Abstract
Objective
Intracranial aneurysms are considered large if >10 mm and giant if >25 mm. The risk of aneurysmal rupture compounds with increase in size of the aneurysm, thus, warranting appropriate intervention. In this study, we have analyzed the outcome and effectiveness of microsurgical procedures in large and giant aneurysms.
Methods
A retrospective analysis of all the patients who underwent microsurgical procedures for large and giant cerebral aneurysms from 2014–2018 in our institute was conducted. There were a total of 52 patients, in which direct clipping was performed in 42 (80.7%) patients, proximal trapping in 3 (5.7%) patients, trapping with bypass in 3 (5.7%) patients, suction decompression in 3 (5.7%) patients, and 1 (1.9%) patient underwent surgical reconstruction.
Results
Among the 52 patients, in the postoperative period, 1 (1.9%) patient became comatose, 1 (1.9%) patient developed hemiplegia, 1 (1.9%) patient had a transient hemiparesis, and 1 (1.9%) patient had transient lower cranial nerve palsy. Two (3.8%) patients had chronic subdural hematoma during the 3-month follow-up. There was no mortality in our series.
Conclusions
There are several treatment strategies available to manage large and giant cerebral aneurysms. In this study, we had minimal morbidity (3.8%), favorable outcome (96.1%), and no mortality. Therefore, we would like to conclude that appropriate microsurgical procedures, in experienced hands, can be considered as first line in the management for large and giant intracranial aneurysms, especially those with complex anatomy, wide neck, mass effect, partial thrombosis, and the presence of critical perforating vessels from the aneurysm wall.
Key words: Bypass, Clipping, DIVA, Giant, Intracranial aneurysm, Outcome, Trapping
Abbreviations and Acronyms: 3D, 3-Dimensional; BTO, Balloon test occlusion; CFD, Computational fluid dynamics; CTA, Computed tomography angiogram; DIVA, Dual image video angiography; DSA, Digital subtraction angiography; GOS, Glasgow Outcome Scale; ICA, Internal cerebral artery; ICG, Indo-cyanine green; MEP, Motor evoked potential; MRI, Magnetic resonance imaging; OA, Occipital artery; PICA, Posterior inferior cerebellar artery; RSD, Retrograde suction decompression; VA, Vertebral artery
Introduction
Large and giant intracranial aneurysms account for approximately 5% of all intracranial aneurysms. They manifest during the fifth to seventh decades of life and have a female predominance. They can lead to neurologic deficits owing to mass effect, ischemia, or subarachnoid hemorrhage. The risk of rupture is as high as 50% in 5 years according to the International Study of Unruptured Intracranial Aneurysms (ISUIA) trial. Available treatment strategies include microsurgical clipping, endovascular therapy, and combined approaches. Most surgical series of large and giant aneurysms were conducted more than a decade ago. Recent advances in techniques and instrumentation have tremendously improved the ability to safely manage such aneurysms by microsurgical procedures.1, 2, 3 Appropriate surgical approach as the first line of management in these lesions are the main stay of this study.
Materials and Methods
A retrospective analysis of 52 patients who were diagnosed with large and giant intracranial aneurysms who underwent microsurgical procedures from 2014–2018 in our institute was conducted. We reviewed the clinical records, preoperative images, and operation videos of all the cases. Appropriate procedures were performed in all the patients depending on the location and morphology of the aneurysm. Direct clipping, proximal trapping, trapping occlusion with bypass, suction decompression, and reconstruction of aneurysm were performed, which are discussed later. Intraoperative doppler ultrasonography, indo-cyanine green (ICG) fluorescence imaging, neuroendoscopy, and intraoperative electrophysiological monitoring (somatosensory evoked potential/motor evoked potential [MEP]) were routinely used. Preoperative computational fluid dynamics (CFD) and intraoperative dual image video angiography (DIVA) were used in most cases. Regular follow-up of all the patients was completed. The Glasgow Outcome Scale (GOS) was used to assess outcomes; good outcome was defined as a final GOS of 4, 5 and poor outcome as a final GOS of <4. We have a fully functioning interventional radiologist team who are performing endovascular procedures routinely. Procedures (microsurgical or endovascular) are planned as a team, which involves neurosurgeons, interventional radiologists, patients, and patient's attendees.
Illustrative Case 1
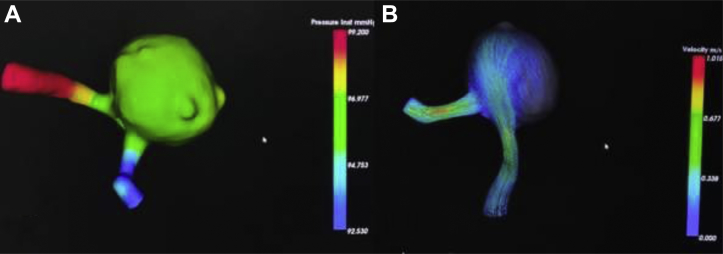
An 83-year-old woman with no history of comorbidities or family history of aneurysms was incidentally detected to have a large left vertebral artery (VA) posterior inferior cerebellar artery (PICA) segment aneurysm 15 mm in size, and its neck of about 10 mm in size (Figure 1) on 3-dimensional (3D) computed tomography angiogram (CTA) of the brain. CFD showed increased wall pressure, decreased wall shear stress pressure, divergent flow, and spiral streamline (Figure 2). After discussion with the interventional team, the patient and the patient's attendees were given the option for an open endovascular procedure. Because they opted for the surgical procedure after knowing both pros and cons, she underwent occipital artery (OA)-PICA bypass followed by trapping of the aneurysm. The aneurysm was trapped completely via direct clipping at its proximal and distal ends after verifying patency of the bypass graft using intraoperative micro-Doppler ultrasound, ICG angiography, and DIVA. Intraoperative DIVA showed good filling via the OA-PICA bypass graft and complete obliteration of the aneurysm (Figure 3), which was confirmed with a postoperative CTA (Figure 4). The intraoperative and postoperative period was uneventful.
