Abstract
Background:
The prevalence of diverticulosis has increased in our aging population, but the risk factors for diverticulosis are not fully understood. The role of hypertension in the risk of diverticulosis remains uncertain. This study investigated whether hypertension is associated with asymptomatic colorectal diverticulosis.
Methods:
This study enrolled asymptomatic patients who received a colonoscopy as part of a health check. Hypertension was defined by actual measured blood pressure. Logistic regression models were used to examine the relationship between hypertension and diverticulosis. In addition, we established three logistic regression models for covariate adjustment, and further stratified patients with hypertension into three subgroups based on their type of hypertension.
Results:
The study group consisted of 2748 participants, including 141 participants with diverticulosis and 2607 participants without diverticulosis. After adjustments for potential covariates, the odds ratio (OR) for having diverticulosis was 1.83 (95% confidence interval, 1.21–2.75, p = 0.004) in the hypertension group compared with the group without hypertension. In subgroup analyses, hypertension without antihypertensive medication use, and hypertension despite the use of antihypertensive medication were also significantly associated with the occurrence of asymptomatic diverticulosis (OR = 1.73, p = 0.028; OR = 2.07, p = 0.013, respectively). Current normal blood pressure under antihypertensive drug therapy was not associated with diverticulosis (OR = 1.74, p = 0.092).
Conclusions:
Our findings suggest a positive association between hypertension and diverticulosis. Participants with poorly controlled blood pressure were found to have a higher risk of asymptomatic diverticulosis. Our study presents epidemiologic evidence for future prevention strategies against diverticulosis.
Keywords: age, diverticulosis, hypertension, obesity
Introduction
Diverticulosis is the formation of single or multiple diverticula in the colon. It is believed that diverticula are caused by increased pressure on the weak side of the bowel wall, as occurs when straining during a bowel movement,1 although a recent cross-sectional study did not confirm this theory.2 Due to ageing of the population, the prevalence of diverticular disease has increased significantly in Japan and China.3–5 The vast majority of patients with diverticula are asymptomatic. During a patient’s lifetime, however, diverticula may be associated with clinical illness, such as diverticulitis, complications of abscess, fistula, obstruction, and lower intestinal hemorrhage.6,7 Although only 1–4% of diverticulosis patients develop diverticulitis,8 diverticular disease constitutes the eighth most common outpatient digestive disease in the United States, accounting for 2.7 million health-care-related visits each year.9
Currently, the etiopathogenesis and risk factors of colonic diverticulosis are not completely understood. Previous studies have indicated that some factors increase the risk of developing diverticulosis. As examples, an unhealthy lifestyle, western dietary patterns, and obesity have been reported to be associated with a higher risk of diverticulosis.10,11 All of these factors are also associated with hypertension.12 In addition, prior studies have shown a dramatic increase in the prevalence of diverticula among elderly people.13 The prevalence of diverticula is as high as 60% in patients older than 70 years but is only 25% among patients less than 40 years old. Although hypertension is prevalent in the older population, it has often been neglected outside the diverticulosis field.
One Danish nationwide study revealed that diverticular disease is a risk factor for stroke and acute myocardial infarction.14 Another study in Italy showed an association between the presence of diverticulosis and cardiovascular disease.15 A few studies have focused on the possible relationship between hypertension and diverticulosis; however, the results are conflicting and based mainly on univariable analyses in which potential confounders were not considered.16,17 One study showed that a prevalence of hypertension of 30% among participants with diverticulosis and 20% among participants without diverticulosis.18 On the other hand, some studies found no significant relationship between hypertension and diverticulosis.3,19,20 In addition, in the studies cited, the definition of hypertension was not sufficiently elucidated.
A better understanding of the potential effects of hypertension on diverticulosis is urgently needed to inform appropriate management. The aim of the present study was to analyze the associations between different subgroups of patients with hypertension and diverticulosis, and to shed light on potential risk factors related to colonic diverticulosis.
