Abstract
Objective:
This study aimed to evaluate the value of the Young’s modulus obtained by transrectal real-time shear wave elastography (SWE) for detection of prostate cancer (PCa).
Methods:
215 patients underwent SWE in six identical planes before biopsy guided with transrectal ultrasonography. The Young’s modulus of the entire prostate gland was defined as the mean of the results in these planes. The measurements were compared with the pathological results, the prostate specific antigen (PSA), and the Gleason score (GS) after biopsy.
Results:
The Young’s modulus of elasticity, including the maximum Young’s modulus (Emax), the mean Young’s modulus (Emean), and the minimum Young’s modulus (Emin), were significantly higher in malignant lesions than those in benign lesions (all p < 0.05). The optimal cut-off values for PCa were 128.48 kPa, 62.27 kPa, and 20.03 kPa, respectively. The sensitivities were 77.88%, 81.42%, and 60.18%, respectively, and the specificities were 85.33%, 74.51 and 63.73 %, respectively. PSA positively correlated with Emax and Emean (r = 0.686 and 0.678, respectively), as did the GS (r = 0.410 and 0.382, respectively).
Conclusion:
The Young’s modulus of entire prostate gland can be used to differentiate benign from malignant prostatic lesions. There were higher Young’s modulus of elasticity and higher risk of malignant lesions. Meanwhile, higher Young’s modulus correlated with higher PSA and GS.
Advances in knowledge:
This study indicates SWE can detect PCa by quantified the stiffness of entire prostate gland whether the lesions have been visible or not on gray-scale and Doppler ultrasound.
Introduction
Prostate cancer (PCa) is one of the most common malignant tumors in males; second in incidence of all malignant tumors in males worldwide.1–3 In recent years, different screening methods for Pca, including digital rectal examination (DRE), prostate specific antigen (PSA) testing, transrectal ultrasonography (TRUS), MRI, and systematic biopsy guided with transrectal ultrasonography have been accepted.
Each method has its own advantages and disadvantages. The accuracy of DRE is easily influenced by the doctor’s experience, and it is not easy to palpate for deep or small lesions.4,5 The specificity of serum PSA testing is relatively low.2,4,6 TRUS has limited sensitivity and specificity for diagnosis of PCa, with a range of 40–50%.7–9 MRI has a relatively high sensitivity but a low specificity for PCa.10 Although biopsy guided with transrectal ultrasonography is the “gold standard” for PCa, its risks include bleeding and infection etc.11
Real-time shear wave elastography (SWE) is a type of of elasticity imaging technique, developed by SuperSonic Imagine (Aix-en-Provence, France), that can measure the stiffness of tissue noninvasively and quantitatively.12,13 Stiffness will change in the presence of pathology,14–16 so the change in tissues stiffness can often indicate diseases, and may be helpful to diagnose the nature of the illness.17,18
Previous studies have used SWE to detect PCa.19–22 Most of them interrogated abnormal nodules in the prostate gland, and obtained the Young’s modulus of elasticity values of benign and malignant prostate lesions. However, the detection rate of PCa foci is not high by gray-scale and Doppler ultrasound.7–9 Thus, only interrogating the abnormal nodules of prostate gland by SWE to differentiate PCa is not easy in practice.
In this study, the Young’s modulus of elasticity of the prostate gland was measured by transrectal SWE in six identical ultrasound planes, looking for abnormal foci that may or may not have been visible on grayscale or Doppler imaging. The Young’s modulus of elasticity of entire prostate gland was defined as the average of measurement results in the six identical ultrasound planes. The aim was to evaluate the diagnostic performance of the Young’s modulus of elasticity measurement in the differentiation of benign from malignant prostate disease.
Methods and materials
Patients
From June 2015 to December 2017, 215 patients were enrolled in this study at Baoji Municipal Centre Hospital (Shanxi, China). They had visited the hospital because of symptoms of frequent micturition, dysuria, and/or urge incontinence. The age range of the patients was 28–90 y (mean, 71.3 ± 8.5 y). Inclusion criteria for enrollment were abnormal serum PSA, a nodule palpated or the entire gland have been rigid on DRE, or one or more nodules detected on TRUS and MRI. Exclusion criteria: males who had undergone prostate biopsy or surgery, or had received endocrine therapy previously. The ethics committee and academic board of our hospital approved all aspects of the study. All patients signed an informed consent before starting the study.
TRUS and SWE examination
TRUS and SWE examinations were performed by one urological radiologist, who has more than 15 years’ experience in urological ultrasonography. The examinations were performed with an ultrasound system (Aixplorer®, SuperSonic Imagine, Aix-en-Provence, France) with an SE12-3 multi frequency intracavitary probe (3–12 MHz).
After serum prostate specific antigen (PSA) testing, all patients underwent TRUS examination. It was necessary to administer an enema before the examination. With the patient lying in the left lateral decubitus position, with hips and knees flexed, the doctor inserted the probe into the patient’s rectum. The entire gland was visualized; any abnormal areas were observed carefully in multiple planes, and aspects such as the size, the shape, the echogenicity and color Doppler flow were noted. It was important whether there are suspicious abnormal areas of echogenicity in entire gland. The SWE mode was activated, Optimized settings should include maximized penetration and appropriate elasticity scale (70–90 kPa). In the transverse plane of the prostate, and the sampling box was placed on both sides of the base, the middle, and the apex of the prostate, respectively. The size of the sampling box was set up according to the size of the prostate gland. A still image was acquired after the probe was fixed for about 3–5 sec. During SWE, the operator was careful to avoid applying too much pressure with the transducer, to prevent creating iatrogenic areas of increased stiffness.
