Abstract
Trimethylamine-N-oxide (TMAO) is a recently identified predictor of cardiovascular and chronic kidney disease. TMAO is primarily generated through gut-microbiome mediated conversion of dietary choline and carnitine to TMA, which is converted to TMAO by hepatic flavin monooxygenase 3 (FMO3) and subsequently undergoes renal elimination. We investigated the role of uptake and efflux drug transporters in TMAO disposition in vitro and in vivo. After screening a large array of uptake transporters, we show organic cation transporter 2 (OCT2) is the key transporter for TMAO cellular uptake. In Oct1/2 knockout mice, we observed increased plasma TMAO levels with reduced renal retention, suggesting the importance of Oct2 in facilitating the uptake of TMAO into renal tubular cells in vivo. Multiple transporters of the ATP-binding cassette (ABC) family, including ABCG2 (BCRP) and ABCB1 (MDR1), were capable of TMAO efflux. In human subjects, clinical, dietary, and pharmacogenetic covariates were evaluated for contribution to TMAO levels in a cohort of dyslipidemic patients (n = 405). Interestingly, genetic variation in ABCG2, but not other transporters, appeared to play a role in modulating TMAO exposure.
Keywords: transporters, TMAO, OCT2, BCRP
Graphical Abstract

INTRODUCTION
The burden of cardiovascular disease (CVD) in Western societies remains significant and steadfast despite the increasing number of modifiable and treatable risk factors, including dyslipidemia, hypertension, type 2 diabetes, and diet. Increased systemic exposure of cholesterol contributes to the well-established development of atherosclerosis, thus placing patients at risk for CVD.1 While the use of pharmacological inhibitors of cholesterol synthesis, primarily statins, has become a common preventative therapy, residual risk of adverse cardiovascular events remains, even with the optimal use of such agents.2,3 Interestingly, a recent unbiased metabolomics screen revealed the association of trimethylamine-N-oxide (TMAO) as a significant independent predictor of CVD risk, likely through promotion of atherogenesis.4 Further, elevated circulating TMAO levels were associated with higher risk of adverse cardiac events including myocardial infarction, stroke, and death.5
Although TMAO can be ingested directly by eating fish, the majority of TMAO is primarily formed through a two-step, multiorganism metabolism pathway that is dependent on the gut microbiome and the host liver.6 Dietary choline and l-carnitine within lipid-rich foods such as eggs, high fat dairy, and red meat are efficiently catabolized by intestinal bacteria to an intermediate precursor trimethylamine (TMA), which is further metabolized to TMAO in the liver.4,7 Hepatic oxidation of TMA to TMAO is dependent on the flavin monooxygenase (FMO) family, of which FMO3 has the greatest role.8–10 The importance of FMO3 to TMAO metabolism is demonstrated by a rare genetic condition known as trimethylaminuria, or fish-(mal)odor syndrome, whereby patients harboring null FMO3 variants are unable to convert the odorous gas, TMA, to TMAO.9–11 Treatment of these patients often involves limiting dietary choline intake suggesting diet as a key contributor of TMAO formation.11
Mechanistically, TMAO appears to be more than a pro-atherosclerotic biomarker of disease and acts to inhibit reverse cholesterol transport (RCT) and upregulate macrophage scavenger receptors leading to foam cell formation.4,7 TMAO has further been shown to modulate proteins involved in bile acid synthesis and transport as well as sterol metabolism suggesting its impact on CVD pathogenesis may be multi-factorial.7,12 In addition to its association with CVD risk, circulating TMAO levels have been identified as a prognostic marker of mortality in chronic kidney disease (CKD) patients due to renal fibrosis and dysfunction.13 Further, we recently demonstrated that elevated TMAO level is an independent risk factor for ischemic CV events in a large cohort (n = 2529) of CKD patients.14
TMAO, a low molecular weight osmolyte, primarily undergoes renal elimination.15–17 Because clearance of TMAO is greater than what can be accounted for by glomerular filtration, its elimination has long been thought to involve active secretion by drug transporters.13,18,19 Additionally, TMAO exists as a zwitterion at physiologic pH further suggesting it requires transport to cross biological membranes.20 However, the identification or the role of drug transporters in TMAO disposition is currently unknown. Transporters are now widely appreciated as a major determinant of interindividual variability of circulating drug levels through both genetic and nongenetic mechanisms (reviewed in refs 21 and 22). Therefore, identification of transporters for TMAO may provide additional molecular and mechanistic explanation of interindividual TMAO variation and may represent a new pharmacologic target for reducing TMAO-associated CVD risk. Here, we carried out an extensive molecular transporter screening as well as an in vivo assessment in mice to characterize the transporters of relevance to TMAO disposition, as well as defined the association between genetic variation in genes governing TMAO transport and metabolism pathways and TMAO levels in a cohort of dyslipidemic patients.
MATERIALS AND METHODS
In vitro TMAO Transporter Screening.
Radiolabeled [3H] trimethylamine-N-oxide (80 Ci/mmol) was custom synthesized by American Radiolabeled Chemicals, Inc. (Saint Louis, MO, USA). Unlabeled trimethylamine-N-oxide was obtained from Sigma-Aldrich (Oakville, Ontario, Canada). Transporter plasmid construction packaged into the pEF6/V5-His-TOPO vector has been previously described.23–25 OCT2 808T was introduced to the reference OCT2-pEF plasmid using the Statagene site-directed mutagenesis kit (primers: forward, TGGTTGCAGTTCACAGTTtCTCTGCCCAA-CTTCTTCTTC; reverse, GAAGAAGAAGTTGGGC-AGAGaAACTGTGAACTGCAACCA). Transient expression of transporters was performed as described previously using a recombinant vaccinia-based transfection system in HeLa cells.23,26,27 Screening of MATE1 transporter was performed as previously described in the uptake transport mode (pH8.0).28 Radiolabeled [3H]-TMAO (~3 × 105 dpm/well) in the absence of unlabeled TMAO and in the presence of 5 μM unlabeled TMAO was used for uptake and efflux transporter screening experiments, respectively. Cellular uptake of radio-labeled TMAO was measured in cell lysates using a liquid scintillation counter (PerKinElmer, Shelton, CT, USA). Transport activity was expressed in percent compared to vector control (parental plasmid lacking insert). Experiments were performed in duplicate on a minimum of 2–3 experimental days.