Methods
Study population
This study enrolled asymptomatic patients who received a colonoscopy as part of a health check at the Tri-Service General Hospital (TSGH) health promotion center between 2010 and 2017. These patients can easily schedule a health check for any type of examination annually if it is economically feasible. Colonoscopies were performed by skilled endoscopy specialists after the participants had fasted overnight (except medications). An oral cathartic was prescribed for bowel preparation according to the protocol for diagnostic colonoscopy. The patients underwent a detailed examined for colorectal mucosal lesions. Patients with evidence of colitis, familial polyposis syndrome, previous colon resection, or previous colon cancer were excluded. The study was approved by the Institutional Review Board (IRB) of TSGH, National Defense Medical Center in Taiwan (#2_106_05_144) in accordance with the Declaration of Helsinki. Since the study used deidentified past health check records, the IRB granted a waiver of informed consent for the study.
Definition of hypertension
Based on the guidelines for management of hypertension from the Taiwan Society of Cardiology and the Taiwan Hypertension Society,21 participants were considered to have hypertension if their blood pressure was ⩾140/90 mmHg or if they were using antihypertensive medication. To prevent elevated blood pressure due to nervousness, the patient’s blood pressure was measured 2 h before the colonoscopy. Blood pressure was measured using a digital sphygmomanometer with automatic cuff inflation after the participants had rested in a chair for at least 10 min. The participants were required to be seated quietly with their elbows at heart level, their forearms comfortably on the table and their palms facing up to ensure an accurate reading. The average of two readings was recorded for analysis.
We also divided the participants with hypertension into three subgroups. Group A was defined as those who had normal blood pressure under antihypertensive medication therapy, group B was defined as those who had hypertension and were not using antihypertensive medication, and group C was defined as those who still had hypertension despite antihypertensive medication therapy. In addition, we classified participants with measured values ⩾120 mmHg for systolic blood pressure (SBP) or ⩾80 mmHg for diastolic blood pressure (DBP) into three categories according to the staging of the Taiwan Society of Cardiology and the Taiwan Hypertension Society.21 Prehypertension was defined as SBP values between 120 mmHg and 139 mmHg, or DBP values between 80 mmHg and 89 mmHg. Measured SBP values between 140 mmHg and 159 mmHg, or DBP values between 90 mmHg and 99 mmHg, were stratified as stage 1 hypertension. We defined an SBP higher than 160 mmHg or a DBP higher than 100 mmHg as stage 2 hypertension.
Study variables
Age, gender, exercise habits, smoking status, alcohol consumption, and medical history were collected from a self-completed questionnaire and identity card. Smoking status was divided into three types: never smoker, former smoker, and current smoker. Exercise habits and alcohol consumption were classified according to the frequency per week. Measurements of body weight and height were completed with a digital scale. Body mass index (BMI) was calculated by dividing a participant’s weight in kilograms by the square of the participant’s height in meters (kg/m2). After fasting for 8 h, blood samples were collected. Total cholesterol, triglycerides, uric acid, creatinine, and fasting glucose were analyzed.
Statistical analysis
Age, BMI, serum total cholesterol, triglycerides, uric acid, creatinine, and fasting glucose were defined as continuous variables. We used Student’s t tests to compare the mean values of continuous variables with a normal distribution. We used Mann–Whitney U tests to compare the mean values of continuous variables without a normal distribution. The other covariates, including gender, exercise habits, smoking status, alcohol consumption, and hypertension, were regarded as categorical variables. Chi-square tests were used to compare these categorical variables according to the baseline characteristics. The effects of hypertension on the risk of diverticulosis were examined using logistic regression models. Three extended models were used for covariate adjustment. Model 1 is the unadjusted model. We adjusted for age, sex, BMI, total cholesterol, triglycerides, uric acid, creatinine, and fasting glucose in Model 2. We further adjusted for the factors in Model 2 plus exercise habits, smoking status, and alcohol consumption in Model 3. Furthermore, we divided the participants with hypertension into group A, group B, and group C, and covariate adjustments were designed as logistic regression models. To further strengthen our results, we also analyzed the data using propensity score matching (matched for age, gender, and BMI). We also classified blood pressure as prehypertension, stage 1 hypertension, and stage 2 hypertension, and designed covariate adjustments as logistic regression models. In addition, we classified the participants into five groups according to their blood pressure values. We compared those higher blood pressure groups with the lowest blood pressure group using logistic regression analysis. Moreover, because age is a substantial risk factor for diverticulosis, we further analyzed the data categories by age.
Furthermore, several sensitivity analyses, including restricting analyses to participants without self-reported cardiovascular disease or without a history of diverticulitis, were performed. Significant differences were indicated when two-sided p values were less than 0.05. All statistical analyses were conducted using SPSS (version 18.0, SPSS Inc., Chicago, IL).