Elasticity of the entire gland was measured by the following steps: Quantitative analysis software (Q-box) was started in each detection plane during SWE. The size of the round region of interest (ROI) was adapted to the sampling box of SWE. Elasticity was measured at both sides of the base, the middle, and the apex of the prostate, respectively. If the suspicious abnormal areas of echogenicity were found in prostate, they should be covered in ROI for measurement. The maximum Young’s modulus of elasticity (Emax), the mean Young’s modulus of elasticity (Emean), and the minimum Young’s modulus of elasticity (Emin) were acquired from each each detection plane. The Young’s modulus of elasticity of the entire gland was equal to the average of the measurement results in the six regions. These were our data to be analyzed.
Biopsy and pathologic examination
After SWE measurement was completed, all patients underwent TRUS-guided systematic biopsy of the prostate immediately. About 5 min before performing biopsies, 10 ml of oxybuprocaine hydrochloride gel (Lvzhou® Pharm, Shenyang, China) was squeezed into the rectum. An 18-gauge, 25 cm puncture biopsy needle and automatic biopsy device (MAGNUM®, C.R. Bard, Inc., Warwick, RI) were used to obtain biopsy cores. Biopsy specimens were generally obtained from 12 cores, which were respectively from the base, the middle, and the apex of bilateral prostate, as far as possible from the peripheral zone and the central zone. Additional biopsies were taken from any foci of abnormal echogenicity first identified on TRUS.
All pathological diagnoses were determined by two pathologists with more than 15 years of experience in prostate pathology after their consultations. They were blinded to the TRUS and SWE results but not to the patients’ clinical and biochemical data. The Gleason score (GS) system was used for histological grading of PCa.
Statistical analysis
Statistical analyses and receiver operating characteristic (ROC) analysis were performed using commercial software (SPSS version 18.0®; SPSS Inc., Chicago, IL; MedCalc for Windows 15.2®; MedCalc Software, Inc, Mariakerke, Belgium). Quantitative data are expressed as mean ± standard deviation (‾x ±s) if normal distribution was achieved. The Young’s modulus and PSA of the two groups were analyzed by two-sample t-tests for independent samples. The pathological diagnoses of prostate biopsies were regarded as the gold standard; ROC curves were plotted according to these, and the areas under the ROC curves (AUCs) were calculated. The Z test was used to compare the AUCs of the different parameters. The optimal cut-off value for each parameter was obtained at the Youden index was maximum (sensitivity + specificity − 1). The correlation between SWE parameters and PSA were analyzed by Pearson’s correlation. The Pearson’s correlation test was also used in patients with PCa to correlate SWE parameters with GS. The Young's moduli of PCa with different GS were compared by ANOVA (analysis of variance), and LSD (least significant difference) – t test was used for paired comparison. Any p values < 0.05 were considered statistically significant.
Results
Patient characteristics
The 215 patients were distributed into two groups according to the pathological diagnoses of their prostate biopsies; 102 were benign and 113 were malignant. The benign diagnoses included benign prostatic hyperplasia (BPH) (n = 93), BPH complicated with prostatitis (n = 8), and tuberculous prostatitis (n = 1). The malignant diagnoses included acinar adenocarcinoma (n = 109), metastases to the prostate (n = 1), sarcomatoid carcinoma (n = 1), urothelial carcinoma (n = 1), and lymphoma (n = 1).
Comparison of elasticity
As outlined in Table 1, the Emax, Emean, and Emin of the entire gland of the malignant group were all significantly higher than those of the benign group, (all p < 0.05) (Figures 1 and 2).
Table 1.
Comparison of Young's modulus between benign and malignant diseases of prostate gland ()
| Group | No. of patients | PSA (ng/ml) | Young’s modulus(kPa) | ||
| Emax | Emean | Emin | |||
| Group of benign | 102 | 14.52 ± 14.03 | 98.44 ± 43.63 | 54.47 ± 25.04 | 20.82 ± 14.71 |
| Group of malignant | 113 | 128.08 ± 108.54 | 175.82 ± 57.64 | 95.52 ± 39.65 | 26.03 ± 21.31 |
| t Value | –11.020 | –11.161 | –9.165 | –2.101 | |
| P Value | <0.001 | <0.001 | <0.001 | 0.037 | |
Emax, maximum Young’s modulus; Emean, mean Young’s modulus; Emin, minimum Young’s modulus;PSA, prostate specific antigen.
Figure 1.
