Abstract
Objective:
The objective is to establish interscan, inter- and intra-rater reproducibility of a multicontrast three-dimensional contrast-enhanced intracranial vessel wall (IVW) MRI protocol with 0.6 mm acquired (0.3 mm interpolated) isotropic resolution in the detection of intracranial atherosclerosis.
Methods:
Subjects with established intracranial atherosclerosis were prospectively recruited and underwent two contrast-enhanced three-dimensional IVW scans within a 2-week period. Four raters with varying degrees of vessel wall imaging interpretation experience, through an iterative training process developed guidelines for plaque identification with no, possible and definite plaque categories. Using these guidelines, the raters reviewed the cases in pairs (consensus rating), while blinded to the interpretations of the other pair, clinical reports and patient history. The rater pairs reviewed 19 segments per patient for the presence and location of atherosclerotic plaques. Inter-scan, inter rater and intra rater reproducibility were assessed.
Results:
19 subjects were scanned twice, with 361 total segments reviewed and 304–324 evaluable segments analyzed in the different reproducibility assessments. Overall inter-rater agreement for possible and definite plaque was 88.9 % [κ = 0.73; 95% confidence interval (CI) (0.62–0.81)], inter-scan/intra-rater agreement was 82.1 % [κ = 0.58; 95% CI (0.48–0.70)] and inter-scan/inter-rater agreement of 84.5% [κ = 0.64; 95% CI (0.51 – 0.76)].
Conclusion:
Contrast-enhanced IVW imaging, with the utilization of detailed plaque definition guidelines for image review, can be a reproducible technique for the evaluation of intracranial atherosclerosis.
Advances in knowledge:
This work is the first to establish reproducibility of IVW for plaque identification with and without contrast. Reproducibility using contrast is important as most IVW applications rely on lesion enhancement.
Intracranial vessel wall (IVW) MRI is an emerging technique for the differentiation1–3 and characterization4–7 of intracranial vasculopathies. IVW has shown accuracy as well as added value in vasculopathy differentiation when compared to luminal imaging alone.1,3 In addition, IVW has shown the ability to characterize vulnerable features associated with aneurysms5 and intracranial atherosclerosis (ICAD)4 that with continued investigation, may help stratify lesion risk of future events.
Non-contrast IVW has been shown to be a relatively reproducible imaging technique when considering inter-scan, inter- and intra-rater reliability for the evaluation of ICAD,8 however, the diagnostic value of IVW heavily relies on utilization of post-contrast imaging, as the pattern, presence and intensity of lesion enhancement plays a role in disease differentiation,2 plaque symptomatology9 and detection. Increased intensity and presence of plaque enhancement has been shown to significantly correlate with culprit plaque status.9,10 In addition, the degree of plaque enhancement in cross-sectional assessment has been shown to correlate with temporal proximity to the stroke event.11 Enhancement characteristics of plaques also play an important role in differentiation of ICAD from other intracranial vasculopathies, including reversible cerebral vasoconstriction syndrome and vasculitis2,3 as well as Moyamoya vasculopathies.1 The current study is the first to prospectively evaluate the reproducibility of a multicontrast three-dimensional (3D) IVW protocol that includes pre- and post-contrast imaging with acquired isotropic voxel size of 0.6 mm (0.3 mm interpolated) in patients with known ICAD.
Methods and materials
Patient population
After institutional review board approval, subjects were prospectively recruited from the radiology database based on the following criteria: (1) presence of intracranial arterial stenosis or irregularity on head CT angiography (CTA), and (2) ≥2 vascular risk factors (hypertension, diabetes mellitus, dyslipidemia, age >50 for males and >60 for females, obesity or smoking). Exclusion criteria were: (1) evidence of cardioembolic disease, (2) clinical suspicion of central nervous system vasculitis, (3) evidence of intracranial arterial dissection on imaging; (4) recent cranial trauma or previous radiation, (5) short-term stenosis reversibility on luminal imaging indicating a vasospastic process, (6) contraindication to MRI, (8) estimated glomerular filtration rate <60 mL/min/1.73 m2, (7) history of allergy to gadolinium contrast, (8) prior intracranial stent placement, (9) pregnancy, or (10) inability to provide informed consent. After identification, patients were contacted about the study and informed consent was obtained.