OCT2 Transport Kinetics of TMAO.
To measure OCT2 transport kinetics of TMAO, [H3] TMAO uptake during the linear phase (first 30 s) was determined in the presence of increasing concentration of unlabeled TMAO (0.5 μM–200 mM). [H3] TMAO uptake in cells transfected with parental plasmid was subtracted from uptake by OCT2-pEF transfected cells. Maximal uptake rate (Vmax) and concentration needed to achieve half-maximal uptake (Km) were estimated using Michaelis–Menten nonlinear curve-fitting.
Transporter Knockout Mice Study.
Wildtype FVB, Oct1/2−/− and Mdr1a/b−/−-Bcrp−/− male mice were purchased from Taconic (Hudson, NY, USA), n = 10–14 in each group. Mice were housed at Western University under a 12 h-on/12 h-off light cycle and fed a standard mouse diet available ad libitum. Wildtype and KO mice (8–10 weeks old) were sacrificed under isofluorane at the same time of day (morning) to account for nocturnal food intake. Blood was collected and centrifuged (14,000 rpm, 10 min, 4 °C), and plasma was aliquoted and stored at −80 °C until analysis. Liver and kidneys were extracted and flash frozen in liquid nitrogen and stored at −80 °C until analysis. All study procedures were approved by the Animal Care and Use Committee at the Western University.
Study Population.
The study population included 405 previously recruited outpatients from the London Health Sciences Center Lipid Clinic from August 2009 to May 2011.29 All subjects provided written informed consent. Each subject was on daily atorvastatin or rosuvastatin therapy at the time of enrollment and provided one blood sample as previously described.29 Data collected included sex, age, ethnicity, and medication use. Creatinine clearance was determined with the modification of diet in renal disease (MDRD) equation using plasma creatinine concentrations measured from the provided blood sample. The study protocol was approved by the Research Ethics Board at Western University.
Genotyping.
Genomic DNA was isolated from blood samples as previously described.29 Genotypes were determined by allelic discrimination TaqMan assays (Applied Biosystems, Carlsbad, CA, USA). The following genotypes were determined: FMO3 E158K (g.21949G>A, rs2266782); FMO3 E308G (g.28225A>G, rs2266780); SLC22A2 c.808G>T (rs316019), ABCB1 c.3435C>T (rs1045642), ABCC2 c.1249G>A (rs2273697), and ABCG2 c.421C>A (rs2231142).
Quantitation of TMAO, Choline, Carnitine, and Creatinine in Human and Mouse Samples.
Plasma and tissue concentrations of TMAO, choline, carnitine, and creatinine were measured simultaneously by ultra performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) using a modified protocol.30 Two mass transitions were monitored for each analyte to ensure lack of interference from other endogenous biomarkers. Liver and kidney samples were thawed on ice then homogenized 1:1 and 1:2, respectively, in water. For plasma and tissue homogenate samples (50 μL), proteins were precipitated by addition of 150 μL of acetonitrile containing internal standard (TMAO-d9, choline-d9, and creatinine-d3). Standards made in water were diluted similarly with internal standards in acetonitrile (all standards were obtained from Toronto Research Chemicals, Toronto, Ontario, Canada). Samples and standards were centrifuged for 30 min at 14,000 rpm, and 150 μL of supernatant was used for analysis. Mobile phases included 95:5 and 5:95 water/acetonitrile both containing 0.05% formic acid and 5 mM ammonium formate. Chromatographic separation was performed using an Agilent Technologies 1290 Infinity LC injector HTS and a HILIC Plus silica column (Agilent Technologies, Mississuaga, Ontario, Canada) with an injection volume of 20 μL, and a gradient over 6 min: 0.5 min of 90:10, from 90:10 to 30:70 over 4.5 min, back to 90:10 over 1 min. The mass spectrometer (Thermo Finnigan TSQ Quantum Ultra) with heated electrospray ionization source operated in positive mode, with a quantitative and qualitative mass transition measured per analyte: TMAO (76 → 58, 76 → 59), choline (104 → 45, 104 → 60), and creatinine (114 → 44, 114 → 86). Values for each analyte were accepted if the concentrations quantitated for the qualitative mass transition were on average within 20% of that quantitated with the quantitative mass transition. Recovery of each analyte was 90–110% in all matrices. The lowest concentration quantitated for each analyte are as follows: TMAO (0.05 μg/mL), choline(0.01 μg/mL), and creatinine (0.05 μg/mL). The coefficient of variation (%) for quality controls were as follows: low QC (TMAO, 5.0%; choline, 6.9%; creatinine, 14.0%), medium QC (TMAO, 4.8%; choline, 7.0%; creatinine, 6.4%), and high QC (TMAO, 7.8%; choline, 1.5%; creatinine, 4.1%).
Statistical Analysis.
Data was analyzed using GraphPad Prism and the statistical software R. Transporter screening data was analyzed using one-way ANOVA with Dunnett’s multiple comparison test or Student’s unpaired t test. TMAO levels measured in plasma and tissue from mice were analyzed using one-way ANOVA with Bonferroni’s multiple comparison test. Plasma TMAO levels stratified by genotype were compared using Kruskal–Wallis with Dunn’s multiple comparison test or Mann–Whitney U-test. Spearman correlations were used to determine relationships between TMAO plasma concentrations and clinical variables. Multiple linear regression analysis was performed to determine significant covariates on the inter-individual variability of log-transformed TAMO levels in dyslipidemic patients. Covariates considered included: age, sex, body mass index (BMI), choline level, carnitine concentration, CrCl, FMO3, OCT2, ABCB1, ABCC2, and ABCG2 genotype. Covariates were assessed individually and were considered for the final model at a significance level of p < 0.2. Covariates meeting the criteria were entered into a multiple linear regression model adjusting for age and sex and remained in the final model if p ≤ 0.1.
RESULTS
TMAO Is a Substrate for Organic Cation Transporter 2 and for Multiple Efflux Transporters.
