Abstract
Introduction
Emerging evidence suggests that effective treatment of glioblastoma (GBM), the most common and deadly form of adult primary brain cancer, will likely require concurrent treatment of multiple aspects of tumor pathobiology to overcome tumor heterogeneity and the complex tumor-supporting microenvironment. Recent studies in non-central nervous system (CNS) tumor cells have demonstrated that oxaliplatin (OXA) can induce multi-faceted anti-tumor effects, in particular at drug concentrations below those required to induce apoptosis. These findings motivated re-investigation of OXA for the treatment of GBM.
Methods
The effects of OXA on murine KR158 and GL261 glioma cells including cell growth, cell death, inhibition of signal transducer and activator of transcription (STAT) activity, O-6-methylguanine-DNA methyltransferase (MGMT) expression, and immunogenic cell death (ICD) initiation, were evaluated by cytotoxicity assays, Western blot analysis, STAT3-luciferase reporter assays, qRT-PCR assays, and flow cytometry. Chemical inhibitors of endoplasmic reticulum (ER) stress were used to investigate the contribution of this cell damage response to the observed OXA effects. The effect of OXA on bone marrowderived macrophages (BMDM) exposed to glioma conditioned media (GCM) was also analyzed by Western blot analysis.
Results
We identified the OXA concentration threshold for induction of apoptosis and from this determined the drug dose and treatment period for sub-cytotoxic treatments of glioma cells. Under these experimental conditions, OXA reduced STAT3 activity, reduced MGMT levels and increased temozolomide sensitivity. In addition, there was evidence of immunogenic cell death (elevated EIF2α phosphorylation and calreticulin exposure) following prolonged OXA treatment. Notably, inhibition of ER stress reversed the OXA-mediated inhibition of STAT3 activity and MGMT expression in the tumor cells. In BMDMs exposed to GCM, OXA also reduced levels of phosphorylated STAT3 and decreased expression of Arginase 1, an enzyme known to contribute to pro-tumor functions in the tumor-immune environment.
Conclusions
OXA can induce notable multi-faceted biological effects in glioma cells and BMDMs at relatively low drug concentrations. These findings may have significant therapeutic relevance against GBM and warrant further investigation.
Keywords: Oxaliplatin, STAT3, MGMT, Glioma, Glioblastoma, Endoplasmic reticulum stress
Introduction
Gliomas are one of the most common primary central nervous system (CNS) tumors, and glioblastoma (GBM) is one of the most deadly and difficult-to-treat glioma types. Key features of GBM include cellular and molecular heterogeneity, tumor cell invasion into functioning brain tissue, and a highly immunosuppressive tumor microenvironment (TME) [1, 2]. GBM is difficult-to-treat in part because many anti-GBM drugs (1) cause major toxicities in the brain and/or body at high doses, (2) do not cross the blood–brain barrier, or (3) fail to concurrently address complex aspects of the disease pathobiology. Recent analyses of pre-clinical and clinical studies strongly suggest that effective GBM treatment will require multi-modal therapeutic effects to counteract heterogeneous, often redundant oncologic processes in the TME [1–4].
Recently, platinum-based drugs, traditionally considered DNA crosslinking chemotherapeutics, have been shown to promote anti-tumor effects independent of inducing DNA damage and apoptosis. Interestingly, these ‘alternative’ effects appear to occur at lower, less toxic drug concentrations and include inhibition of transcription factors such as the signal transducer and activators of transcription (STAT) proteins [5–7], immunomodulation [8, 9], and disruption of ribosome biogenesis [10–12]. Some of the alternative effects were inferred through clinical observations in non-CNS cancers over the past 40 years and are now thought to drive some of the remarkable patient responses that have been observed [8, 13, 14]. In particular, oxaliplatin (OXA) has been found to induce multi-faceted alternative effects more than the other commonly used platinum-based drugs, cisplatin and carboplatin [14, 15]. Accordingly, we sought to re-investigate the potential of lower, less cytotoxic OXA doses for the treatment of GBM given the need for multifaceted anti-GBM effects and the potentially lower risks associated with toxicity in the CNS. Our approach in this study was to begin with in vitro analyses of both glioma cells and glioma conditioned media (GCM)-treated bone marrow-derived macrophages (BMDM), a model for tumor- associated macrophages, given the abundance and pathobiological importance of these cell populations in the GBM microenvironment [16–18].
Materials and methods
Cell culture of KR158-luciferase and GL261-luciferase glioma cells and generation of KR158 STAT3 luciferase reporter cell line
The murine KR158 and KR158-luciferase (luc) glioma cell lines were obtained from Dr. Tyler Jacks (Massachusetts Institute of Technology, Cambridge, MA) and murine GL261-luc glioma cells were from Dr. Michael Lim (Johns Hopkins School of Medicine, Baltimore, MD). Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM L -glutamine and 1% penicillin–streptomycin. KR158 cells were transfected with a STAT3-luciferase reporter plasmid (pGL4.47[luc2/SIE/Hygro]; Promega) using Lipofectamine (Thermofisher) and a stably-transfected pooled cell line was isolated by hygromycin B (Corning) selection (500 0μg/mL).
Cell growth assays
KR158-luc or GL261-luc cells were seeded in triplicate in 96-well cell culture dishes in normal growth conditions. Cells were then left untreated or treated with 50, 100, 200, 300, 400 or 500 μM oxaliplatin (OXA; Sigma-Aldrich) dissolved in DMEM for 24 h and then WST-1 proliferation agent (Sigma-Aldrich) was added to the media. Cell growth was then determined by absorbance readings according to the manufacturer’s recommendations.
Cell death assays
KR158-luc or GL261-luc cells were seeded in triplicate in 96-well cell culture dishes in normal growth conditions. Cells were then left untreated or treated with OXA (same doses as above) for 24 h. Lactate dehydrogenase (LDH) cytotoxicity assay (Sigma-Aldrich) was performed according to the manufacturer’s recommendations. In experiments involving temozolomide (TMZ), KR158-luc cells were seeded in triplicate in 96-well cell culture dishes in normal growth conditions. Cells were then either left untreated, or pre-treated with 200 μM OXA for 9 h. Both untreated and OXA pre-treated cells were then treated with either 0, 0.5, 1, 2, 3, 4, or 5 mM TMZ (Sigma-Aldrich) for 48 h. Cell viability was then measured by MTT assay (Trevigen) according to the manufacturer’s recommendations.