MRI protocol
For each patient, both scans were carried out on a Philips Ingenia 3T (Philips Healthcare; Best, Netherlands) scanner with 32 channel head coil. The 3D Intracranial Wall Imaging protocol (3D-WALLI) consists of four sequences (Table 1) all at 0.6 mm3 acquired resolution (interpolated to 0.3 mm3). T 1 weighting (T 1W) and proton density weighting (PDw) were implemented using a variable flip angle Volume ISotropic Turbo spin echo Acquisition (VISTA) acquisition. PDw VISTA was acquired with Delay Alternating with Nutation for Tailored Excitation preparation for blood and cerebrospinal fluid suppression.12 Before contrast administration, 3D time-of-flight MR angiography (MRA) was also performed. Post-contrast T 1W-VISTA was scanned 3 min after single dose gadolinium contrast injection (0.1 mmol/kg of Prohance at 2 cc/s). Total scan time was approximately 20 min. The imaging parameters are summarized in Table 1. The follow-up IVW scan was performed within 2 weeks of the first MRI study.
Table 1.
MRI imaging parameters
| Sequence | T1W VISTA | PDw DANTE VISTA | Post-T1W VISTA | 3D-TOF |
| Orientation | Coronal | Coronal | Coronal | Axial |
| Field of view | 180 × 180 × 50 0.6 × 0.6 × 0.6 |
190 × 190 × 72 | ||
| Resolution(mm) | 0.6 × 0.6 × 1.2 | |||
| Interpolated Resolution (mm) | 0.3 × 0.3 × 0.3 | 0.6 × 0.6 × 0.6 | ||
| TR (ms) | 1400 | 3000 | 1400 | 15 |
| TE (ms) | 30 | 30 | 30 | 3.5 |
| Flip angle(˚) | Varies | Varies | Varies | 18 |
| Echo train length | 45 | 100 | 45 | NA |
| Averages | 1 | 1 | 1 | 1 |
| Preparation | No | DANTE | No | No |
| Scan time (min:s) | 5:57 | 5:38 | 5:57 | 3:21 |
DANTE, delay alternating with nutation for tailored excitation; PD, proton density; PDw, proton density weighting; TE, echo time; TOF, time of flight; TR, repetition time; T 1W, T 1 weighting; VISTA, volume isotropic turbo spin echo acquisition; min, minutes; mm, millimeters; ms, milliseconds; s, seconds.
Plaque identification criteria
A set of guidelines were developed for IVW review for the determination of presence or absence of atherosclerotic plaques through an iterative development and training process. The raters initially met to draft a preliminary set of guidelines and then independently rated five training cases, separate from the cases acquired for the reproducibility study. After this initial evaluation, raters reconvened to compare results and provide feedback on the guidelines. The guidelines were refined and training was repeated with a new set of five IVW training cases. This process of iteratively rating the training cases and refining criteria was performed four times until raters felt agreement was sufficient. The training cases were all clinically acquired 3D IVW scans with and without contrast using similar imaging parameters to the experimental scans in the current study. These cases all had imaging and clinically established ICAD.
Table 2 lists the review guidelines for identifying plaque. Supplementary Material 1 lists review guidelines established for non-contrast studies for ICAD identification. Depending on the imaging appearance, plaques were classified as possible or definite to characterize confidence in their presence. While non-stenotic plaque can occur,13 there typically is at least some luminal irregularity,6 which is why definite plaque requires the presence of luminal stenosis or irregularity while possible plaque includes wall thickening without any luminal abnormality. Flow artifacts can also occur that can mimic arterial wall lesions, and are more pronounced on post-contrast imaging.14 For this reason, we decided that the presence of wall thickening needed to be present on both pre- and post-contrast VISTA, with lumen shape and size matching between pre-, post-contrast VISTA and TOF MRA. In addition, plaque should show wall thickening on all sequences.15 Intracranial atherosclerotic plaques typically enhance, however, plaques enhance heterogeneously and incompletely, and typically not as bright as the blood pool,2 which should differentiate plaque from the enhancement of the cavernous sinus. Vasa vasorum typically only crosses a few millimeters into the intradural compartment but—with aging, hypertension and development of intracranial atherosclerosis—can grow in along the proximal intradural segments (proximal internal carotid and V4 vertebral arteries) without resultant luminal narrowing.16 This was also included in our possible plaque category as it would be difficult to differentiate from non-stenotic plaque. For each case, raters first reviewed each arterial segment independently in a plane perpendicular to the target lumen on the post-contrast T 1 VISTA sequence to find target lesions with wall thickening and/or enhancement. With identified lesions, the corresponding slices on the TOF MRA and pre-contrast T 1 VISTA were reviewed in tandem with the post-contrast images. In patients without post-contrast imaging, the lesions would be first identified on T 1 VISTA without contrast and then confirmed on Delay Alternating with Nutation for Tailored Excitation PD VISTA and TOF MRA. The reproducibility case review was initiated more than 3 months after the development of the guidelines.
Table 2.