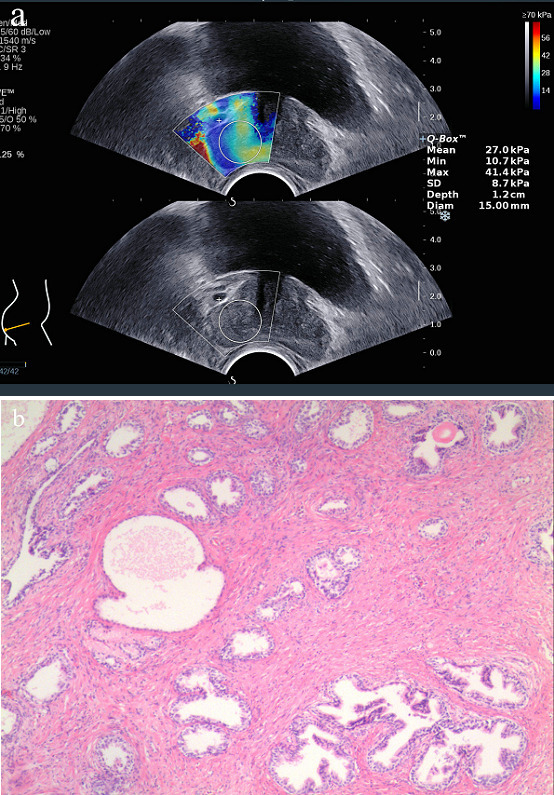
Images obtained from a 65-year-old male with a PSA level of 9.12 ng ml−1. The patient was in hospital because of symptoms of frequent micturition and dysuria, he underwent SWE examinations and TRUS-guided systematic biopsy of the prostate. a. Young’s modulus of the prostate gland were measured by SWE. Emax was 41.4 kPa, Emean was 27.0 kPa and Emin was 10.7 kPa. b.) Histological image of the prostate gland (hematoxylin and eosin stain,×10).Final diagnosis was benign prostatic hyperplasia. Emax = maximum Young’s modulus; Emean = mean Young’s modulus; Emin = minimum Young’s modulus.
Figure 2.
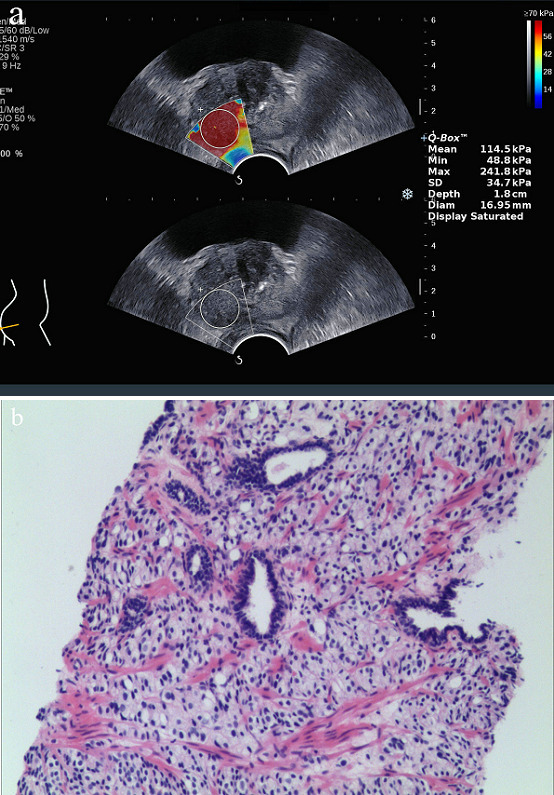
Images obtained from a 60-year-old male with a PSA level of 305.22 ng ml−1. The patient has no special discomfort, only the serum PSA was significantly increased during his healthy physical examination. Then he underwent SWE examinations and TRUS-guided systematic biopsy of the prostate. (a.) Young’s modulus of the prostate gland were measured by SWE. Emax was 241.8 kPa, Emean was 114.5 kPa and Emin was 48.8 kPa. (b.) Histological image of the prostate gland (hematoxylin and eosin stain,×10).Final diagnosis was prostate cancer, and the Gleason scores was 4 + 4=8. Emax = maximum Young’s modulus; Emean = mean Young’s modulus; Emin = minimum Young’s modulus.
Diagnostic performance for PCa
The optimal cut-off value, AUC, sensitivity, specificity, Youden index, positive predictive value (PPV) and negative predictive value (NPV) of the different parameters can be seen in Table 2. After the Z test, the AUCs were not found to be statistically different between the serum PSA and Emax (z = 1.923, p = 0.554). However, the, AUC of serum PSA was greater than that of Emean and Emin, respectively; they were significantly different (z = 2.210 and z = 7.055, p = 0.027 and p < 0.001, respectively). The AUCs were not found to be significantly different between Emax and Emean (z = 1.032, p = 0.302). The AUC of Emax and Emean were both significantly greater than that of Emin (z = 6.316 and z = 6.722, both p < 0.001, respectively) (Figure 3).
Table 2.
The diagnostic performance between malignant and benign prostate lesion for different parameters
| Parameters | AUC 95% CI) | Optimal cut-off value |
Sensitivity
(%) |
Specificity
(%) |
Youden index |
PPV
(%) |
NPV
(%) |
| Emax | 0.855 (0.804–0.905) | 128.48 kPa | 77.88 | 85.33 | 0.6121 | 83.02 | 77.06 |
| Emean | 0.842 (0.788–0.895) | 62.27 kPa | 81.42 | 74.51 | 0.5593 | 77.97 | 78.35 |
| Emin | 0.588 (0.512–0.665) | 20.03 kPa | 60.18 | 63.73 | 0.2390 | 64.15 | 58.72 |
| PSA | 0.910 (0.870–0.951) | 37.01 ng ml−1 | 74.34 | 98.04 | 0.7238 | 96.55 | 77.34 |
AUC, area under the receiver operating characteristic curves; CI, confidence interval; Emax, maximum Young’s modulus; Emean, mean Young’s modulus; Emin, minimum Young’s modulus; NPV, negative predictive value; PPV, positive predictive value; PSA, prostate specific antigen.