Western blot analysis
Cells were harvested using 0.05% trypsin and lysed using radioimmunoprecipitation analysis (RIPA) lysis buffer (Amresco) supplemented with a protease/phosphatase inhibitor cocktail (Cell Signaling Technology (CST)). A bicinchoninic acid (BCA) protein assay (Pierce Protein Biology) was used to determine the protein concentration of each lysate and immunoblotting was performed as described [19] using the following primary antibodies: actin, CD86, cleaved PARP, GAPDH, iNOS, phospho (p) STAT3 (Y-705), STAT3, p-EIF2α (S-51), EIF2 α, pSTAT1 (Y-701), STAT1, pSTAT5 (Y-694), STAT5, pSTAT6 (Y-641), STAT6, and YM1 (all from CST). The O-6-methylguanine-DNA methyl transferase (MGMT) antibody was obtained from Enzo Life Sciences.
STAT3 luciferase reporter assays
KR158 STAT3-luciferase (luc) reporter cells were seeded at a density 2 × 105 and grown in 6-well culture dishes. Cells were then left untreated or treated with 200 μM OXA, cisplatin (CDDP), bis-chloroethylnitrosourea (BCNU), or TMZ unless otherwise indicated for either 9 h or the length of time indicated. All drugs were dissolved in DMEM. Cells were pelleted by spinning in a microcentrifuge at 2000 rpm for 3 min in clear microcentrifuge tubes and lysed using the One-Glo Luciferase Assay System (Promega). Luciferase activity was determined using the One-Glo Luciferase Assay System (Promega) using a Glomax 20/20 luminometer.
Flow cytometry
KR158-luc cells were seeded in 6-well cell culture dishes at a density of 2 × 105 per well and either left untreated or treated with 200 μM OXA for 24 h. Then cells were then collected, incubated with a fluorescent PE-conjugated calreticulin antibody (VWR) at a 1:100 dilution, and analyzed for cell surface calreticulin using a BD LSRFortessa flow cytometer.
Isolation of bone marrow-derived macrophages and treatment conditions
Bone marrow-derived myeloid progenitor cells were isolated by harvesting marrow from the femurs of 5–6-week- old C57BL/6 mice. Non-adherent cells were collected by centrifugation and red blood cells lysed. Cells were then cultured in α-MEM with 20 ng/mL macrophage colony stimulating factor (M-CSF) (R & D Systems) for 3 days to generate bone marrow-derived, M-CSF dependent macrophages (BMDMs). Glioma conditioned media (GCM) was obtained by culturing KR158-luc glioma cells in α-MEM for 72 h. Media was then removed and centrifuged to remove cells and cell debris. Cultured BMDMs were then either left untreated or treated with interferon (IFN)-γ plus lipopolysaccharide (LPS) (to promote M1/ anti-tumorigenic phenotype), IL-4 (to promote M2/protumorigenic phenotype), or GCM for 72 h.
Quantitative real time polymerase chain reaction (qRT-PCR) of MGMT expression
To analyze the expression of MGMT mRNA, RNA was isolated from KR158 cell lysates using an RNAeasy RNA Isolation mini kit (Qiagen). Next, cDNA was generated using a first strand cDNA synthesis kit (ThermoFisher). Finally, qRT-PCR was performed using the Mm01269876_ m1 taqman MGMT gene expression assay (ThermoFisher) and a 7900HT PCR machine (ThermoFisher). Data was normalized to GAPDH expression (Mm99999915_g) (ThermoFisher).
Statistics
Statistical analysis of the data was conducted using a twotailed Student’s t-test using unequal variance for pairwise comparisons. Two-way ANOVA was performed for multiple comparisons. Values are given as mean ± standard deviation. A level of P < 0.05 was considered statistically significant.
Results
OXA dose-response experiments using murine glioma cells reveals a sub-cytotoxic dose threshold
Recent studies involving non-CNS cancers have suggested that OXA may have multi-factorial anti-cancer effects using drug concentrations that are not directly cytotoxic [10, 11]. To determine if OXA could exhibit similar activities in glioma cells, we first defined a dose range that did not induce apoptosis and cell death. Murine KR158-luc and GL261-luc cells were treated with increasing concentrations of OXA for 24 h and cell growth and death assays were conducted. OXA concentrations up to 200 μM minimally affected glioma cell growth (Fig. 1a, b). Additionally, OXA concentrations up to 500 μM did not promote cell death as monitored by LDH release (Fig. 1c, d). To confirm that OXA was not inducing glioma cell apoptosis under these treatment conditions, KR158-luc and GL261- luc cells were treated with increasing OXA concentrations and poly (ADP-ribose) polymerase (PARP) cleavage was monitored by Western blot analysis. PARP cleavage was only detectable at 1 mM OXA, the highest dose we tested (Fig. 1e, f). In consideration of these findings, we used an OXA concentration of 200 μM and a treatment period of less than 24 h in our subsequent experiments to probe potential alternative effects of this drug.
Fig. 1.
