Abstract
Accurate preoperative staging of gastric cancer and the assessment of tumor response to neoadjuvant treatment is of importance for treatment and prognosis. Current imaging techniques, mainly endoscopic ultrasonography (EUS), computed tomography (CT) and 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET), have their limitations. Historically, the role of magnetic resonance imaging (MRI) in gastric cancer has been limited, but with the continuous technical improvements, MRI has become a more potent imaging technique for gastrointestinal malignancies. The accuracy of MRI for T- and N-staging of gastric cancer is similar to EUS and CT, making MRI a suitable alternative to other imaging strategies. There is limited evidence on the performance of MRI for M-staging of gastric cancer specifically, but MRI is widely used for diagnosing liver metastases and shows potential for diagnosing peritoneal seeding. Recent pilot studies showed that treatment response assessment as well as detection of lymph node metastases and systemic disease might benefit from functional MRI (e.g. diffusion weighted imaging and dynamic contrast enhancement). Regarding treatment guidance, additional value of MRI might be expected from its role in better defining clinical target volumes and setup verification with MR-guided radiation treatment.
Gastric cancer is the fifth most common malignancy in the world, with nearly one million new cases of gastric cancer diagnosed every year.1 Curative treatment of gastric adenocarcinoma consists of partial or total resection of the stomach combined with lymphadenectomy.2 Over the last years, multimodality treatment strategies such as neoadjuvant chemo(radio)therapy, perioperative chemotherapy and adjuvant chemotherapy have gained importance in the treatment of gastric cancer by improving the likelihood of a radical tumor resection, disease free survival and overall survival.3–8 Unfortunately, the overall 5 year survival rate still remains poor (35–45%).4,9
Accurate staging of gastric cancer allows for selection of the most appropriate therapy, minimizes unnecessary surgery and maximizes the likelihood of benefit from the selected treatment. After initial diagnosis by gastroscopy with tumor biopsy, diagnostic work-up can consist of endoscopic ultrasonography (EUS), computed tomography (CT) and 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET). However, these techniques all have their limitations. EUS is an invasive, highly operator-dependent technique and does not detect distant metastases.10,11 CT exposes patients to ionizing radiation and has poor soft-tissue contrast. 18F-FDG PET is impaired by the fact that not all gastric carcinomas are 18F-FDG-avid (avidity ranging from 42–96%) and has a low spatial resolution.12
Historically, the role of magnetic resonance imaging (MRI) in gastric cancer has been limited, since relatively long acquisition times and technical challenges of peristaltic motion and respiration artifacts resulted in poor imaging quality.13,14 With the continuous technical improvements in MRI scanning, including fast imaging techniques, (respiratory) motion compensation techniques, use of anti peristaltic agents and the introduction of functional MRI features (such as diffusion-weighted imaging [DWI]), MRI has become a more potent imaging technique for gastrointestinal malignancies.15,16 However, MRI is generally associated with higher costs, longer examination times and a lower robustness compared to other staging modalities.17
Numerous studies have addressed the diagnostic performance of MRI in preoperative staging for gastric cancer in recent literature. However, due to heterogeneity among studies in applied methodology and reported outcomes, the role of MRI for gastric cancer remains controversial. The aim of this review article is to outline the value of different imaging techniques for preoperative staging and treatment response assessment in patients with gastric cancer, with an emphasis on the current role and future potential of MRI.
T- staging
Importance
Accurate assessment of local tumor invasion, or T-staging in the TNM classification system, is of importance to determine treatment and prognosis for individual patients. Understaging might lead to tumor-positive resection margins and futile surgical attempts. Overstaging, however, could impair optimal care when a potentially curable patient is incorrectly categorized as incurable.17 In addition, specific knowledge of potential serosal involvement provides useful information regarding patient prognosis.18–20 Finally, with the development of minimally invasive treatments for early gastric cancer (EGC), such as endoscopic mucosal resection, and the possibilities of neoadjuvant treatment, the necessity of a precise imaging tool to evaluate the tumor invasion depth preoperatively is increasing.21
Current imaging
Endoscopic ultrasound (EUS) is frequently applied for preoperative local staging of gastric cancer in patients without evidence of metastatic disease.22 EUS has the advantage that it can be combined with fine-needle aspiration (FNA) of suspected lymph nodes, but it remains highly operator dependent.23 In a systematic review including 23 EUS studies, diagnostic accuracy for overall T-staging in gastric cancer varied between 65 and 92.1%.22 A pooled meta-analysis including 22 articles confirmed these results, with a pooled accuracy for T-staging with EUS of 75% (95% confidence interval [CI]: 71–80%).24 Sensitivity and specificity for assessing serosal involvement varied between 77.8 and 100% and between 67.9 and 100%, respectively.22 These results are similar for a Cochrane meta-analysis including 50 studies (n = 4397), describing a sensitivity of 86% (95% CI: 81–90%) and specificity of 90% (95% CI: 87–93%) in differentiating between tumors with and without serosal invasion.25
CT is another commonly used technique to assess local tumor invasion. Advantages include short scanning times and visualization of both the thorax and abdomen at the same time. Yet, CT provides poor soft-tissue contrast, requires intravenous contrast material and adequate distention of the stomach for dedicated image evaluation, and is always accompanied by exposure to radiation.22 In terms of diagnostic accuracy in T-staging of gastric cancers, CT achieves similar results to EUS. The performance of CT for overall T-staging based on a review of the results of 6 studies found a diagnostic accuracy varying between 77.1 and 88.9%.22 Sensitivity and specificity for assessing serosal involvement varied between 82.8 and 100% and between 80 and 96.8%, respectively.22
A recent meta-analysis including eight studies (n = 1736) that compared EUS with CT in the same cohort, demonstrates equivalent sensitivity and specificity for T-staging for both modalities, with the exception of a significantly higher sensitivity for T1-staging for EUS (82% vs 41% for CT, p = 0.03).26
18F-FDG PET is currently not routinely indicated for evaluating the exact depth of tumor invasion, partly due to its low spatial resolution. Sensitivity rates for primary detection of gastric cancer using 18F-FDG PET varied between 58 and 94% among seven studies included in a review.27 Specificity ranged from 78–100% in this same review. Assessment of gastric cancer with 18F-FDG PET can be influenced by the absence of 18F-FDG-avidity of some gastric tumors, with percentages varying between 42–96% for 18F-FDG-avidity based on 18 studies included in a review.12 This variance is associated with several clinicopathologic parameters, such as tumor stage, size, location and subtype.12,27
MRI
MRI is a promising technique with high performance in depicting different gastric wall layers and differentiation of tumor tissue from fibrosis, as described in an ex-vivo study using 7.0T MRI.28 Gastric cancers appear as heterogeneous soft tissue masses on T 1 weighted (T1W) MR images and show either decreased or increased enhancement relative to the background stomach on dynamic contrast-enhanced (DCE-)MRI.29 Gastric linitis plastica tends to have a lower signal intensity than normal adjacent stomach tissue on T2W images due to its desmoplastic nature and enhances only modestly after intravenous gadolinium-based contrast. Furthermore, DCE-MRI can aid the identification of transmural spread, including peritoneal involvement.29 There is no worldwide consensus regarding the anatomical criteria that should be used to define tumor invasion on any imaging modality. The classification of the T-stages varies within the studies included in this review, and depends on the degree of enhancement of the tumor and different gastric wall layers.
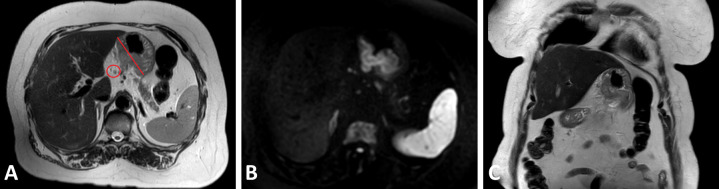
An illustration of a primary gastric tumor on T2W images, as well as on DW-MRI and DCE-MRI, can be found in Figures 1A, B, 2A and B, respectively.
Figure 1.
Axial T 2 weighted magnetic resonance images (A) and corresponding high signal on diffusion-weighted images (b = 800 s/mm²) (B) of the primary gastric tumor and pathologic lymph node (red marking), as well as a coronal T 2 weighted magnetic resonance image in one patient with cT3N1 gastric adenocarcinoma (C).
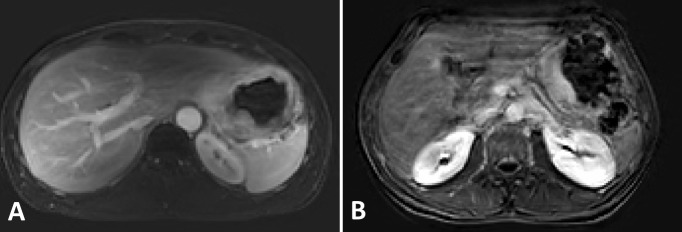
Figure 2.
Preoperative dynamic contrast enhanced (DCE) magnetic resonance images in axial plane approximately 1.25 min after intravenous contrast injection of two patients with gastric cancer (A and B). Figure B shows increased motion-related artifacts compared to Figure A.
In total, 18 prospective studies describing the diagnostic performance of MRI in determining tumor detection and stage are described in Table 1.30–47 Data on accuracy, sensitivity, specificity, predictive values, and over- and understaging are included. All studies used histopathology as reference standard.
The detectability of gastric cancer is strongly influenced by tumor size, T-stage, histologic subtype and enhancement pattern of the gastric wall.44 In one study, both anatomical MRI and DW-MRI were unable to locate the area of pathological tissue in all patients with pT1 tumors,35 another study reported detection in 16% (7/43) of pT1 tumors by anatomical MRI and 21% (7/43) by combined anatomical MRI and DW-MRI.48 This was similar for CT (16%, 7/43) in a direct comparison between both modalities in the latter study.48
The accuracy for correct assessment of T-stage varied from 64 to 88%.32,34–47 With the addition of DWI to anatomical MRI (T1W and T2W) in one study, an increase of 7% in the accuracy of T-staging was reported.35 The accuracy for overall T-staging was significantly better for T2W, DCE and DW-MRI combined, than for T2W with only one of the functional sequences (either DCE or DW-MRI).37 Regarding DCE-MRI, a significant correlation between the parameter Ve (extravascular extracellular volume fraction) and T-stage was found.49 The use of a positive oral contrast agent (gadopentetate dimeglumine) instead of water did not increase diagnostic accuracy of T-staging.45
The detectability of gastric cancers, as well as the correct assessment of T-stage, is likely to be influenced by knowledge of the gastroscopy results on tumor extent and location. In three studies, the readers were informed about the tumor location, which most likely increases the detectability of small and superficial gastric cancers.30,31,37 In two other studies, it was stated that the readers were blinded for clinical findings, however, it was unclear whether this includes gastroscopy results.41,45 All other included studies in Table 1 do no not report any information regarding blinding or non-blinding of the readers for gastroscopy results.
Table 1.
