Abstract
Pancreatic ductal adenocarcinoma (PDAC) is among the most deadly solid tumours. This is due to a generally late-stage diagnosis of a primarily treatment-refractory disease. Several large-scale sequencing and mass spectrometry approaches have identified key drivers of this disease and in doing so highlighted the vast heterogeneity of lower frequency mutations that make clinical trials of targeted agents in unselected patients increasingly futile. There is a clear need for improved biomarkers to guide effective targeted therapies, with biomarker-driven clinical trials for personalised medicine becoming increasingly common in several cancers. Interestingly, many of the aberrant signalling pathways in PDAC rely on downstream signal transduction through the mitogen-activated protein kinase and phosphoinositide 3-kinase (PI3K) pathways, which has led to the development of several approaches to target these key regulators, primarily as combination therapies. The following review discusses the trend of PDAC therapy towards molecular subtyping for biomarker-driven personalised therapies, highlighting the key pathways under investigation and their relationship to the PI3K pathway.
Keywords: pancreatic cancer, clinical trials, cell biology
Introduction
Accounting for ~95% of pancreatic cancers, pancreatic ductal adenocarcinoma (PDAC) has a very poor overall 5-year survival of 8% and is predicted to be the second leading cause of cancer-related deaths in the developed world by 2030.1–3 This has only marginally improved since the introduction of gemcitabine in 1995.4 5 Surgery remains the only curative treatment and is often applied with adjuvant chemotherapy, but as few as 10%–15% of patients are eligible at initial diagnosis.6–9 Most patients with PDAC have few or non-specific symptoms as the tumour develops, and this means that a large proportion are diagnosed at a late stage, already presenting with locally advanced or metastatic disease.10 For those patients that are not immediately eligible for resection, neoadjuvant chemotherapy can be given to reduce borderline tumours prior to resection.11 Recent clinical trials aimed at improving response to chemotherapy have demonstrated improved survival with patients treated with either a combination of gemcitabine and nab-paclitaxel or FOLFIRINOX (folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin).12–16 However, patient tolerability may be limited with such aggressive treatment regimens.16 While improvements in surgical techniques and chemotherapy regimens are providing modest improvements in survival, there is a clear need to better understand this aggressive disease to facilitate both earlier diagnosis and elucidate new targets for combination therapies.
PDAC progression model
The most widely accepted model for PDAC development is the progression model, in which PDAC originates from preinvasive pancreatic intraepithelial neoplasms (PanINs), which occur in distinct pathological stages, namely PanIN-1A, PanIN-1B, PanIN-2 and PanIN-3.17–19 In the early stages (ie, PanIN-1A and PanIN-1B or low-grade), a mucinous epithelium replaces the typically cuboidal morphology of normal pancreatic ducts, with a low level of dysplasia.19–22 Yet, recent work has suggested that pancreatic repair after injury, by the process of acinar-to-ductal metaplasia, may also be involved in PDAC initiation.23–25 As these PanINs progress (ie, to PanIN-2 and PanIN-3 or intermediate-grade and high-grade, respectively), dysplasia increases and is detectable as nuclear irregularity, loss of cell polarity and an increase in intraluminal apoptotic debris.19–22 This progression towards an invasive carcinoma has been shown to occur in parallel with increased proliferation and mutational burden from early preinvasive PanIN stages to metastatic PDAC (figure 1).19 26 Importantly, a mechanism underlying the switch from PanIN to metastatic PDAC remains unclear, but new genetically engineered mice that model multistep carcinogenesis may support the widely accepted stepwise mutational model, where some have suggested that catastrophic genomic events may instead trigger the transformation from preneoplastic lesions.27 28
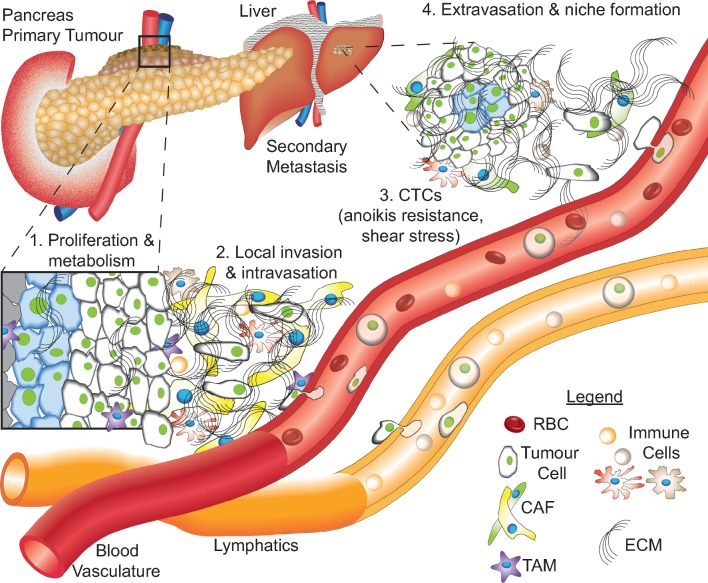
Figure 1.
Schematic representation of PDAC progression from the primary tumour to a locally invasive disease and eventually metastasis. (1) Pancreatic cancer cells proliferate in the primary tumour, metabolising nutrients delivered by the blood vasculature and surrounding stroma. (2) Cancer cells invade through the extracellular matrix (ECM), including cancer-associated fibroblasts (CAFs) and tumour-associated macrophages (TAMs), among other cancer-associated cell types, eventually intravasating or invading into the lymph and travelling to distant sites. (3) Circulating tumour cells (CTCs) must develop resistance to anoikis, as well as shear stress, in order to survive in the circulation with red blood cells (RBCs) and leucocytes. (4) After travelling through the circulation, CTCs extravasate at secondary sites, commonly the liver, establishing a new niche. ECM, extracellular matrix; PDAC, pancreatic ductal adenocarcinoma.
A similar progression model has been proposed for intraductal papillary mucinous neoplasms (IPMNs), which are generally benign, but progress to an invasive carcinoma in up to 25% of cases.29–32 Both IPMNs and mucinous cystic neoplasms are radiologically detectable as macroscopic lesions and are classified according to the Sendai guidelines.33 They are typically distinguished from PDAC at a macroscopic level by mucoid contents and have distinct stromal subtypes at a microscopic level.34 35 Indeed, the mucoid expression itself has been used to subtype IPMNs according to whether the gene expression is gastric or intestinal, which clearly distinguished aggressive disease as the intestinal subtype.35
Attempts have been made to classify PanINs in terms of their mutational burden. Initially, evidence of telomere shortening and mutations in KRAS were found to occur very early in PanIN progression.36 37 This excluded KRAS as a potential marker for PDAC progression but highlights the general classification as the earliest initiator mutation in PDAC, occurring in ~95% of PDAC cases.26 38 Progression through to PanIN-2 and PanIN-3 typically includes additional mutations in TP53, SMAD4 and/or CDKN2A, but the vast molecular heterogeneity of this disease precludes any single mutation as essential for PDAC development.38 39 With this in mind, several large-scale sequencing and mass spectrometry approaches have been implemented to subtype the disease based on these molecular characteristics.40–43 The goal of such work is to better integrate biomarkers into the drug discovery pipeline, where lead compound development is performed hand in hand with biomarker identification (figure 2). This parallel preclinical development aims to foster a more personalised approach to clinical trial development, whereby each patient may be assessed for their respective molecular subtype and treatment is designed based on this result (figure 2).
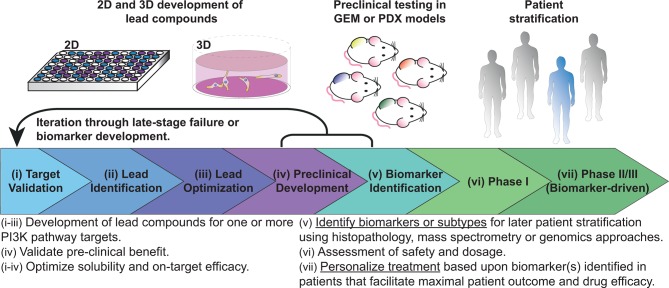
Figure 2.
Adaptable drug development pipeline, demonstrating the progression of lead compounds through target validation, lead compound identification and optimisation, then preclinical validation. The necessary addition to this process is the identification of biomarkers to guide both lead compound development and later stratification in phase II/III clinical trials. These processes may be iterated to improve on-target efficacy, solubility and biomarkers. After safety and tolerability is confirmed in phase I clinical trials, biomarker-driven phase II/III may reduce the high attrition rates of lead compounds if appropriate patient stratification can demonstrate beneficial response in the assessed subsets of patients. These biomarkers may also provide opportunities for retrospective analysis and later iteration into clinical trials. PI3K, phosphoinositide 3-kinase.
Molecular subtyping of PDAC
While several mutations occur at relatively high frequency in PDAC, mutations in the aforementioned genes are not currently associated with clinically actionable phenotypes. The milieu of lower frequency mutations, however, has motivated subtyping based on commonly mutated biological processes, termed gene programmes (GPs). The aim of such work is to develop therapeutic strategies that are selectively effective against specific tumour subtypes.15 44 Early work stratified PDAC according to an activated stromal index, which classified patients according to the ratio of alpha smooth muscle actin (immunohistochemical (IHC) staining) and collagen (stained with the collagen-specific Aniline blue).45 Such an index informs primarily on stromal targeting and alone is not sufficient to guide therapies aimed at complete tumour regression. Indeed, a second study took the opposite approach and removed the stroma by laser microdissection from the PDAC samples, prior to microarray analysis and subtyping of PDAC based on multivariate analysis of transcriptional profiles, namely classical, exocrine-like and quasimesenchymal (QM; table 1, see column ‘Collisson’).40 Such an approach allowed the authors to identify neoplastic epithelial-specific gene expression and to identify pathways involved in PDAC progression. This approach also motivated metabolite profiling within these subtypes, where classical tumours were shown to be lipogenic, while QM tumours were glycolytic.46 With clear subtype-specific metabolic targets, new avenues for combination therapies within a personalised setting are an obvious progression to improve patient responses. Additionally, increasing evidence for the importance of the stroma in disease progression means assessment of either the tumour or stroma in isolation is likely to be too simplistic to provide any lasting improvements in patient survival.47
Table 1.
Molecular subtyping of patients with pancreatic cancer
| Collisson | Moffit | Bailey | |
| Approach | Microarray | Microarray | Expression analysis (RNAseq and microarray) |
| Cohort | 63 primary resected PDAC | 145 primary resected and 61 metastatic PDAC tumours | 96 RNAseq and 242 microarray primary patient samples |
| Tumour/stromal contribution | Microdissection | Multivariate analysis (virtual microdissection) | Macrodissection |
| Tumour subtypes | Classical | Classical | Pancreatic progenitor |
| Immunogenic | |||
| Exocrine-like | ADEX | ||
| QM | Basal like | Squamous | |
| Stromal subtypes | Not assessed | Activated | ESTIMATE |
| Normal |
This pancreatic cancer subtype table is adapted from refs 40–42.
ADEX, Aberrantly Differentiated Endocrine eXocrine; ESTIMATE, Estimation of STromal and Immune cells in MAlignant Tumor tissues using Expression data; PDAC, pancreatic ductal adenocarcinoma; QM, quasimesenchymal.
Physical microdissection approaches rely on IHC to inform stromal activation state and also limit the application of patient subtyping by molecular approaches due to a low sample throughput and smaller sample volume.48 As large datasets become increasingly common, new analytical approaches improve the readouts incurred. A more recent approach to PDAC subtyping involved virtual microdissection of large microarray datasets, facilitating molecular subtyping of both the tumour and the stroma.41 Using multivariate analysis to distinguish tumour and stromal components, the tumour was split into a classical and more aggressive basal-like subtype, and the stroma was classified into activated or normal subtypes (table 1, see column ‘Moffit’). This additional stromal subtyping was also recently applied to PDAC patient-derived xenograft (PDX) tumours, whereby tumours classified as basal or classical were shown to have an ‘echo’ in the mouse stroma.49 They further demonstrated the power of their classifications through inhibition of cholesterol uptake in subtyped PDX models, where basal tumours were highly sensitive to inhibition, but classical tumours were shown to have higher NPC1L1 expression and may require a greater concentration of inhibitor to achieve an equivalent growth inhibition.
Further subtyping was recently performed on a 328 primary patient PDAC cohort using expression analysis from RNAseq (96 patients) and microarrays (232 patients).42 This study included samples with invasive IPMN-associated PDACs and some metastatic tumours and, in contrast to the previous studies, applied macrodissection to excise areas of nonmalignant tissue, maintaining the stromal component in each sample.38 42 50 Tumour purity could then be inferred in terms of stromal and immune infiltration based on the Estimation of STromal and Immune cells in MAlignant Tumor tissues using Expression data approach.51 Beyond purity assessment, this approach facilitated assessment of GPs associated with microenvironmental factors, such as hypoxia, ECM deposition and activated immune pathways.42 The microenvironmental influence on cancer progression is an essential consideration for emerging therapies, where immune cells, cancer-associated fibroblasts and ECM components are regularly associated with cancer progression (figure 1). Inclusion of this stromal contribution, as well as the large breadth of patient samples, allowed the authors to reclassify PDAC into four distinct subtypes (summarised in table 1 (see column ‘Bailey’) and box 1). This is particularly important in light of the high attrition rates for lead compounds currently experienced by the pharmaceutical industry, where more detailed molecular analysis prior to treatment is expected to improve both patient and trial outcomes (figure 2).52–54
Box 1. Pancreatic cancer subtypes.
Molecular subtypes (described in ref 42)
Immunogenic
Patients were classified with this subtype if they showed evidence of high levels of immune infiltrate, which presents an inferred opportunity for emerging immunotherapies.
Pancreatic progenitor
This subtype shares several gene programmes (GPs) with the immunogenic subtype. It is defined by an increased activity of transcriptional networks associated with pancreatic endodermal cell fate. Pancreatic ductal adenocarcinoma (PDAC) associated with IPMN typically fell within this subtype, where an increase in fatty acid metabolism and O-linked glycosylation of mucins was upregulated.
Aberrantly Differentiated Endocrine eXocrine
This subtype presented with higher activity of GPs associated with exocrine secretion, consistent with a more differentiated pancreatic lineage.
Squamous
Describing the most aggressive PDAC tumours, including the KPC GEM PDAC model, this subtype is defined by an upregulation of GPs associated with hypoxia, metabolic reprogramming, ECM deposition, squamous differentiation and proliferation.
Neoantigen quality (described in ref 280)
Antigens encoded by tumour-specific genes (neoantigens) are enriched in long-term PDAC survivors, along with high T cell infiltration. The quality of neoantigen may then provide a biomarker for emerging immunotherapies.
Mass spectrometry subtypes (described in ref 43)
Using 8 or 33 phosphosites as classifiers, the Australian Pancreatic Cancer Genome Initiative patient-derived cell lines or commercially available pancreatic cancer lines from the American Type Culture Collection were grouped into three subtypes based on their pTyr levels. Of these, subtype 3 in both cell line cohorts was enriched for receptor tyrosine kinase (RTK) phosphorylation and showed increased sensitivity to the epidermal growth factor receptor inhibitor erlotinib. This suggests that mass spectrometry approaches may provide a binary system for classifying patients into RTK or phosphoinositide 3-kinase pathway targeted therapies.