OXA concentrations of less than 200 μM minimally impact glioma cell growth and survival in vitro. a KR158-luc cells were either left untreated (NT, no treatment) or treated with the indicated concentrations of OXA for 24 h and then cell growth was determined using WST-1 reagent. b GL261-luc cells were treated as described for panel (a). c KR158-luc cells were treated with the indicated concentrations of OXA for 24 h and then cell death was determined using an LDH cytotoxicity assay. Lysis buffer serves as a positive control and indicates the maximum LDH released, and thus 100% cell death. d GL261-luc cells were treated as described for panel (c). e KR158- luc cells were treated with the indicated concentrations of OXA. Cell lysates were then collected and analyzed for cleaved PARP and GAPDH levels by Western blot. f GL261-luc cells were treated as described for panel (e). In a–d the values shown are the mean ± SD of three individual experiments
OXA treatment of glioma cells reduces STAT3 activity
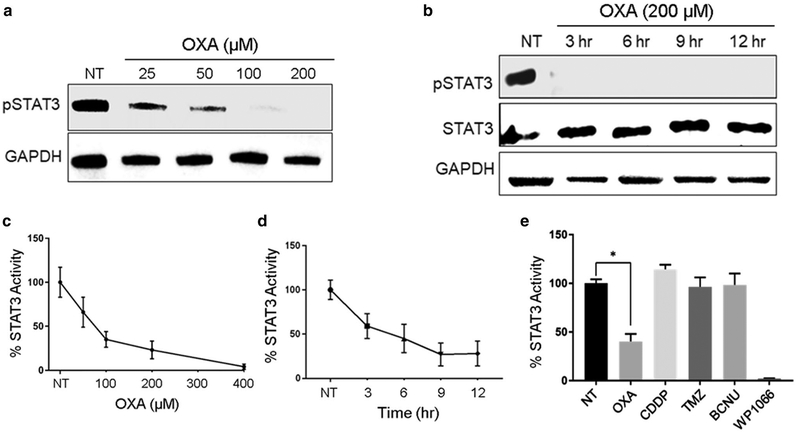
Given the considerable importance of STAT3 signaling in glioma biology [20], we evaluated the effects of OXA exposure on STAT3 activity. First, we performed a doseresponse experiment using KR158-luc cells and drug concentrations ranging from 25 to 200 μM. Cells were harvested at 9 h post-drug addition and phospho (p)-STAT3 levels were examined by Western blot analysis. We found that pSTAT3 levels decreased in a dose-responsive manner (Fig. 2a). To determine the time course of pSTAT3 reduction we treated cells with 200 μM OXA for 3, 6, 9, or 12 h and blotted for pSTAT3. We found that pSTAT3 levels were undetectable by 3 h of OXA treatment, the first time point examined (Fig. 2b). OXA also reduced pSTAT3 levels with a similar time-course when added to GL261- luc cells (Fig. S1).
Fig. 2.
OXA treatment of KR158-luc glioma cells reduces STAT3 phosphorylation and STAT3 activity. a KR158-luc cells were either left untreated (NT, no treatment) or treated with the indicated concentrations of OXA for 9 h, cells were harvested and lysed, and pSTAT3 and GAPDH levels were analyzed by Western blot. b KR158-luc cells were treated with 200 μM OXA for the indicated lengths of time. Cell lysates were analyzed for pSTAT3, STAT3, and GAPDH levels by Western blot. c KR158 STAT3-luc reporter cells were either left untreated or treated with the indicated concentrations of OXA for 9 h, cells were harvested and analyzed for STAT3 activity. d KR158 STAT3-luc reporter cells were either left at the indicated time points untreated or treated with 200 μM OXA. Cells were then harvested and analyzed for STAT3 activity. e KR158 STAT3-luc reporter cells were either left untreated or treated with 200 μM of OXA, cisplatin (CDDP), bis-chloroethylnitrosourea (BCNU), temozolomide (TMZ), or WP1066 for 9 h. Cells were then harvested and analyzed for STAT3 activity. *P < 0.05 compared to NT control by Student t test
Although STAT3 tyrosine phosphorylation is a commonly used read-out for STAT3 transcriptional activity [21], we wanted to confirm that OXA inhibits STAT3 function using an additional experimental approach. To this end, KR158 cells were transfected with a STAT3-luciferase (luc) reporter construct and a stably-transfected cell line was established. We first treated KR158 STAT3-luc reporter cells with increasing concentrations of OXA for 9 h and found that STAT3 activity levels decreased in a dose-responsive manner (Fig. 2c). The KR158 STAT3-luc reporter cells were then treated with 200 μM of OXA for various lengths of time and luciferase activity was compared to that of untreated reporter cells. We found that STAT3 activity was decreased at 3 h after initiation of drug treatment (Fig. 2d).
To determine if other platinum compounds or non-platinum-based chemotherapeutics could also regulate glioma STAT3 activity we treated KR158 STAT3-luc cells with cisplatin (CDDP), bis-chloroethylnitrosourea (BCNU), or temozolomide (TMZ). Cisplatin was the first FDA- approved platinum-based chemotherapeutic but has some notable differences in cellular effects compared to OXA [10]. TMZ and BCNU are the two primary FDA-approved chemotherapeutics used clinically for the treatment of GBM. The Janus kinase (JAK)2/3 inhibitor WP1066 served as a positive control [22]. We found that STAT3 activity was not affected when cells were treated with CDDP, TMZ, or BCNU (Fig. 2e). We then determined whether OXA could reduce JAK2 phosphorylation, the primary JAK implicated in STAT3 activation in glioma cells [20]. KR158-luc cells were treated with OXA and analyzed for pJAK2 levels by Western blot. We did not detect changes in pJAK2 levels after OXA treatment (Fig. S2).
We also analyzed the phosphorylation status of three other STAT family members (STAT1, STAT5, and STAT6) implicated in glioma biology after OXA treatment using the same experimental conditions used for Fig. 2e (200 μM drug for 9 h). We found that pSTAT1 and pSTAT6 protein levels, but not pSTAT5 levels, were reduced after drug exposure (Fig. S3).