Results
Characteristics of the study population
The study group consisted of 2748 asymptomatic participants, including 141 participants with diverticulosis and 2607 participants without diverticulosis, with 1672 men and 1076 women. The clinical characteristics of the study group were classified as shown in Table 1. Among the participants, 60.8% were men, and the mean age was 53.21 (±11.56) years (range 18–89 years). An increase in the prevalence of diverticulosis was observed with increasing age (see Figure 1). Older age, the presence of hypertension, a higher BMI, and higher triglyceride, uric acid, fasting glucose, and creatinine levels were more prominent in the diverticulosis group compared with the nondiverticulosis group (see Supplementary data, Tables S1–S2).
Table 1.
Characteristics of the study participants according to diverticulosis status.
| Baseline characteristics | Diverticulosis
group (n = 141) |
Nondiverticulosis group (n = 2607) | p value |
|---|---|---|---|
| Continuous variables* | |||
| Age (years) | 59.04 ± 10.28 | 52.90 ± 11.55 | <0.001 |
| Body mass index (kg/m2) | 26.21 ± 4.18 | 24.52 ± 3.79 | <0.001 |
| Total cholesterol (mg/dl) | 194.67 ± 36.77 | 194.41 ± 36.86 | 0.936 |
| Triglyceride (mg/dl) | 165.67 ± 102.52 | 144.81 ± 90.60 | 0.001 |
| Uric acid (mg/dl) | 6.34 ± 1.41 | 5.80 ± 1.45 | <0.001 |
| Creatinine (mg/dl) | 1.00 ± 0.83 | 0.85 ± 0.29 | <0.001 |
| Fasting glucose (mg/dl) | 104.21 ± 26.68 | 98.04 ± 27.26 | 0.010 |
| Categorical variables$ | |||
| Sex | <0.001 | ||
| Male | 106 (75.2) | 1566 (60.1) | |
| Female | 35 (24.8) | 1041 (39.9) | |
| Hypertension‡ | <0.001 | ||
| No | 54 (38.3) | 1687 (64.7) | |
| Yes | 87 (61.7) | 920 (35.3) | |
| Exercise | 0.305 | ||
| No exercise | 18 (13.5) | 338 (13.7) | |
| <1 times/week | 52 (39.1) | 1015 (41.2) | |
| 1–2 times/week | 26 (19.5) | 585 (23.8) | |
| 3–5 times/week | 37 (27.8) | 523 (21.3) | |
| Smoking | 0.089 | ||
| Never smoker | 72 (53.3) | 1542 (62.7) | |
| Former smoker | 20 (14.8) | 309 (12.6) | |
| Current smoker | 43 (31.9) | 610 (24.8) | |
| Alcohol consumption | 0.169 | ||
| Never drinking | 60 (48.0) | 1138 (50.0) | |
| 1–2 times/week | 54 (43.2) | 1024 (45.0) | |
| 3–7 times/week | 11 (8.8) | 113 (5.0) |
Values were expressed as mean (standard deviation).
Values in the categorical variables were expressed as number (%).
Hypertension = blood pressure ⩾140/90 mmHg, or if they were using antihypertensive medication.
Figure 1.
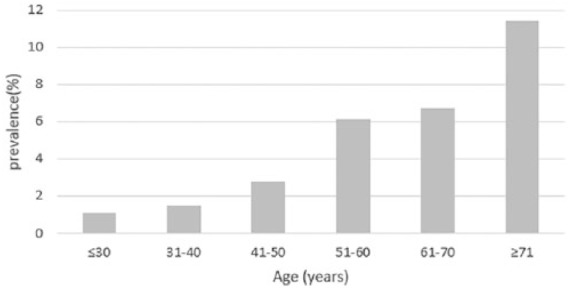
Prevalence of diverticulosis stratified by age.
Association between hypertension and asymptomatic diverticulosis
The results of the logistic regression analyses are presented in Table 2. In Model 1 with all participants, the unadjusted odds ratio (OR) was 2.95 [95% confidence interval (CI), 2.08–4.19, p <0.001]. After adjustments for further covariates in Model 2, the OR was 1.73 (95% CI, 1.16–2.51, p = 0.009). In Model 3, additional adjustments for smoking status, exercise, and alcohol consumption did not affect the statistical significance in our study (OR = 1.83, p = 0.004). In addition, after propensity score matching (see Supplementary data, Table S3), the OR for having diverticulosis was 1.76 (95% CI, 1.07–2.90, p = 0.025) in the hypertension group compared with the group without hypertension.