The goal of this molecular phenotyping is to establish trials, such as IMPaCT, PRECISION-Panc, SHIVA, or biomarker-driven avatar trials (NCT02795650), where actionable molecular data guides therapies.55 56 These trials have established the feasibility of biopsy collection for pancreatic cancer within a clinical setting, where molecular assessment was performed by IHC or genomic approaches. However, biomarker-driven trials for pancreatic cancer remain infrequent, despite increasing evidence for a lack of stratification leading to late-stage failure. This is particularly evident for PI3K pathway inhibitors, where preclinical efficacy is driving their assessment in a clinical setting, but biomarker-driven trials in pancreatic cancer are sorely lacking (table 2). This is in stark contrast to the increase in biomarker-driven trials in other cancers, where biomarkers such as loss of phosphatase and tensin homolog (PTEN), PIK3CA mutation or Akt amplification/mutation are increasingly used to stratify patients for treatment with PI3K pathway inhibitors.57 Further to this goal, the subtyping approaches described above may also provide novel clinically actionable biomarkers or GPs to allow patient-selective assessment of PI3K pathway inhibitors to push PDAC survival beyond the current standard of care.
Table 2.
List of PI3K pathway inhibitors currently undergoing clinical development for pancreatic cancer
| Target | Inhibitor | Phase | Status | Patients | Combination | NIH number | Reference(s) |
| Akt inhibitors | |||||||
| Pan-Akt | MK2206 | I | Completed | AdvST/MST (~9% pancreatic cancer) |
Monotherapy | NCT00670488 | 262 263 |
| I | Completed | AdvST/MST | Selumitinib (MEKi) | NCT01021748 | |||
| I | Completed | PDAC (PTEN loss) | Monotherapy | NCT00848718 | 264 | ||
| I | Completed | Pancreatic cancer | Dinaciclib (CDKi) | NCT01783171 | |||
| II | Completed | Pancreatic cancer | Selumitinib (MEKi) versus mFOLFOX6 |
NCT01658943 | 265 | ||
| Afuresertib (GSK2110183) | I | Completed | AdvST (21% pancreatic cancer) |
Trametinib (MEKi) | NCT01476137 | 266 | |
| II | Ongoing | AdvST | NCT01531894 | ||||
| Uprosertib (GSK2141795) | I | Completed | Pancreatic cancer | Trametinib (MEKi) | NCT01138085 | ||
| I | Completed | AdvST | NCT00920257 | ||||
| Oleandrin (PBI-05204) | I | Completed | AdvST (6% pancreatic cancer) |
NCT00554268 | 267 | ||
| II | Ongoing | Metastatic pancreatic cancer | NCT02329717 | ||||
| Perifosine | II | Completed | Locally advanced or metastatic pancreatic cancer | NCT00053924 | |||
| II | Completed | Locally advanced or metastatic pancreatic cancer | NCT00059982 | 268 | |||
| RX-0201 | II | Completed | Metastatic pancreatic cancer | Gemcitabine | NCT01028495 | ||
| Rapalogs | |||||||
| mTORC1 (FKBP12) | Sirolimus (rapamycin) | I | Completed | Pancreatic cancer | Sunitinib (RTKi) | NCT00583063 | 150 |
| I | Completed | Pancreatic cancer | Sorafenib (RTKi) | NCT00449280 | 150 | ||
| II | Completed | Pancreatic cancer | NCT00499486 | ||||
| II | Completed | Pancreatic cancer | NCT00276744 | ||||
| I/II | Ongoing | PDAC | Metformin | NCT02048384 | |||
| I | Ongoing | Pancreatic cancer | Vismodegib (SMOi) | NCT01537107 | |||
| Temsirolimus (CCI-779, Torisel) | I | Completed | Pancreatic cancer | Lenalidomide | NCT01183663 | ||
| I | Terminated | PDAC | Gemcitabine | NCT00593008 | |||
| I/II | Ongoing | Pancreatic cancer | Nivolumab (PD-1i) | NCT02423954 | |||
| II | Completed | Locally advanced or metastatic pancreatic cancer | NCT00075647 | 95 | |||
| Everolimus (RAD001) | I | Completed | Pancreatic cancer | Sorafenib (RTKi) | NCT00981162 | ||
| I | Completed | Pancreatic cancer | Trametinib (MEKi) | NCT00955773 | 269 | ||
| I/II | Completed | PDAC | Gemcitabine | NCT00560963 | |||
| I/II | Completed | Pancreatic cancer | Cetuximab (EGFRi) and capecitabine | NCT01077986 | 99 | ||
| II | Terminated | Pancreatic cancer | Erlotinib (EGFRi) | NCT00640978 | 95 | ||
| II | Completed | Pancreatic cancer | NCT00409292 | 94 | |||
| I/II | Recruiting | PDAC | Ribociclib (CDKi) | NCT02985125 | |||
| Ridafirolimus | I | Completed | AdvST (12% pancreatic cancer) |
Bevacizumab (VEGFRi) | NCT00781846 | 149 | |
| PI3K inhibitors | |||||||
| PI3K isoform p110α | Alpelisib (BYL719) | I | Ongoing | Pancreatic cancer | Gemcitabine and abraxane | NCT02155088 | |
| Pan-PI3K | Buparlisib (BKM120) | I | Completed | Pancreatic cancer | mFOLFOX6 | NCT01571024 | 270 |
| I | Completed | Pancreatic cancer | LDE225 (SMOi) | NCT01576666 | |||
| I | Completed | Pancreatic cancer | Trametinib (MEKi) | NCT01155453 | 271 | ||
| I | Ongoing | AdvST | MEK163 (MEKi) | NCT01363232 | |||
| PX-866 | I | Completed | AdvST (5% PDAC) |
Docetaxel | NCT01204099 | 272 | |
| ZSTK474 | I | Completed | AdvST | NCT01280487 | |||
| Copanlisib (BAY 80–6946) |
I | Completed | AdvST (18% pancreatic cancer) |
NCT00962611 | 273 | ||
| Dual PI3K pathway inhibitors | |||||||
| mTORC1/2 | Vistusertib (AZD2014) | I | Completed | AdvST | NCT01026402 | 98 | |
| II | Recruiting | AdvST (RICTOR amplified) | NCT03166904 | ||||
| II | Recruiting | AdvST: combination with Selumitinib (MEKi) |
NCT02583542 | ||||
| II | Recruiting | AdvST (TSC1/2 loss or mutation) | NCT03166176 | ||||
| II | Recruiting | AdvST: combination with Olaparib (PARPi) |
NCT02576444 | ||||
| p70-S6K and Akt | LY2780301 | I | Complete | AdvST (~22% pancreatic cancer) |
NCT01115751 | 274 | |
| PI3K and mTOR | Dactolisib (NVP-BEZ235) |
I | Completed | AdvST | MEK162 (MEKi) | NCT01337765 | |
| NVP-BGT226 | I | Completed | AdvST (2% pancreatic cancer) |
NCT00600275 | 275 | ||
| Voxtalisib (SAR245409, XL765) | I | Completed | AdvST (4% pancreatic cancer) |
NCT00485719 | 276 | ||
| SF1126 (LY294002 prodrug) |
I | Completed | AdvST (5% pancreatic cancer) |
NCT00907205 | 277 | ||
| Gedatolisib (PF-05212384, PKI-587) | I | Terminated | AdvST (5% pancreatic cancer) |
Irinotecan | NCT01347866 | 278 | |
| I | Completed | AdvST (4% PDAC) |
NCT00940498 | 279 | |||
| I | Recruiting | AdvST | Palbociclib (CDKi) | NCT03065062 | |||
AdvST, advanced solid tumours (including pancreatic cancer); CDKi, cyclin-dependent kinase inhibitor; EGFR, epidermal growth factor receptor; EGFRi, EGFR inhibitor; MEKi, MAPK/ERK kinase inhibitor inhibitor; mFOLFOX6, modified FOLFOX (ie, 5-fluorouracil and oxaliplatin); MST, metastatic solid tumours (including pancreatic cancer); mTOR, mechanistic target of rapamycin; NIH, National Institutes of Health; PARP, poly (ADP-ribose) polymerase; PARPi, PARP inhibitors; PDAC, pancreatic ductal adenocarcinoma; PD-1, programmed death-1; PD-1i, PD-1 inhibitor; PI3K, phosphoinositide 3-kinase; RTKi, receptor tyrosine kinase inhibitor; SMOi, smoothened inhibitor; VEGFRi, vascular endothelial growth factor receptor inhibitor.
The phosphoinositide 3-kinase (PI3K) pathway
A broad range of cancer types, including pancreatic cancer, have been candidates for targeting of the PI3K pathway, due to amplification, mutation or loss of key regulators.58 59 The PI3K pathway mediates transduction of signals from both extracellular and intracellular sources, including growth factors and nutrients, leading to downstream signalling involved in cancer growth, survival and progression (figure 1).58 60 61 The pathway is also essential for many cancer-associated activities, including endothelial cell sprouting for angiogenesis, macrophage transcriptional reprogramming, T cell differentiation and homeostasis and fibroblast-supported chemoresistance (figure 1).62–65 Collectively, this suggests that application of PI3K pathway inhibitors as a PDAC therapy may provide an opportunity for dual targeting of cancer cells and the deregulated cancer-associated stromal components.
PDAC is regularly associated with increased Akt activity, which has been identified in ~60% of PDAC samples, with amplification of the AKT2 oncogene occurring in 10%–20% of PDAC cases.66–68 Akt is a key effector of the PI3K pathway, downstream of both PI3K and receptor tyrosine kinases (RTKs; table 2). Furthermore, PDAC tumours have been shown to bear an activating mutation in PIK3CA and/or loss of the tumour suppressor PTEN in ~4% and 25%–70% of cases, respectively.50 69–72 Interestingly, patients with low PTEN expression have a much higher incidence of recurrence or metastasis, compared with those with high PTEN.72 Furthermore, it has been shown that PDAC patients with high PI3K pathway activity show a significantly poorer survival than those with low activation of this pathway.73
Several signalling pathways are known to converge on the MAPK and PI3K pathways as effectors of cellular response within the cell. For example, in ~95% of cases, pancreatic cancer is driven by activating mutations in KRAS, which in turn activates PI3K signalling through the p110α subunit, along with another pathway component PDK1, indicating that a large proportion of patients could benefit from effective targeting of this pathway (figure 3).28 73–76 Furthermore, detection of mutations in PIK3CA can be predictive for improved patient response in preclinical models of PDAC and in patients with breast cancer stratified according to detection of mutations in circulating cell-free DNA.74 77–79 Given the varied roles of different PI3K isoforms in both the tumour and associated stromal cells, isoform-specific inhibitors provide isolated targeting of oncogenic signalling and allow redundancy to alleviate off-target side effects in healthy tissues (table 2; reviewed in refs 80 81). Notably, a PI3Kα-specific inhibitor has shown promising efficacy in combination with an EGFRi in PDAC with high EGFR and Akt phosphorylation.82 Interestingly, PIK3CA mutations in breast cancer have also been linked with Akt-independent tumour progression through SGK3 and highlight the importance of all levels of this key signalling cascade.83 Similarly, isoform-specific PI3Kβ inhibition extended PDAC survival beyond mTORC1/2 targeting alone,84 and in other cancers, inhibition of PI3Kβ and PI3Kδ has shown antimetastatic effects and suggests a role of PI3K in tumour metastatic dissemination.85 86 Furthermore, isoform-specific inhibition of PI3Kδ in cancer-associated immune cells was shown to downregulate their tolerance to PDAC, which improved the activity of T cells against the cancer.87 Collectively, we see strong evidence accumulating for the efficacy of upstream isoform-specific targeting of PI3K in emerging PDAC combination therapies.
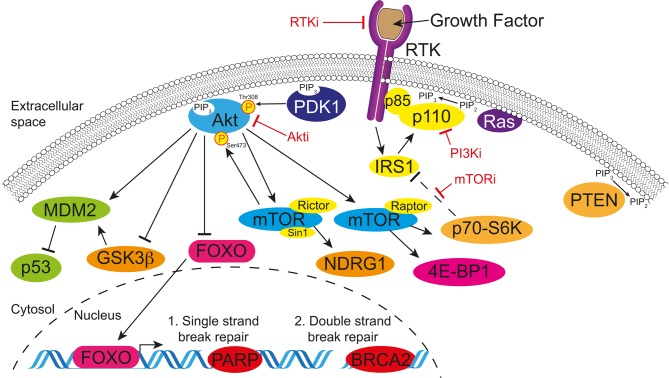
Figure 3.
Simplified schematic of the PI3K pathway, which highlights the common targets for small molecule inhibitors. Briefly, signalling from growth factors activates RTKs and recruits PI3K and other scaffold proteins to the cell membrane, where PIP2 is converted to PIP3. This recruits phosphoinositide-dependent kinase-1 (PDK1) and Akt to the membrane and leads to downstream signalling through the kinase activities of Akt. (1) Single-strand break repair is regulated primarily by PARP and inhibition of PARP can lead to genomic instability. (2) Double-stranded break repair is primarily regulated by a complex with BRCA2, which is lost in familial pancreatic cancer and some PDAC cases and can lead to genomic instability. Genomically unstable tumours require the PI3K pathway to maintain survival pathways and PI3K pathway inhibition may be an emerging option for patients with BRCA2 mutations or in combination with PARP inhibitors. More exhaustive pathway maps can be found in refs 61 80. P13K, phosphoinositide 3-kinase; RTKs, receptor tyrosine kinases.
Concordantly, evidence for the validity of downstream pathway targeting is highlighted in a genetically engineered mouse model of mutant KrasG12D-driven PDAC, which was applied in concert with a sleeping beauty transposon library, both conditionally expressed (ie, LSL-KrasG12D and LSL-SB11) under pancreas-specific Pdx1-Cre.88 89 These approaches identified several genes within the MAPK and PI3K pathways as cooperating mutations for KrasG12D-driven PDAC. Similarly, recent assessment of kinases with the highest levels of absolute and differential expression in a panel of pancreatic cancer cell lines demonstrated significantly reduced cell number after knockdown of EGFR, Akt2, PLK2 or MET.90 This review will focus on PI3K pathway targeting in PDAC (see also table 2, PI3K pathway inhibitors under clinical investigation in PDAC).
