Abstract
Aims:
Most benign breast fibroepithelial lesions (FELs) in adults harbor recurrent somatic MED12 exon 2 mutations, and rare TERT promoter hotspot mutations. We sought to determine the frequency of MED12 exon 2 and TERT promoter hotspot mutations in fibroadenomas (FAs) and benign phyllodes tumors (BePTs) in adolescents and young adults.
Methods:
DNA from 21 consecutive FAs and 8 consecutive BePTs in adolescents and young adults was subjected to Sanger sequencing of the exon 2 of MED12 and the TERT promoter hotspot locus.
Results:
We identified MED12 exon 2 mutations in 62% and 88% of FAs and BePTs, respectively, and no TERT promoter hotspot mutations. The majority of the MED12 exon 2 mutations identified were in-frame deletions (60%).
Conclusions:
As in adults, benign FELs in juvenile patients harbor recurrent MED12 exon 2 mutations.
Keywords: fibroadenoma, phyllodes tumor, sequencing, MED12, telomerase
INTRODUCTION
Fibroadenomas (FAs) and phyllodes tumors (PTs) are breast fibroepithelial lesions (FELs) characterized by an admixture of stromal and epithelial components.[1 2] FAs lie at the benign end of the spectrum and may be managed conservatively. PTs are uncommon lesions, and are classified as benign, borderline or malignant according to the WHO criteria,[2] which include tumor borders, stromal cellularity and atypia, mitotic activity, and the presence of stromal overgrowth and heterologous elements.
The genomic landscape of FELs in adults has been well characterized.[3–5] Highly recurrent somatic Mediator complex subunit 12 (MED12) exon 2 mutations occur in both FAs and PTs in adults,[3–8] supporting their role as early founder events in these lesions. The prevalence of MED12 exon 2 mutations is higher in benign FELs, and shows a stepwise decrease from benign to borderline and to malignant PTs.[5] In addition to MED12 exon 2 mutations, TERT promoter hotspot mutations and gene amplifications are the most frequent somatic genetic alterations found in PTs, and tend to be more common in borderline (57%) and malignant (68%) PTs than in BePTs (18%).[5 7–9], supporting their role in the progression to malignant PTs. In contrast to breast FELs in adults, data on the molecular underpinning of breast FELs in younger women is scarce. A recent study of FAs in a pediatric population revealed MED12 mutations in 54% and 35% of conventional FAs and juvenile FAs, respectively, and did not identify TERT promoter mutations in these lesions.[10] Whether PTs in younger patients have a similar molecular underpinning as in adults is yet to be determined. Here, we sought to determine the frequency of MED12 exon 2 mutations and TERT promoter hotspot mutations in FAs and BePTs in adolescents and young women up to 26 years of age.
METHODS
Following institutional review board (IRB) approval, slides and formalin-fixed paraffin-embedded (FFPE) tissue blocks of 21 consecutive FAs and 8 consecutive BePTs from adolescents and young adults were retrieved from the archives of the Department of Pathology of Memorial Sloan Kettering Cancer Center (MSKCC; NY, USA). Samples were anonymized prior to analysis. All cases were reviewed by four breast pathologists (F.P., M.P.M, D.G. and E.B.) and classified according to the latest World Health Organization (WHO) criteria.[2]
Tumor tissue was microdissected from 8 μm-thick FFPE histological sections under a stereomicroscope (Olympus SZ61) to ensure a tumor stromal cell content >80%. DNA was extracted using the DNAeasy Blood and Tissue Kit (Qiagen) according to manufacturers’ instructions. The assessment of MED12 exon 2 and TERT promoter mutations was performed by Sanger sequencing. PCR amplification was performed using AmpliTaq Gold 360 Master Mix Kit (Life Technologies), as previously described.[5] PCR products were purified with ExoSAP-IT (ThermoFisher Scientific) and subjected to Sanger sequencing using primers sets encompassing exon 2 of MED12 and the TERT promoter hotspot locus (Supplementary Table S1). Sequence electropherograms of the forward and reverse strands were analyzed using Mutation Surveyor (SoftGenetics) and the mutations identified were manually curated. All analyses were performed in duplicate. The functional effect of the MED12 mutations identified was predicted using MutationTaster, Provean, Polyphen-2, and CADD, as previously described.[6]
Fisher’s exact test and Student’s t-test were used for comparison of categorical and continuous variables, respectively. Statistical analyses were performed using SPSS Statistics V25 (IBM). Two tailed p values <0.05 were considered statistically significant.
