Abstract
Objective
Stereotactic body radiation therapy (SBRT) has been used in the treatment of cholangiocarcinoma (CC) but toxicity and clinical results of SBRT in CC are still limited and sparse. Therefore, the aim of this systematic review was to analyze the results of SBRT in the setting of advanced CC.
Methods
A systematic literature search was conducted on PubMed, Scopus, and Cochrane library using the PRISMA methodology. Studies including at least 10 patients with diagnosis of advanced CC regardless of tumor site and other treatments were included. The primary outcome was overall survival (OS) and secondary endpoints were local control (LC) and toxicity rates. The ROBINS-I risk of bias tool was used.
Results
10 studies (231 patients) fulfilled the selection criteria and were included in this review. All but one study showed moderate to serious risk of bias. Median follow up was 15 months (range: 7.8–64.0 months). Pooled 1 year OS was 58.3% (95% CI: 50.2–66.1%) and pooled 2 year OS was 35.5% (95% CI: 22.1–50.1%). Pooled 1 year LC was 83.4%, (95% CI: 76.5–89.4%). The reported toxicities were acceptable and manageable with only one treatment-related death.
Conclusion
The role of SBRT in CC is not yet supported by robust evidence in literature. However, within this limit, preliminary results seem almost comparable to the ones of standard chemotherapy or chemoradiation.
Advances in knowledge
SBRT seems effective in terms of LC with acceptable treatment-related toxicities. Therefore, SBRT can be considered a therapeutic option at least in selected patients with CC, possibly combined with adjuvant chemotherapy (CHT).
Introduction
Cholangiocarcinoma (CC) is an uncommon neoplasm representing 3% of gastrointestinal (GI) cancers and the second most common primary liver malignancy.1 They represent a very heterogeneous group of neoplasm arising from the epithelial cells of the bile duct. CC are classified according to their anatomical location as intra hepatic or extra hepatic. Radical surgery with negative histological margins is the only treatment allowing long-term survival but even after tumor resection, the prognosis is dismal with 5 year overall survival (OS) <20%.1 Moreover, most of these patients have advanced disease at the time of diagnosis and are candidates for non-surgical treatments. Furthermore, in patients undergoing surgery, 15 to 25% microscopic (R1) or macroscopic (R2) residual disease was reported.2
Some studies have demonstrated that external beam radiotherapy (EBRT) with or without systemic chemotherapy (CHT) is a treatment option in unresectable or R1-R2 residual CC with median OS ranging between 10 and 15 months.3–5 Furthermore, a significant correlation between radiotherapy (RT) dose and OS has been reported.6–8 However, the possibility to deliver very high RT dose on this site is limited by the low radiation tolerance of both liver and GI tract.
In the last decade, technological improvements in EBRT delivery accuracy and in respiratory motion compensation has enabled the widespread implementation of stereotactic body radiation therapy (SBRT). Particularly, due to its ability to deliver a high and focused dose in few fractions, SBRT has been proposed for GI tumors of the upper abdomen.9–12 In particular, this technique could be promising in the setting of locally advanced CC given the close proximity to radiosensitive organs. Whereas high level studies in this field are justified, we believe that a review of the available evidence can be useful for the design of these trials. Therefore, this systematic review aimed at analyzing the results of SBRT in CC by reviewing the available data from clinical outcome studies.
Methods and Materials
Our systematic review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, www.crd.york.ac.uk/prospero/) on March 2017 (Registration Number: CRD42017058929).
Bibliographic search
We conducted a systematic search based on PubMed, Scopus, and Cochrane libraries from the earliest data to May 15, 2018. The following search strategy was used on PubMed: stereotactic (All Fields) AND [“human body” (MeSH Terms) OR [“human” (All Fields) AND “body” (All Fields)] OR “human body” (All Fields) OR “body” (All Fields)] AND [“radiotherapy” (Subheading) OR “radiotherapy” (All Fields) OR [“radiation” (All Fields) AND “therapy” (All Fields)] OR “radiation therapy” (All Fields) OR “radiotherapy” (MeSH Terms) OR [“radiation” (All Fields) AND “therapy” (All Fields)] OR “radiation therapy” (All Fields)] AND [“cholangiocarcinoma” (MeSH Terms) OR “cholangiocarcinoma” (All Fields)] OR “Klatskin Tumor” (MeSH Terms).
