Abstract
Background
The success of endoscopic third ventriculostomy (ETV) depends on multiple preoperative and intraoperative factors. The multifactorial influence adds an element of unpredictability to the outcome of the most well-planned procedure. Clinical symptoms and signs may not reflect the status of the ETV stoma postprocedure and the morbidity associated with ETV failure could be catastrophic. In this study, the authors look at the role of early magnetic resonance imaging (MRI) to predict failure to avoid morbidity secondary to malfunction and propose a modified success criterion to guide treatment plan post-ETV failure. Our aim is to prospectively and retrospectively study the use of early postoperative MRI in predicting potential early ETV failure.
Methods
Patients who underwent ETV at Amrita Institute of Medical Sciences from March 2011 to August 2017. The study was a retrospective and prospective observational blinded study. Inclusion criteria included patients with any form of obstructive hydrocephalus who underwent ETV and had undergone an early postoperative MRI in the first 48 hours—or latest by discharge—with a follow-up of at least 3 months. The patient details and the presence of the flow void in the immediate postoperative MRI were documented. Follow-up was for at least 3 months to identify early failures.
Results
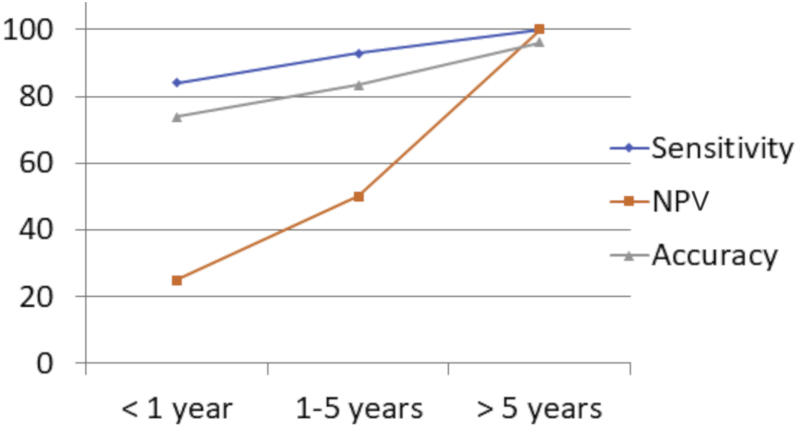
A total of 67 ETVs were performed in 65 patients. At 3 months follow-up, of the 59 ETVs with flow void on MRI, 53 were successful, whereas 6 failed. Of the 8 without flow void, 4 were successful. The overall sensitivity was 93.0, whereas specificity was 40.0. With increasing age, the negative predictive value improved from 25% in age <1 year to 100% in age >5 years, with an accuracy reaching 96% to predict failure of ETV.
Conclusions
An early postoperative MRI is very sensitive to failure of ETV, but not highly specific. The negative predictive value and accuracy of MRI improve significantly with increasing age and in non-hemorrhagic non-infective obstructive etiology.
Key words: Complications, Endoscopic third ventriculostomy, Flow void
Abbreviations and Acronyms: 3D, 3-Dimensional; CSF, Cerebrospinal fluid; ETV, Endoscopic third ventriculostomy; ETVSS, Endoscopic third ventriculostomy success score; FSE, Fast spin echo; MRI, Magnetic resonance imaging; NPV, Negative predictive value; PHH, Posthemorrhagic hydrocephalus; PIH, Postinfective hydrocephalus; PPV, Positive predictive value; TSE, Turbo spin echo; VP, Ventriculoperitoneal
Introduction
The ultimate success of endoscopic third ventriculostomy (ETV) depends on multiple preoperative and intraoperative factors. The multifactorial influence adds an element of unpredictability to the outcome of each of the procedure. Clinical symptoms and signs may not reflect the function of the ETV stoma in the early postoperative period. The morbidity associated with ETV failure could be catastrophic.
The role of magnetic resonance imaging (MRI) in assessing the patency of the ETV stoma is known. Identifying a decrease in ventricular dimension, reduction in third ventricle size to identify flow void at the stoma, cine phase-contrast MRI to detect the stoma flow, and invasive contrast studies to demonstrate the flow are some of the MRI features and techniques that can be used to predict stoma patency.
Failure occurs mostly in the first 3 months following ETV (maximum in the first 2 weeks) with decreasing frequency as time passes. Therefore, follow-up radiologic criteria on children following this procedure are therefore of paramount importance.
In this article, we analyze our protocol of performing an ultra-early postoperative MRI to identify the ETV stoma with the following goals: 1) To identify the potential failure of ETV in the early postoperative period and to take precautionary steps as necessary; and 2) to tailor a strategy in the event of ETV failure.
Aim
To retrospectively and prospectively study the use of early postoperative MRI in predicting potential early ETV failure.
