lys12 for Ala12 amino acid substitution in PYY(1-36)(Lys12PAL) may also have an impact here. However, the overall effect of acylation and subsequent protein binding of PYY(1-36)(Lys12PAL) in vivo may lead to altered kinetics and reduced conversion to PYY(3-36)(Lys12PAL), which would require more detailed study.
Although structural modification of PYY(1-36) may protect against DPP-4 cleavage and therefore be highly influential for biological half-life and receptor specificity,3,18,38 confirmation of preserved bioactivity is still of utmost importance. Consistent with earlier studies,8 PYY(1-36) inhibited both glucose- and alanine-induced insulin secretion. (P3L31P34)PYY(1-36) evoked essentially similar effects, albeit with a reduced magnitude. There were slight differences in efficacy between the 2 peptides regarding the inhibition of glucose- and alanine-induced insulin secretion, and this likely relates to the more distal non-metabolic effects of alanine on beta-cell-induced insulin secretion. Thus, although the Pro3, Leu31, and Pro34 substitutions improved enzymatic stability, this may have resulted in an analogue with decreased biological potency. The presence of functionally important NPYR2 receptors on BRIN BD11 beta-cells,8 unlike primary human beta-cells,2 could also be a factor here, as the increased stability of (P3L31P34)PYY(1-36) will dramatically reduce NPYR2 interactions. Despite this, these data do indicate that (P3L31P34)PYY(1-36) retains affinity for NPY receptors and the ability to activate related signal transduction pathways. Although further studies using CRISPR/Cas9 technology and specific NPYR1 or NPYR2 knockdown, beta-cells would be required to confirm this, as specificity with commercially available NPYR inhibitors could be an issue. In addition, if a specific NPYR1 receptor binding assay was available, it would also be helpful in this regard. Interestingly, PYY(3-36)(Lys12PAL) was devoid of effects on the modulation of insulin secretion. This could be related to reduced levels of ‘free’ peptide due to greater albumin binding of the acylated analogue, as observed for other fatty-acid-derivatised peptides.41 To investigate these concepts further, we decided to examine the effects of all PYY peptides on proliferation and survival in both rodent BRIN BD11 and human 1.1B4 beta-cell lines.
As expected, PYY(1-36) enhanced the growth and survival of both BRIN BD11 and 1.1B4 beta-cell lines,8 presumably through the activation of Y1 receptors.9,10 Importantly, both modified PYY analogues also displayed positive pancreatic beta-cell growth and survival characteristics. However, similar to insulin secretory studies, despite the postulated increased specificity of (P3L31P34)PYY(1-36) and PYY(1-36)(Lys12PAL) towards NPYR1s recognised as being critically important for PYY-mediated beta-cell benefits,9 neither analogue had superior effects than PYY(1-36). Indeed, both analogues were actually significantly less efficacious than the native peptide under many of the test conditions. Thus, the generation of stable and bioactive, NPYR1-specific, PYY peptide analogues appears to be particularly challenging, implying that current structure/function knowledge for PYY requires more detailed investigation. Indeed, the notion that a compound can possess inhibitory actions on insulin secretion while concomitantly imparting beta-cell survival benefits is interesting and now an accepted action for NPYR1 activation by PYY(1-36).10 Moreover, the suggestion that beta-cell rest improves beta-cell function per se, and therefore enduring glucose control could also be a factor here.42 In this regard, a recent study has revealed that C-terminal integrity of PYY peptides is essential for preserved biological activity at the level of the beta-cell,43,44 and this may need to be considered for both (P3L31P34)PYY(1-36) and PYY(1-36)(Lys12PAL).
In conclusion, the present data reveal that the rational amino acid substitution of PYY(1-36), but not simple acylation, leads to the generation of an enzyme-resistant PYY(1-36) analogue. However, the improved stability of (P3L31P34)PYY(1-36), or perceived enhanced circulating half-life of PYY(1-36)(Lys12PAL), was offset by overall reduced biological activity. Thus, further work is required to develop stable, NPYR1-specific, PYY analogues to fully exploit the notable beneficial effects of PYY(1-36) on beta-cell growth and survival.
