Abstract
It has been hypothesized that selective muscarinic acetylcholine receptor (mAChR) M4 subtype activation could provide therapeutic benefits to a number of neurological disorders while minimizing unwanted cholinergic side effects observed due to nonselective mAChR activation. Given the high sequence and structural homology of the orthosteric binding sites among mAChRs, achieving M4 subtype-selective activation has been challenging. Herein, we describe the discovery of a series of M4 subtype-selective agonists bearing novel carbamate isosteres. Comparison of the isosteres’ electrostatic potential isosurface sheds light on key structural features for M4 subtype-selective activation. The identified key features were further illustrated in a proposed receptor–agonist interaction mode.
Keywords: Muscarinic acetylcholine receptor, M4 mAChR, selective mAChR agonist, carbamate isosteres, electrostatic potential isosurface, ensemble docking
The muscarinic acetylcholine receptors (mAChRs) M1–M5 are a family of five class 1 G protein-coupled receptors (GPCRs), with M1 and M4 predominantly expressed in the brain.1 They have emerged as important drug targets for a number of neurological disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and schizophrenia, given the promising procognitive and antipsychotic efficacy demonstrated by xanomeline, an M1/M4-preferring mAChR agonist,2 in two phase II clinical trials.3,4 However, further clinical development of xanomeline was halted due to significant cholinergic adverse events (AEs). It was hypothesized that selective activation of M4, a mAChR subtype primarily expressed in brain, could retain the cognitive and antipsychotic benefits of xanomeline while minimizing cholinergic AEs.5,6 Given that, several M4 subtype-selective mAChR positive allosteric modulators (PAMs) such as LY20332987 and VU01521008,9 have been identified. We were interested in exploring activation of the M4 receptor via a direct agonist approach. Different from PAM, an M4 agonist has the capability of activating the M4 receptor independent of cholinergic tone (the level of endogenous agonist acetylcholine) and thus could be more efficacious than PAM in patients with cholinergic deficits.
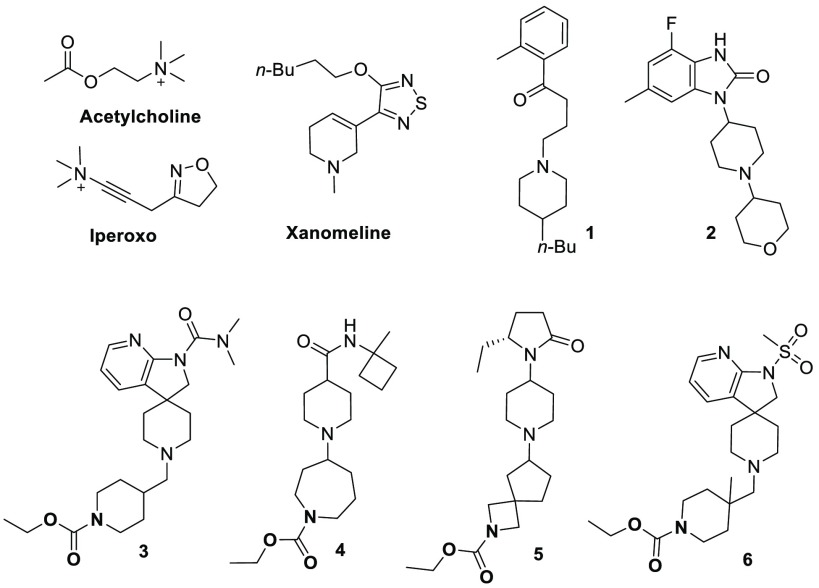
Achieving subtype selectivity for agonists has proven to be highly challenging, due to the highly conserved orthosteric binding site across subtypes. Thus far, only a few mAChR agonists with various degrees of selectivity have been reported, including M1-selective agonists such as compounds 1(10) and 2,11,12 M1 and M4 dual agonists 3(13) and 4,14 and M4-preferred agonists 5,15 as illustrated in Figure 1. Modification of the M1/M4 dual agonist 3, such as through introduction of a quarternary methyl group at the 4-position of piperidine, led to the highly M4 mAChR selective agonist 6,16 which exhibited little agonist activity against M1–M3 and M5. This was highlighted in a recent review by Takai.17 The structural elements required for M1 agonist activity were also studied and reported in our recent publication.18 However, the information on key structural elements for M4 subtype specific activation is still limited since the so-far reported M4 agonists, either M1/M4 dual, M4-preferred, or M4-selective, all share highly similar chemical features: a basic piperidine core and a terminal ethyl carbamate moiety. The ethyl carbamate moiety appears to be critical for M4 subtype agonist activity while for the M1-selective agonists, several noncarbamate moieties such as alkyl or ether groups are tolerated. These subtle SARs suggested that, despite the highly conserved structure and sequence at the orthosteric site, certain specific receptor-agonist interactions could contribute to selective mAChR subtype activation, as demonstrated by the structure and activity difference between compounds 3 and 6. The weight of evidence indicated that the interaction between the ethyl carbamate moiety and the mAChR receptor might be critical for M4 subtype activation but not required for the other subtypes such as M1 or M2. In this report, we describe our efforts on probing the key structural features of the ethyl carbamate moiety for M4 activation, as well as the identification of selective M4 agonists with novel carbamate isosteres. As part of this work, specific interactions driving M4 agonism were proposed based on the observed SAR and the ensemble docking model.
Figure 1.
