Abstract
Aim:
This study validated the RapidArc (RA) delivery using a volumetric ArcCHECK phantom as per the guidelines proposed in Task Group Report 119 from the American Association of Physicists in Medicine Task group 119 (AAPM TG 119). This study also investigated the impact of the Acuros XB (AXB) algorithm in comparison to analytical anisotropic algorithm (AAA) on the RA dose calculations in the homogeneous medium of the ArcCHECK phantom.
Materials and Methods:
A volumetric ArcCHECK phantom along with AAPM TG 119 tests was used to evaluate the RA plans and verify the dose delivery for photon beam of 6 MV energy.
Results:
The RA planning results were comparable and satisfied the planning criteria stated in the TG 119 report for all test cases. The average percentage gamma passing rates for the AAA-calculated plans were 98.5 (standard deviation [SD]: 0.6), 98.5 (SD: 1.3), and 98.1 (SD: 2.0) and for the AXB-calculated plans were 95.1 (SD: 1.8), 96.1 (SD: 1.3), and 94.0 (SD: 0.9) for the Clinac-iX (6 MV) and TrueBeam (TB)-STx (6 MV_filtered beam [FB] and 6 MV_flattening filter-free beam [FFFB]), respectively. For ion chamber measurements, the average percentage dose differences for the AAA-calculated plans were 1.5 (SD: 2.5), 2.7 (SD: 1.4), and 1.4(SD: 2.7) and for AXB-calculated plans were 2.3 (SD: 1.6), 3.2 (SD: 1.5), and 2.3 (SD: 2.0) for Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 MV_FFFB), respectively.
Conclusion:
Thus, the ArcCHECK can successfully be utilized for the validation of the RA delivery. The AXB has potential to perform dose calculations comparable to those of the AAA for RA plans in the homogeneous medium of the ArcCHECK phantom.
Keywords: American Association of Physicists in Medicine Task group 119, Acuros XB, ArcCHECK, RapidArc
INTRODUCTION
Volumetric modulated arc therapy (VMAT) is a novel technique proposed by Otto.[1] The concept of rotational therapy was initially explored by Brahme et al.[2] Later in 1995, Yu[3] proposed the clinical use of intensity modulated arc therapy (IMAT) with field shape modulated per gantry angle on the linear accelerator to produce conformal dose distribution. VMAT is the extension of the IMAT principle.
RapidArc (RA) radiation therapy is one of the most sophisticated technologies available in radiotherapy (RT) departments worldwide. It more precisely targets a cancerous tumor while sparing the healthy surrounding tissues. It also significantly reduces patient treatment times and radiation exposure. In the RA technique, the optimal dose distribution with efficient treatment delivery can be achieved with the interplay of various modulation parameters such as dose rate, gantry rotation speed, multileaf collimator (MLC) movement, and the number of control points.[1,4] However, owing to the physical limitations of hardware operation and misalignment between the dosimetric and mechanical components of the linear accelerator, dose discrepancies can occur in the radiation beam delivery.[5,6] Thus, it is desirable to employ appropriate and extensive quality assurance (QA) at the RA planning and delivery level, as errors at these stages may alter the treatment outcomes. However, it is tedious and laborious to perform RA QA at an individual level because of the various complex parameters involved.
Task group (TG) 119 of the American Association of Physicists in Medicine (AAPM) has proposed a guideline for IMRT commissioning and QA.[7] The goal of this report was to quantify the overall performance of the IMRT systems. The concept of the TG 119 has also been successfully utilized to quantify the overall performance of VMAT or RA delivery by Wen et al.,[8] Mynampati et al.,[9] and Nithya et al.[10]
The present study validated RA delivery using a volumetric ArcCHECK phantom as per the guidelines proposed in TG 119 report. This study also investigated the impact of the Acuros XB (AXB) algorithm on RA plan dose calculations in the homogeneous medium of the ArcCHECK phantom.
MATERIALS AND METHODS
The ArcCHECK (Model 1220, Sun Nuclear Corporation, Melbourne, Florida) is a three-dimensional (3D) beam dosimetry QA system intended for the measurement of RT dose distributions that are delivered as defined by a planning system and compared to the dose distribution as calculated by the planning system.[11] It is a cylindrical water-equivalent phantom with a 3D array of 1386 diode detectors arranged in a spiral pattern with 10-mm sensor spacing. The active area of each detector is 0.8 mm × 0.8 mm, and the sensitivity is 0.4 nC/Gy. There are no time or dose limits for measurements using the ArcCHECK phantom. A temperature sensor measures the ambient temperature of the detector area. Dose measurements from each sensor are updated every 50 milli seconds. The ArcCHECK is also equipped with two inclinometers, to help measuring the angle of rotation about the cylinder axis as well as the tilt of the axis. The center of the phantom (15 cm diameter) is designed to accommodate various accessories such as a solid homogeneous core, a dosimetric core with ion chamber(s) or diode arrays, an imaging QA core, and a core with heterogeneous materials for dose studies.[12]
For point dose verification, a CC 13S (IBA Dosimetry, Germany) ion chamber with a Dose 1 (IBA Dosimetry, Germany) electrometer was used for absolute point dose measurements in conjunction with the ArcCHECK. The ion chamber was placed in the central core of the ArcCHECK at the isocenter of the linear accelerator. Diodes were kept 2.9 cm below the surface of the ArcCHECK; this thickness of the buildup material is equivalent to 3.3 cm of water. Dose fluence maps were acquired at 89.6 cm, i.e., the diode level, and measured in cumulative mode. Dose fluence maps and absolute point dose measurements were performed simultaneously. The ion chamber readings were corrected for temperature and pressure variation. SNC patient software version 6.2 (Sun Nuclear Corporation, Melbourne, Florida) was used to compare the calculated and measured doses.
For analysis purposes, the gamma criteria of 3 mm distance to agreement and 3% dose difference (DD) were used for the evaluation of the RA plans, with a threshold value 10%. A gamma passing rate of 90% and above was considered acceptable.[13] A tolerance limit of ± 5% was considered acceptable for ion chamber measurements in comparison to the treatment planning system (TPS) calculations.
The AAPM TG 119 computed tomography (CT) and radiotherapy (RT) structure sets are available at http://www.aapm.ogr/pubs/tg119/default.asp. The RT structures were imported into Eclipse TPS. Thereafter, these DICOM RT structures were fused and registered on the CT images of a homogeneous ArcCHECK phantom provided by Sun Nuclear. In this study, the following test cases were used for planning and delivery verification: multi-target, prostate, head and neck, C-shape (easy), and C-shape (hard). Full descriptions of these structures are available in the AAPM TG 119 report.