Supplemental Material
Supplemental material, Supplemental_Figures for Effects of 2 Novel PYY(1-36) Analogues, (P3L31P34)PYY(1-36) and PYY(1-36)(Lys12PAL), on Pancreatic Beta-Cell Function, Growth, and Survival by Ryan A Lafferty, Victor A Gault, Peter R Flatt and Nigel Irwin in Clinical Medicine Insights: Endocrinology and Diabetes
Supplemental Material
Supplemental material, Supplemental_Fig_Legends for Effects of 2 Novel PYY(1-36) Analogues, (P3L31P34)PYY(1-36) and PYY(1-36)(Lys12PAL), on Pancreatic Beta-Cell Function, Growth, and Survival by Ryan A Lafferty, Victor A Gault, Peter R Flatt and Nigel Irwin in Clinical Medicine Insights: Endocrinology and Diabetes
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a PhD studentship (awarded to R.A.L.) from the Department for the Economy (DfE) Northern Ireland and University of Ulster strategic research funding.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: V.A.G., P.R.F., and N.I. are named on patents filed by the University of Ulster for exploitation of peptide therapeutics.
Author Contributions: All authors contributed to research design, discussion of data and production of manusciprt. VAG designed both PYY(1-36) analogue peptides and all experimental work was conducted by RAL.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Tan T, Bloom S. Gut hormones as therapeutic agents in treatment of diabetes and obesity. Curr Opin Pharmacol. 2013;13:996-1001. [DOI] [PubMed] [Google Scholar]
- 2. Walther C, Mörl K, Beck-Sickinger AG. Neuropeptide Y receptors: ligand binding and trafficking suggest novel approaches in drug development. J Pept Sci. 2011;17:233-246. [DOI] [PubMed] [Google Scholar]
- 3. Wu T, Rayner CK, Young RL, Horowitz M. Gut motility and enteroendocrine secretion. Curr Opin Pharmacol. 2013;13:928-934. [DOI] [PubMed] [Google Scholar]
- 4. Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY 3–36. N Engl J Med. 2003;349:941-948. [DOI] [PubMed] [Google Scholar]
- 5. Koegler FH, Enriori PJ, Billes SK, et al. Peptide YY(3–36) inhibits morning, but not evening, food intake and decreases body weight in rhesus macaques. Diabetes. 2005;54:3198-3204. [DOI] [PubMed] [Google Scholar]
- 6. Pittner RA, Moore CX, Bhavsar SP, et al. Effects of PYY[3–36] in rodent models of diabetes and obesity. Int J Obes. 2004;28:963-971. [DOI] [PubMed] [Google Scholar]
- 7. Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab. 2006;292:1062-1068. [DOI] [PubMed] [Google Scholar]
- 8. Khan D, Vasu S, Moffett RC, Irwin N, Flatt PR. Islet distribution of Peptide YY and its regulatory role in primary mouse islets and immortalised rodent and human beta-cell function and survival. Mol Cell Endocrinol. 2016;436:102-113. [DOI] [PubMed] [Google Scholar]
- 9. Persaud SJ, Bewick GA. Peptide YY: more than just an appetite regulator. Diabetologia. 2014;57:1762-1769. [DOI] [PubMed] [Google Scholar]
- 10. Lafferty RA, Flatt PR, Irwin N. Emerging therapeutic potential for peptide YY for obesity-diabetes. Peptides. 2018;100:269-274. [DOI] [PubMed] [Google Scholar]
- 11. Ramracheya RD, McCulloch LJ, Clark A, et al. PYY-dependent restoration of impaired insulin and glucagon secretion in type 2 diabetes following Roux-en-Y gastric bypass surgery. Cell Rep. 2016;15:944-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guida C, McCulloch LJ, Godazgar M, et al. Sitagliptin and Roux-en-Y gastric bypass modulate insulin secretion via regulation of intra-islet PYY. Diabetes Obes Metab. 2018;20:571-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sam AH, Gunner DJ, King A, et al. Selective ablation of peptide YY cells in adult mice reveals their role in beta cell survival. Gastroenterology. 2012;143:459-468. [DOI] [PubMed] [Google Scholar]