Materials and Methods
The study included patients who underwent ETV at Amrita Institute of Medical Sciences from March 2011 to August 2017. The design of the study was a prospective and retrospective observational study.
Inclusion criteria included: 1) any form of obstructive hydrocephalus in which ETV was the mode of treatment; 2) all patients who had an early postoperative MRI performed 24–48 hours—or latest by discharge. Our MRI protocol, viz. “hydrocephalus protocol,” to demonstrate a flow void was a focused MRI protocol performed using either 1.5T GE HDxT MRI (GE company, Boston, Massachusetts, USA) or by the GE 3T Discovery 750W system (GE company). Axial and sagittal fast spin echo (FSE) T2 sequences performed with 4 mm slice thickness and 0.5 mm gap, and 3-dimensional (3D) Fast Imaging Employing Steady-state Acquisition (FIESTA) sequence with 1 mm thickness taken at midsagittal sections; and 3) the patients should have a necessary follow-up of at least 3 months. The follow-up was to interpret the fate of the procedure.
Of the study cohort, 40 patients (n = 40) were included in the prospective group, and another group of patients (n = 25) were included after retrospective analyzing of the database, and who satisfied the inclusion criteria correlated with outcome blinded interpretation of the patient MRI by a neuroradiologist.
For the study purpose, early failure of ETV was defined as: 1) Any adverse event post-ETV that is directly attributable to its functioning, for example cerebrospinal fluid (CSF) leak, altered sensorium or progressive macrocephaly leading to shunt surgery or re-ETV; and 2) the event should occur within 3 months of the primary ETV.
On the prospective arm (n = 40), a patient who had ETV underwent postoperative MRI according to the protocol. The data prospectively collected included the demographic data, that is, age, sex, diagnosis and management details. The clinical features and intraoperative findings were documented. The presence or absence of the flow void in the postoperative MRI was documented by an independent assessee. The data were then correlated with the clinical outcome on follow-up.
In the retrospective arm (n = 25), patients who satisfied the inclusion criteria were chosen, and the MRI was interpreted by the blinded assessor and subsequently correlated with the outcome as defined for the study.
The images were analyzed for the presence or absence of flow void at the level of the stoma in both sagittal and axial planes by an independent assessor, blinded to the outcome in the retrospective arm. For the study purpose, outcome follow-up was for 3 months to identify early failures, as we had defined earlier.
Statistical analysis was performed using IBM SPSS software version 20.0 (IBM Corp., Armonk, New York, USA) and Dx test software (MedCalc, Ostend, Belgium). The results are given in mean ± SD for all the continuous variables and frequency (percentage) for categorical variables. The McNemar χ2 test was used to test the statistically significant difference in the postoperative flow void and success of ETV. Diagnostic measures such as sensitivity, specificity, predictive values, and accuracy were calculated for the postoperative imaging.
Results
A total of 67 ETVs were performed in 65 patients. The age ranged from 4 months to 20 years. Twenty-three ETVs were performed in children aged <1 year, 18 ETVs were performed in the 1–5 year age group, and 26 ETVs were performed in the >5 years of age category. The etiology was postinfective hydrocephalus (PIH)/posthemorrhagic hydrocephalus (PHH) in 13 patients, and 54 patients had nonhemorrhagic, noninfective, obstructive etiology (Table 1).
Table 1.
Summary of the Patients Who Underwent Endoscopic Third Ventriculostomy and Magnetic Resonance Imaging Results
| Age Group (Years) | Number of Patients | Etiology PHH or PIH/Obstructive | Success (False Negative)∗ | Failure (False Positive)† | Flow Void Present | Flow Void Absent |
|---|---|---|---|---|---|---|
| Group A <1 | 23 | 4/19 | 19 (3) | 4 (3) | 19 | 4 |
| Group B 1–5 | 18 | 5/13 | 14 (1) | 4 (2) | 15 | 3 |
| Group C >5 | 26 | 4/22 | 24 (0) | 2 (1) | 25 | 1 |
PHH, posthemorrhagic hydrocephalus; PIH, postinfective hydrocephalus.
False negative refers to absent flow void, but the patient had a successful clinical outcome.
False positive refers to the presence of flow void, but patient required a repeat cerebrospinal fluid diversion procedure (repeat endoscopic third ventriculostomy or shunt).
Of the 67 ETVs (n = 65 patients), 59 procedures had a flow void at the level of the stoma, whereas 8 did not show a flow void. Of the 59 ETV with flow void on MRI, 53 were successful at 3 months, whereas 6 procedures failed. Of the 8 procedures without a flow void, 4 were successful, whereas 4 failed by 3 months. The overall sensitivity to detect the stoma was 93.0%, whereas specificity was 40.0%. The overall positive predictive value (PPV) was 89.9%, and the negative predictive value (NPV) was 50%.