Varian linacs, namely, TrueBeam (TB)-STx (60 leaf pairs high-definition [HD] MLC: inner 32 leaf pairs 0.25 cm and outer 28 leaf pairs 0.5 cm resolution at isocenter) and a Clinac-iX (2300CD) (60 leaf pairs millennium MLC: inner 40 leaf pairs 0.5 cm and outer 20 leaf pairs 1.0 cm resolution at isocenter) were used for this study. Eclipse TPS version 11 (Varian Medical System, Palo Alto, USA) was used for RA planning. Aria version 11 (Varian Medical System, Palo Alto, USA) was used for the record and verification system. A filtered beam (FB) of 6 MV from the Clinac-iX linac machine and FB and flattening filter-free beam (FFFB) 6 MV from the TB-STx were used for planning purposes. The dose rates were 600 MU (6 MV_FB) and 1200 MU (6 MV_FFFB) per minute, respectively. Linac calibration was performed under the reference conditions prescribed by Technical Series Report no. 398 recommended by the International Atomic Energy Agency.[14] The linac was calibrated at 1 cGy per MU for a reference source-to-skin distance of 100 cm at the dmax depth for their respective photon beams (Clinac-iX: 6 MV and TB: 6 MV_FB and 6 MV_FFF).
RA planning was performed for all mentioned test sites as prescribed in the AAPM TG 119 report. All treatment plans and dose calculations were performed with Eclipse TPS version 11 for both linacs. The RA plans were optimized using the progressive resolution optimizer 3 algorithm (PRO3, second generation), and the concept of PRO was described by Otto.[1] In this algorithm, the full arc is progressively optimized at 178 control points in four phases. At every iteration level, it optimizes the MLC position and monitor unit (MU) weight within the limitations (MLC speed, gantry speed, dose rate, and mechanical limits) of the delivery unit.[15] During optimization, dose calculation is performed with a simplified multi-resolution dose calculation algorithm. To mitigate leakage due to the tongue and groove effect, the collimator was rotated to 30° from the nominal angle. The final dose calculations were carried out using the analytical anisotropic algorithm (AAA) with a dose grid resolution of 1.5 mm × 1.5 mm × 1.5 mm. Keeping the number of MUs the same, the plans were also recalculated using the AXB algorithm for comparison with the AAA-calculated results. The ArcCHECK phantom was assigned as poly (methyl methacrylate) (PMMA) material with mass density of 1.160 g/cm3 and assigned CT value was 231 Hounsfield unit (HU).
Evaluation parameters
Each RA plan was evaluated with respect to the AAPM TG 119 planning criteria. Apart from the AAPM TG 119 criteria, for intercomparison, these results were also evaluated with respect to MLC type, beam type, and dose calculation algorithm type. The conformity index (CI) was calculated as the ratio between the volume covered by the prescribed isodose and the target volume.[16] The prescription doses (PD) for the C-shape, head and neck, multi-target, and prostate test cases were 50, 50, 50, and 76 Gy, respectively. Similarly, the homogeneity index (HI) was calculated as the DD normalized to the dose prescribed between doses covering 5% (D5) and 95% (D95) of the target.[16]
Further, the dose-spillage volumes were computed to assess the normal tissue sparing outside the target volume. The following virtual structures were assessed for the different dose spillage volumes: (a) high-dose spillage volume (VHS) taking into the account of normal tissue receiving the >90% of the PD, (b) intermediate-dose spillage volume (VIS) taking into account of normal tissue receiving >50% of the PD, (c) low-dose spillage volume (VLS) taking into account of normal tissue receiving >25% of the PD. Finally, the treatment efficiency of each RA plan was calculated as a ratio of the cumulative sum of MUs per fraction to the dose per fraction. A two-sample paired t-test was performed to find the statistical significance of these results. A P-value < 0.05 was considered statistically significant with 95% confidence limit.
RESULTS
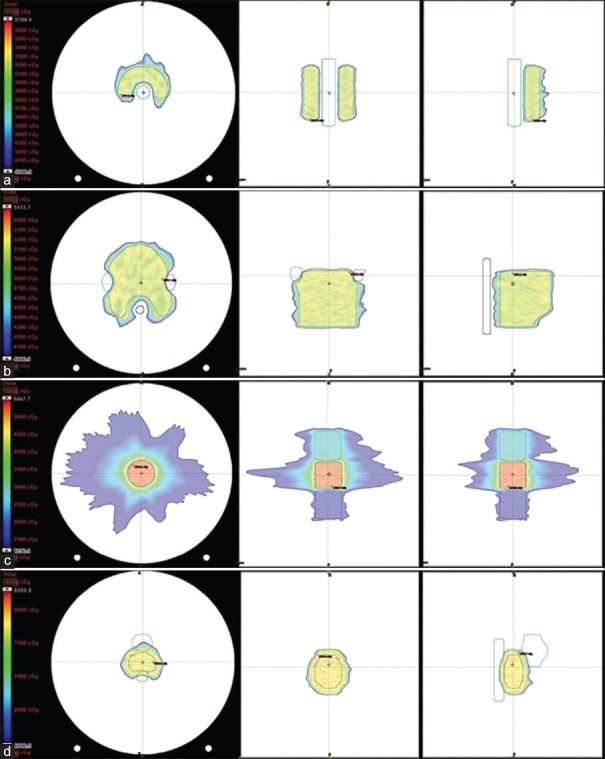
Treatment planning benchmarking is necessary for RT institutes to investigate their planning ability and to facilitate a review of the accuracy of TPSs under local relevant conditions. Table 1 shows the planning results for all test cases: C-shape (easy), C-shape (hard), head and neck, multi-target, and prostate, with planning goals and their respective results reported in the AAPM TG 119 report. The RA plan results were comparable to those of the AAPM TG 119 report and satisfied or exceeded all planning criteria stated in the AAPM TG 119 for all test cases. RA plans were optimized using couch modeling, a set of HUs valid for low and high energy, and the entire couch length was used as couch surface HU: −300 and couch interior HU: −1000. Figure 1 presents the test structures on an ArcCHECK phantom with the planned dose for the RA plans.
Table 1.
