Abstract
Objective—
The activation of valve interstitial cells (VICs) toward an osteogenic phenotype characterizes aortic valve sclerosis, the early asymptomatic phase of calcific aortic valve disease. Osteopontin is a phosphorylated acidic glycoprotein that accumulates within the aortic leaflets and labels VIC activation even in noncalcified asymptomatic patients. Despite this, osteopontin protects VICs against in vitro calcification. Here, we hypothesize that the specific interaction of osteopontin with CD44v6, and the related intracellular pathway, prevents calcium deposition in human-derived VICs from patients with aortic valve sclerosis.
Approach and Results—
On informed consent, 23 patients and 4 controls were enrolled through the cardiac surgery and heart transplant programs. Human aortic valves and VICs were tested for osteogenic transdifferentiation, ex vivo and in vitro. Osteopontin–CD44 interaction was analyzed using proximity ligation assay and the signaling pathways investigated. A murine model based on angiotensin II infusion was used to mimic early pathological remodeling of the aortic valves. We report osteopontin–CD44 functional interaction as a hallmark of early stages of calcific aortic valve disease. We demonstrated that osteopontin–CD44 interaction mediates calcium deposition via phospho-Akt in VICs from patients with noncalcified aortic valve sclerosis. Finally, microdissection analysis of murine valves shows increased cusp thickness in angiotensin II-treated mice versus saline infused along with colocalization of osteopontin and CD44 as seen in human lesions.
Conclusions—
Here, we unveil a specific protein–protein association and intracellular signaling mechanisms of osteopontin. Understanding the molecular mechanisms of early VIC activation and calcium deposition in asymptomatic stage of calcific aortic valve disease could open new prospective for diagnosis and therapeutic intervention.
Keywords: aortic valve, osteopontin
Calcific aortic valve disease (CAVD) is a slowly progressive multifactorial disorder more common with age, without being an inevitable consequence of aging.1–5 Initial phases of the disease include mild thickening of the valve, whereas more advanced stages are associated with impaired leaflet motion and resistance to blood flow.6–9 These conditions are known as aortic valve sclerosis (AVSc) and calcific aortic valve stenosis, respectively. Aortic sclerosis is a hallmark of several cardiovascular conditions ranging from chronic heart failure and myocardial infarction to calcific aortic stenosis.1,2,4,5,8 AVSc is present in 25% to 30% of patients over 65 years of age, and it is characterized by thickening of the leaflets with none or marginal effects on the mechanical proprieties of the valve making its presentation asymptomatic.1,2,4,5,10,11 In addition, aortic sclerosis is challenging to define due to the variable and qualitative nature of its description by echocardiographic evaluation.1,2,4,5,8 Moreover, tissues from asymptomatic patients with aortic sclerosis are generally unavailable to investigators because these valves are not surgically replaced until moderate to severe stenosis occurs. Noteworthy, once aortic sclerosis is detected, there is an increased risk of cardiovascular events.12 At the onset of early symptoms of calcific aortic stenosis, the survival curve declines rapidly from the expected event-free curve, with a dramatic deviation in case of severe symptomatic stenosis.4,11,13 Despite its high prevalence, little is known about the initiating pathogenetic mechanisms determining the activation of valve interstitial cells (VICs) toward osteogenic-like phenotype and calcification.
Our extensive work on the pathogenesis of the early stage of CAVD has demonstrated that noncalcified leaflets from patients with AVSc can be induced to express markers of osteogenic transdifferentiation (osteopontin, osteonectin, runt-related transcription factor 2, α-smooth muscle actin, and alkaline phosphatase).6,7,14,15 Notably, we can promote biomineralization of noncalcified tissues through the combinatory effect of bone morphogenetic protein 4 (BMP4) and mechanical stimulation (15% stretch at 1 Hz)11 or through oxidative stress.14 VIC activation is a hallmark of pathological remodeling of the aortic valve leaflet even in the absence of clinical symptoms.6,14,16–21 Oxidative damage, altered mechanical stress, several cytokines, and growth factors have been recently reported as markers of VIC activation, resulting in the expression of osteogenic markers.6,14,17,18,20–24 Additionally, we reported that human VICs can be induced to transdifferentiate with a repopulated tissue-engineered aortic valve model, based on porcine extracellular matrix scaffold with results showing overexpression of osteopontin and other osteogenic markers.6 Furthermore, we demonstrated an active role of phosphorylated osteopontin as an important inhibitor of in vitro vascular calcification.17,25,26 We previously reported that circulating osteopontin levels are elevated in patients with CAVD when compared with healthy controls.17,26–28 Because the biological function of osteopontin in vitro is to inhibit calcium deposition, it has been proposed that the increasing level of osteopontin in diseased tissue reflects a compensatory mechanism aimed to block biomineralization. Here, we investigate how osteopontin prevents in vitro calcification via a protein–protein interaction with one of its receptors.
The primary receptors for osteopontin are those integrins that present the central integrin binding motif RGD.26 In addition to the integrin, the C-terminal fragment of osteopontin binds directly to CD44v6 in an RGD-independent manner,29 inducing cell migration and regulating calcium deposition.26,30,31 This receptor consists of 20 exons, where the first five and the last five are constant and the others are present in the isoforms. Exons 1 to 16 encode the extracellular domain, exon 17 encodes the transmembrane domain, and exons 18 and 19 encode the cytoplasmic domain. The 10 exons located between 5a and 14 are subjected to alternative splicing, resulting in the generation of CD44 variants. CD44 is a multifunctional receptor, which plays a role in cell adhesion, cell trafficking, presentation of chemokine and growth factors, and transmission of growth signals and signals mediating hematopoiesis and apoptosis.31
Using human-derived tissues, obtained through the heart transplant program and the cardiovascular surgery department at the University of Pennsylvania, we aimed to investigate, ex vivo and in vitro, the role of osteopontin–CD44 interaction governing calcium deposition in valve interstitial cells from patients with noncalcified AVSc. Furthermore, we implemented a murine model based on chronic infusion of angiotensin II (Ang II)32–35 to mimic early aortic valve tissue remodeling and expression of osteogenic markers.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
Osteopontin–CD44 Functional Interaction as a Hallmark of Early Stages of CAVD
On informed consent, 27 patients were selected from our cardiac bioregistry. Four controls and 18 aortic valve sclerotic tissues were obtained through the heart transplant research program. Five aortic valve stenotic tissues were collected during aortic valve replacement procedures from patients with end-stage CAVD (Table). Aortic valve calcification was assessed, and a calcium score of 1 to 4 was assigned for each patient by a single cardiologist based on the method described by Rosenhek et al36: 1, no calcification; 2, mildly calcified (small isolated spots); 3, moderately calcified (multiple larger spots); and 4, severely calcified (extensive thickening and calcification of all cusps; Table).
Table.
Patient Demographics
| Demographics | Controls | Aortic Sclerosis | Aortic Stenosis | P Value |
|---|---|---|---|---|
| n=4 | n=18 | n=5 | ||
| Age | 33.8±12.2 | 58.8±9.1 | 80.2±7.0 | 0.0002 |
| Male subjects | 25% (1) | 50% (9) | 20% (1) | NS |
| Diabetes mellitus | 25% (1) | 28% (5) | 20% (1) | NS |
| Hypertension | 25% (1) | 50% (9) | 100% (5) | NS |
| Cerebral vascular accident | 25% (1) | 17% (3) | 0% (0) | NS |
| Coronary artery disease | 0% (0) | 39% (7) | 40% (2) | NS |
| Congestive heart failure | 0% (0) | 33% (6) | 20% (1) | NS |
| Hyperlipidemia | 0% (0) | 33% (6) | 80% (4) | NS |
| Calcium score | 1±0.0 | 1±0.0 | 3.5±0.58 | <0.0001 |
NS indicates non-significant.