In the age group <1 year, the sensitivity of postoperative MRI to predict successful outcome was 84.0%, whereas specificity was 25.0%. The overall PPV was 84.0%, and the NPV was 25.0%.
In the age group 1–5 years, the sensitivity of postoperative MRI to predict successful outcome was 92.9%, whereas specificity was 50.0%. The overall PPV was 92.9%, and the NPV was 50%.
In the age group >5 years of age, the overall sensitivity of postoperative MRI to predict successful outcome was 100%, whereas specificity was 50.0%. The total PPV was 96.0%, and the NPV was 100% (Table 2).
Table 2.
Analysis of the Endoscopic Third Ventriculostomy Outcome Data (Age Wise) Concerning Magnetic Resonance Imaging Correlation
| Group and Age Range (Years) | Successful Outcome | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|
| A) 0–1 | 16/23 (69.6%) | 84% | 25 | 84 | 25 | 73.91% |
| B) 1–5 | 13/18 (72.2%) | 92.9% | 50 | 92.9 | 50 | 83.33% |
| C) >5 | 24/26 (92.3%) | 100% | 50 | 96 | 100 | 96.15% |
PPV, positive predictive value; NPV, negative predictive value.
The analysis of ETV outcome was done with respect to various aetiologies. For the cohort of children with PHH/PIH as etiology, the sensitivity of MRI to predict failure was 88.9%, whereas specificity was 50.0%. The overall PPV was 80.0%, and the NPV was 66.7%. With non-infective/non-hemorrhagic obstructive hydrocephalus (included other etiologies such as aqueductal stenosis, posterior fossa tumors, fourth ventricular outlet obstruction of non-infective/non-hemorrhagic origin, etc.), the sensitivity improved to 93.8%, whereas specificity was 33.3.0%. The overall PPV was 91.8%, and the NPV was 40% (Table 3).
Table 3.
Analysis of the Endoscopic Third Ventriculostomy Outcome Data (According to the Etiology) Concerning Magnetic Resonance Imaging Correlation
| Diagnosis | Successful Outcome | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|
| PIH/PHH | 8/13 (61.5%) | 88.9 | 50 | 80 | 66.7 | 76.92% |
| Obstructive | 45/54 (83.3%) | 93.8 | 33.3 | 91.8 | 40 | 87.3% |
PIH, postinfective hydrocephalus; PHH, posthemorrhagic hydrocephalus.
Discussion
The success of ETV is highly variable and exact outcome unpredictable in a best-decided scenario despite complemented by a precise technique. The most dreadful postoperative complications related to failure or malfunction are CSF leak and hydrocephalus.1 CSF leak can lead to meningitis and is related morbidity.
Early clinical detection of ETV failures are difficult, especially in infants in which the only change might be a gradual but abnormally increasing trend of head circumference. However, patients may show signs of failure despite a patent ETV stoma. Hydrocephalus secondary to malfunction can be damaging in the short- and long-term. Predicting failure can be delayed until critical stages, especially in the very young.2 Therefore, precisely predicting failure, especially noninvasively, is a desirable goal.
Failure occurs mostly in the first 3 months following the procedure (maximum in the first 2 weeks) with decreasing frequency as time passes. The cumulative probability of a failure presenting itself during the first 16 days after ETV was found to be 90%.3, 4, 5, 6, 7, 8
Failure of ETV is owing to obstruction at the level of the stoma because of closure and is related to ostomy diameter, the formation of secondary arachnoid membranes, and hemorrhagic or infectious events, including blood clots or cellular debris. Functionally reduced CSF absorption, scarring of the fenestrated floor, or a tumor spreading are other causes of ETV failure.9, 10, 11, 12
The role of MRI as a non-invasive tool to assess the suitability of ETV for CSF diversion is well accepted. Various MRI motion-sensitive and cisternographic MRI techniques (spin echo, turbo spin echo (TSE), steady-state free precession, 3D constructive interference in the steady state, reverse fast imaging with steady-state precession, spatial modulation of magnetization, and cine phase contrast) have been applied. Three-dimensional constructive interference in steady-state or equivalents, TSE or FSE, and cine phase contrast have gained a place in evaluating the CSF flow and anatomy of the cisterns.13, 14 Heavily T2-weighted sequences (such as 3D constructive interference in steady-state) have been reported to be useful in evaluating CSF-containing and the integrity of the third ventricle walls, but these sequences provided no physiological information, and thus, are inadequate for diagnosis. Phase contrast MRI requires spaces technical expertise and is (relatively) time consuming requiring patient cooperation. Additionally, it requires experience, fails to provide morphological information, and may give poor results depending on the technical limitations of the technique used.13, 15, 16, 17, 18, 19
Some other invasive techniques (including contrast-material enhanced magnetic resonance cisternography and magnetic resonance ventriculography) are used in certain circumstances. The same methods can be used in follow-up after ETV, but it is evident that being invasive is not practical for routine use.