Overview of clinical studies assessing the diagnostic performance of MRI for T-staging in patients with gastric cancer
| Author | Year | n | Age(years), mean [range] |
Male
n (%) |
MR image acquisition | Definition of end point a | Overstaging, % (95% CI) | Under staging, % (95% CI) | Accuracy, % (95% CI) |
PPV, %
(95% CI) |
NPV, %
(95% CI) |
Sensitivity, % (95% CI) | Specificity,% (95% CI) |
| Arslan30 | 2017 | 51 | 61 [35-82] | 37 (73) | 1.5T, T2W, DWI (b-values 0, 400, 800) | Correct T2-stage | - | - | NA | 47 (NA) | 91 (NA) | 73 (NA) | 78 (NA) |
| Correct T3-stage | - | - | NA | 85 (NA) | 64 (NA) | 71 (NA) | 80 (NA) | ||||||
| Correct T4-stage | - | - | NA | 63 (NA) | 91 (NA) | 56 (NA) | 93 (NA) | ||||||
| Giganti,31 b | 2016 | 52 | 69 [43-85] | 33 (63) | 1.5T, T2W, DCE, DWI (b-values 0–600) | Local invasion (T1-3 versus T4a-b) |
- | - | 85 (75–94) | 76 (65–88) | 88 (80–97) | 76 (64–88) | 89 (80–97) |
| Liang32 | 2015 | 35 | 58 [38-75] | 21 (60) | 3.0T, DWI (b-value 800) | Correct T-stage | 11 (3–27) | 6 (1–19) | 83 (66–93) | - | - | - | - |
| Serosal invasion | - | - | 100 (90–100) | 100 (0–100) | 100 (90–100) | 100 (0–100) | 100 (90–100) | ||||||
| Early gastric cancer | - | - | 94 (81–99) | 75 (19–99) | 97 (83–100) | 75 (19–99) | 97 (83–100) | ||||||
| Joo33 | 2015 | 49 | 62 [38-81] | 33 (67) | 3.0T, T1W, T2W, DCE, DWI (b-values 0, 100, 500, 1000) | Serosal invasion | - | - | 77 (62–88) | 55 (23–83) | 83 (67–94) | 50 (21–79) | 86 (70–95) |
| Liu34 | 2014 | 45 | 63 [28-82] | 28 (62) | 3.0T, DWI (b-values 0, 1000) | Correct T-stage | 33 (20–49) | 2 (0–12) | 64 (50–78) | - | - | - | - |
| Serosal invasion | - | - | 93 (82–99) | 70 (35–93) | 100 (90–100) | 100 (59–100) | 92 (79–98) | ||||||
| Caivano35 | 2014 | 31 | 67 [29-84] | 18 (58) | 3.0T, T1W, T2W, DWI (b-values 0, 350, 750) | Correct T-stage | 7 (1–22) | 13 (4–30) | 80 (61–92) | - | - | - | - |
| Serosal invasion | - | - | 93 (78–99) | 100 (63–100) | 91 (71–99) | 80 (44–97) | 100 (83–100) | ||||||
| Huo36 | 2014 | 30 | 60 [50-69] | 19 (63) | 1.5T, T1W, T2W, DCE, DWI (b-values not reported) | Correct T-stage | 10 (2–27) | 10 (2–27) | 80 (61–92) | - | - | - | - |
| Early gastric cancer | - | - | 96 (82–100) | 75 (19–99) | 100 (86–100) | 100 (29–100) | 96 (80–100) | ||||||
| Liu37 | 2014 | 51 | 62 [28-82] | 33 (65) | 3.0T, T2W, DCE, DWI (b-values 0, 1000) | Correct T-stage | NA | NA | 88 (76–96) | - | - | - | - |
| Serosal invasion | - | - | 96 (87–100) | 98 (88–100) | 86 (42–100) | 98 (88–100) | 86 (42–100) | ||||||
| Early gastric cancer | - | - | 96 (87–100) | 100 (69–100) | 95 (83–99) | 83 (52–98) | 100 (91–100) | ||||||
| Lei38 | 2013 | 38 | 52 [31-82] | 26 (68) | 1.5T, T1W, T2W, DCE, DWI (b-value 600) | Correct T-stage | 21 (9–36) | 7 (2–21) | 71 (55–85) | - | - | - | - |
| Serosal invasion | - | - | 92 (79–98) | 67 (22–96) | 97 (84–100) | 80 (28–99) | 94 (80–99) | ||||||
| Early gastric cancer | - | - | 92 (79–98) | 100 (59–100) | 91 (75–98) | 70 (35–93) | 100 (88–100) | ||||||
| Anzidei39 | 2009 | 40 | 54 [NA] | 26 (65) | 1.5T, T2W | Correct T-stage | 0 (0–9) | 20 (9–36) | 80 (64–91) | - | - | - | - |
| Serosal invasion | - | - | 92 (81–99) | 100 (80–100) | 89 (67–99) | 89 (67–99) | 100 (80–100) | ||||||
| Early gastric cancer | - | - | 92 (81–99) | 67 (22–96) | 100 (88–100) | 100 (40–100) | 94 (79–99) | ||||||
| Zhong40 c | 2005 | 15 | 63 [46-81] | 9 (60) | 1.0T, T1W, T2W, MR hydrography | Correct T-stage | 14 (2–43) | 21 (5–51) | 64 (35–87) | ||||
| Serosal invasion | - | - | 86 (57–98) | 90 (56–100) | 75 (19–99) | 90 (56–100) | 75 (19–99) | ||||||
| Early gastric cancer | - | - | 100 (77–100) | 100 (0–100) | 100 (75–100) | 100 (0–100) | 100 (75–100) | ||||||
| Kim,41d | 2000 | 26 | 56 [23-77] | 17 (65) | 1.0T, T1W, T2W | Correct T-stage | 19 (7–39) | 0 (0–13) | 81 (61–93) | - | - | - | - |
| Serosal invasion | - | - | 81 (61–93) | 69 (41–89) | 100 (69–100) | 100 (72–100) | 67 (38–88) | ||||||
| Wang42 | 2000 | 30 | 51 [23-78] | 18 (60) | 1.5T, T2W | Correct T-stage | 6 (1–20) | 6 (1–20) | 88 (72–97) | - | - | - | - |
| Serosal invasion | - | - | 91 (76–98) | 96 (80–100) | 71 (29–96) | 93 (76–99) | 83 (36–100) | ||||||
| Early gastric cancer | - | - | 100 (89–100) | 100 (0–100) | 100 (89–100) | 100 (0–100) | 100 (89–100) | ||||||
| Sohn43 | 2000 | 30 | 55 [37-77] | 19 (63) | 1.5T, T1W, T2W | Correct T-stage | 7 (1–22) | 20 (8–39) | 73 (54–88) | - | - | - | - |
| Serosal invasion | - | - | 93 (78–99) | 100 (80–100) | 85 (55–98) | 89 (67–99) | 100 (72–100) | ||||||
| Early gastric cancer | - | - | 93 (78–99) | 88 (47–100) | 95 (77–100) | 88 (47–100) | 95 (77–100) | ||||||
| Kang44 | 2000 | 46 | 54 [26-69] | 34 (74) | 1.5T, T1W, DCE | Correct T-stage | 2 (0–11.5) | 15 (6–29) | 83 (69–92) | - | - | - | - |
| Serosal invasion | - | - | 93 (82–99) | 96 (82–100) | 88 (64–99) | 93 (77–99) | 94 (70–100) | ||||||
| Early gastric cancer | - | - | 96 (85–99) | 60 (15–95) | 100 (91–100) | 100 (29–100) | 95 (84–99) | ||||||
| Kim,45d | 2000 | 15 | 53 [24-75] | 10 (67) | 1.0T, T2W | Correct T-stage | 13 (2–41) | 0 (0–22) | 87 (60–98) | - | - | - | - |
| Oi,46d | 1997 | 37 | 60 [41-77] | 26 (70) | 1.5T, T1W, DCE | Correct T-stage | 11 (3–25) | 8 (2–22) | 81 (65–92) | - | - | - | - |
| Serosal invasion | - | - | 86 (71–95) | 90 (73–98) | 71 (29–96) | 93 (77–100) | 63 (25–91) | ||||||
| Matsushita,47 c | 1994 | 48 | 59 [27-78] | 35 (73) | 1.5T, T1W, DCE | Correct T-stage | 6 (1–17) | 6 (1–17) | 88 (75–95) | - | - | - | - |
| Serosal invasion | - | - | 92 (80–98) | 92 (78–98) | 92 (62–100) | 97 (85–100) | 79 (49–95) |
CI, confidence interval; DWI, diffusion weighted imaging; NA, not available based on the results reported by the study; NPV, negative predictive value; PPV, positive predictive value; T, Tesla; T1W, T 1 weighted MRI; T2W, T 2 weighted MRI; n, number of patients.
Note. All studies used histopathology as a reference standard.
Serosal invasion was defined as correct assessment of ≥T3 versus ≤T2 for studies that used T-staging according to the AJCC TNM staging sixth edition and ≥T4a versus ≤T3 for studies that used T-staging according to the AJCC TNM staging seventh edition. Early gastric cancer was defined as correct assessment of ≤T1 (versus advanced gastric cancer;≥T2).
Results are directly deduced from article, no raw data was reported so no 2-by-2 contingency tables could be reconstructed.
Study included 15 patients, with 1 patient having two gastric cancer lesions resulting in a total of 16 cases.
Only advanced gastric cancer cases (≥T2) were included in this study.
The reported percentages of over- and understaging of the local tumor extent with MRI ranged between 0–33% and 0–21% across study populations, respectively (Table 1). Correct diagnosis of invasion of adjacent structures on MRI varied from 40% (2/5) to 80% (8/10) in earlier studies, partly due to overlooking the invasion of the mesocolon, transverse colon and pancreas in some cases.43,44 In one of these studies, only 20% of tumors that invaded adjacent structures were correctly identified by CT.43
The accuracy of MRI for correct identification of serosal invasion varied between 77 and 100%, with positive predictive values (PPV), negative predictive values (NPV), sensitivities and specificities ranging from 67–100%, 71–100%, 50–100% and 63–100%, respectively.32–35,37–43,46,47
The performance of MRI in T-staging was directly compared with CT in three independent studies.31,41,43 Accuracies were generally higher for MRI, however this difference was only proven to be statistically significant in one of these studies (85% for MRI versus 83% for CT [p = 1.00],31 73% for MRI versus 67% for helical CT [p > 0.05]43 and 81% for MRI versus 73% for spiral CT [p < 0.05].41 In addition, the performance of MRI was also directly compared with EUS in one of these studies.31 Although not statistically significant, a lower accuracy was demonstrated for EUS (85% for MRI versus 71% for EUS, p = 0.12).31 Sensitivity rates of MRI and CT were significantly lower than of EUS (76% for MRI versus 94% for EUS [p = 0.08], and 65% for CT versus 94% for EUS [p = 0.02]). On the other hand, specificity rates were significantly higher for MRI and CT compared with EUS (89% for MRI versus 60% for EUS [p < 0.01], and 91% for CT versus 60% for EUS [p < 0.01]).31 The addition of either MRI or combined 18F-FDG PET/MRI to CT or EUS did not result in a significant increase in diagnostic performance.31,50 Integrating PET with MRI will most likely be clinically relevant in cases where the soft-tissue contrast of MRI outperforms that of CT.51
Overall, the accuracy of MRI is similar or slightly better when compared to the currently most frequently used imaging modalities (i.e. EUS and CT) in the evaluation of T-staging. However, its limited availably and higher costs would only make MRI an alternative imaging modality when CT is contraindicated or when CT results are ambiguous. Table 2 provides an overview of the imaging modalities and their indications, advantages and limitations in the management of gastric cancer.
Table 2. .
Imaging modalities and their indications, advantages and limitations in the management of gastric cancer
| Clinical indications | Advantages | Limitations | ||||
| T-staging | N-staging | M-staging | Treatment response assessment | |||
| CT | Primary staging modality | Primary staging modality | Primary staging modality | All are currently insufficient to justify changes in treatment decision making |
|
|
| EUS | Evaluation of possibility of endoscopic mucosal resection of early, node-negative, gastric cancer | Affirmation of N0 in patients with N0 as diagnosed by primary staging (CT) to guide neoadjuvant treatment decisions | None |
|
|
|
| 18F-FDG PET | None | Metabolic information improves diagnostic accuracy of CT | No conclusive evidence regarding additional diagnostic information over CT, EUS and laparoscopy |
|
|
|
| MRI | Comparable diagnostic performance to CT, so indicated when CT is contraindicated or when CT results are ambiguous | Comparable performance to CT, so only indicated when CT is contraindicated or when CT results are ambiguous | Limited evidence, but can aid characterization of small liver metastases (≤10 mm). |
|
|
|
DCE, dynamic contrast-enhanced; DWI, diffusion-weighted imaging; EUS, endoscopic ultrasound; 18F-FDG PET, 18F-fluorodeoxyglucose positron emission tomography; FNA, fine needle aspiration; MRI, magnetic resonance imaging.
N-staging
Importance
Preoperative assessment of lymph node involvement in patients with gastric cancer is of great importance for indicating prognosis,52–56 and selecting the appropriate treatment strategy, especially when assessing lymph node involvement outside the intended resection field.57,58 Accurate mapping of the anatomic location of positive lymph node metastases could lead to a focused extended lymphadenectomy, or to omission of surgery when the location of the lymph node metastases makes the lymph node metastases oncologically equivalent to distant metastases.59 In patients with N0 gastric cancer, the 5 year survival rate after surgical treatment is 86.1%, whereas in patients with N1, N2 and N3 gastric cancer, survival rates dramatically decrease to 58.1%, 23.3 and 5.9%, respectively.19 Moreover, lymph node metastases are an independent risk factor for gastric cancer recurrence in patients following curative resection.60 Therefore, adequate lymph node assessment is important to prevent understaging and subsequently determine eligibility for adjuvant therapy.61
Current imaging
Regional lymph node involvement is currently most frequently evaluated using EUS, CT and/or 18F-FDG PET/CT. The basic strategy for diagnosing metastatic lymph nodes on imaging is measuring size, of which no conclusive criteria exist. Using size criteria may induce false negative and false positive findings because pathological nodes are not always enlarged and lymph nodes can be enlarged due to inflammation instead of malignancy, respectively.62,63
The performance of EUS is not optimal for confirmation or exclusion of regional lymph node involvement: a recent Cochrane meta-analysis of 44 studies (n = 3573) showed a pooled sensitivity and specificity for EUS of 83% (95% CI: 79–87%) and 67% (95% CI: 61–72%), respectively.25 An advantage of EUS is that cytological material can be obtained via FNA.64 However, no studies directly comparing EUS with EUS-FNA within one cohort of gastric cancer patients are available thus far.
CT is a frequently used imaging modality to evaluate the presence of lymph node metastases in patients with gastric cancer. Sensitivity ranges from 62.5–91.9% (median 80.0%) and specificity ranges from 50.0–87.9% (median 77.8%) according to a systematic review including 10 studies.65 Since the detection of lymph node metastases on CT is anatomy-based, non-enlarged tumor-harboring lymph nodes and enlarged inflammatory nodes impair both sensitivity and specificity. Integrated 18F-FDG PET/CT provides better diagnostic accuracy for the detection of distant lymph node metastases through the addition of metabolic information. The high positive predictive value (>90%) makes 18F-FDG PET/CT useful when CT findings are equivocal, however, 18F-FDG PET is shown to have a relatively low sensitivity varying from 41 to 80% for diagnosis of nodal involvement.66–68
Another, not so frequently used technique for evaluating lymph node status is abdominal ultrasound. According to a recent systematic review and meta-analysis that evaluated six studies, the performance of abdominal ultrasound is rather disappointing, with reported sensitivities ranging between 12.2–80% (median 39.9%) and specificity of 56.3–100% (median 81.8%).65
MRI
An overview of the current literature on MRI with reported or calculated predictive values, sensitivity, specificity and accuracy for the assessment of nodal involvement is shown in Table 3.30–33,35,38,40,41,43,44,69–73 All 15 studies were prospective in nature and used histopathology as reference standard. As with other imaging modalities, size was the most frequently applied criterion on MRI to diagnose metastatic lymph nodes. The definition of pathologic lymph nodes on anatomical MRI varies from a short-axis diameter of >5 mm to >10 mm within the included studies. This definition will in turn influence sensitivity and specificity (i.e. a smaller threshold will increase sensitivity at the expense of specificity, and vice versa).59 On DWI, lymph nodes were generally considered metastatic when showing high signal intensity. An illustration of pathologic lymph nodes of gastric cancer on T2W images, as well as on DW-MRI, can be found in Figures 1A, B, 3A and B.