Criteria for plaque assessment for reproducibility assessment
| Not assessable |
|
| No plaque |
|
| Possible plaque |
|
| Definite plaque |
|
CSF, cerebrospinal fluid; IVW, intracranial vessel wall; MRA, MR angiography; TOF, time of flight; VISTA, volume isotropic turbo spin echo acquisition.
Image analysis
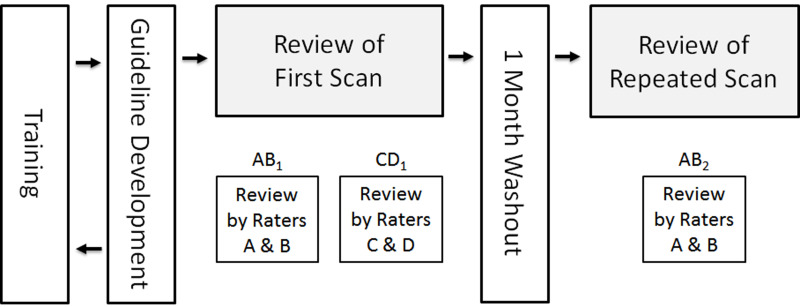
After criteria development, there was a 3 month washout period before image analysis started. The 19 cases were reviewed by four raters with 1–8 years of experience interpreting IVW using RadiAnt DICOM viewer software (Medixant; Poznan, Poland), blinded to clinical reports and patient history. Cases were reviewed in pairs with one rater performing a primary review and the second rater performing a peer review. Any disagreements between raters was resolved through consensus. Image review was performed in two major rounds, separated by a 1-month washout period. In Round 1, the first scan of each case was randomized twice to produce two copies with different identifiers. These scans were randomly assigned to rater pairs such that the two copies of each case were assigned to non-overlapping rater pairs, for 38 paired reviews total of the 19 cases. The two distinct rater pairs for each case performed their reviews independently of the other pair so inter rater agreement could be estimated. In Round 2, the second scan of each case was randomized and randomly assigned to rater pairs such that the rater pair that read the second scan also read one of the copies of the first scan, for 19 paired reviews total of the 19 cases. This allowed both inter-scan/intra-rater agreement (the same rater pair reviewed the first and second scans with a 1-month washout period in between) and inter scan/inter rater agreement (two non-overlapping rater pairs independently reviewed the first and second scans) to be estimated. The study design is illustrated in Figure 1.
Figure 1.
Flow chart of study design. The first scan of each subject was reviewed independently by two distinct rater pairs (A, B and C, D). The four study readers were randomly assigned to the A–D slots for each subject. The repeated scan was reviewed at least 1 month after the first scan by one of the same rater pairs. Inter-rater reproducibility was assessed by comparing the results of AB1 and CD1. Inter-scan/intra-reader reproducibility was assessed by comparing AB1 and AB2 while inter-scan–inter-reader reproducibility was assessed by comparing CD1 and AB2.
Each case was reviewed on a segment-by-segment basis (19 segments per case), with review including: paired A1 and A2 anterior (ACA), M1 and M2 middle (MCA) and P1 and P2 posterior (PCA) cerebral arteries, paired V4 vertebral arteries, cavernous and supraclinoid internal carotid arteries (ICA) and the basilar artery. These segments were selected for review as ICAD most frequently involves these segments. For each segment, the raters determined (1) how many plaques were present; (2) whether the lesion was possible or definite based on the criteria described in Table 2 (Figure 2); (3) whether the plaque involved the proximal, middle or distal portion of the arterial segment; and (4) the radial location of the lesion along the segment.
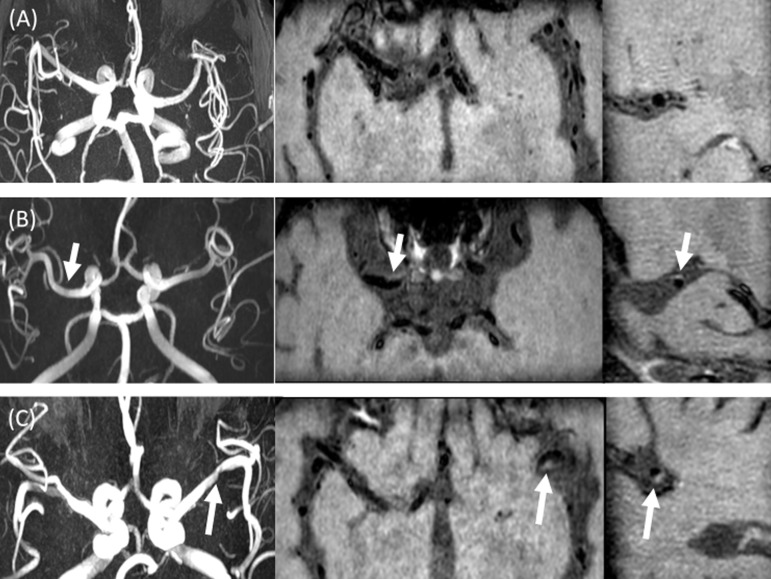
Figure 2.