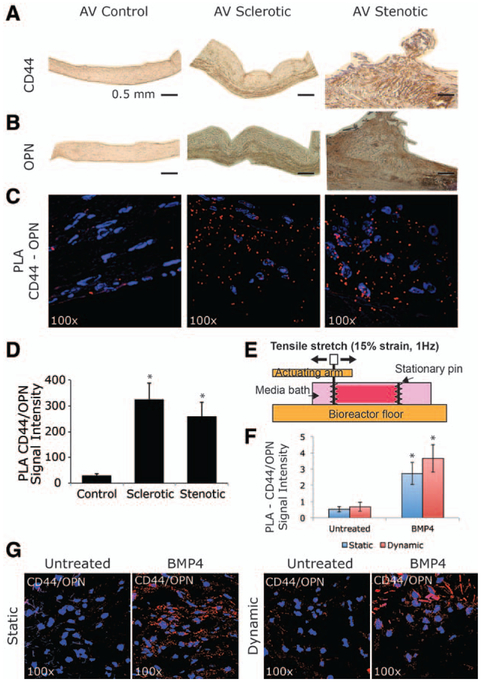
In control aortic valves, both CD44 and osteopontin expression are barely detectable throughout the entire leaflets (Figure 1A and 1B). By contrast, in sclerotic and stenotic aortic valves, the expression of both proteins is higher when compared with healthy controls. Moreover, the stenotic tissues have a uniform expression, whereas the sclerotic ones have a major con-centration of CD44 and osteopontin in the ventricularis region (Figure 1A and 1B). According to echocardiographic analysis, controls and AVSc do not show calcium within the leaflets, whereas aortic valve stenosis shows side-specific calcium accumulation (Figure I in the online-only Data Supplement). To test ex vivo functional interaction, between osteopontin and CD44, proximity ligation assay was performed as described in the Material and Methods section. Reaction products (red dots), representing the direct binding between osteopontin and CD44, show the extracellular functional interaction of these 2 proteins in both sclerotic and stenotic tissues (≈10-fold increment) when compared with control aortic valves (Figure 1C and 1D).
Figure 1.
CD44–osteopontin (OPN) functional interaction as a hallmark of early stages of calcific aortic valve disease (A and B). Representative images showing histological analysis of human aortic valve (AV; controls, sclerosis, and stenosis). Immunohistochemistry staining for CD44 and OPN. Bar represents 0.5 mm. C, Proximity ligation assay (PLA), red fluorescent dots indicate reaction product showing extracellular binding between CD44 and OPN. Magnification ×100. D, Bar graph representing PLA quantification in controls, sclerotic, and stenotic aortic valves. *P<0.05. E, Configuration of testing sample in tension bioreactor. F, Bar graph representing PLA quantification in sclerotic aortic valves under static or dynamic (15% stretch at 1 Hz) conditions in the absence or presence of bone morphogenetic protein 4 (BMP4; 100 ng/mL). *P<0.05. G, PLA showing CD44/OPN binding in AV sclerotic tissue at 6 days under static or dynamic (15% stretch at 1 Hz) conditions in the absence or presence of bone morphogenetic protein 4 (BMP4; 100 ng/mL).
We previously showed that BMP4 and mechanical stimulation induce both osteogenic markers expression and biomineralization of human noncalcified aortic valve sclerotic tissues,6 and we tested whether osteopontin–CD44 binding was also regulated by biomechanical stimulation. We therefore applied 15% stretch at 1 Hz in the presence or absence of BMP4 (100 ng/mL), using a tensile bioreactor to mimic the stress on the valve during the cardiac cycle (Figure 1E).6 As an internal control, native tissues were treated statically in the presence or absence of BMP4 (100 ng/mL). Cyclic stretch of the leaflets does not damage leaflet morphology and maintains native extracellular matrix structure and cellular composition.37,38 As shown in Figure 1F and 1G, the osteopontin–CD44 binding was influenced only by BMP4, independently of the mechanical stimuli.
Osteopontin and CD44 Protect Human Sclerotic Valve Interstitial Cells From Calcification Induced by BMP4
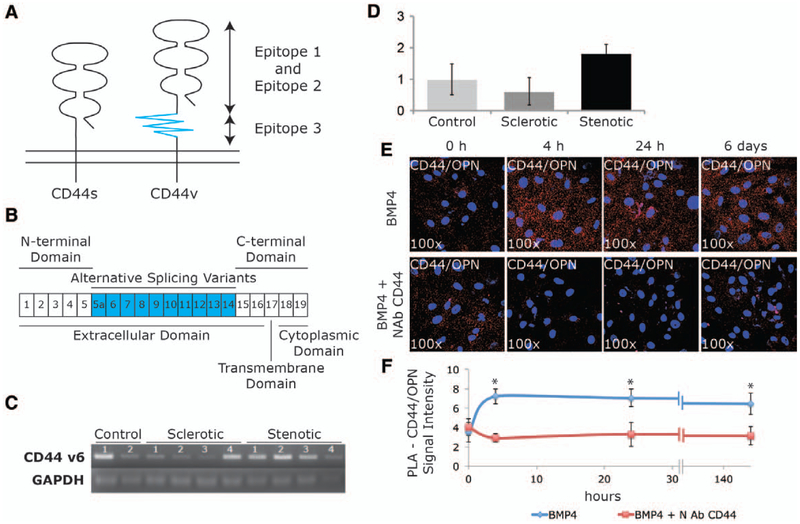
To test the role of osteopontin–CD44, in the regulation of VIC osteogenic transdifferentiation and calcification under BMP4, we isolated primary interstitial cells from 10 patients (2 controls, 4 sclerotic, and 4 stenotic) according to the methods described by Branchetti et al.14 Phenotypic stability was assessed after isolation, and VICs were used between passage 2 and 4. Figure 2A and 2B shows a schematic representation of structure, epitopes, domains, and exons of CD44 standard isoform (CD44s) and its possible variants (CD44v). It has been shown that osteopontin binds the variant 6 of CD44 (CD44v6).29 We therefore tested whether CD44v6 was present in aortic valve–derived VICs of our 3 cohorts of patients. As shown in Figure 2C and 2D, all the tested human isolated VICs from controls, sclerotic, or stenotic patients express CD44v6 isoform, independently of the progression of the disease.
Figure 2.
CD44–osteopontin (OPN) interaction on isolated valve interstitial cells (VICs) from asymptomatic patients with aortic valve sclerosis (AVSc). A, Schematic structure of CD44s and CD44v that has an additional longer stem, which contains the variant exon(s). B, CD44 gene consists of 20 exons, exons 1 to 5 (N-terminal domain) and exons 15 to 19 (C-terminal domain). Exons 5a to 14 (v1–v10) generate CD44 variants through alternative splicing. C, Polymerase chain reaction (PCR) product obtained using primers CD44v6 fw and CD44v6 rv on human isolated VICs, 2 controls, 4 patients with sclerosis, and 4 patients with stenosis. D, Bar graph quantification of PCR products for controls, AVSc, and patients with aortic valve stenosis. E, Proximity ligation assay (PLA) showing CD44/OPN binding in aortic valve sclerotic VICs at 0 hours, 4 hours, 24 hours, and 6 days after in vitro treatments (100 ng/mL) in the presence or absence of neutralizing antibody (NAb) CD44 (5 μg/mL). Magnification ×100. F, Line graph represents PLA quantification. *P<0.05. BMP4 indicates bone morphogenetic protein 4.