Table 3. .
Overview of clinical studies assessing the diagnostic performance of MRI for N-staging in patients with gastric cancer
| Author | Year | n | Age (years), mean[range] |
Male
n (%) |
MR image acquisition | Definition of pathologic lymph nodes a | Accuracy, % (95% CI) |
PPV, %
(95% CI) |
NPV, %
(95% CI) |
Sensitivity, % (95% CI) |
Specificity,
% (95% CI) |
|
| Differentiation between N0 and N+ (patient based values) | ||||||||||||
| Giganti,31 , b | 2016 | 52 | 69 [43-85] | 33 (63) | 1.5T, T2W, DCE, DWI (b-values 0–600) | Ø>6 mm and high SI on DWI | 71 (59–83) | 72 (60–84) | 70 (58–83) | 69 (57–82) | 73 (61–85) | |
| Liang32 | 2015 | 35 | 58 [38-75] | 21 (60) | 3.0T, DWI (b-value 800) | Not specified | 83 (66–93) | 94 (71–100) | 72 (47–90) | 76 (53–92) | 93 (66–100) | |
| Hasbahceci69 | 2015 | 23 | 59 [NR] | 11 (48) | 1.5T, T1W, T2W, DWI (b-values 50, 400, 800) | Ø≥5 mm and heterogenous SI, low ADC value (<1.1×10−3 mm2/s) | 65 (43–83) | 81 (54–96) | 29 (4–71) | 72 (47–90) | 40 (5–85) | |
| Joo33 | 2015 | 49 | 62 [38-81] | 33 (67) | 3.0T, T1W, T2W, DCE, DWI (b-values 0, 100, 500, 1000) | Ø≥8 mm or higher SI than muscle on DWI | 77 (62–88) | 79 (61–91) | 71 (42–92) | 87 (69–96) | 59 (33–82) | |
| Caivano35 | 2014 | 30 | 67 [29-84]c | 18 (58)c | 3.0T, T1W, T2W, DWI (b-values 0, 350, 750) | Ø>8 mm and signal characteristics like gastric neoplastic lesion | 100 (88–100) | 100 (85–100) | 100 (63–100) | 100 (85–100) | 100 (63–100) | |
| Lei38 | 2013 | 38 | 52 [31-82] | 26 (68) | 1.5T, T1W, T2W, DCE, DWI (b-value 600) | Perigastric Ø > 5 mm Distalis perigastric Ø > 6 mm |
85 (69–94) | 91 (71–99) | 77 (50–93) | 83 (63–95) | 87 (60–98) | |
| Kang44 | 2000 | 46 | 54 [26-69] | 34 (74) | 1.5T, T1W, DCE | Regional LN >8 mm or enhanced | 83 (69–92) | 91 (75–98) | 64 (35–87) | 85 (69–95) | 75 (43–95) | |
| Kim41 | 2000 | 26 | 56 [23-77] | 17 (65) | 1.0T, T1W, T2W | Ø>8 mm | 81 (61–93) | 100 (72–100) | 67 (38–88) | 69 (41–89) | 100 (69–100) | |
| Differentiation between N-stages (N0, N1, N2 or N3) | ||||||||||||
| Arslan,30 , b | 2017 | 51 | 61 [35-82] | 37 (73) | 1.5T, T2W, DWI (b-values 0, 400, 800) |
Ø≥8 mm or higher SI than muscle on DWI | N1 | NA | 60 (NA) | 92 (NA) | 75 (NA) | 85 (NA) |
| N2 | NA | 82 (NA) | 74 (NA) | 79 (NA) | 77 (NA) | |||||||
| N3 | NA | 86 (NA) | 91 (NA) | 60 (NA) | 98 (NA) | |||||||
| Zhong40 | 2005 | 15 | 63 [46-81] | 9 (60) | 1.0T, T1W, T2W, MR hydrography |
Ø>8 mm | 57 (29–82) | NR | NR | NR | NR | |
| Sohn43 | 2000 | 30 | 55 [37-77] | 19 (63) | 1.5T, T1W, T2W | Ø>8 mm | 55 (36–74) | NR | NR | NR | NR | |
| Differentiation between positive or negative individual lymph nodes (lymph node based values) | ||||||||||||
| Zhong70 , b | 2016 | 82 | 52 [24-77] | 65 (79) | 3.0T, T2W, DWI (b-values 0, 1000) | Ø>5 mm and/or central necrosis and/or indistinct border | NA | 64 (NA) | 79 (NA) | 73 (NA) | 70 (NA) | |
| iso-high/high signal intensity in DWI and iso-low/ low signal intensity on ADC map | NA | 65 (NA) | 81 (NA) | 77 (NA) | 70 (NA) | |||||||
| Cheng71 | 2013 | 28 | 64 [43-81] | 22 (79) | 1.5T, T1W, T2W, DCE, DWI (b-values 0, 400, 800) | Border irregularity T2W | 53 (41–65) | 57 (39–74) | 46 (31–63) | 48 (32–64) | 56 (38–73) | |
| Ø≥6 mm on conventional MR images | NA | 66 (NA) | 62 (NA) | 74 (NA) | 53 (NA) | |||||||
| Enhancement pattern (contrast-enhanced T1W) | 72 (61–82) | 89 (71–98) | 63 (48–77) | 57 (41–72) | 91 (76–98) | |||||||
| Size, border regularity and enhancement pattern | NA | 91 (NA) | 63 (NA) | 56 (NA) | 94 (NA) | |||||||
| ADC value (<1.39×10−3 mm2/s) | NA | 84 (NA) | 82 (NA) | 86 (NA) | 79 (NA) | |||||||
| ADC, size, border irregularity and enhanced pattern | NA | 88 (NA) | 77 (NA) | 78 (NA) | 89 (NA) | |||||||
| Tokuhara72 | 2008 | 31 | 68 [51-84] | 29 (94) | 1.5T, T2W, UPSIO | USPIO enhancement pattern d | 93 (91–95) | 76 (68–83) | 99 (97–100) | 96 (90–99) | 93 (90–95) | |
| Tatsumi73 | 2006 | 17 | 59 [52-75] | 14 (82) | 1.5T, T2W, USPIO | USPIO enhancement pattern d | 95 (91–98) | 86 (75–93) | 100 (97–100) | 100 (94–100) | 93 (87–96) | |
Ø, Short-axis diameter of lymph node considered metastatic; CI, confidence interval; DCE, dynamic contrast-enhanced; DWI, diffusion-weighted imaging; MR, magnetic resonance; NA, not available based on the results reported by the study; NPV, negative predictive value; NR, not reported; PPV, positive predictive value; SI, signal intensity; T, Tesla; T1W, T 1-weighted MRI; T2W, T 2-weighted MRI; USPIO, ultrasmall superparamagnetic iron oxide; n, number of patients.
Note. All studies used histopathology as a reference standard.
Definition of lymph nodes considered metastatic on MRI.
Results are directly deduced from article, no raw data was reported so no 2-by-2 contingency tables could be reconstructed.
In total, 31 patients were included in this study, however histology was only obtained in 30 patients. Age and sex are based on total study group (n = 31).
Lymph nodes with partial high signal intensity due to partial uptake or no blackening of nodes due to lack of uptake of USPIO were considered metastatic.
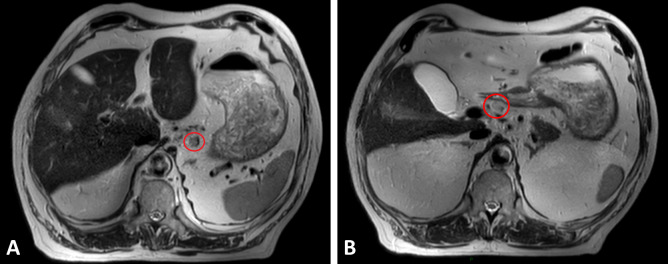
Figure 3.
Preoperative T 2-weighted magnetic resonance images in axial planes with pathologic lymph nodes (red markings) in one patient with gastric cancer.
The accuracy of MRI for correct differentiation between node-negative and node-positive patients varied between 65 and 100%, with PPV, NPV, sensitivities and specificities ranging from 72–100%, 29–100%, 69–100% and 40–100%, respectively. The accuracy for correct differentiation between N-stages (N0 versus N1 versus N2 versus N3) was moderate (55–57%) for two older studies that did not use DWI.40,43 A recent study using solely DWI showed higher PPV, NPV, sensitivities and specificities up to 86%, 91%, 79 and 98%, respectively.30
Four studies directly compared diagnostic performance of high resolution T2W and DWI, all demonstrated a higher accuracy for DWI.33,35,70,71 Measurements of the apparent diffusion coefficient (ADC), as determined with DWI, performed better than combined morphological criteria including short-axis diameter, border irregularity and DCE-enhancement patterns.71 Metastatic lymph nodes showed significantly lower median ADC values (1.28 × 10−3 mm2/s) compared to that of benign nodes (1.55 × 10−3 mm2/s). With a cut-off value of 1.39 × 10−3 mm2/s, the ADC measurement showed a sensitivity of 85.7% and specificity of 79.4% in distinguishing metastatic nodes.71 However, another study did not find a significant difference in ADC values between non-metastatic and metastatic lymph nodes.69
On DCE-MRI scans, voxelwise parametric maps of the volume transfer coefficient (Ktrans), reverse reflux rate constant (Kep), Ve and initial area-under the-gadolinium-concentration-curve during the first 60 sec (iAUC) of the primary tumor did not show significant differences between lymph node negative and lymph node positive patients.49
Overall, the diagnostic performance tended to increase with higher N-stages30,38 and although no direct comparison was available, 3.0T MRI resulted in a higher accuracy for lymph node staging than studies performed on 1.5T MRI (93% [28/30]35 versus 52% [24/46],44 respectively).
Two studies used ultrasmall superparamagnetic iron oxide (USPIO) enhancement instead of size to determine whether lymph nodes were metastatic or not and reported remarkably higher accuracies, PPV, NPV, sensitivities and specificities compared to other studies.72,73 However, to date, USPIO is only approved as a therapeutic agent, and a greater awareness of its adverse event profile has evolved which limits its current use as MRI contrast agent.74 The same studies stress the limitations of the use of size as a criterion to diagnose metastatic lymph nodes on imaging. According to their findings, 61.0% (36/59) of the histologically proven metastatic lymph nodes were normal-sized (<10 mm) or even less than 5 mm in size (13.6%, 8/59) on MRI. Also, smaller lymph node metastases are more difficult to detect, and detection highly depends on the resolution of the scans. On MRI, only 12.7% of lymph nodes < 5 mm could be identified, 42.8% of lymph nodes 5–10 mm and 68.9% of lymph nodes > 10 mm.73
The performance of MRI for the detection of metastatic lymph nodes was directly compared with EUS and/or CT in six studies.30,31,33,38,41,43 Two out of three studies that compared T2W and DW-MRI to CT reported (non-significant) higher diagnostic performance for MRI,30,33 whereas two studies that compared anatomical T2W to CT reported slightly lower accuracies for MRI compared to CT (however, also not significant).41,43 Accuracies for CT and EUS (77 and 75%, respectively) did not significantly differ from the accuracy of MRI (71%) in a study that directly compared all three imaging modalities.31 However, EUS showed a significantly superior sensitivity (92%) in the depiction of pathological nodes compared to CT (73%) and anatomical and functional (DCE and DWI) MRI (69%).31 Specificities of MRI (73%) and CT (81%) were higher compared to EUS (58%, MRI versus EUS p = 0.15, CT versus EUS p = 0.03). A second study compared the performance of anatomical and functional (DCE and DWI) MRI with EUS in correct assessment of N-stage and found the highest accuracy when combining both modalities, compared to MRI or EUS alone (71.1% vs 68.4% versus 65.8%, respectively).38 Lastly, when integrated 18F-FDG PET/MRI was compared with CT, the diagnostic performance for N-staging was not significantly different.50
Several studies describe that the lymph nodes identified by imaging were not exactly matched to those assessed by histopathology.33,41,69 Therefore, there could be a mismatch between the lymph nodes that were considered metastatic on MRI versus the lymph nodes that were histologically proven metastatic, resulting in a correctly assessed preoperative N-stage. This way of assessing accuracy of staging with imaging is, however, the closest resemblance to regular clinical practice. So far, only two studies applied a node-by-node comparison.72,73
In general, there were no statistically significant differences reported in the included studies between MRI and CT or EUS for correct detection of lymph node metastases.30,31,33,38,41,43 Thus, when contraindications such as renal insufficiency exist for the most commonly used lymph node staging modality (contrast-enhanced CT) or when CT results are ambiguous, MRI and EUS could be used to provide similar diagnostic information (Table 2).