Figure 1.
(A) Left vertebral artery, posterior inferior cerebellar artery (PICA) segment aneurysm. (B) PICA originating just proximal to the aneurysm. VA, vertebral artery.
Figure 2.
(A and B) Computational fluid dynamics showing increased wall pressure and a spiral streamline flow.
Figure 3.
(A) Dual image video angiography (DIVA) showing filling of the aneurysm, occipital artery (OA)-posterior inferior cerebellar artery (PICA) bypass completed. (B) Post-trapping DIVA shows no flow in the aneurysm and good flow in the OA-PICA. VA, vertebral artery.
Figure 4.
(A) Left transcondylar approach. (B) Postoperative image with clips in situ and complete exclusion of the aneurysm from the circulation.
Illustrative Case 2
A 64-year-old woman presented with a visual field defect in the left eye lasting for 3 months. She had no comorbidities or family history of aneurysms. On evaluation with 3D CTA and magnetic resonance imaging (MRI)-CTA of the brain, she was discovered to have a large left internal carotid artery ophthalmic segment aneurysm measuring approximately 15 mm in diameter with an 8 mm neck (Figure 5). She underwent a standard left pterional craniotomy and suction decompression followed by clipping of the aneurysms. The ipsilateral common carotid artery was used as a proximal control. Common carotid artery, external carotid artery distal to the superior thyroid artery, and internal cerebral artery (ICA) distal to the aneurysm were clamped. The superior thyroid artery was then catheterized, and retrograde suction decompression (RSD) of the aneurysm was completed (Figure 6A). The aneurysm was effectively deflated. Definitive clipping of the aneurysm was performed with 3 permanent clips (Figure 6B). Postclipping DIVA showed complete occlusion of the aneurysm (Figure 6C). The postoperative period was uneventful, and there was a significant improvement in the field defect postoperatively.
Figure 5.
(A and B) Magnetic resonance imaging-computed tomography angiogram fusion image showing left internal carotid artery-ophthalmic segment large aneurysm.
Figure 6.
(A) Catheterization of the carotid artery with suction catheter. (B) Suction decompression of the large aneurysm with temporary clips in situ. (C) Postclipping dual image video angiography shows complete occlusion of the aneurysm. CC, common carotid artery; EC, external carotid artery; ICA, internal cerebral artery.
Illustrative Case 3
A 50-year-old man presented with a history of headache and dizziness for the previous 3 years. He was diagnosed to have a fusiform left VA aneurysm that was partially thrombosed. He had undergone multiple endovascular procedures (coiling and stenting) in another hospital. He developed progressive diplopia, left facial weakness, dysarthria, and dizziness for 6 months following the endovascular intervention. Serial MRIs showed a progressively increasing, large partially thrombosed aneurysm in the left VA causing significant brain stem compression (Figure 7). In view of the progressive mass effect, surgery was planned and preceded. Using left transcondylar approach, removal of coils, partial thrombectomy, aneurysmorrhaphy, and arterial reconstruction was completed. Extensive vasa vasorum was identified in the aneurysmal wall that was probably supplying the aneurysm, even after stenting, and causing regrowth or recurrence of the aneurysm (Figure 8). Postoperative CTA showed complete obliteration of the aneurysm (Figure 9), and his neurologic symptoms improved on follow-up.
Figure 7.
(A) Magnetic resonance imaging of the brain shows a large partially thrombosed aneurysm in the left vertebral artery (VA). (B) Computed tomography angiogram shows a large fusiform aneurysm of the left VA involving the posterior inferior cerebellar artery segment.
Figure 8.
(A) Giant vertebral artery aneurysm with dilated vasa vasorum (circled), (B) neuroendoscopic view of the aneurysm, (C) retrieval of the intra-aneurysmal coil, (D) reconstruction of the aneurysmal wall and clipping. An, aneurysm; LCN, lower cranial nerves; PICA, posterior inferior cerebellar artery; VA, vertebral artery.
Figure 9.

Postclipping computed tomography angiogram shows exclusion of the aneurysm from the circulation.
Illustrative Case 4
A 78-year-old woman with no history of comorbidities or family history of aneurysms was incidentally detected to have giant right ICA cavernous segment aneurysm 25 mm in size (Figure 10). She underwent right pterional craniotomy and trapping occlusion of the aneurysm, with a low flow rescue bypass of superficial temporal artery with M2 segment of the middle cerebral artery, followed by a definitive external carotid artery to M4 segment of the middle cerebral artery high-flow bypass using radial artery graft. Two bypasses were planned preoperatively considering the time taken to perform a high-flow bypass and previous experiences; we planned a rescue low-flow bypass as a prophylactic measure to prevent ischemic brain injury. Intraoperative DIVA showed good flow in both bypasses (Figure 11), and the postoperative period was uneventful.
