Abstract
Low-dose environmental chemicals including endocrine-disrupting chemicals can disturb endocrine, nervous and immune systems. Traditional chemical-focused approaches, strict regulation and avoidance of exposure sources, can help protect humans from individual or several chemicals in the high-dose range, but their value in the low-dose range is questionable. First, exposure sources to problematic environmental chemicals are omnipresent, and many common pollutants present no safe level. In this situation, the value of any effort focusing on individual chemicals is very limited. Second, critical methodological issues, including the huge number of environmental chemicals, biological complexity of mixtures and non-linearity, make it difficult for risk assessment-based regulation to provide reliable permissible levels of individual chemicals. Third, the largest exposure source is already internal; human adipose tissue contains the most complex chemical mixtures. Thus, in the low-dose range, a paradigm shift is required from a chemical-focused to a human-focused approach for health protection. Two key questions are (1) how to control toxicokinetics of chemical mixtures to decrease their burden in critical organs and (2) how to mitigate early harmful effects of chemical mixtures at cellular levels. Many lifestyles can be evaluated for these purposes. Although both the chemical-focused and human-focused approaches are needed to protect humans, the human-focused holistic approach must be the primary measure in the low-dose range of environmental chemicals.
Keywords: adipose tissue, chemical mixtures, EDCs, epidemiology, evolution, paradigm shift, regulation
Introduction
Over several decades, evidence about possible harms of low-dose environmental chemicals has accumulated in various research fields. Environmental chemicals can disturb hormone, nervous and immune systems through multiple mechanisms at low doses, thus contributing to the development of many diseases.1–4
Among them, the most studied chemicals are ones which disrupt the endocrine system, called endocrine disrupting chemicals (EDCs).2 Hormone-disrupting effects of chemicals can act at low doses which are missed in traditional risk assessment,5 Therefore, EDCs can have effects at doses that are assumed to be safe under the current risk assessment. How to protect humans from EDCs has been a major health challenge.
Conventional approaches to environmental chemicals to protect humans are (1) avoidance of exposure sources and (2) strict regulation based on risk assessment. In the high-dose range, these methods are important to protect humans from harms of individual or several chemicals. However, it is becoming clear that these approaches cannot effectively protect humans from possible harms of low-dose environmental chemicals.
In this debate article, we discuss why the current approaches cannot work in the low-dose range and propose the necessity of a new paradigm to protect humans. Also, we will argue for the importance of chronic exposure to environmental chemicals after birth, although exposure during critical developmental periods is currently the key concern. In this article, EDCs will be used as a typical example of environmental chemicals to which humans are exposed daily. However, our arguments can be applied to other low-dose environmental chemicals which are not formally classified as EDCs.
Reality of human exposure to EDCs
Tip of the iceberg
Humans are continuously exposed to a variety of EDCs through food, air, water and consumer products.6 The list of suspected EDCs is rapidly growing; the number in the Endocrine Disruption Exchange database was 881 in 2011, but 1419 in 2017.7 Chemicals that are classified as EDCs have very dissimilar structures and diverse biochemical properties.8 Even nanoparticles are reported to be EDCs in in-vitro and in-vivo studies.9
While there has been a great deal of research effort to identify EDCs and evaluate biological effects of EDCs over the past two decades, many uncertainties still remain. It is worthy of note that ‘the tip of the iceberg’ is the title of one section in the summary for decision-makers of the 2012 WHO/United Nations Environment Programme (UNEP) report on EDCs, despite the long list of references.10 Current EDC screening programmes assess the endocrine disrupting potential only focusing on oestrogen, androgen, thyroid or steroidogenesis systems, and target chemicals with high production volumes.11 However, there are many other hormones and chemicals can disrupt the endocrine system through other pathways besides direct agonists or antagonists for hormone receptors.12 Given the diverse and complex endocrine system, the current list of EDCs is highly likely to be only a small piece of the whole picture.