Chemical structures of representative mAChR agonists. Nonselective mAChR agonists: acetylcholine, iperoxo, and xanomeline; M1-selective agonists: 1 (AC-42) and 2 (GSK1034702); M1/M4 dual agonists: 3 (derivative 1 from ref (13)) and 4 (HTL9936); M4-preferred agonist: 5 (example from WO2017021728 A1); and M4-selective agonist 6 (compound 1 from ref (16)).
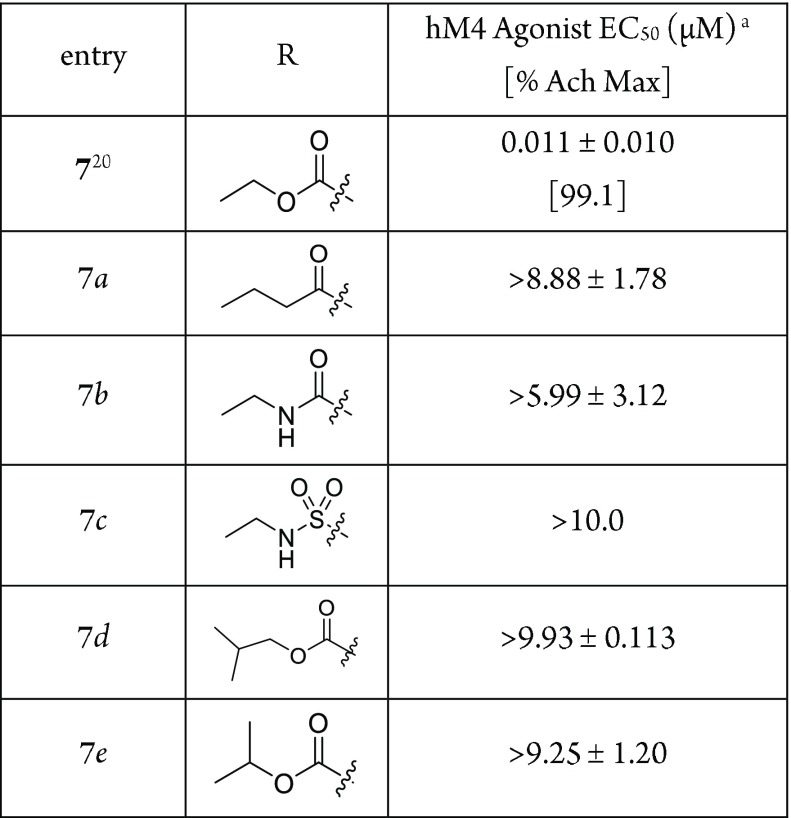
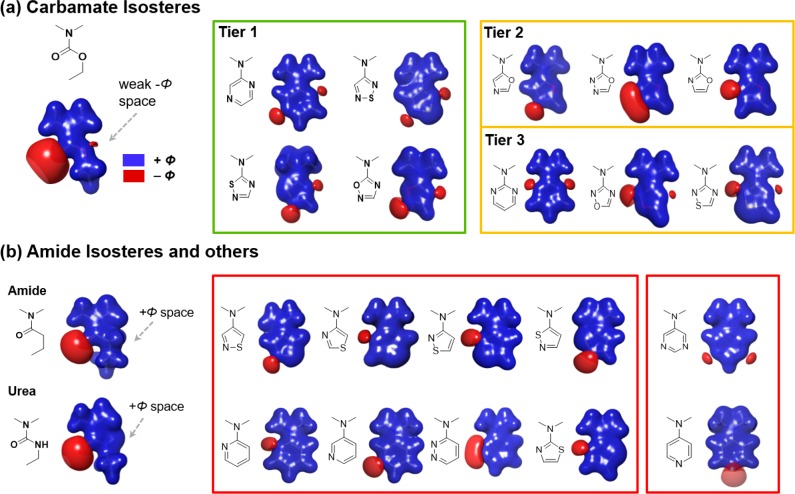
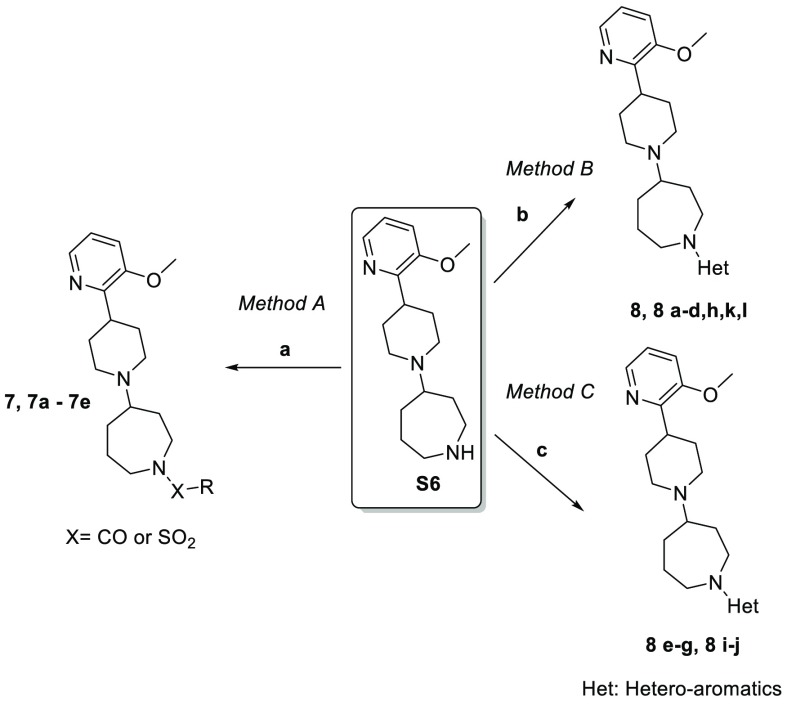
Our initial attempts to develop novel M4-selective agonists (Scheme 1) started from commonly used carbamate isosteres, such as amide, urea, or sulfonamide (7a–7c). This proved to be challenging, as such changes led to complete loss of M4 agonist activities (Table 1). Modification of the ethyl group was also not productive: methyl was reported to retain some of the M4 activity,15,19 but the sterically bulkier isopropyl (7e) and isobutyl (7d) analogues were both inactive in our study. Given such steep SAR, we investigated further difference in the shape and electrostatics between carbamate and alternative fragments. Figure 2 shows the electrostatic potential isosurfaces of carbamate, urea, amide, and their heteroaromatic isosteres. Interestingly, when comparing the electrostatic potential of the carbamate, amide, and urea moieties, we found that there was a weak negative potential around the ether oxygen of the carbamate moiety, while there was a strong positive potential around the corresponding amide CH2 and urea NH. This unique electrostatic potential difference prompted us to explore various heteroaromatics as potential isosteres (Scheme 2), using this observation as a major criterion to determine whether a given heteroaromatic moiety was ethyl carbamate-like or amide/urea-like.
