Abstract
Background:
Androgenetic alopecia (AGA) refers to the appearance of the common nonscarring progressive patterned loss of terminal hair on the frontal scalp and/or vertex of the scalp in both men and women, seen with increasing age in genetically predisposed individuals. Until recently, a scalp biopsy was the only objective tool to diagnose and monitor the disease severity. Trichoscopy of scalp is a new noninvasive technique applied to facilitate the diagnosis of hair and scalp disorders using a manual or video dermatoscope. We found a significant difference in some of the variables such as brown peripilar sign (BPPS), white peripilar sign (WPPS), focal atrichia which may aid in the diagnosis of early and late stages of both male and female AGA along with its clinical correlation. No significant difference in the occipital area was found in all AGA patients.
Aims:
This study aims to study the trichoscopic findings of AGA and to correlate their relationship with disease severity in our tertiary care hospital.
Settings and Design:
This was a prospective, observational study.
Subjects and Methods:
A total of 91 patients (66 males and 25 females) of the age group between 18 and 70 years, were included in the study at the outpatient department of dermatology in 1 year. Each patient underwent a detailed general physical, systemic, and dermatological examination. The diagnosis of AGA was based on clinical grounds. The type of hair loss in each patient was recorded. Trichoscopic evaluation and capture of trichoscopic images was performed using an eScope Oitez Digital Microscope.
Ethics:
In accordance with the Helsinki Declaration of 1975 (revised in 2000), the study was approved by Ethical and Scientific Research committees of Care Institute of Medical Sciences, Hyderabad.
Statistical Analysis Used:
Statistical analysis was carried out with R-studio. Statistical significance in the difference in the outcome variables between the stages was assessed by Fisher's exact test. The statistical test was considered statistically significant at P < 0.05.
Results:
A positive correlation between clinical and trichoscopic findings with respect to disease severity was seen in some of the variables in our study. Both male and female AGA patients have hair shaft thickness heterogeneity as the most common feature. BPPS is seen in early grades of AGA (P < 0.01); WPPS and focal atrichia are seen in later grades of AGA (P < 0.01). Scalp honeycomb pigmentation was most commonly seen in all stages and is not correlated to the disease severity of AGA.
Conclusion:
As trichoscopy can reveal early variations in hair follicle diameter long before hair loss becomes clinically visible and has the advantage of examining larger areas in a relatively short duration makes it a practical choice for clinic set up. It adds new easily recognizable images for visual teledermatology. Besides, the easy documentation allows the doctor and patient to view the video graphics images simultaneously and helps in evaluating a therapeutic response by comparing it with pre-treatment images.
Key words: Female androgenetic alopecia, male androgenetic alopecia, trichoscopy
INTRODUCTION
Androgenetic alopecia (AGA) refers to the appearance of the common nonscarring progressive patterned loss of terminal hair on the frontal scalp and/or vertex of the scalp in both men and women, seen with increasing age in genetically predisposed individuals.[1] The conversion of terminal to vellus follicle is the central event in the pathogenesis of AGA.[2] Hamilton and Norwood classified male pattern hair loss clinically into seven grades.[3] Ludwig scale used 3-stage grading scale for female pattern hair loss (FPHL).[1,4]
Until recently, a scalp biopsy was the only objective tool to diagnose and monitor the disease severity. Trichoscopy of the scalp is a new noninvasive technique applied to facilitate the diagnosis of hair and scalp disorders using a manual or video dermatoscope with lenses ranging from ×20 to ×1000 magnifications.[5,6,7] The usual working magnification is ×10 to ×100 fold.[8]
SUBJECTS AND METHODS
Male and female patients of age group between 18 and 70 years, each patient underwent a detailed general physical, systemic, and dermatological examination. The diagnosis of AGA was made based on (H-N) grades in males and Ludwig stages in females clinically. The type of hair loss in each patient was recorded.
Study design
This was a prospective, observational study at the outpatient department of Dermatology, Care Hospitals, Banjara Hills, Hyderabad, in 1 year.
Sample size
A total of 91 patients both male and females clinically diagnosed as AGA were included in the study after receiving appropriate informed consent.
Exclusion criteria
Other hair loss diseases such as
Alopecia areata
Telogen effluvium
Hair loss as a result of surgery, trauma, or radiotherapy
Other scalp disorders-psoriasis, seborrhoeic dermatitis, and tinea capitis.