Unpredictability of EDC mixtures
Humans live with simultaneous exposure to a tremendous number of EDCs, but most laboratory research about EDCs deal with individual EDCs. As strong synergic effects of several similarly acting EDCs were reported in in-vitro studies,13 researchers tend to assume that biological effects of EDC mixtures would be synergic or at least additive.14 However, net effects of real-world mixture of EDCs may be closer to unpredictable because they exist as a mixture of ‘agonists’ and ‘antagonists’ to various hormones which cross-talk with each other.15 Supporting this argument, experimental studies demonstrated that combination effects of only a few EDCs with different endocrine disrupting properties (eg, estrogenic plus antiandrogenic) were unpredictable.16–18
Researchers now consider the exposome, the measure of all life-time environmental exposures as assessed using biomarkers, personal sensors and omics technologies, as a new methodology to solve uncertainty around human studies of EDC mixtures.19 However, biological unpredictability cannot be overcome with sophisticated methodology. Besides the issue about EDC mixtures, human studies of EDCs suffer from insurmountable methodological limitations which render the value of human studies on EDCs dubious. They include low reliability of exposure assessment of common EDCs, non-linearity, non-existence of an unexposed group and complicated interactions with diet and obesity.20 Blind optimism about the utility of the exposome needs to be reconsidered before too much resource is invested in this field.
EDC mixtures in human adipose tissue
One neglected, but important aspect of the human exposure to EDCs is that adipose tissue plays a role as the most important exposure source to EDCs in modern society.21 Although a countless number of exposure sources of EDCs exist around us, contemporary human adipose tissue contains the most complex EDC mixtures because many EDCs are lipophilic and humans are at the top of food chain.21 The most common EDCs in adipose tissue are strong lipophilic chemicals such as persistent organic pollutants (POPs).22 Less lipophilic chemicals such as bisphenol A, polyaromatic hydrocarbons, synthetic musk compounds, triclosan and nonylphenol, are also widely detected in human adipose tissue.23 24 Accumulation of EDC mixtures in adipose tissue canreflect the history of exposure to environmental chemicals during the whole lifetime.
EDCs in adipose tissue are slowly and continuously released to circulation during lipolysis.21 If EDCs in adipose tissue are released to circulation at more than the usual rate and/or if they are not properly metabolised and eliminated, their chance to reach critical organs increases. As increased uncontrolled lipolysis is a feature of hypertrophic dysfunctional adipocytes,21 the release of EDC mixtures to circulation can be more serious in obese persons than lean persons.
Why conventional recommendation on environmental chemicals cannot protect humans in the low-dose range
Although both avoidance of exposure sources and stricter regulations are commonly recommended, the effectiveness of these approaches is generally restricted to prevention of high-dose toxicity of individual chemicals. Understanding why these conventional approaches cannot work to protect human from possible harms of low-dose environmental chemicals is important for researchers, policy-makers and public.
Can avoidance of EDC exposure be effective?
Certain lifestyles such as living without plastic can decrease exposure levels of several well-known EDCs.25 However, the decreased exposure to several individual EDCs cannot ensure less harm from EDCs. First, there is still exposure to a huge number of EDCs from both the external environment and the internal adipose tissue. Second, the non-monotonic dose–response relationship observed with EDCs literally means that lowering the exposure levels cannot assure less harm or can even be more harmful. Third, under the omnipresence of EDCs, a lifestyle trying to avoid EDC exposures may induce chronic anxiety and mental stress which itself is related to various diseases.26
Can strict regulation be effective?
In the face of difficulties in avoiding exposure source of EDCs, as the next solution, preventing exposures through more effective regulation on EDCs is recommended.27 As processes leading to regulation are extremely slow due to conflicts among stakeholders,28 this issue is often discussed from a political perspective. However, this issue should be discussed from a scientific viewpoint as well.
First and foremost, if the value of a lifestyle that tries to avoid exposure to several individual compounds in the world of EDC mixtures is questionable as we discussed above, how can strict regulation on several EDCs be valuable? In addition, many common environmental chemicals such as lead, air pollution and POPs have shown no safe level with non-linear dose-response relationships.29–32 Researchers may think of policies to reduce exposure levels to a minimum. However, the lower the existing level for any chemical, the harder it is to further reduce through regulation due to diverse, often vague, sources of exposure and food-chain contamination.