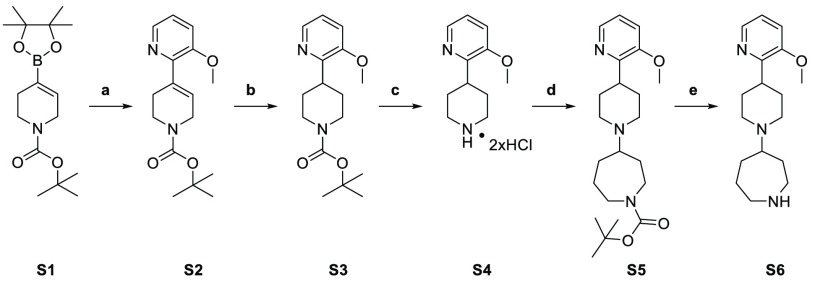
Scheme 1. Synthesis of Template Compound 7.
Reagents and conditions: (a) 2-bromo-3-methoxypyridine, Pd(dppf)Cl2, Na2CO3, dioxane, H2O, 90 °C, 18 h; (b) palladium on carbon (10%), H2, methanol, 12 °C, 16 h; (c) 4 M HCl in methanol, methanol, 0° to 12 °C, 16 h; (d) N-Boc-hexahydro-1H-azepin-4-one, triethylamine, sodium triacetoxyborohydride, dichloromethane, 45 °C, 6 h; (e) 4 M HCl in ethyl acetate, dichloromethane, 0 to 25 °C, 4 h.
Table 1. Structure and M4 Activitiy of Carbamate, Amide, Urea, and Sulfonyl Urea Analogues.
![]()
Figure 2.
Electrostatic potential isosurface of (a) ethyl carbamate-like heteroaromatics, (b) amide and urea-like heteroaromatics, and others. Blue surface: positive electrostatic potential contoured at σ = 0.05; red surface: negative electrostatic potential contoured at σ = 0.03. Electrostatic potential was calculated with B3LYP/6-311+G** in Jaguar.26
Scheme 2. General Synthesis of Analogues Bearing Heteroaromatic Carbamate Isosteres.
Reagents and conditions: (a) acyl chloride or sulfonyl chloride, triethylamine, dichloromethane, 0 to 20 °C, 3 h; (b) aryl halide, diisopropylethylamine, DMSO, 105 to 150 °C, 18–20 h; (c) aryl halide, Xphos-Pd-G3 or RuPhos-Pd-G2, sodium tert-butoxide, tert-amyl alcohol, 110 °C, 16 h.
A series of heteroaromatics, shown in Figure 2, were calculated and categorized into four groups based on how similar their electrostatic potential isosurfaces are to the ethyl carbamate moiety. Figure 2a includes the heteroaromatics bearing most similarity to ethyl carbamate, further broken down into three tiers: the first tier includes pyrazine, 1,2,5-thiadiazole, 1,2,4-thiadiazole, and 1,2,4-oxadiazole. These three heteroaromatics have strong negative electrostatic potential regions at each side of the ring that match well with those of ethyl carbamate. The second tier includes two oxazole isomers and an oxadiazole. This group of heteroaromatics has strong negative potential that matches the carbonyl of the ethyl carbamate but lacks negative potential on the other side to match the ether oxygen. Tier 3 includes pyrimidine, 1,2,4-oxadiazole, and thiadiazole. Although fragments in this group have negative potential on both sides, neither of the negative potential domains match that of the carbamate carbonyl, which is believed to be essential for interaction with the conserved Asn417 of mAChR, as illustrated in Figure 4 and Figure 5. Figure 2b shows the heteroaromatics that share high electrostatic similarity to amide or urea, such as 2-pyridine, pyrimidine, thiazole, isothiazole, and pyridazine. These examples have negative potential on one side but positive potential on the other side.
Figure 4.