RapidArc planning on an ArcCHECK phantom for all structures in comparison to the American Association of Physicists in Medicine task group 119 report for the Clinac-iX (6 MV) and TB-STx (6 MV_filtered beam and 6 MV flattening filter-free beam), respectively
| Parameters | Plan goal (cGy) | Clinac-iX | TB-STx | TG 119 6 MV Mean±SD |
||||
|---|---|---|---|---|---|---|---|---|
| 6MV FB | 6MV FB | 6 MV FFFB | ||||||
| AAA | AXB | AAA | AXB | AAA | AXB | |||
| C Shape (easier) | ||||||||
| PTV D95 | 5000 | 5001.4 | 4951.8 | 5003.8 | 4939.3 | 5005.1 | 4895.5 | 5010±17 |
| PTV D10 | <5500 | 5299.2 | 5260.5 | 5243.9 | 5170.5 | 5241.1 | 5132.7 | 5440±52 |
| CORE D10 | <1000 | 2271.1 | 2229.1 | 2088.6 | 1932.2 | 2131.2 | 1963.9 | 2200±314 |
| C Shape (hard) | ||||||||
| PTV D95 | 5000 | 5003.2 | 4950.7 | 5000.5 | 4939.7 | 5000.5 | 4907.1 | 5011±16.5 |
| PTV D10 | <5500 | 5362.3 | 5326.1 | 5310.9 | 5237.7 | 5310.0 | 5212.2 | 5702±220 |
| CORE D10 | <1000 | 1715.3 | 1648.7 | 1418.3 | 1322.6 | 1447.9 | 1300.5 | 1630±307 |
| H and N | ||||||||
| PTV D90 | 5000 | 5001.8 | 4963.2 | 5001.4 | 4915.7 | 5003.3 | 4895.0 | 5028±58 |
| PTV D99 | >4650 | 4846.7 | 4786.5 | 4849.6 | 4780.3 | 4854.2 | 4761.9 | 4704±52 |
| PTV D20 | <5500 | 5143.5 | 5096.5 | 5143.2 | 5053.9 | 5150.2 | 5041.5 | 5299±93 |
| Cord (dmax) | <4000 | 3408.3 | 3359.4 | 3417.2 | 3250.8 | 3487.6 | 3297.1 | 5741±250 |
| Rt Parotid D50 | <2000 | 1769.6 | 1754.3 | 1744.9 | 1638.1 | 1749.8 | 1655.1 | 1798±184 |
| Lt Parotid D50 | <2000 | 1860.9 | 1819.8 | 1723.4 | 1612.2 | 1715.9 | 1613.3 | 1798±184 |
| Multi-target | ||||||||
| Central D99 | >5000 | 4897.9 | 4759.2 | 4977.9 | 4852.1 | 4979.3 | 4808.6 | 4955±162 |
| Central D10 | <5300 | 5296.3 | 5303.1 | 5287.7 | 5285.7 | 5296.1 | 5239.4 | 5455±173 |
| Superior D99 | >2500 | 2573.3 | 2570.1 | 2598.9 | 2530.2 | 2591.5 | 2497.8 | 2516±85 |
| Superior D10 | <3500 | 2910.5 | 2892.1 | 2935.1 | 2867.9 | 2912.6 | 2869.3 | 3412±304 |
| Inferior D99 | >1250 | 1320.7 | 1286.5 | 1295.5 | 1218.9 | 1312.7 | 1252.7 | 1407±185 |
| Inferior D10 | <2500 | 1680.1 | 1649.9 | 1666.4 | 1620.8 | 1669.4 | 1606.1 | 2418±272 |
| Prostate | ||||||||
| Prostate D95 | >7560 | 7628.3 | 7531.9 | 7584.1 | 7508.8 | 7571.9 | 7467.8 | 7566±21 |
| Prostate D5 | <8300 | 8107.5 | 8086.1 | 7993.9 | 7905.3 | 7982.3 | 7861.4 | 8143±156 |
| Rectum D30 | <7000 | 4890.3 | 4810.8 | 4661.8 | 4509.6 | 4738.5 | 4572.1 | 6536±297 |
| Rectum D10 | <7500 | 7437.7 | 7247.3 | 7383.9 | 7244.5 | 7375.2 | 7207.9 | 7303±150 |
| Bladder D30 | <7000 | 2944.9 | 2880.1 | 2760.3 | 2646.3 | 2781.3 | 2669.2 | 4304±878 |
| Bladder D10 | <7500 | 5096.4 | 5045.5 | 4857.3 | 4751.8 | 4685.1 | 4563.1 | 6269±815 |
AAA: Analytical anisotropic algorithm, AXB: Acuros XB, FFF: Flattening filter-free, SD: Standard deviation, TB: TrueBeam
Figure 1.
Structures (a) C-shape, (b) head and neck, (c) multi-target, and (d) prostate on an ArcCHECK phantom with planned doses for RapidArc planning in transverse, frontal, and sagittal planes
Analytical anisotropic algorithm-calculated plans
For the C-shape test cases in Table 1, the PTV D95 and PTV D10 for the Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 MV_FFFB) were comparable to the AAPM TG 119 results. The core D10 results were not achieved in the C-shape (hard) test cases. However, these results were comparable to the AAPM TG 119, which also reported higher values. Figures 2 and 3 show the dose-volume histogram (DVH) comparison for C-shape (easy) and C-shape (hard) test cases from RA plans calculated using the AAA and AXB algorithms for the Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 MV_FFFB), respectively.
Figure 2.
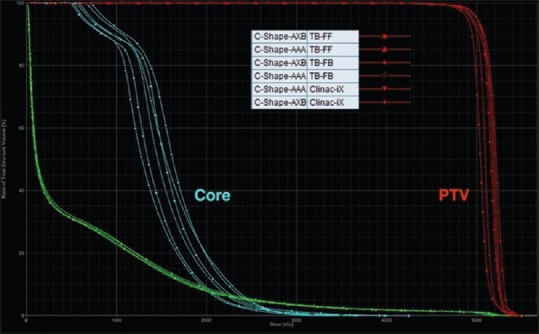
Dose-volume histogram comparisons of the C-shape (easy) case from RapidArc plans calculated using the analytical anisotropic algorithm and Acuros XB algorithm for the Clinac-iX (6 MV) and TrueBeam-STx (6 MV_filtered beam and 6 MV_flattening filter-free beam), respectively
Figure 3.