Figure 10.
Right internal carotid artery giant cavernous segment aneurysm. R.ICA, right internal carotid artery.
Figure 11.
Intraoperative images. (A) Low flow superficial temporal artery M4 bypass. (B) High flow external carotid artery M2 bypass and postanastomosis dual image video angiography with good flow. EC, external carotid artery; M2/M4, segments of the middle cerebral artery; STA, superficial temporal artery.
Results
Demography
Among the 52 patients, there were 18 (34.6%) men and 34 (65.3%) women. The mean age of the patients was 66.07 (41–88) years. The mean size of the aneurysm was 13.38 mm. Forty-one (78.8%) aneurysms were anterior circulation and 11 (21.1%) posterior circulation aneurysms (Table 1). Five patients in this series had multiple aneurysms, of which only the large and giant (>10 mm) aneurysms were considered.
Table 1.
Location of the Aneurysms
| Aneurysmal Vessels | Number |
|---|---|
| Anterior cerebral artery | 3 |
| Anterior communicating artery | 12 |
| Middle cerebral artery | 10 |
| Internal carotid artery – bifurcation | 2 |
| Internal carotid artery – Pcom segment | 6 |
| Internal carotid artery – ophthalmic segment | 5 |
| Internal carotid artery – Ant.choroidal segment | 1 |
| Internal carotid artery – cavernous segment | 2 |
| Basilar artery | 3 |
| Vertebral artery | 8 |
| Total | 52 |
Ant.choroidal, anterior choroidal artery; Pcom, posterior communicating artery.
Clinical Presentation and Investigations
Most of the aneurysms were incidental, that is, 46 (88.4%) aneurysms out of 52 patients. Two (3.8%) patients presented with subarachnoid hemorrhage (Hunt and Hess grade 2). One (1.9%) patient had an intracranial hemorrhage. Three (5.7%) patients had cranial nerve deficits in the form of facial nerve palsy, occulomotor nerve palsy, and visual field defects, respectively. All the patients underwent complete preoperative workup with MRI brain scan, 3D CTA of the brain, digital subtraction angiography (DSA), and CFD (Table 2).
Table 2.
Clinical Presentation
| Clinical Presentation | Number |
|---|---|
| Incidental | 46 |
| Headache + SAH | 2 |
| Facial weakness | 1 |
| Occulomotor nerve palsy | 1 |
| Visual field defect | 1 |
| Intracranial hemorrhage | 1 |
| Total | 52 |
SAH, sub arachnoid hemorrhage.
Procedures Performed
Direct clipping of the aneurysm, without any added procedures, was completed in 42 (80.7%) patients. Proximal trapping was completed in 3 (5.7%) patients, suction decompression with clipping in 3 (5.7%) patients, trapping with bypass in 3 (5.7%) patients, 1 patient underwent low-flow OA-PICA bypass, and 2 patients underwent high-flow extracranial–intracranial bypass. Surgical reconstruction with clipping was performed in 1 (1.9%) patient. Intraoperative ICG and MEP was used in all patients. Intraoperative DIVA and neuroendoscope was used in most of the patients. Postoperative angiogram revealed complete occlusion in 46 (88.4%) aneurysms and residual neck in 6 (11.5%) aneurysms. One (1.9%) patient developed transient hemiparesis, possibly owing to vasospasm, which was treated appropriately, and the patient improved with physiotherapy. One (1.9%) patient developed transient lower cranial palsy that resolved. One (1.9%) patient had intraoperative rupture of the aneurysm with brain bulge initially, who became comatose after successful clipping of the aneurysm owing to continuous postoperative cerebral edema. There was no mortality in the entire series. Of the 6 incompletely clipped aneurysms, 3 cases needed endovascular intervention for complete occlusion of the residual aneurysm, 1 patient underwent re-surgery for clipping of the residual neck, and other 2 patients were followed up with serial imaging. They showed no increase in size of the residual neck, therefore, were managed conservatively with regular follow-up (Table 3).
Table 3.
Microsurgical Procedures Performed
| Procedures Performed | Number |
|---|---|
| Direct clipping of aneurysm | 42 |
| Proximal trap | 3 |
| Trapping occlusion with bypass | 3 (1 low flow; 2 high flow) |
| Suction decompression + clipping | 3 |
| Surgical reconstruction + clipping | 1 |
Follow-Up and Outcome
All 52 patients were followed-up regularly. The patient who was comatose (GOS-2) remains comatose. One (1.9%) patient had hemiplegia and is on regular physiotherapy, and 2 (3.8%) patients had chronic subdural hematoma during the 3- and 6-month follow-up for which they underwent surgical evacuation (Table 4). Permanent morbidity was found in 2 (3.8%) patients. There was no mortality in our series.
Table 4.
Complications
| Complications | Number |
|---|---|
| Coma | 1 |
| Hemiplegia | 1 |
| Chronic subdural hematoma | 2 |
| Transient hemiparesis | 1 |
| Transient lower cranial nerve palsy | 1 |
| Total | 6 |
GOS was used to assess the outcome, and was determined by the patient's neurologic examination and functional status at discharge. Classification was as follows: 1, deceased; 2, vegetative state; 3, severely disabled; 4, moderately disabled; and 5, mildly or not disabled.1, 4 Out of 52 patients, 50 patients had a GOS score of 5, 1 patient had a GOS score of 4, and 1 patient had a GOS score of 2 (Table 5).