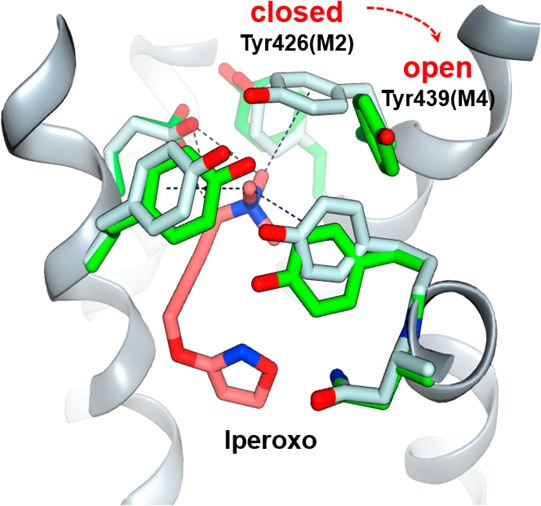
Comparison of Tyr-lid closed form (PDB: 4MQS; tyrosine side chain carbons are colored in gray) and modeled Tyr-lid open form (described in the experimental section in the SI; side chains are colored in green). Nonselective orthosteric agonist iperoxo is colored in red.
Figure 5.
(a) Docking model of ethyl carbamate 7. (b) Docking model of pyrazine 8 at the orthosteric site of the M4 receptor. The binding site surface is colored by electrostatic potential. The protein backbone is rendered as a gray ribbon, and side chain carbons are in green. (c) 2D diagram of the pyrazine–receptor interactions.
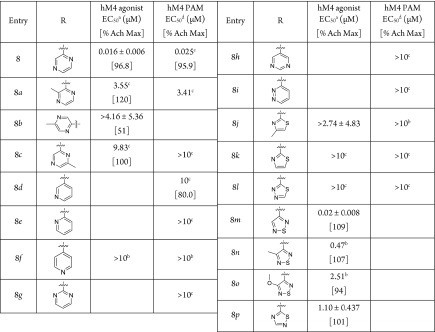
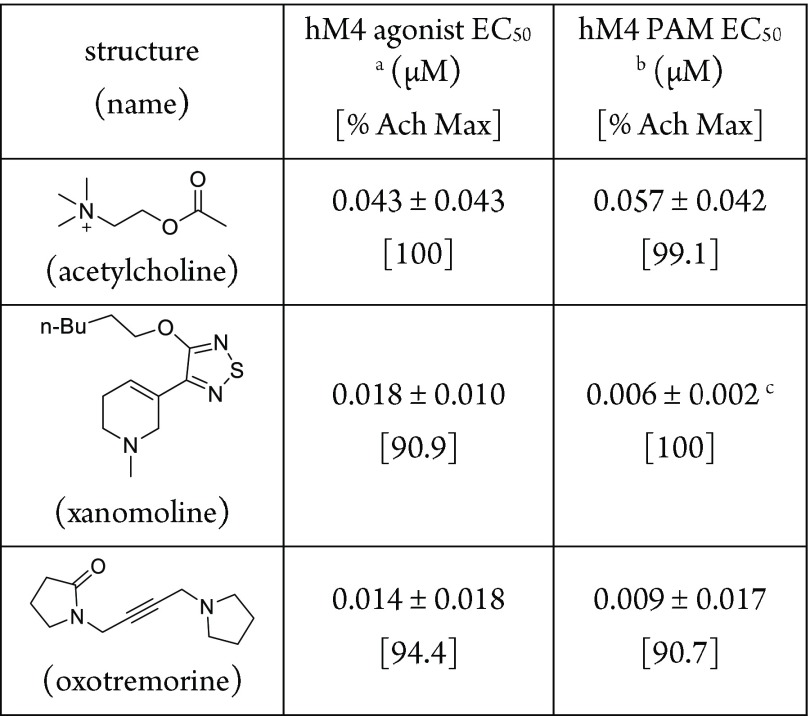
The first batch of heteroaromatic analogues, 8, 8a–8l, as shown in Table 3, were made through parallel medicinal chemistry and screened in M4 functional assays, where the activation EC50 was determined either in the presence of an EC20 level of acetylcholine (PAM mode) or in the absence of acetylcholine (agonist mode). The EC50 determined under PAM mode and agonist mode should be equivalent for an agonist, as an agonist does not potentiate the activity of acetylcholine. This hypothesis is supported by known full muscarinic agonists such as acetylcholine, xanomoline, and oxotremorine: their EC50 values determined under agonist and PAM mode are essentially equivalent, as shown in Table 2. Among these analogues, only pyrazine 8 was active, with an EC50 of 25 nM in the PAM mode and an EC50 of 16 nM in the agonist mode. Small substituents were tolerated at ortho- or para-positions, albeit with drops in potency, such as 2-methyl pyrazine (8a) (EC50 = 3.55 μM) and 4-methyl pyrazine (8b) (EC50 = 4.16 μM). Substitution at the meta-position led to complete loss of activity (8c, inactive). The heteroaromatics in Figure 2b, such as pyridines 8d–8f, pyrimidines 8g and 8h, and pyridazine 8i showed no M4 activity in PAM mode. (Compounds 8d, 8e, and 8g–8i were not tested in agonist mode due to the limited amount of material available.) Compound 8f’s inactivity was confirmed in agonist mode as well. Thiazoles 8j and 8k, as well as 1,3,4-thiadiazole 8l, showed no M4 activity in either agonist or PAM mode, consistent with the proposed electrostatic potential similarity comparison. It was interesting to note that the 2-pyrimidine 8g, which was one of the most frequently used carbamate isosteres, did not show any M4 agonist activity. To further test this hypothesis, analogues bearing 1,2,5- and 1,2,4-thiadiazoles from the first group were synthesized as singletons. We were gratified to see that the 1,2,5-thiadiazole analogue (8m) retained M4 activity (EC50 = 20 nM), comparable to that of pyrazine 8. A similar trend was also observed with the 1,2,5-thiadiazole ring: ortho-methyl 8n (EC50 = 470 nM) and ortho-methoxy 8o (EC50 = 2.5 μM) analogues both showed activity, albeit weaker. The 1,2,4-thiadiazole 8p was only moderately active, with an EC50 of 1.1 μM, potentially due to the different distribution of positive potential around the nitrogen at the 2-position. In summary, our initial effort to make new analogues yielded pyrazine as a promising ethyl carbamate isostere. Subsequent chemistry guided by the electrostatic potential signature yielded additional ethyl carbamate isosteres such as 1,2,5-thiadiazole. These experimental results validated our hypothesis that appropriate electrostatic potential distribution plays an important role in M4 mAChR activation.