With the recognition of importance of chemical mixtures, regulatory agencies have begun to develop new methodologies on risk assessment of chemical mixtures. For example, an integrated approach of in-vivo studies, in-vitro studies and in-silico quantitative analysis of large networks of molecular and functional changes, together with systematic reviews or meta-analysis of high-quality epidemiological studies, is suggested to provide a stronger basis for regulatory decisions.33 However, the possibility of unpredictability of EDC mixtures does not seem to be seriously recognised by laboratory researchers in the field of risk assessment of chemical mixtures. Also, often overlooked is that there are overwhelming methodological issues with human studies on EDC which make reliable human evidence about harms of EDC mixtures unobtainable.20
Reconsideration of chronic exposure to EDCs
At present, early-life exposure to EDCs is considered the main concern due to the powerful Developmental Origin of Health and Disease (DOHaD) theory and multigenerational effects.34 Meanwhile, EDC exposure during non-critical periods has not gained attention as much as EDC exposure during critical periods. Although there is no question that the developmental stage is the most susceptible period to toxicity of environmental chemicals, there are other aspects of EDC exposure during critical and non-critical periods which need an attention from researchers.
Evolutionary aspects of epigenetic programming due to EDCs during critical periods
Irreversible epigenetic programming due to the exposure to EDCs during critical periods is considered to be harmful because of increasing susceptibility to disease later in life and transgenerational effects.35 However, an evolutionary aspect of epigenetic programming triggered by various environmental stressors has largely been dismissed in the field of EDCs. In fact, epigenetic programming during critical periods is a key mechanism for a developing organism to buffer itself from an ever-changing environment.36 37 Epigenetic changes adapt faster than genetic changes to various environmental stimuli.38
If offspring have to live under conditions after birth similar to the in-utero condition, their overall survival can be enhanced by virtue of the epigenetic programming.39 However, a mismatch with the predicted environment could lead to adverse health and fitness effects. The degree of mismatch between the prenatal and postnatal environment is suggested to be a major determinant of risk of future disease which is called the ‘predictive adaptive response’ (PAR) hypothesis.40
Although many short-lived species provide clear evidence for predictive developmental adaptation,40 there is a question whether the PAR hypothesis can be applied to humans, given their long life span.41 However, the comparison of those exposed to the Dutch Hunger Winter with babies born after the siege of Leningrad suggests that humans may not be exceptional in this respect. In the Dutch famine birth cohort, study of which gave rise to the DOHaD theory,42 nutritional deficits during fetal development and consequent low birth weight were connected to increased risks for obesity, diabetes and cardiovascular disease.43 However, a similar famine in Leningrad failed to replicate the findings from the Netherlands.44 One difference between these two countries was nutritional status after birth. In the Netherlands, many starved fetuses were promptly well-nourished in postnatal life, but the Leningrad cohort experienced similar poor nutritional status even after birth.44
The PAR hypothesis would not be confined only to nutrition. EDCs also require investigation from the viewpoint of the PAR hypothesis. Diethylstilbestrol (DES), the first synthetic oestrogen used to reduce the risk of premature delivery and pregnancy complications, is a representative of delayed adverse effects of exposure to an oestrogenic chemical during development.45 Increased risk of a rare reproductive tract cancer, infertility, reproductive complications and obesity were reported among adult offspring born to mothers prescribed DES.45 However, DES can be also seen as an example of mismatch because EDCs were exposed only during in-utero period.
At present, these two well-known cases of DOHaD in humans, the Dutch famine birth cohort and DES, fit the theory of mismatch between the prenatal and postnatal environment. It is surprising to notice that many laboratory studies of prenatal or perinatal exposure to EDCs treat these chemicals only during specific periods of development.46 Thus, this experimental design artificially creates a mismatch between the prenatal and postnatal environment. However, the reality is that the exposure to most EDCs is continuous from conception to death in all living organisms.
Importance of chronic exposure to EDCs during non-critical periods
Continuous exposure to EDCs can be harmful to humans by disturbing homeostasis of the endocrine system.47 The longer the period of disturbance, the higher the risk of hormone-related diseases is. Recent human studies linking POPs to metabolic diseases including type 2 diabetes have been performed among adults, focusing on the chronic exposure during non-critical periods.48 49 Importantly, unlike the early-life exposure to EDCs which produces irreversible epigenetic programming, epigenetic modulation from the exposure during non-critical period can be reversible if the insult is removed.2 50 These points offer a potential opportunity for interventions to reverse possible harms due to the chronic exposure to EDCs during non-critical periods.
Suggestion of a new paradigm
If low-dose EDC mixtures play a role in the development of many diseases in humans, but traditional approaches to chemicals and innovative scientific approaches do not work, what can we do?