RESULTS
Fibroadenomas and benign phyllodes tumors in adolescents and young adults harbor frequent MED12 exon 2 mutations.
The median age at diagnosis of FEL was 19 years of age (range 13–26; Supplementary Table S2). The FELs consisted of 21 FAs and 8 PTs (Fig 1a-1b). Two of the FAs (JF16 and JF25) included in this series displayed features of juvenile FAs,[11] such as stromal and epithelial hyperplasia, without stromal atypia (Supplementary Table S3). FAs and BePTs had a median tumor size of 2.5 cm (range 0.9–10.5) and 1.4 cm (range 0.7–4.4), respectively (Supplementary Table S2). Most FAs displayed an intracanalicular growth pattern (11/21; 52%), followed by a pericanalicular growth pattern (8/21; 38%), and a mixed growth pattern (2/21; 10%). (Supplementary Table S3). All PTs in this study (8/8) displayed well defined borders, low stomal cellularity with mild stromal atypia, up to 2 mitoses per 10 high power fields, and no stromal overgrowth or heterologous elements, and were classified as benign.[2]
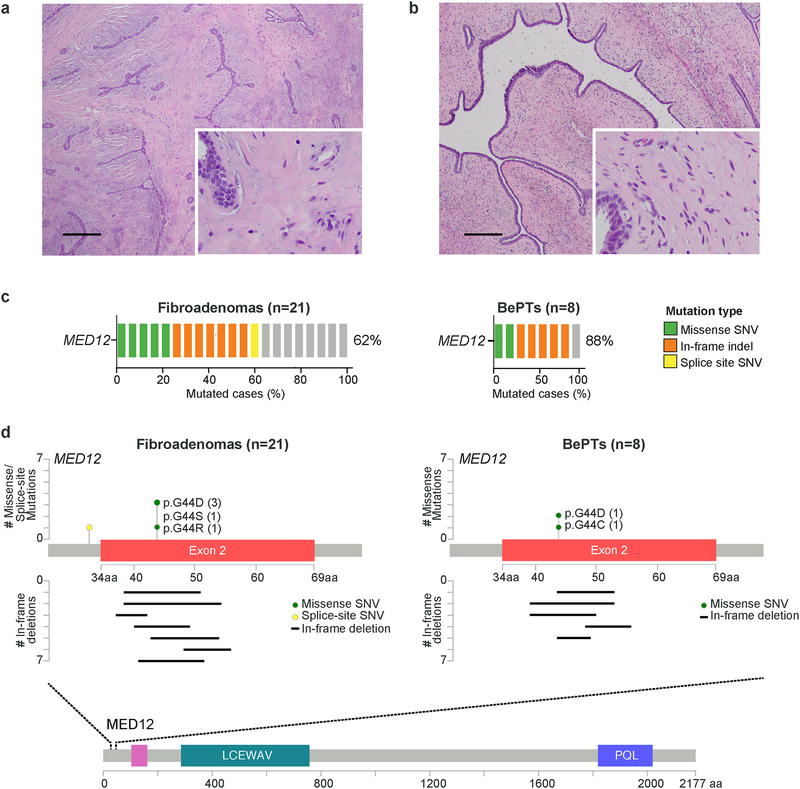
Figure 1. MED12 and TERT mutational frequency and spectrum of MED12 exon 2 mutations in fibroepithelial lesions in young patients.
Representative photomicrographs of (a) a fibroadenoma (FA) and (b) a benign phyllodes tumor (BePT) from this series. Scale bars, 100 μm (c) Frequency of MED12 exon 2 mutations in FAs (n=21) and BePTs (n=8) in adolescents and young adults, identified by Sanger sequencing. (d) MED12 protein domain structure and detailed view of exon 2, depicting the 13 and 7 mutations identified in the 21 FAs and 8 BePTs from this study, respectively. aa, aminoacid; BePTs, benign phyllodes tumors; SNV, single-nucleotide variant.