Figure 3.
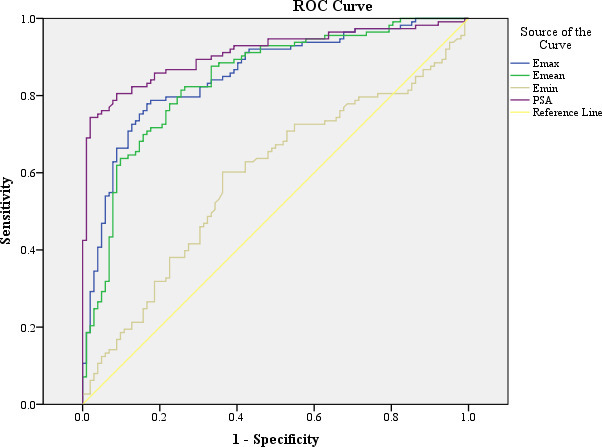
Receiver operating characteristic curves for Young’s modulus and prostate specific antigen. The areas under the curves are 0.910, 0.855, 0.842 and 0.588 for PSA, Emax, Emean and Emin, respectively. PSA = prostate specific antigen; Emax = maximum Young’s modulus; Emean = mean Young’s modulus; Emin = minimum Young’s modulus.
Correlation between Young’s modulus of elasticity and serum PSA concentration or GS
The serum PSA level positively correlated with Emax and Emean (r = 0.686 and r = 0.678, respectively), (both p < 0.001) (Figure 4). The serum PSA level also positively correlated with Emin (r = 0.180) (p = 0.008). The GS positively correlated with Emax and Emean (r = 0. 410 and r = 0.382, respectively), (both p < 0.001) (Figure 5). There was no correlation between Emin and GS (r = 0.067), (p = 0.496). The Young's moduli of PCa with different GS satisfied homogeneity of variance (all p > 0.05). As outlined in Table 3, the Emax and Emean of PCa with GS >7 were significantly higher than those of GS ≤7, (all p < 0.05).
Figure 4.
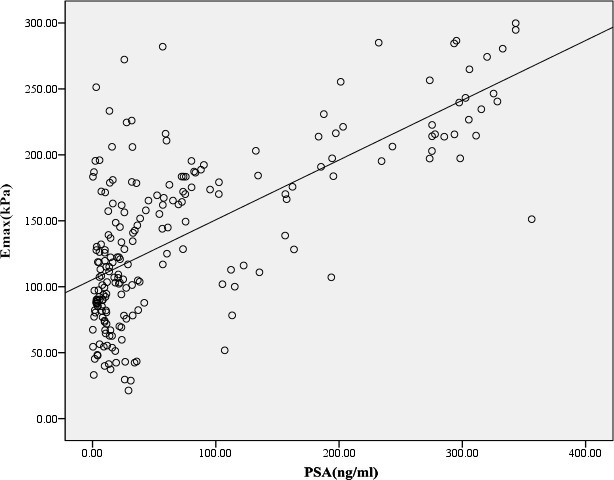
The scatter plot between PSA and Emax. PSA was positive correlation with Emax, and the Pearson correlation coefficient was 0.686. PSA = prostate specific antigen; Emax = maximum Young’s modulus.
Figure 5.
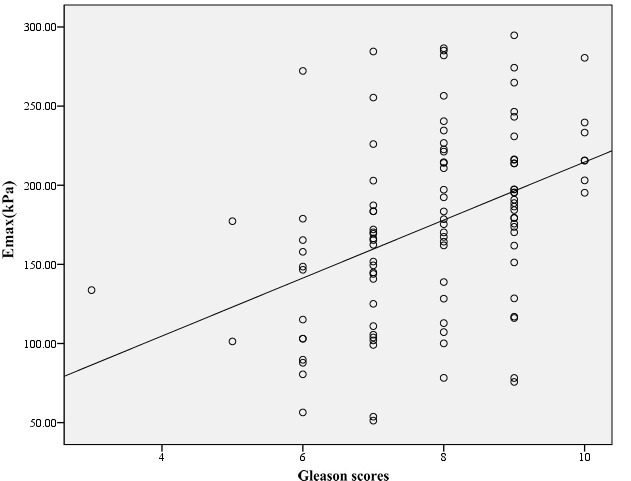
The scatter plot between Gleason scores and Emax. Gleason scores was positive correlation with Emax, and the Pearson correlation coefficient was 0.410. Emax = maximum Young’s modulus.
Table 3.
Comparison of Young's modulus among different gleason scores of PCa ()
| Gleason score | No. of patients | Young’s modulus (kPa) | ||
| Emax | Emean | Emin | ||
| GS < 7 | 16 | 132.35 ± 52.39 | 64.96 ± 17.24 | 20.70 ± 9.73 |
| GS = 7 | 26 | 154.28 ± 54.10 a | 83.70 ± 40.72 a | 25.93 ± 24.91 |
| GS > 7 | 67 | 195.26 ± 53.03bc | 108.45 ± 38.46bc | 27.84 ± 22.19 |
| F Value | 11.94 | 10.98 | 0.713 | |
| P Value | <0.001 | <0.001 | 0.492 | |
Emax, maximum Young’s modulus; Emean, mean Young’s modulus; Emin, minimum Young’s modulus;GS, Gleason score.