PI3K pathway inhibitors in the clinic
After the discovery and isolation of rapamycin on the island of Rapa Nui from Streptomyces hygroscopicus, over 30 years of research continues to find new therapeutic applications for this compound.91 For example, the mTOR inhibitor (mTORi) rapamycin was recently assessed in PDAC driven by activated PI3K/AKT signalling via PTEN loss, where targeting of mTORC1 by rapamycin significantly reduced the onset and progression of the disease (figure 3).92 Work to improve the solubility and bioavailability of rapamycin-based compounds (rapalogs) has seen modification at the C-42 position through addition of an ester, ether or phosphonate group to generate temsirolimus, everolimus and ridaforolimus, respectively.60 Clinical trials in pancreatic neuroendocrine tumours (pNETs) have demonstrated a clear benefit for rapalogs as single agents (table 2);93 however, no significant improvements have been identified for rapalogs as single agents in PDAC.94 95 This has been attributed to an upstream feedback loop where inhibition of mTORC1 alone relieves the inhibitory phosphorylation of insulin receptor substrate 1 (IRS1)by p70-S6K and mTORC1, leading to an upregulation of Akt phosphorylation (figure 3).96 97 Hence, while trials of rapalogs may benefit from stratification for patients with high PI3K pathway activity, newer agents that target both mTORC1 and mTORC2, or other pathway components, allow the negation of this feedback loop with promising therapeutic potential (table 2).84 98 Importantly, new combination therapies with rapalogs should consider the combined toxicity with other targeted compounds. For example, combination of everolimus with the RTKi cetuximab was found to be too toxic for patients with PDAC in a phase I/II clinical trial, while the single agents show minimal toxicity.94 99 100 With this in mind, trials are still ongoing in PDAC using rapalogs in combination therapies (table 2).
Next-generation dual PI3K pathway inhibitors are being developed that take advantage of the homology of the kinase domains from class I, II and III PI3Ks and those of phosphoinositide 3-kinase-related kinases, such as mTOR, ATM and DNA-PK (figure 3, table 2).101 However, these dual inhibitors have been linked with drug-related dosage-dependent toxicities, such as hyperglycaemia, nausea, vomiting and diarrhoea, consistent with PI3K isoform targeting, and reinforce the need for preclinical assessment of the additive or synergistic toxicities when developing novel combination therapies.81 Moving forward, improvements in solubility are driving greater oral bioavailability, where lower drug dosages can show equivalent drug efficacy and gastrointestinal toxicities are readily reduced.102 103 One exciting example of a dual PI3K pathway inhibitor is AZD2014, which was developed by iterative structure–activity relationship medicinal chemistry approaches to have high aqueous solubility and a potent inhibitory effect against both mTORC1 and mTORC2.104 Recent preclinical work by our group has demonstrated potent antiproliferative and anti-invasive effects in the KPC (LSL-KrasG12D, LSL-Trp53R172H and Pdx1-Cre) GEM PDAC model84 105 and after a promising phase I clinical trial in advanced solid tumours (AdvSTs), AZD2014 has progressed to phase II biomarker-driven clinical trials either alone or in combination with a MAPK/ERK kinase inhibitor (MEKi) (table 2).98 The additional anti-invasive role for AZD2014 is consistent with increasing evidence for the emerging antimetastatic and anti-invasive effect of PI3K pathway targeting (figure 1).105–109 Indeed, the opposing roles for the different Akt isoforms in cell motility have identified an invasion and metastasis promoting role of Akt2 but an inhibitory role for Akt1.110–114 The dual role of Akt1 in either promoting tumour growth or metastasis was recently shown to be regulated by the inositol polyphosphate 5-phosphatase (PIPP), where PIPP ablation resulted in reduced metastasis but increased tumour growth.108 Similarly, mTORC1 and mTORC2 have been shown to regulate migration and invasion through Rac1 and RhoA.58 80 115 Furthermore, mTOR inhibition dramatically reduced metastasis in prostate cancer, highlighting the broader potential of PI3K pathway therapeutics as antimetastatic agents.116 Intriguingly, the mTOR inhibitor, everolimus, resulted in a partial response in a patient with pancreatic cancer that was induced by Peutz-Jeghers syndrome (PJS).117 PJS is caused by a tumour-suppressor gene mutation in the serine threonine kinase 11 gene (STK11, also known as LKB1), which results in ~11%–36% of patients with PJS developing pancreatic cancer.117 This loss of STK11 leads to a loss of suppression of mTOR signalling and raises the tantalising possibility that mTOR inhibition could have monotherapy efficacy in PDAC in selected cases with a similar genetic background.
Emerging opportunities for combination therapies
Opportunities for patients with RTK amplification or mutation
The RTK family comprises several subfamilies that are not limited to ErbB, fibroblast growth factor receptors (FGFRs), insulin and insulin-like growth factor receptors, platelet-derived growth factor receptor (PDGFR), vascular endothelial growth factor (VEGF) receptor (VEGFR) and Axl and the Ephrin receptors. Inhibition of these receptors using RTK inhibitors (RTKi) generally takes one of three forms: antibody or recombinant protein inhibition of the extracellular ligand binding domain, inhibition of the ligand itself, or targeting of the cytoplasmic tyrosine kinase domain.
In recent work, 19 PDAC cell lines from the American Type Culture Collection and 17 patient-derived cell lines (PDCLs) from the Australian Pancreatic Cancer Genome Initiative collection, sequenced as part of the International Cancer Genome Consortium (ICGC), were used to assess global phosphotyrosine (pTyr) profiles in PDAC by mass spectrometry.43 This approach allowed the authors to define two sets of classifier mutations (8 and 33 pTyr sites) that predicted three PDAC subtypes over the two cell line panels (box 1). Interestingly, when RTK activity was enriched, the cell lines showed an enhanced sensitivity to the EGFRi erlotinib, suggesting that this subtyping approach may provide a method to stratify patients for RTK-targeted therapies. While the somatic mutation profiles did not correlate with the pTyr-based subtyping, similar GPs were identified between both the genomic and mass spectrometry studies.42 43 However, at the mass spectrometry level, it was possible to identify the activation status of kinase networks and receptors. This lends weight to the overlapping use of both genomic and mass spectrometry approaches to assess aberrant pathway expression, mutation status and, importantly, activation state and provides clear motivation for the incorporation of both techniques into the drug discovery pipeline (figure 2).
The ErbB family
RTKs are transmembrane receptors that communicate signals from ligands outside of the cell by activating their cytoplasmic tyrosine kinase domains, which facilitate downstream signalling within the cell, typically through activation of the MAPK and PI3K pathways. The ErbB family contains four RTKs structurally related to the epidermal growth factor receptor (EGFR; human epidermal growth factor receptor (HER) 1 and ErbB-1). EGFR expression is observed in normal pancreatic ducts but has been shown to increase from the early stages of PanIN development through to PDAC.118–120 Targeting of the EGFR receptor with the small molecule inhibitor erlotinib in combination with gemcitabine resulted in a statistically significant, but clinically modest, improvement in overall survival compared with gemcitabine monotherapy in patients with metastatic disease and has also been evaluated in the adjuvant setting.121 122 These studies subsequently motivated the assessment of predictive markers that would stratify patients for this treatment.123–126 These studies found conflicting evidence for KRAS mutational status as a predictive or prognostic marker for erlotinib response but suggested that mutations or amplification of EGFR may be sufficient to stratify patients for therapy. Interestingly, expression of ErbB-3 (HER3) has been associated with sensitivity to erlotinib treatment in pancreatic cancer cell lines and therefore may prove an effective biomarker for adjuvant erlotinib for patients with PDAC.127–129 ErbB-3 requires heterodimerisation for downstream signalling through the PI3K pathway and expression in PDAC is a poor prognostic factor for survival.127–129 Another emerging personalised approach to PDAC therapy comes from the success of targeting ErbB-2 (neu and HER2) amplified tumours with a humanised monoclonal antibody.130 ErbB-2 amplification in PDAC has a relatively low prevalence of 2%;50 131 132 however, clinical trials with trastuzumab (Herceptin) in combination with chemotherapy have shown beneficial responses in metastatic PDAC patients with ErbB-2 amplification,133 134 and studies are still ongoing in metastatic or recurrent PDAC.56 ErbB-4 (HER4) is the last member of the ErbB family but is only weakly expressed in PDAC.135 136 However, given the established importance of the other ErbB family members in PDAC progression, they may also prove effective biomarkers for inhibition of the PI3K pathway, which is less sensitive to changes in receptor dimerisation.
FGFR, PDGFR and VEGFR stromal targeted therapies and biomarkers
The FGFR, PDGFR and VEGFR families share sufficient structural homology that targeting of these receptors often has overlapping responses. In PDAC, overactivation of FGFR signalling has been associated with 2% of patients, and targeting of this receptor in PDAC using dovitinib has recently completed a phase I clinical trial in combination with chemotherapy, after a promising preclinical study, where dovitinib was found to exert its effect through decreased Akt activity (NCT01497392).50 137 Furthermore, FGFR and PDGFR upregulation in a proof-of-principle study using Kras-deficient PDAC was recently linked with increased sensitivity to PI3K pathway targeting highlighting the essential supportive role this pathway plays in PDAC progression and the potential of RTKs as biomarkers for patient stratification.138 Interestingly, inhibition of FGFR alone or in combination with PDGFR inhibition was not sufficient to decrease cancer cell proliferation to the same degree as PI3K pathway inhibitors, indicating that multiple RTK pathways feed into PI3K activation in PDAC and that PI3K inhibition may provide an opportunity for targeting of multiple de-regulated RTK pathways simultaneously (figure 3).138
Due to the highly desmoplastic reaction characteristic of PDAC, it is important to consider the stromal responses to therapies and even look for new targets within this compartment. Moreover, the effect of FGFR targeting in stromal pancreatic stellate cells has also demonstrated a beneficial outcome by reducing cancer cell invasion and hence better containing the tumour.139 This suggests that PI3K pathway inhibition may also have an antistromal effect that reduces the protumourigenic role of the activated cancer-associated fibroblasts and stellate cells, but as yet, this effect has not been assessed. Interestingly, overexpression of FGFR has also been used in a less conventional approach, where targeting this cell-surface receptor with an antibody-conjugated adenovirus specifically delivered a viral gene.140 This viral gene then predisposed these cells to antiviral therapy by ganciclovir. While this work has not progressed beyond preclinical models, other alternative therapies, such as antibody-conjugated nanoparticles, toxins, viruses or CAR-Ts,141–144 highlight the variety of emerging therapies that could potentially combat this primarily treatment-refractory disease.
PDGFR is less commonly mutated in PDAC, but upregulation of PDGFR signalling has been implicated as a mechanism for metastatic progression in p53-mutated tumours.42 145 146 Interestingly, one patient with PDAC who responded well to AZD2014 therapy in a phase I trial was found to have a PDGFR1A mutation, and this may present a novel biomarker for therapies aimed at PI3K pathway inhibition.98 An important function of PDGFR signalling is an overlapping role with VEGFR signalling for angiogenesis, which has been extensively assessed as a target in PDAC.147 After promising clinical trials led to approval of the mTORC1 inhibitor rapamycin and the broad-spectrum RTKi Sunitinib in pNETs, which are typically highly vascularised, several clinical trials began looking at the effectiveness of these inhibitors in PDAC (table 2).93 148 However, the antiangiogenic effects in PDAC provided minimal clinical benefit and future clinical trials are looking at the application of RTKi as part of combination therapies (table 2).149–152 One interesting target that has emerged from VEGFR targeting strategies is the discovery that placental growth factor, a VEGF homologue, is specifically upregulated in tumour vasculature and provides a target for disease-specific angiogenesis, without affecting normal healthy vessels.153 However, the effectiveness of this strategy remains controversial and has yet to progress to the clinic.154
Ephrin receptors as predictive biomarkers or novel targets
The largest known RTK family is that of the Ephrin receptors, of which both the EphrinA and EphrinB subfamilies are associated with poorer survival in patients with PDAC and are predictive of tumour proliferative and growth capacity.155 156 Indeed, increased activity of EphrinA2 has been associated with Kras-driven PDAC progression and knockdown in a mouse model of PDAC decreased metastasis.43 157 Furthermore, axon guidance GPs in which EphrinA5 and EphrinA7 play a role have been implicated in PDAC development, providing further motivation for application of the EphrinA/EphrinB receptors as predictive biomarkers for aggressive disease.38 Their continued association with PDAC has led to several approaches to therapeutically target these receptors.158 For example, a recent toxin-conjugated monoclonal antibody against the EphrinA2 receptor MEDI-547 completed phase I clinical trials in treatment-refractory solid tumours.159 Similarly, the broad-spectrum small molecule tyrosine kinase inhibitor dasatinib has an established inhibitory effect on the intracellular kinase domains of Ephrin receptors and provides a parallel approach for targeting of other RTKs.160 After promising preclinical studies, dasatinib has progressed to clinical trials for metastatic PDAC in combination with FOLFOX (NCT01652976) or gemcitabine/erlotinib (NCT01660971) chemotherapy.161–163 However, dasatinib in combination with gemcitabine did not improve overall compared with gemcitabine and placebo in locally advanced, non-metastatic PDAC.164 Another common approach to target upregulated Ephrin signalling is to inhibit the downstream pathways, such as the MAPK or PI3K pathways.158 Importantly, as PDAC therapy necessarily turns towards predictive biomarkers to guide personalised therapies, upregulation of Ephrin family members may predict response to RTK, MAPK or PI3K pathway inhibition in PDAC.
Canonical and non-canonical inhibition of aberrant transforming growth factor β (TGFβ) signalling
The membrane-bound TGFβ receptor is mutated at a relatively low frequency in PDAC.50 70 However, disruptions in other pathway components occur in ~47% of patients, including mutations in SMAD4, SMAD3, TGFBR1, TFGBR2, ACVR1B and ACVR2A.42 There is a complex relationship between TGFβ signalling and either tumour suppression or metastatic spread.165 Indeed, loss of SMAD4 is indicative of a poorer prognosis, while TGFβ pathway activation is associated with an epithelial-to-mesenchymal transition, one of the driving factors for metastatic dissemination.165 166 This has made TGFβ signalling the focus of recent clinical trials combining TGFβ receptor inhibition with gemcitabine (NCT02154646 and NCT01373164) or immunotherapy (NCT02734160). However, these trials are not biomarker driven and hence are not stratified for SMAD4 mutational status, which is associated with failure of adjuvant chemotherapies in PDAC.167 168 The role of SMAD4 in TGFβ signalling is primarily tumour suppressive, and this function may limit application of TGFβ receptor inhibitors, where they would best be applied to patients with SMAD4 deletion.169 A key non-canonical mediator of TGFβ signalling is the PI3K pathway, which was shown to be inhibited by TGFβ receptor inhibitors and activated by endogenous TGFβ.165 170 171 Hence, an alternative route, independent of the tumour-suppressive functions of TGFβ signalling, may be through inhibition of these non-canonical signalling pathways.
Targeting DNA damage repair defective tumours
Aberration in DNA damage repair pathways, such as mutations in BRCA1, BRCA2, PALB2 or ATM, are commonly associated with increased risk of familial pancreatic cancer,172 173 but also occur in the later stages of PanIN development and PDAC.18 20 42 56 Loss of these DNA repair proteins leads to genomic instability and predisposes patients to breast, ovarian, prostate and pancreatic cancers.174–176 Patients with mutations in this pathway in other cancers have shown beneficial responses to PARPi, and recent clinical trials in PDAC have been performed to assess the beneficial role of second-line olaparib monotherapy in BRCA1/2-deficient patients, following failure on gemcitabine.177 PARPi work on the basis of synthetic lethality whereby tumours with defects in double-stranded DNA repair pathways become dependent on PARP to repair the resultant collapsed replication forks and maintain chromosomal stability and cell cycle progression.178–180 Another option for patients with mutations in DNA repair pathways is by causing further DNA damage in these defective cells by either platinum-based therapies or mitomycin C.56 181 Furthermore, the PI3K pathway has a well-established role in DNA damage repair, and promising combination therapies in endometrial and breast cancers have motivated clinical trials in PDAC to assess the effect of PARPi in combination with PI3K pathway inhibitors (table 2, figure 3).182–184 The clear responses seen in patients presenting with these DNA repair defects provides a promising personalised approach to therapy when standard of care is found to be ineffective.