M-staging
Importance
Preoperative diagnosis of distant metastatic disease such as peritoneal metastases or liver metastases guides treatment strategies in gastric cancer and is essential in order to avoid unnecessary surgery in patients who would not benefit from gastrectomy. This is illustrated by the fact that around a third of patients (29%) present with liver metastases at diagnosis75 and that approximately 23% of patients clinically and radiologically free of distant metastases appeared to have peritoneal metastases upon surgery, which underlines that there is still significant room for improvement of preoperative diagnostic evaluation.76 Detecting these metastases would divert patients from a futile attempt at curative local therapy, and prevent the potential reduced quality of life and increased health care costs associated with such treatment strategies.
Current imaging
The modality of first choice for M-staging is currently CT of the abdomen and pelvis.77 A recent review of four studies reported sensitivities for the detection of M1 disease on CT ranging from 14.3–59.1%, and specificities ranging from 93.3–99.8%.78 Sensitivity of CT for the detection of peritoneal metastasis was only 28.3% (15 of 53), with a specificity of 98.9% (440 of 445).79
A diagnostic accuracy of EUS for M-staging (location of distant metastasis not otherwise specified) of 90.0% was reported, with a very limited sensitivity of 10.6% but excellent specificity of 99.6%.80 Ascites detected by EUS increased the probability of the presence of peritoneal metastases in two studies, with a sensitivity of 61–73% and specificity of 84–99%.81,82 Direct comparison of CT and EUS in diagnosing ascites favored CT regarding sensitivity (59.1% vs 10.6%), whereas specificity did not significantly differ (99.8% vs 99.6%).80
Although widely used in oncology for the evaluation of metastatic disease, 18F-FDG PET/CT is not routinely indicated yet for gastric adenocarcinoma.77 However, recent studies showed significantly higher accuracy in the detection of distant lymph node metastases with 18F-FDG PET/CT compared to contrast-enhanced CT (CECT) alone in 106 patients with locally advanced gastric cancer (86.0% vs 75.6%, respectively).67 No statistically significant difference in the performance of CECT and the combination of CECT and 18F-FDG PET/CT was seen for diagnosis of overall distant metastases.67 Another study, however, reported that 18F-FDG PET/CT provides additional diagnostic information over standard staging (CT, EUS and laparoscopy), leading to a reduction of 10% in futile attempts of surgical exploration in patients that were found to have metastatic disease.83
Lastly, a review including 15 studies reporting on the performance of diagnostic laparoscopy for M staging, reported an overall accuracy, sensitivity, and specificity ranging from 85.0–98.9%, 64.3–94%, and 80.0–100%, respectively. The use of a diagnostic laparoscopy altered treatment in 8.5–59.6% of cases, avoiding laparotomy in 8.5–43.8% of cases.84 As such, laparoscopy with or without peritoneal washings for malignant cells to exclude occult metastatic disease is recommended in all advanced stage (i.e. stage IB-III) gastric cancers that are considered to be potentially resectable.85
MRI
The currently available literature describing the diagnostic performance of MRI in determining metastatic disease is limited. The few studies that have been conducted to assess the performance of MRI mostly have a low prevalence of metastatic disease and did not specifically focus on gastric cancer metastases.
For detection of peritoneal seeding in gastrointestinal and gastric cancer cases, the diagnostic performance of (DW-)MRI did not significantly differ from 18F-FDG PET/CT or CT.33,86 When directly comparing the performance of DW-MRI to 18F-FDG PET/CT in a study concerning 30 patients with gastrointestinal malignancies, of which five primary gastric cancers, accuracy, PPV, NPV, sensitivity and specificity for detection of peritoneal seeding were respectively 80%, 84%, 73%, 84 and 73% for 18F-FDG PET/CT and 83%, 89%, 75%, 84 and 82% for DW-MRI.86 This supports the fact that diagnosis of peritoneal seeding remains a challenge for imaging techniques because of its variable appearance and the small size of lesions.
The performance of MRI for the assessment of liver metastases is only reported by a few studies, including a small number of gastric cancer metastases. DW-MRI was able to differentiate liver metastases from adjacent liver parenchyma, based on ADC values, in two cases of liver metastases from gastric cancer in one of the previously mentioned studies.35 In another study that did not specifically focus on gastric cancer (49 patients with primary adenocarcinomas of the gastrointestinal tract, of which four patients with gastric cancer), MRI was proven to be significantly superior to 18F-FDG PET/CT for the detection of small liver metastases (≤10 mm).87 In a recent pilot study of 12 patients with colorectal cancer (n = 9) or gastric cancer (n = 3), chemotherapy-induced focal hepatopathy (which could mimic metastases in patients with gastrointestinal malignancy during chemotherapy) could be differentiated from metastases on the basis of DCE and DW-MRI findings.88 Furthermore, DW-MRI could aid in the prediction of response of liver metastases to chemotherapy as researched in a cohort of 86 patients with liver metastases from gastrointestinal tract cancers (of which 28 patients with primary gastric cancers), resulting in a sensitivity of 94.3% and specificity of 76.7% using a cutoff value of 1.14 × 10 mm2/s for the ADC value of the liver metastases before treatment.89
Lastly, even though the combination of 18F-FDG PET/MRI did not improve diagnostic accuracy in T- and N-staging in a group of 42 patients compared to CT as mentioned before, it did significantly improve correct preoperative M-staging compared to CT (92.9% vs 73.9–81.0%).50 However, it was not reported whether this improvement derived from the information of the 18F-FDG PET scan or MRI scan. However, it is possible that M-staging with 18F-FDG PET/MRI will benefit from MRI accuracy, especially in the brain and the liver.51
Overall, for the evaluation of systemic disease, CT is currently the primary staging tool for distant metastases, with a relatively low sensitivity (ranging from 14.3–59.1%), but high specificity (ranging from 93.3–99.8%).78 The results of MRI for M-staging in gastric cancer specifically are limited to date, but the addition of DWI might be promising in the future33,35,86,88,89 as well as the recent integration of PET and MRI hardware.50 Also, DW-MRI and DCE-MRI have been proven valuable for M-staging in other cancers, such as the detection of liver metastases of colorectal and gynecologic cancers.90–93
Treatment response assessment
Importance
Although the optimal way to integrate chemo(radio)therapy within the treatment of gastric cancer has not been globally established yet, the benefit of multimodality treatment has become evident.7,94,95 Neoadjuvant chemotherapy improves microscopically radical resections, reduces residual tumor-positive lymph nodes and improves survival.27 Currently up to 30–40% of gastric carcinoma patients respond to the available chemotherapy regimens as defined by any form of tumor regression.96,97 For preoperative chemoradiotherapy, radical resection rates of 67–92% and pathologic complete response rates of 5–29% have been reported.98 Accurate differentiation between responders and non-responders could assist in individualized therapeutic decision-making. Ineffective chemo(radio)therapy regimens could potentially be omitted, terminated early or switched to more effective regimens. Furthermore, reliable treatment response assessment regarding metastatic disease, for example by assessment of peritoneal cytology (as obtained by staging laparoscopy) before and after neoadjuvant treatment, could guide clinical decision-making with respect to the consideration of hyperthermic intraperitoneal chemotherapy (HIPEC) procedures.99 Since pathological complete response to neoadjuvant therapy is not frequently observed in gastric cancer, correct assessment of pathologic complete response with the goal to pursue organ-preserving strategies (without surgery) does not seem realistic in the near future for gastric cancer patients.4,96,100
Current imaging
Anatomical as well as molecular imaging modalities have been used for tumor response assessment to neoadjuvant chemotherapy, including endoscopy, EUS, contrast-enhanced ultrasonography, CT, 18F-FDG PET and combined 18F-FDG PET/CT. Assessment of dimensional changes in tumor volume according to the response evaluation criteria in solid tumors (RECIST) is frequently used.101 These criteria require the presence of a measurable lesion, which is not always the case in diffuse growing gastric cancers. Also, volume analysis can be affected by tumor shape irregularity, different grades of visceral distension and the inability of dimensional criteria to differentiate residual viable tumor from therapy-induced fibrosis.102
Overall, studies concerning CT and/or EUS in the assessment of response to neoadjuvant chemotherapy demonstrate that there is an association between anatomical tumor response (i.e. volume changes of the primary tumor) and histopathological response.103,104 However, these volume changes take time to become apparent. Alternatives for anatomical changes include morphological changes of the primary lesions evaluated by endoscopy,105 perfusion parameters on CT106 or a molecular imaging approach such as 18F-FDG PET. Proportional changes in tumor glucose consumption assessed by 18F-FDG PET have been found to be associated with neoadjuvant chemotherapy induced response and survival,96,97 but these findings are equivocal.103 Moreover, the use of 18F-FDG PET could be somewhat limited since not all gastric carcinomas are 18F-FDG-avid.12
MRI
Tumor response evaluation to (neo)adjuvant therapy with the use of DW-MRI has been subject of research for a great variation of cancer types.107–113 In two relatively small studies (n = 32 and n = 17, respectively) focusing on the relation between ADC of the primary tumor and response to neoadjuvant chemotherapy in patients with gastric cancer, significantly higher ADC values were found in responders (defined as tumor regression grades [TRG] 1–3 at histopathology) compared to non-responders after neoadjuvant treatment.102,114 The significant increase in ADC values in responders to neoadjuvant therapy can be explained by the presence of necrosis and fibrosis after successful treatment, which should correspond to an increase in water diffusivity and, consequently, in ADC values.114
With regard to MRI, the same limitations apply to anatomical measurements to evaluate tumor response as mentioned earlier for CT and EUS. In a study of 32 patients, tumor volume changes using DW-MRI was not found to be of value in assessing response to neoadjuvant chemotherapy in gastric cancer.102
When assessing the response of histologically proven metastatic lymph nodes to neoadjuvant chemotherapy in patients with advanced gastric cancer on DW-MRI, all lymph node groups showed an increase in ADC values during chemotherapy.115 This effect was visible after the third day of chemotherapy, which occurred earlier than change in lymph node diameter.115 However, no statistically significant difference was found between complete responders, partial responders and the stable disease group based on RECIST criteria when comparing mean ADC values of lymph nodes.115
Fully-integrated 18F-FDG PET/MRI could add to the performance of MRI in evaluating treatment response in the future: one preliminary study (n = 11) reports the feasibility in patients with unresectable gastric cancer.116 However, no significant difference was found in ADC and SUVmax values prior to treatment between responders and non-responders, as determined based on follow-up CT scans after 2–3 cycles of chemotherapy.116
Overall, all currently available imaging modalities show only moderate sensitivity and specificity with regard to response assessment in gastric cancer, generally making them insufficient to justify changes in treatment decision-making. In this context, the performance of (functional) MRI is currently a topic of research. However, convincing high-quality studies regarding differentiation of responders and non-responders based on MRI are lacking so far. Lastly, since the included studies do not report on oncological outcomes, it is unclear if earlier of more accurate assessment of response to treatment changes outcome in patients.
Treatment guidance
Currently, radiotherapy does not have a clearly defined role in the treatment of gastric cancer.85 Although postoperative chemoradiotherapy is an evidence-based strategy,3 perioperative chemotherapy is considered standard treatment.4 Recently however, there is growing interest to evaluate the clinical advantages of preoperative chemoradiotherapy to induce tumor downstaging and improve surgical results (i.e. the TOPGEAR trial117 and the CRITICS-2 trial [ClinicalTrials.gov Identifier NCT02931890]). These regimens strongly rely on accurate delineation of the clinical target volumes, as one of the greatest challenges is to deliver radiation dose accurately to the tumor while minimizing toxicity. The stomach is surrounded by a number of critical organs that are at risk and considered dose-limiting during radiation therapy. To deliver a tumoricidal dose of radiation, large volumes of healthy tissues in the abdomen are also irradiated (including pancreas, duodenum, great vessels, and vertebrae). With the recent development of an integrated MR system with a radiotherapy accelerator however, MRI-guided adaptive radiotherapy could allow for more precise delineation of clinical target volumes, radiation treatment delivery, and even dose escalation in the near future.118–121 Especially in preoperative (chemo)radiotherapy for gastric cancer, MRI evaluation of setup accuracy could be of great benefit.122 Daily adaptation of treatment plans based on intra- and interfraction anatomical variation becomes possible, allowing better normal tissue sparing and/or radiation dose escalation. A recent case-report already provided insightful results on large inter fraction variation and deformations that were observed during MRI-guided radiotherapy for a gastric cancer patient.123 However, whether MRI actually contributes to a better definition of target volumes for radiotherapy planning and delivery remains to be established.. An illustration of clinical target volume delineation on MRI and CT images in a patient with gastric cancer can be found in Figure 4.
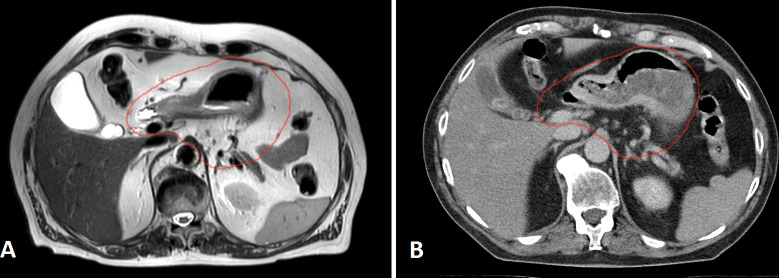
Figure 4.