Compared with GS <7, p > 0.05.
Compared with GS <7, p < 0.05.
Compared with GS = 7, p < 0.05.
Discussion
This study indicates that the Young’s modulus of elasticity of the entire prostate gland measured by SWE technique can be used as a new indicator for the diagnosis of PCa, irrespective of focal lesions on gray-scale and Doppler ultrasound. The higher the Young’s modulus of elasticity, the more likely the diagnosis of PCa. It is feasible to detect PCa with SWE.
Previous studies have demonstrated that nearly 58% of PCAs are sporadic or multifocal, and progress along the capsule of the prostate gland, such that it may not appear as a well-defined nodule like other malignant tumors.23 The sonographic findings of PCas are complex and variable, and depend on many factors. Thus, it is difficult to detect lesions of PCa accurately using gray-scale ultrasound.24,25 Meanwhile, Doppler ultrasound dose not substantially improve the diagnostic accuracy.7 Moreover, both the performance of ultrasound instruments and the operator dependence could influence the detection and identification of lesions. Therefore, it is necessary to find an ultrasound method to improve diagnostic accuracy for PCa. Ophir et al26 had considered that the changes of texture for PCa lesions may be earlier than the changes in their morphology and blood flow distribution. A study by Hoyt et al27 has shown that tissue of PCa has a higher stiffness value compared with normal prostate tissue. Thus, the stiffness of prostate gland has certain value for diagnosis of PCa.
SWE is a more recently developed ultrasonic elastography imaging technique, which can transmit the radiation force impulse by the transducer,13 and continuously focuses on different tissue depths, producing shear waves based on the Mach Cone principle.28,29 It can obtain the absolute value of the elastic modulus by detecting the velocity of shear waves in human tissue, which is Young’s modulus of elasticity.30,31 Young’s modulus of elasticity increases directly with stiffness and vice versa. Compared with other elastic imaging techniques, such as transient elastography (TE), acoustic radiation force impulse (ARFI) and strain elastography (SE), SWE has several advantages, including real time two-dimensional sonogram, the area of the sampling box can be adjusted appropriately, and the absolute value of tissue elasticity can be obtained.
For the above reasons, to determine whether or not the patients had PCa, Young’s modulus of elasticity of the entire prostate gland was measured and calculated by SWE in this study. We mainly analyzed the elastic changes in the entire prostate gland in addition to focusing on the elastic changes in the focal nodules or abnormal echogenicity areas in the prostate sonogram. It is tantamount to quantify the information obtained by DRE.
In our study, the prostatic Young’s modulus of elasticity of the malignant group, including Emax, Emean, and Emin, were all significantly higher than those of the benign group (all p < 0.05).This shows that the stiffness of the entire prostate gland is significantly higher than that of benign prostate tissue (such as BPH et al) when the prostate gland is involved with cancer. The result was similar to previous studies by Sarfraz et al32 and Sungmin et al.19 And it isn’t relating with the specific parts of the prostate lesion (at the base, the mid or the apex of the prostate). In this group, the sensitivity and specificity of the prostatic Young’s modulus of elasticity were higher than that of conventional ultrasound (40%–50%)7–9 for PCa. It shows that SWE can improve the diagnostic performance of conventional ultrasound for PCa.
After ROC curves were plotted, we found there was no significant difference in AUC between the serum PSA and the Emax (0.910 vs 0.855, p = 0.054). However, the AUC of serum PSA was greater than that of Emean and Emin, respectively (0.910 vs 0.842 and 0.588, p = 0.027 and p < 0.001). In this group, when the optimal cut-off values of the Emax, the Emean, and the Emin for PCa were 128.48 kPa, 62.27 kPa and 20.03 kPa, the sensitivity was 77.88%, 81.42 and 60.18%, and the specificity was 85.33%, 74.51 and 63.73%, respectively. When using 37.01 ng ml−1 as the optimal cut-off values of the serum PSA, the sensitivity and the specificity were 74.34 and 98.04%, respectively. This indicates that the Young’s modulus of elasticity is of value in the diagnosis of PCa, but its diagnostic performance is not higher than that of serum PSA. There are two possible reasons for the result: 1) In this study, the Young’s modulus of elasticity of the entire prostate gland was regarded as the object, however the entire stiffness of prostate gland is positively correlated with the lesion size of PCa, only when the lesions of PCa grow to a certain size or infiltrates prostate tissue to a certain extent, the stiffness of entire prostate gland would be increased. 2) The stiffness of the prostate gland is also influenced by other factors, such as age, BPH and calcification.33,34
In this study, the serum PSA concentration positively correlated with the Young’s modulus of elasticity of the prostate gland, including Emax, Emean, and Emin. It indicates that the stiffness of the cancerous prostate gland is greater, and the serum PSA concentration is higher. The GS also positively correlated with the Young’s modulus of elasticity of the prostate gland, and the Emax and Emean of PCa with GS >7 were significantly higher than those of GS ≤7 (all p < 0.05). Previous studies by Sumura et al35 and Correas et al20 have also demonstrated these results. Several studies have shown that the GS had a good correlation with biological behavior and prognosis of PCa,36,37 and the level of serum PSA also had a certain correlation with pathological grades of PCa.38,39 These findings pointed out that the values of the Young’s modulus of elasticity may have a certain correlation with aggressiveness and clinical prognosis of PCa. To demonstrate this deduction, we need to increase the quantity of the samples for further study.