The trichoscopic evaluation was done on every patient. The capture of trichoscopic images was performed using an eScope Oitez Digital Microscope (with optical magnification 10–40×, 200×, resolution 640 × 480–3488 × 2616 pixels with 8 LED Illumination with 2 stage control and 9.0 Megapixel CMOS Sensor with polarizing filter). Trichoscopic patterns of disease were recorded, and the necessary pictures were saved.
Trichoscopy image capturing was performed by a single person to avoid diversification. The selection of the trichoscopic variables included in the evaluation process was based on the available literature data and expertise.
Fields examined on trichoscope
Male androgenetic alopecia
Hair loss regions including vertex, frontal, and temporal hairline, as well as genetically spared occipital area, were observed, and images were captured for analysis.
Female androgenetic alopecia
Frontal, temporal, crown areas, and occipital area were observed, images were captured for analysis.
Parameters seen in trichoscopic examination[8,9,10]
Hair shaft thickness heterogeneity (HSTH): >20% in Male AGA (MAGA) and >10% in Female AGA (FAGA) which corresponds to vellus hair transformation is the feature of AGA
Brown peripilar sign (BPPS): is brown halo around the emergent hair shaft
White peripilar sign (WPPS): larger in size as white halo at the follicular ostium
Yellow dots are round or polycyclic best seen under polarized light. Reflect empty hair follicle
Focal atrichia: They are areas of total hair loss on scalp, usually in a size of a pencil eraser
Scalp honeycomb pigmentation (SHCP): which corresponds to melanotic rete ridges.
RESULTS
Both male and female AGA patients have HSTH as most common feature. BPPS is seen in early grades of AGA (P < 0.01), WPPS and focal atrichia are seen in later grades of AGA (P < 0.01).
SHCP was most commonly seen in all stages and is not correlated to the disease severity of AGA.
DISCUSSION
Of 91 cases, 66 (73%) cases were males, 25 (27%) females. Out of 66 male patients, 39 (59.09%) patients belonged to the age group of 18–29 years which was the highest in our study. Of 25 female patients, 9 (36%) patients belonged to the age group of 31–40 years which was the highest in our study. The mean age of MAGA in our study was 30 years (standard deviation [SD] ±9); FAGA was 37 years (SD ± 13). Mean duration of hair loss was 4 years in both genders with (SD ± 3.5) in males and (SD ± 3) in females in our study.
Distribution of cases of H-N grades in male androgenetic alopecia
Out of 66 MAGA cases, 21 (31.8%) cases were in H-N Grade III, which was the highest in our study.
Distribution of cases of Ludwig stages in female androgenetic alopecia
Out of 25 FAGA cases, 17 (68%) cases were in Ludwig stage II, which was the highest in our study.
The mean age of MAGA in our study was 30 years (SD ± 9), FAGA was 37 years (SD ± 13) which is nearer to the studies done in Indian population by Grover[11] in which mean age of MAGA was 30–39 years and Krupa Shankar et al.[12] with a mean age of 37.05 (SD ± 6) years in MAGA. Similarly, in a study by Hu et al.[8] done on the Chinese population had the mean age of 30.6 (SD ± 7.4) and 35.8 (SD ± 11.6) years in MAGA and FAGA, respectively.
The most common grade in our study was H-N Grade III 21 cases (31.8%) followed by Grade II 17 cases (25.8%) similar to that observed by Sehgal et al.[13], Grover[11] in Indian population and by Paik et al. in the Korean population.[14] However, in a study by Krupa Shankar et al. in Indian population[12] Grade II was (27.27%) followed by Grade I (22.12%) and Grade III (21.78%). Whereas the Chinese study by Wang et al. had Grade IV as the most common type.[15]
HSTH is regarded as a hallmark of AGA in previous reports.[16,17,18,19] Similarly, we found that both male and female AGA patients have HSTH as most common feature this is in accordance with observations of Hu et al.,[8] and that of major criteria of FAGA established by Rakowska et al.[20] Another study done on 89 FPHL patients by Galliker and Trüeb[21] showed that HSTH is seen in 72% of early and 100% in advanced FAGA.
BPPS is seen 89.4% of MAGA and 40% of FAGA in early stages with P < 0.01 in our study. The previous Asian research done by Inui et al.[16] detected 66% and 20% in male and female patients, respectively.