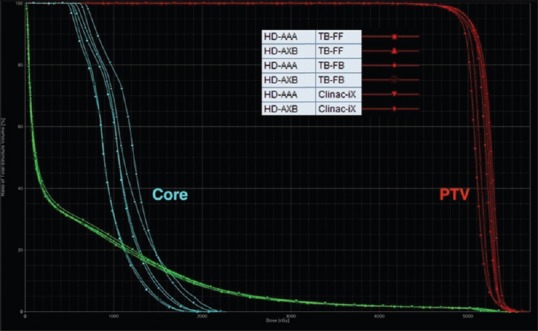
Dose-volume histogram comparisons of the C-shape (hard) case from RapidArc plans calculated using the analytical anisotropic algorithm and Acuros XB algorithm for the Clinac-iX (6 MV) and TrueBeam-STx (6 MV_filtered beam and 6 MV_flattening filter-free beam), respectively
For the head-and-neck test case, the target coverage PTV D90 was comparable to the AAPM TG 119 for all plans. Cord (dmax) and parotid D50 also satisfied the planning criteria stated in the AAPM TG 119 report for all RA plans. Figure 4 presents the DVH comparison for the head-and-neck test case from the RA plans calculated using the AAA and AXB algorithm for the Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 MV_FFFB), respectively.
Figure 4.
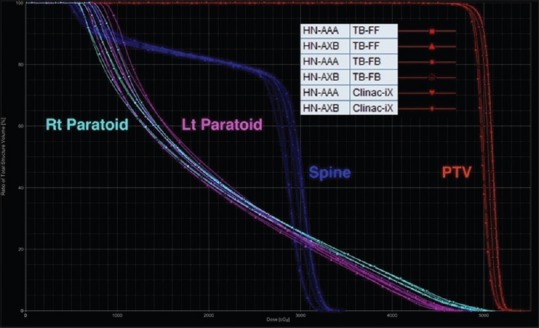
Dose-volume histogram comparisons of the head-and-neck case from RapidArc plans calculated using analytical anisotropic algorithm and Acuros XB algorithm for the Clinac-iX (6 MV) and TrueBeam-STx (6 MV_filtered beam and 6 MV_flattening filter-free beam), respectively
For the multi-target test case in Table 1, all plans achieved the planning goals and were comparable to the AAPM TG 119 except for the central D99, which was slightly lower than the AAPM TG 119 but comparable to the standard deviation (SD) in TG 119 results (1.62 Gy). Figure 5 shows the DVH comparison for the multi-target test case from RA plans calculated using the AAA and AXB algorithms for the Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 MV_FFFB), respectively.
Figure 5.
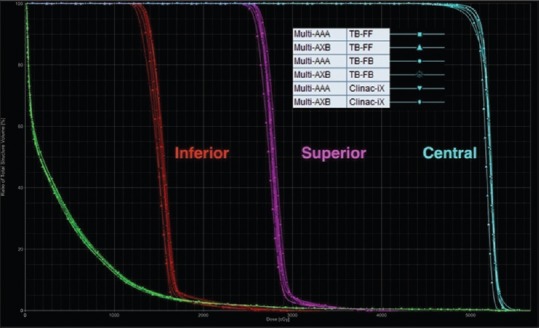
Dose-volume histogram comparisons of the multi-target case from RapidArc plans calculated using analytical anisotropic algorithm and Acuros XB algorithm for the Clinac-iX (6 MV) and TrueBeam-STx (6 MV_filtered beam and 6 MV_flattening filter-free beam), respectively
For the prostate case in Table 1, the target coverage was comparable to those of the AAPM TG 119 results for all plans. Bladder dose (D30 and D10) and rectum dose (D30) were lower than those of the AAPM TG 119. The rectum dose D10 was comparable to that of the AAPM TG 119. Figure 6 shows the DVH comparison for the prostate test case from the RA plans calculated using AAA and AXB algorithm for the Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 MV_FFFB), respectively.
Figure 6.
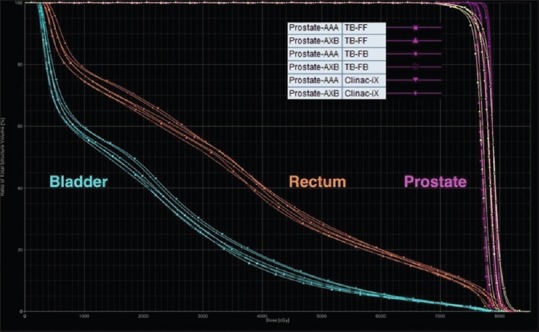
Dose-volume histogram comparisons of the prostate case from RapidArc plans calculated using analytical anisotropic algorithm and Acuros XB algorithm for the Clinac-iX (6MV) and TrueBeam-STx (6MV_ filtered beam and 6MV_flattening filter-free beam), respectively
Acuros XB-calculated plans
This study also investigated the impact of the AXB dose calculation algorithm in RA treatment delivery. The results calculated with the AXB algorithm are shown in Table 1 in comparison to the AAA-calculated results. The AXB showed a lower dose to target structures by average percentage DDs of 1.05 (SD: 0.83), 1.89 (SD: 1.32), and 2.33 (SD: 1.02) for the Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 MV_FFFB), respectively. Similarly, the AXB showed a lower dose to the organ-at-risk (OARs) structures by average percentage DDs of 0.39 (SD: 2.28), 4.79 (SD: 2.05), and 5.26 (SD: 2.55) for the Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 FFFB), respectively.
Tables 2 and 3 show the average CI and HI for RA plans calculated using the AAA and AXB algorithms, respectively. The average CIs were 1.010 (SD: 0.055), 1.011 (SD: 0.057), and 1.013 (SD: 0.058), and the average HIs were 0.061 (SD: 0.011), 0.052 (SD: 0.010), and 0.056 (SD: 0.011) using the AAA calculation for the Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 MV_FFFB), respectively. The average CIs were 0.966 (SD: 0.020), 0.878 (SD: 0.221), and 0.791 (SD: 0.234) and the average HIs were 0.066 (SD: 0.013), 0.053 (SD: 0.008), and 0.059 (SD: 0.013) using the AXB algorithm calculation for the Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 FFFB), respectively. The calculated P-values for CI and HI were > 0.05 for the AAA and AXB-calculated plans, which were not statistically significant.
Table 2.