Examples of plaque classifications. Figure 2A shows an example of “no plaque” category. There is no luminal stenosis on MRA (A, left), without evidence of wall thickening on axial T 1 pre-contrast (A, middle) and sagittal T 1 post-contrast MPR (A, right). Figure 2B shows an example of “possible plaque”. There is no evidence of luminal stenosis on MRA (B, left), with evidence of wall thickening (arrow) on axial T 1 pre-contrast (B, middle) and sagittal T 1 post-contrast MPR (B, right). Figure 2C shows an example of “definite plaque”. On MRA MIP image (C, left), there is focal stenosis of the left M1 MCA (arrow), with corresponding luminal narrowing and wall thickening (arrows) on axial T 1 pre-contrast (C, middle) and sagittal MPR T 1 post-contrast (C, right) images. MCA, middle cerebral artery; MIP, maximum intensity projection; MPR, multiplanar reformat; MRA, MR angiography.
All image analysis data were collected and managed using REDCap electronic data capture tools hosted at the University of Washington.17 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
Statistical analysis
Inter-rater and inter-scan reproducibility of detecting any plaque (possible or definite plaque) and detecting definite plaque were summarized using percent agreement and Cohen’ κ for both the rater training and reproducibility components of the study. Κ-values of agreement between 0 and 0.20 were defined as poor agreement; 0.21 and 0.40 as fair agreement; 0.41 and 0.60 as moderate agreement; 0.61 and 0.80 as good agreement; 0.81 and 1.0 as excellent agreement.18 Throughout, the unit of analysis was the arterial segment (up to 19 per patient). Reproducibility was summarized overall using all segments as well as summarized for individual segment groups: ICA segments (cavernous and supraclinoid segments), proximal segments (A1, M1, and P1), distal segments (A2, M2, and P2), and vertebrobasilar. The non-parametric bootstrap was used to calculate confidence intervals for κ and to compare κ estimates between groups of segments. Resampling was performed by subject to account for non-independence of segments from the same subject. The Bonferroni-corrected significance level α = 0.0083 was used when comparing the four segment groups to account for six pairwise statistical comparisons. All statistical calculations were conducted with the statistical computing language R (v. 3.1.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
Guideline development
Four rounds of rating for training were performed with the four reviewers rating the same five cases per round during the guideline development process. Inter-reader agreement improved for each successive round. For Round 1, overall agreement was 59.8% (κ = 0.22). For round 2, overall agreement was 68.6% (κ = 0.36). For round 3, overall agreement was 71.7% (κ = 0.32), and for the final round overall agreement was 76.2% (κ = 0.52).
Patient demographics
19 patients were prospectively recruited and included in the study. Clinical characteristics are summarized in Table 3. Post-contrast T 1W-VISTA was not available for four subjects. Contrast was not administered for at least one scan of two subjects due to either low estimated glomerular filtration rate detected immediately prior to imaging or technical difficulties with venous access. Contrast was administered for remaining two subjects but there was a technical failure during acquisition or archiving which prevented the post-contrast T 1W-VISTA images from being included in the review.
Table 3.
Subject demographics and risk factors
| Variable | Value |
| Male sex | 13 (68.4) |
| Age, years | 62 ± 12 |
| Race | |
| Asian | 2 (10.5) |
| Black or African American | 3 (15.8) |
| Native Hawaiian or Other Pacific Islander | 2 (10.5) |
| White | 12 (63.2) |
| History of hypertension | 16 (84.2) |
| History of hyperlipidemia | 13 (68.4) |
| History of diabetes | 10 (52.6) |
| Smoking status | |
| Current | 1 (5.3) |
| Former | 11 (57.9) |
| Never | 7 (36.8) |
A total of 361 segments from 19 patients were reviewed. There were 315 segments included in the inter rater analysis after excluding 46 segments were marked non-evaluable by at least one rater (20 by both raters). Similarly, there were 324 segments included in the inter scan/intra rater analysis after 37 were marked as non-evaluable by at least one rater (21 on both scans). Lastly, 304 segments were included in the inter scan/inter rater analysis after 57 segments were marked as non-evaluable by at least one rater (21 by both raters).