Table 3. Structure–Activity Relationships (SAR) of Various Heteroaromatics.
![]()
cAMP assays with human M4/HEK cells; values represent means ± STD of three (n = 3) independent experiments;
n = 2.
n = 1.
Tested in the presence of an EC20 level of acetylcholine (see experimental section in the SI).
Table 2. Activity of Known mAchR Agonists.
cAMP assays with human M4/HEK cells; values represent geometric means ± STD (standard deviation) of greater than three independent experiments.
Tested in the presence of an EC20 level of acetylcholine (see experimental section in the SI).
n = 3.
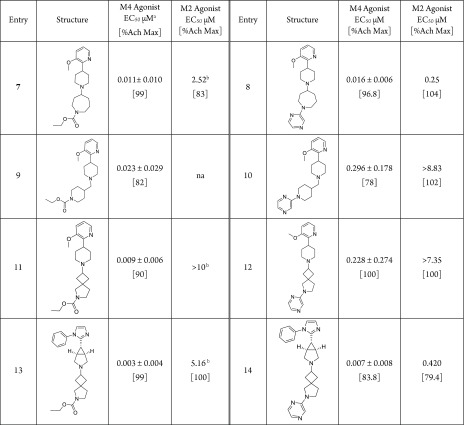
With pyrazine identified as the best ethyl carbamate isostere, we next explored whether such SAR could be transferable across multiple scaffolds. As shown in Table 4, M4 potencies were generally retained in various scaffolds, ranging from slightly weaker potencies using methylene piperidine (9 vs 10) or azaspiro[3,4]octane tail (11 vs 12) to comparable potencies using the azepane tail (7 vs 8) or 3,4-spiro tail with a [3.1.0] bicyclic linker and a phenyl-imidazole headgroup (13 vs 14). It is interesting to note that there appear to be sensitivity differences to the changes in the other parts of the molecule between the pyrazine and ethyl carbamate series, as demonstrated by the pronounced potency jump observed for the pair of pyrazine analogues 12 (EC50 = 228 nM) and 14 (EC50 = 7 nM) with structural changes in the headgroup: such a pronounced effect was not observed in the corresponding ethyl carbamate scaffold (compound 11 vs compound 13). Similar to the ethyl carbamate, the mAChR selectivity of pyrazine analogues can be modulated by changes in the headgroup, yielding agonists with a high level of selectivity.
Table 4. Matched Molecular Pair Comparisons between Ethyl Carbamate and Pyrazine Analogues.
cAMP assay with human M4/HEK cells; values represent means ± STD of three (n = 3) independent experiments.
n = 1.
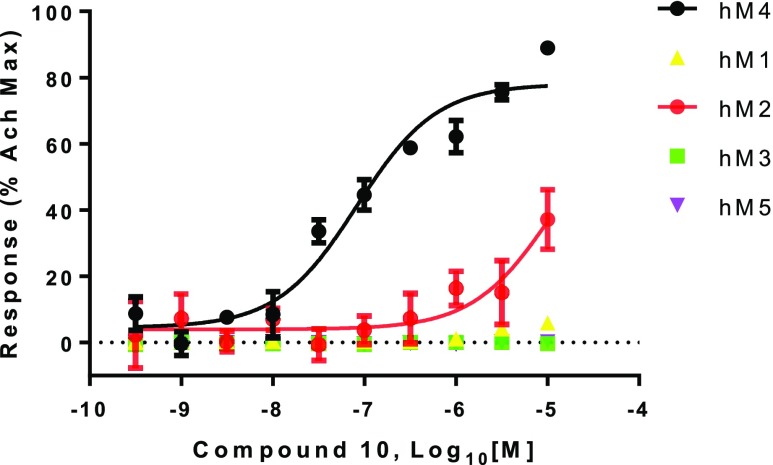
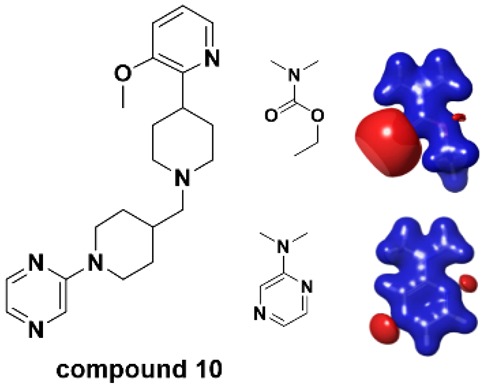
Dose response curves for the mAChR M1–M5 subtype agonist activity of compound 10 are shown in Figure 3. The measured Pgp efflux ratio22 of Compound 10 is 3.89, and the BCRP efflux ratio23 is 1.62. Based on that, the calculated human steady-state ratio of free brain (Cb,u) to free plasma (Cp,u) concentration24 is 0.77, which predicts high brain permeability for compound 10. Although further investigation is necessary, the favorable predicted brain permeability and muscarinic subtype selectivity suggested that compound 10 could be a useful tool compound for studying the physiological role of M4 mAChR in vivo.