Various parameters have been analyzed as an indicator of successful ETV with a patent stoma; however, their use in practical application has not been evaluated enough to rely on confidently. Volume variations of the ventricles and the subarachnoid spaces are a good indicator of the efficacy of the ETV. Compared to extrathecal shunts, the changes in the ventricular volumes are more subtle and may be seen only in the minority20 after ETV. Additionally, these may be difficult to appreciate on 2-dimensional MRI,21 do not occur uniformly, may be delayed, and the rate depends on the preoperative status and chronicity.22 The ventricles may not return to normal size even after successful ETV; therefore, the predictive value of ventricular size is doubtful.9, 23
Protons in the motion cause signal loss, and are seen as “flow void” in a TSE sequence.13 Functional analysis of ETV stoma patency and the assessment of the procedural success by MRI can be demonstrated by flow void or flow signal in the floor of the third ventricle.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 24 The triage of patients with failed ETV solely depends on whether the stoma of ETV is functional or not, considering that the former requires shunt insertion, whereas the latter may be explored for redo ETV.6, 11 The method has been subject to both physiological and technical fallacies. The absence of flow void, despite the presence of flow at stoma, can be due to slow flow. Some strategies such as flow compensation and implementation of presaturation pulse have been introduced to suppress or enhance the flow-related artefact in the TSE sequence. Some operator-dependent sequence parameters such as time of echo, slice thickness, spatial resolution, receiver bandwidth, the number of average, and echo train length could affect the flow-related artefact.13 Section thickness and imaging planes are the most effective operator-dependent parameters of TSE sequences, as the thinner the slice, the more enhanced the visibility of the flow void.13 Standard axial 5-mm TSE T2-weighted image is an exception, with high sensitivity to flow void. The perpendicularity could explain this between the flow direction and the imaging plane. The axial imaging plane also enhances every kind of flow-related artefact, including turbulence, because of obstruction in the cisterns, as well as flow through the fenestration resulting in a decreased specificity. Even in cases deemed clinical failures, sometimes patency of the ETV site has been demonstrated. This has been reported with an incidence as high as 50% in some series.20, 25, 26
Our MRI hydrocephalus protocol to demonstrate a flow void consists of using axial and sagittal FSE T2 sequences with 4 mm slice thickness and 0.5 mm gap combined with 3D FIESTA sequence of 1 mm thickness taken at midsagittal sections. All 3 sequences together require <10 minutes of scan time. Moreover, this protocol can be performed with ease in children who are asleep and occasionally with short sedation. We opine combining a sagittal image with the axial image can obviate fallacy associated with axial imaging (i.e., flow void in basal cisterns).
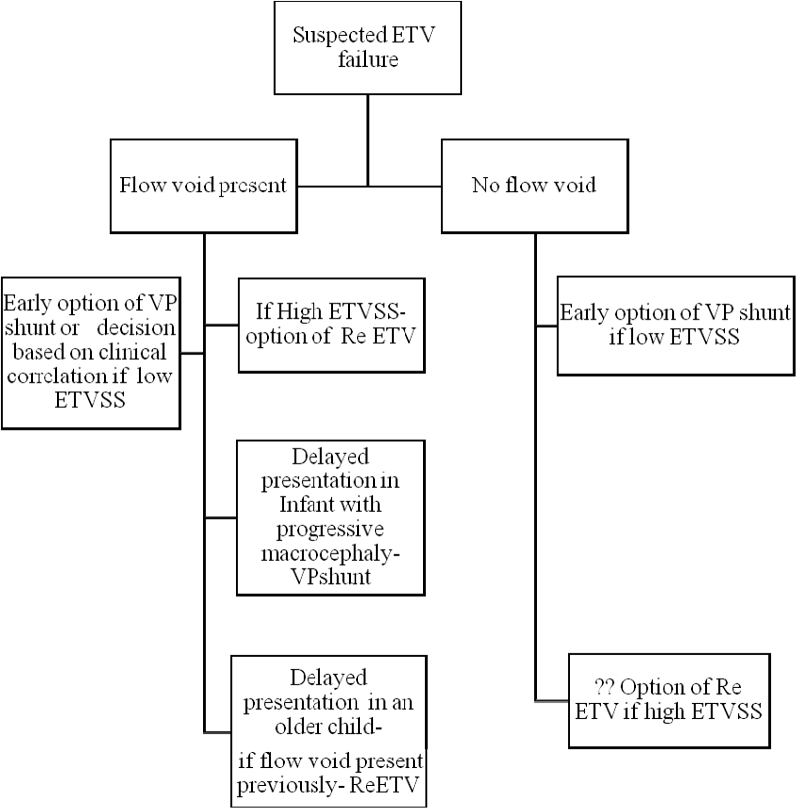
Our protocol of performing an early postoperative MRI has been used to demonstrate the ETV functioning and can chart a treatment strategy in the event of an adverse event (Figure 1).