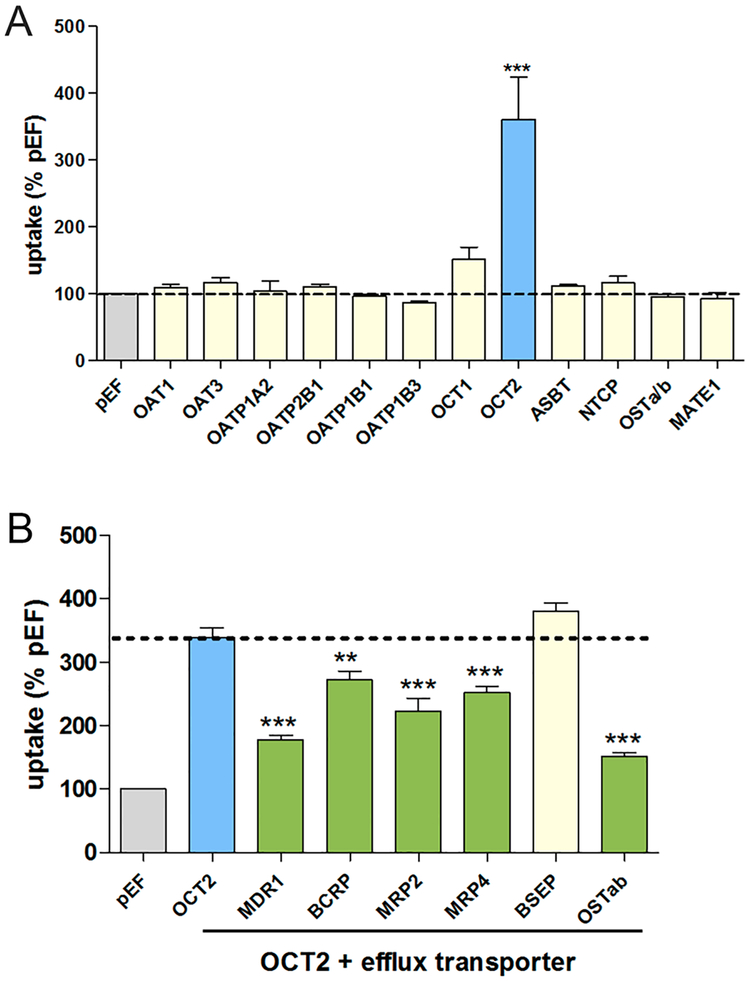
To identify TMAO drug transporters, we individually expressed a panel of membrane uptake transporter expression plasmids in HeLa cells using a heterologous gene expression system. Radiolabeled TMAO was measured in cell lysates following 10 min incubation. TMAO uptake was significantly greater in cells expressing the organic cation transporter 2 (OCT2) (Figure 1A). Additional uptake transporters including organic anion transporters (OATs), organic anion transporting polypeptides (OATPs), multidrug and toxic compound extrusion transporter (MATE), and bile acid transporters were screened and did not exhibit TMAO uptake (Figure 1A).
Figure 1.
Uptake and efflux transport of TMAO. A panel of uptake membrane transporters was screened for [H3] TMAO transport activity above vector control at 10 min in the absence of unlabeled TMAO (A). OCT2 was the only transporter assessed capable of transporting TMAO in vitro. A panel of efflux membrane transporters was screened for TMAO transport activity in the presence of OCT2 expression, and transport was determined by a significant decrease in [H3] TMAO OCT2-mediated uptake (B). Efflux experiments were performed in the presence of 5 μM unlabeled TMAO. Data are expressed as mean ± SE **, p < 0.01; ***, p < 0.001.
Screening of TMAO transport by efflux transporters was evaluated using HeLa cells that were dually transfected with OCT2 and efflux transporter expression plasmids. Compared with cells transfected with OCT2 alone, uptake was significantly lower in cells also expressing MDR1(ABCB1), BCRP (ABCG2), MRP2 (ABCC2), MRP4 (ABCC4), and OSTαβ (Figure 1B), indicating TMAO to be a substrate of multiple efflux transporters. TMAO transport was not observed in cells expressing the bile salt export pump (BSEP).
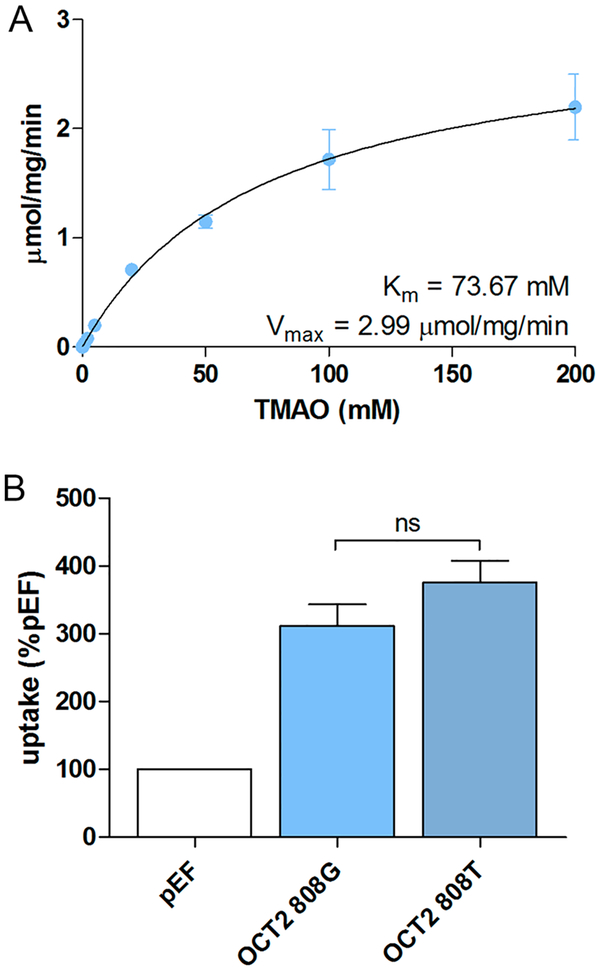
To further characterize TMAO transport, kinetic and genetic analysis of OCT2-mediated TMAO uptake was evaluated. Pharmacokinetic parameters were assessed by measuring radiolabeled TMAO uptake by OCT2-transfected cells in the presence of increasing concentrations (0.5 μM–200 mM) of unlabeled TMAO. Nonlinear curve fitting indicated this to be a low-affinity, high-capacity system of TMAO transport (Km 73.67 mM, Vmax 2.99 μmol/mg/min) (Figure 2A). We evaluated the effect of a common single nucleotide polymorphism (SNP) in the gene encoding OCT2 (SLC22A2 c.808G>T, rs316019), which has been shown to confer reduced metformin renal clearance.31,32 Here, we did not observe differences in [H3] TMAO uptake between cells expressing the reference allele (OCT2 808G) and those expressing the reduced function allele (OCT2 808T) (Figure 2B).