Sanger sequencing analysis revealed frequent MED12 exon 2 mutations in FAs (62%; 13/21) and BePTs (88%; 7/8), and no TERT promoter hotspot mutations (Fig. 1c and Supplementary Table 2). The observed MED12 and TERT mutational frequencies of FAs and BePTs in this cohort are similar to those reported in benign breast FELs in adults by our group and others,[3 4 6 8 12] and in a recent study of FAs in a pediatric population.[10] Our analyses identified five FAs (24%; 5/21) harboring missense mutations in the hotspot codon 44 of MED12, including p.G44D (n=3), p.G44S (n=1) and p.G44R (n=1). Seven FAs harbored MED12 exon 2 in-frame deletions, with five encompassing codon 44. A splice-site SNV targeting the first intron of MED12 was identified in one FA (Fig. 1c-1d, Fig. 2 and Supplementary Table S2). Two BePTs harbored missense mutations targeting the hotspot codon 44 of MED12 (p.G44D and p.G44C), and five BePTs harbored MED12 exon 2 in-frame deletions, four of which encompassed codon 44 (Fig 1c-1d, Fig 2 and Supplementary Table S2). All identified mutations were predicted to be deleterious (Supplementary Table S2). Whilst most MED12 exon 2 mutations in adult benign FELs have been found to be missense,[3–6] we observed that the majority of MED12 exon 2 mutations in benign FELs adolescents and young adults were in-frame deletions (60%; 12/20), followed by missense mutations (35%; 7/19) and a splice site mutation (5%; 1/20).
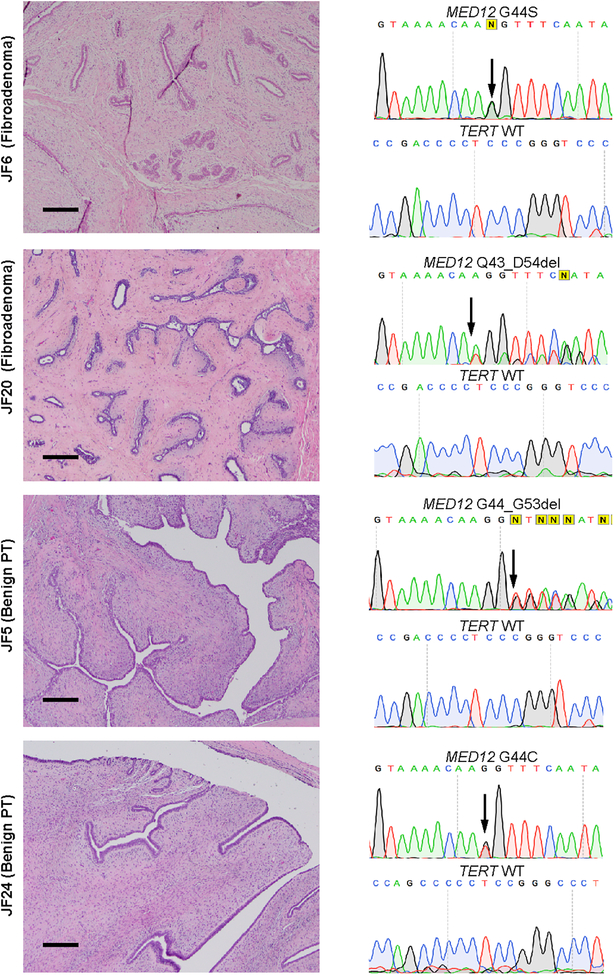
Figure 2. MED12 exon 2 mutations identified by Sanger sequencing in fibroadenomas and benign phyllodes tumor in young female patients.
Representative photomicrographs and sequence electropherograms of different missense SNVs and in-frame deletions targeting exon 2 of MED12 in fibroadenomas and benign phyllodes tumors identified in young female patients by Sanger sequencing. The aminoacid change is indicated for each case. Arrows point to the altered base. Scale bars, 100 μm.