Preoperative T 2-weighted magnetic resonance images (A) and planning CT images (B) of one patient with gastric cancer in axial planes. The red contouring reflects the clinical target volume (CTV) that could be used for preoperative radiation therapy.
MRI protocols for gastric cancer
Visualization of the gastric wall strongly depends on its diameter, MRI scanner characteristics, organ motion, and distention of the gastric wall. To overcome some of these obstacles, a consistent approach to MR imaging of the stomach described in the literature involves gastric distention by drinking water (up to 1000 ml), administration of scopolamine or glucagon to reduce artifacts from peristalsis, the use of breath-hold MR techniques, and multiplanar image acquisition.29 Especially adequate distention of the gastric wall is crucial to differentiate between wall layers, and thus specification of the exact depth of tumor invasion.35 Gadolinium-based contrast agents are currently the most frequently used for MR imaging. However, they lack specificity for target organs and have a short imaging lifetime.124 In the future, gastric tumor visualization on MRI might be improved with new contrast agents that are subject of in vitro and in vivo research, such as coupled Gd-DPTA and anti EGFR-iRGD (a recombinant protein).124
Since MRI is not yet widely accepted as a standard imaging modality for staging gastric cancer, there are no generally accepted protocols for gastric MRI.125 In our institution, we compared several anatomical and functional sequences on a 3T MR system (Ingenia; Philips Medical Systems, Best, The Netherlands).126 To improve stomach distention and to suppress signal from physiologic stomach filling, approximately 400 ml pineapple juice was given orally. Gadolinium was used as the intravenous contrast agent. As an anatomical sequence, an exhale navigator triggering during the acquisition of a high resolution T2W turbo spin echo MRI, rather than respiratory sensor triggering, provided excellent contrast with limited motion artifacts in both axial and coronal view. For functional MRI imaging, which can be used for staging and treatment response assessment, free-breathing, single-shot echo planar DWI using b-values of 0, 200 and 800 s/mm2, and a free-breathing, 4D THRIVE DCE provided good temporal resolution and limited motion artifacts. For the purpose of radiation treatment guidance and delivery, we furthermore developed a sequence for treatment planning and intra fraction motion monitoring. For treatment planning purposes, a fast 3D high resolution mDixon with a large field of view and a high signal to noise ratio within one exhale breathhold is feasible. For motion monitoring, 4D T2W MRI with retrospective self-sorting reconstruction resulted in a high resolution, high signal to noise ratio and good slice ordering. For intra fraction motion, turbo spin echo cine-MRI resulted in a better signal to noise ratio and high resolution without artifacts compared to a 2D T1W dynamic turbo field echo or fast field echo. Figures 1–4 represent images from our institution, in accordance with the abovementioned protocol.
Challenges and future perspectives in gastric cancer staging
Despite advances in the staging and treatment of gastric cancer, several challenges still lie ahead. First, there is no worldwide consensus regarding the anatomical criteria that should be used to define tumor invasion or a metastatic lymph node on any imaging modality. In case of lymph node assessment, imaging techniques for detection of lymph node metastases rely on uncertain size criteria, except for 18F-FDG PET/CT, which is in turn accompanied by a limited sensitivity. With adequate mapping of pathologic lymph nodes during staging, a more precise lymphadenectomy might become reality in combination with the possibilities of intraoperative lymphatic drainage imaging.127 However, a better imaging technique for accurate detection of lymph node metastases, and subsequent individualized treatment based on these findings, is yet to be found.
Second, there is an unmet need for standardization of reliable criteria to accurately evaluate response to perioperative therapy, as well as for the evaluation of oncological outcomes after treatment response assessment. Given the absence of reliable criteria for evaluating the treatment response, most multidisciplinary teams will continue treatment with perioperative therapy in patients without evidence of disease progression on imaging. As a result, overtreatment occurs in a substantial part of patients, leading to a reduction in quality of life and an increase in health care costs. However, if accurate detection of non-response without disease progression would be possible, these patients would not be exposed to the side-effects of continued perioperative therapy without the benefit. Accurate assessment of response might furthermore increase compliance in the responding patients, as currently only around 40% of patients completes the entire perioperative chemotherapy regimen.4,128
Third, accurate diagnosis of the presence of distant metastases (especially peritoneal metastases) in patients presenting with gastric cancer remains challenging. Currently a diagnostic laparoscopy has the highest performance. As a consequence, patients are subjected to an invasive surgical procedure, which also puts pressure on health resources.
In the upcoming years, the preoperative staging and treatment response assessment in gastric cancer might benefit from imaging biomarkers derived from functional MR imaging such as DWI.129 DWI depends on the mobility of water protons within tissues, which is measured with ADC values and can provide specific information about cellular density of tumors.102 ADC values help to differentiate between normal gastric wall and pathological tissue,35,37,48,69,114,130,131 gastric adenocarcinoma and lymphoma,130 and liver metastases and adjacent liver parenchyma.35 Furthermore ADC values increase gradually with the degree of histologic tumor differentiation32,37,132 and there is an inverse correlation between the ADC value and the T- and N-stage.133,134 Its correlation with the Her2Neu status of gastric tumors has been described135,136 and it could also be useful in response prediction to neoadjuvant treatment as discussed before.133 Lastly, lower tumor ADC values are associated with a negative prognosis (i.e. overall survival) and could potentially serve as prognostic factor in the evaluation of aggressiveness of gastric cancer.134,137,138
One of the challenges specifically for the use of ADC values of DW-MRI in clinical practice, is that there is no consensus how to calculate and interpret ADC values. ADC values are calculated based on a region of interest (ROI), but the approach of determination of this ROI varies greatly between studies. An ROI can either be manually drawn or semi-automatic, and can be based on T2W images or DWI images of varying b-values. This can especially be relevant when using ADC values for diagnosis of lymph node metastases or peritoneal seeding, since the small size of the nodes/nodules influence the setting of the ROI, which in turn could influence the ADC values measured.35 Furthermore, some authors suggest the use of minimum ADC-values and others have taken into account mean ADC. Thereby, direct comparison of DW-MRI results across studies is greatly impaired, which underlines the need for standardization of scan protocols, image analysis and careful review of reproducibility of measurement across centers before implementation.
Conclusion
Gastric cancer management requires a multimodality staging strategy in which CT remains the primary staging modality due to its relatively high accuracy rates and wide availability. To date, preoperative locoregional staging of gastric cancer does not significantly benefit from the use of MRI, despite its high contrast resolution and characteristic soft-tissue contrast.
In addition, this review demonstrates that additional value of MRI might be expected for detection of lymph node metastases and systemic disease, for defining clinical target volumes and setup verification with MR-guided radiation treatment, and for treatment response prediction, especially with continuous technical improvements (e.g. organ-motion compensation techniques) and the possibilities of functional MRI (e.g. diffusion weighted imaging and dynamic contrast enhancement). Further, large studies are needed to establish the role of MRI for these applications in clinical practice.
Essentials
Gastric cancer management requires a multimodality staging strategy in which CT remains the primary staging modality due to its relatively high accuracy rates and wide availability.
The accuracy of MRI for T- and N-staging of gastric cancer is similar to EUS and CT, making MRI a suitable alternative when contraindications are present for the primary staging modalities.
MRI is widely used for diagnosing liver metastases and shows potential for diagnosing peritoneal seeding.
Treatment response assessment remains challenging and all imaging modalities are currently insufficient to justify changes in treatment decision making.
Treatment response assessment as well as detection of lymph node metastases and systemic disease might benefit from imaging biomarkers derived from functional MRI (e.g. diffusion weighted imaging and dynamic contrast enhancement) in the future.
Additional value of MRI might be expected from its role in better defining clinical target volumes and treatment setup verification for preoperative radiation treatment.
Footnotes
Acknowledgment: The authors gratefully acknowledge Vivian van Pelt from the Department of Radiation Oncology, Netherlands Cancer Institute - Antoni van Leeuwenhoek (NKI-AVL), Amsterdam, for her assistance with the MRI figures.
The authors Alicia S. Borggreve and Lucas Goense contributed equally to the work.
Ethics approval: This study did not involve human and animal subjects, and therefore an institutional review board approval and a waiver of informed consent were not required.
Competing interests: The authors report no competing interests.
Disclosure: No conflict of interest was reported by any of the authors.
Contributor Information
Alicia S. Borggreve, Email: a.s.borggreve@umcutrecht.nl.
Lucas Goense, Email: l.goense-2@umcutrecht.nl.
Hylke J.F. Brenkman, Email: h.j.f.brenkman@umcutrecht.nl.
Stella Mook, Email: s.mook-2@umcutrecht.nl.
Gert J. Meijer, Email: g.j.meijer@umcutrecht.nl.
Frank J. Wessels, Email: f.j.wessels-3@umcutrecht.nl.
Marcel Verheij, Email: m.verheij@nki.nl.
Edwin P.M. Jansen, Email: epm.jansen@nki.nl.
Richard van Hillegersberg, Email: r.vanhillegersberg@umcutrecht.nl.
Peter S.N. van Rossum, Email: p.s.n.vanrossum-2@umcutrecht.nl.
Jelle P. Ruurda, Email: j.p.ruurda@umcutrecht.nl.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Ervik M, et al . GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer . 2013. . Available from: http://globocan.iarc.fr [ July 27, 2017 ].