Figure 2.
OCT2 kinetics of TMAO. TMAO kinetics was assessed for OCT2-mediated transport and Michaelis–Menten-type nonlinear curve-fitting performed (A). OCT2-mediated uptake transport of TMAO was compared between OCT2 c.808G and T alleles (B).
Knockout of Oct1/2 Increases TMAO Plasma Concentrations.
The contribution of transporters to TMAO disposition in vivo was examined using knockout (KO) mice. Plasma TMAO levels were increased approximately 2-fold in Oct1/2 KO mice compared to wildtype mice (Figure 3A). As Oct1 is primarily expressed in the liver with limited expression in the kidney and Oct2 is predominantly renally expressed we determined the tissue to plasma TMAO ratio for these organs.21 We observed no difference in the liver to plasma TMAO ratio in Oct1/2 KO mice compared to wildtype (Figure 3B). However, a 2-fold decrease in the kidney to plasma TMAO ratio was observed in Oct1/2 KO mice (Figure 2C), suggesting that Oct2 plays a role in the renal clearance of TMAO. To assess the relevance of MDR1 and BCRP to TMAO disposition, triple KO mice lacking Mdr1a, Mdr1b, and Bcrp were evaluated. No difference was detected in TMAO plasma levels or liver and kidney partitioning in MDR1a/1bBcrp KO mice compared with wildtype mice (Figure 3).
Figure 3.
TMAO disposition in transporter knockout mice. TMAO levels were measured in plasma, liver, and kidney from wildtype (FVB), Oct1/2 KO, and Mdr1a/1b/Bcrp KO mice. TMAO levels are significantly increased in Oct1/2 KO mice (A). Tissue to plasma ratios were calculated for liver (B) and kidney (C) showing a decreased kidney to plasma ratio of TMAO in Oct1/2 KO mice compared to wildtype. *p < 0.05, **p < 0.01.
Transporter Polymorphisms Have Minimal Effect on TMAO Level.
To assess the contribution of transporters to variation in TMAO exposure we analyzed a cohort of dyslipidemic patients (n = 405) currently managed on statin therapy that has been previously described.29 We observed a nearly 2000-fold range in TMAO plasma concentrations upon random sampling in this cohort. The transporters identified as capable of TMAO uptake and efflux, with known polymorphisms, were correlated with TMAO levels. Mean plasma levels stratified by genotype for each transporter are presented in Table 1. In concordance with our in vitro data, univariate analysis showed no effect of SLC22A2 c.808T, encoding OCT2 on TMAO plasma concentrations (Table 1). Similarly, no effect was observed for ABCB1 c.3435T and ABCC2 c.1249A. An increased trend of TMAO levels was observed in ABCG2 c.421A allele carriers (p = 0.116, Table 1). Together, this data suggests that transporter polymorphisms likely do not play a major role in TMAO disposition.
Table 1.
Association of Genetic Variation in Transporters with Plasma TMAO Concentration
| genotype | no. of patients | TMAO, μM mean (SD) | pa |
|---|---|---|---|
| OCT2 (SLC22A2) c.808G>T | |||
| G/G | 337 | 5.74 (5.68) | 0.582 |
| G/T; T/T | 70 | 5.77 (5.89) | |
| ABCB1 c.3435C>T | |||
| C/C | 76 | 5.97 (5.79) | 0.854 |
| C/T | 226 | 5.62 (5.93) | |
| T/T | 105 | 5.86 (5.20) | |
| ABCC2 c.1249G>A | |||
| G/G | 248 | 5.87 (6.03) | 0.216 |
| G/A | 138 | 5.71 (5.24) | |
| A/A | 21 | 4.54 (4.86) | |
| ABCG2 c.421C>A | |||
| C/C | 332 | 5.45 (4.80) | 0.116 |
| C/A; A/A | 74 | 7.15 (8.62) | |
p value calculated using Mann–Whitney U test or Kruskal–Wallis test with Dunn’s Multiple Comparison test as appropriate.
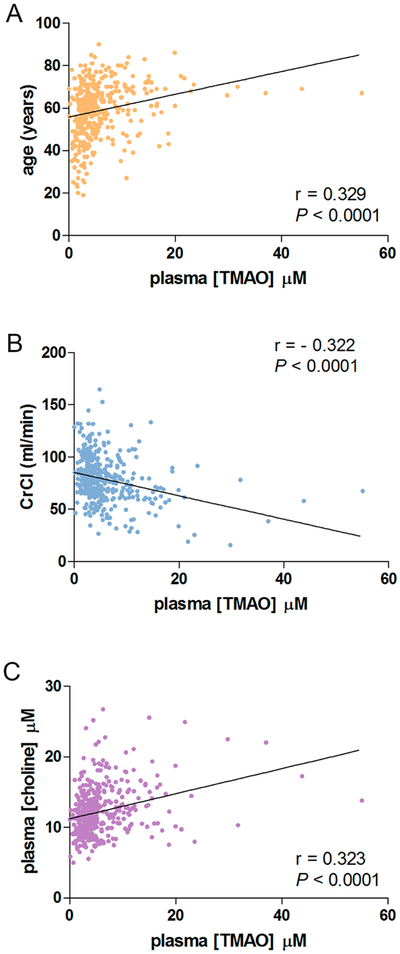
We further examined additional genetic and nongenetic factors that have been suggested to contribute to TMAO formation or elimination. Patient age was significantly correlated with increased TMAO levels (r = 0.329, p < 0.0001, Figure 4A), while no effect was observed for sex (Table 2). An increase in TMAO levels was marginally correlated with BMI (p = 0.05, Table 2). We observed a significant negative correlation between creatinine clearance (CrCl) and TMAO levels (r = −0.322, p < 0.0001, Figure 4B), which is not unexpected as TMAO is renally excreted. Dietary factors, including choline and carnitine, which are converted within the gut to the TMAO precursor TMA,4,7,33 were evaluated. Plasma choline levels were significantly associated with increased TMAO level (r = 0.323, p < 0.0001, Figure 4C), while no effect was observed for carnitine (Table 2). Two common SNPs associated with decreased FMO3 enzyme activity were examined. A decreased trend in TMAO was seen in FMO3 E158K A allele carriers (p = 0.07), while no association was observed for FMO3 E308G (Table 2).