Table 2.
Risks of diverticulosis in the hypertension group.
| Hypertension group | Odds ratio (95% confidence interval) |
p value |
|---|---|---|
| Total (n = 1007)* | ||
| Model 1$ | 2.95 (2.08–4.19) | <0.001 |
| Model 2 | 1.71 (1.16–2.52) | 0.009 |
| Model 3 | 1.83 (1.21–2.75) | 0.004 |
| Group A (n = 255) | ||
| Model 1 | 2.66 (1.56–4.52) | <0.001 |
| Model 2 | 1.60 (0.88–2.93) | 0.125 |
| Model 3 | 1.74 (0.91–3.31) | 0.092 |
| Group B (n = 516) | ||
| Model 1 | 2.48 (1.62–3.81) | <0.001 |
| Model 2 | 1.51 (0.94–2.43) | 0.087 |
| Model 3 | 1.73 (1.06–2.83) | 0.028 |
| Group C (n = 236) | ||
| Model 1 | 4.38 (2.73–7.03) | <0.001 |
| Model 2 | 2.20 (1.28–3.76) | 0.004 |
| Model 3 | 2.07 (1.17–3.67) | 0.013 |
Total = blood pressure ⩾140/90 mmHg, or if they were using antihypertensive medication; Group A = current normal blood pressure under antihypertensive drugs therapy; Group B = hypertension without antihypertensive medication control; Group C = current hypertension under antihypertensive medication control.
Adjusted covariates: Model 1 = unadjusted; Model 2 = age, sex, BMI, total cholesterol, triglyceride, uric acid, creatinine, fasting glucose; Model 3 = Model 2+ (exercise, smoking, alcohol consumption).
Sensitivity analysis
We further examined the effect of hypertension on the occurrence of colonic diverticulosis by separating the participants with hypertension into three groups based on the definition of hypertension (see Table 2). After multivariable analysis in the fully adjusted model, group B and group C were significantly associated with the presence of asymptomatic diverticulosis. The OR was 1.73 (p = 0.028) for group B and 2.07 (p = 0.013) for Group C. Group C showed the highest OR among the three groups; however, in group A, in which blood pressure was well controlled by antihypertensive drug treatment, a notable association between hypertension and asymptomatic diverticulosis was observed only in Model 1 as the significance was lost in adjusted Models 2 and 3 (OR = 1.60, p = 0.125; OR = 1.74, p = 0.092, respectively).
Furthermore, we stratified stages of hypertension according to blood pressure values. Overall, participants with stage 1 and stage 2 hypertension had a significantly higher risk of diverticulosis (see Table 3). The OR was 2.30 (p = 0.005) for stage 1 hypertension and 2.62 (p = 0.011) for stage 2 hypertension. Participants with prehypertension exhibited no significant relation with the occurrence of diverticulosis. We further stratified participants by age. The significant association between hypertension stage and the risk of diverticulosis mentioned above was observed only in participants older than 50 years and not in participants younger than 50 years. Among the older participants, the OR was 2.40 (p = 0.007) for those with stage 1 hypertension and 2.66 (p = 0.017) for those with stage 2 hypertension. In addition, after dividing the participants with hypertension into five groups, the OR was 2.38 (p = 0.006) for the top quintile compared with the bottom quintile of hypertension (see Supplementary data, Table S4).
Table 3.
Risks of diverticulosis with hypertension stage.