- 2. Japanese Gastric Cancer Association . Japanese Gastric cancer treatment guidelines 2014 (VER. 4) . Gastric Cancer 2017. ; 20 : 1 – 19 . doi: 10.1007/s10120-016-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Macdonald JS , Smalley SR , Benedetti J , Hundahl SA , Estes NC , Stemmermann GN , et al. . Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction . N Engl J Med 2001. ; 345 : 725 – 30 . doi: 10.1056/NEJMoa010187 [DOI] [PubMed] [Google Scholar]
- 4. Cunningham D , Allum WH , Stenning SP , Thompson JN , Van de Velde CJH , Nicolson M , et al. . Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer . N Engl J Med 2006. ; 355 : 11 – 20 . doi: 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 5. Ychou M , Boige V , Pignon J-P , Conroy T , Bouché O , Lebreton G , et al. . Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial . J Clin Oncol 2011. ; 29 : 1715 – 21 . doi: 10.1200/JCO.2010.33.0597 [DOI] [PubMed] [Google Scholar]
- 6. Ronellenfitsch U , Schwarzbach M , Hofheinz R , Kienle P , Kieser M , Slanger TE , et al. . Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: systematic review with meta-analysis combining individual patient and aggregate data . Eur J Cancer 2013. ; 49 : 3149 – 58 . doi: 10.1016/j.ejca.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 7. Diaz-Nieto R , Orti-Rodríguez R , Winslet M , Orti-Rodríguez R , Winslet M . Post-surgical chemotherapy versus surgery alone for resectable gastric cancer . Cochrane Database Syst Rev 2013. ; (9) : CD008415 . doi: 10.1002/14651858.CD008415.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al-Batran S-E , Homann N , Pauligk C , Illerhaus G , Martens UM , Stoehlmacher J , et al. . Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIO-FLOT3 trial . JAMA Oncol 2017. ; 3 : 1237 . doi: 10.1001/jamaoncol.2017.0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartgrink HH , van de Velde CJH , Putter H , Bonenkamp JJ , Klein Kranenbarg E , Songun I , et al. . Extended lymph node dissection for gastric cancer: who may benefit? final results of the randomized Dutch Gastric Cancer Group trial . J Clin Oncol 2004. ; 22 : 2069 – 77 . doi: 10.1200/JCO.2004.08.026 [DOI] [PubMed] [Google Scholar]
- 10. Wani S , Coté GA , Keswani R , Mullady D , Azar R , Murad F , et al. . Learning curves for EUS by using cumulative sum analysis: implications for American Society for gastrointestinal endoscopy recommendations for training . Gastrointest Endosc 2013. ; 77 : 558 – 65 . doi: 10.1016/j.gie.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 11. Park CH , Park JC , Kim EH , Jung DH , Chung H , Shin SK , et al. . Learning curve for EUS in gastric cancer T staging by using cumulative sum analysis . Gastrointest Endosc 2015. ; 81 : 898 – 905 . doi: 10.1016/j.gie.2014.08.024 [DOI] [PubMed] [Google Scholar]
- 12. Kaneko Y , Murray WK , Link E , Hicks RJ , Duong C . Improving patient selection for 18F-FDG PET scanning in the staging of gastric cancer . J Nucl Med 2015. ; 56 : 523 – 9 . doi: 10.2967/jnumed.114.150946 [DOI] [PubMed] [Google Scholar]
- 13. Arocena MG , Barturen A , Bujanda L , Casado O , Ramírez MM , Oleagoitia JM , et al. . MRI and endoscopic ultrasonography in the staging of gastric cancer . Rev. esp. enferm. dig. 2006. ; 98 : 582 – 90 . doi: 10.4321/S1130-01082006000800003 [DOI] [PubMed] [Google Scholar]
- 14. Keogan MT , Edelman RR . Technologic advances in abdominal MR imaging . Radiology 2001. ; 220 : 310 – 20 . doi: 10.1148/radiology.220.2.r01au22310 [DOI] [PubMed] [Google Scholar]
- 15. van Rossum PSN , van Hillegersberg R , Lever FM , Lips IM , van Lier ALHMW , Meijer GJ , et al. . Imaging strategies in the management of oesophageal cancer: what's the role of MRI? Eur Radiol 2013. ; 23 : 1753 – 65 . doi: 10.1007/s00330-013-2773-6 [DOI] [PubMed] [Google Scholar]
- 16. Mortensen MB . Novel imaging strategies for upper gastrointestinal tract cancers . Expert Rev Gastroenterol Hepatol 2015. ; 9 : 295 – 303 . doi: 10.1586/17474124.2015.959928 [DOI] [PubMed] [Google Scholar]
- 17. Seevaratnam R , Cardoso R , Mcgregor C , Lourenco L , Mahar A , Sutradhar R , et al. . How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis . Gastric Cancer 2012. ; 15 ( S1 ): 3 – 18 . doi: 10.1007/s10120-011-0069-6 [DOI] [PubMed] [Google Scholar]
- 18. Dhar DK , Kubota H , Tachibana M , Kotoh T , Tabara H , Watanabe R , et al. . Long-term survival of transmural advanced gastric carcinoma following curative resection: multivariate analysis of prognostic factors . World J Surg 2000. ; 24 : 588 – 94 . doi: 10.1007/s002689910099 [DOI] [PubMed] [Google Scholar]
- 19. Zhang X-F , Huang C-M , Lu H-S , Wu X-Y , Wang C , Guang G-X , et al. . Surgical treatment and prognosis of gastric cancer in 2,613 patients . World J Gastroenterol 2004. ; 10 : 3405 – 8 . doi: 10.3748/wjg.v10.i23.3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin LX , Moses LE , Squires MH , Poultsides GA , Votanopoulos K , Weber SM , et al. . Factors associated with recurrence and survival in lymph node-negative gastric adenocarcinoma: a 7-Institution study of the US gastric cancer collaborative . Ann Surg 2015. ; 262 : 999 – 1005 . doi: 10.1097/SLA.0000000000001084 [DOI] [PubMed] [Google Scholar]
- 21. Min YW , Min B-H , Lee JH , Kim JJ . Endoscopic treatment for early gastric cancer . World J Gastroenterol 2014. ; 20 : 4566 – 73 . doi: 10.3748/wjg.v20.i16.4566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwee RM , Kwee TC . Imaging in local staging of gastric cancer: a systematic review . J Clin Oncol 2007. ; 25 : 2107 – 16 . doi: 10.1200/JCO.2006.09.5224 [DOI] [PubMed] [Google Scholar]
- 23. Tharian B , Tsiopoulos F , George N , Pietro SD , Attili F , Larghi A . Endoscopic ultrasound fine needle aspiration: technique and applications in clinical practice . World J Gastrointest Endosc 2012. ; 4 : 532 – 44 . doi: 10.4253/wjge.v4.i12.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cardoso R , Coburn N , Seevaratnam R , Sutradhar R , Lourenco LG , Mahar A , et al. . A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer . Gastric Cancer 2012. ; 15 ( Suppl 1 ): 19 – 26 . doi: 10.1007/s10120-011-0115-4 [DOI] [PubMed] [Google Scholar]
- 25. Mocellin S , Pasquali S . Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer . Cochrane Database Syst Rev 2015. ; 2 : CD009944 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nie R-C , Yuan S-Q , Chen X-J , Chen S , Xu L-P , Chen Y-M , et al. . Endoscopic ultrasonography compared with multidetector computed tomography for the preoperative staging of gastric cancer: a meta-analysis . World J Surg Oncol 2017. ; 15 . doi: 10.1186/s12957-017-1176-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dassen AE , Lips DJ , Hoekstra CJ , Pruijt JFM , Bosscha K . FDG-PET has no definite role in preoperative imaging in gastric cancer . Eur J Surg Oncol 2009. ; 35 : 449 – 55 . doi: 10.1016/j.ejso.2008.11.010 [DOI] [PubMed] [Google Scholar]
- 28. Yamada I , Miyasaka N , Hikishima K , Kato K , Kojima K , Kawano T , et al. . Gastric carcinoma: ex vivo MR imaging at 7.0 T-Correlation with histopathologic findings . Radiology 2015. ; 275 : 841 – 8 . doi: 10.1148/radiol.14141878 [DOI] [PubMed] [Google Scholar]
- 29. Motohara T , Semelka RC . MRI in staging of gastric cancer . Abdom Imaging 2002. ; 27 : 376 – 83 . doi: 10.1007/s00261-001-0118-4 [DOI] [PubMed] [Google Scholar]
- 30. Arslan H , Fatih Özbay M , Çallı İskan , Doğan E , Çelik S , Batur A , et al. . Contribution of diffusion weighted MRI to diagnosis and staging in gastric tumors and comparison with multi-detector computed tomography . Radiol Oncol 2017. ; 51 : 23 – 9 . doi: 10.1515/raon-2017-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giganti F , Orsenigo E , Arcidiacono PG , Nicoletti R , Albarello L , Ambrosi A , et al. . Preoperative locoregional staging of gastric cancer: is there a place for magnetic resonance imaging? prospective comparison with EUS and multidetector computed tomography . Gastric Cancer 2016. ; 19 : 216 – 25 . doi: 10.1007/s10120-015-0468-1 [DOI] [PubMed] [Google Scholar]
- 32. Liang J , Lv H , Liu Q , Li H , Wang J , Cui E . Role of diffusion-weighted magnetic resonance imaging and apparent diffusion coefficient values in the detection of gastric carcinoma . Int J Clin Exp Med 2015. ; 8 : 15639 – 47 . [PMC free article] [PubMed] [Google Scholar]
- 33. Joo I , Lee JM , Kim JH , Shin C-I , Han JK , Choi BI . Prospective comparison of 3T MRI with diffusion-weighted imaging and MDCT for the preoperative TNM staging of gastric cancer . J Magn Reson Imaging 2015. ; 41 : 814 – 21 . doi: 10.1002/jmri.24586 [DOI] [PubMed] [Google Scholar]
- 34. Liu S , He J , Guan W , Li Q , Zhang X , Mao H , et al. . Preoperative T staging of gastric cancer: comparison of diffusion- and T2-weighted magnetic resonance imaging . J Comput Assist Tomogr 2014. ; 38 ( Id ): 544 – 50 . doi: 10.1097/RCT.0000000000000090 [DOI] [PubMed] [Google Scholar]
- 35. Caivano R , Rabasco P , Lotumolo A , D' Antuono F , Zandolino A , Villonio A , et al. . Gastric cancer: the role of diffusion weighted imaging in the preoperative staging . Cancer Invest 2014. ; 32 : 184 – 90 . doi: 10.3109/07357907.2014.896014 [DOI] [PubMed] [Google Scholar]
- 36. Huo X , Yuan K , Shen Y , Li M , Wang Q , Xing L , et al. . Clinical value of magnetic resonance imaging in preoperative T staging of gastric cancer and postoperative pathological diagnosis . Oncol Lett 2014. ; 8 : 275 – 80 . doi: 10.3892/ol.2014.2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu S , He J , Guan W , Li Q , Yu H , Zhou Z , et al. . Added value of diffusion-weighted MR imaging to T2-weighted and dynamic contrast-enhanced MR imaging in T staging of gastric cancer . Clin Imaging 2014. ; 38 : 122 – 8 . doi: 10.1016/j.clinimag.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 38. Lei C , Huang L , Wang Y , Huang Y , Huang Y . Comparison of MRI and endoscope ultrasound detection in preoperative T/N staging of gastric cancer . Mol Clin Oncol 2013. ; 1 : 699 – 702 . doi: 10.3892/mco.2013.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anzidei M , Napoli A , Zaccagna F , Di Paolo P , Zini C , Cavallo Marincola B , et al. . Diagnostic performance of 64-MDCT and 1.5-T MRI with high-resolution sequences in the T staging of gastric cancer: a comparative analysis with histopathology . Radiol Med 2009. ; 114 : 1065 – 79 . doi: 10.1007/s11547-009-0455-x [DOI] [PubMed] [Google Scholar]
- 40. Zhong L , Li L , Sun JH , Xu JR . Preoperative diagnosis of gastric cancer using 2-D magnetic resonance imaging with 3-D reconstruction techniques . Chin J Dig Dis 2005. ; 6 : 159 – 64 . doi: 10.1111/j.1443-9573.2005.00224.x [DOI] [PubMed] [Google Scholar]
- 41. Kim AY , Han JK , Seong CK , Kim TK , Choi BI . MRI in staging advanced gastric cancer: is it useful compared with spiral CT? J Comput Assist Tomogr 2000. ; 24 : 389 – 94 . doi: 10.1097/00004728-200005000-00006 [DOI] [PubMed] [Google Scholar]
- 42. Wang CK , Kuo YT , Liu GC , Tsai KB , Huang YS . Dynamic contrast-enhanced subtraction and delayed MRI of gastric tumors: radiologic-pathologic correlation . J Comput Assist Tomogr 2000. ; 24 : 872 – 7 . doi: 10.1097/00004728-200011000-00009 [DOI] [PubMed] [Google Scholar]
- 43. Sohn KM , Lee JM , Lee SY , Ahn BY , Park SM , Kim KM . Comparing MR imaging and CT in the staging of gastric carcinoma . AJR Am J Roentgenol 2000. ; 174 : 1551 – 7 . doi: 10.2214/ajr.174.6.1741551 [DOI] [PubMed] [Google Scholar]
- 44. Kang BC , Kim JH , Kim KW , Lee DY , Baek SY , Lee SW , et al. . Value of the dynamic and delayed Mr sequence with Gd-DTPA in the T-staging of stomach cancer: correlation with the histopathology . Abdom Imaging 2000. ; 25 : 14 – 24 . doi: 10.1007/s002619910003 [DOI] [PubMed] [Google Scholar]
- 45. Kim AY , Han JK , Kim TK , Park SJ , Choi BI . MR imaging of advanced gastric cancer: comparison of various Mr pulse sequences using water and gadopentetate dimeglumine as oral contrast agents . Abdom Imaging 2000. ; 25 : 7 – 13 . doi: 10.1007/s002619910002 [DOI] [PubMed] [Google Scholar]
- 46. Oi H , Matsushita M , Murakami T , Nakamura H . Abdominal imaging dynamic MR imaging for extraserosal invasion of advanced gastric cancer . Imaging 1997. ; 40 : 35 – 40 . [DOI] [PubMed] [Google Scholar]
- 47. Matsushita M , Oi H , Murakami T , Takata N , Kim T , Kishimoto H , et al. . Extraserosal invasion in advanced gastric cancer: evaluation with MR imaging . Radiology 1994. ; 192 : 87 – 91 . doi: 10.1148/radiology.192.1.8208971 [DOI] [PubMed] [Google Scholar]