Sensitisation of cell cycle defective tumours to cyclin-dependent kinase (CDK) inhibitors as combination therapy
Mutations in CDKN2A, CCND1 and/or CDK4/6 commonly occur in PDAC, and recent work has demonstrated that the reliance of some tumours on this pathway may sensitise them to CDK inhibitors (CDKi).50 In recent work, the sensitivity of a panel of PDCLs was assessed for their response to a CDKi, which identified PDCLs with high expression of retinoblastoma protein and low expression of p16INK4A were significantly correlated with improved response to CDKi, in combination with gemcitabine.185 This is in line with work in breast and ovarian cancers, and melanoma, where this same expression pattern is common.186–188 Furthermore, it has been shown that in PDAC and other cancers, combinations of CDKi with PI3K pathway inhibition in subsets of patients can have an even greater response, thus stratification in this setting may warrant further investigation (table 2).189 190
Histone deacetylases (HDACs) and mutant p53 inhibitors
Loss or mutation of the tumour suppressor p53 occurs in ~75% of patients with PDAC, where gain-of-function mutations occur at a higher prevalence and are thought to provide a growth advantage, as well as driving metastatic progression.50 191 192 The primary role of p53 is to bind DNA as a transcriptional activator or repressor, mediating transcriptional networks responsible for cell death and replicative senescence in response to genotoxic or oncogenic stress.193 194 HDACs work by regulating gene expression at an epigenetic level and have been associated with upregulation of mutant p53 in several cancers, including PDAC.195 196 Furthermore, several HDACs are overexpressed in PDAC, prompting assessment of the clinical benefit of their inhibition.197 198 Recently, a phase I clinical trial of vorinostat with chemoradiation in PDAC showed promising overall survival benefits.199 In parallel, emerging studies in other AdvSTs demonstrated promising synergistic benefits when combining vorinostat with the broad-spectrum RTKi sorafenib200 201 and subsequently led to a new phase I trial of vorinostat and sorafenib with chemotherapy in PDAC (NCT02349867).
One of the key mediators of p53 protein stability is mouse double minute 2 (MDM2), which is responsible for ubiquitination and subsequent degradation of p53 by the proteasome.202 Mutation of CDKN2A occurs in 35% of PDAC tumours leading to loss of expression of the tumour suppressors p16INK4a and p14ARF (p19ARF in murine tumours).50 ARF inhibits MDM2, and hence loss of this tumour suppressor leads to increased levels of MDM2 and a decrease in p53 pathway activity.203 Another key mediator of MDM2 activation is Akt, which activates MDM2 in parallel with other survival pathways (figure 3).61 Furthermore, the tumour suppressor PTEN has been shown to bypass MDM2 and stabilise p53 protein levels, leading to downstream activity.204 205 In the complex mutational landscape of PDAC, it is unclear how PI3K pathway inhibitors may affect the already overexpressed levels of mutant p53.206 However, ~26% of PDAC tumours retain wild-type p53, and recent work demonstrated that MDM2 inhibitors were able to reactivate wild-type p53 pathway signalling in pancreatic cancer.207 This may suggest that PI3K pathway inhibition in PDAC could reduce MDM2 levels and facilitate activation of the tumour-suppressive functions of p53 and lead to apoptosis.208 209 Caution may need to be taken however, for patients with p53 mutations where upregulation of this mutant protein may enhance tumourigenesis.210 The complex relationship between these pathways includes GSK-3β, which has been implicated in MDM2 activation and p53 degradation.211 212 GSK-3β is a canonical Akt substrate and is inactivated by this phosphorylation interaction (figure 3).61 This complex regulation of MDM2 by different members of the PI3K pathway may explain the complex responses of p53 wild-type or deficient tumours when applying PI3K pathway inhibitors as radiosensitising agents.213–216
Development of small molecule inhibitors for oncogenic KRAS and MAPK signalling
Mutations in KRAS occur in ~95% of PDAC cases, and this has prompted several efforts to target both mutant KRAS and the resultant aberrant downstream signalling.26 38 The predominant KRAS mutations in PDAC are KRASG12D and KRASG12V, where KRAS G12D accounts for 83% of KRAS mutations in PDAC and has been shown to classify into more aggressive molecular subtypes.41 217 This aggressive classification may be due to the downstream signalling cascades that have been linked to specific KRAS mutants, where KRASG12D predominantly activates the MAPK and PI3K pathways, whereas KRASG12V predominantly activates Ral signalling.218 Several attempts have been made to inhibit oncogenic Ras isoforms by either competitive inhibition of GTP binding or by preventing membrane translocation but have so far failed to successfully inhibit Ras at a low enough dose for clinical efficacy.217 Similarly, the farnesyl transferase inhibitor, tipifarnib, did not prolong overall survival compared with gemcitabine alone in advanced PDAC.219 Interestingly, RNA interference approaches have been efficaciously applied to PDAC tumours that were metabolically reprogrammed by mutant Ras, where inhibition of the mutant isoform was sufficient to delay tumour growth.220–222 With this in mind and by successfully delivering small inhibitory RNAs (siRNAs), one group was able to demonstrate the in vivo application of a miniature biodegradable polymeric matrix for delivery of a KRASG12D-targeted siRNA.221 Knockdown of oncogenic KRASG12D at the transcript level effectively inhibited downstream pathways and reduced in vivo tumour burden. Another recent approach to deliver siRNAs against oncogenic KRASG12D to PDAC tumours is using fibroblast-derived exosomes, termed iExosomes, which maintain CD47 expression and hence show increased bioavailability and tumour uptake.220
As an alternative approach to inhibition of oncogenic KRAS, innumerable inhibitors have been developed to target the key signalling cascades immediately downstream, namely the MAPK and PI3K pathways. Several clinical trials have been performed using MEKi in combination with gemcitabine,223 224 but these have so far failed to demonstrate significant improvements in survival, compared with gemcitabine alone. Inhibition of the MAPK pathway is regularly associated with an increase in PI3K pathway activity.225 226 Hence, new treatment strategies have emerged that aim at inhibiting both of these key effector pathways (table 2). While these follow from promising preclinical studies, where the combined efficacy of dual MAPK and PI3K pathway inhibition provides significant tumour growth inhibition, the combined toxicity of this approach can present a strong limiting factor.226 227 Notably, the sequential effect of targeting these pathways may increase tumour susceptibility to inhibition while potentially minimising toxicity.228
Microenvironmental influences on drug response
PDAC is characterised by a highly desmoplastic reaction, commonly associated with high levels of stromal infiltration, ECM deposition and tumour hypoxia.47 229 Targeting of the ECM or associated stroma has shown some efficacy in PDAC, by improving drug delivery and sensitising tumours to chemotherapy,230–232 although the viability of targeting the stroma in PDAC remains controversial. Complete stromal ablation in PDAC was shown to enhance cancer aggressiveness,233 234 which calls for more subtle and targeted approaches to normalising instead of completely ablating the tumour-associated stroma.47 235 This effect is partly thought to occur due to a normalisation of the tumour vascular network and manipulation of the ECM/stroma, improving drug efficacy in the tumour.231 235 236 Another common feature resultant from enhanced desmoplasia is the development of a hypoxic environment (figure 4). Hypoxia is strongly associated with increased radioresistance, chemoresistance and metastasis,237–239 and PDAC is among those cancers with a propensity for high levels of tumour hypoxia, which is predictive of poorer patient prognosis.229 240
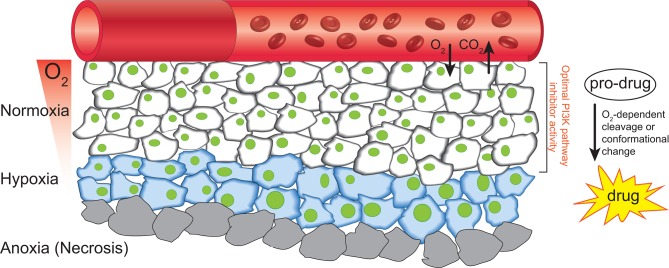
Figure 4.
Schematic of the formation of a hypoxic environment and the potential targeting of this microenvironment with HAPs. RBCs transport oxygen through the blood vasculature, and hypoxia forms when this diffusion-limited process delivers insufficient oxygen to cells distant to the vasculature (blue cells). The extreme case of anoxia (grey cells) regularly results in necrotic cell death. HAPs take advantage of the hypoxic environment of tumours to deliver cytotoxic compounds to these tumour regions, where the prodrug is either enzymatically cleaved by the cells metabolic machinery or undergoes a conformational change in response to the low oxygen partial pressure. HAPs, hypoxia-activated prodrugs; RBCs, red blood cells.
Reduced oxygen consumption and increased glycolysis were recently identified by mitochondrial genome sequencing in PDAC PDCLs.241 This is a key aspect of the Warburg effect, which predicts tumours to rely more heavily on glycolysis for their metabolism.242 While this presents an advantage for tumours that experience reduced vascularity and oxygen levels, it also presents an opportunity to potentially starve the tumour in these hypoxic regions.243 One of the first steps for tumours to switch to glycolytic metabolism is an increase in lactate dehydrogenase (LDH) activity, which converts pyruvate into lactate.244 Inhibition of LDH has recently been shown to synergise with gemcitabine in vitro and may provide a novel strategy for PDAC.245 Another important aspect of PDAC metabolism is the metabolic reprogramming resultant from KRAS mutation, which upregulates glucose uptake and biomass synthesis.246 Furthermore, the upregulation of MUC1 during PDAC progression, along with HIF1α in hypoxic tumour regions, has been shown to cooperate by upregulating anabolic metabolism through the pentose phosphate pathway, resulting in gemcitabine resistance.247 The glycolytic switch in hypoxia can lead to a decrease in pH, and hence pH-regulating proteins are also an important downstream target of the cellular hypoxic response.248 Inhibitors of these pH-regulatory components are currently being assessed, after promising preclinical work, for their role in limiting tumour growth.249 250 The PI3K pathway also plays an important role in glucose uptake, amino acid metabolism and response to cellular stress.58 60 61 In hypoxia, Akt activity is upregulated, along with glucose transporters, to facilitate the switch to anaerobic metabolism.105 214 238 243 Moreover, treatment of PDAC with PI3K pathway inhibitors is less effective in hypoxia, highlighting an important microenvironmental consideration for future stratified clinical trials.105 251
With this in mind, we recently demonstrated that a hypoxia-activated prodrug (HAP) could alleviate hypoxia-induced resistance to a PI3K pathway inhibitor in a combination therapy.105 HAPs are typically metabolised by enzymatic reduction in hypoxia from a primarily inactive form to an active form while having limited effects on normoxic or healthy tissues, making them ideal for development of combination therapies (figure 4; reviewed in ref 238). This approach was also found to be effective in renal cell carcinoma, where combination with rapalogs and the HAP TH-302 significantly improved survival in preclinical models.252 The efficacy of these HAPs began to be realised with tirapazamine, which showed a high differential toxicity in hypoxia, compared with normoxia, which made it an ideal candidate for combination with radiotherapy.253 254 However, despite promising phase II clinical trials in squamous cell carcinoma, a phase III trial failed to show improved efficacy over chemoradiotherapy alone.255 Notably, this trial was not biomarker driven and hence did not stratify patients based on hypoxic tumour burden, which may have disguised any potential efficacy in a subset of patients. This is a common issue in clinical trials and is also implicated in the phase III failure of TH-302 after promising phase II results (NCT01746979).256 The preclinical promise and potential applications of HAPs in combination therapies means that despite the phase III setbacks, improved tirapazamine analogues, such as SN3000 and SN29751, as well as other recently developed HAPs, including PR-104 and AQ4N, are all under preclinical/clinical investigation.257–259
Recent work by our group demonstrated that a hypoxic gene signature was associated with a poorer prognosis for patients with PDAC.229 While this is a promising approach, application in the clinic may be limited by the collection of patient biopsies, which may not adequately represent the extent of hypoxia within the whole tumour. The necessary stratification of patients for future clinical trials calls for a method to assess the whole tumour non-invasively (figure 2). To this end, positron emission tomography approaches have been developed based on radiolabelled 2-nitroimidazoles or antibodies, which can be coupled with 18F–fluorodeoxyglucose imaging to first identify malignant lesions.238 Similarly, non-invasive imaging of oxygen partial pressure using electron paramagnetic resonance imaging or assessment of pyruvate metabolism by MRI have also been used to stratify PDAC tumours for treatment with TH-302 and radiation.260
Moving forward, the design and synthesis of HAPs with defined molecular targets are emerging for specific applications. For example, hypoxia-activated chk1 inhibitors were recently developed as proof-of-principle molecules for targeting the hypoxic compartment of tumours, where chk1 is an important component of the DNA damage response and cell cycle progression.261 From these studies, it is clear that the emerging application of microenvironmental-targeted agents in combination therapies can improve patient outcomes, and as newer generation inhibitors are developed, we are likely to see a wider application of these agents entering the clinic.
Conclusions
Given the lagging improvements in therapy, there is a dire need to find new biomarkers and targets to move pancreatic cancer towards personalised medicine approaches (figure 2). To guide clinical success, emerging combinations would benefit from a preclinical platform of evidence in at least one in vivo model, as well as optimisation of solubility for reduced toxicity and, importantly, identification of at least one suitable biomarker for patient stratification at the level of clinical trials (figure 2). The emerging efficacy of PI3K pathway inhibitors for PDAC and the convergence of several aberrantly expressed signalling cascades highlights a clear progression towards their application for this disease. For example, patients with aberrant DNA damage repair pathways have responded well to PI3K pathway inhibition as part of combination therapies, and trials are already underway in PDAC. Furthermore, given the complex dimerisation of the ErbB family of RTKs and the association of Ephrin receptors with more aggressive PDAC subtypes, RTKs may provide biomarkers for patients that would respond efficaciously to PI3K pathway inhibition. Moving forward, one of the key goals of the ICGC2 is to link bioinformatics approaches, such as molecular subtyping of patients, to clinical data, and we expect this to drive an increase in biomarker-driven clinical trials (proposed in box 2). This is a necessary step to decrease the attrition of lead compounds in the pharmaceutical industry and to ensure that next-generation inhibitors progress to patients that are appropriately subtyped for maximum benefit.
Box 2. What may improve clinical trials?
Patient subtyping from tumour biopsies by genomic and/or mass spectrometry approaches.
Biomarker identification prior to progression to phase II/III studies to ensure appropriate patient stratification for maximal benefit (circulating cell-free DNA/genomic approaches/IHC).
Incorporation of non-invasive imaging for hypoxic tumour burden, such as electron paramagnetic resonance imaging, MRI or positron emission tomography with 18F-fluorodeoxyglucose.
Testing of promising lead compounds against stratified patient-derived xenograft/Avatar cohorts prior to phase I clinical trials.