Overall reproducibility
Results of the three reproducibility analyses are summarized in Table 4. From the inter-rater analysis, there were 75 segments (23.8%) in total determined by both groups of raters to have any plaque (possible or definite), and 52 segments (16.5%) to have definite plaque. There was good inter-rater agreement for any plaque (88.9% agreement; κ = 0.73, 95% confidence interval CI 0.62–0.81) and for definite plaque (85.4% agreement; κ = 0.61, 95% CI 0.46–0.71).
Table 4.
Overall inter-rater and inter-scan reproducibility
| Inter-rater reproducibility (N = 315 segments) |
Inter-scan/Intra-rater
reproducibility (N = 324 segments) |
Inter-scan/Inter-rater
reproducibility (N = 304 segments) |
|||||||||||||
| Variable |
No. (%)
by both |
No. (%)
by either |
% Agree | κ | (95% CI) |
No. (%)
by both |
No. (%)
by either |
% Agree | κ | (95% CI) |
No. (%)
by both |
No. (%)
by either |
% Agree | κ | (95% CI) |
| Any plaque | 75 (23.8) | 110 (34.9) | 280 (88.9%) | 0.73 | (0.62, 0.81) | 71 (21.9) | 129 (39.8) | 266 (82.1) | 0.58 | (0.48, 0.70) | 70 (23.0) | 117 (38.5) | 257 (84.5) | 0.64 | (0.51, 0.76) |
| Definite plaque | 52 (16.5) | 98 (31.1) | 269 (85.4%) | 0.61 | (0.46, 0.71) | 56 (17.3) | 100 (30.9) | 280 (86.4) | 0.63 | (0.49, 0.75) | 53 (17.4) | 96 (31.6) | 261 (85.9) | 0.62 | (0.48, 0.74) |
CI, confidence interval.
Inter-scan/intra-rater reproducibility was moderate for any plaque (82.1% agreement; κ = 0.58, 95% CI 0.48–0.70) and good for definite plaque (86.4% agreement; κ = 0.63, 95% CI 0.49–0.75). For inter-scan/inter-rater reproducibility, there was good agreement for both any plaque (84.5% agreement; κ = 0.64, 95% CI 0.51–0.76) and definite plaque only (85.9% agreement; κ = 0.62, 95% CI 0.48–0.74). Inter-rater and inter-scan reproducibility were largely similar in the groups with and without post-contrast T 1w-VISTA (Supplementary Material 1).
Segment-level agreement
Details on segment-level inter rater reproducibility are described in detail in Supplementary Material 1. Inter rater reproducibility for any and definite plaque was moderate for the ICA segments (κ = 0.52–0.59), good for the proximal segments (κ = 0.67–0.75), and poor-to-fair for vertebrobasilar segments (κ = 0.13–0.26). For the distal segments, reproducibility was good for any plaque (κ = 0.60) yet poor for definite plaque (κ = 0.14). Reproducibility was significantly lower for any plaque in the vertebrobasilar segments than in the proximal segments (p < 0.0083). There were no other statistically significant differences in inter rater reproducibility between the segment groups.
Segment-level inter scan/intra rater reproducibility for any plaque and definite plaque was largely moderate-to-good across all segment groups (κ = 0.36–0.62, Supplementary Material 1), with no significant differences between groups. Inter scan/inter reader reproducibility for any plaque and definite plaque were moderate-to-good for ICA and proximal segments (κ = 0.44–0.73) and fair-to-moderate for distal and vertebrobasilar segments (κ = 0.29–0.58) (Supplementary Material 1). However, there were no statistically significant differences between segment groups.
Discussion
Evaluation of the reproducibility of contrast-enhanced IVW techniques for the detection of ICAD has not been performed, and to our knowledge, the current study is the first to evaluate the inter scan, inter rater and intra rater reproducibility of a multi contrast, contrast enhanced IVW MRI protocol in the setting of ICAD. In addition, through an iterative process, this study developed guidelines for the identification of ICAD that helped progressively improve agreement between raters with highly variable degrees of experience. These guidelines could be used in future studies and to help train new raters for IVW studies.
In the current study, overall inter-rater and inter-scan reproducibility were moderate-to-good for any plaque and definite plaque categories (κ: 0.58–0.73). This level of agreement indicates that through adequate training and utilization of guidelines defining plaque presence, contrast-enhanced IVW using a multicontrast can be considered reliable for the detection of ICAD.