- 48. Jang KM , Kim SH , Lee SJ , Lee MW , Choi D , Kim KM . Upper abdominal gadoxetic acid-enhanced and diffusion-weighted MRI for the detection of gastric cancer: comparison with two-dimensional multidetector row CT . Clin Radiol 2014. ; 69 : 827 – 35 . doi: 10.1016/j.crad.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 49. Joo I , Lee JM , Han JK , Yang H-K , Lee H-J , Choi BI . Dynamic contrast-enhanced MRI of gastric cancer: correlation of the perfusion parameters with pathological prognostic factors . J Magn Reson Imaging 2015. ; 41 : 1608 – 14 . doi: 10.1002/jmri.24711 [DOI] [PubMed] [Google Scholar]
- 50. Lee DH , Kim SH , Joo I , Hur BY , Han JK . Comparison between 18F-FDG PET/MRI and MDCT for the assessment of preoperative staging and resectability of gastric cancer . Eur J Radiol 2016. ; 85 : 1085 – 91 . doi: 10.1016/j.ejrad.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 51. Buchbender C , Heusner TA , Lauenstein TC , Bockisch A , Antoch G . Oncologic PET/MRI, part 1: Tumors of the brain, head and neck, chest, abdomen, and pelvis . J Nucl Med 2012. ; 53 : 928 – 38 . [DOI] [PubMed] [Google Scholar]
- 52. Siewert JR , Böttcher K , Stein HJ , Roder JD . Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer study . Ann Surg 1998. ; 228 : 449 – 61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Takagane A , Terashima M , Abe K , Araya M , Irinoda T , Yonezawa H , et al. . Evaluation of the ratio of lymph node metastasis as a prognostic factor in patients with gastric cancer . Gastric Cancer 1999. ; 2 : 122 – 8 . doi: 10.1007/s101200050034 [DOI] [PubMed] [Google Scholar]
- 54. Yokota T , Kunii Y , Teshima S , Yamada Y , Saito T , Takahashi M , et al. . Significant prognostic factors in patients with early gastric cancer . Int Surg 2000. ; 85 : 286 – 90 . [PubMed] [Google Scholar]
- 55. Deng J , Liang H , Sun D , Pan Y . The prognostic analysis of lymph node-positive gastric cancer patients following curative resection . J Surg Res 2010. ; 161 : 47 – 53 . doi: 10.1016/j.jss.2008.12.019 [DOI] [PubMed] [Google Scholar]
- 56. Deng J-Y , Liang H . Clinical significance of lymph node metastasis in gastric cancer . World J Gastroenterol 2014. ; 20 : 3967 – 75 . doi: 10.3748/wjg.v20.i14.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. De Vita F , Giuliani F , Galizia G , Belli C , Aurilio G , Santabarbara G , et al. . Neo-adjuvant and adjuvant chemotherapy of gastric cancer . Ann Oncol 2007. ; 18 ( Suppl6 ): vi120 – 3 . doi: 10.1093/annonc/mdm239 [DOI] [PubMed] [Google Scholar]
- 58. Hartgrink HH , Jansen EPM , van Grieken NCT , van de Velde CJH . Gastric cancer . Lancet 2009. ; 374 : 477 – 90 . doi: 10.1016/S0140-6736(09)60617-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vergadis C , Schizas D . Is accurate N - staging for gastric cancer possible? Front Surg 2018. ; 5 : 41 . doi: 10.3389/fsurg.2018.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shiraishi N , Inomata M , Osawa N , Yasuda K , Adachi Y , Kitano S . Early and late recurrence after gastrectomy for gastric carcinoma. univariate and multivariate analyses . Cancer 2000. ; 89 : 255 – 61 . [DOI] [PubMed] [Google Scholar]
- 61. Coburn NG , Swallow CJ , Kiss A , Law C . Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer . Cancer 2006. ; 107 : 2143 – 51 . doi: 10.1002/cncr.22229 [DOI] [PubMed] [Google Scholar]
- 62. Noda N , Sasako M , Yamaguchi N , Nakanishi Y . Ignoring small lymph nodes can be a major cause of staging error in gastric cancer . Br J Surg 1998. ; 85 : 831 – 4 . doi: 10.1046/j.1365-2168.1998.00691.x [DOI] [PubMed] [Google Scholar]
- 63. Mönig SP , Zirbes TK , Schröder W , Baldus SE , Lindemann DG , Dienes HP , et al. . Staging of gastric cancer: correlation of lymph node size and metastatic infiltration . AJR Am J Roentgenol 1999. ; 173 : 365 – 7 . doi: 10.2214/ajr.173.2.10430138 [DOI] [PubMed] [Google Scholar]
- 64. Dumonceau J-M , Deprez PH , Jenssen C , Iglesias-Garcia J , Larghi A , Vanbiervliet G , et al. . Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated January 2017 . Endoscopy 2017. ; 49 : 695 – 714 . doi: 10.1055/s-0043-109021 [DOI] [PubMed] [Google Scholar]
- 65. Kwee RM , Kwee TC . Imaging in assessing lymph node status in gastric cancer . Gastric Cancer 2009. ; 12 : 6 – 22 . doi: 10.1007/s10120-008-0492-5 [DOI] [PubMed] [Google Scholar]
- 66. Kim EY , Lee WJ , Choi D , Lee SJ , Choi JY , Kim B-T , et al. . The value of PET/CT for preoperative staging of advanced gastric cancer: comparison with contrast-enhanced CT . Eur J Radiol 2011. ; 79 : 183 – 8 . doi: 10.1016/j.ejrad.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 67. Kawanaka Y , Kitajima K , Fukushima K , Mouri M , Doi H , Oshima T , et al. . Added value of pretreatment (18)F-FDG PET/CT for staging of advanced gastric cancer: Comparison with contrast-enhanced MDCT . Eur J Radiol 2016. ; 85 : 989 – 95 . doi: 10.1016/j.ejrad.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 68. Yang Q-M , Kawamura T , Itoh H , Bando E , Nemoto M , Akamoto S , et al. . Is PET-CT suitable for predicting lymph node status for gastric cancer? Hepatogastroenterology 2008. ; 55 ( 82-83 ): 782 – 5 . [PubMed] [Google Scholar]
- 69. Hasbahceci M , Akcakaya A , Memmi N , Turkmen I , Cipe G , Yildiz P , et al. . Diffusion MRI on lymph node staging of gastric adenocarcinoma . Quant Imaging Med Surg 2015. ; 5 : 392 – 400 . doi: 10.3978/j.issn.2223-4292.2015.03.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhong J , Zhao W , Ren F , Qi S , Wang X , Lv T , et al. . Lymph node metastasis in patients with gastric cancer: a multi-modality, morphologic and functional imaging study . Am J Transl Res 2016. ; 8 : 5601 – 9 . [PMC free article] [PubMed] [Google Scholar]
- 71. Cheng J , Wang Y , Deng J , McCarthy RJ , Wang G , Wang H , et al. . Discrimination of metastatic lymph nodes in patients with gastric carcinoma using diffusion-weighted imaging . J Magn Reson Imaging 2013. ; 37 : 1436 – 44 . doi: 10.1002/jmri.23925 [DOI] [PubMed] [Google Scholar]
- 72. Tokuhara T , Tanigawa N , Matsuki M , Nomura E , Mabuchi H , Lee S-W , et al. . Evaluation of lymph node metastases in gastric cancer using magnetic resonance imaging with ultrasmall superparamagnetic iron oxide (USPIO): diagnostic performance in post-contrast images using new diagnostic criteria . Gastric Cancer 2008. ; 11 : 194 – 200 . doi: 10.1007/s10120-008-0480-9 [DOI] [PubMed] [Google Scholar]
- 73. Tatsumi Y , Tanigawa N , Nishimura H , Nomura E , Mabuchi H , Matsuki M , et al. . Preoperative diagnosis of lymph node metastases in gastric cancer by magnetic resonance imaging with ferumoxtran-10 . Gastric Cancer 2006. ; 9 : 120 – 8 . doi: 10.1007/s10120-006-0365-8 [DOI] [PubMed] [Google Scholar]
- 74. Vasanawala SS , Nguyen K-L , Hope MD , Bridges MD , Hope TA , Reeder SB , et al. . Safety and technique of ferumoxytol administration for MRI . Magn Reson Med 2016. ; 75 : 2107 – 11 . doi: 10.1002/mrm.26151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dixon M , Mahar AL , Helyer LK , Vasilevska-Ristovska J , Law C , Coburn NG . Prognostic factors in metastatic gastric cancer: results of a population-based, retrospective cohort study in Ontario . Gastric Cancer 2016. ; 19 : 150 – 9 . doi: 10.1007/s10120-014-0442-3 [DOI] [PubMed] [Google Scholar]
- 76. Sarela AI , Miner TJ , Karpeh MS , Coit DG , Jaques DP , Brennan MF . Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma . Ann Surg 2006. ; 243 : 189 – 95 . doi: 10.1097/01.sla.0000197382.43208.a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Coburn N , Seevaratnam R , Paszat L , Helyer L , Law C , Swallow C , et al. . Optimal management of gastric cancer: results from an international RAND/UCLA expert panel . Ann Surg 2014. ; 259 : 102 – 8 . doi: 10.1097/SLA.0b013e318288dd2b [DOI] [PubMed] [Google Scholar]
- 78. Kwee RM , Kwee TC . Modern imaging techniques for preoperative detection of distant metastases in gastric cancer . World J Gastroenterol 2015. ; 21 : 10502 – 9 . doi: 10.3748/wjg.v21.i37.10502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim SJ , Kim H-H , Kim YH , Hwang SH , Lee HS , Park DJ , et al. . Peritoneal metastasis: detection with 16- or 64-detector row CT in patients undergoing surgery for gastric cancer . Radiology 2009. ; 253 : 407 – 15 . doi: 10.1148/radiol.2532082272 [DOI] [PubMed] [Google Scholar]
- 80. Feng X-Y , Wang W , Luo G-Y , Wu J , Zhou Z-W , Li W , et al. . Comparison of endoscopic ultrasonography and multislice spiral computed tomography for the preoperative staging of gastric cancer - results of a single institution study of 610 Chinese patients . PLoS One 2013. ; 8 : e78846 . doi: 10.1371/journal.pone.0078846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chu K-M , Kwok K-F , Law S , Wong K-H . A prospective evaluation of catheter probe EUS for the detection of ascites in patients with gastric carcinoma . Gastrointest Endosc 2004. ; 59 : 471 – 4 . doi: 10.1016/S0016-5107(03)02873-6 [DOI] [PubMed] [Google Scholar]
- 82. Lee YT , Ng EKW , Hung LCT , Chung SCS , Ching JYL , Chan WY , et al. . Accuracy of endoscopic ultrasonography in diagnosing ascites and predicting peritoneal metastases in gastric cancer patients . Gut 2005. ; 54 : 1541 – 5 . doi: 10.1136/gut.2004.055772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Smyth E , Schöder H , Strong VE , Capanu M , Kelsen DP , Coit DG , et al. . A prospective evaluation of the utility of 2-deoxy-2-[(18) F] fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer . Cancer 2012. ; 118 : 5481 – 8 . doi: 10.1002/cncr.27550 [DOI] [PubMed] [Google Scholar]
- 84. Leake P-A , Cardoso R , Seevaratnam R , Lourenco L , Helyer L , Mahar A , et al. . A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer . Gastric Cancer 2012. ; 15 ( S1 ): 38 – 47 . doi: 10.1007/s10120-011-0047-z [DOI] [PubMed] [Google Scholar]
- 85. Smyth EC , Verheij M , Allum W , Cunningham D , Cervantes A , Arnold D , et al. . Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up . Ann Oncol 2016. ; 27 ( suppl_5 ): v38 – 49 . doi: 10.1093/annonc/mdw350 [DOI] [PubMed] [Google Scholar]
- 86. Soussan M , Des Guetz G , Barrau V , Aflalo-Hazan V , Pop G , Mehanna Z , et al. . Comparison of FDG-PET/CT and MR with diffusion-weighted imaging for assessing peritoneal carcinomatosis from gastrointestinal malignancy . Eur Radiol 2012. ; 22 : 1479 – 87 . doi: 10.1007/s00330-012-2397-2 [DOI] [PubMed] [Google Scholar]
- 87. Maegerlein C , Fingerle AA , Souvatzoglou M , Rummeny EJ , Holzapfel K . Detection of liver metastases in patients with adenocarcinomas of the gastrointestinal tract: comparison of (18)F-FDG PET/CT and MR imaging . Abdom Imaging 2015. ; 40 : 1213 – 22 . doi: 10.1007/s00261-014-0283-x [DOI] [PubMed] [Google Scholar]
- 88. Han NY , Park BJ , Sung DJ , Kim MJ , Cho SB , Lee CH , et al. . Chemotherapy-induced focal hepatopathy in patients with gastrointestinal malignancy: gadoxetic Acid–enhanced and diffusion-weighted MR imaging with clinical-pathologic correlation . Radiology 2014. ; 271 : 416 – 25 . doi: 10.1148/radiol.13131810 [DOI] [PubMed] [Google Scholar]
- 89. Zheng D-X , Meng S-C , Liu Q-J , Li C-T , Shang X-D , Zhu Y-S , et al. . Predicting liver metastasis of gastrointestinal tract cancer by diffusion-weighted imaging of apparent diffusion coefficient values . World J Gastroenterol 2016. ; 22 : 3031 . doi: 10.3748/wjg.v22.i10.3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chandarana H , Taouli B , Diffusion-Weighted MRI , Metastases L . Diffusion-weighted MRI and liver metastases . Magn Reson Imaging Clin N Am 2010. ; 18 : 451 – 64 . doi: 10.1016/j.mric.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 91. Cantwell CP , Setty BN , Holalkere N , Sahani DV , Fischman AJ , Blake MA . Liver lesion detection and characterization in patients with colorectal cancer: a comparison of low radiation dose non-enhanced PET/CT, contrast-enhanced PET/CT, and liver MRI . J Comput Assist Tomogr 2008. ; 32 : 738 – 44 . doi: 10.1097/RCT.0b013e3181591d33 [DOI] [PubMed] [Google Scholar]
- 92. Hardie AD , Naik M , Hecht EM , Chandarana H , Mannelli L , Babb JS , et al. . Diagnosis of liver metastases: value of diffusion-weighted MRI compared with gadolinium-enhanced MRI . Eur Radiol 2010. ; 20 : 1431 – 41 . doi: 10.1007/s00330-009-1695-9 [DOI] [PubMed] [Google Scholar]
- 93. Galea N , Cantisani V , Taouli B . Liver lesion detection and characterization: role of diffusion-weighted imaging . J Magn Reson Imaging 2013. ; 37 : 1260 – 76 . doi: 10.1002/jmri.23947 [DOI] [PubMed] [Google Scholar]
- 94. Xiong B-H , Cheng Y , Ma L , Zhang C-Q . An updated meta-analysis of randomized controlled trial assessing the effect of neoadjuvant chemotherapy in advanced gastric cancer . Cancer Invest 2014. ; 32 : 272 – 84 . doi: 10.3109/07357907.2014.911877 [DOI] [PubMed] [Google Scholar]