-
•
If an adverse event occurs in a patient in which the presence of flow void was previously documented in a postoperative MRI and if the patient has high success criteria (endoscopic third ventriculostomy success score [ETVSS]), this may support a conservative strategy.27, 28 The conservative strategy may be in the form of lumbar drainage,29 if it occurs in the early postoperative period, or an option of repeat ETV if it occurs later in follow-up.
-
•
In the absence of a flow void and low success criteria (ETVSS), early resort to ventriculoperitoneal (VP) shunt has merit.
-
•
Late failures accompanied by the absence of flow void can have an option of revision of ETV, especially if flow void was present previously.
-
•
An infant is an exception in that even late failures may suggest a failure of ETV and may still require resort to VP shunt.
-
•
Low ETV success criteria (ETVSS) but with a presence of flow void may present a clinical dilemma in the event of ETV failure and may have to be resolved on the basis of clinical instincts.
Figure 1.
A flow chart depicting the recommended treatment strategy in the event of an early suspected endoscopic third ventriculostomy failure. We recommend the following treatment strategy on the basis of the presence or absence of flow void and the preoperative endoscopic third ventriculostomy success score (success criteria). ETV, endoscopic third ventriculostomy; ETVSS, endoscopic third ventriculostomy success score; VP, ventriculoperitoneal.
Figure 1 is a flow chart depicting the recommended treatment strategy in the event of an early suspected ETV failure. We recommend the following treatment strategy on the basis of the presence or absence of flow void and the preoperative ETVSS score (success criteria).
It is routine practice to do follow-up imaging after ETV. The protocols are varying and dependent on the unit and logistics, only that we do an early MRI as protocol to identify the stoma. We did not find any study in the literature analyzing the role of early imaging post-ETV.
In group A (groups defined in Table 1), the sensitivity of MRI to predict successful outcome was 84.0%, whereas specificity was 25.0%. The overall PPV was 84.0%, and the NPV was 25.0%. ETV has shown successful outcome varying between 56% and 89% in infants, with an increase in children aged >2 years.25 In this subgroup, with low specificity, low NPV, and low ETVSS we propose VP shunt at the first instance of failure. Additionally, morbidity in this subgroup has a greater implication; therefore, an early resolution is merited. Presence of flow void in MRI may differentiate patients (especially infants i.e., group A) who may “enjoy” a temporary lumbar CSF diversion and convert a “failure” to a “success.”
In group B, the sensitivity of postoperative MRI to predict successful outcome was 92.9%, whereas specificity was 50.0%. The overall PPV was 92.9%, and the NPV was 50%. With the NPV being 50%, we propose the option of being conservative in the presence of flow void and if the criteria for ETV success is high. In the absence of flow void and low ETVSS, VP shunt may be indicated.
We would like to highlight that 3 infants and 1 patient in group B did not have flow void but had successful clinical parameters and they remained successful at longer follow-up (reflected in the low specificity and PPV).
We chose to follow them more closely. Being infants (n = 3, only 1 was a non-infant) sudden death was not anticipated. Additionally, the presence or absence of flow void has multifactorial influence, infant physiology being 1 of them.
In group C, the overall sensitivity of postoperative MRI to predict outcome was 100%, whereas specificity was 50.0%. The overall PPV was 96.0, and the NPV was 100. In this subgroup, considering that the sensitivity and the NPV is 100%, we propose conservative management in all situations other than in the absence of flow void.
Success rates can also differ depending on the type of underlying pathology (Figure 2, Figure 3). In idiopathic aqueductal stenosis, which is considered an ideal indication for ETV, success rate, as mentioned in literature, starts from 40% in children aged <2 years and 71% in children >2 years.30 In children with PHH, success rates decrease to 55.6%–71.4%.12, 31, 32 Our analysis carried over the cohort of children with PHH/PIH, the sensitivity of MRI to predict failure was 88.9%, whereas its specificity was 50.0. The overall NPV was 66.7%. Although with non-infective/non-hemorrhagic obstructive hydrocephalus, the sensitivity improved to 93.8%, whereas specificity was 33.3%. The NPV for this cohort was 40% (Table 3).
Figure 2.
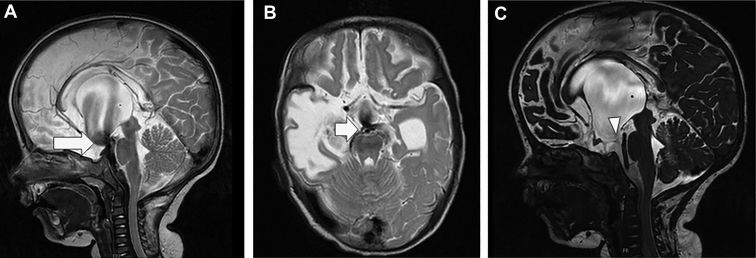
An infant who underwent endoscopic third ventriculostomy for obstructive hydrocephalus, with postoperative magnetic resonance imaging showing intense flow void (arrows in A and B) in the sagittal and axial images (A and B) and Sag CISS (C), The arrow head shows the defect of the floor with a subtle flow void. Sag CISS, sagittal CISS.