| HTN Stage | Total
(n = 2748) |
Age ⩽ 50 years (n
= 999) |
Age > 50 years
(n = 1749) |
|||
|---|---|---|---|---|---|---|
| OR* (95% CI) | p value | OR* (95% CI) | p value | OR* (95% CI) | p value | |
| Normal (reference) | 1 | – | 1 | – | 1 | – |
| Pre-HTN | 1.56 (0.88–2.74) | 0.125 | 2.16 (0.64–7.35) | 0.216 | 1.33 (0.70–2.52) | 0.381 |
| Stage 1 HTN | 2.30 (1.29–4.12) | 0.005 | 1.06 (0.20–5.55) | 0.948 | 2.40 (1.28–4.51) | 0.007 |
| Stage 2 HTN | 2.62 (1.25–5.52) | 0.011 | 1.20 (0.11–13.01) | 0.879 | 2.66 (1.20–5.92) | 0.017 |
HTN, Hypertension; Pre-HTN [systolic blood pressure (SBP): 120–139 mmHg or diastolic blood pressure (DBP) 80–89 mmHg]; Stage 1 HTN, Stage 1 hypertension (SBP: 140–159 mmHg or DBP 90–99 mmHg); Stage 2 HTN, Stage 2 hypertension (SBP: ⩾160 mmHg or DBP ⩾100 mmHg).
Adjusted for age, sex, BMI, total cholesterol, triglyceride, uric acid, creatinine, fasting glucose, exercise, smoking, alcohol consumption.
In addition, we repeated the analyses excluding participants (n = 93) with self-reported cardiovascular disease and excluding participants (n = 1) with a history of diverticulitis (see Supplementary data, Tables S5–S6). The similar results obtained from these analyses increased the robustness of our original findings.
Discussion
To the best of our knowledge, the present study is the first to analyze the association between hypertension and diverticulosis, not only considering potential covariates but also stratification of the participants with hypertension into different groups. Participants with hypertension were more likely to have colonic diverticulosis compared with those without hypertension. In addition, participants with poorly controlled blood pressure have a higher risk of asymptomatic diverticulosis.
The results of the present study showed a significant association between hypertension and diverticulosis. Few studies have focused on evaluating the impact of hypertension on asymptomatic diverticulosis, and the results of these studies are contradictory. A cross-sectional analysis by Sakuta and colleagues reported a relationship between hypertension and asymptomatic diverticulosis only in middle-aged Japanese men.18 One Korean study found that diverticulosis was associated with elevated blood pressure, increased alcohol consumption and a high-fat diet.16 Another study including both symptomatic and asymptomatic participants reported that colonic diverticulosis was associated with hypertension, adenomatous polyps, and diabetes mellitus.17 None of the above studies examined confounders as in our study. Confounders such as age, sex, BMI, alcohol consumption, smoking status, and other physical conditions may be responsible for the conflicting data in the literature. For example, a population-based study in Japan revealed a positive association between hypertension and diverticulosis in a univariable analysis; however, this association was attenuated after adjusting for confounders.3 Fully adjusted potential confounders strengthen the results of our study. In addition, we also note that participants of older age, with a higher BMI, and higher triglyceride, uric acid, fasting glucose, and creatinine levels tended to have concomitant colonic diverticulosis, in accordance with previous studies.3,11,16,19,22
We further stratified hypertension into different groups, and the results corroborated our hypothesis that hypertension plays an important role in diverticulosis. In Group C, we found that poorly controlled hypertension had the strongest association with diverticulosis. Group B showed that untreated hypertension also represents a risk factor for asymptomatic diverticulosis. Group A, however, demonstrated that the effect of hypertension on the diverticulum diminished after successful blood pressure control, and this result is in line with the results of a previous Japanese study in which the definition of hypertension included participants who used antihypertensive medication, and the authors found that hypertension had no influence on diverticulosis.23 To enhance our point, we analyzed the association between diverticulosis and three stages of hypertension (prehypertension, stage 1 hypertension and stage 2 hypertension). Notably, participants with higher blood pressure had a significantly higher risk of diverticulosis, especially participants older than 50 years. To conclude, our multivariable analysis with separate hypertension groups revealed that blood pressure control may play an important role in preventing asymptomatic diverticulosis.
Although the mechanisms that link hypertension to diverticulosis are not well known, a plausible connection may be attributed to vascular changes. Vasa recta are straight arteries that arise from arcades to supply blood to the colonic mucosa. The sites where the vasa recta pass through the circular muscle layer form weak points (loci minoris resistentiae) along the colonic wall.24 Morphologically, colonic diverticulosis has been reported to be present at these relatively vulnerable spots.25,26 Manifestations of aging arteries include endothelial dysfunction,27 inflammation,28,29 remodeling, calcification, and increased stiffness.30 Others have also reported that hypertension causes endothelial injury and arteriosclerosis.31,32 In other words, atherogenesis was described as having a significant relationship with vascular elastin fragmentation.33 Thus, the degeneration of the blood vessels leads to a decrease in blood supply, and the weak point of the colonic wall more became more fragile, leading to structural changes in the colon wall. These findings, which are related to relevant biological mechanisms, suggest that increased blood pressure might induce structural changes in the colon wall.