Conformity index for the task group 119 test cases from the analytical anisotropic algorithm and Acuros XB algorithm-calculated RapidArc plans for the Clinac-iX (6 MV) and TB-STx (6MV_filtered beam and 6MV_flattening filter-free beam), respectively
| Structure | CI | |||||
|---|---|---|---|---|---|---|
| Clinac-iX (6MV FB) | TB-STx (6MV FB) | TB-STx (6MV FFFB) | ||||
| AAA | AXB | AAA | AXB | AAA | AXB | |
| C Shape (easier) | 1.037 | 0.970 | 1.035 | 0.953 | 1.033 | 0.785 |
| C Shape (hard) | 1.055 | 0.997 | 1.046 | 0.974 | 1.049 | 0.911 |
| H and N | 0.923 | 0.955 | 0.929 | 0.492 | 0.935 | 0.399 |
| Multi-target | 1.045 | 0.963 | 1.069 | 1.048 | 1.077 | 1.010 |
| Prostate | 0.989 | 0.943 | 0.977 | 0.923 | 0.971 | 0.851 |
| Mean±SD | 1.010±0.055 | 0.966±0.020 | 1.011±0.057 | 0.878±0.221 | 1.013±0.058 | 0.791±0.234 |
TB: TrueBeam, FB: Filtered beam, FFFB: Flattening filter-free beam, CI: Conformity index, AAA: Analytical anisotropic algorithm, AXB: Acuros XB, SD: Standard deviation
Table 3.
Homogeneity index for task group 119 test cases from the analytical anisotropic algorithm and Acuros XB algorithm-calculated RapidArc plans for the Clinac-iX (6 MV) and TB-STx (6 MV_ filtered beam and 6 MV_ flattening filter-free beam), respectively
| Structure | HI | |||||
|---|---|---|---|---|---|---|
| Clinac-iX (6MV FB) | TB-STx (6MV FB) | TB-STx (6MV FFFB) | ||||
| AAA | AXB | AAA | AXB | AAA | AXB | |
| C shape (easier) | 0.064 | 0.065 | 0.052 | 0.052 | 0.054 | 0.057 |
| C shape (hard) | 0.076 | 0.079 | 0.067 | 0.067 | 0.070 | 0.072 |
| H and N | 0.046 | 0.046 | 0.042 | 0.046 | 0.047 | 0.048 |
| Multi-target | 0.055 | 0.069 | 0.044 | 0.047 | 0.045 | 0.047 |
| Prostate | 0.063 | 0.073 | 0.053 | 0.052 | 0.063 | 0.073 |
| Mean±SD | 0.061±0.011 | 0.066±0.013 | 0.052±0.010 | 0.053±0.008 | 0.056±0.011 | 0.059±0.013 |
HI: Homogeneity index, TB: TrueBeam, FB: Filtered beam, FFFB: Flattening filter-free beam, AAA: Analytical anisotropic algorithm, AXB: Acuros XB, SD: Standard deviation
Millennium versus high-definition multileaf collimator analysis
This study also investigated the impact of the different type of multileaf collimator (MLC) used in RA planning for photon beams of 6 MV energy generated using Clinac-iX and TB-STx, respectively. The results for these MLC systems are shown in Table 1 for AAA and AXB calculation. For AAA calculations, the average percentage DDs found in target coverage were 0.22 (SD: 0.94) and for OARs DD was 5.58 (SD: 5.30) between millennium and HD-MLC, respectively. Similarly, for AXB calculations, the average percentage DDs found in target coverage was 1.07 (SD: 1.54) and for OARs DD was 8.29 (SD: 5.85) between millennium and HD-MLC, respectively. There was no significant difference (P > 0.05) found in CI for RA planning using millennium and HD-MLC systems for AAA and AXB dose calculation algorithms, respectively. However, there was significant difference (P < 0.05) found in HI for RA planning using millennium and HD-MLC systems for AAA and AXB dose calculation algorithms, respectively. In overall, HD-MLC produces comparable CI and superior HI in comparison to millennium MLC system.
Filtered beam versus flattening filter-free beam analysis
In addition, this study investigated the impact of the different type of photon beams of 6 MV energy generated using TB-STx in flattening filter and flattening filter-free mode, respectively. The results for these different beam types are shown in Table 1 for AAA and AXB calculation. For AAA calculations, the average percentage DDs found in target coverage was 0.04 (SD: 0.43) and for OARs DD was 0.53 (SD: 1.82) between FB and FFFB, respectively. Similarly, for AXB calculations, the average percentage DDs found in target coverage was 0.40 (SD: 0.94) and for OARs DD was 0.03 (SD: 1.85) between FB and FFFB, respectively. There was no significant difference (P > 0.05) found in CI and HI using FB and FFFB of 6 MV energy for AAA and AXB dose calculation algorithms, respectively, except CI (P < 0.05) for AXB calculation.
Dose spillage volume analysis
Dose spillage volumes normalized to target volume are shown in Table 4, along with their P values comparing for different MLC and beam type using AAA, AXB calculation for Clinac-iX (6 MV), and TB-STx (6 FB and 6 FFFB), respectively. There was significant difference (P < 0.05) found in VHS, VIS, and VLS for the millennium and HD-MLC system using AAA and AXB calculation, respectively. For AAA calculations, there was no significant difference (P > 0.05) found in dose spillage volume VHS and VIS, except VLS (P < 0.05) between millennium and HD-MLC system for 6 MV photon beam. For AXB calculations, there was significant difference (P < 0.05) found in dose-spillage volume VHS, VIS, and VLS between millennium and HD-MLC system for 6 MV photon beam. There was no significant difference (P > 0.05) found in dose spillage volume VHS, VIS, and VLS between FB and FFFB for both algorithm calculations.
Table 4.