Table 5.
Outcome Analysis Based on the Glasgow Coma Scale
| Glasgow Coma Scale | Number of Patients |
|---|---|
| 1 | 0 |
| 2 | 1 (1.9%) |
| 3 | 0 |
| 4 | 1 (1.9%) |
| 5 | 50 (96.1) |
Discussion
Large or giant intracranial aneurysms can be saccular, fusiform, or dissecting types. Saccular aneurysms are berry-like outpunching that occurs most commonly at arterial bifurcations. These are thought to arise due to hemodynamic stress and repeated endothelial damage from turbulent blood flow. Most giant aneurysms are of the saccular type.
Fusiform aneurysms involve the circumference of the arterial wall and lack a definable neck. These tend to involve longer arterial segments, predominantly secondary to atherosclerotic degeneration, and are more common in the posterior circulation.5 Yamaura6 reported that 28% of intracranial aneurysms occurring in the VA and PICA are dissecting aneurysms, and 13% are atherosclerotic fusiform aneurysms. Previously, it was thought that larger aneurysms were less prone to rupture due to the concentric bands of thrombus that were believed to form a protective sheath. However, natural history studies demonstrated that the risk of aneurysm rupture increases significantly with aneurysm size.1, 7
Giant aneurysms constitute 3%–13.5% of all intracranial aneurysms, with an incidence averaging 5% in various series.8, 9 Unruptured giant aneurysms have a higher probability of subsequent rupture and thrombosis.10, 11
Treatment strategy is planned by assessing factors such as age, comorbidities, size, location, morphology, projection of the aneurysm, neck-to-dome ratio, aneurysmal characteristics such as thrombosis and calcification, collateral circulation, and the presence of critical perforating vessels arising from the aneurysm wall.1, 12
Thorough preoperative imaging is mandatory in these lesions. CT brain is useful in detecting calcification and skull base erosion in cases of large or giant aneurysms with mass effect.13, 14 MRI helps in evaluating ischemia, perianeurysmal hemorrhage, and the relationship of the aneurysm with the surrounding neurovascular structures.15 3D DSA is very useful and imminent in planning surgery, as it permits visualization of the entire aneurysmal sac, neck, parent vessel, and perforators from all directions.16 CFD enables assessment of the hemodynamic environment within the parent vessel and the aneurysm, thus, it helps to understand and relate hemodynamic characteristics with aneurysm initiation, growth, and rupture.17, 18 Intraoperative correlation of preoperative CFD images helps the surgeon to identify the weaker or thrombosed aneurismal wall, so that manipulation of those weaker spot are avoided or performed with extra care.
Intraoperative adjuncts like ICG, DIVA, and endoscope were used to clearly demarcate the aneurysm from the surrounding structures in both pre- and postclipping scenarios. They also help in identifying residual aneurysm, inclusion of the parent artery, or perforators in the clips; therefore, readjustment of the clips can be completed immediately. ICG gives adequate information regarding the neck and parent or branching artery involvement in >90% of cases. Perforators as small as 0.5 mm in diameter can also be identified with this technique.19 DIVA uses the intravenous ICG with near-infrared fluorescence, which are visualized in a single monitor, thus, making it easier to understand the anatomic relationships between various intracranial structures and provide better assessment of the depth-of-field when compared to plain ICG.20, 21 The neuroendoscope with its higher magnification and additional illumination can provide additional details of the microsurgical anatomy of deeper structures that may not be visualized with the microscope alone.22 Electrophysiological monitoring, like MEP, makes it possible to detect real-time cerebral ischemia in cases of aneurysmal surgery involving the anterior circulation,23, 24, 25 thus, these techniques helps in preventing postoperative neurologic complications and eliminating the need for further surgeries.
Large and giant aneurysms can be managed with various treatment options like open surgery, endovascular therapy, or a combined approach. Although endovascular modalities like stenting, coiling, and flow diverters have favorable clinical and angiographic outcomes, surgical clipping continues to be the first management option in experienced hands. In our series, all the patients underwent specific and appropriate microsurgery pertaining to the lesion as described with a good clinical outcome. Sughrue et al.26 in his study with 140 patients with 141 giant aneurysms were treated surgically. The mortality rate was 13%, morbidity 9%, and favorable outcome in 81%. Sharma et al.4 reported a mortality rate of 9%, and a morbidity rate of 12% for giant aneurysms treated surgically, with favorable outcomes in 86% of patients. Our series also had comparable results with a favorable outcome in 96.1% with 0% mortality.
Management
The following are various techniques in the management of large and giant intracranial aneurysms.
Surgical Procedures
Microsurgical procedures for large and giant aneurysms depend on the aneurysmal characteristics like wide neck, mass effect, partial thrombosis, and the presence of critical perforating vessels arising from the aneurysm wall. Proximal control is mandatory in the management of large and giant aneurysms. Neck control in ICA aneurysms, proximal vessel control with temporary clips in the middle cerebral artery, anterior cerebral artery, anterior communicating artery, and others, and cardiac standstill are techniques that aid in a safe dissection, prevent intraoperative rupture, facilitates successful clip ligation and arterial reconstruction. Owall et al.27 and Bebawy et al.28 have explained the use of adenosine in intracranial aneurysm clipping. An adenosine infusion of 0.14 mg/kg/min lead to a decrease in mean arterial pressure by 43% (82–46 mmHg), and a mean hypotensive period of 32 minutes without signs of tachyphylaxis.