In a time-course experiment, we show that in AVSc-derived VICs, the binding between osteopontin and CD44 is highly increased in response to BMP4 treatment (Figure 2E and 2F). The proximity ligation assay products (red dots), representing the interaction between the extracellular portion of the transmembrane receptor CD44 and osteopontin, increases after 4 hours of BMP4 treatment and persists ≤6 days. A neutralizing antibody against CD44 (NAb-CD44), recognizing the epitope 2 (conserved region in the extracellular domain, Figure 2A), competes with osteopontin binding site to CD44 and can prevent the osteopontin–CD44 interaction induced by BMP4 (Figure 2E and 2F).
We then treated isolated sclerotic VICs for 12 days in the presence or absence of BMP4 (100 ng/mL) following protocols previously described.6 Using quantitative real-time polymerase chain reaction, we analyzed the expression of osteopontin (3.3-fold increase) and alkaline phosphatase (2.2-fold increase) under BMP4 treatment to demonstrate its role in controlling the VICs activation toward an osteogenic-like phenotype (P<0.05; Figure 3A). In a time-course experiment, AVSc-derived VICs were treated for 3, 6, and 12 days with BMP4 (100 ng/mL) and analyzed for CD44(v6) and osteopontin expression. As shown in Figure 3B to 3F, and according to our previous results,6 BMP4 treatment increases osteopontin expression in a time-dependent fashion either by real-time polymerase chain reaction (Figure 3A and 3B) or Western blotting (Figure 3C and 3D) while CD44 protein expression (Figure 3C and 3D) and CD44v6 mRNA (Figure 3E and 3F) were not significantly affected by BMP4 treatment and remain constant through the 12 days (Western blotting and real time).
Figure 3.
CD44 and osteopontin (OPN) protect human sclerotic valve interstitial cells (VICs) from calcification induced by bone morphogenetic protein 4 (BMP4). A, Bar graph representing fold change in gene expression of OPN and alkaline phosphatase (ALP) in aortic valve sclerotic VICs after 12 days of in vitro treatment of BMP4 (100 ng/mL). B, Osteopontin quantification by real-time polymerase chain reaction (RT-PCR) of AVSc-derived VICs under BMP4 treatments at 0, 3, 6, and 12 days. C, Western blotting of protein extracts from AVSc-derived VICs treated with BMP4 (100ng/mL) for 3, 6, and 12 days. Antibodies against CD44, OPN, and GAPDH were used. D, Quantification of Western blotting. E, RT-PCR for CD44v6 and GAPDH of total RNA extracted from AVSc-derived VICs treated with BMP4 (100 ng/mL). F, RT quantification. G, Bar graph represents calcium deposition on aortic valve sclerotic VICs after 12 days of treatment with BMP4 (100 ng/mL), neutralizing antibody (NAb) OPN (5 μg/mL), NI-IgG (5 μg/mL), and their combinations. *P=0.05. H, Alizarin red staining representing calcium deposition (red) on VICs after 12 days treatment with BMP4 (100 ng/mL), NAb CD44 (5 μg/mL), NAb OPN (5 μg/mL), and their combination.
It has been widely demonstrated that osteopontin is able to prevent calcium deposition in vitro.26,39 In this study, we tested whether, under BMP4 stimulation, osteopontin is able to prevent calcium deposition by binding to CD44 in VICs isolated from patients with AVSc. AVSc-derived VICs were treated with BMP4 (100 ng/mL) for 12 days: despite the increased level of alkaline phosphatase (Figure 3A). AVSc-derived VICs do not show calcium accumulation (Figure 3G and 3H). However, blocking osteopontin–CD44 interaction (by neutralizing antibody against either CD44 or osteopontin) induced calcium deposition, +3.4 and +1.9 (P<0.05) fold increase, respectively, when compared with BMP4 treatment alone (Figure 3G and 3H). These results suggest that the functional interaction between osteopontin and CD44 protects AVSc-VICs from calcium deposition.
Akt Phosphorylation Induced by Osteopontin–CD44 Is Required to Protect Human Sclerotic Valve Interstitial Cells From Calcium Deposition
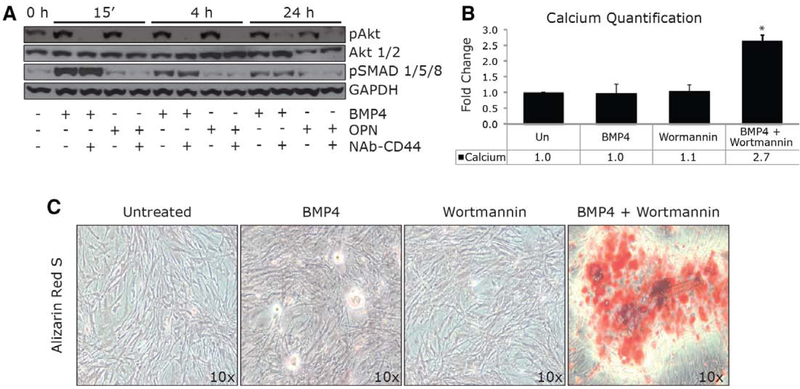
We then investigated the intracellular signaling pathway activated by BMP4 and osteopontin–CD44 in human-derived VICs. Extensive work has reported a direct activation of MAPK (mitogen-activated protein kinase) signaling pathway by osteopontin in different cell lines.30,40,41 We previously demonstrated that osteopontin mediates calcium deposition independently of p38 and ERK1/2 activation.26 Here, we tested Akt phosphorylation induced by BMP4 through osteopontin. Phosphorylation of SMAD1/5/8 was also tested as an independent signaling pathway activated by BMP4. In our experiment, phospho-Akt is seen by Western blot after 15 minutes, 4 hours, and 24 hours of BMP4 and osteopontin treatments; however, Akt phosphorylation is prevented by pretreatment with a neutralizing antibody against CD44 (NAb–CD44). Furthermore, the phosphorylation of SMAD1/5/8 is BMP4 dependent, and the presence of NAb– CD44 does not affect the SMAD phosphorylation (Figure 4A).
Figure 4.
Akt phosphorylation induced by CD44/osteopontin (OPN) is required to protect human sclerotic valve interstitial cells (VICs) from calcium deposition. A, Western blotting of pAkt, Akt 1/2, pSMAD 1/5/8, and GAPDH at 0 hours, 15 minutes, 4 hours, and 24 hours after bone morphogenetic protein 4 (BMP4; 100 ng/mL) in combination with neutralizing antibody (NAb) CD44 (5 μg/mL) or NAb OPN (5 μg/mL). B, Bar graph represents calcium deposition on aortic valve sclerotic VICs after 12 days treatment with BMP4 (100 ng/mL) in the absence or presence of Wortmannin (0.2 mmol/L). *P=0.05. C, Alizarin red staining representing calcium deposition (red) on VICs after 12 days treatment with BMP4 (100 ng/mL) in the presence or absence of Wortmannin (0.2 mmol/L).
We finally evaluated the role of phospho-Akt, induced by osteopontin–CD44 binding, on regulating calcium deposition of VICs derived from patients with noncalcified AVSc. We therefore treated human isolated sclerotic VICs with BMP4 (100 ng/mL) in the presence or absence of Wortmannin (200 nmol/L), a known phosphoinositide-3-kinase/Akt cascade inhibitor. As presented in Figure 4B and 4C, the combination of BMP4 and Wortmannin resulted in an increment of calcium deposits measured by colorimetric assay and Alizarin red staining. These results suggest that Akt phosphorylation induced by osteopontin–CD44 is required to protect human sclerotic valve interstitial cells from calcium deposition.