Development of new prodrugs to use in combination therapies with reduced off-target effects.
Raising the bar when defining preclinical ‘success’.
Acknowledgments
The authors would like to thank Dr Marina Pajic, Dr David R Croucher and Kendelle J Murphy for critical reading of the manuscript.
Footnotes
Contributors: JRWC and PT developed the main concept of the manuscript. All authors wrote the manuscript. JPM and PT wrote grant applications that funded the work.
Funding: This work was supported by an Nation Health and Medical Research (NHMRC) project grant, an NHMRC fellowship, an Nation Breast Cancer Foundation (NBCF) grant, an Australian Research Council (ARC) Future fellowship, a Len Ainsworth Pancreatic Cancer Fellowship, Cancer Council NSW grant, a Tour de Cure grant and Cancer Research UK (CRUK) core funding (A17196 and A21139). This project was made possible by an Avner Pancreatic Cancer Foundation Grant.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 2009;6:699–708. 10.1038/nrgastro.2009.177 [DOI] [PubMed] [Google Scholar]
- 2. Rahib L, Smith BD, Aizenberg R, et al. . Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. 10.1158/0008-5472.CAN-14-0155 [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA: a cancer journal for clinicians, 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 4. Greenlee RT, Murray T, Bolden S, et al. . Cancer statistics. CA: A Cancer Journal for Clinicians;2000:50:7–33. [DOI] [PubMed] [Google Scholar]
- 5. Burris HA, Moore MJ, Andersen J, et al. . Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403–13. 10.1200/JCO.1997.15.6.2403 [DOI] [PubMed] [Google Scholar]
- 6. Winter JM, Brennan MF, Tang LH, et al. . Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol 2012;19:169–75. 10.1245/s10434-011-1900-3 [DOI] [PubMed] [Google Scholar]
- 7. Neoptolemos JP, Stocken DD, Friess H, et al. . A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med Overseas Ed 2004;350:1200–10. 10.1056/NEJMoa032295 [DOI] [PubMed] [Google Scholar]
- 8. Oettle H, Neuhaus P, Hochhaus A, et al. . Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473–81. 10.1001/jama.2013.279201 [DOI] [PubMed] [Google Scholar]
- 9. Chang DK, Johns AL, Merrett ND, et al. . Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol 2009;27:2855–62. 10.1200/JCO.2008.20.5104 [DOI] [PubMed] [Google Scholar]
- 10. Donahue TR, Reber HA. Surgical management of pancreatic cancer–pancreaticoduodenectomy. Semin Oncol 2015;42:98–109. 10.1053/j.seminoncol.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 11. Parikh PY, Lillemoe KD. Surgical management of pancreatic cancer–distal pancreatectomy. Semin Oncol 2015;42:110–22. 10.1053/j.seminoncol.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 12. Ahn DH, Krishna K, Blazer M, et al. . A modified regimen of biweekly gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer is both tolerable and effective: a retrospective analysis. Ther Adv Med Oncol 2017;9:75–82. 10.1177/1758834016676011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hidalgo M. Pancreatic cancer. N Engl J Med Overseas Ed 2010;362:1605–17. 10.1056/NEJMra0901557 [DOI] [PubMed] [Google Scholar]
- 14. Von Hoff DD, Ervin T, Arena FP, et al. . Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–703. 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol 2015;12:319–34. 10.1038/nrclinonc.2015.53 [DOI] [PubMed] [Google Scholar]
- 16. Conroy T, Desseigne F, Ychou M, et al. . FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med Overseas Ed 2011;364:1817–25. 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 17. Hruban RH, Goggins M, Parsons J, et al. . Progression model for pancreatic cancer. Clin Cancer Res 2000;6:2969–72. [PubMed] [Google Scholar]
- 18. Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer 2002;2:897–909. 10.1038/nrc949 [DOI] [PubMed] [Google Scholar]
- 19. Klein WM, Hruban RH, Klein-Szanto AJ, et al. . Direct correlation between proliferative activity and dysplasia in pancreatic intraepithelial neoplasia (PanIN): additional evidence for a recently proposed model of progression. Mod Pathol 2002;15:441–7. 10.1038/modpathol.3880544 [DOI] [PubMed] [Google Scholar]
- 20. Makohon-Moore A, Iacobuzio-Donahue CA. Pancreatic cancer biology and genetics from an evolutionary perspective. Nat Rev Cancer 2016;16:553–65. 10.1038/nrc.2016.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol 2008;1:306–16. [PMC free article] [PubMed] [Google Scholar]
- 22. Basturk O, Hong SM, Wood LD, et al. . A revised classification system and recommendations from the baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol 2015;39:1730–41. 10.1097/PAS.0000000000000533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pinho AV, Rooman I, Reichert M, et al. . Adult pancreatic acinar cells dedifferentiate to an embryonic progenitor phenotype with concomitant activation of a senescence programme that is present in chronic pancreatitis. Gut 2011;60:958–66. 10.1136/gut.2010.225920 [DOI] [PubMed] [Google Scholar]
- 24. Jensen JN, Cameron E, Garay MVR, et al. . Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology 2005;128:728–41. 10.1053/j.gastro.2004.12.008 [DOI] [PubMed] [Google Scholar]
- 25. Shi C, Hong SM, Lim P, et al. . KRAS2 mutations in human pancreatic acinar-ductal metaplastic lesions are limited to those with PanIN: implications for the human pancreatic cancer cell of origin. Mol Cancer Res 2009;7:230–6. 10.1158/1541-7786.MCR-08-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanda M, Matthaei H, Wu J, et al. . Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012;142:730–3. 10.1053/j.gastro.2011.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Notta F, Chan-Seng-Yue M, Lemire M, et al. . A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature 2016;538:378–82. 10.1038/nature19823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schönhuber N, Seidler B, Schuck K, et al. . A next-generation dual-recombinase system for time- and host-specific targeting of pancreatic cancer. Nat Med 2014;20:1340–7. 10.1038/nm.3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maitra A, Fukushima N, Takaori K, et al. . Precursors to invasive pancreatic cancer. Adv Anat Pathol 2005;12:81–91. 10.1097/01.pap.0000155055.14238.25 [DOI] [PubMed] [Google Scholar]
- 30. Castellano-Megías VM, Andrés CI, López-Alonso G, et al. . Pathological features and diagnosis of intraductal papillary mucinous neoplasm of the pancreas. World J Gastrointest Oncol 2014;6:311–24. 10.4251/wjgo.v6.i9.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matthaei H, Schulick RD, Hruban RH, et al. . Cystic precursors to invasive pancreatic cancer. Nat Rev Gastroenterol Hepatol 2011;8:141–50. 10.1038/nrgastro.2011.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Biankin AV, Kench JG, Biankin SA, et al. . Pancreatic intraepithelial neoplasia in association with intraductal papillary mucinous neoplasms of the pancreas: implications for disease progression and recurrence. Am J Surg Pathol 2004;28:1184–92. [DOI] [PubMed] [Google Scholar]
- 33. Tanaka M, Chari S, Adsay V, et al. . International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006;6:17–32. 10.1159/000090023 [DOI] [PubMed] [Google Scholar]
- 34. Haugk B. Pancreatic intraepithelial neoplasia-can we detect early pancreatic cancer? Histopathology 2010;57:503–14. 10.1111/j.1365-2559.2010.03610.x [DOI] [PubMed] [Google Scholar]
- 35. Yonezawa S, Higashi M, Yamada N, et al. . Significance of mucin expression in pancreatobiliary neoplasms. J Hepatobiliary Pancreat Sci 2010;17:108–24. 10.1007/s00534-009-0174-7 [DOI] [PubMed] [Google Scholar]
- 36. van Heek NT, Meeker AK, Kern SE, et al. . Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol 2002;161:1541–7. 10.1016/S0002-9440(10)64432-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res 1997;57:2140–3. [PubMed] [Google Scholar]
- 38. Biankin AV, Waddell N, Kassahn KS, et al. . Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012;491:399–405. 10.1038/nature11547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heinmöller E, Dietmaier W, Zirngibl H, et al. . Molecular analysis of microdissected tumors and preneoplastic intraductal lesions in pancreatic carcinoma. Am J Pathol 2000;157:83–92. 10.1016/S0002-9440(10)64520-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Collisson EA, Sadanandam A, Olson P, et al. . Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med 2011;17:500–3. 10.1038/nm.2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moffitt RA, Marayati R, Flate EL, et al. . Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 2015;47:1168–78. 10.1038/ng.3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bailey P, Chang DK, Nones K, et al. . Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47–52. 10.1038/nature16965 [DOI] [PubMed] [Google Scholar]
- 43. Humphrey ES, Su SP, Nagrial AM, et al. . Resolution of novel pancreatic ductal adenocarcinoma subtypes by global phosphotyrosine profiling. Mol Cell Proteomics 2016;15:2671–85. 10.1074/mcp.M116.058313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jones S, Zhang X, Parsons DW, et al. . Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008;321:1801–6. 10.1126/science.1164368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Erkan M, Michalski CW, Rieder S, et al. . The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol 2008;6:1155–61. 10.1016/j.cgh.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 46. Daemen A, Peterson D, Sahu N, et al. . Metabolite profiling stratifies pancreatic ductal adenocarcinomas into subtypes with distinct sensitivities to metabolic inhibitors. Proc Natl Acad Sci U S A 2015;112:E4410–E4417. 10.1073/pnas.1501605112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vennin C, Murphy KJ, Morton JP, et al. . Reshaping the Tumor Stroma for Treatment of Pancreatic Cancer. Gastroenterology 2018;154:820–38. 10.1053/j.gastro.2017.11.280 [DOI] [PubMed] [Google Scholar]
- 48. Iacobuzio-Donahue CA, Maitra A, Olsen M, et al. . Exploration of global gene expression patterns in pancreatic adenocarcinoma using cDNA microarrays. Am J Pathol 2003;162:1151–62. 10.1016/S0002-9440(10)63911-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nicolle R, Blum Y, Marisa L, et al. . Pancreatic adenocarcinoma therapeutic targets revealed by tumor-stroma cross-talk analyses in patient-derived xenografts. Cell Rep 2017;21:2458–70. 10.1016/j.celrep.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Waddell N, Pajic M, Patch AM, et al. . Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495–501. 10.1038/nature14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yoshihara K, Shahmoradgoli M, Martínez E, et al. . Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun 2013;4:2612 10.1038/ncomms3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Conway JR, Carragher NO, Timpson P. Developments in preclinical cancer imaging: innovating the discovery of therapeutics. Nat Rev Cancer 2014;14:314–28. 10.1038/nrc3724 [DOI] [PubMed] [Google Scholar]
- 53. Biankin AV, Piantadosi S, Hollingsworth SJ. Patient-centric trials for therapeutic development in precision oncology. Nature 2015;526:361–70. 10.1038/nature15819 [DOI] [PubMed] [Google Scholar]
- 54. Crane CH, Iacobuzio-Donahue CA. Keys to personalized care in pancreatic oncology. J Clin Oncol 2012;30:4049–950. 10.1200/JCO.2012.45.1799 [DOI] [PubMed] [Google Scholar]
- 55. Le Tourneau C, Delord JP, Gonçalves A, et al. . Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol 2015;16:1324–34. 10.1016/S1470-2045(15)00188-6 [DOI] [PubMed] [Google Scholar]
- 56. Chantrill LA, Nagrial AM, Watson C, et al. . Precision medicine for advanced pancreas cancer: The individualized Molecular Pancreatic Cancer Therapy (IMPaCT) Trial. Clin Cancer Res 2015;21:2029–37. 10.1158/1078-0432.CCR-15-0426 [DOI] [PubMed] [Google Scholar]
- 57. Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol 2018;15:273–91. 10.1038/nrclinonc.2018.28 [DOI] [PubMed] [Google Scholar]
- 58. Liu P, Cheng H, Roberts TM, et al. . Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 2009;8:627–44. 10.1038/nrd2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer 2011;11:289–301. 10.1038/nrc3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol 2010;7:209–19. 10.1038/nrclinonc.2010.21 [DOI] [PubMed] [Google Scholar]
- 61. Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 2007;129:1261–74. 10.1016/j.cell.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Duluc C, Moatassim-Billah S, Chalabi-Dchar M, et al. . Pharmacological targeting of the protein synthesis mTOR/4E-BP1 pathway in cancer-associated fibroblasts abrogates pancreatic tumour chemoresistance. EMBO Mol Med 2015;7:735–53. 10.15252/emmm.201404346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Graupera M, Guillermet-Guibert J, Foukas LC, et al. . Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature 2008;453:662–6. 10.1038/nature06892 [DOI] [PubMed] [Google Scholar]
- 64. Kaneda MM, Cappello P, Nguyen AV, et al. . Macrophage PI3Kγ Drives Pancreatic Ductal Adenocarcinoma Progression. Cancer Discov 2016;6:870–85. 10.1158/2159-8290.CD-15-1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol 2012;12:325–38. 10.1038/nri3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ruggeri BA, Huang L, Wood M, et al. . Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol Carcinog 1998;21:81–6. [DOI] [PubMed] [Google Scholar]
- 67. Cheng JQ, Ruggeri B, Klein WM, et al. . Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A 1996;93:3636–41. 10.1073/pnas.93.8.3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schlieman MG, Fahy BN, Ramsamooj R, et al. . Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer 2003;89:2110–5. 10.1038/sj.bjc.6601396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Janku F, Hong DS, Fu S, et al. . Assessing PIK3CA and PTEN in early-phase trials with PI3K/AKT/mTOR inhibitors. Cell Rep 2014;6:377–87. 10.1016/j.celrep.2013.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Witkiewicz AK, McMillan EA, Balaji U, et al. . Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun 2015;6:6744 10.1038/ncomms7744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ying H, Elpek KG, Vinjamoori A, et al. . PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-κB-cytokine network. Cancer Discov 2011;1:158–69. 10.1158/2159-8290.CD-11-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Foo WC, Rashid A, Wang H, et al. . Loss of phosphatase and tensin homolog expression is associated with recurrence and poor prognosis in patients with pancreatic ductal adenocarcinoma. Hum Pathol 2013;44:1024–30. 10.1016/j.humpath.2012.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kennedy AL, Morton JP, Manoharan I, et al. . Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol Cell 2011;42:36–49. 10.1016/j.molcel.2011.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Eser S, Reiff N, Messer M, et al. . Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell 2013;23:406–20. 10.1016/j.ccr.2013.01.023 [DOI] [PubMed] [Google Scholar]