- 14. Matveyenko AV, Butler PC. Relationship between β-cell mass and diabetes onset. Diabetes Obes Metab. 2008;10:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gault VA, O’Harte FPM, Harriott P, Flatt PR. Characterization of the cellular and metabolic effects of a novel enzyme-resistant antagonist of glucose-dependent insulinotropic polypeptide. Biochem Biophys Res Commun. 2002;290:1420-1426. [DOI] [PubMed] [Google Scholar]
- 16. Gault VA, Hunter K, Irwin N, et al. Characterisation and glucoregulatory actions of a novel acylated form of the (Pro3)GIP receptor antagonist in type 2 diabetes. Biol Chem. 2007;388:173-179. [DOI] [PubMed] [Google Scholar]
- 17. Parker JC, Irwin N, Lavery KS, et al. Metabolic effects of sub-chronic ablation of the incretin receptors by daily administration of (Pro3)GIP and exendin(9–39)amide in obese diabetic (ob/ob) mice. Biol Chem. 2007;388:221-226. [DOI] [PubMed] [Google Scholar]
- 18. Keire DA, Mannon P, Kobayashi M, Walsh JH, Solomon TE, Reeve JR. Primary structures of PYY, [Pro 34]PYY, and PYY-(3–36) confer different conformations and receptor selectivity. Am J Physiol Gastrointest Liver Physiol. 2000;279:G126-G131. [DOI] [PubMed] [Google Scholar]
- 19. Dumont Y, Fournier A, St-Pierre S, Quirion R. Characterization of neuropeptide Y binding sites in rat brain membrane preparations using [125I][Leu31, Pro34]peptide YY and [125I]peptide YY3–36 as selective Y1 and Y2 radioligands. J Pharmacol Exp Ther. 1995;272:673-680. [PubMed] [Google Scholar]
- 20. Fuhlendorff J, Gether U, Aakerlund L, et al. [Leu31, Pro34]neuropeptide Y: a specific Y1 receptor agonist. Proc Natl Acad Sci U S A. 1990;87:182-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gehlert DR, Schober DA, Gackenheimer SL, et al. [125I]Leu31, Pro34-PYY is a high affinity radioligand for rat PP1/Y4 and Y1 receptors: evidence for heterogeneity in pancreatic polypeptide receptors. Peptides. 1997;18:397-401. [DOI] [PubMed] [Google Scholar]
- 22. Green BD, Gault VA, Mooney MH, et al. Degradation, receptor binding, insulin secreting and antihyperglycaemic actions of palmitate-derivatised native and Ala8-substituted GLP-1 analogues. Biol Chem. 2004;385:169-177. [DOI] [PubMed] [Google Scholar]
- 23. Irwin N, Green BD, Mooney MH, et al. A novel, long-acting agonist of glucose-dependent insulinotropic polypeptide suitable for once-daily administration in type 2 diabetes. J Pharmacol Exp Ther. 2005;314:1187-1194. [DOI] [PubMed] [Google Scholar]
- 24. Irwin N, Green BD, Gault VA, et al. Degradation, insulin secretion, and antihyperglycemic actions of two palmitate-derivitized N-terminal pyroglutamyl analogues of glucose-dependent insulinotropic polypeptide. J Med Chem. 2005;48:1244-1250. [DOI] [PubMed] [Google Scholar]
- 25. Irwin N, Clarke GC, Green BD, et al. Evaluation of the antidiabetic activity of DPP IV resistant N-terminally modified versus mid-chain acylated analogues of glucose-dependent insulinotropic polypeptide. Biochem Pharmacol. 2006;72:719-728. [DOI] [PubMed] [Google Scholar]
- 26. Irwin N, O’Harte FP, Gault VA, et al. GIP(Lys16PAL) and GIP(Lys37PAL): novel long-acting acylated analogues of glucose-dependent insulinotropic polypeptide with improved antidiabetic potential. J Med Chem. 2006;49:1047-1054. [DOI] [PubMed] [Google Scholar]
- 27. Kerr BD, Irwin N, O’Harte FPM, Bailey CJ, Flatt PR, Gault VA. Fatty acid derivatised analogues of glucose-dependent insulinotropic polypeptide with improved antihyperglycaemic and insulinotropic properties. Biochem Pharmacol. 2009;78:1008-1016. [DOI] [PubMed] [Google Scholar]
- 28. O’Harte FP, Parthsarathy V, Hogg C, Flatt PR. Long-term treatment with acylated analogues of apelin-13 amide ameliorates diabetes and improves lipid profile of high-fat fed mice. PLoS ONE. 2018;13:e0202350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O’Harte FPM, Parthsarathy V, Hogg C, Flatt PR. Acylated apelin-13 amide analogues exhibit enzyme resistance and prolonged insulin releasing, glucose lowering and anorexic properties. Biochem Pharmacol. 2018;146:165-173. [DOI] [PubMed] [Google Scholar]