Microsurgical Direct Clipping
Selection of an appropriate surgical approach is critical for a successful surgical outcome.4 Adequate craniotomies such as pterional, bifrontal, and transcondylar resection allows visualization of the parent artery and its branches with minimal parenchymal retraction. Incision of the dural ring, resection of the lateral wall of the optic canal, optic strut, and the anterior clinoid process are useful in proximal ICA aneurysms.29, 30
When a giant aneurysm cannot be clipped directly, alternative surgical techniques like suction decompression of the aneurysm before clipping, parent artery occlusion with or without bypass, reconstruction of aneurysmal sac, endovascular techniques such as coiling of the residual aneurysm, stent-assisted coiling, stenting, or flow diverter should be considered.31
RSD
In cases of large and giant and large paraclinoid ICA aneurysms, the success of surgical treatment depends on adequate exposure of the aneurysm neck and obliteration of the aneurysm with minimal manipulation of the adjacent neurovascular. In 1994, Batjer et al.32 described the RSD technique by direct cannulation of the cervical ICA with an 18-gauge angiocatheter and aspiration using a 20-mL syringe. They were able to successfully collapse and clip >40 aneurysms using this technique. Mattingly et al.33 used the same technique with a positive outcome over a 17-year period. In our series, 3 patients underwent RSD and clipping. We introduced the suction catheter through the superior thyroid artery and decompressed the large ICA aneurysm, permanent clips were then applied, and the aneurysm was occluded completely in 2 patients. One (1.9%) aneurysm that was partially extradural could not be occluded completely. The intradural part was occluded with clips but the extradural part then required endovascular coiling.
Proximal Trapping
Therapeutic ICA or VA occlusion is a well-established technique in the management of giant aneurysms. This treatment modality can be used only after the patient is able to successfully tolerate a balloon test occlusion (BTO), as the risk of stroke is as high as 33% in unselected patients.1 Trapping of an aneurysm is a very effective procedure but it must be done with great caution, for 2 collateral systems are involved, that of the parent vessel and that of any vessel arising from the trapped segment. According to Drake,8 collaterals can be assessed by puncturing the aneurysm after temporary proximal clipping. If there is brisk bleeding, it can be safely assumed that the collateral circulation is good. With the newer intraoperative imaging modalities like ICG and DIVA, not only the status of collaterals or perforators can be determined, but also the assessment of the occlusion status of the aneurysm can be determined accurately. Three (5.7%) of our patients needed proximal trapping. All of them had posterior circulation aneurysms (VA), which were trapped successfully. In 1893, Matas34 was the first to discuss VA sacrifice in large and giant aneurysms. VA BTO was performed in all 3 patients prior to parent artery trapping. BTO provides valuable details regarding the cross flow and collaterals that helps in decision-making regarding parent vessel sacrifice.35
Parent Artery Occlusion with Bypass
Complete trapping of the parent artery with bypass is another modality of management in cases of large or giant cavernous ICA or VA aneurysms. Bypasses are of 2 types: low-flow bypass (<50 mL/min) (superficial temporal or OA), and high-flow bypass (>50 mL/min) grafts using either the radial artery or saphenous vein. Hamada et al.36 and various other authors proposed that patients with a VA dissecting aneurysm involving a PICA origin should undergo PICA revascularization by low-flow OA-PICA bypass.1, 37, 38 Mohit et al.39 described a high-flow bypass procedure in basilar top and ICA giant aneurysms. In our series, we performed parent artery occlusion with low-flow bypass for 1 patient with a VA-PICA large aneurysm, in which OA was anastomosed with PICA, and parent artery occlusion with high-flow bypass for 2 patients using radial artery graft. Complete exclusion of aneurysms from the circulation and good patency of bypassed vessels were maintained with no postoperative complications. Ischemic complications should always be anticipated in the treatment of giant or complex intracranial aneurysms treated by parent artery occlusion, either by surgical or endovascular procedures, even if pre- and intraoperative blood flow studies indicate sufficient collateralization. Pancucci et al.40 and Lanterna et al.41 described rescue bypasses that may be low flow or high flow in cases of hypoperfusion, ischemia, or neurologic deficit following parent artery sacrifice procedures.