- 75. Wu CY, Carpenter ES, Takeuchi KK, et al. . PI3K regulation of RAC1 is required for KRAS-induced pancreatic tumorigenesis in mice. Gastroenterology 2014;147:1405–16. 10.1053/j.gastro.2014.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Baer R, Cintas C, Dufresne M, et al. . Pancreatic cell plasticity and cancer initiation induced by oncogenic Kras is completely dependent on wild-type PI 3-kinase p110α. Genes Dev 2014;28:2621–35. 10.1101/gad.249409.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kodahl AR, Ehmsen S, Pallisgaard N, et al. . Correlation between circulating cell-free PIK3CA tumor DNA levels and treatment response in patients with PIK3CA-mutated metastatic breast cancer. Mol Oncol 2018;12:925–35. 10.1002/1878-0261.12305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Payne SN, Maher ME, Tran NH, et al. . PIK3CA mutations can initiate pancreatic tumorigenesis and are targetable with PI3K inhibitors. Oncogenesis 2015;4:e169 10.1038/oncsis.2015.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Frenel JS, Carreira S, Goodall J, et al. . Serial next-generation sequencing of circulating cell-free dna evaluating tumor clone response to molecularly targeted drug administration. Clin Cancer Res 2015;21:4586–96. 10.1158/1078-0432.CCR-15-0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer 2015;15:7–24. 10.1038/nrc3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yap TA, Bjerke L, Clarke PA, et al. . Drugging PI3K in cancer: refining targets and therapeutic strategies. Curr Opin Pharmacol 2015;23:98–107. 10.1016/j.coph.2015.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wong MH, Xue A, Julovi SM, et al. . Cotargeting of epidermal growth factor receptor and PI3K overcomes PI3K-Akt oncogenic dependence in pancreatic ductal adenocarcinoma. Clin Cancer Res 2014;20:4047–58. 10.1158/1078-0432.CCR-13-3377 [DOI] [PubMed] [Google Scholar]
- 83. Gasser JA, Inuzuka H, Lau AW, et al. . SGK3 mediates INPP4B-dependent PI3K signaling in breast cancer. Mol Cell 2014;56:595–607. 10.1016/j.molcel.2014.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Driscoll DR, Karim SA, Sano M, et al. . mTORC2 Signaling drives the development and progression of pancreatic cancer. Cancer Res 2016;76:6911–23. 10.1158/0008-5472.CAN-16-0810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Khalil BD, Hsueh C, Cao Y, et al. . GPCR Signaling Mediates Tumor Metastasis via PI3Kβ. Cancer Res 2016;76:2944–53. 10.1158/0008-5472.CAN-15-1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang Z, Liu J, Wang Y, et al. . Phosphatidylinositol 3-kinase β and δ isoforms play key roles in metastasis of prostate cancer DU145 cells. The FASEB Journal 2018:fj.201800183R 10.1096/fj.201800183R [DOI] [PubMed] [Google Scholar]
- 87. Ali K, Soond DR, Pineiro R, et al. . Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature 2014;510:407–11. 10.1038/nature13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mann KM, Ward JM, Yew CC, et al. . Sleeping Beauty mutagenesis reveals cooperating mutations and pathways in pancreatic adenocarcinoma. Proc Natl Acad Sci U S A 2012;109:5934–41. 10.1073/pnas.1202490109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pérez-Mancera PA, Rust AG, van der Weyden L, et al. . The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature 2012;486:266–70. 10.1038/nature11114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kothari V, Wei I, Shankar S, et al. . Outlier kinase expression by RNA sequencing as targets for precision therapy. Cancer Discov 2013;3:280–93. 10.1158/2159-8290.CD-12-0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Seto B. Rapamycin and mTOR: a serendipitous discovery and implications for breast cancer. Clin Transl Med 2012;1:29 10.1186/2001-1326-1-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Morran DC, Wu J, Jamieson NB, et al. . Targeting mTOR dependency in pancreatic cancer. Gut 2014;63:1481–9. 10.1136/gutjnl-2013-306202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yao JC, Shah MH, Ito T, et al. . Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med Overseas Ed 2011;364:514–23. 10.1056/NEJMoa1009290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wolpin BM, Hezel AF, Abrams T, et al. . Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol 2009;27:193–8. 10.1200/JCO.2008.18.9514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Javle MM, Shroff RT, Xiong H, et al. . Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies. BMC Cancer 2010;10:368 10.1186/1471-2407-10-368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sun SY, Rosenberg LM, Wang X, et al. . Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 2005;65:7052–8. 10.1158/0008-5472.CAN-05-0917 [DOI] [PubMed] [Google Scholar]
- 97. O’Reilly KE, Rojo F, She QB, et al. . mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 2006;66:1500–8. 10.1158/0008-5472.CAN-05-2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Basu B, Dean E, Puglisi M, et al. . First-in-human pharmacokinetic and pharmacodynamic study of the dual m-TORC 1/2 inhibitor AZD2014. Clin Cancer Res 2015;21:3412–9. 10.1158/1078-0432.CCR-14-2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kordes S, Richel DJ, Klümpen HJ, et al. . A phase I/II, non-randomized, feasibility/safety and efficacy study of the combination of everolimus, cetuximab and capecitabine in patients with advanced pancreatic cancer. Invest New Drugs 2013;31:85–91. 10.1007/s10637-012-9802-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cartwright TH, Cohn A, Varkey JA, et al. . Phase II study of oral capecitabine in patients with advanced or metastatic pancreatic cancer. J Clin Oncol 2002;20:160–4. 10.1200/JCO.2002.20.1.160 [DOI] [PubMed] [Google Scholar]
- 101. Andrs M, Korabecny J, Jun D, et al. . Phosphatidylinositol 3-Kinase (PI3K) and Phosphatidylinositol 3-Kinase-related Kinase (PIKK) inhibitors: importance of the morpholine ring. J Med Chem 2015;58:41–71. 10.1021/jm501026z [DOI] [PubMed] [Google Scholar]
- 102. Liu Y, Wan WZ, Li Y, et al. . Recent development of ATP-competitive small molecule phosphatidylinostitol-3-kinase inhibitors as anticancer agents. Oncotarget 2017;8:7181–200. 10.18632/oncotarget.12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kawada H, Ebiike H, Tsukazaki M, et al. . Optimization of the phenylurea moiety in a phosphoinositide 3-kinase (PI3K) inhibitor to improve water solubility and the PK profile by introducing a solubilizing group and ortho substituents. Bioorg Med Chem 2016;24:2897–906. 10.1016/j.bmc.2016.04.060 [DOI] [PubMed] [Google Scholar]
- 104. Pike KG, Malagu K, Hummersone MG, et al. . Optimization of potent and selective dual mTORC1 and mTORC2 inhibitors: the discovery of AZD8055 and AZD2014. Bioorg Med Chem Lett 2013;23:1212–6. 10.1016/j.bmcl.2013.01.019 [DOI] [PubMed] [Google Scholar]
- 105. Conway JRW, Warren SC, Herrmann D, et al. . Intravital Imaging to Monitor Therapeutic Response in Moving Hypoxic Regions Resistant to PI3K Pathway Targeting in Pancreatic Cancer. Cell Rep 2018;23:3312–26. 10.1016/j.celrep.2018.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yoshikawa Y, Takano O, Kato I, et al. . Ras inhibitors display an anti-metastatic effect by downregulation of lysyl oxidase through inhibition of the Ras-PI3K-Akt-HIF-1α pathway. Cancer Lett 2017;410:82–91. 10.1016/j.canlet.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 107. Zhao L, Li C, Liu F, et al. . A blockade of PD-L1 produced antitumor and antimetastatic effects in an orthotopic mouse pancreatic cancer model via the PI3K/Akt/mTOR signaling pathway. Onco Targets Ther 2017;10:2115–26. 10.2147/OTT.S130481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ooms LM, Binge LC, Davies EM, et al. . The Inositol Polyphosphate 5-Phosphatase PIPP Regulates AKT1-Dependent Breast Cancer Growth and Metastasis. Cancer Cell 2015;28:155–69. 10.1016/j.ccell.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 109. Rumman M, Jung KH, Fang Z, et al. . HS-173, a novel PI3K inhibitor suppresses EMT and metastasis in pancreatic cancer. Oncotarget 2016;7:78029–47. 10.18632/oncotarget.12871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Arboleda MJ, Lyons JF, Kabbinavar FF, et al. . Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res 2003;63:196–206. [PubMed] [Google Scholar]
- 111. Yoeli-Lerner M, Yiu GK, Rabinovitz I, et al. . Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell 2005;20:539–50. 10.1016/j.molcel.2005.10.033 [DOI] [PubMed] [Google Scholar]
- 112. Zhou GL, Tucker DF, Bae SS, et al. . Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. J Biol Chem 2006;281:36443–53. 10.1074/jbc.M600788200 [DOI] [PubMed] [Google Scholar]
- 113. Irie HY, Pearline RV, Grueneberg D, et al. . Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol 2005;171:1023–34. 10.1083/jcb.200505087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hutchinson JN, Jin J, Cardiff RD, et al. . Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res 2004;64:3171–8. 10.1158/0008-5472.CAN-03-3465 [DOI] [PubMed] [Google Scholar]
- 115. Gulhati P, Bowen KA, Liu J, et al. . mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res 2011;71:3246–56. 10.1158/0008-5472.CAN-10-4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hsieh AC, Liu Y, Edlind MP, et al. . The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 2012;485:55–61. 10.1038/nature10912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Klümpen HJ, Queiroz KC, Spek CA, et al. . mTOR inhibitor treatment of pancreatic cancer in a patient With Peutz-Jeghers syndrome. J Clin Oncol 2011;29:e150–e153. 10.1200/JCO.2010.32.7825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yamanaka Y, Friess H, Kobrin MS, et al. . Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res 1993;13:565–9. [PubMed] [Google Scholar]
- 119. Korc M, Chandrasekar B, Yamanaka Y, et al. . Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest 1992;90:1352–60. 10.1172/JCI116001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ueda S, Ogata S, Tsuda H, et al. . The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas 2004;29:e1–8. [DOI] [PubMed] [Google Scholar]
- 121. Moore MJ, Goldstein D, Hamm J, et al. . Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960–6. 10.1200/JCO.2006.07.9525 [DOI] [PubMed] [Google Scholar]
- 122. Sinn M, Bahra M, Liersch T, et al. . Conko-005: Adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after R0 resection of pancreatic cancer: A multicenter randomized phase III trial. J Clin Oncol 2017;35:3330–7. 10.1200/JCO.2017.72.6463 [DOI] [PubMed] [Google Scholar]
- 123. Boeck S, Jung A, Laubender RP, et al. . KRAS mutation status is not predictive for objective response to anti-EGFR treatment with erlotinib in patients with advanced pancreatic cancer. J Gastroenterol 2013;48:544–8. 10.1007/s00535-013-0767-4 [DOI] [PubMed] [Google Scholar]
- 124. Boeck S, Jung A, Laubender RP, et al. . EGFR pathway biomarkers in erlotinib-treated patients with advanced pancreatic cancer: translational results from the randomised, crossover phase 3 trial AIO-PK0104. Br J Cancer 2013;108:469–76. 10.1038/bjc.2012.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. da Cunha Santos G, Dhani N, Tu D, et al. . Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: National Cancer Institute of Canada Clinical Trials Group Study PA.3. Cancer 2010;116:5599–607. 10.1002/cncr.25393 [DOI] [PubMed] [Google Scholar]
- 126. Wang JP, Wu CY, Yeh YC, et al. . Erlotinib is effective in pancreatic cancer with epidermal growth factor receptor mutations: a randomized, open-label, prospective trial. Oncotarget 2015;6:18162–73. 10.18632/oncotarget.4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Frolov A, Schuller K, Tzeng CW, et al. . ErbB3 expression and dimerization with EGFR influence pancreatic cancer cell sensitivity to erlotinib. Cancer Biol Ther 2007;6:548–54. 10.4161/cbt.6.4.3849 [DOI] [PubMed] [Google Scholar]
- 128. Buck E, Eyzaguirre A, Haley JD, et al. . Inactivation of Akt by the epidermal growth factor receptor inhibitor erlotinib is mediated by HER-3 in pancreatic and colorectal tumor cell lines and contributes to erlotinib sensitivity. Mol Cancer Ther 2006;5:2051–9. 10.1158/1535-7163.MCT-06-0007 [DOI] [PubMed] [Google Scholar]
- 129. Friess H, Yamanaka Y, Kobrin MS, et al. . Enhanced erbB-3 expression in human pancreatic cancer correlates with tumor progression. Clin Cancer Res 1995;1:1413–20. [PubMed] [Google Scholar]
- 130. Gingras I, Gebhart G, de Azambuja E, et al. . HER2-positive breast cancer is lost in translation: time for patient-centered research. Nat Rev Clin Oncol 2017;14:669–81. 10.1038/nrclinonc.2017.96 [DOI] [PubMed] [Google Scholar]
- 131. Chou A, Waddell N, Cowley MJ, et al. . Clinical and molecular characterization of HER2 amplified-pancreatic cancer. Genome Med 2013;5:78 10.1186/gm482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Safran H, Steinhoff M, Mangray S, et al. . Overexpression of the HER-2/neu oncogene in pancreatic adenocarcinoma. Am J Clin Oncol 2001;24:496–9. 10.1097/00000421-200110000-00016 [DOI] [PubMed] [Google Scholar]
- 133. Harder J, Ihorst G, Heinemann V, et al. . Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br J Cancer 2012;106:1033–8. 10.1038/bjc.2012.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Safran H, Iannitti D, Ramanathan R, et al. . Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest 2004;22:706–12. 10.1081/CNV-200032974 [DOI] [PubMed] [Google Scholar]
- 135. Mill CP, Gettinger KL, Riese II DJ. Ligand stimulation of ErbB4 and a constitutively-active ErbB4 mutant result in different biological responses in human pancreatic tumor cell lines. Exp Cell Res 2011;317:392–404. 10.1016/j.yexcr.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Normanno N, De Luca A, Bianco C, et al. . Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006;366:2–16. 10.1016/j.gene.2005.10.018 [DOI] [PubMed] [Google Scholar]
- 137. Zhang H, Hylander BL, LeVea C, et al. . Enhanced FGFR signalling predisposes pancreatic cancer to the effect of a potent FGFR inhibitor in preclinical models. Br J Cancer 2014;110:320–9. 10.1038/bjc.2013.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Muzumdar MD, Chen PY, Dorans KJ, et al. . Survival of pancreatic cancer cells lacking KRAS function. Nat Commun 2017;8:1090 10.1038/s41467-017-00942-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Coleman SJ, Chioni AM, Ghallab M, et al. . Nuclear translocation of FGFR1 and FGF2 in pancreatic stellate cells facilitates pancreatic cancer cell invasion. EMBO Mol Med 2014;6:467–81. 10.1002/emmm.201302698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kleeff J, Fukahi K, Lopez ME, et al. . Targeting of suicide gene delivery in pancreatic cancer cells via FGF receptors. Cancer Gene Ther 2002;9:522–32. 10.1038/sj.cgt.7700464 [DOI] [PubMed] [Google Scholar]
- 141. Jindal V, Arora E, Masab M, et al. . Chimeric antigen receptor T cell therapy in pancreatic cancer: from research to practice. Med Oncol 2018;35:84 10.1007/s12032-018-1145-0 [DOI] [PubMed] [Google Scholar]
- 142. Kratschmer C, Levy M. Targeted delivery of auristatin-modified toxins to pancreatic cancer using aptamers. Mol Ther Nucleic Acids 2018;10:227–36. 10.1016/j.omtn.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Man YKS, Davies JA, Coughlan L, et al. . THe novel oncolytic adenoviral mutant Ad5-3Δ-A20T retargeted to αvβ6 integrins efficiently eliminates pancreatic cancer cells. Mol Cancer Ther 2018;17:575–87. 10.1158/1535-7163.MCT-17-0671 [DOI] [PubMed] [Google Scholar]