- 30. Gault VA, Martin CMA, Flatt PR, Parthsarathy V, Irwin N. Xenin-25[Lys13PAL]: a novel long-acting acylated analogue of xenin-25 with promising antidiabetic potential. Acta Diabetol. 2015;52:461-471. [DOI] [PubMed] [Google Scholar]
- 31. Bloom SR. Novel compounds and their effects on feeding behavior. Patent application WO2008003947A1, USA, 2006. [Google Scholar]
- 32. Pathak V, Vasu S, Gault VA, Flatt PR, Irwin N. Sequential induction of beta cell rest and stimulation using stable GIP inhibitor and GLP-1 mimetic peptides improves metabolic control in C57BL/KsJ db/db mice. Diabetologia. 2015;58:2144-2153. [DOI] [PubMed] [Google Scholar]
- 33. McClenaghan NH, Barnett CR, Ah-Sing E, et al. Characterization of a novel glucose-responsive insulin-secreting cell line, BRIN-BD11, produced by electrofusion. Diabetes. 1996;45:1132-1140. [DOI] [PubMed] [Google Scholar]
- 34. Flatt PR, Bailey CJ. Plasma glucose and insulin responses to glucagon and arginine in Aston ob/ob mice: evidence for a selective defect in glucose-mediated insulin release. Horm Metab Res Suppl. 1982;14:127-130. [DOI] [PubMed] [Google Scholar]
- 35. Green AD, Vasu S, Flatt PR. Cellular models for beta-cell function and diabetes gene therapy. Acta Physiol (Oxf). 2017;222:e13012. [DOI] [PubMed] [Google Scholar]
- 36. McCluskey JT, Hamid M, Guo-Parke H, McClenaghan NH, Gomis R, Flatt PR. Development and functional characterization of insulin-releasing human pancreatic beta cell lines produced by electrofusion. J Biol Chem. 2011;286:21982-21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650-654. [DOI] [PubMed] [Google Scholar]
- 38. Söll RM, Dinger MC, Lundell I, Larhammer D, Beck-Sickinger AG. Novel analogues of neuropeptide Y with a preference for the Y1-receptor. Eur J Biochem. 2001;268:2828-2837. [DOI] [PubMed] [Google Scholar]
- 39. Grandt D, Dahms P, Schimiczek M, Eysselein VE, Reeve JR, Jr, Mentlein R. Proteolytic processing by dipeptidyl aminopeptidase IV generates receptor selectivity for peptide YY (PYY). Med Klin (Munich). 1993;88:143-145. [PubMed] [Google Scholar]
- 40. Germain N, Minnion JS, Tan T, et al. Analogs of pancreatic polypeptide and peptide YY with a locked PP-fold structure are biologically active. Peptides. 2013;39:6-10. [DOI] [PubMed] [Google Scholar]
- 41. Li Y, Wang Y, Wei Q, et al. Variant fatty acid-like molecules Conjugation, novel approaches for extending the stability of therapeutic peptides. Sci Rep. 2015;5:18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hara M, Fowler JL, Bell GI, Philipson LH. Resting beta-cells – a functional reserve? Diabetes Metab. 2016;42:157-161. [DOI] [PubMed] [Google Scholar]
- 43. Lafferty RA, Flatt PR, Irwin N. C-terminal degradation of PYY peptides in plasma abolishes effects on satiety and beta-cell function. Biochem Pharmacol. 2018;158:95-102. [DOI] [PubMed] [Google Scholar]
- 44. Toräng S, Bojsen-Møller KN, Svane MS, et al. In vivo and in vitro degradation of peptide YY3–36 to inactive peptide YY3–34 in humans. Am J Physiol Regul Integr Comp Physiol. 2016;310:R866-R874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Figures for Effects of 2 Novel PYY(1-36) Analogues, (P3L31P34)PYY(1-36) and PYY(1-36)(Lys12PAL), on Pancreatic Beta-Cell Function, Growth, and Survival by Ryan A Lafferty, Victor A Gault, Peter R Flatt and Nigel Irwin in Clinical Medicine Insights: Endocrinology and Diabetes
Supplemental material, Supplemental_Fig_Legends for Effects of 2 Novel PYY(1-36) Analogues, (P3L31P34)PYY(1-36) and PYY(1-36)(Lys12PAL), on Pancreatic Beta-Cell Function, Growth, and Survival by Ryan A Lafferty, Victor A Gault, Peter R Flatt and Nigel Irwin in Clinical Medicine Insights: Endocrinology and Diabetes