OXA treatment of glioma cells reduces MGMT expression and sensitizes cells to TMZ exposure
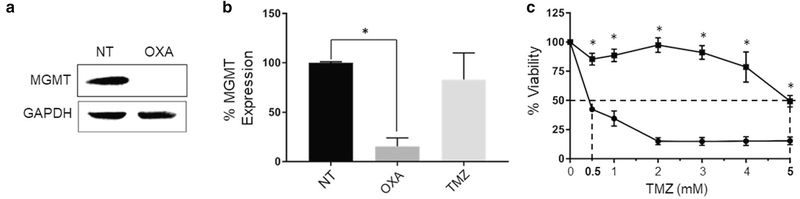
We next determined if OXA altered expression of the DNA repair enzyme O-6-methylguanine-DNA methyltransferase (MGMT), which is largely responsible for resistance to the GBM standard-of-care chemotherapeutic TMZ [23, 24]. We found that OXA treatment of KR158-luc cells reduced MGMT protein (Fig. 3a) and mRNA (Fig. 3b) levels. In contrast, TMZ treatment had no effect on MGMT mRNA expression (Fig. 3b). We then investigated the potential for OXA to sensitize KR158-luc cells to TMZ. KR158-luc cells were either left untreated or pre-treated with 200 μM OXA for 9 h. Both untreated cells and OXA pre-treated cells were then treated with increasing concentrations of TMZ for 48 h and cell viability was determined. OXA alone did not affect cell viability, as expected, while TMZ alone only had an effect on viability at high doses (Fig. 3c). However, combined OXA and TMZ treatment significantly decreased cell viability compared to TMZ alone (reduction in IC50 from 4.5 to 0.33 mM) (Fig. 3c).
Fig. 3.
OXA reduces MGMT gene expression and sensitizes KR158- luc glioma cells to TMZ. a KR158-luc cells were either left untreated (NT, no treatment) or treated with 200 μM OXA for 9 h and MGMT and GAPDH expression was analyzed by Western blot. b KR158- luc cells were either left untreated (NT, no treatment), treated with 200 μM OXA or 200 μM TMZ for 9 h. Cell lysates were then collected, RNA was isolated, cDNA generated, and qRT-PCR was performed for MGMT and GAPDH mRNA expression. Data was normalized to GAPDH expression and MGMT expression was compared to the untreated control. Values shown are the mean ± SD of three individual experiments. *P < 0.05, by Student t test. c KR158-luc cells were either left untreated or treated with the indicated concentrations of TMZ alone (square) or pre-treated with 200 μM OXA for 9 h and then treated with 200 μM OXA and the indicated concentrations of TMZ (circle) for 48 h. Cell viability was determined by MTT assay. *P < 0.01 compared to OXA + TMZ by Student t test
Endoplasmic reticulum stress is required for OXA-mediated reduction of pSTAT3 levels, downregulation of MGMT expression, and initiation of immunogenic cell death in glioma cells
Recent studies using non-CNS cancer cells have identified cellular stress as a key mediator of the chemotherapeutic effects of OXA [10, 25, 26]. Indeed, a high degree of endoplasmic reticulum (ER) stress induction may distinguish this drug from other platinum-based chemotherapeutics [10]. Therefore, to determine if protecting cells from ER stress would prevent STAT3 inhibition by OXA, we first treated KR158-luc cells with salubrinal (an ER stress inhibitor [27]), OXA, or both salubrinal and OXA for 9 h. Cells were harvested and pSTAT3 levels examined by Western blot analysis. We found that OXA did not reduce pSTAT3 levels in the presence of salubrinal (Fig. 4a), suggesting that protecting the cell from ER stress prevents the reduction in STAT3 activity by OXA. To confirm that this was the case, we treated cells with 4-phenylbutyric acid (4-PBA), which protects cells from ER stress by directly reducing misfolded proteins within the ER [28]. Similar to the results using salubrinal, protecting the cell from ER stress using 4-PBA prevents the reduction in pSTAT3 levels by OXA (Fig. 4b). ER stress has been shown to reduce MGMT expression [29]. Therefore, to determine if the downregulation of MGMT expression by OXA was also dependent on ER stress, we treated KR158-luc cells with either OXA, salubrinal, or OXA and salubrinal for 9 h and analyzed MGMT mRNA expression by qRT-PCR. Salubrinal alone did not affect MGMT mRNA levels (Fig. 4c), but the combination of salubrinal and OXA prevented the OXA-mediated reduction in MGMT levels, suggesting a similar requirement for ER stress as noted for STAT3 inhibition.
Fig. 4.
Endoplasmic reticulum stress is essential for alternative OXA effects. a KR158-luc cells were left untreated or treated with 200 μM OXA and/or 50 μM salubrinal. Cells were harvested, lysed and analyzed for pSTAT3, STAT3, and GAPDH levels by Western blot. b KR158-luc cells were left untreated or treated with 200 μM OXA and/or 5 mM 4-phenylbutyric acid (4-PBA). Cells were harvested, lysed and analyzed for pSTAT3, STAT3, and GAPDH protein levels by Western blot. c KR158-luc cells were either left untreated (NT, no treatment), treated with 200 μM OXA, 50 μM salubrinal, or both 200 μM OXA and 50 μM salubrinal for 9 h. Cell lysates were then collected, RNA was isolated, cDNA generated, and qRT-PCR was performed for MGMT and GAPDH mRNA expression. Data was normalized to GAPDH expression and MGMT expression was compared to the untreated control. Values shown are the mean ± SD of three individual experiments. *P < 0.05, Student t test. d KR158- luc cells were left untreated or treated as in Panel a above and analyzed for pEIF2α, EIF2α, and actin levels by Western blot. e KR158-luc cells were either left untreated (NT, no treatment) or treated with 200 μM OXA for 24 h. Cells were then harvested and analyzed for cell surface calreticulin expression by flow cytometry. Mean cell fluorescence of the OXA-treated cells was normalized to untreated controls for each experiment. Values shown are the mean ± SD of three individual experiments. *P < 0.05, Student t test. f Schematic summary of OXA treatment of glioma cells results. (A) Sub-cytotoxic concentrations of OXA inhibit STAT3 phosphorylation and transcriptional activity in an ER stress-dependent manner. (B) Prolonged exposure of glioma cells to OXA leads to EIF2α phosphorylation and translocation of calreticulin (blue hexagons) to the cell surface, which is a key component of the process leading to immunogenic cell death (ICD). EIF2α modulates protein translation following ER stress and may therefore link the induction of ER stress by OXA to translational disruption following OXA treatment. (C) OXA reduces MGMT expression in an ER stress-dependent manner
OXA is reported to induce immunogenic cell death (ICD), a form of cell death resulting in increased immunogenicity of the dying cell, through ER stress [11, 12]. Therefore, we also investigated whether OXA treatment of glioma cells could activate pathways important for ICD. A crucial early step leading to ICD after OXA treatment is the phosphorylation of eukaryotic translation initiation factor 2α (EIF2α) [30]. To evaluate if OXA exposure altered EIF2α phosphorylation, KR158-luc cells were treated with salubrinal, OXA, or both salubrinal and OXA for 9 h. Cells were harvested and pEIF2α levels examined by Western blot analysis. We detected increased pEIF2α levels at 9 h of OXA treatment, and reduced pEIF2α levels with salubrinal cotreatment (Fig. 4d).