Figure 4.
Age, kidney function, and diet influence circulating TMAO levels. Spearman correlations were evaluated and determined TMAO levels were positively correlated with age (A) and plasma choline levels (C). Plasma levels of TMAO were inversely correlated with creatinine clearance (B).
Table 2.
Univariate Analysis for Effect on Log-Transformed TMAO Concentrationa
| predictor variable | estimate | standard error | p |
|---|---|---|---|
| age | 0.009 | 0.001 | <0.001 |
| sex (female) | −0.012 | 0.037 | 0.750 |
| BMI | 0.006 | 0.003 | 0.050 |
| choline concentration | 1.021 | 0.144 | <0.001 |
| carnitine concentration | 0.087 | 0.094 | 0.352 |
| CrCl | −0.911 | 0.128 | <0.001 |
| FMO3 E158K (G/A, A/A) | −0.072 | 0.039 | 0.070 |
| FMO3 E308G (A/G, G/G) | 0.005 | 0.387 | 0.901 |
BMI, body mass index; CrCl, creatinine clearance.
Multiple linear regression modeling for effect of covariates on log-transformed TMAO levels was performed (Table 3). The covariates considered in our model accounted for only 18.5% of the variability in plasma TMAO levels (adjusted R squared: 0.185). Demographic variables including age, sex, and BMI explained 10% of the variation in TMAO levels. Of the individual covariates considered, CrCl explained the largest percentage (5.6%) of variation while choline concentration accounted for 2% of the variability. ABCG2 c.421C>A and FMO3 E158K had minimal effect on TMAO concentration explaining only 1% of the total observed variation (Table 3). Together, this data suggests that genetic variation in transporters has minimal effect on plasma concentrations of TMAO in a dyslipidemic patient population.
Table 3.
Multiple Linear Regression Model for Effect on Log-Transformed TMAO Concentration (Adjusted R Squared: 0.185)a
| predictor variable | estimate | standard error | p | Adjusted r2 |
|---|---|---|---|---|
| intercept | 0.570 | 0.397 | 0.152 | |
| age | 0.006 | 0.001 | <0.001 | |
| sex (female) | −0.018 | 0.036 | 0.615 | |
| BMI | 0.005 | 0.003 | 0.070 | 0.100 |
| CrCl | −0.512 | 0.145 | <0.001 | 0.156 |
| choline concentration | 0.524 | 0.166 | <0.010 | 0.176 |
| ABCG2 (C/A, A/A) | 0.083 | 0.043 | 0.051 | 0.181 |
| FMO3 E158K (G/A, A/A) | −0.059 | 0.036 | 0.104 | 0.185 |
BMI, body mass index; CrCl, creatinine clearance.
DISCUSSION
Although circulating TMAO has been identified as a risk factor for CVD, the molecular mechanisms that can adequately account for the large observed interindividual variation are not completely defined. Dietary factors including choline and carnitine and the gut microbiota have been implicated as contributory to variation in TMAO levels.4,5,7 More recently, the importance of renal elimination as a factor affecting TMAO levels has come to light as increased concentrations of TMAO were observed in patients with end-stage renal disease or CKD.13,16 Additionally, TMAO level was identified as an independent risk factor for ischemic CV events in patients with CKD suggesting the need to determine modifiable or targetable determinants of TMAO levels.14 While TMAO can undergo glomerular filtration, evidence of decreased TMAO renal clearance in conjunction with elevated systemic exposure upon increasing doses of l-carnitine suggests saturation of an active secretion process.18,34 As such, we investigated the role of transporters to TMAO disposition providing the first report of active TMAO uptake and efflux.
We identified TMAO as a substrate of the organic cation transporter OCT2. OCT2 is highly expressed on the basolateral membrane of renal tubule cells.21 Kinetic assessment of OCT2-mediated TMAO uptake suggest that OCT2 is a high capacity transport system for TMAO given the remarkably high Km value (Figure 2). However, even at the lowest tested substrate concentration (0.5 μM), overall uptake by OCT2 was far greater than other uptake transporters (Figure 1). Therefore, it is likely that OCT2 is physiologically relevant to TMAO renal tubular uptake across all concentrations observed in humans (Figure 4). In vivo confirmation of such a role is supported by our data generated using Oct1/2 deficient mice, where we see increased plasma TMAO levels and decreased kidney/plasma TMAO ratio in the knockout mice. Although TMAO synthesis via the FMO3 pathway is known to be reduced in adult male mice, the goal of our study was to demonstrate the effect of OCT2 transporter deficiency to TMAO renal clearance regardless of absolute circulating amount of TMAO.8 Indeed, given the high capacity nature of TMAO transport by OCT2, we believe the kidney/plasma TMAO ratio would remain similar even with higher FMO3 activity or dietary intake of TMAO.
In human subjects, decreased OCT2 activity due to presence of SCL22A2 c.808T variant allele has been previously associated with reduced renal clearance of metformin in certain ethnicities, but its clinical relevance remains controversial as it may be substrate and/or race-dependent.31,32,35 We evaluated this common variant but failed to observe an association with decreased transport in vitro (Figure 2B) or an effect on plasma TMAO levels in vivo in our patient cohort (Table 1). While pharmacogenomics may not play a large role in OCT2-mediated TMAO transport other mechanisms including post-transcriptional regulation and/or drug interactions may be of greater relevance and provide novel targets for pharmacotherapy. As renal elimination of TMAO is the primary route of excretion, we were not surprised to observe a significant inverse association between CrCl and TMAO levels suggesting that TMAO clearance likely involves both filtration and active secretion processes. The involvement of transport processes, which can be modulated in the presence of exogenous drugs particularly in the aging population where polypharmacy is commonplace, may contribute to increased TMAO levels and CVD risk. In fact, prolonged exposure to higher TMAO levels resulting from a choline- or TMAO-rich diet has recently been shown to lead to progressive renal fibrosis and dysfunction in animal models.13
We determined that TMAO appears to be a substrate of several efflux transporters, many of which are expressed in the liver. As the liver is the primary site of conversion of TMA to TMAO it is not surprising that TMAO may use multiple transporters for hepatic clearance. In mice we observed a low TMAO liver to plasma ratio suggesting hepatic efflux to be an efficient process. In vivo, circulating levels of TMAO may fluctuate directly as a result of basolateral efflux via MRP4 or indirectly through biliary excretion, involving MDR1, MRP2, and BCRP, expressed on the canalicular membrane, and subsequent intestinal reabsorption.21 Using MDR1a/1bBcrp KO mice we observed no difference in TMAO liver partitioning compared to wildtype mice, suggesting the redundant function of biliary efflux transporters or alternatively indicating basolateral efflux to be the primary route of hepatic exit. Consistent with this, the amount of TMAO excreted into the bile was negligible (0.18%) in rats following intravenous dosing of TMAO.36 However, TMAO can be detected in human bile along with betaine, choline, and carnitine,37 suggesting species specific differences in TMAO biliary efflux.