There appeared to be some variation in the level of agreement for individual segment groups. Across the three different reproducibility analyses, reproducibility was moderate-to-good for the ICA and proximal segments (κ: 0.44–0.75) but tended to be lower for the vertebrobasilar segments in particular (κ: 0.13–0.46) and the distal segments to some extent (κ: 0.14–0.60). These results should be interpreted cautiously because the per-group sample size tended to be small, so confidence intervals were wide and most differences did not reach statistical significance after accounting for the number of comparisons. For vertebrobasilar evaluation, one potential reason for lower agreement was due to the vertebral artery segments being along the edge of the imaging field. In addition, the prepontine cistern is a common location for hyperdynamic cerebrospinal fluid flow, resulting in artifact making evaluation of the basilar artery more difficult. For distal segments, agreement may have been lower due to the smaller caliber of the arteries, and increased potential for cerebrospinal fluid flow artifact, especially along M2 MCA branches within the sylvian fissure being confused for lesions.
Qiao et al8 evaluated the inter-scan and inter-rater reliability of 102 participants of the Atherosclerosis Risk in Communities cohort that underwent non-contrast T 1 IVW (0.5 mm resolution) and TOF MRA, with oversampling of subjects with cognitive impairment. For presence of plaque, the study found overall moderate agreement for inter-rater reproducibility (κ = 0.59), and good agreement for intra-rater (κ = 0.70) and inter-scan (κ = 0.68) reproducibility. These results are similar to our findings of good inter-rater (κ = 0.73), moderate inter-scan/intra-rater (κ = 0.58), and good inter-scan/inter-rater (κ = 0.64) agreement. One difference between these two studies is the evaluation of reproducibility of ICAD detection in non-contrast exams versus contrast-enhanced IVW studies. In addition, our study incorporated a multicontrast protocol in the evaluation for reproducibility. Inclusion of T 2 weighted or PD-weighted IVW sequences into the protocol can more accurately depict plaque composition, including detection of fibrous cap and lipid rich necrotic core as well as aid in vasculopathy differentiation.2,15,19
Establishing the reproducibility of contrast-enhanced IVW is important as most studies focused on plaque identification,13,20 differentiation of vasculopathies2 and evaluation of plaque vulnerability9,11,21,22 rely on the presence and degree of plaque enhancement. In the evaluation of intracranial vasculopathies with luminal abnormalities on angiographic imaging, Mossa-Basha et al2 found that the intensity and pattern of lesion enhancement contributed to the differentiation of ICAD, vasculitis and reversible cerebral vasoconstriction, as did other factors including the pattern of wall involvement and lesion T 2 signal characteristics. In the evaluation of 78 plaques in 20 patients with acute ischemic stroke, it was found that plaque enhancement greater than or equal to the enhancement of the pituitary infundibulum was associated with culprit lesions [odds ratio 34.6; 95% CI (4.5–266.5). Culprit plaques showed a higher degree of enhancement compared to non-culprit lesions (25.9% ± 13.4 vs 13.6% ± 12.3, p = .003) and culprit lesions always enhanced.9 These studies, in addition to many others, emphasize the importance of utilization of contrast in IVW scans.
There are a number of limitations to the current study. First, we relied on luminal imaging to identify ICAD based on the presence of luminal stenosis or irregularity, though non-stenotic plaque can occur.13,20 However, in our experience, non-stenotic plaques typically do present with some degree of luminal irregularity, so our concern was that if the inclusion criteria was relaxed, the number of artifactual lesions identified would be much greater than the number of actual non-stenotic plaques. Second, not all patients in the current study received contrast due to a drop in kidney function discovered at the time of imaging or technical reasons. However, overall reproducibility did not appear to differ between the contrast-enhanced and four non-contrast IVW studies. In addition, through the evaluation of contrast-enhanced and non-contrast studies, this bolsters the plaque identification guidelines developed in the current study that can contribute to clinical or investigative training for ICAD detection. Third, our agreement estimates correspond to the performance of paired/consensus reviews, which could differ from the performance of individual raters. This approach, however, has been used in multiple previous studies evaluating rater reproducibility in vessel wall imaging evaluation.23–26 Fourth, there is no histological confirmation of the presence of ICAD in the identified lesions, which is a problem in most IVW studies. Reliance on luminal imaging for the confirmation of lesions and our strict inclusion criteria to exclude other vascular diseases mitigate this limitation. Lastly, the same raters were used to develop the criteria and rate studies, which could limit generalizability to other raters. To mitigate this limitation, we selected reviewers with variable degrees of experience, and kept the criteria development and rating processes independent and separated by a 3 month washout period.
Conclusion
Contrast-enhanced high-resolution IVW imaging, with the utilization of detailed plaque definition guidelines for image review, can be a reproducible technique for the evaluation of intracranial atherosclerosis.
Footnotes
Acknowledgment: The study was supported by NIH grants R01 NS092207 01A1 and R56 NS092207 01A1 funded by the National Institute of Neurodegenerative Diseases and Stroke (NINDS).
The authors of this manuscript declare relationships with the following companies:
Mahmud Mossa-Basha, Tom Hatsukami and Chun Yuan have research grants supported by Philips Healthcare.