A key requirement for ICD is the pre-apoptotic translocation of the ER chaperone calreticulin to the cell surface [30]. Exposure of calreticulin on the cell surface acts as an “eat-me” signal for phagocytic cells and is required for ICD to occur [30]. Therefore, to confirm OXA regulation of pathways leading to ICD in glioma cells, KR158-luc cells were either left untreated or treated with 200 μM OXA for 24 h and then evaluated for cell surface calreticulin expression by flow cytometry. We found that OXA treatment resulted in a 49% increase in mean cell calreticulin staining (Fig. 4e). Our findings suggest ER stress is required for the multifaceted effects observed after OXA treatment of glioma cells (Fig. 4f).
Glioma conditioned media treatment of BMDMs increases pSTAT3 and Arginase-1 levels and OXA treatment inhibits this cellular response
A significant portion of the GBM tumor mass is composed of infiltrating non-neoplastic cells. Infiltrating monocytes from the circulation and the brain resident macrophage–microglia—are the primary non-neoplastic cells in gliomas and these two immune cell populations compose as much as 30% of the tumor mass [16–18]. Factors released within the tumor microenvironment recruit and alter the phenotype and function of these myeloid cells, resulting in their polarization to a tumor supporting, pro-tumorigenic state [16–18, 31–33]. A major transcription factor controlling the co-option of tumor infiltrating myeloid cells is STAT3 [31–33]. We therefore sought to determine the impact of OXA on BMDM STAT3 phosphorylation and polarization after exposure to glioma conditioned media (GCM).
First, BMDMs were either left untreated, treated with LPS + IFNγ (to induce an M1, anti-tumorigenic macrophage phenotype), IL-4 (to induce an M2, pro-tumorigenic macrophage phenotype) or GCM. As expected, LPS + IFN-γ increased CD86 expression (an M1 marker), while IL-4 increased expression of arginase-1, Ym-1, and pSTAT6 (M2 markers) (Fig. 5a). Exposure of BMDMs to KR158- luc GCM resulted in increased pSTAT3 levels as well as increased expression of arginase-1 and Ym-1, changes which indicate a shift to a tumor-supporting state (Fig. 5a). We also found that OXA treatment of GCM-exposed BMDMs reduced pSTAT3 levels (Fig. 5b). Considering the impact of STAT3 on macrophage phenotype and function [34], we examined phenotypic markers of macrophage polarization after OXA treatment. OXA treatment had no effect on CD86 expression but reduced expression of arginase-1, an indicator of a pro-tumorigenic macrophage phenotype (Fig. 5c). Our results regarding the effect of OXA treatment on murine glioma cells and GCM-exposed murine BMDMs are summarized in Fig. 5d.
Fig. 5.
OXA treatment of GCM-exposed BMDMs reduces pSTAT3 levels and Arg1 expression. a BMDMs were then either left untreated (NT, no treatment) or treated with LPS + IFNγ (M1/anti-tumor phenotype control), IL-4 (M2/pro-tumor phenotype control), or KR158- luc GCM for 72 h. Cells were harvested and analyzed for CD86, Arg- 1, Ym-1, pSTAT3, pSTAT6, and GAPDH levels by Western blot. b BMDMs were either left untreated (NT, no treatment), treated with KR158-luc GCM for 72 h, or treated with KR158-luc GCM for 72 h and then 200 μM OXA for 9 h. Cells were harvested and pSTAT3, STAT3, and GAPDH levels analyzed by Western blot. c BMDMs were either left untreated (NT, no treatment) or treated as in panel b, cell lysates were prepared, and, CD86, Arg-1, and GAPDH levels analyzed by Western blot. d Summary of OXA effects against glioma cells and GAMs. In glioma cells, OXA treatment inhibited STAT3 activity, induced ICD pathways including increased cell surface exposure of calreticulin (CALR), and downregulated MGMT expression. In GCM-exposed macrophages, OXA treatment reduced pSTAT3 levels and expression of arginase-1, a phenotypic marker of a pro-tumor macrophage
Discussion
In this study, we found that OXA promotes a spectrum of biological effects independent from inducing apoptosis including inhibition of STAT3 activity, downregulation of MGMT expression, and initiation of ICD in glioma cells. We discovered that these first two effects were at least partially mediated by ER stress—a cell damage response process known to alter major cellular signaling nodes [12, 35]. Consistent with the MGMT expression data, pretreatment of tumor cells with OXA resulted in a significant increase in sensitivity to TMZ. Interestingly, we found that OXA also reduced pSTAT3 levels in GCM-exposed BMDMs and altered the expression of the enzyme arginase-1 in these cells, suggesting a shift in functional polarization toward an anti-tumor state. Taken together, these findings reveal intriguing multi-faceted therapeutic potential of OXA in the treatment of gliomas in this dosing context.
The major hurdle limiting successful use of platinumbased chemotherapeutics for glioma therapy is thought to be dose-limiting toxicities and minimal delivery into the CNS due to the blood brain barrier [14, 36]. Numerous efforts are underway to generate less-toxic “protected” platinum drug formulations such as Lipoplatin (liposomal cisplatin) for use against CNS and other tumors [37]. Despite this work and some promising results observed in clinical trials, numerous prior efforts to apply platinum-based chemotherapeutics for glioma therapy have been disappointing [38–41]. New findings in this current study and others [5, 6, 10] related to the potential therapeutic effects of OXA at lower less toxic doses suggest that a major consideration in re-formulations of OXA should be maximizing the potential for multi-faceted alternative effects, rather than maximizing cytotoxic drug concentrations in tumor tissues.