Many efflux transporters screened are also expressed on the brush border membrane of the kidney.21 Therefore, it is likely that a combined role of various efflux transporters may account for the efflux transport of TMAO out of renal tubular cells. No difference in kidney/plasma ratio was observed in MDR1a/1bBcrp KO mice compared to wildtype mice, suggesting other efflux transporters can compensate for their absence in mice. Indeed, in addition to ABCB1 (MDR1) and ABCG2 (BCRP), other ABC transporters such as ABCC2 (MRP2) and ABCC4 (MRP4) are also known to be expressed on the apical cell membrane domain of renal tubular cells.21
Interestingly, while absence of Bcrp (Abcg2) did not affect TMAO disposition in mice, we observed a trend toward increased plasma concentrations in human subjects harboring the ABCG2 c.421A allele, which approached significance (p = 0.051) in our multivariate analysis. In fact, the mean TMAO concentration for ABCG2 variant carriers (7.15 μM) was above the highest quartile level (>6.18 μM) that was documented to be associated with significant risk for myocardial infarction, stroke, and death.5 As ABCG2 is highly expressed on the apical domain of multiple organs, including the intestine, liver, and kidney, reduced ABCG2 expression or activity would be predicted to result in higher circulating TMAO level.
It is becoming increasingly evident that TMAO, cholesterol, and bile acid metabolism and transport are complex and likely involve multiple levels of regulation.12 FMO3 is responsible for the hepatic oxidation of TMA to TMAO, and null activity of this enzyme results in the medical condition trimethylaminuria.8,11,38 However, a lack of genetic association between FMO3 and TMAO levels previously and here suggests that FMO3 activity is not likely a driving factor in observed TMAO variation.39 Mechanistically, TMAO has been shown to inhibit RCT and upregulate scavenger receptors on macrophages leading to inflammation and foam cell formation modulating bile acid and sterol metabolism.4,7 However, FMO3 was also recently identified as an integral factor in regulating cholesterol balance by impacting lipid metabolism and inflammation in a manner distinct from TMAO.40 Decreased drug transporter mRNA expression, including MRP2 and other bile acid transporters was also noted in mice supplemented with a TMAO diet.7 The pathways leading to dysregulation of bile acid synthesis and sterol metabolism directed by TMAO, FMO3, or both likely involve the nuclear hormone receptors, farnesoid X receptor (FXR) and liver X receptor (LXR), leading to the transcriptional regulation of drug metabolizing enzymes, transporters, and pro-inflammatory cytokines.8,40–42 Further work is needed to better understand the complex and likely interweaving pathways leading to variation in TMAO levels and its subsequent clinical manifestation as increased CVD risk.
Evaluation of clinical, dietary, and genetic factors, including FMO3 and drug transporter polymorphisms, explained a relatively small portion (18.5%) of the observed variability in TMAO levels. Together, patient age and kidney function as measured by CrCl explained the majority of the variation in the model (15.6%) following adjustment for sex and BMI. The contribution of dietary choline accounted for 2% of the explained variation, while the effect of l-carnitine was negligible. As blood sampling was random and not in fasted patients, it is possible the effect of diet on TMAO levels is underestimated in this study. Additionally, FMO3 and genetic variation in drug transporters appear to have a relatively small effect on the variation in TMAO exposure. Previous studies in mice and man came to similar conclusions following genome-wide association studies.39 However, given that patients with CVD risk factors are typically older and on several medications, the potential for drug–drug interactions at the level of transporters may add an additional level of complexity to understanding variation in TMAO level. The effect of concomitant use of drugs inhibiting OCT2 and/or efflux transporters on TMAO plasma concentration was not accounted for in this study and should be examined in future studies.
We note that the gut microbiome was not assessed in this study, whether in mice or in human subjects, thus limiting any interpretation of its effect here on TMAO exposure. It has been well established that TMAO levels are largely dependent on the production of its precursor TMA by gut bacteria.4,6,7 Variation in the bacterial phylum within the intestinal tract, which can be modified acutely and chronically by diet (vegetarian, vegan, or omnivore) and during disease states likely has the greatest effect on circulating TMAO levels. We recently identified TMAO as a potential biomarker of inflammatory bowel disease (IBD)43 as IBD patients had significantly lower TMAO levels compared to age and sex matched healthy controls. More specifically, patients experiencing active ulcerative colitis (UC) had lower TMAO levels compared to inactive UC, while no difference was noted between active vs inactive Crohn’s disease.43 These results further support the importance of the gut microbiota and suggest that the colon, which has the largest amount of bacteria, may be the prominent site of TMA formation providing a therapeutic target. In fact, pharmacological inhibition of TMA formation through the use a choline structural analogue was recently shown to decrease TMAO levels and inhibit the development of atherosclerotic lesions in mice, suggesting a potential new strategy for reducing CV risk.44
CONCLUSIONS
We identified OCT2 as the key uptake transporter for TMAO. In addition, it would appear a number of efflux transporters are also capable of TMAO transport. In humans, genetic variation of transporters did not appear to contribute significantly, perhaps with the exception of ABCG2, to the observed variation in circulating TMAO levels likely due to redundancy in TMAO transport. Since elevated TMAO level is now widely accepted as an independent risk factor for cardiovascular disease/events, TMAO transporters should be considered as a new drug target for reducing TMAO levels in humans.