- 144. Qian C, Wang Y, Chen Y, et al. . Suppression of pancreatic tumor growth by targeted arsenic delivery with anti-CD44v6 single chain antibody conjugated nanoparticles. Biomaterials 2013;34:6175–84. 10.1016/j.biomaterials.2013.04.056 [DOI] [PubMed] [Google Scholar]
- 145. Weissmueller S, Manchado E, Saborowski M, et al. . Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor β signaling. Cell 2014;157:382–94. 10.1016/j.cell.2014.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kurahara H, Maemura K, Mataki Y, et al. . Impact of p53 and PDGFR-β Expression on Metastasis and Prognosis of Patients with Pancreatic Cancer. World J Surg 2016;40:1977–84. 10.1007/s00268-016-3477-2 [DOI] [PubMed] [Google Scholar]
- 147. Pàez-Ribes M, Allen E, Hudock J, et al. . Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009;15:220–31. 10.1016/j.ccr.2009.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Raymond E, Dahan L, Raoul J-L, et al. . Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med Overseas Ed 2011;364:501–13. 10.1056/NEJMoa1003825 [DOI] [PubMed] [Google Scholar]
- 149. Nemunaitis J, Hochster HS, Lustgarten S, et al. . A phase I trial of oral ridaforolimus (AP23573; MK-8669) in combination with bevacizumab for patients with advanced cancers. Clin Oncol 2013;25:336–42. 10.1016/j.clon.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 150. Gangadhar TC, Cohen EE, Wu K, et al. . Two drug interaction studies of sirolimus in combination with sorafenib or sunitinib in patients with advanced malignancies. Clin Cancer Res 2011;17:1956–63. 10.1158/1078-0432.CCR-10-2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Martínez-Bosch N, Guerrero PE, Moreno M, et al. . The pancreatic niche inhibits the effectiveness of sunitinib treatment of pancreatic cancer. Oncotarget 2016;7:48265–79. 10.18632/oncotarget.10199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. O’Reilly EM, Niedzwiecki D, Hall M, et al. . A Cancer and Leukemia Group B phase II study of sunitinib malate in patients with previously treated metastatic pancreatic adenocarcinoma (CALGB 80603). Oncologist 2010;15:1310–9. 10.1634/theoncologist.2010-0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Fischer C, Jonckx B, Mazzone M, et al. . Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 2007;131:463–75. 10.1016/j.cell.2007.08.038 [DOI] [PubMed] [Google Scholar]
- 154. Hedlund EM, Yang X, Zhang Y, et al. . Tumor cell-derived placental growth factor sensitizes antiangiogenic and antitumor effects of anti-VEGF drugs. Proc Natl Acad Sci U S A 2013;110:654–9. 10.1073/pnas.1209310110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lu Z, Zhang Y, Li Z, et al. . Overexpression of the B-type Eph and ephrin genes correlates with progression and pain in human pancreatic cancer. Oncol Lett 2012;3:1207–12. 10.3892/ol.2012.650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Giaginis C, Tsourouflis G, Zizi-Serbetzoglou A, et al. . Clinical significance of ephrin (eph)-A1, -A2, -a4, -a5 and -a7 receptors in pancreatic ductal adenocarcinoma. Pathol Oncol Res 2010;16:267–76. 10.1007/s12253-009-9221-6 [DOI] [PubMed] [Google Scholar]
- 157. Gundry C, Marco S, Rainero E, et al. . Phosphorylation of Rab-coupling protein by LMTK3 controls Rab14-dependent EphA2 trafficking to promote cell:cell repulsion. Nat Commun 2017;8:14646 10.1038/ncomms14646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Boyd AW, Bartlett PF, Lackmann M. Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov 2014;13:39–62. 10.1038/nrd4175 [DOI] [PubMed] [Google Scholar]
- 159. Annunziata CM, Kohn EC, LoRusso P, et al. . Phase 1, open-label study of MEDI-547 in patients with relapsed or refractory solid tumors. Invest New Drugs 2013;31:77–84. 10.1007/s10637-012-9801-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Chang Q, Jorgensen C, Pawson T, et al. . Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br J Cancer 2008;99:1074–82. 10.1038/sj.bjc.6604676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Li J, Rix U, Fang B, et al. . A chemical and phosphoproteomic characterization of dasatinib action in lung cancer. Nat Chem Biol 2010;6:291–9. 10.1038/nchembio.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Shi H, Zhang CJ, Chen GY, et al. . Cell-based proteome profiling of potential dasatinib targets by use of affinity-based probes. J Am Chem Soc 2012;134:3001–14. 10.1021/ja208518u [DOI] [PubMed] [Google Scholar]
- 163. Morton JP, Karim SA, Graham K, et al. . Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 2010;139:292–303. 10.1053/j.gastro.2010.03.034 [DOI] [PubMed] [Google Scholar]
- 164. Evans TRJ, Van Cutsem E, Moore MJ, et al. . Phase 2 placebo-controlled, double-blind trial of dasatinib added to gemcitabine for patients with locally-advanced pancreatic cancer. Ann Oncol 2017;28:354–61. 10.1093/annonc/mdw607 [DOI] [PubMed] [Google Scholar]
- 165. Neuzillet C, de Gramont A, Tijeras-Raballand A, et al. . Perspectives of TGF-β inhibition in pancreatic and hepatocellular carcinomas. Oncotarget 2014;5:78–94. 10.18632/oncotarget.1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Blackford A, Serrano OK, Wolfgang CL, et al. . SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res 2009;15:4674–9. 10.1158/1078-0432.CCR-09-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Iacobuzio-Donahue CA, Fu B, Yachida S, et al. . DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806–13. 10.1200/JCO.2008.17.7188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Herman JM, Fan KY, Wild AT, et al. . Correlation of Smad4 status with outcomes in patients receiving erlotinib combined with adjuvant chemoradiation and chemotherapy after resection for pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys 2013;87:458–9. 10.1016/j.ijrobp.2013.06.2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Subramanian G, Schwarz RE, Higgins L, et al. . Targeting endogenous transforming growth factor beta receptor signaling in SMAD4-deficient human pancreatic carcinoma cells inhibits their invasive phenotype1. Cancer Res 2004;64:5200–11. 10.1158/0008-5472.CAN-04-0018 [DOI] [PubMed] [Google Scholar]
- 170. Serova M, Tijeras-Raballand A, Dos Santos C, et al. . Effects of TGF-beta signalling inhibition with galunisertib (LY2157299) in hepatocellular carcinoma models and in ex vivo whole tumor tissue samples from patients. Oncotarget 2015;6:21614–27. 10.18632/oncotarget.4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hamidi A, Song J, Thakur N, et al. . TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci Signal 2017;10:eaal4186 10.1126/scisignal.aal4186 [DOI] [PubMed] [Google Scholar]
- 172. Roberts NJ, Norris AL, Petersen GM, et al. . Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discov 2016;6:166–75. 10.1158/2159-8290.CD-15-0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Petersen GM. Familial pancreatic adenocarcinoma. Hematol Oncol Clin North Am 2015;29:641–53. 10.1016/j.hoc.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer 2007;96:11–15. 10.1038/sj.bjc.6603535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 1999;91:1310–6. 10.1093/jnci/91.15.1310 [DOI] [PubMed] [Google Scholar]
- 176. Goggins M, Hruban RH, Kern SE. BRCA2 is inactivated late in the development of pancreatic intraepithelial neoplasia: evidence and implications. Am J Pathol 2000;156:1767–71. 10.1016/S0002-9440(10)65047-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. . Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244–50. 10.1200/JCO.2014.56.2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Farmer H, McCabe N, Lord CJ, et al. . Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917–21. 10.1038/nature03445 [DOI] [PubMed] [Google Scholar]
- 179. Murai J, Huang SY, Das BB, et al. . Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res 2012;72:5588–99. 10.1158/0008-5472.CAN-12-2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Bryant HE, Schultz N, Thomas HD, et al. . Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005;434:913–7. 10.1038/nature03443 [DOI] [PubMed] [Google Scholar]
- 181. Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, et al. . Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol Cancer Ther 2011;10:3–8. 10.1158/1535-7163.MCT-10-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Philip CA, Laskov I, Beauchamp MC, et al. . Inhibition of PI3K-AKT-mTOR pathway sensitizes endometrial cancer cell lines to PARP inhibitors. BMC Cancer 2017;17:638 10.1186/s12885-017-3639-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Juvekar A, Burga LN, Hu H, et al. . Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov 2012;2:1048–63. 10.1158/2159-8290.CD-11-0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Ibrahim YH, García-García C, Serra V, et al. . PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov 2012;2:1036–47. 10.1158/2159-8290.CD-11-0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Chou A, Froio D, Nagrial AM, et al. . Tailored first-line and second-line CDK4-targeting treatment combinations in mouse models of pancreatic cancer. Gut 2017:gutjnl-2017-315144 10.1136/gutjnl-2017-315144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Cristofanilli M, Turner NC, Bondarenko I, et al. . Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–39. 10.1016/S1470-2045(15)00613-0 [DOI] [PubMed] [Google Scholar]
- 187. Konecny GE, Winterhoff B, Kolarova T, et al. . Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin Cancer Res 2011;17:1591–602. 10.1158/1078-0432.CCR-10-2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Sheppard KE, McArthur GA. The cell-cycle regulator CDK4: an emerging therapeutic target in melanoma. Clin Cancer Res 2013;19:5320–8. 10.1158/1078-0432.CCR-13-0259 [DOI] [PubMed] [Google Scholar]
- 189. Hu C, Dadon T, Chenna V, et al. . Combined inhibition of cyclin-dependent kinases (Dinaciclib) and AKT (MK-2206) blocks pancreatic tumor growth and metastases in patient-derived xenograft models. Mol Cancer Ther 2015;14:1532–9. 10.1158/1535-7163.MCT-15-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Chiron D, Di Liberto M, Martin P, et al. . Cell-cycle reprogramming for PI3K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov 2014;4:1022–35. 10.1158/2159-8290.CD-14-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Morton JP, Timpson P, Karim SA, et al. . Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A 2010;107:246–51. 10.1073/pnas.0908428107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Muller PA, Caswell PT, Doyle B, et al. . Mutant p53 drives invasion by promoting integrin recycling. Cell 2009;139:1327–41. 10.1016/j.cell.2009.11.026 [DOI] [PubMed] [Google Scholar]
- 193. Abraham AG, O’Neill E. PI3K/Akt-mediated regulation of p53 in cancer. Biochem Soc Trans 2014;42:798–803. 10.1042/BST20140070 [DOI] [PubMed] [Google Scholar]
- 194. Nayak SK, Panesar PS, Kumar H. p53-Induced apoptosis and inhibitors of p53. Curr Med Chem 2009;16:2627–40. 10.2174/092986709788681976 [DOI] [PubMed] [Google Scholar]
- 195. Yan W, Liu S, Xu E, et al. . Histone deacetylase inhibitors suppress mutant p53 transcription via histone deacetylase 8. Oncogene 2013;32:599–609. 10.1038/onc.2012.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Stojanovic N, Hassan Z, Wirth M, et al. . HDAC1 and HDAC2 integrate the expression of p53 mutants in pancreatic cancer. Oncogene 2017;36:1804–15. 10.1038/onc.2016.344 [DOI] [PubMed] [Google Scholar]
- 197. Giaginis C, Damaskos C, Koutsounas I, et al. . Histone deacetylase (HDAC)-1, -2, -4 and -6 expression in human pancreatic adenocarcinoma: associations with clinicopathological parameters, tumor proliferative capacity and patients' survival. BMC Gastroenterol 2015;15:148 10.1186/s12876-015-0379-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Koutsounas I, Giaginis C, Theocharis S. Histone deacetylase inhibitors and pancreatic cancer: are there any promising clinical trials? World J Gastroenterol 2013;19:1173–81. 10.3748/wjg.v19.i8.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Chan E, Arlinghaus LR, Cardin DB, et al. . Phase I trial of vorinostat added to chemoradiation with capecitabine in pancreatic cancer. Radiother Oncol 2016;119:312–8. 10.1016/j.radonc.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zhang G, Park MA, Mitchell C, et al. . Vorinostat and sorafenib synergistically kill tumor cells via FLIP suppression and CD95 activation. Clin Cancer Res 2008;14:5385–99. 10.1158/1078-0432.CCR-08-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Dasari A, Gore L, Messersmith WA, et al. . A phase I study of sorafenib and vorinostat in patients with advanced solid tumors with expanded cohorts in renal cell carcinoma and non-small cell lung cancer. Invest New Drugs 2013;31:115–25. 10.1007/s10637-012-9812-z [DOI] [PubMed] [Google Scholar]
- 202. Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer 2013;13:83–96. 10.1038/nrc3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Sherr CJ. Divorcing ARF and p53: an unsettled case. Nat Rev Cancer 2006;6:663–73. 10.1038/nrc1954 [DOI] [PubMed] [Google Scholar]
- 204. Mayo LD, Dixon JE, Durden DL, et al. . PTEN protects p53 from Mdm2 and sensitizes cancer cells to chemotherapy. J Biol Chem 2002;277:5484–9. 10.1074/jbc.M108302200 [DOI] [PubMed] [Google Scholar]
- 205. Freeman DJ, Li AG, Wei G, et al. . PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 2003;3:117–30. 10.1016/S1535-6108(03)00021-7 [DOI] [PubMed] [Google Scholar]
- 206. Suh YA, Post SM, Elizondo-Fraire AC, et al. . Multiple stress signals activate mutant p53 in vivo. Cancer Res 2011;71:7168–75. 10.1158/0008-5472.CAN-11-0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Azmi AS, Philip PA, Aboukameel A, et al. . Reactivation of p53 by novel MDM2 inhibitors: implications for pancreatic cancer therapy. Curr Cancer Drug Targets 2010;10:319–31. 10.2174/156800910791190229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Gottlieb TM, Leal JF, Seger R, et al. . Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene 2002;21:1299–303. 10.1038/sj.onc.1205181 [DOI] [PubMed] [Google Scholar]
- 209. Zhou BP, Liao Y, Xia W, et al. . HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol 2001;3:973–82. 10.1038/ncb1101-973 [DOI] [PubMed] [Google Scholar]