The median age of patients with MED12WT FAs was similar to the one of those with MED12MUT FAs (18 years vs. 20 years; p=0.11, Student’s t-test; Supplementary Table S4), and the size of MED12WT FAs and MED12MUT FAs was also comparable (3.2 cm vs 2.5 cm; p=0.25, Student’s t-test; Supplementary Table S4). As previously reported for FELs in adults[12–14], we observed a correlation between MED12 exon 2 mutational status and FA growth pattern, as most MED12MUT FAs displayed an intracanalicular growth pattern (84.6%, 11/13) and the majority of MED12WT FAs (87.5%, 7/8) had a pericanalicular growth pattern (p<0.001, Fisher’s exact test; Supplementary Table S4). All BePTs in this study displayed a well-defined border, low or low to intermediate stromal cellularity, mild stromal atypia, <=2 mitoses/10 high power fields (HPF) and no stromal overgrowth or heterologous elements. The sole MED12-wild type BePT in our cohort did not differ histologically from the BePTs harboring MED12 exon 2 mutations (Supplementary Tables S5 and S6).
DISCUSSION
Benign FELs in adult women have been shown to harbor highly recurrent somatic MED12 exon 2 mutations restricted to their stromal component.[3–6 8 12] Benign FELs, including FAs and BePTs, are the most common type of breast tumor in adolescent and young females.[15] A recent study revealed that, akin to FAs in adults, FAs in children and adolescents harbor recurrent MED12 exon 2 mutations and lack TERT promoter mutations.[10] Whether the same is true for BePTs in young patients is yet to be determined. We investigated the presence of MED12 exon 2 mutations and TERT promoter hotspot mutations in benign breast FELs, including FAs and BePTs, in adolescents and young adults. Our analyses revealed, in a way akin to breast FELs in adults,[3–8] a high frequency of MED12 exon 2 mutations in FAs (62%) and BePTs (88%), along with a lack of TERT promoter hotspot mutations. These findings corroborate the role of MED12 exon 2 mutations as founder events in the genesis of benign FELs, also in a juvenile population.
Overall, we identified four distinct missense mutations targeting the hotspot codon 44 of MED12, 12 distinct in-frame deletions, out of which nine encompassed codon 44 of MED12, and a splice-site mutation targeting the first intron of MED12 (Fig 1c-1d, Supplementary Table S2). The splice-site mutation and four of the 12 in-frame deletions had not been previously reported (Supplementary Table S2). Most benign FELs in adults harbor MED12 exon 2 missense mutations, and in-frame indels account for only 4.9%−38% and 14%−41% of MED12 exon 2 mutations in FAs and BePTs, respectively[3–6]. Tan et al[10] reported on a higher frequency of missense MED12 exon 2 mutations (75%) over in-frame indels (20%) and frameshift mutations (5%) in the pediatric FAs they studied. We observed a slightly higher proportion of MED12 exon 2 in-frame indels in FAs (7/13; 54%), over missense mutations (5/13; 39%) and splice-site mutations (1/13; 8%). Similarly, we observed that the MED12 exon 2 mutations in BePTs were predominantly in-frame deletions (71%; 5/7), followed by missense mutations (29%; 2/7). Notably, all identified MED12 exon 2 in-frame deletions were predicted to have a deleterious effect by bioinformatics approaches assessing the functional impact of the given variant (Supplementary Table 2).
We observed an association of MED12 exon 2 mutations with an intracanalicular growth pattern in FAs in this series of juvenile patients, consistent with similar findings in previous studies evaluating FELs in adults.[6 12 13] All PTs included in the current series were BePTs, and 88% harbored MED12 exon 2 mutations, consistent with the notion that PTs may evolve from FAs[16 17].
Our study has several limitations. Our cohort is relatively small, which reflects the rarity of breast tumors in younger patients. The assessment of mutations was restricted to MED12 exon 2 and to the TERT promoter hotspot locus, and no other genomic alterations were studied. Our cohort includes only one MED12WT BePT, precluding any conclusions to be drawn regarding the relationship between MED12 status and morphologic features in BePTs. Furthermore, our study cohort does not include any borderline or malignant PT, and we cannot comment on the role of TERT promoter mutations in the progression of benign to malignant FELs in young patients. Despite the aforementioned limitations, our study supports the notion that recurrent MED12 exon 2 mutations underpin benign FELs in younger patients, akin to benign FELs in adults, lending support to similar management approaches regardless of the patient age.