Endovascular Treatment
Because of the complex anatomy and the inherent risks associated with microsurgery, endovascular techniques are increasingly used to treat aneurysms. This includes Guglielmi detachable coils, stenting, and flow diverters. Endovascular therapy of giant intracranial aneurysms has been suggested to be the first option because of its lower procedure-related morbidity and mortality rates by some authors.42, 43 A recent meta-analysis of flow-diverter treatment for 225 posterior circulation aneurysms noted an 84% occlusion rate at 6 months postoperation, with overall good outcomes in 79% of patients.44 More randomized studies with long-term follow-up will be needed to determine if all types of flow diversion ultimately lead to a lasting cure. The need for antiplatelet prophylaxis will probably limit the use of flow diverters in anatomically complex and dissecting aneurysms in patients after spontaneous intracranial hemorrhage. Although attractive and minimally invasive, endovascular procedures also have high rates of incomplete occlusion and recanalization, especially in large and giant aneurysms.45 In cases of thrombosed aneurysms, there is a significant rate of coil migration into the thrombus. Microsurgery was preferred over endovascular procedure as the first line of management in our series because of the larger size, mass effect, and irregular shapes of the aneurysms in DSA images.46, 47, 48
Combined Surgical and Endovascular Procedure
In the case of giant aneurysms, symptoms commonly result from the mass effect on the adjacent neurovascular structures by the aneurysm itself, ischemic stroke, or aneurysm rupture. Most cases reported in the literature describe a progressive enlargement of the aneurysm with clinical deterioration, even after repeated endovascular treatments. Although this enlargement phenomenon has been extensively studied, there is no consensus regarding the mechanisms of continuous growth of the aneurysm. Surgery may represent too high a risk; however, embolization alone does not guarantee the reduction of the mass effect.49, 50 Although endovascular intervention plays an important role in fusiform posterior circulation aneurysms, it cannot completely cure the cause besides offering palliative care, neither is surgery alone superior to any other intervention. Combined approaches must be planned considering various parameters like age, comorbidities, aneurysm morphology, collateral circulation, and the presence of critical perforating vessels from the aneurysm wall, to obliterate the aneurysm completely from the circulation and provide a better outcome with less morbidity and mortality.
Conclusions
There are several treatment strategies like microsurgical procedures, endovascular intervention, and combined approaches available to manage large and giant cerebral aneurysms. In this retrospective single-center study in the microsurgical procedures of 52 patients of large and giant aneurysms, we had minimal morbidity (3.8%), favorable outcome (96.1%), and no mortality. Therefore, we would like to conclude that appropriate microsurgical intervention in experienced hands, can be considered as the first line of management for large and giant intracranial aneurysms, especially in cases with complex anatomy, wide neck, mass effect, partial thrombosis, and the presence of critical perforating vessels from the aneurysm wall.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Chalouhi N., Thakkar V., Tjoumakaris S. Microsurgical clipping of large and giant cerebral aneurysms: a single-center contemporary experience. J Clin Neurosci. 2014;21:1424–1427. doi: 10.1016/j.jocn.2013.11.052. [DOI] [PubMed] [Google Scholar]
- 2.Lawton M.T., Spetzler R.F. Surgical strategies for giant intracranial aneurysms. In: Langmoen I.A., Lundar T., Aaslid R., Reulen H.J., editors. Vol. 72. Springer; Vienna: 1999. pp. 141–156. (Neurosurgical Management of Aneurysmal Subarachnoid Haemorrhage, Acta Neurochirurgica Supplements). [DOI] [PubMed] [Google Scholar]
- 3.Symon L., Vajda J. Surgical experiences with giant intracranial aneurysms. J Neurosurg. 1984;61:1009–1028. doi: 10.3171/jns.1984.61.6.1009. [DOI] [PubMed] [Google Scholar]
- 4.Sharma B.S., Gupta A., Ahmad F.U., Suri A., Mehta V.S. Surgical management of giant intracranial aneurysms. Clin Neurol Neurosurg. 2008;110:674–681. doi: 10.1016/j.clineuro.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Mehta R.I., Salamon N., Zipser B.D., Mehta R.I. Giant intracranial aneurysm. Radiographics. 2010;30:1133–1138. doi: 10.1148/rg.304095199. [DOI] [PubMed] [Google Scholar]
- 6.Yamaura A. Diagnosis and treatment of vertebral aneurysms. J Neurosurg. 1988;69:345–349. doi: 10.3171/jns.1988.69.3.0345. [DOI] [PubMed] [Google Scholar]
- 7.UCAS Japan Investigators. Morita A., Kirino T. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–2482. doi: 10.1056/NEJMoa1113260. [DOI] [PubMed] [Google Scholar]
- 8.Drake C.G. Giant intracranial aneurysms: experience with surgical treatment in 174 patients. Clin Neurosurg. 1979;26:12–95. doi: 10.1093/neurosurgery/26.cn_suppl_1.12. [DOI] [PubMed] [Google Scholar]