Dose-spillage volumes normalized to target volume, along with their P values comparing for different multileaf collimator and beam type using analytical anisotropic algorithm, Acuros XB calculation for Clinac-iX (6 MV) and TB-STx (6MV_filtered beam and 6MV_falttening filter-free beam), respectively
| Dose spillage volume | Millennium-120 (FB) | P | HD-120 (FB) | P | HD-120 (FFFB) | P | AAA P Mill versus HD |
AXB P Mill versus HD |
AAA P FB versus FFFB |
AXB P FB versus FFFB |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AAA | AXB | AAA | AXB | AAA | AXB | ||||||||
| VHS | 0.11±0.05 | 0.09±0.04 | 0.004 | 0.11±0.06 | 0.08±0.04 | 0.028 | 0.10±0.06 | 0.06±0.04 | 0.007 | 0.828 | 0.034 | 0.485 | 0.327 |
| VIS | 2.54±1.03 | 2.44±0.99 | 0.005 | 2.46±1.09 | 2.27±0.99 | 0.010 | 2.44±1.08 | 2.22±0.98 | 0.011 | 0.171 | 0.021 | 0.653 | 0.357 |
| VLS | 12.91±4.51 | 11.84±4.34 | 0.019 | 11.53±4.63 | 10.95±4.36 | 0.016 | 11.61±4.59 | 10.97±4.36 | 0.019 | 0.026 | 0.010 | 0.535 | 0.904 |
FB: Filtered beam, FFFB: Flattening filter-free beam, AAA: Analytical anisotropic algorithm, AXB: Acuros XB, HD: High definition
Treatment efficiency
For the evaluation of the treatment efficiency of RA plans for filtered and flatting filter-free photon beams of 6MV using millennium and HD-MLC system, the numbers of MUs per dose fraction were also studied. The MUs were 4.30 ± 1.54, 4.32 ± 1.62, and 5.00 ± 1.90 for Clinac-iX (Millennium, 6 MV) and TB-STx (HD, 6 MV_FB and 6 MV_FFFB), respectively. There was no significant difference found in MU per dose fraction for FB of 6 MV energy generated using Clinac-iX (Millennium) and TB-STx (HD), respectively. On the other side, there was a significant increase in MU per dose fraction for FFFB of 6 MV energy over FB of 6 MV energy generated using TB-STx (HD), respectively.
Gamma and dose difference evaluation
Tables 5 and 6 show the results of the gamma evaluation and ion chamber measurement for all RA plans, respectively. The average percentage gamma passing rates for the AAA-calculated plans were 98.5 (SD: 0.6), 98.5 (SD: 1.3), and 98.1 (SD: 2.0) for the Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 MV_FFFB), respectively. The average percentage gamma passing rates for the AXB-calculated plans were 95.1 (SD: 1.8), 96.1 (SD: 1.3), and 94.0 (SD: 0.9) for the Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 MV_FFFB), respectively. For ion chamber measurements, the average percentage DDs for the AAA-calculated plans were 1.5 (SD: 2.5), 2.7 (SD: 1.4), and 1.4(SD: 2.7) for the Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 MV_FFFB), respectively. The average percentage DDs for the AXB-calculated plans were 2.3 (SD: 1.6), 3.2 (SD: 1.5), and 2.3 (SD: 2.0) for the Clinac-iX (6 MV) and TB-STx (6 MV_FB and 6 MV_FFFB), respectively.
Table 5.
Gamma analysis of the task group 119 test cases from the analytical anisotropic algorithm and Acuros XB algorithm-calculated RapidArc plans for the Clinac-iX (6 MV) and TB-STx (6MV_filtered beam and 6MV_flattening filter-free beam), respectively
| Structure | Gamma (3%/3mm TH: 10%) | |||||
|---|---|---|---|---|---|---|
| Clinac-iX (6MV FB) | TB-STx (6MV FB) | TB-STx (6MV FFFB) | ||||
| AAA | AXB | AAA | AXB | AAA | AXB | |
| C shape (easier) | 98.1 | 93.9 | 98.1 | 95.1 | 98.6 | 93.2 |
| C shape (hard) | 98.5 | 96.6 | 98.7 | 96.4 | 99.2 | 95.3 |
| H and N | 99.1 | 93.5 | 99.9 | 98.3 | 99.5 | 94.4 |
| Multi-target | 97.7 | 94.1 | 96.6 | 95.4 | 94.6 | 93.5 |
| Prostate | 99.0 | 97.4 | 99.4 | 95.2 | 98.8 | 93.4 |
| Mean±SD | 98.5±0.6 | 95.1±1.8 | 98.5±1.3 | 96.1±1.3 | 98.1±2.0 | 94.0±0.9 |
TH: Threshold, FB: Filtered beam, FFF: Flattening filter free, AAA: Analytical anisotropic algorithm, AXB: Acuros XB, SD: Standard deviation
Table 6.
Ion chamber measurements in comparison to the treatment planning system-calculated dose at the ArcCHECK isocenter for the task group 119 test cases from the analytical anisotropic algorithm and Acuros XB algorithm-calculated RapidArc plans for the Clinac-iX (6 MV) and TB-STx (6MV_filtered beam and 6MV_flattening filter-free beam), respectively
| Structure | IC versus TPS calculation (% difference) | |||||
|---|---|---|---|---|---|---|
| Clinac-iX (6MV FB) | TB-STx (6MV FB) | TB-STx (6MV FFFB) | ||||
| AAA | AXB | AAA | AXB | AAA | AXB | |
| C shape (easier) | 3.4 | 4.7 | 4.1 | 4.6 | 4.4 | 4.9 |
| C shape (hard) | −2.7 | 2.5 | 2.2 | 2.3 | 3.2 | 3.6 |
| H and N | 2.4 | 2.6 | 1.6 | 3.5 | −0.7 | 1.7 |
| Multitarget | 1.4 | 1.2 | 1.3 | 1.1 | −2.0 | −0.3 |
| Prostate | 2.8 | 0.5 | 4.1 | 4.6 | 2.0 | 1.9 |
| Mean±SD | 1.5±2.5 | 2.3±1.6 | 2.7±1.4 | 3.2±1.5 | 1.4±2.7 | 2.3±2.0 |
TPS: Treatment planning system, IC: Ion Chamber, TB: TrueBeam, FFF: Flattening filter free, AAA: Analytical anisotropic algorithm, AXB: Acuros XB, SD: Standard deviation
DISCUSSION
This study evaluated the RA treatment delivery on a volumetric ArcCHECK phantom as per AAPM TG 119 recommendations and also investigated the impact of the AXB algorithm on RA plan calculations and delivery. The RA planning results for both algorithms satisfied the AAPM TG 119 criteria. Avgousti et al.[17] also reported similar findings using a volumetric Delta4 phantom device for the evaluation of IMRT delivery as per AAPM TG 119. Ezzell et al.,[7] Mynampati et al.,[9] and Kaushik et al.[18] also reported similar results based on the measurements on the rectangular slab geometries. The results showed that both algorithms were comparable in homogeneous medium of ArcCHECK phantom due to the same multiple source model used in the configuration phase of these algorithms. Hence, this substantiates the proper configuration and implementation of AXB in the TPS.[19] This study reveals that the AXB plans had a lower dose to the target and OARs as compared to those of the AAA-calculated plans. Fogliata et al.[20] reported that the AAA predicts an average 1.6% higher dose in the muscles for 6 MV in 3D-conformal planning as compared to the AXB. In another study, Fogliata et al.[21] reported in soft tissues that the mean PTV dose was lower for the AXB, with a range of 0.4% ± 0.6% for IMRT and 1.3% ± 0.2% for RA for a 6MV photon beam. Similar findings were also reported by Rana et al.