Whereas it was seen in 90% of male and 86% of female Caucasian AGA patients in a study done by Deloche et al.,[22] in contrast, 44.0% of MAGA and 44.5% of FAGA was observed in Chinese study done by Hu et al.[8] In another study, 31.7% was observed in FAGA done by Zhang et al.[9] We found that it was seen in early AGA patients with a higher density of hair.
WPPS is seen 100% from H-N Grade IV to VII bringing the total to 60.6% of males and 68% of females in Ludwig Stages II and III in our study. In contrast to our study, 20.7% of male and 15.0% of female AGA patients have WPPS in Chinese study done by Hu et al.[8] They found it in 72.4% in H-N Grade VII followed by 65% in Grade VI and 37% in Grade V. In another study done by Zhang et al.[9] it was observed in 26.7% of FAGA patients with advanced stages. We suppose that this sign was related to perifollicular fibrosis in the late stage of AGA.[9]
Yellow dots found in 92.4% of males and 88% of female in our study were seen in both early and late stages with P < 0.01. In contrast, 20.1% of male and 24.0% of female AGA patients with advanced stages of AGA was seen in the Chinese study done by Hu et al.[8] and in another study done by Zhang et al.[9] on Chinese females showed 1.67% in late stages of FAGA patients. The presence of yellow dots of different sizes with a variable number in AGA contains mainly sebum[18] was categorized as one of the major criteria in the diagnosis of FPHL in a recent study by Rakowska et al.[20] This disparity in findings can be explained by the difference in ethnic group enrolled in studies which implies variation in sebaceous gland activity as well as in the degree of pigmentation of the scalp in late stages. We believe that the differences regarding the frequency of this finding are due to skin phototypes, as well as different shampoo habits between cultures.
Focal atrichia which is usually referred to as “pencil-erased” focal loss of hair was found in 21.2% of MAGA and 24% of FAGA patients in our study and showed a positive correlation with disease severity, similar to the study by Hu et al.[8] who observed it in 28% of MAGA and 56.5% of FAGA patients with late stages and also in a study by Zhang et al.[9] who observed, (34/60) 56.7% in FAGA patients which correlated with advancing stage of AGA.
Scalp honeycomb pigmentation was found in 87.9% in males and 80% in female AGA in our study, whereas in a Chinese study by Hu et al. SHCP was seen in 33.2% of male and 30.5% of female patients[8] in late stages of AGA and a study by Zhang et al.[9] observed it in 61.7% of FAGA patients with late stages.
We found a significant difference in some of the variables of trichoscopy such as BPPS, WPPS, focal atrichia which may aid in the diagnosis of early and late stages of AGA along with its clinical correlation [Tables 1–3].
Table 1.
Clinical and trichoscopic correlation in H-N grades of male androgenetic alopecia n (%) and P value
| Trichoscopic Diagnosis | I n-14 (21.2%) | II n-17 (25.8%) | III n-21 (31.8%) | IV n-7 (10.6%) | V n-4 (6.1%) | VI n-1 (1.5%) | VII n-2 (3%) | P |
|---|---|---|---|---|---|---|---|---|
| HSTH | 14 (100%) | 17 (100%) | 21 (100%) | 7 (100%) | 4 (100%) | 1 (100%) | 2 (100%) | |
| BPPS | 14 (100%) | 17 (100%) | 19 (90.5%) | 6 (85.7%) | 3 (75%) | 0 (0.0%) | 0 (0.0%) | <0.01 |
| WPPS | 1 (7.1%) | 8 (47.1%) | 17 (81%) | 7 (100%) | 4 (100%) | 1 (100%) | 2 (100%) | <0.01 |
| Yellow dots | 13 (92.9) | 16 (94.1%) | 18 (85.7%) | 7 (100%) | 4 (100%) | 1 (100%) | 2 (100%) | 0.86 |
| Focal atrichia | 1 (7.1%) | 1 (5.9%) | 3 (14.3%) | 5 (71.4%) | 2 (50%) | 0 (0.0%) | 2 (100%) | 0.88 |
| Scalp honey comb pigmentation | 7 (50%) | 16 (94.1%) | 21 (100%) | 7 (100%) | 4 (100%) | 1 (100%) | 2 (100%) | <0.01 |
Table 3.