Figure 3.
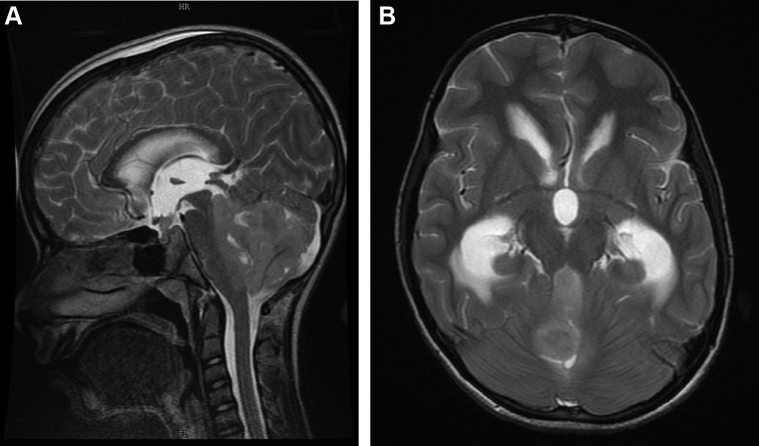
An 11-year-old boy with posterior fossa SOL and leptomeningeal disease with his sagittal magnetic resonance imaging (A) and axial images (B) showing lack of flow void postendoscopic third ventriculostomy. His contrast magnetic resonance imaging identified the leptomeningeal disease. He had to undergo an early ventriculoperitoneal shunt when he presented with signs of raised intracranial pressure. SOL, space occupying lesion.
Other advantages of our protocol to perform an immediate postoperative MRI after ETV are: 1) The technique is useful in as early as the first postoperative day when the assessment of the size of the ventricles or periventricular ooze may be flawed; 2) it is non-invasive and can be done with minimal or no sedation, especially when the subjects are children; 3) the accuracy is very high in specific subgroups, that is, non-hemorrhagic/noninfective obstructive hydrocephalus and in others good enough to guide management protocols when combined with ETV success criteria; and 4) the protocol does not have economic implications as imaging is a part of follow-up of most units that perform ETV, only that we insist and incorporate the decision early in the timeline.
A repeat MRI close to the adverse event may add objective support for the event showing lack of flow void.
Fallacies and Limitations
Our study of using MRI to predict early failure of ETV and guide management has some limitations and fallacies: 1) the interpretation of the presence of flow void on a technically appropriately performed MRI is observer-dependent and occasionally be variably interpreted. Therefore, we suggest the sagittal images should be combined with axial image corroborated by clinical follow-up, or, if in doubt, in case of an event, a repeat imaging or an additional technique like the phase contrast flow study. This makes it more sensitive and avoids false-positive results because of misinterpretation of axial image flow void owing to cisternal flow; and 2) in infants, the failure of ETV can become clinically obvious in a delayed manner, probably attributable to their immature physiology. Additionally, in infants the functioning may not be apparent despite a patent stoma. We have had a low threshold in using VP shunt in this subgroup of patients in case of doubt, especially with low success score.
As the success of ETV improves, proportionately the sensitivity of an early MRI to identify the flow at the stoma improves (Figure 4). This trend is probably a functional attribute, explained by the equally robust flow across the stoma in a successful ETV.
Figure 4.
The trend of the demonstration of flow void regarding age and the sensitivity, negative predictive value, and accuracy to predict endoscopic third ventriculostomy failure. NPV, negative predictive value.
We realize that the results, interpretation of ultra-early MRI presence or absence of flow void and its applicability to management protocol is complex, but the goal is to identify a non-cumbersome and non-invasive imaging modality to anticipate morbidity and have a treatment plan in the event of morbidity. Because MRI are performed shortly after the ETV (before failure is obvious), they potentially reflect the “functioning” of an ETV not only the technical part (that is clear from the intraoperative observations).