After we compared the diagnostic performances of Emax, Emean, and Emin for PCa, the AUC of Emin was only 0.588, meanwhile, the sensitivity and the specificity were only 60.18 and 63.73%, respectively. Emin has a very low correlation with the serum PSA concentration and the GS (r = 0.180 and r = 0.067, respectively). And there was no significant difference in Emin of PCa with different GS. Thus, we think that Emin has a limited diagnostic value for PCa. In clinical practice, we should chiefly use Emax and Emean as the reference for detecting PCa.
In this study, the optimal cut-off values for the Young’s modulus of elasticity of the prostate gland were observably higher than those in a previous study by Correas et al.20 This difference might be for several reasons: First, the method of measuring the Young’s modulus of elasticity was different. In our study, the ROIs were placed on the base, the middle and the apex on both sides of the prostate. The Young’s modulus of elasticity values of the prostate gland were equal to the average of the measurement results at these planes, and not only measured the Young’s modulus of elasticity of abnormal echo lesions in the prostate sonogram. Second, physical differences between Chinese and Europeans may have caused the difference in experimental results. Additionally, the patients’ older age may have been an influencing factor.
There are some limitations in our study: (1) The pathological diagnoses of prostate biopsies were regarded as the “gold standard,” rather than the pathological findings of surgical specimens, which may elevate the misdiagnosis rate. (2) In this study, the Young’s modulus of elasticity values of the entire prostate gland was equal to the average of the measuring results, which obtained from the base, the mid and the apex of the bilateral prostate. When the volume of prostate gland was larger and the size of PCa lesions were small, maybe the measurements were not carried out at the sections where the lesions of PCa were located. Therefore, the method probably underestimated the stiffness of the prostate gland. (3) The stiffness of the entire prostate gland maybe influenced by BPH, inflammation, calcification and the lesion size, etc. It might reduce the accuracy of SWE for detection of PCa.
Conclusion
Our preliminary results indicated that there is a difference in the Young’s modulus of the entire prostate gland between benign and malignant prostatic diseases. The risk of PCa increases with increasing Young’s modulus of elasticity. SWE can quantitate the stiffness of the prostate gland, which is convenient and objective. It provides a new ultrasonic method for the differential diagnosis of benign and malignant prostatic diseases, and it is helpful to improve the diagnostic performance of conventional ultrasound for PCa. In the future, comparing the diagnostic performance of SWE and multi parameter MRI for PCa will be our research direction, the aimed to evaluate whether SWE can reduce healthy cost without reducing the accuracy of PCa screening.
Footnotes
Acknowledgment: We thank Libby Cone, MD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Conflicts of Interest Statement: All the authors had read and approved the final version of the manuscript. The authors have no potential conflicts of interest related with this article.
Funding: This study was supported by a research grant from the Baoji Municipal Centre Hospital (Shanxi Province, China).
Contributor Information
Yonghao Ji, Email: xiaoyaozi982@126.com.
Litao Ruan, Email: ruanlitao@163.com.
Wei Ren, Email: renwei1962@126.com.
Guoliang Dun, Email: baojius@126.com.
Jianxue Liu, Email: liu198206229@126.com.
Yaoren Zhang, Email: 13772691503@163.com.
Qinyun Wan, Email: 373254327@qq.com.
REFERENCES
- 1. Center MM , Jemal A , Lortet-Tieulent J , Ward E , Ferlay J , Brawley O , et al. . International variation in prostate cancer incidence and mortality rates . Eur Urol 2012. ; 61 : 1079 – 92 . doi: 10.1016/j.eururo.2012.02.054 [DOI] [PubMed] [Google Scholar]
- 2. Mottet N , Bellmunt J , Bolla M , Briers E , Cumberbatch MG . EAU-ESTRO-SIOG guidelines on prostate cancer . Eur Urol . 2016. . Available from: https://uroweb.org/individual-guidelines/oncology-guidelines . [DOI] [PubMed]
- 3. Torre LA , Bray F , Siegel RL , Ferlay J , Lortet-Tieulent J , Jemal A , et al. . CA Cancer J Clin 2012. ; 2015 : 87 – 108 . [DOI] [PubMed] [Google Scholar]