Limitations
Our study revealed a statistically significant relationship between hypertension and diverticulosis; however, some limitations exist. First, a few traditional covariates, such as dietary fiber intake and constipation, were not assessed, although conflicting literature reports exist currently on the role of fiber intake and constipation in diverticulosis.2,34 For the most part, dietary fiber has been considered to be inversely associated with the risk of diverticulosis.35,36 However, Song and colleagues found no differences in the consumption of dietary fiber between participants with and without diverticulosis.16 Moreover, another study conducted by Peery and colleagues found that dietary fiber intake was significantly increased in participants with diverticulosis.37 It is difficult to confirm the true effect size and direction of dietary fiber on the risk of diverticulosis because of the subjective data on fiber intake and the long-term duration of impact. Therefore, it might be a little biased to insert unsophisticated diet scores into statistical models. Second, our investigation had a cross-sectional design, and we therefore could not confirm a causal relationship between hypertension and colonic diverticulosis. However, the strong correlation of hypertension with diverticulosis underlines the potential role of hypertension in prevention strategies. Third, we obtained medical history information using a questionnaire, and participants may not have recalled their medical histories reliably. Fourth, the overall prevalence of diverticulosis in our study was low relative to other studies. This discrepancy may be mainly attributed to different race and lifestyle factors. In addition, all our participants underwent health examinations at their own expense, which may indicate that they were of higher socioeconomic status and healthier. Future studies in other populations are needed. Moreover, although colonoscopy is very useful for detecting mucosal abnormalities and distinguishing colitis, it is less sensitive than radiological techniques in diagnosing diverticulosis.38
Conclusion
We conclude that hypertension exhibits a positive correlation with diverticulosis. Furthermore, participants with poorly controlled blood pressure, regardless of the use of antihypertensive medications, were found to have a significantly higher risk of asymptomatic diverticulosis than those without hypertension. Therefore, prevention and management of hypertension may be useful for reducing the risk of colonic diverticulosis and may also be helpful for decreasing the incidence of diverticular diseases, such as diverticulitis. Additional longitudinal and interventional studies are required to verify this relationship.
Supplemental Material
Supplemental material, Supplementary_Material for Hypertension control and risk of colonic diverticulosis by Li-Xian Yeo, Tzu-Hsiang Tseng, Wei-Liang Chen, Tung-Wei Kao, Li-Wei Wu, Wen-Hui Fang, Yaw-Wen Chang and Tao-Chun Peng in Therapeutic Advances in Gastroenterology
Footnotes
Author contributions: Study design: Li-Xian Yeo, Tzu-Hsiang Tseng, Wei-Liang Chen, Tung-Wei Kao, Tao-Chun Peng
Data collection: Li-Xian Yeo, Tzu-Hsiang Tseng, Li-Wei Wu, Wen-Hui Fang, Yaw-Wen Chang, Tao-Chun Peng
Data analysis: Li-Xian Yeo, Tzu-Hsiang Tseng, Li-Wei Wu, Wen-Hui Fang, Tao-Chun Peng
Drafting of the manuscript: Li-Xian Yeo, Tzu-Hsiang Tseng, Wei-Liang Chen, Li-Wei Wu, Tao-Chun Peng
All authors read and approved the final version of the manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Tao-Chun Peng  https://orcid.org/0000-0003-1336-288X
https://orcid.org/0000-0003-1336-288X
Contributor Information
Li-Xian Yeo, Division of Family Medicine, Department of Family and Community Medicine, Tri-Service General Hospital; and School of Medicine, National Defense Medical Center, Taipei.
Tzu-Hsiang Tseng, Division of Family Medicine, Department of Family and Community Medicine, Tri-Service General Hospital; and School of Medicine, National Defense Medical Center, Taipei.
Wei-Liang Chen, Division of Family Medicine, Department of Family and Community Medicine, Tri-Service General Hospital; and School of Medicine, National Defense Medical Center, Taipei; Division of Geriatric Medicine, Department of Family and Community Medicine, Tri-Service General Hospital; and School of Medicine, National Defense Medical Center, Taipei; Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei.