Conclusions
An early postoperative MRI is very sensitive to failure of ETV but not highly specific. MRI demonstration of flow void at the stoma can influence the strategy in case of an adverse event. The NPV and accuracy of MRI improve significantly with increasing age and in non-hemorrhagic/non-infective obstructive etiology.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Bouras T., Sgouros S. Complications of endoscopic third ventriculostomy: a systematic review. Acta Neurochir Suppl. 2012;113:149–153. doi: 10.1007/978-3-7091-0923-6_30. [DOI] [PubMed] [Google Scholar]
- 2.Fischbein N.J., Ciricillo S.F., Barr R.M. Endoscopic third ventriculocisternostomy: MR assessment of patency with 2-D cine phase-contrast versus T2-weighted fast spin echo technique. Pediatr Neurosurg. 1998;28:70–78. doi: 10.1159/000028624. [DOI] [PubMed] [Google Scholar]
- 3.Feng H., Huang G., Liao X. Endoscopic third ventriculostomy in the management of obstructive hydrocephalus: an outcome analysis. J Neurosurg. 2004;100:626–633. doi: 10.3171/jns.2004.100.4.0626. [DOI] [PubMed] [Google Scholar]
- 4.Fritsch M.J., Kienke S., Ankermann T., Padoin M., Mehdorn H.M. Endoscopic third ventriculostomy in infants. J Neurosurg. 2005;103:50–53. doi: 10.3171/ped.2005.103.1.0050. [DOI] [PubMed] [Google Scholar]
- 5.Dusick J.R., McArthur D.L., Bergsneider M. Success and complication rates of endoscopic third ventriculostomy for adult hydrocephalus: a series of 108 patients. Surg Neurol. 2008;69:5–15. doi: 10.1016/j.surneu.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Jenkinson M.D., Hayhurst C., Al-Jumaily M., Kandasamy J., Clark S., Mallucci C.L. The role of endoscopic third ventriculostomy in adult patients with hydrocephalus. J Neurosurg. 2009;110:861–866. doi: 10.3171/2008.10.JNS17667. [DOI] [PubMed] [Google Scholar]
- 7.Navarro R., Gil-Parra R., Reitman A.J., Olavarria G., Grant J.A., Tomita T. Endoscopic third ventriculostomy in children: early and late complications and their avoidance. Childs Nerv Syst. 2006;22:506–513. doi: 10.1007/s00381-005-0031-1. [DOI] [PubMed] [Google Scholar]
- 8.Warf B.C., Campbell J.W. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment of hydrocephalus for infants with myelomeningocele: long-term results of a prospective intent-to-treat study in 115 East African infants. J Neurosurg Pediatr. 2008;2:310–316. doi: 10.3171/PED.2008.2.11.310. [DOI] [PubMed] [Google Scholar]
- 9.Hellwig D., Giordano M., Kappus C. Redo third ventriculostomy. World Neurosurg. 2013;79(2 suppl):S22.e13–S22.e20. doi: 10.1016/j.wneu.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Fukuhara T., Luciano M.G., Kowalski R.J. Clinical features of third ventriculostomy failures classified by fenestration patency. Surg Neurol. 2002;58:102–110. doi: 10.1016/s0090-3019(02)00773-5. [DOI] [PubMed] [Google Scholar]
- 11.Mahapatra A., Mehr S., Singh D., Tandon M., Ganjoo P., Singh H. Ostomy closure and the role of repeat endoscopic third ventriculostomy (re-ETV) in failed ETV procedures. Neurol India. 2011;59:867–873. doi: 10.4103/0028-3886.91367. [DOI] [PubMed] [Google Scholar]
- 12.Siomin V., Weiner H., Wisoff J. Repeat endoscopic third ventriculostomy: is it worth trying? Childs Nerv Syst. 2001;17:551–555. doi: 10.1007/s003810100475. [DOI] [PubMed] [Google Scholar]
- 13.Dincer A., Yildiz E., Kohan S., Memet Ozek M. Analysis of endoscopic third ventriculostomy patency by MRI: value of different pulse sequences, the sequence parameters, and the imaging planes for investigation of flow void. Childs Nerv Syst. 2011;27:127–135. doi: 10.1007/s00381-010-1219-6. [DOI] [PubMed] [Google Scholar]
- 14.Lev S., Bhadelia R.A., Estin D., Heilman C.B., Wolpert S.M. Functional analysis of third ventriculostomy patency with phase-contrast MRI velocity measurements. Neuroradiology. 1997;39:175–179. doi: 10.1007/s002340050387. [DOI] [PubMed] [Google Scholar]
- 15.Algin O. Role of complex hydrocephalus in unsuccessful endoscopic third ventriculostomy. Childs Nerv Syst. 2010;26:3–4. doi: 10.1007/s00381-009-1001-9. [author reply: 5-6] [DOI] [PubMed] [Google Scholar]