- 210. Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell 2014;25:304–17. 10.1016/j.ccr.2014.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Kulikov R, Boehme KA, Blattner C. Glycogen synthase kinase 3-dependent phosphorylation of Mdm2 regulates p53 abundance. Mol Cell Biol 2005;25:7170–80. 10.1128/MCB.25.16.7170-7180.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Boehme KA, Kulikov R, Blattner C. p53 stabilization in response to DNA damage requires Akt/PKB and DNA-PK. Proc Natl Acad Sci U S A 2008;105:7785–90. 10.1073/pnas.0703423105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Meek DW, Hupp TR. The regulation of MDM2 by multisite phosphorylation-opportunities for molecular-based intervention to target tumours? Semin Cancer Biol 2010;20:19–28. 10.1016/j.semcancer.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 214. Leszczynska KB, Foskolou IP, Abraham AG, et al. . Hypoxia-induced p53 modulates both apoptosis and radiosensitivity via AKT. J Clin Invest 2015;125:2385–98. 10.1172/JCI80402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Potiron VA, Abderrahmani R, Abderrhamani R, et al. . Radiosensitization of prostate cancer cells by the dual PI3K/mTOR inhibitor BEZ235 under normoxic and hypoxic conditions. Radiother Oncol 2013;106:138–46. 10.1016/j.radonc.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 216. Kuger S, Flentje M, Djuzenova CS. Simultaneous perturbation of the MAPK and the PI3K/mTOR pathways does not lead to increased radiosensitization. Radiat Oncol 2015;10:214 10.1186/s13014-015-0514-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Cox AD, Fesik SW, Kimmelman AC, et al. . Drugging the undruggable RAS: Mission possible? Nat Rev Drug Discov 2014;13:828–51. 10.1038/nrd4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ihle NT, Byers LA, Kim ES, et al. . Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst 2012;104:228–39. 10.1093/jnci/djr523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Van Cutsem E, van de Velde H, Karasek P, et al. . Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 2004;22:1430–8. 10.1200/JCO.2004.10.112 [DOI] [PubMed] [Google Scholar]
- 220. Kamerkar S, LeBleu VS, Sugimoto H, et al. . Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017;546:498–503. 10.1038/nature22341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Zorde Khvalevsky E, Gabai R, Rachmut IH, et al. . Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:20723–8. 10.1073/pnas.1314307110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Singh A, Greninger P, Rhodes D, et al. . A gene expression signature associated with "K-Ras addiction" reveals regulators of EMT and tumor cell survival. Cancer Cell 2009;15:489–500. 10.1016/j.ccr.2009.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Ko AH, Bekaii-Saab T, Van Ziffle J, et al. . A multicenter, open-label phase ii clinical trial of combined mek plus egfr inhibition for chemotherapy-refractory advanced pancreatic adenocarcinoma. Clin Cancer Res 2016;22:61–8. 10.1158/1078-0432.CCR-15-0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Infante JR, Somer BG, Park JO, et al. . A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer 2014;50:2072–81. 10.1016/j.ejca.2014.04.024 [DOI] [PubMed] [Google Scholar]
- 225. Abel EV, Basile KJ, Kugel CH, et al. . Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. J Clin Invest 2013;123:2155–68. 10.1172/JCI65780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Pettazzoni P, Viale A, Shah P, et al. . Genetic events that limit the efficacy of MEK and RTK inhibitor therapies in a mouse model of KRAS-driven pancreatic cancer. Cancer Res 2015;75:1091–101. 10.1158/0008-5472.CAN-14-1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Shimizu T, Tolcher AW, Papadopoulos KP, et al. . The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin Cancer Res 2012;18:2316–25. 10.1158/1078-0432.CCR-11-2381 [DOI] [PubMed] [Google Scholar]
- 228. Koplev S, Longden J, Ferkinghoff-Borg J, et al. . Dynamic rearrangement of cell states detected by systematic screening of sequential anticancer treatments. Cell Rep 2017;20:2784–91. 10.1016/j.celrep.2017.08.095 [DOI] [PubMed] [Google Scholar]
- 229. Miller BW, Morton JP, Pinese M, et al. . Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol Med 2015;7:1063–76. 10.15252/emmm.201404827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Olive KP, Jacobetz MA, Davidson CJ, et al. . Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457–61. 10.1126/science.1171362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Jacobetz MA, Chan DS, Neesse A, et al. . Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013;62:112–20. 10.1136/gutjnl-2012-302529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Provenzano PP, Cuevas C, Chang AE, et al. . Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418–29. 10.1016/j.ccr.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. . Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719–34. 10.1016/j.ccr.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Rhim AD, Oberstein PE, Thomas DH, et al. . Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014;25:735–47. 10.1016/j.ccr.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Vennin C, Chin VT, Warren SC, et al. . Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci Transl Med 2017;9:eaai8504 10.1126/scitranslmed.aai8504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Nobis M, McGhee EJ, Morton JP, et al. . Intravital FLIM-FRET imaging reveals dasatinib-induced spatial control of src in pancreatic cancer. Cancer Res 2013;73:4674–86. 10.1158/0008-5472.CAN-12-4545 [DOI] [PubMed] [Google Scholar]
- 237. Sullivan R, Paré GC, Frederiksen LJ, et al. . Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor-1 activity. Mol Cancer Ther 2008;7:1961–73. 10.1158/1535-7163.MCT-08-0198 [DOI] [PubMed] [Google Scholar]
- 238. Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011;11:393–410. 10.1038/nrc3064 [DOI] [PubMed] [Google Scholar]
- 239. Rofstad EK, Galappathi K, Mathiesen B, et al. . Fluctuating and diffusion-limited hypoxia in hypoxia-induced metastasis. Clin Cancer Res 2007;13:1971–8. 10.1158/1078-0432.CCR-06-1967 [DOI] [PubMed] [Google Scholar]
- 240. Chang Q, Jurisica I, Do T, et al. . Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer Res 2011;71:3110–20. 10.1158/0008-5472.CAN-10-4049 [DOI] [PubMed] [Google Scholar]
- 241. Hardie RA, van Dam E, Cowley M, et al. . Mitochondrial mutations and metabolic adaptation in pancreatic cancer. Cancer Metab 2017;5:2 10.1186/s40170-017-0164-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol 1927;8:519–30. 10.1085/jgp.8.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 2008;8:705–13. 10.1038/nrc2468 [DOI] [PubMed] [Google Scholar]
- 244. Cohen R, Neuzillet C, Tijeras-Raballand A, et al. . Targeting cancer cell metabolism in pancreatic adenocarcinoma. Oncotarget 2015;6:16832–47. 10.18632/oncotarget.4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Maftouh M, Avan A, Sciarrillo R, et al. . Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer 2014;110:172–82. 10.1038/bjc.2013.681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Ying H, Kimmelman AC, Lyssiotis CA, et al. . Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012;149:656–70. 10.1016/j.cell.2012.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Shukla SK, Purohit V, Mehla K, et al. . MUC1 and HIF-1alpha Signaling Crosstalk Induces Anabolic Glucose Metabolism to Impart Gemcitabine Resistance to Pancreatic Cancer. Cancer Cell 2017;32:71–87. 10.1016/j.ccell.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Parks SK, Cormerais Y, Durivault J, et al. . Genetic disruption of the pHi-regulating proteins Na+/H+ exchanger 1 (SLC9A1) and carbonic anhydrase 9 severely reduces growth of colon cancer cells. Oncotarget 2017;8:10225–37. 10.18632/oncotarget.14379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Ward C, Meehan J, Mullen P, et al. . Evaluation of carbonic anhydrase IX as a therapeutic target for inhibition of breast cancer invasion and metastasis using a series of in vitro breast cancer models. Oncotarget 2015;6:24856–70. 10.18632/oncotarget.4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Carta F, Vullo D, Osman SM, et al. . Synthesis and carbonic anhydrase inhibition of a series of SLC-0111 analogs. Bioorg Med Chem 2017;25:2569–76. 10.1016/j.bmc.2017.03.027 [DOI] [PubMed] [Google Scholar]
- 251. Faes S, Planche A, Uldry E, et al. . Targeting carbonic anhydrase IX improves the anti-cancer efficacy of mTOR inhibitors. Oncotarget 2016;7:36666–80. 10.18632/oncotarget.9134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Sun JD, Ahluwalia D, Liu Q, et al. . Combination treatment with hypoxia-activated prodrug evofosfamide (TH-302) and mTOR inhibitors results in enhanced antitumor efficacy in preclinical renal cell carcinoma models. Am J Cancer Res 2015;5:2139–55. [PMC free article] [PubMed] [Google Scholar]
- 253. Brown JM. SR 4233 (tirapazamine): a new anticancer drug exploiting hypoxia in solid tumours. Br J Cancer 1993;67:1163–70. 10.1038/bjc.1993.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Jones GD, Weinfeld M. Dual action of tirapazamine in the induction of DNA strand breaks. Cancer Res 1996;56:1584–90. [PubMed] [Google Scholar]
- 255. Rischin D, Peters LJ, O’Sullivan B, et al. . Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol 2010;28:2989–95. 10.1200/JCO.2009.27.4449 [DOI] [PubMed] [Google Scholar]
- 256. Borad MJ, Reddy SG, Bahary N, et al. . Randomized phase II trial of gemcitabine plus TH-302 versus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2015;33:1475–81. 10.1200/JCO.2014.55.7504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Guise CP, Abbattista MR, Singleton RS, et al. . The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res 2010;70:1573–84. 10.1158/0008-5472.CAN-09-3237 [DOI] [PubMed] [Google Scholar]
- 258. Hicks KO, Siim BG, Jaiswal JK, et al. . Pharmacokinetic/pharmacodynamic modeling identifies SN30000 and SN29751 as tirapazamine analogues with improved tissue penetration and hypoxic cell killing in tumors. Clin Cancer Res 2010;16:4946–57. 10.1158/1078-0432.CCR-10-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Albertella MR, Loadman PM, Jones PH, et al. . Hypoxia-selective targeting by the bioreductive prodrug AQ4N in patients with solid tumors: results of a phase I study. Clin Cancer Res 2008;14:1096–104. 10.1158/1078-0432.CCR-07-4020 [DOI] [PubMed] [Google Scholar]
- 260. Matsumoto S, Kishimoto S, Saito K, et al. . Metabolic and physiologic imaging biomarkers of the tumor microenvironment predict treatment outcome with radiation or a hypoxia-activated prodrug in mice. Cancer Res 2018;78:3783–92. 10.1158/0008-5472.CAN-18-0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. O’Connor LJ, Cazares-Körner C, Saha J, et al. . Design, synthesis and evaluation of molecularly targeted hypoxia-activated prodrugs. Nat Protoc 2016;11:781–94. 10.1038/nprot.2016.034 [DOI] [PubMed] [Google Scholar]
- 262. Yap TA, Yan L, Patnaik A, et al. . First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol 2011;29:4688–95. 10.1200/JCO.2011.35.5263 [DOI] [PubMed] [Google Scholar]
- 263. Yap TA, Yan L, Patnaik A, et al. . Interrogating two schedules of the AKT inhibitor MK-2206 in patients with advanced solid tumors incorporating novel pharmacodynamic and functional imaging biomarkers. Clin Cancer Res 2014;20:5672–85. 10.1158/1078-0432.CCR-14-0868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Molife L, Yan L, Vitfell-Rasmussen J, et al. . Phase 1 trial of the oral AKT inhibitor MK-2206 plus carboplatin/paclitaxel, docetaxel, or erlotinib in patients with advanced solid tumors. J Hematol Oncol 2014;7:1 10.1186/1756-8722-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Chung V, McDonough S, Philip PA, et al. . Effect of selumetinib and mk-2206 vs oxaliplatin and fluorouracil in patients with metastatic pancreatic cancer after prior therapy: Swog s1115 study randomized clinical trial. JAMA Oncol 2017;3:516–22. 10.1001/jamaoncol.2016.5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Tolcher AW, Patnaik A, Papadopoulos KP, et al. . Phase I study of the MEK inhibitor trametinib in combination with the AKT inhibitor afuresertib in patients with solid tumors and multiple myeloma. Cancer Chemother Pharmacol 2015;75:183–9. 10.1007/s00280-014-2615-5 [DOI] [PubMed] [Google Scholar]
- 267. Hong DS, Henary H, Falchook GS, et al. . First-in-human study of pbi-05204, an oleander-derived inhibitor of akt, fgf-2, nf-κΒ and p70s6k, in patients with advanced solid tumors. Invest New Drugs 2014;32:1204–12. 10.1007/s10637-014-0127-0 [DOI] [PubMed] [Google Scholar]
- 268. Marsh RW, Rocha Lima CM, Levy DE, et al. . A phase II trial of perifosine in locally advanced, unresectable, or metastatic pancreatic adenocarcinoma. Am J Clin Oncol 2007;30:26–31. 10.1097/01.coc.0000251235.46149.43 [DOI] [PubMed] [Google Scholar]
- 269. Tolcher AW, Bendell JC, Papadopoulos KP, et al. . A phase IB trial of the oral MEK inhibitor trametinib (GSK1120212) in combination with everolimus in patients with advanced solid tumors. Ann Oncol 2015;26:58–64. 10.1093/annonc/mdu482 [DOI] [PubMed] [Google Scholar]
- 270. McRee AJ, Sanoff HK, Carlson C, et al. . A phase I trial of mFOLFOX6 combined with the oral PI3K inhibitor BKM120 in patients with advanced refractory solid tumors. Invest New Drugs 2015;33:1225–31. 10.1007/s10637-015-0298-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Bedard PL, Tabernero J, Janku F, et al. . A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin Cancer Res 2015;21:730–8. 10.1158/1078-0432.CCR-14-1814 [DOI] [PubMed] [Google Scholar]
- 272. Bowles DW, Ma WW, Senzer N, et al. . A multicenter phase 1 study of PX-866 in combination with docetaxel in patients with advanced solid tumours. Br J Cancer 2013;109:1085–92. 10.1038/bjc.2013.474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Patnaik A, Appleman LJ, Tolcher AW, et al. . First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin’s lymphomas. Ann Oncol 2016;27:1928–40. 10.1093/annonc/mdw282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Azaro A, Rodon J, Calles A, et al. . A first-in-human phase I trial of LY2780301, a dual p70 S6 kinase and Akt Inhibitor, in patients with advanced or metastatic cancer. Invest New Drugs 2015;33:710–9. 10.1007/s10637-015-0241-7 [DOI] [PubMed] [Google Scholar]
- 275. Markman B, Tabernero J, Krop I, et al. . Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Ann Oncol 2012;23:2399–408. 10.1093/annonc/mds011 [DOI] [PubMed] [Google Scholar]
- 276. Papadopoulos KP, Tabernero J, Markman B, et al. . Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245409 (XL765), a novel, orally administered PI3K/mTOR inhibitor in patients with advanced solid tumors. Clin Cancer Res 2014;20:2445–56. 10.1158/1078-0432.CCR-13-2403 [DOI] [PubMed] [Google Scholar]
- 277. Mahadevan D, Chiorean EG, Harris WB, et al. . Phase I pharmacokinetic and pharmacodynamic study of the pan-PI3K/mTORC vascular targeted pro-drug SF1126 in patients with advanced solid tumours and B-cell malignancies. Eur J Cancer 2012;48:3319–27. 10.1016/j.ejca.2012.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Wainberg ZA, Alsina M, Soares HP, et al. . A Multi-Arm Phase I Study of the PI3K/mTOR Inhibitors PF-04691502 and Gedatolisib (PF-05212384) plus Irinotecan or the MEK Inhibitor PD-0325901 in Advanced Cancer. Target Oncol 2017;12:775–85. 10.1007/s11523-017-0530-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Shapiro GI, Bell-McGuinn KM, Molina JR, et al. . First-in-Human Study of PF-05212384 (PKI-587), a Small-Molecule, Intravenous, Dual Inhibitor of PI3K and mTOR in Patients with Advanced Cancer. Clin Cancer Res 2015;21:1888–95. 10.1158/1078-0432.CCR-14-1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Balachandran VP, Łuksza M, Zhao JN, et al. . Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 2017;551:512–6. 10.1038/nature24462 [DOI] [PMC free article] [PubMed] [Google Scholar]