Figure 3.
mAChR subtype selectivity profile of compound 10. The dose responses were normalized using the maximum efficacy of acetylcholine for the corresponding mAChR subtypes. Assay details are described in the SI.
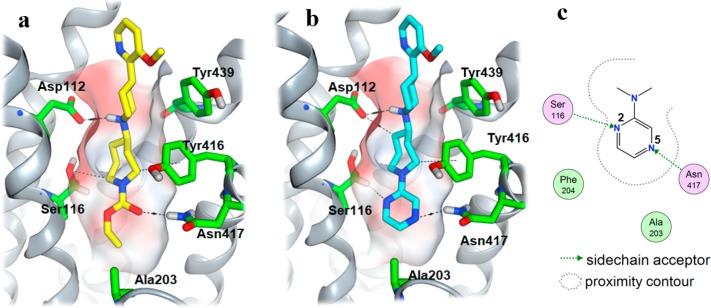
With this information in hand, the proposed binding conformation for M4-selective agonists was developed through ensemble cross-docking. As described in the experimental section in the SI, the protein conformers that led to the most consistent binding poses for active M4 agonists were selected as the activated receptor templates. It is notable that, in all the selected templates, Tyr439 adopted an “open” conformation, which was different from what was observed in the reported X-ray structure of the M2-activated form,25 where the corresponding M2 Tyr426 was in a “closed” conformation, forming a “tyrosine-lid” together with Tyr104, Tyr403, and Tyr430 on the top of the orthosteric binding site in M2. These two different conformations are shown in Figure 4. Except for Tyr439, there was little significant conformational change in other orthosteric amino acid side chains.
The docked binding modes of ethyl carbamate compound 7 and its pyrazine analogue 8 are shown in Figure 5. Overall, they exhibit similar interactions with the receptor at the orthosteric site, where the basic piperidine amine interacts with the conserved Asp112. In the modeled activated M4, the open “tyrosine-lid” creates a passage for the agonist’s piperidine linker, allowing the methoxy-2-pyridyl headgroup to extend beyond the acetylcholine binding site and form interactions with the aromatic side chains of Tyr92 and Trp106.
The ethyl carbamate binds deep at the orthosteric site and interacts with Asn417 through strong hydrogen bonding between carbonyl and NH (Figure 5a). Although there is no obvious strong protein interaction observed with the carbamate ether oxygen, the β carbon of Ser116 locates closely to the ether oxygen, resulting in both an extremely narrow space and potential Cβ H–O interaction with the ether oxygen. This specific Ser116-carbamate interaction feature explains the exclusive preference of carbamate over urea or amide, wherein the urea NH or amide CH2 would collide with the Ser116 Cβ hydrogen. The space around the ethyl group is also strictly limited because of the surrounding hydrophobic amino acids: Val120, Ala203, and Phe204. This limited binding space around the ethyl group results in limited substitution options as discussed previously, where only methyl was tolerated, and larger or longer alkyl groups were inactive (7d and 7e in Table 1).
In the docking model of the corresponding pyrazine analogue 8 (Figure 5b), the 5-nitrogen of pyrazine forms a strong hydrogen bond with the Asn417 NH and the 2-nitrogen of pyrazine acts similarly to the ether oxygen on the carbamate, forming a favorable interaction with the Cβ hydrogen of Ser116, as illustrated in the 2D interaction diagram in Figure 5c. This proposed Ser116–pyrazine interaction is consistent with the observed SAR, as the 2-nitrogen is required for M4 agonist activity. Heteroaromatics without a hydrogen bond acceptor at the 2-position to interact with Ser116 were all inactive.
In conclusion, several novel heteroaromatics have been identified as effective ethyl carbamate isosteres, yielding a series of novel M4 selective agonists. The electrostatic potential isosurface analysis proved to be a useful method to differentiate closely related heteroaromatics, yielding both pyrazine and 1,2,5-thiadiazole as effective ethyl carbamate replacements. The pyrazine motif has further been incorporated into different scaffolds, yielding M4 subtype-selective mAChR agonists. The subsequent M4 docking model provided structure-based explanations for the preference of ethyl carbamate as well as isosteres such as pyrazine and thiadiazoles, revealing hydrogen bonds with the Asn417 amide NH and Ser116 Cβ hydrogen as the most likely key interactions for M4 activation.
Acknowledgments
We thank Scot Mente, Xinjun Hou, and Michael Brodney for helpful discussions. We also thank HD Biosciences Co. for technique support.
Glossary
Abbreviations
- mAChR
muscarinic acetylcholine receptor
- ACh
acetylcholine
- ECL
extracellular loop
- PAM
positive allosteric modulator
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.9b00106.