It is increasingly recognized that effective treatment of GBM will require multi-modal or combination strategies that concurrently counteract numerous aspects of the disease biology and TME. The two most abundant cells types in the TME are glioma cells and macrophages/microglia [16–18]. For this reason, we focused on these cells in this study. Our findings suggest that OXA may act at critical nodes of GBM pathobiology through induction of ER stress, leading to disruption of STAT3 activity, reduced expression of the DNA repair enzyme MGMT, and enhanced immunogenicity through ICD. While OXA has been shown previously to induce ER stress leading to ICD [15], this study links OXA-induced ER stress to the inhibition of STAT3 and downregulation of MGMT, highlighting additional and important alternative effects of OXA. MGMT diminishes the effectiveness of TMZ, the standard chemotherapy for GBM, by repairing TMZ-induced DNA damage [23, 24]. As a result, MGMT has become a major therapeutic target for overcoming tumor resistance to the standard GBM treatment [23, 24]. Although, STAT3 inhibition in glioma cells has been shown to post-transcriptionally down-regulate MGMT expression [42], we found OXA reduces MGMT mRNA expression, revealing that OXA may not only reduce MGMT independent of STAT3, but may also be therapeutically valuable as an MGMT inhibitor, especially considering our findings that OXA sensitizes tumor cells to TMZ.
An important next step is the evaluation of the multifaceted therapeutic effects of OXA in vivo using immunocompetent mouse or rat GBM models. Several studies have evaluated the effect of platinum-based chemotherapeutics, including OXA, after convection-enhanced delivery (CED), which significantly improves the delivery of drug to glioma cells compared to other delivery routes. However, local platinum-based drug delivery has been associated with adverse drug toxicity [43–46]. To potentially overcome this toxicity, several studies have used nanoparticle formulations of platinum-based chemotherapeutics in combination with CED. Such formulations have been demonstrated to reduce toxicity and improve therapeutic efficacy in preclinical glioma models [43, 44, 46]. The in vivo evaluation of the multifaceted effects of OXA in vivo may need to include both nanoformulations and advanced delivery strategies, which in addition to improving therapeutic delivery and lowering toxicity, may enhance OXA effects by providing sustained OXA levels at the tumor site [43, 44].
While this study uncovered new, potentially valuable antiglioma effects of OXA, there are some notable limitations. First, the two mouse glioma cell lines used here, GL261 (a chemically induced cancer line) and KR158 (derived from a tumor formed in NF1 and p53 conditional knockout mice), have been characterized extensively and used in numerous pre-clinical GBM studies [47–49]; however, they are not human glioma cells. Second, while GCM-exposed BMDMs are frequently used as a model for tumor-associated macrophages [50, 51], the broad and complex population of macrophages in human tumors [52] is not adequately represented by this simplified model. Lastly, the full extent of potentially positive and negative effects of OXA was not investigated here. Additional studies exploring transcriptomic and proteomic alterations in vivo, other potential mechanisms of these and other alternative OXA effects, and therapeutic efficacy experiments in vivo would be valuable. Ongoing and future drug re-purposing efforts aimed at new clinical applications of platinum-based drugs will likely benefit from considering strategies that harness the therapeutic potential of both alternative and direct cytotoxic effects.
Supplementary Material
Acknowledgements
We thank Rebeca Galisteo for technical assistance. We also thank Dr. Michael Lim for providing GL261-luc cells and Dr. Tyler Jacks for providing KR158-luc cells.
Funding This work was supported in part by the National Institutes of Health (K08 NS090430 [GW], F30 CA216970 [NR]; R56 AI076736 [ADK]; R25 CA186872 [JRH]; P30 CA134274 [JRH]), the American Cancer Society (128970-RSG-16–012-01-CDD [GW]), Merit Review Award Number I01 BX001850 from the US Department of Veterans Affairs Biomedical R&D Service [ADK] and the UMMS Foundation Nathan Schnaper Fund [JRH].
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
Ethical approval All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11060-018-2979-1) contains supplementary material, which is available to authorized users.