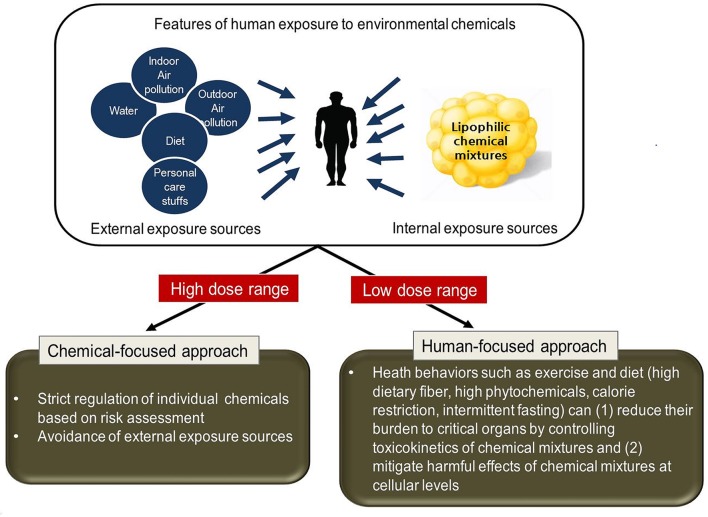
As a consequence of all the problems we discussed above, a paradigm shift is required from a chemical-focused to a human-focused (holistic) approach in the low-dose range (figure 1). The starting point is to recognise that the key is EDC mixtures, not several individual EDCs. The next point is that the most important source of EDC mixtures is already inside us, not outside us. In this situation, how to efficiently control EDC mixtures which are released from adipose tissue to circulation through lipolysis is critical. The third point is that all living organisms have the ability to excrete xenobiotics51 and possess innate repair and self-recovery mechanisms such as autophagy and DNA repair.52 53
Figure 1.
Suggestion of shifting paradigm from a chemical-focused to a human-focused approach in the low-dose range of chemical mixtures. Traditional approaches such as strict regulation or avoidance of exposure sources are effective to protect humans from harms of individual or several chemicals in the high-dose range. In the low-dose range, methods to control or fight against them in the human body should be investigated. Exercise and healthy diet (high dietary fibre, high phytochemicals, calorie restriction and intermittent fasting) can be considered for these purposes.
Therefore, the human-focused approach can be seen as methods (1) to decrease body burden of chemicals by increasing the excretion of chemical mixtures from the body and (2) to mitigate early harmful effects of chemical mixtures at cellular levels by the activation of repair and self-recovery systems.
Interestingly, many lifestyles can be used for these purposes. For example, chronic physical activity can increase the metabolism and elimination of chemical mixtures by increasing biotransformation enzyme activity in the liver54 and/or increasing biliary clearance.55 High intake of dietary fibre can increase the excretion of strong lipophilic chemicals in faeces.56 In addition, diets high in anti-inflammatory bioactive food components can modulate the toxicity of environmental pollutants.57 Furthermore, more comprehensively, evolutionarily adapted health behaviours such as exercise, calorie restriction, intermittent fasting, cognitive stimulation and phytochemicals can be used to mitigate harms of low-dose chemical mixtures at the cellular level.58 These mild stress-inducing health behaviours can augment cellular innate defence, maintenance and repair system by the activation of mitohormesis or xenohormesis.59–63 Future studies are needed to estimate the quantitative impact of the human-focused approach. Also, researches on more specific methods which can promote the excretion of chemical mixtures or active the repair and self-recovery system are desirable.
In fact, these lifestyles are generally considered as healthy without any consideration of environmental chemicals. However, it is highly plausible that the link with environmental chemicals can be an additional beneficial mechanism. Studies of mechanisms and intervention studies on this topic would be worthwhile. Also, further research on possible methods which can more effectively modulate the excretion and biological effects of chemical mixtures should be performed
Conclusion
The current prevailing individual chemical-focused reductionism-based approach is insufficient because life without exposure to EDCs is no longer possible, and the value of regulation is also limited. Additionally, the human-focused holistic approach can be deliberated as a practical way against EDCs based on the fact that the most significant exposure source of EDC mixtures is human adipose tissue. Also, evolutionary aspects of epigenetic programming during critical periods should be investigated. Harms due to chronic exposure to EDCs should receive more attention because they can be mitigated by adopting a human-focused approach.
Footnotes
Patient consent for publication: Not required.
Contributors: LD-H: conceiving the original idea, writing the draft. DRJ: discussing the main idea, revising the draft.
Funding: This work was supported by The Environmental Health Action Program (2016001370002), funded by the Korea Ministry of Environment of the Republic of Korea.