- 16.Algin O., Hakyemez B., Parlak M. Phase-contrast MRI and 3D-CISS versus contrast-enhanced MR cisternography for the detection of spontaneous third ventriculostomy. J Neuroradiol. 2011;38:98–104. doi: 10.1016/j.neurad.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Algin O., Ucar M., Ozmen E. Assessment of third ventriculostomy patency with the 3D-SPACE technique: a preliminary multicenter research study. J Neurosurg. 2015;122:1347–1355. doi: 10.3171/2014.10.JNS14298. [DOI] [PubMed] [Google Scholar]
- 18.Bargallo N., Olondo L., Garcia A.I., Capurro S., Caral L., Rumia J. Functional analysis of third ventriculostomy patency by quantification of CSF stroke volume by using cine phase-contrast MR imaging. AJNR Am J Neuroradiol. 2005;26:2514–2521. [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder H.W., Schweim C., Schweim K.H., Gaab M.R. Analysis of aqueductal cerebrospinal fluid flow after endoscopic aqueductoplasty by using cine phase-contrast magnetic resonance imaging. J Neurosurg. 2000;93:237–244. doi: 10.3171/jns.2000.93.2.0237. [DOI] [PubMed] [Google Scholar]
- 20.Goumnerova L.C., Frim D.M. Treatment of hydrocephalus with third ventriculocisternostomy: outcome and CSF flow patterns. Pediatr Neurosurg. 1997;27:149–152. doi: 10.1159/000121242. [DOI] [PubMed] [Google Scholar]
- 21.Di Rocco F., Grevent D., Drake J.M. Changes in intracranial CSF distribution after ETV. Childs Nerv Syst. 2012;28:997–1002. doi: 10.1007/s00381-012-1752-6. [DOI] [PubMed] [Google Scholar]
- 22.Preul C., Tittgemeyer M., Lindner D., Trantakis C., Meixensberger J. Quantitative assessment of parenchymal and ventricular readjustment to intracranial pressure relief. AJNR Am J Neuroradiol. 2004;25:377–381. [PMC free article] [PubMed] [Google Scholar]
- 23.Kulkarni A.V., Drake J.M., Armstrong D.C., Dirks P.B. Imaging correlates of successful endoscopic third ventriculostomy. J Neurosurg. 2000;92:915–919. doi: 10.3171/jns.2000.92.6.0915. [DOI] [PubMed] [Google Scholar]
- 24.Wilcock D.J., Jaspan T., Worthington B.S., Punt J. Neuro-endoscopic third ventriculostomy: evaluation with magnetic resonance imaging. Clin Radiol. 1997;52:50–54. doi: 10.1016/s0009-9260(97)80306-6. [DOI] [PubMed] [Google Scholar]
- 25.Cinalli G., Sainte-Rose C., Chumas P. Failure of third ventriculostomy in the treatment of aqueductal stenosis in children. Neurosurg Focus. 1999;6:e3. doi: 10.3171/foc.1999.6.4.6. [DOI] [PubMed] [Google Scholar]
- 26.Buxton N., Macarthur D., Mallucci C., Punt J., Vloeberghs M. Neuroendoscopic third ventriculostomy in patients less than 1 year old. Pediatr Neurosurg. 1998;29:73–76. doi: 10.1159/000028693. [DOI] [PubMed] [Google Scholar]
- 27.Kulkarni A.V., Drake J.M., Kestle J.R. Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J Neurosurg Pediatr. 2010;6:310–315. doi: 10.3171/2010.8.PEDS103. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni A.V., Riva-Cambrin J., Browd S.R. Use of the ETV Success Score to explain the variation in reported endoscopic third ventriculostomy success rates among published case series of childhood hydrocephalus. J Neurosurg Pediatr. 2011;7:143–146. doi: 10.3171/2010.11.PEDS10296. [DOI] [PubMed] [Google Scholar]
- 29.Ozisik P., Roth J., Beni-Adani L., Constantini S. Continuous spinal drain following endoscopic third ventriculostomy: a proposal to change the definition of failure. Childs Nerv Syst. 2011;27:1973–1978. doi: 10.1007/s00381-011-1562-2. [DOI] [PubMed] [Google Scholar]
- 30.Boschert J., Hellwig D., Krauss J.K. Endoscopic third ventriculostomy for shunt dysfunction in occlusive hydrocephalus: long-term follow up and review. J Neurosurg. 2003;98:1032–1039. doi: 10.3171/jns.2003.98.5.1032. [DOI] [PubMed] [Google Scholar]
- 31.Smyth M.D., Tubbs R.S., Wellons J.C., 3rd, Oakes W.J., Blount J.P., Grabb P.A. Endoscopic third ventriculostomy for hydrocephalus secondary to central nervous system infection or intraventricular hemorrhage in children. Pediatr Neurosurg. 2003;39:258–263. doi: 10.1159/000072871. [DOI] [PubMed] [Google Scholar]
- 32.Di Rocco C., Massimi L., Tamburrini G. Shunts vs endoscopic third ventriculostomy in infants: are there different types and/or rates of complications? A review. Childs Nerv Syst. 2006;22:1573–1589. doi: 10.1007/s00381-006-0194-4. [DOI] [PubMed] [Google Scholar]