References
- 1.Rolle CE, Sengupta S, Lesniak MS (2012) Mechanisms of immune evasion by gliomas. Adv Exp Med Biol 746:53–76 [DOI] [PubMed] [Google Scholar]
- 2.Aum DJ, Kim DH, Beaumont TL, Leuthardt EC, Dunn GP, Kim AH (2014) Molecular and cellular heterogeneity: the hallmark of glioblastoma. Neurosurg Focus 37(6):E11. [DOI] [PubMed] [Google Scholar]
- 3.Shah AH, Graham R, Bregy A, Thambuswamy M, Komotar RJ (2014) Recognizing and correcting failures in glioblastoma treatment. Cancer Invest 32(6):299–302 [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Xu T, Jiang Y, Xu H, Yan Y, Fu D, Chen J (2015) The challenges and the promise of molecular targeted therapy in malignant gliomas. Neoplasia 17(3):239–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross-Knorr S, Lu S, Perez K, Guevara S, Brilliant K, Pisano C, Quesenberry PJ, Resnick MB, Chatterjee D (2013) RKIP phosphorylation and STAT3 activation is inhibited by oxaliplatin and camptothecin and are associated with poor prognosis in stage II colon cancer patients. BMC Cancer 13:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesterhuis WJ, Punt CJ, Hato SV, Eleveld-Trancikova D, Jansen BJ, Nierkens S, Schreibelt G, de Boer A, Van Herpen CM, Kaanders JH, van Krieken JH, Adema GJ, Figdor CG, de Vries IJ (2011) Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest 121(8):3100–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hato SV, Figdor CG, Takahashi S, Pen AE, Halilovic A, Bol KF, Vasaturo A, Inoue Y, de Haas N, Verweij D, Van Herpen CML, Kaanders JH, van Krieken J, Van Laarhoven HWM, Hooijer GKJ, Punt CJA, Asai A, de Vries IJM, Lesterhuis WJ (2017) Direct inhibition of STAT signaling by platinum drugs contributes to their anti-cancer activity. Oncotarget 8(33):54434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hato SV, de Vries IJM, Lesterhuis WJ (2012) STATing the importance of immune modulation by platinum chemotherapeutics. Oncoimmunology 1(2):234–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Wu L, Zhang J, Wu H, Han E, Guo Q (2017) Chemoimmunotherapy by combining oxaliplatin with immune checkpoint blockades reduced tumor burden in colorectal cancer animal model. Biochem Biophys Res Commun 487(1):1–7 [DOI] [PubMed] [Google Scholar]
- 10.Bruno PM, Liu Y, Park GY, Murai J, Koch CE, Eisen TJ, Pritchard JR, Pommier Y, Lippard SJ, Hemann MT (2017) A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med 23(4):461–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiring-helli F, Aymeric L, Michaud M, Apetoh L, Barault L, Mendiboure J, Pignon JP, Jooste V, van Endert P, Ducreux M, Zitvogel L, Piard F, Kroemer G (2010) Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 29(4):482–491 [DOI] [PubMed] [Google Scholar]
- 12.Kepp O, Menger L, Vacchelli E, Locher C, Adjemian S, Yamazaki T, Martins I, Sukkurwala AQ, Michaud M, Senovilla L, Galluzzi L, Kroemer G, Zitvogel L (2013) Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev 24(4):311–318 [DOI] [PubMed] [Google Scholar]
- 13.Hato SV, Khong A, de Vries IJ, Lesterhuis WJ (2014) Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res 20(11):2831–2837 [DOI] [PubMed] [Google Scholar]
- 14.Roberts NB, Wadajkar AS, Winkles JA, Davila E, Kim AJ, Wood-worth GF (2016) Repurposing platinum-based chemotherapies for multi-modal treatment of glioblastoma. Oncoimmunology 5(9):e1208876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, Michaud M, Menger L, Gdoura A, Tajeddine N, Tesniere A, Zitvogel L, Kroemer G (2011) Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene 30(10):1147–1158 [DOI] [PubMed] [Google Scholar]
- 16.Carvalho da Fonseca AC, Badie B (2013) Microglia and macrophages in malignant gliomas: recent discoveries and implications for promising therapies. Clin Dev Immunol 2013:264124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson SD, Srinivasan VM, Heimberger AB (2015) The role of STAT3 in tumor-mediated immune suppression. J Neurooncol 123(3):385–394 [DOI] [PubMed] [Google Scholar]
- 18.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K (1979) Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg 50(3):305–311 [DOI] [PubMed] [Google Scholar]
- 19.Hersh DS, Peng S, Dancy JG, Galisteo R, Eschbacher JM, Castellani RJ, Heath JE, Legesse T, Kim AJ, Woodworth GF, Tran NL, Winkles JA (2018) Differential expression of the TWEAK receptor Fn14 in IDH1 wild-type and mutant gliomas. J Neurooncol 138(2):241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouédraogo ZG, Biau J, Kemeny J-L, Morel L, Verrelle P, Chautard E (2017) Role of STAT3 in genesis and progression of human malignant gliomas. Mol Neurobiol 54(8):5780–5797 [DOI] [PubMed] [Google Scholar]
- 21.Zhong Z, Wen Z, Darnell JE Jr, (1994) Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264(5155):95–98 [DOI] [PubMed] [Google Scholar]
- 22.Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, Kondo S, Priebe W, Kondo Y (2007) A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene 26(17):2435–2444 [DOI] [PubMed] [Google Scholar]
- 23.Jiang G, Jiang AJ, Xin Y, Li LT, Cheng Q, Zheng JN (2014) Progression of O(6)-methylguanine-DNA methyltransferase and temozolomide resistance in cancer research. Mol Biol Rep 41(10):6659–6665 [DOI] [PubMed] [Google Scholar]
- 24.Fan CH, Liu WL, Cao H, Wen C, Chen L, Jiang G (2013) O6-methylguanine DNA methyltransferase as a promising target for the treatment of temozolomide-resistant gliomas. Cell Death Dis 4:e876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi S, Tan P, Yan B, Gao R, Zhao J, Wang J, Guo J, Li N, Ma Z (2016) ER stress and autophagy are involved in the apoptosis induced by cisplatin in human lung cancer cells. Oncol Rep 35(5):2606–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Y, Tang B, Yu P-W, Tang B, Hao Y-X, Lei X, Luo H-X, Zeng D-Z (2012) Autophagy protects against oxaliplatin-induced cell death via ER stress and ROS in Caco-2 cells. PLoS ONE 7(11):e51076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J (2005) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307(5711):935–939 [DOI] [PubMed] [Google Scholar]
- 28.Kubota K, Niinuma Y, Kaneko M, Okuma Y, Sugai M, Omura T, Uesugi M, Uehara T, Hosoi T, Nomura Y (2006) Suppressive effects of 4-phenylbutyrate on the aggregation of Pael receptors and endoplasmic reticulum stress. J Neurochem 97(5):1259–1268 [DOI] [PubMed] [Google Scholar]