- 95. Ronellenfitsch U , Schwarzbach M , Hofheinz R , Kienle P , Kieser M , Slanger TE , et al. . Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus . Cochrane Database Syst Rev 2013. ; 29 . doi: 10.1002/14651858.CD008107.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ott K , Fink U , Becker K , Stahl A , Dittler H-J , Busch R , et al. . Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial . J Clin Oncol 2003. ; 21 : 4604 – 10 . doi: 10.1200/JCO.2003.06.574 [DOI] [PubMed] [Google Scholar]
- 97. Di Fabio F , Pinto C , Rojas Llimpe FL , Fanti S , Castellucci P , Longobardi C , et al. . The predictive value of 18F-FDG-PET early evaluation in patients with metastatic gastric adenocarcinoma treated with chemotherapy plus cetuximab . Gastric Cancer 2007. ; 10 : 221 – 7 . doi: 10.1007/s10120-007-0438-3 [DOI] [PubMed] [Google Scholar]
- 98. Trip AK , Verheij M , van Sandick JW , Boot H M , Jansen EP , Cats A . Emerging issues in multimodality treatment of gastric cancer . Transl Gastrointest Cancer 2015. ; 4 : 154 – 73 . [Google Scholar]
- 99. Jamel S , Markar SR , Malietzis G , Acharya A , Athanasiou T , Hanna GB . Prognostic significance of peritoneal lavage cytology in staging gastric cancer: systematic review and meta-analysis . Gastric Cancer 2018. ; 21 : 10 – 18 . doi: 10.1007/s10120-017-0749-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cho H , Nakamura J , Asaumi Y , Yabusaki H , Sakon M , Takasu N , et al. . Long-term survival outcomes of advanced gastric cancer patients who achieved a pathological complete response with neoadjuvant chemotherapy: a systematic review of the literature . Ann Surg Oncol 2015. ; 22 : 787 – 92 . doi: 10.1245/s10434-014-4084-9 [DOI] [PubMed] [Google Scholar]
- 101. Eisenhauer EA , Therasse P , Bogaerts J , Schwartz LH , Sargent D , Ford R , et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1 . Eur J Cancer 2009. ; 45 : 228 – 47 . doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 102. De Cobelli F , Giganti F , Orsenigo E , Cellina M , Esposito A , Agostini G , et al. . Apparent diffusion coefficient modifications in assessing gastro-oesophageal cancer response to neoadjuvant treatment: comparison with tumour regression grade at histology . Eur Radiol 2013. ; 23 : 2165 – 74 . doi: 10.1007/s00330-013-2807-0 [DOI] [PubMed] [Google Scholar]
- 103. Kwee RM , Kwee TC . Role of imaging in predicting response to neoadjuvant chemotherapy in gastric cancer . World J Gastroenterol 2014. ; 20 : 1650 . doi: 10.3748/wjg.v20.i7.1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lee SM , Kim SH , Lee JM , Im S-A , Bang Y-J , Kim WH , et al. . Usefulness of CT volumetry for primary gastric lesions in predicting pathologic response to neoadjuvant chemotherapy in advanced gastric cancer . Abdom Imaging 2009. ; 34 : 430 – 40 . doi: 10.1007/s00261-008-9420-8 [DOI] [PubMed] [Google Scholar]
- 105. Tahara T , Shibata T , Okubo M , Kawamura T , Horiguchi N , Yoshida D , et al. . Evaluations of primary lesions by endoscopy clearly distinguishes prognosis in patients with gastric cancer who receive chemotherapy . PLoS One 2017. ; 12 : e0173663 : e0173663 . doi: 10.1371/journal.pone.0173663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lundsgaard Hansen M , Fallentin E , Lauridsen C , Law I , Federspiel B , Bæksgaard L , et al. . Computed tomography (CT) perfusion as an early predictive marker for treatment response to neoadjuvant chemotherapy in gastroesophageal junction cancer and gastric cancer--a prospective study . PLoS One 2014. ; 9 : e97605 . doi: 10.1371/journal.pone.0097605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Koh D-M , Scurr E , Collins D , Kanber B , Norman A , Leach MO , et al. . Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients . AJR Am J Roentgenol 2007. ; 188 : 1001 – 8 . doi: 10.2214/AJR.06.0601 [DOI] [PubMed] [Google Scholar]
- 108. Harry VN . Novel imaging techniques as response biomarkers in cervical cancer . Gynecol Oncol 2010. ; 116 : 253 – 61 . doi: 10.1016/j.ygyno.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 109. Hein PA , Kremser C , Judmaier W , Griebel J , Pfeiffer K-P , Kreczy A , et al. . Diffusion-weighted magnetic resonance imaging for monitoring diffusion changes in rectal carcinoma during combined, preoperative chemoradiation: preliminary results of a prospective study . Eur J Radiol 2003. ; 45 : 214 – 22 . doi: 10.1016/S0720-048X(02)00231-0 [DOI] [PubMed] [Google Scholar]
- 110. Kim SH , Lee JY , Lee JM , Han JK , Choi BI . Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer . Eur Radiol 2011. ; 21 : 987 – 95 . doi: 10.1007/s00330-010-1989-y [DOI] [PubMed] [Google Scholar]
- 111. Cui Y , Zhang X-P , Sun Y-S , Tang L , Shen L . Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases . Radiology 2008. ; 248 : 894 – 900 . doi: 10.1148/radiol.2483071407 [DOI] [PubMed] [Google Scholar]
- 112. Park SH , Moon WK , Cho N , Song IC , Chang JM , Park I-A , et al. . Diffusion-weighted MR imaging: pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer . Radiology 2010. ; 257 : 56 – 63 . doi: 10.1148/radiol.10092021 [DOI] [PubMed] [Google Scholar]
- 113. van Rossum PSN , van Lier ALHMW , van Vulpen M , Reerink O , Lagendijk JJW , Lin SH , et al. . Diffusion-weighted magnetic resonance imaging for the prediction of pathologic response to neoadjuvant chemoradiotherapy in esophageal cancer . Radiother Oncol 2015. ; 115 : 163 – 70 . doi: 10.1016/j.radonc.2015.04.027 [DOI] [PubMed] [Google Scholar]
- 114. Giganti F , De Cobelli F , Canevari C , Orsenigo E , Gallivanone F , Esposito A , et al. . Response to chemotherapy in gastric adenocarcinoma with diffusion-weighted MRI and 18 F-FDG-PET/CT: Correlation of apparent diffusion coefficient and partial volume corrected standardized uptake value with histological tumor regression grade . J. Magn. Reson. Imaging 2014. ; 40 : 1147 – 57 . doi: 10.1002/jmri.24464 [DOI] [PubMed] [Google Scholar]
- 115. Zhong J , Zhao W , Ma W , Ren F , Qi S , Zheng J , et al. . DWI as a quantitative biomarker in predicting chemotherapeutic efficacy at Multitime points on gastric cancer lymph nodes metastases . Medicine 2016. ; 95 : e3236 . doi: 10.1097/MD.0000000000003236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lee DH , Kim SH , Im S-A , Oh D-Y , Kim T-Y , Han JK . Multiparametric fully-integrated 18-FDG PET/MRI of advanced gastric cancer for prediction of chemotherapy response: a preliminary study . Eur Radiol 2016. ; 26 : 2771 – 8 . doi: 10.1007/s00330-015-4105-5 [DOI] [PubMed] [Google Scholar]
- 117. Leong T , Smithers BM , Michael M , Gebski V , Boussioutas A , Miller D , et al. . TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG . BMC Cancer 2015. ; 15 : 532 . doi: 10.1186/s12885-015-1529-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lagendijk JJW , Raaymakers BW , Raaijmakers AJE , Overweg J , Brown KJ , Kerkhof EM , et al. . MRI/linac integration . Radiother Oncol 2008. ; 86 : 25 – 9 . doi: 10.1016/j.radonc.2007.10.034 [DOI] [PubMed] [Google Scholar]
- 119. Pollard JM , Wen Z , Sadagopan R , Wang J , Ibbott GS . The future of image-guided radiotherapy will be Mr guided . Br J Radiol 2017. ; 90 : 20160667 . doi: 10.1259/bjr.20160667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Raaymakers BW , Lagendijk JJW , Overweg J , Kok JGM , Raaijmakers AJE , Kerkhof EM , et al. . Integrating a 1.5 T MRI scanner with a 6 mV accelerator: proof of concept . Phys Med Biol 2009. ; 54 : N229 – N237 . doi: 10.1088/0031-9155/54/12/N01 [DOI] [PubMed] [Google Scholar]
- 121. Kerkmeijer LGW , Fuller CD , Verkooijen HM , Verheij M , Choudhury A , Harrington KJ , et al. . The MRI-Linear accelerator Consortium: evidence-based clinical introduction of an innovation in radiation oncology connecting researchers, methodology, data collection, quality assurance, and technical development . Front Oncol 2016. ; 6 : 215 . doi: 10.3389/fonc.2016.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yan Y , Yang J , Beddar S , et al. . A methodology to investigate the impact of image distortions on the radiation dose when using magnetic resonance images for planning . Phys Med Biol. March 2018. . doi: 10.1088/1361-6560/aab5c3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mittauer K , Paliwal B , Hill P , Bayouth JE , Geurts MW , Baschnagel AM , et al. . A new era of image guidance with magnetic resonance-guided radiation therapy for abdominal and thoracic malignancies . Cureus 2018. ; 10 : e2422 . doi: 10.7759/cureus.2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Xin X , Sha H , Shen J , Zhang B , Zhu B , Liu B . Coupling Gd‑DTPA with a bispecific, recombinant protein anti‑EGFR‑iRGD complex improves tumor targeting in MRI . Oncol Rep 2016. ; 35 : 3227 – 35 . doi: 10.3892/or.2016.4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Choi J-I , Joo I , Lee JM . State-of-the-art preoperative staging of gastric cancer by MDCT and magnetic resonance imaging . World J Gastroenterol 2014. ; 20 : 4546 – 57 . doi: 10.3748/wjg.v20.i16.4546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Van Pelt VWJ , Kruis MF , Van de Lindt T , Ter Beek LC , Verheij M , Van der Heide UA . PO-0904: development of an MRI-protocol for radiotherapy treatment guidance in gastric cancer . Radiotherapy and Oncology 2017. ; 123 : S499 – S500 . doi: 10.1016/S0167-8140(17)31341-5 [DOI] [Google Scholar]
- 127. Tummers QRJG , Boogerd LSF , de Steur WO , Verbeek FPR , Boonstra MC , Handgraaf HJM , et al. . Near-infrared fluorescence sentinel lymph node detection in gastric cancer: a pilot study . World J Gastroenterol 2016. ; 22 : 3644 – 51 . doi: 10.3748/wjg.v22.i13.3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cats A , Jansen EPM , van Grieken NCT , Sikorska K , Lind P , Nordsmark M , et al. . Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (critics): an international, open-label, randomised phase 3 trial . Lancet Oncol 2018. ; 19 : 616 – 28 . doi: 10.1016/S1470-2045(18)30132-3 [DOI] [PubMed] [Google Scholar]
- 129. Giganti F , Tang L , Baba H . Gastric cancer and imaging biomarkers: Part 1 – a critical review of DW-MRI and CE-MDCT findings . Eur Radiol 2018. : 1 – 11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Avcu S , Arslan H , Unal O , Kotan C , Izmirli M . The role of diffusion-weighted MR imaging and ADC values in the diagnosis of gastric tumors . JBR-BTR 2012. ; 95 : 1 – 5 . doi: 10.5334/jbr-btr.62 [DOI] [PubMed] [Google Scholar]
- 131. Onur MR , Ozturk F , Aygun C , Poyraz AK , Ogur E . Role of the apparent diffusion coefficient in the differential diagnosis of gastric wall thickening . J Magn Reson Imaging 2012. ; 36 : 672 – 7 . doi: 10.1002/jmri.23698 [DOI] [PubMed] [Google Scholar]
- 132. Zhang Y , Chen J , Liu S , Shi H , Guan W , Ji C , et al. . Assessment of histological differentiation in gastric cancers using whole-volume histogram analysis of apparent diffusion coefficient maps . J Magn Reson Imaging 2017. ; 45 : 440 – 9 . doi: 10.1002/jmri.25360 [DOI] [PubMed] [Google Scholar]
- 133. Liu S , Wang H , Guan W , Pan L , Zhou Z , Yu H , et al. . Preoperative apparent diffusion coefficient value of gastric cancer by diffusion-weighted imaging: correlations with postoperative TNM staging . J Magn Reson Imaging 2015. ; 42 : 837 – 43 . doi: 10.1002/jmri.24841 [DOI] [PubMed] [Google Scholar]
- 134. Giganti F , Ambrosi A , Chiari D , Orsenigo E , Esposito A , Mazza E , et al. . Apparent diffusion coefficient by diffusion-weighted magnetic resonance imaging as a sole biomarker for staging and prognosis of gastric cancer . Chinese J Cancer Res 2017. ; 29 : 118 – 26 . doi: 10.21147/j.issn.1000-9604.2017.02.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. He J , Shi H , Zhou Z , Chen J , Guan W , Wang H , et al. . Correlation between apparent diffusion coefficients and HER2 status in gastric cancers: pilot study . BMC Cancer 2015. ; 15 : 749 . doi: 10.1186/s12885-015-1726-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ji C , Zhang Q , Guan W , Guo T , Chen L , Liu S , et al. . Role of intravoxel incoherent motion MR imaging in preoperative assessing HER2 status of gastric cancers . Oncotarget 2017. ; 8 : 49293 – 302 . doi: 10.18632/oncotarget.17570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Giganti F , Orsenigo E , Esposito A , Chiari D , Salerno A , Ambrosi A , et al. . Prognostic role of diffusion-weighted MR imaging for resectable gastric cancer . Radiology 2015. ; 276 : 444 – 52 . doi: 10.1148/radiol.15141900 [DOI] [PubMed] [Google Scholar]
- 138. Liu S , Zheng H , Zhang Y , et al. . Whole-volume apparent diffusion coefficient-based entropy parameters for assessment of gastric cancer aggressiveness . J Magn Reson Imaging 2017. . [DOI] [PubMed] [Google Scholar]