Trichoscopic patterns observed in our study and their clinical correlations
| Trichoscopic pattern | Description | Trichoscopic picture Correlate | Clinical correlation |
|---|---|---|---|
| 1. HSTH | Corresponds to vellus hair transformation and is the hallmark feature of AGA |
 [Figure-1a] [Figure-1a] |
Seen in all grades of male and female AGA |
| 2. BPPS | Characterized by brown halo around the emergent hair shaft with a diameter of approximately 1mm, seen in early stages |
 [Figure-1b] [Figure-1b] |
Early grades of male and female AGA |
| 3. WPPS | White halo at the follicular ostium |
 [Figure-1c] [Figure-1c] |
Seen in severe grades |
| 4. Yellow dots | Are round or polycyclic appear as yellow dots reflect empty hair follicles distended with sebum in AGA |
 [Figure-1d] [Figure-1d] |
Seen in both early and severe grades |
| 5. Focal atrichia | Areas of total hair loss on scalp, usually in a size of a pencil eraser |
 [Figure-1e] [Figure-1e] |
Seen in late stages of FAGA and MAGA |
| 6. Scalp honeycomb pigmentation | Formed as hypomelanotic areas bordered by hyperchromic lines (melanin of rete ridges) |
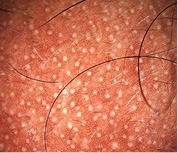 [Figure-1f] [Figure-1f] |
Commonly seen in all grades of AGA in bald areas |
HSTH [Figure 1a], BPPS [Figure 1b], WPPS [Figure 1c], yellow dots [Figure 1d], focal atrichia [Figure 1e], scalp honeycomb pigmentation [Figure 1f and Table 3]. HSTH – Hair shaft thickness heterogeneity; BPPS – Brown peripilar sign; WPPS – White peripilar sign; AGA – Androgenetic alopecia; MAGA – Male androgenetic alopecia; FAGA – Female androgenetic alopecia
Table 2.
The summary of clinical and trichoscopic correlation of female androgenetic alopecia in Ludwig stages n (%) and P
| Trichoscopic variables | F-I n-7 (28%) | F-II n-17 (68%) | F-III n-1 (4%) | P |
|---|---|---|---|---|
| HSTSH >10% | 7 (100%) | 16 (96%) | 1 (100%) | 0.19 |
| BPPS | 7 (100%) | 3 (17.6%) | 0 (0.0%) | <0.01 |
| WPPS | 0 (0.0%) | 16 (94.1%) | 1 (100%) | <0.01 |
| Yellow dots | 5 (71.4%) | 17 (100%) | 0 (0.0%) | <0.01 |
| Focal atrichia | 1 (14.3%) | 5 (29.4%) | 0 (0.0%) | 0.62 |
| Scalp Honey comb pigmentation | 4 (57.1%) | 15 (88.2%) | 1 (100%) | 0.19 |
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.McMichael A. Female Pattern Hair Loss (Androgenetic Alopecia in Women): Pathogenesis, Clinical Features, and Diagnosis. Up-To-Date. 2016. [Last accessed on 2016 Jul 6]. pp. 383–8. Available from: http://www.uptodate.com.pbidi.unam.mx:8080/contents/female-pattern-hair-loss-androgenetic-alopecia-in-women-pathogenesisclinical-features-and-diagnosis?source=see_link§ionName=DIAGNOSIS&anchor=H109567572#H109567572 .
- 2.Sinclair RD. Rook's Textbook of Dermatology. Oxford: Blackwell Scientific; 1982. Common baldness and androgenetic alopecia; pp. 16–31. [Google Scholar]
- 3.Donovan J, Goldstein BG, Goldstein AO, Hordinsky M, et al., editors. Androgenetic Alopecia in Men: Pathogenesis, Clinical Features, and Diagnosis. 2016. [Last accessed on 2016 Mar 09]. Available from: www.uptodate.com .