General methods for the synthesis, modeling, and in vitro characterization of all compounds (PDF)
Author Contributions
The manuscript was written through contributions of all authors. Q.Y., E.A.L., D.W., N.M.K., J.T.L., C.R.B., and L.Z. drafted and proofread the manuscript. D.W. and E.A.L. performed the chemical syntheses. Q.Y., E.A.L., D.W., N.M.K., J.T.L., C.R.B., and L.Z. oversaw the target selection and data interpretation. M.P. and J.T.L. performed the in vitro molecular pharmacology studies.
The authors declare the following competing financial interest(s): The authors hold patents on developing M4 agonists for the treatment of neurological disorders.
Supplementary Material
References
- Lebois E. P.; Thorn C.; Edgerton J. R.; Popiolek M.; Xi S. Muscarinic receptor subtype distribution in the central nervous system and relevance to aging and Alzheimer’s disease. Neuropharmacology 2018, 136, 362–373. 10.1016/j.neuropharm.2017.11.018. [DOI] [PubMed] [Google Scholar]
- Thorn C. A.; Moon J.; Bourbonais C. A.; Harms J.; Edgerton J. R.; Stark E.; Steyn S. J.; Butler C. R.; Lazzaro J. T.; O’Connor R. E.; Popiolek M. Striatal, Hippocampal, and Cortical Networks Are Differentially Responsive to the M4- and M1-Muscarinic Acetylcholine Receptor Mediated Effects of Xanomeline. ACS Chem. Neurosci. 2019, 10, 1753–1764. 10.1021/acschemneuro.8b00625. [DOI] [PubMed] [Google Scholar]
- Bodick N. C.; Offen W. W.; Levey A. I.; Cutler N. R.; Gauthier S. G.; Satlin A.; Shannon H. E.; Tollefson G. D.; Rasmussen K.; Bymaster F. P.; Hurley D. J.; Potter W. Z.; Paul S. M. Effects of Xanomeline, a Selective Muscarinic Receptor Agonist, on Cognitive Function and Behavioral Symptoms in Alzheimer Disease. Arch. Neurol. 1997, 54, 465–473. 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- Shekhar A.; Potter W. Z.; Lightfoot J.; Lienemann J.; Dubé S.; Mallinckrodt C.; Bymaster F. P.; McKinzie D. L.; Felder C. C. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry 2008, 165, 1033–1039. 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- Bubser M.; Bridges T. M.; Dencker D.; Gould R. W.; Grannan M.; Noetzel M. J.; Lamsal A.; Niswender C. M.; Daniels J. S.; Poslusney M. S.; Melancon B. J.; Tarr J. C.; Byers F. W.; Wess J.; Duggan M. E.; Dunlop J.; Wood M. W.; Brandon N. J.; Wood M. R.; Lindsley C. W.; Conn P. J.; Jones C. K. Selective Activation of M4Muscarinic Acetylcholine Receptors Reverses MK-801-Induced Behavioral Impairments and Enhances Associative Learning in Rodents. ACS Chem. Neurosci. 2014, 5, 920–942. 10.1021/cn500128b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popiolek M.; Mandelblat-Cerf Y.; Young D.; Garst-Orozco J.; Lotarski S. M.; Stark E.; Kramer M.; Butler C. R.; Kozak R. Vivo Modulation of Hippocampal Excitability by M4Muscarinic Acetylcholine Receptor Activator: Implications for Treatment of Alzheimer’s Disease and Schizophrenic Patients. ACS Chem. Neurosci. 2019, 10, 1091–1098. 10.1021/acschemneuro.8b00496. [DOI] [PubMed] [Google Scholar]
- Valant C.; Felder C. C.; Sexton P. M.; Christopoulos A. Probe Dependence in the Allosteric Modulation of a G Protein-Coupled Receptor: Implications for Detection and Validation of Allosteric Ligand Effects. Mol. Pharmacol. 2012, 81, 41–52. 10.1124/mol.111.074872. [DOI] [PubMed] [Google Scholar]
- Brady A. E.; Jones C. K.; Bridges T. M.; Kennedy J. P.; Thompson A. D.; Heiman J. U.; Breininger M. L.; Gentry P. R.; Yin H.; Jadhav S. B.; Shirey J. K.; Conn P. J.; Lindsley C. W. Centrally Active Allosteric Potentiators of the M4Muscarinic Acetylcholine Receptor Reverse Amphetamine-Induced Hyperlocomotor Activity in Rats. J. Pharmacol. Exp. Ther. 2008, 327, 941–953. 10.1124/jpet.108.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencker D.; Weikop P.; Sørensen G.; Woldbye D. P. D.; Wörtwein G.; Wess J.; Fink-Jensen A. An allosteric enhancer of M4 muscarinic acetylcholine receptor function inhibits behavioral and neurochemical effects of cocaine. Psychopharmacology 2012, 224, 277–287. 10.1007/s00213-012-2751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzik B.; Cooper D. G.; Forbes I. T.; Garzya V.; Jin J.; Shi D.; Smith P. W.; Walker G.. Compounds Which Have Activity at M1 Receptor and Their Uses in Medicine. 2008, US 2008/0255195.