Ang II Infusion Provokes Remodeling of the Aortic Valve Tissue in Mice
We recently reported that reactive oxygen species (ROS) accumulation is leading VICs activation ex vivo and in vitro,14 with results showing that adenoviral delivery of antioxidant enzymes ameliorates VICs activation and calcification. We therefore used a previously developed32,33 murine model based on chronic Ang II infusion to reproduce ROS accumulation in the cardiac tissue. This model was first developed to induce ascending aortic aneurysm.32–35 The choice of this model has been justified by the role of Ang II in activating constitutive nitric oxide synthase with production of ROS (H2O2 and ONOO) via angiotensin II type I receptors (AT1R) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Using this model, we tested whether ROS accumulation was inducing valve remodeling and how it controlled osteopontin–CD44 expression in vivo. Male wild-type mice (C57BL/6J) were fed with enriched diet and infused with saline (n=12) or Ang II (1000 ng/kg per minute; n=28) for 28 days. Echocardiographic and immunohistological analysis were performed on each animal to test for ROS and osteogenic marker accumulation, osteopontin–CD44 expression, and extracellular matrix remodeling (Table I in the online-only Data Supplement).
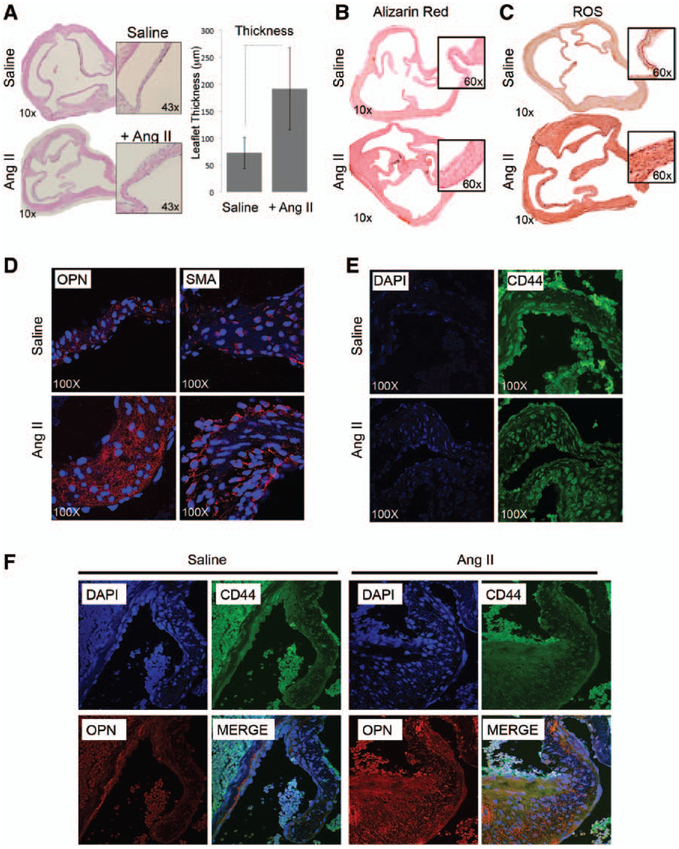
Microdissection analysis of murine valve tissue shows increased (P<0.01) cusp thickness in Ang II-treated mice versus saline infused (Figure 5A) without calcium accumulation (Figure 5B), resembling human early aortic valve remodeling.6,14,17 Histological and protein quantification analysis also revealed increased ROS (nitrotyrosine staining) in mice cusps (Figure 5C) as seen in human lesions.14 We then tested the expression of osteopontin, CD44, and other osteogenic markers in our murine model, with results showing that Ang II infusion promotes overexpression of osteopontin and smooth muscle actin (Figure 5D), while—according to previous data—similar expression levels of CD44 were detected (Figure 5E). Finally, we performed coimmunofluorescence experiments to test the colocalization of osteopontin and CD44 on murine aortic valve tissues. As shown in Figure 5F, our confocal microscope analysis shows colocalization of osteopontin–CD44 in tissue exposed to Ang II.
Figure 5.
Angiotensin II (Ang II) infusion provokes remodeling of the aortic valve tissue in mice. A, Hematoxylin–eosin staining of cross section of mice hearts harvested 28 days after saline or Ang II infusion (magnification, ×10 and ×43). Bar graph representing leaflet thickness (μm). B, Alizarin red staining showing minimal calcium accumulation in the aortic valve of Ang II-treated mice. C, Nitrotyrosine staining showing oxidative damage accumulation in the aortic valve of Ang II-treated mice (magnification, ×10 and ×60). D and E, Osteopontin (OPN), smooth muscle actin (SMA), and CD44 expression detected by immunofluorescence in aortic valve leaflets. Images were taken using confocal microscopy (magnification, ×100). F, Coimmunofluorescence showing OPN (red) and CD44 (green) colocalization in aortic valve leaflets (magnification, ×60). DAPI indicates 4’,6-diamidino-2-phenylindole; and ROS, reactive oxygen species.
Discussion
Over the last decade, several clinical trials, mostly extensions of atherosclerosis-related studies (collectively referred as statin trials), have been performed to halt the progression of CAVD with contradictory results. The early enthusiastic findings documenting a reduction in the progression of CAVD have been questioned by later randomized studies, which show substantial equivalence between treatments and placebo.42–46 It has been proposed that CAVD therapy in these trials may have been initiated too late in the course of the disease to be effective.47–50 Once sclerosis is detectable, there is an increased risk of cardiovascular events. In addition, human aortic sclerotic tissues are generally not available to investigators because these valves are not surgically replaced until moderate to severe aortic stenosis occurs.1,11,23,51,52 At the onset of even mild symptoms, survival rate decreases from the event-free population, with a dramatic decline in case of severe symptomatic aortic valve stenosis.11,13 We now understand that aortic valve stenosis is just the end stage of a disease that progresses from the microscopic early changes of aortic sclerosis to, in a subset of patients, asymptomatic and then symptomatic severe calcific stenosis. In vitro and in vivo studies have shown that valve calcification is an active process controlled by inflammatory mediators and regulators of osteogenic-like differentiation of VIC in the fibrosa layer of leaflets. We have extensively investigated the early asymptomatic stage of CAVD using a human bioregistry of tissue and VIC-derived cells.6,7,14,15,26,28 Because of its asymptomatic presentation, little is known about the molecular mechanisms underlying tissue remodeling and VIC activation in patients with AVSc. Here, we unveil the specific functional association of osteopontin with CD44v6 and the intracellular signaling mechanisms controlling calcium deposition on VIC derived from noncalcified aortic valve tissues.