- 4. Borley N , Feneley MR . Prostate cancer: diagnosis and staging . Asian J Androl 2009. ; 11 : 74 – 80 . doi: 10.1038/aja.2008.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhai L , Madden J , Foo W-C , Mouraviev V , Polascik TJ , Palmeri ML , et al. . Characterizing stiffness of human prostates using acoustic radiation force . Ultrason Imaging 2010. ; 32 : 201 – 13 . doi: 10.1177/016173461003200401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heidenreich A , Bellmunt J , Bolla M , Joniau S , Mason M , Matveev V , et al. . EAU guidelines on prostate cancer. Part I: screening, diagnosis, and treatment of clinically localised disease . Actas Urol Esp 2011. ; 35 : 501 – 14 . doi: 10.1016/j.acuro.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 7. Beerlage HP , Aarnink RG , Ruijter ET , Witjes JA , Wijkstra H , Van De Kaa CA , et al. . Correlation of transrectal ultrasound, computer analysis of transrectal ultrasound and histopathology of radical prostatectomy specimen . Prostate Cancer Prostatic Dis 2001. ; 4 : 56 – 62 . doi: 10.1038/sj.pcan.4500495 [DOI] [PubMed] [Google Scholar]
- 8. Junker D , Schäfer G , Kobel C , Kremser C , Bektic J , Jaschke W , et al. . Comparison of real-time elastography and multiparametric MRI for prostate cancer detection: a whole-mount step-section analysis . AJR Am J Roentgenol 2014. ; 202 : W263 – W269 . doi: 10.2214/AJR.13.11061 [DOI] [PubMed] [Google Scholar]
- 9. Junker D , De Zordo T , Quentin M , Ladurner M , Bektic J , Horniger W , et al. . Real-time elastography of the prostate . Biomed Res Int 2014. ; 2014 : 1 – 11 . doi: 10.1155/2014/180804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pinto F , Totaro A , Calarco A , Sacco E , Volpe A , Racioppi M , et al. . Imaging in prostate cancer diagnosis: present role and future perspectives . Urol Int 2011. ; 86 : 373 – 82 . doi: 10.1159/000324515 [DOI] [PubMed] [Google Scholar]
- 11. Chiang I-N , Chang S-J , Pu Y-S , Huang K-H , Yu H-J , Huang C-Y . Major complications and associated risk factors of transrectal ultrasound guided prostate needle biopsy: a retrospective study of 1875 cases in Taiwan . J Formos Med Assoc 2007. ; 106 : 929 – 34 . doi: 10.1016/S0929-6646(08)60063-7 [DOI] [PubMed] [Google Scholar]
- 12. Arda K , Ciledag N , Aktas E , Aribas BK , Köse K . Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography . AJR Am J Roentgenol 2011. ; 197 : 532 – 6 . doi: 10.2214/AJR.10.5449 [DOI] [PubMed] [Google Scholar]
- 13. Bercoff J , Tanter M , Fink M . Supersonic shear imaging: a new technique for soft tissue elasticity mapping . IEEE Trans Ultrason Ferroelectr Freq Control 2004. ; 51 : 396 – 409 . doi: 10.1109/TUFFC.2004.1295425 [DOI] [PubMed] [Google Scholar]
- 14. Athanasiou A , Tardivon A , Tanter M , Sigal-Zafrani B , Bercoff J , Deffieux T , et al. . Breast lesions: quantitative elastography with supersonic shear imaging–preliminary results . Radiology 2010. ; 256 : 297 – 303 . doi: 10.1148/radiol.10090385 [DOI] [PubMed] [Google Scholar]
- 15. Syversveen T , Brabrand K , Midtvedt K , Strøm EH , Hartmann A , Jakobsen JA , et al. . Assessment of renal allograft fibrosis by acoustic radiation force impulse quantification--a pilot study . Transpl Int 2011. ; 24 : 100 – 5 . doi: 10.1111/j.1432-2277.2010.01165.x [DOI] [PubMed] [Google Scholar]
- 16. Aigner F , Pallwein L , Junker D , Schäfer G , Mikuz G , Pedross F , et al. . Value of real-time elastography targeted biopsy for prostate cancer detection in men with prostate specific antigen 1.25 ng/ml or greater and 4.00 ng/ml or less . J Urol 2010. ; 184 : 913 – 7 . doi: 10.1016/j.juro.2010.05.026 [DOI] [PubMed] [Google Scholar]
- 17. Dudea SM , Giurgiu CR , Dumitriu D , Chiorean A , Ciurea A , Botar-Jid C , et al. . Value of ultrasound elastography in the diagnosis and management of prostate carcinoma . Med Ultrason 2011. ; 13 : 45 – 53 . [PubMed] [Google Scholar]
- 18. Ginat DT , Destounis SV , Barr RG , Castaneda B , Strang JG , Rubens DJ . Us elastography of breast and prostate lesions . Radiographics 2009. ; 29 : 2007 – 16 . doi: 10.1148/rg.297095058 [DOI] [PubMed] [Google Scholar]
- 19. Woo S , Kim SY , Cho JY , Kim SH , Sungmin W , Sang YK . Shear wave elastography for detection of prostate cancer: a preliminary study . Korean J Radiol 2014. ; 15 : 346 – 55 . doi: 10.3348/kjr.2014.15.3.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Correas J-M , Tissier A-M , Khairoune A , Vassiliu V , Méjean A , Hélénon O , et al. . Prostate cancer: diagnostic performance of real-time shear-wave elastography . Radiology 2015. ; 275 : 280 – 9 . doi: 10.1148/radiol.14140567 [DOI] [PubMed] [Google Scholar]
- 21. Rouvière O , Melodelima C , Hoang Dinh A , Bratan F , Pagnoux G , Sanzalone T , et al. . Stiffness of benign and malignant prostate tissue measured by shear-wave elastography: a preliminary study . Eur Radiol 2017. ; 27 : 1858 – 66 . doi: 10.1007/s00330-016-4534-9 [DOI] [PubMed] [Google Scholar]