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Bansal A, Henao-Mejia J, Simmons RA. Immune system: an emerging player in mediating effects of endocrine disruptors on metabolic health. Endocrinology 2018;159:32–45. 10.1210/en.2017-00882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gore AC, Chappell VA, Fenton SE, et al. EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev 2015;36:E1–50. 10.1210/er.2015-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marchetti C. Interaction of metal ions with neurotransmitter receptors and potential role in neurodiseases. Biometals 2014;27:1097–113. 10.1007/s10534-014-9791-y [DOI] [PubMed] [Google Scholar]
- 4. Henkler F, Luch A. Adverse health effects of environmental chemical agents through non-genotoxic mechanisms. J Epidemiol Community Health 2011;65:1–3. 10.1136/jech.2008.083881 [DOI] [PubMed] [Google Scholar]
- 5. Welshons WV, Thayer KA, Judy BM, et al. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 2003;111:994–1006. 10.1289/ehp.5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wagner M. Know thy unknowns: why we need to widen our view on endocrine disruptors. J Epidemiol Community Health 2017;71:209–12. 10.1136/jech-2016-207259 [DOI] [PubMed] [Google Scholar]
- 7. TEDX. TEDX list of potential endocrine disruptors, 2017. [Google Scholar]
- 8. Stojić N, Erić S, Kuzmanovski I. Prediction of toxicity and data exploratory analysis of estrogen-active endocrine disruptors using counter-propagation artificial neural networks. J Mol Graph Model 2010;29:450–60. 10.1016/j.jmgm.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 9. Iavicoli I, Fontana L, Leso V, et al. The effects of nanomaterials as endocrine disruptors. Int J Mol Sci 2013;14:16732–801. 10.3390/ijms140816732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. WHO/UNEP, et al. : Bergman Å HJ, Jobling S, Kidd KA, Zoeller RT,. The state-of-the-science of endocrine disrupting chemicals 2012. Geneva: WHO/UNEP, 2013. [Google Scholar]
- 11. US EPA. Weight-of-evidence Guidance Document: Evaluating Results of EDSP Tier 1 Screening to Identify Candidate Chemicals for Tier 2 Testing. 2010. Draft for Public Comment.
- 12. Kretschmer XC, Baldwin WS. CAR and PXR: xenosensors of endocrine disrupters? Chem Biol Interact 2005;155:111–28. 10.1016/j.cbi.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 13. Kortenkamp A. Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect 2007;115(Suppl 1):98–105. 10.1289/ehp.9357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 2009;30:293–342. 10.1210/er.2009-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duarte-Guterman P, Navarro-Martín L, Trudeau VL. Mechanisms of crosstalk between endocrine systems: regulation of sex steroid hormone synthesis and action by thyroid hormones. Gen Comp Endocrinol 2014;203:69–85. 10.1016/j.ygcen.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 16. Carnevali O, Notarstefano V, Olivotto I, et al. Dietary administration of EDC mixtures: A focus on fish lipid metabolism. Aquat Toxicol 2017;185:95–104. 10.1016/j.aquatox.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 17. Hu Y, Wang R, Xiang Z, et al. Antagonistic effects of a mixture of low-dose nonylphenol and di-n-butyl phthalate (monobutyl phthalate) on the Sertoli cells and serum reproductive hormones in prepubertal male rats in vitro and in vivo. PLoS One 2014;9:e93425 10.1371/journal.pone.0093425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sárria MP, Santos MM, Reis-Henriques MA, et al. The unpredictable effects of mixtures of androgenic and estrogenic chemicals on fish early life. Environ Int 2011;37:418–24. 10.1016/j.envint.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 19. Lioy PJ, Rappaport SM. Exposure science and the exposome: an opportunity for coherence in the environmental health sciences. Environ Health Perspect 2011;119:A466–7. 10.1289/ehp.1104387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee DH, Jacobs DR. Methodological issues in human studies of endocrine disrupting chemicals. Rev Endocr Metab Disord 2015;16:289–97. 10.1007/s11154-016-9340-9 [DOI] [PubMed] [Google Scholar]
- 21. Lee YM, Kim KS, Jacobs DR, et al. Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes Rev 2017;18:129–39. 10.1111/obr.12481 [DOI] [PubMed] [Google Scholar]