Supplementary Material
FUNDING
Research reported in this publication was funded in part by a Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute (grant No P30CA008748). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
COMPETING FINANCIAL INTERESTS
J.S. Reis-Filho reports personal/consultancy fees from VolitionRx, Page.AI and Goldman Sachs, Indengene, Grail and Ventana Medical Systems, outside the submitted work. All other authors have no competing interests to declare.
REFERENCES
- 1.Krings G, Bean GR, Chen YY. Fibroepithelial lesions; The WHO spectrum. Semin Diagn Pathol 2017;34(5):438–52. [DOI] [PubMed] [Google Scholar]
- 2.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO Classification of Breast Tumors. IARC: Lyon, 2012. [Google Scholar]
- 3.Tan J, Ong CK, Lim WK, et al. Genomic landscapes of breast fibroepithelial tumors. Nat Genet 2015;47(11):1341–5. [DOI] [PubMed] [Google Scholar]
- 4.Lim WK, Ong CK, Tan J, et al. Exome sequencing identifies highly recurrent MED12 somatic mutations in breast fibroadenoma. Nat Genet 2014;46(8):877–80. [DOI] [PubMed] [Google Scholar]
- 5.Piscuoglio S, Ng CK, Murray M, et al. Massively parallel sequencing of phyllodes tumours of the breast reveals actionable mutations, and TERT promoter hotspot mutations and TERT gene amplification as likely drivers of progression. J Pathol 2016;238(4):508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piscuoglio S, Murray M, Fusco N, et al. MED12 somatic mutations in fibroadenomas and phyllodes tumours of the breast. Histopathology 2015;67(5):719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cani AK, Hovelson DH, McDaniel AS, et al. Next-Gen Sequencing Exposes Frequent MED12 Mutations and Actionable Therapeutic Targets in Phyllodes Tumors. Mol Cancer Res 2015;13(4):613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida M, Ogawa R, Yoshida H, et al. TERT promoter mutations are frequent and show association with MED12 mutations in phyllodes tumors of the breast. Br J Cancer 2015;113(8):1244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nozad S, Sheehan CE, Gay LM, et al. Comprehensive genomic profiling of malignant phyllodes tumors of the breast. Breast Cancer Res Treat 2017;162(3):597–602. [DOI] [PubMed] [Google Scholar]
- 10.Tay TKY, Guan P, Loke BN, et al. Molecular insights into paediatric breast fibroepithelial tumours. Histopathology 2018. [DOI] [PubMed] [Google Scholar]
- 11.Pike AM, Oberman HA. Juvenile (cellular) adenofibromas. A clinicopathologic study. Am J Surg Pathol 1985;9(10):730–6. [DOI] [PubMed] [Google Scholar]
- 12.Pfarr N, Kriegsmann M, Sinn P, et al. Distribution of MED12 mutations in fibroadenomas and phyllodes tumors of the breast--implications for tumor biology and pathological diagnosis. Genes Chromosomes Cancer 2015;54(7):444–52. [DOI] [PubMed] [Google Scholar]
- 13.Mishima C, Kagara N, Tanei T, et al. Mutational analysis of MED12 in fibroadenomas and phyllodes tumors of the breast by means of targeted next-generation sequencing. Breast Cancer Res Treat 2015;152(2):305–12. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida M, Sekine S, Ogawa R, et al. Frequent MED12 mutations in phyllodes tumours of the breast. Br J Cancer 2015;112(10):1703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross DS, Giri DD, Akram MM, et al. Fibroepithelial Lesions in the Breast of Adolescent Females: A Clinicopathological Study of 54 Cases. Breast J 2017;23(2):182–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng CKY, Bidard FC, Piscuoglio S, et al. Genetic Heterogeneity in Therapy-Naïve Synchronous Primary Breast Cancers and Their Metastases. Clin Cancer Res 2017;23(15):4402–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piscuoglio S, Geyer FC, Burke KA, et al. Massively parallel sequencing analysis of synchronous fibroepithelial lesions supports the concept of progression from fibroadenoma to phyllodes tumor. NPJ Breast Cancer 2016;2:16035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.