High levels of osteopontin in native human calcified aortic valves from patients undergoing aortic valve replacements have been shown by immunohistochemistry, reverse transcription– polymerase chain reaction, Western blotting, and in situ hybridization analyses.7,30,40 In addition to native valves, increased osteopontin levels were also found by immunohistochemistry in calcified valve allografts and in areas of calcification in glutaraldehyde-pretreated bioprosthetic porcine valves.53,54 Elevated circulating osteopontin levels are also reported in other cardiovascular diseases, such as atherosclerosis, ischemic heart disease, heart failure, and rheumatic mitral stenosis.30 Osteopontin is of special interest for CAVD because it is the only described biomarker directly involved in the ectopic and dystrophic calcification phenomenon that occurs during native and bioprosthetic valve degeneration. Interestingly, circulating levels of osteopontin are also elevated in patients with signs of AVSc, suggesting that osteopontin could also be used as biomarker to label patients at risk to develop severe stenosis.17,28 We have reported the role of osteopontin on VICs, vascular smooth muscle cells, and endothelial cells.26 Our analysis showed that osteopontin activates the same intracellular signaling pathways in cultured endothelial and smooth muscle cells and in excised aortic valve tissues. In addition, our data support the concept that the biological function of osteopontin is mediated by CD44.26
This study provides several new insights into the specific interaction of osteopontin with CD44 and the intracellular signaling controlling calcium deposition in human sclerotic-derived VICs. As discussed, tissue from early stages of AVSc is extremely difficult to obtain because these valves are mechanically functional and are not indicated for repair or replacement. However, our collaborations with the Gift of Life program and with the heart transplant program allow us to rely on this unique collection of human specimens. Using this major resource, we demonstrated a specific osteopontin–CD44 functional interaction in early asymptomatic stage of CAVD. We then show, in vitro, that preventing osteopontin–CD44 interaction results in calcium accumulation of VIC obtained from noncalcified patients with AVSc after treatments with BMP4. Finally, using neutralizing antibodies and general inhibitors of intracellular signaling pathways, we unveil that Akt phosphorylation induced by osteopontin–CD44 is required to protect human sclerotic valve interstitial cells from calcium deposition. Our data provide novel insights into the intracellular mechanisms controlling osteopontin, and in general VIC activation and calcification, in human AVSc-derived cells. Specifically, in healthy valve pro-osteogenic markers such as BMP4 and osteopontin are barely detectable. We, and others, have shown that with the development of early stages, there is an accumulation of oxidative damage, TGF-β-related cytokines, and changes in the biomechanical proprieties of the aortic valve leaflets, resulting in increased levels of pro-osteogenic factors, such as BMP4.6,14,17,21,23,24 These pro-osteogenic conditions trigger the expression of osteopontin that has been widely reported to protect against calcium accumulation in vitro.17,26,40,55–57 Here, we demonstrate that one of the mechanisms by which osteopontin is preventing BMP4-mediated biomineralization is through the functional interaction with CD44v6. We show that the disruption of CD44–osteopontin functional interaction (blocking osteopontin, CD44, or their downstream signaling pathway [pAkt]) results in calcium deposition in vitro.
To conclude, it is important to discuss the central role of osteopontin and other matricellular proteins in controlling valve homeostasis. Matricellular proteins are a class of nonstructural and secreted proteins that exert regulatory functions through direct binding to cell surface receptors, other matrix proteins, and soluble extracellular factors.58 Over the past few years, several novel matricellular proteins have been indentified: SIBLING (osteopontin,17,30,58 DMP1,58,59 and BSP58,60,61), CCN (Cyr61,58,62,63 CTGF,58,62,64 and Nov58,65), and other matricelllar proteins (osteonectin,58,66 thrombospondin 1 and 2,67,68 and tenascin69,70) are involved in various physiological and pathological conditions.61,71,72 Matricellular proteins perform their regulatory role on extracellular matrix by sequestering growth factors, binding ions like Ca2+, and inhibiting proteases by direct binding. Matricellular proteins are also involved in promoting cellular differentiation by activating cytokines, binding cell-surface receptors, and mediating the biomechanical transduction. However, despite their dual role of mediators of cell and matrix homeostasis, the functional role of these molecules on the cellular components and extracellular matrix microstructure of the aortic valve progression of CAVD has not been fully elucidated.
Osteopontin is involved in several processes central for maintaining and controlling both endothelial and interstitial cell function.26,30,40 Osteopontin–αvβ3 interaction is important for osteoclast migration and resorption as well as smooth muscle cell migration and adhesion. In addition, osteopontin acts as a cytokine playing important roles in the migration and invasion of several tumor cells.61,73 In addition, osteopontin is regarded to be an important angiogenic factor in several pathologies.30,40 The pleiotropic function of osteopontin is tightly regulated at different levels, including protein–protein association and transcriptional and post-translational levels. It is possible that different osteopontin post-translational modifications enable the protein to bind different receptors with variable affinity and therefore regulate a differential biological response within the aortic valve leaflets.7,26,74,75 It would therefore be important to investigate how these osteopontin variants affect the functional binding with CD44.
In a recent work, we have also reported a direct effect of oxidative stress on the expression of osteopontin and other markers of VICs activation,14 with adenoviral delivery of antioxidant enzymes ameliorating this effect. We could therefore hypothesize that the molecular mechanisms presented in this study represent a general mechanism of regulation of VIC activation. Therefore, we developed a murine model of VIC activation based on Ang II accumulation. The choice of this model has been justified by the role of Ang II in activating constitutive nitric oxide synthase with production of ROS (H2O2 and ONOO) via AT1R and NADPH oxidase. Using this model, we tested whether ROS accumulation was inducing valve remodeling and how it controlled osteopontin–CD44 expression in vivo. Our data show that ROS accumulation in murine aortic valve tissue is associated with cusp remodeling, resembling human early lesion and expression of osteogenic markers, such as osteopontin, and its receptor CD44, smooth muscle actin, and SOX9. These data are in accordance with the expression of these molecules in the human lesions.14 Several limitations are associated with this model: first, Ang II is not specific for aortic valve remodeling, but it induces remodeling of different cardiac and vascular tissues; second, the functional interaction of osteopontin with CD44v6 was not successfully detected under the tested experimental conditions in the murine model. This model is, however, potentially important because it could be used to test in vivo specific peptides or small molecules targeting directly osteopontin, CD44, or both. Understanding the functional role of osteopontin and other matricellular proteins in controlling VIC activation and calcium deposition in early asymptomatic stage of CAVD could open new prospective for diagnosis and therapeutic intervention.
Materials and Methods
Patient population and definition of aortic valve sclerosis.
Patients were enrolled in the study (Table 1) following Institutional Review Board (IRB) approved guidelines of University of Pennsylvania Perelman School of Medicine (protocol #809349) and according to the Declaration of Helsinki. Control and aortic valve sclerotic tissues were obtained through collaborations with the heart transplant research program at the University of Pennsylvania Perelman School of Medicine and with The Gift of Life Program. Aortic valve sclerosis was defined as irregular, non-uniform thickening of portions of the aortic valve leaflets or commissures, or both; thickened portions of the aortic valve without an appearance suggesting calcification (i.e., bright echoes); non-restricted or minimally restricted opening of the aortic leaflets; and peak continuous wave Doppler velocity across the valve < 2 m/s. Four aortic control valves, 18 aortic sclerotic valves and 5 aortic stenotic valves were used.
Isolation of aortic valve interstitial cells (VICs).
Isolation of aortic VICs was performed using a modified method described by Branchetti et al1. Briefly, aortic leaflets were placed in 2 mg/ml type II collagenase (Worthington Biochemical Corp., Worthington, VA) in Dulbecco’s modified Eagle’s medium containing 1% Penicillin/Streptomycin solution and incubated in a shaker for 20 min at 37°C. Loosened endothelial layer was removed by wiping the leaflet surfaces with sterile cotton swabs. Tissues were then finely minced and dissociated in type II collagenase (1 mg/ml) and hyaluronidase (100 U/ml) for 4 h at 37°C. The resulting VICs were seeded in tissue culture plates in Advanced DMEM media and maintained at 37°C and 5% CO2. VICs growth medium contained Advanced DMEM supplemented with 10% Fetal Bovine Serum (Thermo Scientific, Hudson, NH), and 1% Penicillin/Streptomycin solution (Life Technologies, Carlsbad, CA). All the experiments were performed on cultured cells between their second and fourth passages. Isolated cells were banked in liquid nitrogen for further studies.
Reagents.
Ex vivo and in vitro experiments were performed using human recombinant BMP4 protein (Peprotech, Rocky Hill, NJ), Neutralizing antibody anti CD44 (Ancell, Bayport, MN), Neutralizing antibody anti OPN (R&D Systems, Minneapolis, MN) and Wortmannin (Sigma, St. Louis, MO).