- 9.Smith R.R. Surgical management of neurovascular disease. Neurosurgery. 1997;40:418. doi: 10.1097/0006123-199702000-00048. [DOI] [Google Scholar]
- 10.Khurana V.G., Piepgras D.G., Whisnant J.P. Ruptured giant intracranial aneurysms. Part I. A study of rebleeding. J Neurosurg. 1998;88:425–429. doi: 10.3171/jns.1998.88.3.0425. [DOI] [PubMed] [Google Scholar]
- 11.Nagahiro S., Takada A., Goto S., Kai Y., Ushio Y. Thrombosed growing giant aneurysms of the vertebral artery: growth mechanism and management. J Neurosurg. 1995;82:796–801. doi: 10.3171/jns.1995.82.5.0796. [DOI] [PubMed] [Google Scholar]
- 12.Hauck E.F., Wohlfeld B., Welch B.G., White J.A., Samson D. Clipping of very large or giant unruptured intracranial aneurysms in the anterior circulation: an outcome study. J Neurosurg. 2008;109:1012–1018. doi: 10.3171/JNS.2008.109.12.1012. [DOI] [PubMed] [Google Scholar]
- 13.Fisher A., Som P.M., Mosesson R.E., Lidov M., Liu T.-H. Giant intracranial aneurysms with skull base erosion and extracranial masses. J Comput Assist Tomogr. 1994;18:939–942. doi: 10.1097/00004728-199411000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Choi I.S., David C. Giant intracranial aneurysms: development, clinical presentation and treatment. Eur J Radiol. 2003;46:178–194. doi: 10.1016/s0720-048x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 15.Farahmand M., Farahangiz S., Yadollahi M. Diagnostic accuracy of magnetic resonance angiography for detection of intracranial aneurysms in patients with acute subarachnoid hemorrhage: a comparison to digital subtraction angiography. Bull Emerg Trauma. 2013;1:147–151. [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis A.I., Scalzo D., Tew J.M., Jr., Smith R.R. Surgical management of giant aneurysms of the anterior circulation. In: Schmidek H.H., Sweet W., editors. 4th ed. Vol.1. WB Saunders; Philadephia, PA: 2000. pp. 1205–1220. (Operative Neurosurgical Techniques). [Google Scholar]
- 17.Sforza D.M., Putman C.M., Cebral J.R. Computational fluid dynamics in brain aneurysms. Int J Numer Method Biomed Eng. 2012;28:801–808. doi: 10.1002/cnm.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cebral J., Mut F., Sforza D. Clinical application of image-based CFD for cerebral aneurysms. Int J Numer Method Biomed Eng. 2011;27:977–992. doi: 10.1002/cnm.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balamurugan S., Agrawal A., Kato Y., Sano H. Intra operative indocyanine green video-angiography in cerebrovascular surgery: an overview with review of literature. Asian J Neurosurg. 2011;6:88–93. doi: 10.4103/1793-5482.92168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feletti A., Wang X., Tanaka R. Dual-image videoangiography during intracranial microvascular surgery. World Neurosurg. 2017;99:572–579. doi: 10.1016/j.wneu.2016.12.070. [DOI] [PubMed] [Google Scholar]
- 21.Sato T., Suzuki K., Sakuma J. Development of a new high-resolution intraoperative imaging system (dual-image videoangiography, DIVA) to simultaneously visualize light and near-infrared fluorescence images of indocyanine green angiography. Acta Neurochir (Wien) 2015;157:1295–1301. doi: 10.1007/s00701-015-2481-x. [DOI] [PubMed] [Google Scholar]
- 22.Yoshioka H., Kinouchi H. The roles of endoscope in aneurysmal surgery. Neurol Med Chir (Tokyo) 2015;55:469–478. doi: 10.2176/nmc.ra.2014-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada Y., Kato Y., Nouri M. Predictive value of motor evoked potential monitoring during surgery of unruptured anterior circulation cerebral aneurysms. Asian J Neurosurg. 2017;12:644–647. doi: 10.4103/ajns.AJNS_135_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irie T., Yoshitani K., Ohnishi Y. The efficacy of motor-evoked potentials on cerebral aneurysm surgery and new-onset postoperative motor deficits. J Neurosurg Anesthesiol. 2010;22:247–251. doi: 10.1097/ANA.0b013e3181de4eae. [DOI] [PubMed] [Google Scholar]
- 25.Horiuchi K., Suzuki K., Sasaki T. Intraoperative monitoring of blood flow insufficiency during surgery of middle cerebral artery aneurysms. J Neurosurg. 2005;103:275–283. doi: 10.3171/jns.2005.103.2.0275. [DOI] [PubMed] [Google Scholar]
- 26.Sughrue M.E., Saloner D., Rayz V.L., Lawton M.T. Giant intracranial aneurysms. Neurosurgery. 2011;69:1261–1271. doi: 10.1227/NEU.0b013e31822bb8a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owall A., Gordon E., Lagerkranser M., Lindquist C., Rudehill A., Sollevi A. Clinical experience with adenosine for controlled hypotension during cerebral aneurysm surgery. Surv Anesthesiol. 1987;31:272. [PubMed] [Google Scholar]
- 28.Bebawy J.F., Gupta D.K., Bendok B.R. Adenosine-induced flow arrest to facilitate intracranial aneurysm clip ligation. Anesth Analg. 2010;110:1406–1411. doi: 10.1213/ANE.0b013e3181d65bf5. [DOI] [PubMed] [Google Scholar]
- 29.Lawton M.T., Spetzler R.F. Surgical management of giant intracranial aneurysms: experience with 171 patients. Clin Neurosurg. 1995;42:245–266. [PubMed] [Google Scholar]