Tung-Wei Kao, Division of Family Medicine, Department of Family and Community Medicine, Tri-Service General Hospital; and School of Medicine, National Defense Medical Center, Taipei; Division of Geriatric Medicine, Department of Family and Community Medicine, Tri-Service General Hospital; and School of Medicine, National Defense Medical Center, Taipei; Graduate Institute of Clinical Medicine, College of Medicine, National Taiwan University, Taipei.
Li-Wei Wu, Division of Family Medicine, Department of Family and Community Medicine, Tri-Service General Hospital; and School of Medicine, National Defense Medical Center, Taipei; Division of Geriatric Medicine, Department of Family and Community Medicine, Tri-Service General Hospital; and School of Medicine, National Defense Medical Center, Taipei; Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei.
Wen-Hui Fang, Division of Family Medicine, Department of Family and Community Medicine, Tri-Service General Hospital; and School of Medicine, National Defense Medical Center, Taipei.
Yaw-Wen Chang, Division of Family Medicine, Department of Family and Community Medicine, Tri-Service General Hospital; and School of Medicine, National Defense Medical Center, Taipei; Division of Geriatric Medicine, Department of Family and Community Medicine, Tri-Service General Hospital; and School of Medicine, National Defense Medical Center, Taipei.
Tao-Chun Peng, Division of Family Medicine, Department of Family and Community Medicine, Tri-Service General Hospital; and School of Medicine, National Defense Medical Center, Taipei; Division of Geriatric Medicine, Department of Family and Community Medicine, Tri-Service General Hospital; and School of Medicine, National Defense Medical Center, Taipei.
References
- 1. Stollman N, Raskin JB. Diverticular disease of the colon. Lancet 2004; 363: 631–639. [DOI] [PubMed] [Google Scholar]
- 2. Peery AF, Sandler RS, Ahnen DJ, et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol 2013; 11: 1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamamichi N, Shimamoto T, Takahashi Y, et al. Trend and risk factors of diverticulosis in Japan: age, gender, and lifestyle/metabolic-related factors may cooperatively affect on the colorectal diverticula formation. PLoS One 2015; 10: e0123688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bharucha AE, Parthasarathy G, Ditah I, et al. Temporal trends in the incidence and natural history of diverticulitis: a population-based study. Am J Gastroenterol 2015; 110: 1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang F, Zheng Y, Jiang X, et al. Sex differences in risk factors of uncomplicated colonic diverticulosis in a metropolitan area from Northern China. Sci Rep 2018; 8: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strate LL, Modi R, Cohen E, et al. Diverticular disease as a chronic illness: evolving epidemiologic and clinical insights. Am J Gastroenterol 2012; 107: 1486–1493. [DOI] [PubMed] [Google Scholar]
- 7. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum 2014; 57: 284–294. [DOI] [PubMed] [Google Scholar]
- 8. Shahedi K, Fuller G, Bolus R, et al. Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol 2013; 11: 1609–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015; 149: 1731–1741.e1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strate LL, Keeley BR, Cao Y, et al. Western dietary pattern increases, and prudent dietary pattern decreases, risk of incident diverticulitis in a prospective cohort study. Gastroenterology 2017; 152: 1023–1030.e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wijarnpreecha K, Ahuja W, Chesdachai S, et al. Obesity and the Risk of Colonic Diverticulosis: A Meta-analysis. Dis Colon Rectum 2018; 61: 476–483. [DOI] [PubMed] [Google Scholar]
- 12. Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010; 121: 586–613. [DOI] [PubMed] [Google Scholar]
- 13. De Cecco CN, Ciolina M, Annibale B, et al. Prevalence and distribution of colonic diverticula assessed with CT colonography (CTC). Eur Radiol 2016; 26: 639–645. [DOI] [PubMed] [Google Scholar]
- 14. Strate LL, Erichsen R, Horvath-Puho E, et al. Diverticular disease is associated with increased risk of subsequent arterial and venous thromboembolic events. Clin Gastroenterol Hepatol 2014; 12: 1695–1701 e1691. [DOI] [PubMed] [Google Scholar]
- 15. Dore MP, Pes GM, Marras G, et al. Risk factors associated with colonic diverticulosis among patients from a defined geographic area. Tech Coloproctol 2016; 20: 177–183. [DOI] [PubMed] [Google Scholar]