- 29.Xipell E, Aragon T, Martinez-Velez N, Vera B, Idoate MA, Martinez-Irujo JJ, Garzon AG, Gonzalez-Huarriz M, Acanda AM, Jones C, Lang FF, Fueyo J, Gomez-Manzano C, Alonso MM (2016) Endoplasmic reticulum stress-inducing drugs sensitize glioma cells to temozolomide through downregulation of MGMT, MPG, and Rad51. Neuro Oncol 18(8):1109–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, Yuan J, Zitvogel L, Madeo F, Williams DB, Kroemer G (2009) Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. Embo j 28(5):578–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Alizadeh D, Van Handel M, Kortylewski M, Yu H, Badie B (2009) Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia 57(13):1458–1467 [DOI] [PubMed] [Google Scholar]
- 32.Fujiwara Y, Komohara Y, Kudo R, Tsurushima K, Ohnishi K, Ikeda T, Takeya M (2011) Oleanolic acid inhibits macrophage differentiation into the M2 phenotype and glioblastoma cell proliferation by suppressing the activation of STAT3. Oncol Rep 26(6):1533–1537 [DOI] [PubMed] [Google Scholar]
- 33.Yao Y, Ye H, Qi Z, Mo L, Yue Q, Baral A, Hoon DSB, Vera JC, Heiss JD, Chen CC, Zhang J, Jin K, Wang Y, Zang X, Mao Y, Zhou L (2016) B7-H4(B7x)-mediated cross-talk between gliomainitiating cells and macrophages via the IL6/JAK/STAT3 pathway lead to poor prognosis in glioma patients. Clin Cancer Res 22(11):2778–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luwor RB, Stylli SS, Kaye AH (2013) The role of Stat3 in glioblastoma multiforme. J Clin Neurosci 20(7):907–911 [DOI] [PubMed] [Google Scholar]
- 35.Kadowaki H, Nishitoh H (2013) Signaling pathways from the endoplasmic reticulum and their roles in disease. Genes (Basel) 4(3):306–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckner JC, Ballman KV, Michalak JC, Burton GV, Cascino TL, Schomberg PJ, Hawkins RB, Scheithauer BW, Sandler HM, Marks RS, O’Fallon JR (2006) Phase III trial of carmustine and cisplatin compared with carmustine alone and standard radiation therapy or accelerated radiation therapy in patients with glioblastoma multiforme: North Central Cancer Treatment Group 93–72-52 and Southwest Oncology Group 9503 Trials. J Clin Oncol 24(24):3871–3879 [DOI] [PubMed] [Google Scholar]
- 37.Boulikas T (2004) Low toxicity and anticancer activity of a novel liposomal cisplatin (Lipoplatin) in mouse xenografts. Oncol Rep 12(1):3–12 [PubMed] [Google Scholar]
- 38.Jeremic B, Grujicic D, Jevremovic S, Stanisavljevic B, Milojevic L, Djuric L, Mijatovic L (1992) Carboplatin and etoposide chemotherapy regimen for recurrent malignant glioma: a phase II study. J Clin Oncol 10(7):1074–1077 [DOI] [PubMed] [Google Scholar]
- 39.Peterson K, Harsh Gt, Fisher PG, Adler J, Le Q (2001) Daily low-dose carboplatin as a radiation sensitizer for newly diagnosed malignant glioma. J Neurooncol 53(1):27–32 [DOI] [PubMed] [Google Scholar]
- 40.Brandes AA, Rigon A, Zampieri P, Ermani M, Carollo C, Altavilla G, Turazzi S, Chierichetti F, Florentino MV (1998) Carboplatin and teniposide concurrent with radiotherapy in patients with glioblastoma multiforme: a phase II study. Cancer 82(2):355–361 [DOI] [PubMed] [Google Scholar]
- 41.Jeremic B, Shibamoto Y, Acimovic L, Milicic B, Milisavljevic S, Nikolic N, Dagovic A, Aleksandrovic J, Radosavljevic-Asic G (2001) Hyperfractionated radiation therapy and concurrent low-dose, daily carboplatin/etoposide with or without weekend carboplatin/etoposide chemotherapy in stage III non-small-cell lung cancer: a randomized trial. Int J Radiat Oncol Biol Phys 50(1):19–25 [DOI] [PubMed] [Google Scholar]
- 42.Kohsaka S, Wang L, Yachi K, Mahabir R, Narita T, Itoh T, Tanino M, Kimura T, Nishihara H, Tanaka S (2012) STAT3 inhibition overcomes temozolomide resistance in glioblastoma by downregulating MGMT expression. Mol Cancer Ther 11(6):1289. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C, Nance EA, Mastorakos P, Chisholm J, Berry S, Eberhart C, Tyler B, Brem H, Suk JS, Hanes J (2017) Convection enhanced delivery of cisplatin-loaded brain penetrating nanoparticles cures malignant glioma in rats. J Control Release 263:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arshad A, Yang B, Bienemann AS, Barua NU, Wyatt MJ, Woolley M, Johnson DE, Edler KJ, Gill SS (2015) Convection-enhanced delivery of carboplatin PLGA nanoparticles for the treatment of glioblastoma. PLoS ONE 10(7):e0132266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi M, Fortin D, Sanche L, Paquette B (2015) Convection-enhancement delivery of platinum-based drugs and Lipoplatin(TM) to optimize the concomitant effect with radiotherapy in F98 glioma rat model. Invest New Drugs 33(3):555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi M, Fortin D, Paquette B, Sanche L (2016) Convection- enhancement delivery of liposomal formulation of oxaliplatin shows less toxicity than oxaliplatin yet maintains a similar median survival time in F98 glioma-bearing rat model. Invest New Drugs 34(3):269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maes W, Van Gool SW (2011) Experimental immunotherapy for malignant glioma: lessons from two decades of research in the GL261 model. Cancer Immunol Immunother 60(2):153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Zhang L, Zhang IY, Liang J, Wang H, Ouyang M, Wu S, da Fonseca ACC, Weng L, Yamamoto Y, Yamamoto H, Natarajan R, Badie B (2014) RAGE expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Res 74(24):7285–7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T (2000) Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat Genet 26(1):109–113 [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, Lin Y, Dietz AB, Forsyth PA, Yong VW, Parney IF (2010) Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol 12(4):351–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergamin LS, Braganhol E, Figueiro F, Casali EA, Zanin RF, Sevigny J, Battastini AM (2015) Involvement of purinergic system in the release of cytokines by macrophages exposed to glioma- conditioned medium. J Cell Biochem 116(5):721–729 [DOI] [PubMed] [Google Scholar]
- 52.Hambardzumyan D, Gutmann DH, Kettenmann H (2016) The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 19(1):20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.