- Jones C. K.; Brady A. E.; Davis A. A.; Xiang Z.; Bubser M.; Tantawy M. N.; Kane A. S.; Bridges T. M.; Kennedy J. P.; Bradley S. R.; Peterson T. E.; Ansari M. S.; Baldwin R. M.; Kessler R. M.; Deutch A. Y.; Lah J. J.; Levey A. I.; Lindsley C. W.; Conn P. J. Novel Selective Allosteric Activator of the M1Muscarinic Acetylcholine Receptor Regulates Amyloid Processing and Produces Antipsychotic-Like Activity in Rats. J. Neurosci. 2008, 28, 10422–10433. 10.1523/JNEUROSCI.1850-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan P. J.; Watson J.; Lund J.; Davies C. H.; Peters G.; Dodds C. M.; Swirski B.; Lawrence P.; Bentley G. D.; O’Neill B. V.; Robertson J.; Watson S.; Jones G. A.; Maruff P.; Croft R. J.; Laruelle M.; Bullmore E. T. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int. J. Neuropsychopharmacol. 2013, 16, 721–731. 10.1017/S1461145712000752. [DOI] [PubMed] [Google Scholar]
- Takai K.; Inoue Y.; Konishi Y.; Suwa A.; Uruno Y.; Matsuda H.; Nakako T.; Sakai M.; Nishikawa H.; Hashimoto G.; Enomoto T.; Kitamura A.; Uematsu Y.; Kiyoshi A.; Sumiyoshi T. Discovery of N-substituted 7-azaindoline derivatives as potent, orally available M1 and M4 muscarinic acetylcholine receptors selective agonists. Bioorg. Med. Chem. Lett. 2014, 24, 3189–3193. 10.1016/j.bmcl.2014.04.085. [DOI] [PubMed] [Google Scholar]
- Collingwood S. P.; Ratcliffe A. J.; Pryde D.; Porter R. Recent disclosures of clinical candidates highlights from the society of medicines research symposium. Drugs Future 2015, 40, 81–91. 10.1358/dof.2015.040.01.2273384. [DOI] [Google Scholar]
- Brown G. A.; Cansfield J.; Pickworth M.; Tehan B. G.; Teobold B. J.. Muscarinic agonists. 2017, WO 2017/021729.
- Suwa A.; Konishi Y.; Uruno Y.; Takai K.; Nakako T.; Sakai M.; Enomoto T.; Ochi Y.; Matsuda H.; Kitamura A.; Uematsu Y.; Kiyoshi A.; Sumiyoshi T. Discovery of N-sulfonyl-7-azaindoline derivatives as potent, orally available and selective M4 muscarinic acetylcholine receptor agonists. Bioorg. Med. Chem. Lett. 2014, 24, 2909–2912. 10.1016/j.bmcl.2014.04.083. [DOI] [PubMed] [Google Scholar]
- Takai K.; Enomoto T. Discovery and Development of Muscarinic Acetylcholine M4 Activators as Promising Therapeutic Agents for CNS Diseases. Chem. Pharm. Bull. 2018, 66, 37–44. 10.1248/cpb.c17-00413. [DOI] [PubMed] [Google Scholar]
- Lee A. A.; Yang Q.; Bassyouni A.; Butler C. R.; Hou X.; Jenkinson S.; Price D. A. Ligand biological activity predicted by cleaning positive and negative chemical correlations. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 3373. 10.1073/pnas.1810847116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. A.; Cansfield J. E.; Congreve M. S.; O’Brien M. A.; Pickworth M.; Rackham M. D.; Tehan B. G.; Teobold B. J.. Bicyclic aza compounds as muscarinic M1 receptor agonists. 2015, WO 2015/118342.
- Livermore D.; White K.; Congreve M.; Brown G.; O’Brien M.. Piperidine-1-yl and azepin-1-yl carboxylates as muscarinic M4 receptor agonists. 2014, WO 2014/122474 A1.
- The EC50 values listed in tables are geometric means. The %ACh max are arithmetic means. For the cases where the geometric EC50 value was greater than a value less than 10 μM, it means the number had a mix of >10 μM and actual EC50 values from independent experiments, from which a geometric mean was calculated substituting 10 for >10; the “greater than” was then added to the result.
- Ratio of permeability, measured as a rate in 10–6 cm/s in and out (BA/AB) of a Madin–Darby canine kidney (MDCK) epithelial cell line transfected with the MDR1 gene encoding the P-gp efflux transporter.
- Ratio of permeability, measured as a rate in 10–6 cm/s in and out (BA/AB) of a Madin–Darby canine kidney (MDCK) epithelial cell line with doxycycline induced expressed transfected mouse Bcrp.
- Trapa P. E.; Belova E.; Liras J. L.; Scott D. O.; Steyn S. J. Insights From an Integrated Physiologically Based Pharmacokinetic Model for Brain Penetration. J. Pharm. Sci. 2016, 105, 965–971. 10.1016/j.xphs.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Kruse A. C.; Ring A. M.; Manglik A.; Hu J.; Hu K.; Eitel K.; Hübner H.; Pardon E.; Valant C.; Sexton P. M.; Christopoulos A.; Felder C. C.; Gmeiner P.; Steyaert J.; Weis W. I.; Garcia K. C.; Wess J.; Kobilka B. K. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 2013, 504, 101–106. 10.1038/nature12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochevarov A. D.; Harder E.; Hughes T. F.; Greenwood J. R.; Braden D. A.; Philipp D. M.; Rinaldo D.; Halls M. D.; Zhang J.; Friesner R. A. Jaguar: A high-performance quantum chemistry software program with strengths in life and materials sciences. Int. J. Quantum Chem. 2013, 113, 2110–2142. 10.1002/qua.24481. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.