- 22. Kim KS, Lee YM, Kim SG, et al. Associations of organochlorine pesticides and polychlorinated biphenyls in visceral vs. subcutaneous adipose tissue with type 2 diabetes and insulin resistance. Chemosphere 2014;94:151–7. 10.1016/j.chemosphere.2013.09.066 [DOI] [PubMed] [Google Scholar]
- 23. Moon HB, Lee DH, Lee YS, et al. Occurrence and accumulation patterns of polycyclic aromatic hydrocarbons and synthetic musk compounds in adipose tissues of Korean females. Chemosphere 2012;86:485–90. 10.1016/j.chemosphere.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 24. Geens T, Neels H, Covaci A, et al. triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 2012;87:796–802. [DOI] [PubMed] [Google Scholar]
- 25. Hutter HP, Kundi M, Hohenblum P, et al. Life without plastic: a family experiment and biomonitoring study. Environ Res 2016;150:639–44. 10.1016/j.envres.2016.05.028 [DOI] [PubMed] [Google Scholar]
- 26. Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol 2005;1:607–28. 10.1146/annurev.clinpsy.1.102803.144141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kortenkamp A. Endocrine disruptors: The burden of endocrine-disrupting chemicals in the USA. Nat Rev Endocrinol 2016;13:6–7. 10.1038/nrendo.2016.198 [DOI] [PubMed] [Google Scholar]
- 28. Gross L, Birnbaum LS. Regulating toxic chemicals for public and environmental health. PLoS Biol 2017;15:e2004814 10.1371/journal.pbio.2004814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bowe B, Xie Y, Li T, et al. The 2016 global and national burden of diabetes mellitus attributable to PM 2·5 air pollution. Lancet Planet Health 2018;2:e301–12. 10.1016/S2542-5196(18)30140-2 [DOI] [PubMed] [Google Scholar]
- 30. Lanphear BP. Low-level toxicity of chemicals: No acceptable levels? PLoS Biol 2017;15:e2003066 10.1371/journal.pbio.2003066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lanphear BP, Rauch S, Auinger P, et al. Low-level lead exposure and mortality in US adults: a population-based cohort study. Lancet Public Health 2018;3:e177–84. 10.1016/S2468-2667(18)30025-2 [DOI] [PubMed] [Google Scholar]
- 32. Lee DH, Porta M, Jacobs DR, et al. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev 2014;35:557–601. 10.1210/er.2013-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hernández AF, Tsatsakis AM. Human exposure to chemical mixtures: Challenges for the integration of toxicology with epidemiology data in risk assessment. Food Chem Toxicol 2017;103:188–93. 10.1016/j.fct.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 34. Walker CL. Minireview: Epigenomic Plasticity and Vulnerability to EDC Exposures. Mol Endocrinol 2016;30:848–55. 10.1210/me.2016-1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hou L, Zhang X, Wang D, et al. Environmental chemical exposures and human epigenetics. Int J Epidemiol 2012;41:79–105. 10.1093/ije/dyr154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duncan EJ, Gluckman PD, Dearden PK. Epigenetics, plasticity, and evolution: How do we link epigenetic change to phenotype? J Exp Zool B Mol Dev Evol 2014;322:208–20. 10.1002/jez.b.22571 [DOI] [PubMed] [Google Scholar]
- 37. Herman JJ, Spencer HG, Donohue K, et al. How stable ’should' epigenetic modifications be? Insights from adaptive plasticity and bet hedging. Evolution 2014;68:632–43. 10.1111/evo.12324 [DOI] [PubMed] [Google Scholar]
- 38. Giuliani C, Bacalini MG, Sazzini M, et al. The epigenetic side of human adaptation: hypotheses, evidences and theories. Ann Hum Biol 2015;42:1–9. 10.3109/03014460.2014.961960 [DOI] [PubMed] [Google Scholar]
- 39. Sultan SE. Developmental plasticity: re-conceiving the genotype. Interface Focus 2017;7:20170009 10.1098/rsfs.2017.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gluckman PD, Hanson MA, Beedle AS, et al. Predictive adaptive responses in perspective. Trends Endocrinol Metab 2008;19:109–10. author reply 12 10.1016/j.tem.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 41. Kuzawa CW. Which environments matter in studies of early life developmental plasticity? Evol Med Public Health 2017;2017:188–90. 10.1093/emph/eox024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ravelli AC, van der Meulen JH, Michels RP, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998;351:173–7. 10.1016/S0140-6736(97)07244-9 [DOI] [PubMed] [Google Scholar]