- 4.Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247–54. doi: 10.1111/j.1365-2133.1977.tb15179.x. [DOI] [PubMed] [Google Scholar]
- 5.Jain N, Doshi B, Khopkar U. Trichoscopy in alopecias: Diagnosis simplified. Int J Trichology. 2013;5:170–8. doi: 10.4103/0974-7753.130385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tosti A. Hair Shaft Disorders. Dermoscopy of Hair and Scalp: Pathological and Clinical Correlation. 2007. [Last accessed on 2019 Mar 08]. pp. 51–3. Available from: http://www.google.com/books?hl=tr&lr=&id=mQQABxyVWGUC&oi=fnd&pg=PP1&dq=tosti+A.+Dermoscopy+of+hair+and+scalp+disorders+with+clinical+and+pathological+correlations&ots=osEXxd_eeG&sig=QyQ5oxAh9In8hPupUXZFzY2wy7Q .
- 7.Tosti A, Duque-Estrada B. Dermoscopy in Hair Disorders. J Egypt Women Dermatol Soc. 2010;7:1–4. [Google Scholar]
- 8.Hu R, Xu F, Han Y, Sheng Y, Qi S, Miao Y, et al. Trichoscopic findings of androgenetic alopecia and their association with disease severity. J Dermatol. 2015;42:602–7. doi: 10.1111/1346-8138.12857. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Caulloo S, Zhao Y, Zhang B, Cai Z, Yang J, et al. Female pattern hair loss: Clinico-laboratory findings and trichoscopy depending on disease severity. Int J Trichology. 2012;4:23–8. doi: 10.4103/0974-7753.96082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo HG, Kim JS, Lee SR, Pyo HK, Moon HI, Lee JH, et al. Perifollicular fibrosis: Pathogenetic role in androgenetic alopecia. Biol Pharm Bull. 2006;29:1246–50. doi: 10.1248/bpb.29.1246. [DOI] [PubMed] [Google Scholar]
- 11.Grover S. A study of patterns of androgenetic alopecia in men: An Indian perspective. Br J Dermatol. 2005;152:572–4. doi: 10.1111/j.1365-2133.2005.06442.x. [DOI] [PubMed] [Google Scholar]
- 12.Krupa Shankar D, Chakravarthi M, Shilpakar R. Male androgenetic alopecia: Population-based study in 1,005 subjects. Int J Trichology. 2009;1:131–3. doi: 10.4103/0974-7753.58556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sehgal VN, Kak R, Aggarwal A, Srivastava G, Rajput P. Male pattern androgenetic alopecia in an Indian context: A perspective study. J Eur Acad Dermatol Venereol. 2007;21:473–9. doi: 10.1111/j.1468-3083.2006.01920.x. [DOI] [PubMed] [Google Scholar]
- 14.Paik JH, Yoon JB, Sim WY, Kim BS, Kim NI. The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol. 2001;145:95–9. doi: 10.1046/j.1365-2133.2001.04289.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang TL, Zhou C, Shen YW, Wang XY, Ding XL, Tian S, et al. Prevalence of androgenetic alopecia in china: A community-based study in six cities. Br J Dermatol. 2010;162:843–7. doi: 10.1111/j.1365-2133.2010.09640.x. [DOI] [PubMed] [Google Scholar]
- 16.Inui S, Nakajima T, Itami S. Scalp dermoscopy of androgenetic alopecia in Asian people. J Dermatol. 2009;36:82–5. doi: 10.1111/j.1346-8138.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 17.Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67:1040–8. doi: 10.1016/j.jaad.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Inui S. Trichoscopy for common hair loss diseases: Algorithmic method for diagnosis. J Dermatol. 2011;38:71–5. doi: 10.1111/j.1346-8138.2010.01119.x. [DOI] [PubMed] [Google Scholar]
- 19.Karadaǧ Köse Ö, Güleç AT. Clinical evaluation of alopecias using a handheld dermatoscope. J Am Acad Dermatol. 2012;67:206–14. doi: 10.1016/j.jaad.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Dermoscopy in female androgenic alopecia: Method standardization and diagnostic criteria. Int J Trichology. 2009;1:123–30. doi: 10.4103/0974-7753.58555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galliker NA, Trüeb RM. Value of trichoscopy versus trichogram for diagnosis of female androgenetic alopecia. Int J Trichology. 2012;4:19–22. doi: 10.4103/0974-7753.96080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deloche C, de Lacharrière O, Misciali C, Piraccini BM, Vincenzi C, Bastien P, et al. Histological features of peripilar signs associated with androgenetic alopecia. Arch Dermatol Res. 2004;295:422–8. doi: 10.1007/s00403-003-0447-y. [DOI] [PubMed] [Google Scholar]