- 30.Petraglia A., Moravan M., Jahromi B. Unilateral subfrontal approach to anterior communicating artery aneurysms: a review of 28 patients. Surg Neurol Int. 2011;2:124. doi: 10.4103/2152-7806.85056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lv X., Jiang C., Li Y., Yang X., Zhang J., Wu Z. Treatment of giant intracranial aneurysms. Interv Neuroradiol. 2009;15:135–144. doi: 10.1177/159101990901500201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batjer H.H., Kopitnik T.A., Giller C.A., Samson D.S. Surgery for paraclinoidal carotid artery aneurysms. J Neurosurg. 1994;80:650–658. doi: 10.3171/jns.1994.80.4.0650. [DOI] [PubMed] [Google Scholar]
- 33.Mattingly T., Kole M.K., Nicolle D., Boulton M., Pelz D., Lownie S.P. Visual outcomes for surgical treatment of large and giant carotid ophthalmic segment aneurysms: a case series utilizing retrograde suction decompression (the “Dallas technique”) J Neurosurg. 2013;118:937–946. doi: 10.3171/2013.2.JNS12735. [DOI] [PubMed] [Google Scholar]
- 34.Matas R. Traumatisms and traumatic aneurisms of the vertebral artery and their surgical treatment with the report of a cured case. Ann Surg. 1893;18:477–521. [PMC free article] [PubMed] [Google Scholar]
- 35.Sorteberg A., Bakke S.J., Boysen M., Sorteberg W. Angiographic balloon test occlusion and therapeutic sacrifice of major arteries to the brain. Neurosurgery. 2008;63:651–660. doi: 10.1227/01.NEU.0000325727.51405.D5. [DOI] [PubMed] [Google Scholar]
- 36.Hamada J., Kai Y., Morioka M., Yano S., Todaka T., Ushio Y. Multimodal treatment of ruptured dissecting aneurysms of the vertebral artery during the acute stage. J Neurosurg. 2003;99:960–966. doi: 10.3171/jns.2003.99.6.0960. [DOI] [PubMed] [Google Scholar]
- 37.Park W., Ahn J.S., Park J.C., Kwun B.D., Kim C.J. Occipital artery-posterior inferior cerebellar artery bypass for the treatment of aneurysms arising from the vertebral artery and its branches. World Neurosurg. 2014;82:714–721. doi: 10.1016/j.wneu.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 38.Chiriac A., Ion G., Faiyad Z., Poeată I. Microsurgical clipping of giant thrombosed aneurysm. Rom Neurosurg. 2017;31:146–151. [Google Scholar]
- 39.Mohit A.A., Sekhar L.N., Natarajan S.K., Britz G.W., Ghodke B. High-flow bypass grafts in the management of complex intracranial aneurysms. Neurosurgery. 2007;60(2 suppl 1):105–123. doi: 10.1227/01.NEU.0000249243.25429.EE. [DOI] [PubMed] [Google Scholar]
- 40.Pancucci G., Potts M.B., Rodríguez-Hernández A., Andrade H., Guo L., Lawton M.T. Rescue bypass for revascularization after ischemic complications in the treatment of giant or complex intracranial aneurysms. World Neurosurg. 2015;83:912–920. doi: 10.1016/j.wneu.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Lanterna L.A., Lunghi A., Brembilla C., Gritti P., Bernucci C. Extraintracranial bypass as a rescue therapy for symptomatic flow diverter thrombosis. Case Rep Neurol Med. 2015;2015:1–5. doi: 10.1155/2015/204387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lubicz B., Balériaux D., Lefranc F., Brotchi J., Bruneau M., Levivier M. Endovascular treatment of intracranial aneurysms as the first thérapeutic option. J Neuroradiol. 2007;34:250–259. doi: 10.1016/j.neurad.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Molyneux A.J., Kerr R.S., Yu L.-M. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 44.Rajah G., Narayanan S., Rangel-Castilla L. Update on flow diverters for the endovascular management of cerebral aneurysms. Neurosurg Focus. 2017;42:E2. doi: 10.3171/2017.3.FOCUS16427. [DOI] [PubMed] [Google Scholar]
- 45.Ferns S.P., van Rooij W.J., Sluzewski M., van den Berg R., Majoie C.B. Partially thrombosed intracranial aneurysms presenting with mass effect: long-term clinical and imaging follow-up after endovascular treatment. AJNR Am J Neuroradiol. 2010;31:1197–1205. doi: 10.3174/ajnr.A2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H.-W., Sun Z.-H., Wu C., Xue Z., Yu X.-G. Surgical management of recurrent aneurysms after coiling treatment. Br J Neurosurg. 2017;31:96–100. doi: 10.1080/02688697.2016.1226255. [DOI] [PubMed] [Google Scholar]
- 47.Charbel F.T., Meglio G., Amin-Hanjani S. Superficial temporal artery-to-middle cerebral artery bypass. Oper Neurosurg. 2005;56:186–190. doi: 10.1227/01.neu.0000144487.85531.fd. [DOI] [PubMed] [Google Scholar]
- 48.Choi H., Koh S.-H., Park B., Lee K.-Y., Lee Y.J. Giant aneurysm of the internal carotid artery. Arch Neurol. 2012;69:409. doi: 10.1001/archneurol.2011.1138. [DOI] [PubMed] [Google Scholar]
- 49.Capo G., Vescovi M., Toniato G., Petralia B., Gavrilovic V., Skrap M. Giant vertebral aneurysm: a case report detailing successful treatment with combined stenting and surgery. Surg Neurol Int. 2018;9:6. doi: 10.4103/sni.sni_170_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox B., Humphries W.E., Doss V.T., Hoit D., Elijovich L., Arthur A.S. Rupture of giant vertebrobasilar aneurysm following flow diversion: mechanical stretch as a potential mechanism for early aneurysm rupture. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-011325. bcr2014011325. [DOI] [PMC free article] [PubMed] [Google Scholar]