[22] This study also revealed that HD-MLC system spares more OARs and has lower spillage volumes outside the target comparing to the millennium-MLC system for similar target coverage. This is due to the difference in leaf width at isocenter, source to MLC distance, and leaf transmission-and-leakage. For an MLC system, leaf transmission-and-leakage also depends on leaf material, leaf height, and tongue-and-groove effect of MLC. Tanyi et al.[23] had reported that HD-MLC spares the normal tissue and reduces the dose-spillage volumes outside the target in comparison to millennium-MLC system. This study reveals that flattening filter-free photon beam has significantly inferior treatment efficiency in terms of MUs comparing to filtered photon beam for similar target coverage. This is due to the forward peak nature of flattening filter-free photon beam, as the intensity of FFF beam abruptly decreases with off-axis distance for field sizes larger than and equal to 10 cm × 10 cm, which requires the off-axis distance-dependence intensity modulation of FFF photon beam. This necessitates large number of MUs to deliver uniform dose to the target.[24,25]
This study showed that the AXB-calculated plans had lower gamma passing rates as compared to those of the AAA-calculated plans. Regarding the calculation algorithm, in a multicenter audit of VMAT planning and pretreatment verification, Jurado-Bruggeman et al.[26] were unable to correlate the results of gamma passing rates with the algorithm itself or the software version. This could be influenced by various unidentified causes including the pre-measurement absolute dose calibration of ArcCHECK based on the TPS-calculated dose. Ion chamber measurements can also introduce bias, as the ion chamber was calibrated in a water medium, and measurements were made inside the PMMA cavity. Furthermore, there are differences in the dose computation approaches used in these algorithms. The AXB calculation is sensitive to medium composition and characterization as it calculates radiation transport in medium. In contrast, the AAA models the medium as water of different densities.
The AAPM TG 119 tests performed with a volumetric ArcCHECK phantom are useful for the validation of RA treatment planning and delivery. The TPS, the dose delivery system, and the dose measurement process are the main sources of deviation between the planned and measured doses. TPS is a major source of deviation, contributing approximately 50% of the total deviations between planned and measured results.[7,27] McVicker et al.[28] investigated the sensitivity of the TG 119 in finding the error involved in any stage of commissioning by creating an additional beam model in the TPS by introducing intentional errors in the original beam model. They concluded that the TG 119 commissioning criteria are effective in detecting errors. The European Society for Therapeutic Radiation Oncology guidelines[29] on the verification of IMRT have recommended that “more information is urgently required about the accuracy of IMRT treatment delivery by conducting independent audit or inter-comparison programmes.” Similar recommendation has been reported by other publications also.[27,30,31,32] This may reveal unidentified errors in local treatment and delivery systems and hence lead to improved quality of treatment.
The limitation of the present study was that the ArcCHECK phantom is a homogeneous phantom; thus, the algorithm's ability to correctly model heterogeneities, which is more desirable for clinical environments, could not be assessed.
CONCLUSION
The AAPM TG 119 test cases were successfully applied on an ArcCHECK phantom. The ArcCHECK phantom has been proven as an easy, quick, and reliable system for RA delivery verification following the TG 119 recommendations. The AXB has potential to perform dose calculations comparable to those of the AAA for RA plans in the homogeneous medium of the ArcCHECK phantom. Therefore, the AAPM TG 119 report can be used as an effective tool for the quick evaluation of RA planning and delivery systems.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank the management of Rajiv Gandhi Cancer Institute and Research Centre, New Delhi, India, for their continued support and encouragement to complete this research work.
REFERENCES
- 1.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–7. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 2.Brahme A, Roos JE, Lax I. Solution of an integral equation encountered in rotation therapy. Phys Med Biol. 1982;27:1221–9. doi: 10.1088/0031-9155/27/10/002. [DOI] [PubMed] [Google Scholar]
- 3.Yu CX. Intensity-modulated arc therapy with dynamic multileaf collimation: An alternative to tomotherapy. Phys Med Biol. 1995;40:1435–49. doi: 10.1088/0031-9155/40/9/004. [DOI] [PubMed] [Google Scholar]
- 4.Bortfeld T, Webb S. Single-arc IMRT? Phys Med Biol. 2009;54:N9–20. doi: 10.1088/0031-9155/54/1/N02. [DOI] [PubMed] [Google Scholar]
- 5.Rangaraj D, Oddiraju S, Sun B, Santanam L, Yang D, Goddu S, et al. Fundamental properties of the delivery of volumetric modulated arc therapy (VMAT) to static patient anatomy. Med Phys. 2010;37:4056–67. doi: 10.1118/1.3453575. [DOI] [PubMed] [Google Scholar]
- 6.Han Z, Ng SK, Bhagwat MS, Lyatskaya Y, Zygmanski P. Evaluation of MatriXX for IMRT and VMAT dose verifications in peripheral dose regions. Med Phys. 2010;37:3704–14. doi: 10.1118/1.3455707. [DOI] [PubMed] [Google Scholar]
- 7.Ezzell GA, Burmeister JW, Dogan N, LoSasso TJ, Mechalakos JG, Mihailidis D, et al. IMRT commissioning: Multiple institution planning and dosimetry comparisons, a report from AAPM Task Group 119. Med Phys. 2009;36:5359–73. doi: 10.1118/1.3238104. [DOI] [PubMed] [Google Scholar]
- 8.Wen N, Zhao B, Kim J, Chin-Snyder K, Bellon M, Glide-Hurst C, et al. IMRT and RapidArc commissioning of a TrueBeam linear accelerator using TG-119 protocol cases. J Appl Clin Med Phys. 2014;15:4843. doi: 10.1120/jacmp.v15i5.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mynampati DK, Yaparpalvi R, Hong L, Kuo HC, Mah D. Application of AAPM TG 119 to volumetric arc therapy (VMAT) J Appl Clin Med Phys. 2012;13:3382. doi: 10.1120/jacmp.v13i5.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nithya L, Raj NA, Rathinamuthu S, Pandey MB. Analyzing the performance of the planning system by use of AAPM TG 119 test cases. Radiol Phys Technol. 2016;9:22–9. doi: 10.1007/s12194-015-0328-z. [DOI] [PubMed] [Google Scholar]
- 11.ArcCHECKTM user's guide: The ultimate 4D QA solution. [Last accessed on 2018 Oct 20]. Available from: http//:www.sunnuclear.com .