- 43. Barker DJ, Gluckman PD, Robinson JS. Conference report: fetal origins of adult disease--report of the First International Study Group, Sydney, 29-30 October 1994. Placenta 1995;16:317–20. 10.1016/0143-4004(95)90118-3 [DOI] [PubMed] [Google Scholar]
- 44. Stanner SA, Yudkin JS. Fetal programming and the Leningrad Siege study. Twin Res 2001;4:287–92. 10.1375/twin.4.5.287 [DOI] [PubMed] [Google Scholar]
- 45. Hoover RN, Hyer M, Pfeiffer RM, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med 2011;365:1304–14. 10.1056/NEJMoa1013961 [DOI] [PubMed] [Google Scholar]
- 46. Schug TT, Janesick A, Blumberg B, et al. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol 2011;127:204–15. 10.1016/j.jsbmb.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frye CA, Bo E, Calamandrei G, et al. Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J Neuroendocrinol 2012;24:144–59. 10.1111/j.1365-2826.2011.02229.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee YM, Jacobs DR, Lee DH. Persistent Organic Pollutants and Type 2 Diabetes: A Critical Review of Review Articles. Front Endocrinol 2018;9:712 10.3389/fendo.2018.00712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taylor KW, Novak RF, Anderson HA, et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect 2013;121:774–83. 10.1289/ehp.1205502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. López-León M, Goya RG. The emerging view of aging as a reversible epigenetic process. Gerontology 2017;63:426–31. 10.1159/000477209 [DOI] [PubMed] [Google Scholar]
- 51. Croom E. Metabolism of xenobiotics of human environments. Prog Mol Biol Transl Sci 2012;112:31–88. 10.1016/B978-0-12-415813-9.00003-9 [DOI] [PubMed] [Google Scholar]
- 52. Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009;461:1071–8. 10.1038/nature08467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. Nature 2008;451:1069–75. 10.1038/nature06639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yiamouyiannis CA, Sanders RA, Watkins JB, et al. Chronic physical activity: hepatic hypertrophy and increased total biotransformation enzyme activity. Biochem Pharmacol 1992;44:121–7. 10.1016/0006-2952(92)90045-K [DOI] [PubMed] [Google Scholar]
- 55. Watkins JB, Crawford ST, Sanders RA. Chronic voluntary exercise may alter hepatobiliary clearance of endogenous and exogenous chemicals in rats. Drug Metab Dispos 1994;22:537–43. [PubMed] [Google Scholar]
- 56. Sera N, Morita K, Nagasoe M, et al. Binding effect of polychlorinated compounds and environmental carcinogens on rice bran fiber. J Nutr Biochem 2005;16:50–8. 10.1016/j.jnutbio.2004.09.005 [DOI] [PubMed] [Google Scholar]
- 57. Hoffman JB, Hennig B. Protective influence of healthful nutrition on mechanisms of environmental pollutant toxicity and disease risks. Ann N Y Acad Sci 2017;1398:99–107. 10.1111/nyas.13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim SA, Lee YM, Choi JY, et al. Evolutionarily adapted hormesis-inducing stressors can be a practical solution to mitigate harmful effects of chronic exposure to low dose chemical mixtures. Environ Pollut 2018;233:725–34. 10.1016/j.envpol.2017.10.124 [DOI] [PubMed] [Google Scholar]
- 59. Wiegant FA, de Poot SA, Boers-Trilles VE, et al. Hormesis and cellular quality control: a possible explanation for the molecular mechanisms that underlie the benefits of mild stress. Dose Response 2012;11:413–30. 10.2203/dose-response.12-030.Wiegant [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Calabrese EJ. Hormesis: path and progression to significance. Int J Mol Sci 2018;19:2871 10.3390/ijms19102871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martins I, Galluzzi L, Kroemer G. Hormesis, cell death and aging. Aging 2011;3:821–8. 10.18632/aging.100380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ristow M, Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of Reactive Oxygen Species (ROS). Dose-Response 2014;12:dose-response.1–341. 10.2203/dose-response.13-035.Ristow [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Surh YJ. Xenohormesis mechanisms underlying chemopreventive effects of some dietary phytochemicals. Ann N Y Acad Sci 2011;1229:1–6. 10.1111/j.1749-6632.2011.06097.x [DOI] [PubMed] [Google Scholar]