- 22. Richard GB , David C , Marko B , Vito C , Jean MC , Arnoud WP , et al. . Wfumb guidelines and recommendations on the clinical use of ultuasound elastography: part 5.prostate . Ultrasound in Med. & Biol 2017. ; 43 : 27 – 48 . [DOI] [PubMed] [Google Scholar]
- 23. McNeal JE , Redwine EA , Freiha FS , Stamey TA . Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread . Am J Surg Pathol 1988. ; 12 : 897 – 906 . [DOI] [PubMed] [Google Scholar]
- 24. Nakashima K , Shiina T , Sakurai M , Enokido K , Endo T , Tsunoda H , et al. . JSUM ultrasound elastography practice guidelines: breast . J Med Ultrason 2013. ; 40 : 359 – 91 . doi: 10.1007/s10396-013-0457-0 [DOI] [PubMed] [Google Scholar]
- 25. Smith JA . Transrectal ultrasonography for the detection and staging of carcinoma of the prostate . J Clin Ultrasound 1996. ; 24 : 455 – 61 . doi: [DOI] [PubMed] [Google Scholar]
- 26. Ophir J , Alam SK , Garra B , Kallel F , Konofagou E , Krouskop T , et al. . Elastography: Ultrasonic estimation and imaging of the elastic properties of tissues . Proc Inst Mech Eng H 1999. ; 213 : 203 – 33 . doi: 10.1243/0954411991534933 [DOI] [PubMed] [Google Scholar]
- 27. Hoyt K , Castaneda B , Zhang M , Nigwekar P , di Sant'agnese PA , Joseph JV , et al. . Tissue elasticity properties as biomarkers for prostate cancer . Cancer Biomark 2008. ; 4 ( 4-5 ): 213 – 25 . doi: 10.3233/CBM-2008-44-505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dewall RJ . Ultrasound elastography: principles, techniques, and clinical applications . Crit Rev Biomed Eng 2013. ; 41 : 1 – 19 . doi: 10.1615/CritRevBiomedEng.2013006991 [DOI] [PubMed] [Google Scholar]
- 29. Sporea I , Lie I . Shear wave elastography . Ultraschall Med 2012. ; 33 : 393 – 4 . [PubMed] [Google Scholar]
- 30. Chai C-K , Burd HJ , Wilde GS . Shear modulus measurements on isolated human lens nuclei . Exp Eye Res 2012. ; 103 : 78 – 81 . doi: 10.1016/j.exer.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 31. Bouillard K , Hug F , Guével A , Nordez A . Shear Elastic modulus can be used to estimate an index of individual muscle force during a submaximal isometric fatiguing contraction . J Appl Physiol 2012. ; 113 : 1353 – 61 . doi: 10.1152/japplphysiol.00858.2012 [DOI] [PubMed] [Google Scholar]
- 32. Ahmad S , Cao R , Varghese T , Bidaut L , Nabi G , Sarfraz A . Transrectal quantitative shear wave elastography in the detection and characterisation of prostate cancer . Surg Endosc 2013. ; 27 : 3280 – 7 . doi: 10.1007/s00464-013-2906-7 [DOI] [PubMed] [Google Scholar]
- 33. Zheng X-Z , Ji P , Mao H-W , Zhang X-Y , Xia E-H , Xing- Gu , et al. . A novel approach to assessing changes in prostate stiffness with age using virtual touch tissue quantification . J Ultrasound Med 2011. ; 30 : 387 – 90 . doi: 10.7863/jum.2011.30.3.387 [DOI] [PubMed] [Google Scholar]
- 34. Correas J-M , Tissier A-M , Khairoune A , Khoury G , Eiss D , Hélénon O . Ultrasound elastography of the prostate: state of the art . Diagn Interv Imaging 2013. ; 94 : 551 – 60 . doi: 10.1016/j.diii.2013.01.017 [DOI] [PubMed] [Google Scholar]
- 35. Sumura M , Shigeno K , Hyuga T , Yoneda T , Shiina H , Igawa M . Initial evaluation of prostate cancer with real-time elastography based on step-section Pathologic analysis after radical prostatectomy: a preliminary study . Int J Urol 2007. ; 14 : 811 – 6 . doi: 10.1111/j.1442-2042.2007.01829.x [DOI] [PubMed] [Google Scholar]
- 36. Delahunt B , Lamb DS , Srigley JR , Murray JD , Wilcox C , Samaratunga H , et al. . Gleason scoring: a comparison of classical and modified (International Society of urological pathology) criteria using nadir PSA as a clinical end point . Pathology 2010. ; 42 : 339 – 43 . doi: 10.3109/00313021003787924 [DOI] [PubMed] [Google Scholar]
- 37. Tollefson MK , Blute ML , Rangel LJ , Bergstralh EJ , Boorjian SA , Karnes RJ . The effect of Gleason score on the predictive value of prostate-specific antigen doubling time . BJU Int 2010. ; 105 : 1381 – 5 . doi: 10.1111/j.1464-410X.2009.08976.x [DOI] [PubMed] [Google Scholar]
- 38. Pannek J , Rittenhouse HG , Chan DW , Epstein JI , Walsh PC , Partin AW . The use of percent free prostate specific antigen for staging clinically localized prostate cancer . J Urol 1998. ; 159 : 1238 – 42 . doi: 10.1016/S0022-5347(01)63571-7 [DOI] [PubMed] [Google Scholar]
- 39. Brawer MK . Prostate-specific antigen: current status . CA Cancer J Clin 1999. ; 49 : 264 – 81 . doi: 10.3322/canjclin.49.5.264 [DOI] [PubMed] [Google Scholar]