Daniel Hippe has research grants supported by Philips Healthcare, GE Healthcare, Toshiba and Siemens Healthineers.
Contributor Information
Mahmud Mossa-Basha, Email: mmossab@uw.edu.
Hiroko Watase, Email: hiroko7@uw.edu.
Jie Sun, Email: sunjie@uw.edu.
Dean K. Shibata, Email: shibatad@uw.edu.
Daniel S. Hippe, Email: dhippe@uw.edu.
Niranjan Balu, Email: ninja@uw.edu.
Thomas Hatsukami, Email: tomhat@uw.edu.
Chun Yuan, Email: cyuan@uw.edu.
REFERENCES
- 1. Mossa-Basha M , de Havenon A , Becker KJ , Hallam DK , Levitt MR , Cohen WA , et al. . Added value of vessel wall magnetic resonance imaging in the differentiation of moyamoya vasculopathies in a non-Asian cohort . Stroke 2016. ; 47 : 1782 – 8 . doi: 10.1161/STROKEAHA.116.013320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mossa-Basha M , Hwang WD , De Havenon A , Hippe D , Balu N , Becker KJ , et al. . Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes . Stroke 2015. ; 46 : 1567 – 73 . doi: 10.1161/STROKEAHA.115.009037 [DOI] [PubMed] [Google Scholar]
- 3. Mossa-Basha M , Shibata DK , Hallam DK , de Havenon A , Hippe DS , Becker KJ , et al. . Added value of vessel wall magnetic resonance imaging for differentiation of Nonocclusive intracranial vasculopathies . Stroke 2017. ; 48 : 3026 – 33 . doi: 10.1161/STROKEAHA.117.018227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Havenon A , Mossa-Basha M , Shah L , Kim S-E , Park M , Parker D , et al. . High-resolution vessel wall MRI for the evaluation of intracranial atherosclerotic disease . Neuroradiology 2017. ; 59 : 1193 – 202 . doi: 10.1007/s00234-017-1925-9 [DOI] [PubMed] [Google Scholar]
- 5. Lehman VT , Brinjikji W , Mossa-Basha M , Lanzino G , Rabinstein AA , Kallmes DF , et al. . Conventional and high-resolution vessel wall MRI of intracranial aneurysms: current concepts and new horizons . J Neurosurg 2018. ; 128 : 969 – 81 . doi: 10.3171/2016.12.JNS162262 [DOI] [PubMed] [Google Scholar]
- 6. Mandell DM , Mossa-Basha M , Qiao Y , Hess CP , Hui F , Matouk C , et al. . Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology . AJNR Am J Neuroradiol 2017. ; 38 : 218 – 29 . doi: 10.3174/ajnr.A4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mossa-Basha M , Alexander M , Gaddikeri S , Yuan C , Gandhi D . Vessel wall imaging for intracranial vascular disease evaluation . J Neurointerv Surg 2016. ; 8 : 1154 – 9 . doi: 10.1136/neurintsurg-2015-012127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qiao Y , Guallar E , Suri FK , Liu L , Zhang Y , Anwar Z , et al. . MR imaging measures of intracranial atherosclerosis in a population-based study . Radiology 2016. ; 280 : 860 – 8 . doi: 10.1148/radiol.2016151124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qiao Y , Zeiler SR , Mirbagheri S , Leigh R , Urrutia V , Wityk R , et al. . Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images . Radiology 2014. ; 271 : 534 – 42 . doi: 10.1148/radiol.13122812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vakil P , Vranic J , Hurley MC , Bernstein RA , Korutz AW , Habib A , et al. . T1 gadolinium enhancement of intracranial atherosclerotic plaques associated with symptomatic ischemic presentations . AJNR Am J Neuroradiol 2013. ; 34 : 2252 – 8 . doi: 10.3174/ajnr.A3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skarpathiotakis M , Mandell DM , Swartz RH , Tomlinson G , Mikulis DJ . Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke . AJNR Am J Neuroradiol 2013. ; 34 : 299 – 304 . doi: 10.3174/ajnr.A3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang J , Helle M , Zhou Z , Börnert P , Hatsukami TS , Yuan C . Joint blood and cerebrospinal fluid suppression for intracranial vessel wall MRI . Magn Reson Med 2016. ; 75 : 831 – 8 . doi: 10.1002/mrm.25667 [DOI] [PubMed] [Google Scholar]