RT and Real Time PCR.
Extraction of RNA was performed from valve interstitial cells (VIC) using the RNeasy kit (QIAGEN, Valencia, CA). RNA was quantified using Nanodrop and used for two steps PCR amplification. One milligram of total RNA was converted into cDNA. Real Time PCR (RT-qPCR) was performed on ABI Prism 7500 HT (Applied Biosystems, Carlsbad, CA), according to the manufacturer’s instructions. Dissociation curves were generated after each run to control formation of primer dimers and 18S gene expression was used for normalization. Primers: CD44v6 Fw (5’- TCC CAG TAT GAC ACA TAT TGC -3’); CD44v6 Rv (5’- CCC ACA TGC CAT CTG TTG CC -3’); OPN Fw (5’- TTG CAG CCT TCT CAG CCA A -3’; OPN Rv (5’- CGA GGC AAA AGC AAA TCA CTG -3’); ALP Fw (5’- GCT GGC AGT GGT CAG ATG TT -3’); ALP Rv (5’- CTA TCC TGG CTC CGT GCT C -3’); GAPDH Fw (5’- CCC ATC ACC ATC TTC CAG GAG -3’); GAPDH Rv (5’- CTT CTC CAT GGT GGT GAA GAC G -3’).
Proximity Ligation Assay.
Protein–protein interaction was analyzed using the Duolink reagent kit (Olink Biosciences, Uppsala, Sweden) following manufacturer’s instruction. Briefly, tissues or cells were incubated with specific antibodies, CD44 and OPN, overnight at 4°C with gentle agitation. PLUS and MINUS secondary Proximity Ligation Assay (PLA) probes against rabbit and mouse IgG (Olink Bioscience) were added, and the samples were incubated at 37°C for 1 hour with gentle agitation, followed by incubation with ligation mix for 30 minutes at 37°C. Amplification mix was then applied for 100 minutes at 37°C. The coverslips were mounted on microscope slides with Doulink Mounting medium with DAPI, and the samples were photographed under a fluorescence microscope.
Immunohystochemistry, Western blotting and immunofluorescence.
Protein expression was demonstrated by immunohistochemistry, western blot and immunofluorescence techniques using specific antibodies against CD44 (Novus Biologicals, Littleton CO), CD44, Akt 1/2, pSMAD 1/5/8 (Cell Signaling, Danvers, MA), Osteopontin (Genway Biotech, San Diego, CA,), pAkt (Santa Cruz Biotechnologies, Dallas, TX), and GAPDH (Abcam, Cambridge, MA) following standard protocols.
Calcium quantification.
After the specific treatment VICs were washed with PBS 1x and then incubated overnight at 4°C in 0.6N HCl. Calcium was estimated using the calcium assay kit (Biovision, Mountain View, CA). After removing all the HCl, VICs were washed twice with PBS 1x, proteins were harvested using 1M NaOH + 0.1% SDS and total protein content was estimated using BCA Protein Assay (Thermo Scientific, Waltham, MA). Amount of total calcium was expressed as fold change (Treated/Untreated) calculated using μg of calcium/μg of proteins.
Bioreactor design and tissue preparation.
The tension bioreactor used in this study is similar to the previously described flexure bioreactor used for flexural stimulation of engineered valve tissue as described earlier2,3. The bioreactor consists of two chambers, each with multiple media baths; each bath has stationary pins press-fit into the bottom for tissue anchorage. The opposing pins are fixed to an actuating arm that is attached to a cross-arm which is connected to a central motorized piston. Intact human aortic valves excised in operating room were collected in Advanced DMEM medium and maintained at 4°C to assure maximal cell survival during transport. Each leaflet was trimmed to form a tissue strip, measuring 20 mm circumferentially and 8 mm radially. An attempt was made to keep endothelial cells viable through limited handling. The tissue strip was threaded into the springs and inserted into the tension bioreactor and used for the experiments. To each well 5 ml of Advanced DMEM ± BMP4 (100 ng/ml) was added and subsequently changed every 3 days. At day 6, tissues were removed, embedded in OCT for preparing frozen sections.
Angiotensin II chronic infusion.
C57BL/6J male mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Experiments were conducted under the approved IACUC protocol #804440. Seven weeks old male mice were fed with hypercolesterolaemic diet and infused with saline (n=10) or Angiotensin II (AngII) (n=20; 1000 ng/kg/min) using osmotic pumps (Alzet) for 28 days as previously described4,5. At baseline and 28 days after AngII infusion, B-mode ultrasound imaging was performed on the operated mice to assess the heart function and aortic expansion as previously described (Cardiovascular Vascular Institute Physiology Core Facility Service, UPenn). Measurements were accomplished using random selection of each data set (Supplemental Table I). Heart function and aortic expansion were measured using echocardiographic evaluation cross-sectional, 2-D and Doppler TTE were performed by experienced sonographers using a Visual Sonics Vevo 770 Imaging System.
Statistical analysis.
The data were analyzed using SPSS software (version 15; SPSS). Continuous variables were expressed as mean ± standard error of mean. Comparisons of continuous variables between groups were performed with nonparametric (Mann-Whitney U test). A value of p<0.05 was considered to be statistically significant. Comparisons between more than two groups were performed using Kruskal-Wallis test, with post-hoc pair wise Mann-Whitney tests using the Bonferroni correction to determine significance of difference between individual groups.
Supplementary Material
Significance.
This article is aimed to investigate the functional interaction of osteopontin and CD44 in human-derived interstitial cell from patients with aortic valve sclerosis, the early asymptomatic phase of calcific aortic valve disease. Calcific aortic valve disease, the main cause of aortic valve replacement in the United States, is a multifactorial process more common with age, although not an inevitable consequence of aging. Because of the shift in demographic by the aging population, surgical replacement of the aortic valve is projected to reach >850 000 cases worldwide by 2050, compared with 300 000 aortic valve replacements that take place currently. Although final stages of the disease (aortic valve stenosis) are well described, the cellular mechanisms associated with initiating phases remain largely unknown due to the asymptomatic presentation of aortic valve sclerosis. In this study, conducted entirely using human-derived interstitial cells from patients with noncalcified aortic valve sclerosis, we demonstrated that osteopontin–CD44 interaction protects the interstitial cells from calcium deposition via Akt phosphorylation.
Sources of Funding
This project was supported by the Harrison Memorial Fund of the University of Pennsylvania Perelman School of Medicine (G. Ferrari). This work was also partially supported by a collaborative research grant sponsored by the Valley Hospital Foundation–Marjorie G. Bunnell Charitable Fund (G. Ferrari and J.B. Grau).
Nonstandard Abbreviations and Acronyms
- Ang II
angiotensin II
- AVSc
aortic valve sclerosis
- BMP4
bone morphogenetic protein 4
- CAVD
calcific aortic valve disease
- ROS
reactive oxygen species
- VICs
valve interstitial cells
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.113.303017/-/DC1.
Disclosures
None.