ACKNOWLEDGMENTS
R.B.K. is supported by the Wolfe Medical Research Chair in Pharmacogenomics and by grants from the Canadian Institutes of Health Research (MOP-89753) and the Drug Safety and Effectiveness Network (DSEN-PREVENT, FRN-117588) A.W. is supported by the Clinician-Investigator Program at Schulich School of Medicine & Dentistry at Western, and the Canadian Institutes of Health Research/Crohn’s Colitis Canada/Canadian Association of Gastroenterology Joint Research Fellowship (inflammatory bowel disease priority area) (201411IBD). R.H.H. is supported by a grant from the National Institutes of Health (R01 GM099924).
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Steinberg D Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: part II: the early evidence linking hypercholesterolemia to coronary disease in humans. J. Lipid Res. 2005, 46 (2), 179–90. [DOI] [PubMed] [Google Scholar]
- (2).Pisaniello AD; Scherer DJ; Kataoka Y; Nicholls SJ Ongoing challenges for pharmacotherapy for dyslipidemia. Expert Opin. Pharmacother 2015, 16 (3), 347–56. [DOI] [PubMed] [Google Scholar]
- (3).Ladeiras-Lopes R; Agewall S; Tawakol A; Staels B; Stein E; Mentz RJ; Leite-Moreira A; Zannad F; Koenig W Atherosclerosis: Recent trials, new targets and future directions. Int. J. Cardiol 2015, 192, 72–81. [DOI] [PubMed] [Google Scholar]
- (4).Wang Z; Klipfell E; Bennett BJ; Koeth R; Levison BS; Dugar B; Feldstein AE; Britt EB; Fu X; Chung YM; Wu Y; Schauer P; Smith JD; Allayee H; Tang WH; DiDonato JA; Lusis AJ; Hazen SL Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472 (7341), 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Tang WH; Wang Z; Levison BS; Koeth RA; Britt EB; Fu X; Wu Y; Hazen SL Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med 2013, 368 (17), 1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Brown JM; Hazen SL Metaorganismal nutrient metabolism as a basis of cardiovascular disease. Curr. Opin. Lipidol 2014, 25 (1), 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Koeth RA; Wang Z; Levison BS; Buffa JA; Org E; Sheehy BT; Britt EB; Fu X; Wu Y; Li L; Smith JD; DiDonato JA; Chen J; Li H; Wu GD; Lewis JD; Warrier M; Brown JM; Krauss RM; Tang WH; Bushman FD; Lusis AJ; Hazen SL Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med 2013, 19 (5), 576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Bennett BJ; de Aguiar Vallim TQ; Wang Z; Shih DM; Meng Y; Gregory J; Allayee H; Lee R; Graham M; Crooke R; Edwards PA; Hazen SL; Lusis AJ Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 2013, 17 (1), 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Dolphin CT; Janmohamed A; Smith RL; Shephard EA; Phillips IR Missense mutation in flavin-containing mono-oxygenase 3 gene, FMO3, underlies fish-odour syndrome. Nat. Genet 1997, 17 (4), 491–4. [DOI] [PubMed] [Google Scholar]
- (10).Treacy EP; Akerman BR; Chow LM; Youil R; Bibeau C; Lin J; Bruce AG; Knight M; Danks DM; Cashman JR; Forrest SM Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication. Hum. Mol. Genet 1998, 7 (5), 839–45. [DOI] [PubMed] [Google Scholar]
- (11).Messenger J; Clark S; Massick S; Bechtel M A review of trimethylaminuria: (fish odor syndrome). J. Clin. Aesthet. Dermatol 2013, 6 (11), 45–8. [PMC free article] [PubMed] [Google Scholar]
- (12).Wilson A; McLean C; Kim RB Trimethylamine-N-oxide: a link between the gut microbiome, bile acid metabolism, and atherosclerosis. Curr. Opin. Lipidol 2016, 27 (2), 148–154. [DOI] [PubMed] [Google Scholar]
- (13).Tang WH; Wang Z; Kennedy DJ; Wu Y; Buffa JA; Agatisa-Boyle B; Li XS; Levison BS; Hazen SL Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res 2015, 116 (3), 448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kim RB; Morse BL; Djurdjev O; Tang M; Muirhead N; Barrett B; Holmes DT; Madore F; Clase CM; Rigatto C; Levin A Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016, 89 (5), 1144–52. [DOI] [PubMed] [Google Scholar]
- (15).Al-Waiz M; Mitchell SC; Idle JR; Smith RL The metabolism of 14C-labelled trimethylamine and its N-oxide in man. Xenobiotica 1987, 17 (5), 551–8. [DOI] [PubMed] [Google Scholar]
- (16).Bain MA; Faull R; Fornasini G; Milne RW; Evans AM Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol., Dial., Transplant 2006, 21 (5), 1300–4. [DOI] [PubMed] [Google Scholar]
- (17).Bell JD; Lee JA; Lee HA; Sadler PJ; Wilkie DR; Woodham RH Nuclear magnetic resonance studies of blood plasma and urine from subjects with chronic renal failure: identification of trimethylamine-N-oxide. Biochim. Biophys. Acta, Mol. Basis Dis 1991, 1096 (2), 101–7. [DOI] [PubMed] [Google Scholar]
- (18).Smith JL; Wishnok JS; Deen WM Metabolism and excretion of methylamines in rats. Toxicol. Appl. Pharmacol 1994, 125 (2), 296–308. [DOI] [PubMed] [Google Scholar]
- (19).Forster RP; Berglund F; Rennick BR Tubular secretion of creatine, trimethylamine oxide, and other organic bases by the aglomerular kidney of Lophius americanus. J. Gen. Physiol 1958, 42 (2), 319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Singh R; Haque I; Ahmad F Counteracting osmolyte trimethylamine N-oxide destabilizes proteins at pH below its pKa. Measurements of thermodynamic parameters of proteins in the presence and absence of trimethylamine N-oxide. J. Biol. Chem 2005, 280 (12), 11035–42. [DOI] [PubMed] [Google Scholar]
- (21).DeGorter MK; Xia CQ; Yang JJ; Kim RB Drug transporters in drug efficacy and toxicity. Annu. Rev. Pharmacol. Toxicol 2012, 52, 249–73. [DOI] [PubMed] [Google Scholar]