- 12.Thiyagarajan R, Nambiraj A, Sinha SN, Yadav G, Kumar A, Subramani V, et al. Analyzing the performance of arcCHECK diode array detector for VMAT plan. Rep Pract Oncol Radiother. 2016;21:50–6. doi: 10.1016/j.rpor.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Low DA, Moran JM, Dempsey JF, Dong L, Oldham M. Dosimetry tools and techniques for IMRT. Med Phys. 2011;38:1313–38. doi: 10.1118/1.3514120. [DOI] [PubMed] [Google Scholar]
- 14.An International Code of Practice for Dosimetry based on Absorbed dose to Water, IAEA Tech. Series No. 398, Absorbed Dose Determination in External Beam Radiotherapy. Vienna: IAEA; 2000. [Google Scholar]
- 15.Vanetti E, Nicolini G, Nord J, Peltola J, Clivio A, Fogliata A, et al. On the role of the optimization algorithm of RapidArc(®) volumetric modulated arc therapy on plan quality and efficiency. Med Phys. 2011;38:5844–56. doi: 10.1118/1.3641866. [DOI] [PubMed] [Google Scholar]
- 16.Kumar L, Yadav G, Raman K, Bhushan M, Pal M. The dosimetric impact of different photon beam energy on RapidArc radiotherapy planning for cervix carcinoma. J Med Phys. 2015;40:207–13. doi: 10.4103/0971-6203.170787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avgousti R, Armpilia C, Floros I, Antypas C. Evaluation of intensity modulated radiation therapy delivery system using a volumetric phantom on the basis of the task group 119 report of American Association of Physicists in Medicine. J Med Phys. 2017;42:33–41. doi: 10.4103/0971-6203.202419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaushik S, Tyagi A, Kumar L, Singh MP, Kundu RS, Punia R. Validation of intensity-modulated radiotherapy commissioning as per recommendations in test plans of the American Association of Physicists in Medicine Task Group 119 report. Radiat Prot Environ. 2016;39:138–45. [Google Scholar]
- 19.Fogliata A, Nicolini G, Clivio A, Vanetti E, Mancosu P, Cozzi L. Dosimetric validation of the Acuros XB advanced dose calculation algorithm: Fundamental characterization in water. Phys Med Biol. 2011;56:1879–904. doi: 10.1088/0031-9155/56/6/022. [DOI] [PubMed] [Google Scholar]
- 20.Fogliata A, Nicolini G, Clivio A, Vanetti E, Cozzi L. On the dosimetric impact of inhomogeneity management in the Acuros XB algorithm for breast treatment. Radiat Oncol. 2011;6:103. doi: 10.1186/1748-717X-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fogliata A, Nicolini G, Clivio A, Vanetti E, Cozzi L. Critical appraisal of acuros XB and anisotropic analytic algorithm dose calculation in advanced non-small-cell lung cancer treatments. Int J Radiat Oncol Biol Phys. 2012;83:1587–95. doi: 10.1016/j.ijrobp.2011.10.078. [DOI] [PubMed] [Google Scholar]
- 22.Rana S, Rogers K, Lee T, Reed D, Biggs C. Dosimetric impact of Acuros XB dose calculation algorithm in prostate cancer treatment using RapidArc. J Cancer Res Ther. 2013;9:430–5. doi: 10.4103/0973-1482.119328. [DOI] [PubMed] [Google Scholar]
- 23.Tanyi JA, Summers PA, McCracken CL, Chen Y, Ku LC, Fuss M. Implications of a high-definition multileaf collimator (HD-MLC) on treatment planning techniques for stereotactic body radiation therapy (SBRT): A planning study. Radiat Oncol. 2009;4:22. doi: 10.1186/1748-717X-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma SD. Unflattened photon beams from the standard flattening filter free accelerators for radiotherapy: Advantages, limitations and challenges. J Med Phys. 2011;36:123–5. doi: 10.4103/0971-6203.83464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar L, Yadav G, Samuvel KR, Bhushan M, Kumar P, Suhail M, et al. Dosimetric influence of filtered and flattening filter free photon beam on rapid arc (RA) radiotherapy planning in case of cervix carcinoma. Rep Pract Oncol Radiother. 2017;22:10–8. doi: 10.1016/j.rpor.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurado-Bruggeman D, Hernández V, Sáez J, Navarro D, Pino F, Martínez T, et al. Multi-centre audit of VMAT planning and pre-treatment verification. Radiother Oncol. 2017;124:302–10. doi: 10.1016/j.radonc.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Ibbott GS, Followill DS, Molineu HA, Lowenstein JR, Alvarez PE, Roll JE. Challenges in credentialing institutions and participants in advanced technology multi-institutional clinical trials. Int J Radiat Oncol Biol Phys. 2008;71:S71–5. doi: 10.1016/j.ijrobp.2007.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McVicker D, Yin FF, Adamson JD. On the sensitivity of TG-119 and IROC credentialing to TPS commissioning errors. J Appl Clin Med Phys. 2016;17:34–48. doi: 10.1120/jacmp.v17i1.5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alber M. Guidelines for Verification of IMRT. Brussels, Belgium: ESTRO; 2008. [Google Scholar]
- 30.Gillis S, De Wagter C, Bohsung J, Perrin B, Williams P, Mijnheer BJ. An inter-centre quality assurance network for IMRT verification: Results of the ESTRO QUASIMODO project. Radiother Oncol. 2005;76:340–53. doi: 10.1016/j.radonc.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Budgell G, Berresford J, Trainer M, Bradshaw E, Sharpe P, Williams P. A national dosimetric audit of IMRT. Radiother Oncol. 2011;99:246–52. doi: 10.1016/j.radonc.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Clark CH, Hussein M, Tsang Y, Thomas R, Wilkinson D, Bass G, et al. Amulti-institutional dosimetry audit of rotational intensity-modulated radiotherapy. Radiother Oncol. 2014;113:272–8. doi: 10.1016/j.radonc.2014.11.015. [DOI] [PubMed] [Google Scholar]