References
- 1.Towler DA. Molecular and cellular aspects of calcific aortic valve disease. Circ Res. 2013;113:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. [DOI] [PubMed] [Google Scholar]
- 3.Freeman RV, Otto CM. Management of asymptomatic valvular aortic stenosis. Indian Heart J. 2002;54:31–38. [PubMed] [Google Scholar]
- 4.Kurtz CE, Otto CM. Aortic stenosis: clinical aspects of diagnosis and management, with 10 illustrative case reports from a 25-year experience. Medicine (Baltimore). 2010;89:349–379. [DOI] [PubMed] [Google Scholar]
- 5.Beckmann E, Grau JB, Sainger R, Poggio P, Ferrari G. Insights into the use of biomarkers in calcific aortic valve disease. J Heart Valve Dis. 2010;19:441–452. [PMC free article] [PubMed] [Google Scholar]
- 6.Poggio P, Sainger R, Branchetti E, Grau JB, Lai EK, Gorman RC, Sacks MS, Parolari A, Bavaria JE, Ferrari G. Noggin attenuates the osteogenic activation of human valve interstitial cells in aortic valve sclerosis. Cardiovasc Res. 2013;98:402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grau JB, Poggio P, Sainger R, Vernick WJ, Seefried WF, Branchetti E, Field BC, Bavaria JE, Acker MA, Ferrari G. Analysis of osteopontin levels for the identification of asymptomatic patients with calcific aortic valve disease. Ann Thorac Surg. 2012;93:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gharacholou SM, Karon BL, Shub C, Pellikka PA. Aortic valve sclerosis and clinical outcomes: moving toward a definition. Am J Med. 2011;124:103–110. [DOI] [PubMed] [Google Scholar]
- 9.Pflederer T, Achenbach S. Aortic valve stenosis: CT contributions to diagnosis and therapy. J Cardiovasc Comput Tomogr. 2010;4:355–364. [DOI] [PubMed] [Google Scholar]
- 10.Fisher CI, Chen J, Merryman WD. Calcific nodule morphogenesis by heart valve interstitial cells is strain dependent. Biomech Model Mechanobiol. 2013;12:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heistad DD, Shanahan C, Demer LL. Introduction to the Compendium on calcific aortic valve disease. Circ Res. 2013;113:176–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. [DOI] [PubMed] [Google Scholar]
- 13.Akerström F, Barderas MG, Rodríguez-Padial L. Aortic stenosis: a general overview of clinical, pathophysiological and therapeutic aspects. Expert Rev Cardiovasc Ther. 2013;11:239–250. [DOI] [PubMed] [Google Scholar]
- 14.Branchetti E, Sainger R, Poggio P, Grau JB, Patterson-Fortin J, Bavaria JE, Chorny M, Lai E, Gorman RC, Levy RJ, Ferrari G. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler Thromb Vasc Biol. 2013;33:e66–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sainger R, Grau JB, Poggio P, Branchetti E, Bavaria JE, Gorman JH III, Gorman RC, Ferrari G. Dephosphorylation of circulating human osteopontin correlates with severe valvular calcification in patients with calcific aortic valve disease. Biomarkers. 2012;17:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng Q, Song R, Ao L, Weyant MJ, Lee J, Xu D, Fullerton DA, Meng X. Notch1 promotes the pro-osteogenic response of human aortic valve interstitial cells via modulation of ERK1/2 and nuclear factor-κB activation. Arterioscler Thromb Vasc Biol. 2013;33:1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grau JB, Poggio P, Sainger R, Vernick WJ, Seefried WF, Branchetti E, Field BC, Bavaria JE, Acker MA, Ferrari G. Analysis of osteopontin levels for the identification of asymptomatic patients with calcific aortic valve disease. Ann Thorac Surg. 2012;93:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JH, Chen WL, Sider KL, Yip CY, Simmons CA. β-catenin mediates mechanically regulated, transforming growth factor-β1-induced myofibroblast differentiation of aortic valve interstitial cells. Arterioscler Thromb Vasc Biol. 2011;31:590–597. [DOI] [PubMed] [Google Scholar]
- 19.Xu S, Liu AC, Kim H, Gotlieb AI. Cell density regulates in vitro activation of heart valve interstitial cells. Cardiovasc Pathol. 2012;21:65–73. [DOI] [PubMed] [Google Scholar]
- 20.Mulholland DL, Gotlieb AI. Cell biology of valvular interstitial cells. Can J Cardiol. 1996;12:231–236. [PubMed] [Google Scholar]
- 21.Hagler MA, Hadley TM, Zhang H, Mehra K, Roos CM, Schaff HV, Suri RM, Miller JD. TGF-β signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovasc Res. 2013;99:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yip CY, Blaser MC, Mirzaei Z, Zhong X, Simmons CA. Inhibition of pathological differentiation of valvular interstitial cells by C-type natriuretic peptide. Arterioscler Thromb Vasc Biol. 2011;31:1881–1889. [DOI] [PubMed] [Google Scholar]
- 23.Gould ST, Srigunapalan S, Simmons CA, Anseth KS. Hemodynamic and cellular response feedback in calcific aortic valve disease. Circ Res. 2013;113:186–197. [DOI] [PubMed] [Google Scholar]
- 24.Merryman WD, Youn I, Lukoff HD, Krueger PM, Guilak F, Hopkins RA, Sacks MS. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol. 2006;290:H224–H231. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari G, Sainger R, Beckmann E, Keller G, Yu PJ, Monti MC, Galloway AC, Weiss RL, Vernick W, Grau JB. Validation of plasma biomarkers in degenerative calcific aortic stenosis. J Surg Res. 2010;163:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poggio P, Grau JB, Field BC, Sainger R, Seefried WF, Rizzolio F, Ferrari G. Osteopontin controls endothelial cell migration in vitro and in excised human valvular tissue from patients with calcific aortic stenosis and controls. J Cell Physiol. 2011;226:2139–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sainger R, Grau JB, Poggio P, Branchetti E, Bavaria JE, Gorman JH III, Gorman RC, Ferrari G. Dephosphorylation of circulating human osteopontin correlates with severe valvular calcification in patients with calcific aortic valve disease. Biomarkers. 2012;17:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sainger R, Grau JB, Branchetti E, Poggio P, Lai E, Koka E, Vernick WJ, Gorman RC, Bavaria JE, Ferrari G. Comparison of transesophageal echocardiographic analysis and circulating biomarker expression profile in calcific aortic valve disease. J Heart Valve Dis. 2013;22:156–165. [PMC free article] [PubMed] [Google Scholar]
- 29.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto H Osteopontin and cardiovascular system. Mol Cell Biochem. 2007;300:1–7. [DOI] [PubMed] [Google Scholar]
- 31.Zhao L, Hall JA, Levenkova N, Lee E, Middleton MK, Zukas AM, Rader DJ, Rux JJ, Puré E. CD44 regulates vascular gene expression in a proatherogenic environment. Arterioscler Thromb Vasc Biol. 2007;27:886–892. [DOI] [PubMed] [Google Scholar]
- 32.Daugherty A, Rateri DL, Charo IF, Owens AP, Howatt DA, Cassis LA. Angiotensin II infusion promotes ascending aortic aneurysms: attenuation by CCR2 deficiency in apoE−/− mice. Clin Sci (Lond). 2010;118:681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Branchetti E, Poggio P, Sainger R, Shang E, Grau JB, Jackson BM, Lai EK, Parmacek MS, Gorman RC, Gorman JH, Bavaria JE, Ferrari G. Oxidative stress modulates vascular smooth muscle cell phenotype via CTGF in thoracic aortic aneurysm. Cardiovasc Res. 2013;100:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–H1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruemmer D, Daugherty A, Lu H, Rateri DL. Relevance of angiotensin II-induced aortic pathologies in mice to human aortic aneurysms. Ann N Y Acad Sci. 2011;1245:7–10. [DOI] [PubMed] [Google Scholar]
- 36.Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. [DOI] [PubMed] [Google Scholar]