- (22).Gong IY; Kim RB Impact of genetic variation in OATP transporters to drug disposition and response. Drug Metab. Pharmacokinet 2013, 28 (1), 4–18. [DOI] [PubMed] [Google Scholar]
- (23).Ho RH; Tirona RG; Leake BF; Glaeser H; Lee W; Lemke CJ; Wang Y; Kim RB Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology 2006, 130 (6), 1793–806. [DOI] [PubMed] [Google Scholar]
- (24).Tirona RG; Leake BF; Merino G; Kim RB Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J. Biol. Chem 2001, 276 (38), 35669–75. [DOI] [PubMed] [Google Scholar]
- (25).Lee HH; Leake BF; Teft W; Tirona RG; Kim RB; Ho RH Contribution of hepatic organic anion-transporting polypeptides to docetaxel uptake and clearance. Mol. Cancer Ther. 2015, 14 (4), 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Cvetkovic M; Leake B; Fromm MF; Wilkinson GR; Kim RB OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab. Dispos 1999, 27 (8), 866–71. [PubMed] [Google Scholar]
- (27).Kim RB; Leake B; Cvetkovic M; Roden MM; Nadeau J; Walubo A; Wilkinson GR Modulation by drugs of human hepatic sodium-dependent bile acid transporter (sodium taurocholate cotransporting polypeptide) activity. J. Pharmacol. Exp. Ther 1999, 291 (3), 1204–9. [PubMed] [Google Scholar]
- (28).Meyer zu Schwabedissen HE; Verstuyft C; Kroemer HK; Becquemont L; Kim RB Human multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter: functional characterization, interaction with OCT2 (SLC22A2), and single nucleotide polymorphisms. Am. J. Physiol. Renal Physiol 2010, 298 (4), F997–F1005. [DOI] [PubMed] [Google Scholar]
- (29).DeGorter MK; Tirona RG; Schwarz UI; Choi YH; Dresser GK; Suskin N; Myers K; Zou G; Iwuchukwu O; Wei WQ; Wilke RA; Hegele RA; Kim RB Clinical and pharmacogenetic predictors of circulating atorvastatin and rosuvastatin concentrations in routine clinical care. Circ.: Cardiovasc. Genet 2013, 6 (4), 400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Lenky CC; McEntyre CJ; Lever M Measurement of marine osmolytes in mammalian serum by liquid chromatography-tandem mass spectrometry. Anal. Biochem 2012, 420 (1), 7–12. [DOI] [PubMed] [Google Scholar]
- (31).Wang ZJ; Yin OQ; Tomlinson B; Chow MS OCT2 polymorphisms and in-vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet. Genomics 2008, 18 (7), 637–45. [DOI] [PubMed] [Google Scholar]
- (32).Song IS; Shin HJ; Shim EJ; Jung IS; Kim WY; Shon JH; Shin JG Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin. Pharmacol. Ther 2008, 84 (5), 559–62. [DOI] [PubMed] [Google Scholar]
- (33).Tang WH; Hazen SL The contributory role of gut microbiota in cardiovascular disease. J. Clin. Invest 2014, 124 (10), 4204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Bain MA; Faull R; Milne RW; Evans AM Oral L-carnitine: metabolite formation and hemodialysis. Curr. Drug Metab. 2006, 7 (7), 811–6. [DOI] [PubMed] [Google Scholar]
- (35).Chen Y; Li S; Brown C; Cheatham S; Castro RA; Leabman MK; Urban TJ; Chen L; Yee SW; Choi JH; Huang Y; Brett CM; Burchard EG; Giacomini KM Effect of genetic variation in the organic cation transporter 2 on the renal elimination of metformin. Pharmacogenet. Genomics 2009, 19 (7), 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Mitchell SC; Zhang AQ; Noblet JM; Gillespie S; Jones N; Smith RL Metabolic disposition of [14C]-trimethylamine N-oxide in rat: variation with dose and route of administration. Xenobiotica 1997, 27 (11), 1187–97. [DOI] [PubMed] [Google Scholar]
- (37).Duarte IF; Legido-Quigley C; Parker DA; Swann JR; Spraul M; Braumann U; Gil AM; Holmes E; Nicholson JK; Murphy GM; Vilca-Melendez H; Heaton N; Lindon JC Identification of metabolites in human hepatic bile using 800 MHz 1H NMR spectroscopy, HPLC-NMR/MS and UPLC-MS. Mol. BioSyst 2009, 5 (2), 180–90. [DOI] [PubMed] [Google Scholar]
- (38).Phillips IR; Shephard EA Flavin-containing monooxygenases: mutations, disease and drug response. Trends Pharmacol. Sci 2008, 29 (6), 294–301. [DOI] [PubMed] [Google Scholar]
- (39).Hartiala J; Bennett BJ; Tang WH; Wang Z; Stewart AF; Roberts R; McPherson R; Lusis AJ; Hazen SL; Allayee H; Consortium CA Comparative Genome-Wide Association Studies in Mice and Humans for Trimethylamine N-Oxide, a Proatherogenic Metabolite of Choline and l-Carnitine. Arterioscler., Thromb., Vasc. Biol 2014, 34 (6), 1307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Warrier M; Shih DM; Burrows AC; Ferguson D; Gromovsky AD; Brown AL; Marshall S; McDaniel A; Schugar RC; Wang Z; Sacks J; Rong X; Vallim TA; Chou J; Ivanova PT; Myers DS; Brown HA; Lee RG; Crooke RM; Graham MJ; Liu X; Parini P; Tontonoz P; Lusis AJ; Hazen SL; Temel RE; Brown JM The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep. 2015, 10, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Tirona RG; Kim RB Nuclear receptors and drug disposition gene regulation. J. Pharm. Sci 2005, 94 (6), 1169–86. [DOI] [PubMed] [Google Scholar]
- (42).Urquhart BL; Tirona RG; Kim RB Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J. Clin. Pharmacol 2007, 47 (5), 566–78. [DOI] [PubMed] [Google Scholar]
- (43).Wilson A; Teft WA; Morse BM; Choi YH; Woolsey S; DeGorter MK; Hegele RA; Tirona RG; Kim RB Trimethylamine-N-oxide: a novel biomarker for the identification of inflammatory bowel disease. Dig. Dis. Sci 2015, 60, 3620. [DOI] [PubMed] [Google Scholar]
- (44).Wang Z; Roberts AB; Buffa JA; Levison BS; Zhu W; Org E; Gu X; Huang Y; Zamanian-Daryoush M; Culley MK; DiDonato AJ; Fu X; Hazen JE; Krajcik D; DiDonato JA; Lusis AJ; Hazen SL Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163 (7), 1585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]