- 16. Song JH, Kim YS, Lee JH, et al. Clinical characteristics of colonic diverticulosis in Korea: a prospective study. Korean J Intern Med 2010; 25: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Azzam N, Aljebreen AM, Alharbi O, et al. Prevalence and clinical features of colonic diverticulosis in a Middle Eastern population. World J Gastrointest Endosc 2013; 5: 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sakuta H, Suzuki T. Prevalence rates of type 2 diabetes and hypertension are elevated among middle-aged Japanese men with colonic diverticulum. Environ Health Prev Med 2007; 12: 97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang FW, Chuang HY, Tu MS, et al. Prevalence and risk factors of asymptomatic colorectal diverticulosis in Taiwan. BMC Gastroenterol 2015; 15: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kopylov U, Ben-Horin S, Lahat A, et al. Obesity, metabolic syndrome and the risk of development of colonic diverticulosis. Digestion 2012; 86: 201–205. [DOI] [PubMed] [Google Scholar]
- 21. Chiang CE, Wang TD, Ueng KC, et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J Chin Med Assoc 2015; 78: 1–47. [DOI] [PubMed] [Google Scholar]
- 22. Comstock SS, Lewis MM, Pathak DR, et al. Cross-sectional analysis of obesity and serum analytes in males identifies sRAGE as a novel biomarker inversely associated with diverticulosis. PLoS One 2014; 9: e95232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Green J, Nagata N, Niikura R, et al. Alcohol and smoking affect risk of uncomplicated colonic diverticulosis in Japan. PLoS ONE 2013; 8: e81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brian West A. The pathology of diverticulosis: classical concepts and mucosal changes in diverticula. J Clin Gastroenterol 2006; 40(Suppl. 3): S126–131. [DOI] [PubMed] [Google Scholar]
- 25. Ludeman L, Warren BF, Shepherd NA. The pathology of diverticular disease. Best Pract Res Clin Gastroenterol 2002; 16:543–562. [DOI] [PubMed] [Google Scholar]
- 26. Wedel T, Barrenschee M, Lange C, et al. Morphologic basis for developing diverticular disease, diverticulitis, and diverticular bleeding. Viszeralmedizin 2015; 31: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taddei S, Virdis A, Mattei P, et al. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 1995; 91: 1981–1987. [DOI] [PubMed] [Google Scholar]
- 28. Bruunsgaard H, Skinhoj P, Pedersen AN, et al. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol 2000; 121: 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Csiszar A, Labinskyy N, Smith K, et al. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol 2007; 170: 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang L, Zhang J, Monticone RE, et al. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension 2012; 60: 1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panza JA, Quyyumi AA, Brush JE, Jr, et al. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 1990; 323: 22–27. [DOI] [PubMed] [Google Scholar]
- 32. Cengiz M, Yavuzer S, Kilickiran Avci B, et al. Circulating miR-21 and eNOS in subclinical atherosclerosis in patients with hypertension. Clin Exp Hypertens 2015; 37: 643–649. [DOI] [PubMed] [Google Scholar]
- 33. West AB, Losada M. The pathology of diverticulosis coli. J Clin Gastroenterol 2004; 38: S11–16. [DOI] [PubMed] [Google Scholar]
- 34. Tursi A. Dietary pattern and colonic diverticulosis. Curr Opin Clin Nutr Metab Care 2017; 20: 409–413. [DOI] [PubMed] [Google Scholar]
- 35. Crowe FL, Appleby PN, Allen NE, et al. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): prospective study of British vegetarians and non-vegetarians. BMJ 2011; 343: d4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crowe FL, Balkwill A, Cairns BJ, et al. Source of dietary fibre and diverticular disease incidence: a prospective study of UK women. Gut 2014; 63: 1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peery AF, Barrett PR, Park D, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology 2012; 142: 266–272.e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Halligan S, Saunders B. Imaging diverticular disease. Best Pract Res Clin Gastroenterol 2002; 16: 595–610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Material for Hypertension control and risk of colonic diverticulosis by Li-Xian Yeo, Tzu-Hsiang Tseng, Wei-Liang Chen, Tung-Wei Kao, Li-Wei Wu, Wen-Hui Fang, Yaw-Wen Chang and Tao-Chun Peng in Therapeutic Advances in Gastroenterology