- 37.Merryman W, Lukoff H, Long R, Engelmayrjr G, Hopkins R, Sacks M. Synergistic effects of cyclic tension and transforming growth factor-β1 on the aortic valve myofibroblast. Cardiovasc Pathol. 2007;16:268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allison DD, Drazba JA, Vesely I, Kader KN, Grande-Allen KJ. Cell viability mapping within long-term heart valve organ cultures. J Heart Valve Dis. 2004;13:290–296. [PubMed] [Google Scholar]
- 39.Yu PJ, Skolnick A, Ferrari G, Heretis K, Mignatti P, Pintucci G, Rosenzweig B, Diaz-Cartelle J, Kronzon I, Perk G, Pass HI, Galloway AC, Grossi EA, Grau JB. Correlation between plasma osteopontin levels and aortic valve calcification: potential insights into the pathogenesis of aortic valve calcification and stenosis. J Thorac Cardiovasc Surg. 2009;138:196–199. [DOI] [PubMed] [Google Scholar]
- 40.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. [DOI] [PubMed] [Google Scholar]
- 41.Dai J, Peng L, Fan K, Wang H, Wei R, Ji G, Cai J, Lu B, Li B, Zhang D, Kang Y, Tan M, Qian W, Guo Y. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene. 2009;28:3412–3422. [DOI] [PubMed] [Google Scholar]
- 42.Parolari A, Tremoli E, Cavallotti L, Trezzi M, Kassem S, Loardi C, Veglia F, Ferrari G, Pacini D, Alamanni F. Do statins improve outcomes and delay the progression of non-rheumatic calcific aortic stenosis? Heart. 2011;97:523–529. [DOI] [PubMed] [Google Scholar]
- 43.Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Gonçalves F, Rajamannan NM. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. [DOI] [PubMed] [Google Scholar]
- 45.Benton JA, Kern HB, Leinwand LA, Mariner PD, Anseth KS. Statins block calcific nodule formation of valvular interstitial cells by inhibiting alpha-smooth muscle actin expression. Arterioscler Thromb Vasc Biol. 2009;29:1950–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Bärwolf C, Holme I, Kesäniemi YA, Malbecq W, Nienaber CA, Ray S, Skjærpe T, Wachtell K, Willenheimer R, SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. [DOI] [PubMed] [Google Scholar]
- 47.Aikawa E, Otto CM. Look more closely at the valve: imaging calcific aortic valve disease. Circulation. 2012;125:9–11. [DOI] [PubMed] [Google Scholar]
- 48.Rosenhek R, Iung B, Tornos P, Antunes MJ, Prendergast BD, Otto CM, Kappetein AP, Stepinska J, Kaden JJ, Naber CK, Acartürk E, Gohlke-Bärwolf C. ESC Working Group on Valvular Heart Disease Position Paper: assessing the risk of interventions in patients with valvular heart disease. Eur Heart J. 2012;33:822–828, 828a, 828b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenhek R, Klaar U, Schemper M, Scholten C, Heger M, Gabriel H, Binder T, Maurer G, Baumgartner H. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J. 2004;25:199–205. [DOI] [PubMed] [Google Scholar]
- 50.Baumgartner H, Otto CM. Aortic stenosis severity: do we need a new concept? J Am Coll Cardiol. 2009;54:1012–1013. [DOI] [PubMed] [Google Scholar]
- 51.Coté N, Mahmut A, Bosse Y, Couture C, Pagé S, Trahan S, Boulanger MC, Fournier D, Pibarot P, Mathieu P. Inflammation is associated with the remodeling of calcific aortic valve disease. Inflammation. 2013;36:573–581. [DOI] [PubMed] [Google Scholar]
- 52.Dweck MR, Khaw HJ, Sng GK, Luo EL, Baird A, Williams MC, Makiello P, Mirsadraee S, Joshi NV, van Beek EJ, Boon NA, Rudd JH, Newby DE. Aortic stenosis, atherosclerosis, and skeletal bone: is there a common link with calcification and inflammation? Eur Heart J. 2013;34:1567–1574. [DOI] [PubMed] [Google Scholar]
- 53.Shen M, Marie P, Farge D, Carpentier S, De Pollak C, Hott M, Chen L, Martinet B, Carpentier A. Osteopontin is associated with bioprosthetic heart valve calcification in humans. C R Acad Sci III. 1997;320:49–57. [DOI] [PubMed] [Google Scholar]
- 54.Shen M, Carpentier SM, Berrebi AJ, Chen L, Martinet B, Carpentier A. Protein adsorption of calcified and noncalcified valvular bioprostheses after human implantation. Ann Thorac Surg. 2001;71(5 Suppl):S406–S407. [DOI] [PubMed] [Google Scholar]
- 55.Wada T, McKee MD, Steitz S, Giachelli CM. Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Circ Res. 1999;84:166–178. [DOI] [PubMed] [Google Scholar]
- 56.Giachelli CM. Inducers and inhibitors of biomineralization: lessons from pathological calcification. Orthod Craniofac Res. 2005;8:229–231. [DOI] [PubMed] [Google Scholar]
- 57.O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–2168. [DOI] [PubMed] [Google Scholar]
- 58.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pirotte S, Lamour V, Lambert V, Alvarez Gonzalez ML, Ormenese S, Noël A, Mottet D, Castronovo V, Bellahcène A. Dentin matrix protein 1 induces membrane expression of VE-cadherin on endothelial cells and inhibits VEGF-induced angiogenesis by blocking VEGFR-2 phosphorylation. Blood. 2011;117:2515–2526. [DOI] [PubMed] [Google Scholar]
- 60.Kaden JJ, Bickelhaupt S, Grobholz R, Vahl CF, Hagl S, Brueckmann M, Haase KK, Dempfle CE, Borggrefe M. Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J Heart Valve Dis. 2004;13:560–566. [PubMed] [Google Scholar]
- 61.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. [DOI] [PubMed] [Google Scholar]
- 62.Perbal B CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. [DOI] [PubMed] [Google Scholar]
- 63.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119(Part 23):4803–4810. [DOI] [PubMed] [Google Scholar]
- 64.Matsui Y, Morimoto J, Uede T. Role of matricellular proteins in cardiac tissue remodeling after myocardial infarction. World J Biol Chem. 2010;1:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dhar A, Ray A. The CCN family proteins in carcinogenesis. Exp Oncol. 2010;32:2–9. [PubMed] [Google Scholar]
- 66.Yiu GK, Chan WY, Ng SW, Chan PS, Cheung KK, Berkowitz RS, Mok SC. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol. 2001;159:609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. [DOI] [PubMed] [Google Scholar]
- 68.Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, Detmar M. Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci U S A. 1999;96:14888–14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Minear MA, Crosslin DR, Sutton BS, et al. Polymorphic variants in tenascin-C (TNC) are associated with atherosclerosis and coronary artery disease. Hum Genet. 2011;129:641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen JH, Simmons CA. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circ Res. 2011;108:1510–1524. [DOI] [PubMed] [Google Scholar]
- 71.Schellings M Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc Res. 2004;64:24–31. [DOI] [PubMed] [Google Scholar]
- 72.Alford AI, Hankenson KD. Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38:749–757. [DOI] [PubMed] [Google Scholar]
- 73.Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90:1877–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de las Fuentes L, Gu CC, Mathews SJ, Reagan JL, Ruthmann NP, Waggoner AD, Lai CF, Towler DA, Dávila-Román VG. Osteopontin promoter polymorphism is associated with increased carotid intima-media thickness. J Am Soc Echocardiogr. 2008;21:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shao JS, Sierra OL, Cohen R, Mecham RP, Kovacs A, Wang J, Distelhorst K, Behrmann A, Halstead LR, Towler DA. Vascular calcification and aortic fibrosis: a bifunctional role for osteopontin in diabetic arteriosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:1821–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merryman WD, Liao J, Parekh A, Candiello JE, Lin H, Sacks MS. Differences in tissue-remodeling potential of aortic and pulmonary heart valve interstitial cells. Tissue Eng. 2007;13:2281–2289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.