- 13. de Havenon A , Yuan C , Tirschwell D , Hatsukami T , Anzai Y , Becker K , et al. . Nonstenotic culprit plaque: the utility of high-resolution vessel wall MRI of intracranial vessels after ischemic stroke . Case Rep Radiol 2015. ; 2015 : 1 – 4 . doi: 10.1155/2015/356582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lindenholz A , van der Kolk AG , Zwanenburg JJM , Hendrikse J . The use and pitfalls of intracranial vessel wall imaging: how we do it . Radiology 2018. ; 286 : 12 – 28 . doi: 10.1148/radiol.2017162096 [DOI] [PubMed] [Google Scholar]
- 15. Jiang Y , Peng W , Tian B , Zhu C , Chen L , Wang X , et al. . Identification and quantitative assessment of different components of intracranial atherosclerotic plaque by ex vivo 3T high-resolution Multicontrast MRI . AJNR Am J Neuroradiol 2017. ; 38 : 1716 – 22 . doi: 10.3174/ajnr.A5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Portanova A , Hakakian N , Mikulis DJ , Virmani R , Abdalla WMA , Wasserman BA . Intracranial vasa vasorum: insights and implications for imaging . Radiology 2013. ; 267 : 667 – 79 . doi: 10.1148/radiol.13112310 [DOI] [PubMed] [Google Scholar]
- 17. Harris PA , Taylor R , Thielke R , Payne J , Gonzalez N , Conde JG . Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support . J Biomed Inform 2009. ; 42 : 377 – 81 . doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Curvo-Semedo L , Lambregts DMJ , Maas M , Thywissen T , Mehsen RT , Lammering G , et al. . Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy--conventional MR volumetry versus diffusion-weighted MR imaging . Radiology 2011. ; 260 : 734 – 43 . doi: 10.1148/radiol.11102467 [DOI] [PubMed] [Google Scholar]
- 19. Jiang Y , Zhu C , Peng W , Degnan AJ , Chen L , Wang X , et al. . Ex-vivo imaging and plaque type classification of intracranial atherosclerotic plaque using high resolution MRI . Atherosclerosis 2016. ; 249 : 10 – 16 . doi: 10.1016/j.atherosclerosis.2016.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim TH , Choi JW , Roh HG , Moon W-J , Moon SG , Chun YI , et al. . Atherosclerotic arterial wall change of non-stenotic intracracranial arteries on high-resolution MRI at 3.0T: correlation with cerebrovascular risk factors and white matter hyperintensity . Clin Neurol Neurosurg 2014. ; 126 : 1 – 6 . doi: 10.1016/j.clineuro.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 21. Dieleman N , Yang W , Abrigo JM , Chu WCW , van der Kolk AG , Siero JCW , et al. . Magnetic resonance imaging of plaque morphology, burden, and distribution in patients with symptomatic middle cerebral artery stenosis . Stroke 2016. ; 47 : 1797 – 802 . doi: 10.1161/STROKEAHA.116.013007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vergouwen MDI , Silver FL , Mandell DM , Mikulis DJ , Swartz RH . Eccentric narrowing and enhancement of symptomatic middle cerebral artery stenoses in patients with recent ischemic stroke . Arch Neurol 2011. ; 68 : 338 – 42 . doi: 10.1001/archneurol.2011.20 [DOI] [PubMed] [Google Scholar]
- 23. Li D , Zhao H , Chen X , Chen S , Qiao H , He L , et al. . Identification of intraPlaque haemorrhage in carotid artery by simultaneous non-contrast angiography and intraPlaque haemorrhage (SNAP) imaging: a magnetic resonance vessel wall imaging study . Eur Radiol 2018. ; 28 : 1681 – 6 . doi: 10.1007/s00330-017-5096-1 [DOI] [PubMed] [Google Scholar]
- 24. Sun J , Balu N , Hippe DS , Xue Y , Dong L , Zhao X , et al. . Subclinical carotid atherosclerosis: short-term natural history of lipid-rich necrotic core--a multicenter study with MR imaging . Radiology 2013. ; 268 : 61 – 8 . doi: 10.1148/radiol.13121702 [DOI] [PubMed] [Google Scholar]
- 25. Sun J , Canton G , Balu N , Hippe DS , Xu D , Liu J , et al. . Blood pressure is a major modifiable risk factor implicated in pathogenesis of intraplaque hemorrhage: an in vivo magnetic resonance imaging study . Arterioscler Thromb Vasc Biol 2016. ; 36 : 743 – 9 . doi: 10.1161/ATVBAHA.115.307043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun J , Zhao X-Q , Balu N , Neradilek MB , Isquith DA , Yamada K , et al. . Carotid plaque lipid content and fibrous cap status predict systemic cv outcomes: the MRI substudy in AIM-HIGH . JACC Cardiovasc Imaging 2017. ; 10 : 241 – 9 . doi: 10.1016/j.jcmg.2016.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]