Inclusion criteria
Human studies of any design (prospective or retrospective) with at least 10 enrolled patients with diagnosis of CC and treated with SBRT were included regardless of the tumor site. Studies on hepatocarcinoma were not excluded if they reported differentiated data on at least 10 CC patients. Only studies published in English language were considered in this review. No restrictions about total delivered dose, Biological Effective Dose (BED) and SBRT technique were imposed.
Outcome measures
The primary outcome was OS. Secondary outcomes were local control (LC) and treatment-related toxicity.
Study selection and quality assessment
We used the PRISMA guidelines as a guide to select the items to be included in the review.13,14 The title, abstract, and keywords of the identified articles were independently analyzed by two researchers (RF, GM) and disagreements were resolved by a third senior researcher (AGM). Potentially eligible studies were retrieved and full-text evaluation was performed based on the inclusion and exclusion criteria by two different authors (MB, SB) with disagreements resolved by consensus-based discussion. The following data were collected independently by two authors (RF, MB) from each article with disagreements resolved by the senior author (AGM): authors name and year of publication, study design, accrual period, patients and tumor features, other treatments before and after SBRT, technical components of treatment planning and delivery, total dose and fractionation, BED, outcomes, and toxicity. In the studies where BED was not reported, the value was calculated according to the following equation BED = d *[(1 + (d/n ÷ α/β)], assuming an α/β ratio of 10 for the tumor (n= number of fractions, d = total dose).15 Papers were evaluated based on the ROBINS-I Risk of Bias tool.16 Two reviewers (RF, MB) assessed the quality of the included studies and discrepancies were resolved on agreement.
Statistical analysis
1 year, 2 year OS, and 1 year LC percentages were pooled by means of a random effects model in case of heterogeneity across studies; otherwise, a fixed-effect model was used.17 Statistical heterogeneity was estimated with the I2 statistic (high heterogeneity level:>50%) and tested using the Q2 test (statistical significance level: p < 0.1). The survival percentages were reported as estimates and 95% CI.The analysis was performed with MedCalc statistical software (MedCalc®, Ostend, Belgium).
Results
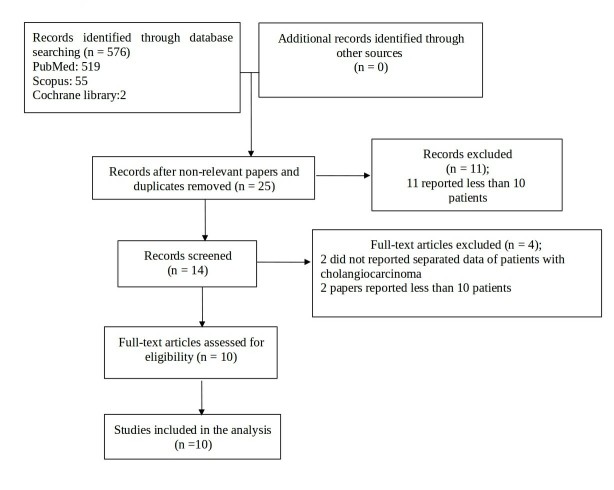
10 articles18–27 fulfilled the inclusion criteria for this review. A detailed analysis of these studies is reported in Tables 1–3 while in Figure 1, the flowchart of the systematic literature search process is represented. Nine studies were retrospective18–26 and one was a prospective Phase I study.27 No randomized controlled trial was found. All but one were considered to have moderate to serious risk of bias according to the ROBINS-I tool.17 Supplementary Material 1 shows the overall risk of bias rating per study according to ROBINS-I.
Table 1.
Characteristics of the studies included in the systematic review
| Study | Study design | Accrual period | No of patients | Median follow up (months) | Tumor features % | Biliary stent % | Treatment | |
| Before SBRT % | After SBRT % | |||||||
| Barney et al. 201224 | Retrospective | 2009–2011 | 10 | 14 | Intra hepatic: 60.0; extra hepatic: 40.0; primary: 50.0; recurrence: 50.0; | NR | Surgery: 50.0; CHT: 40.0; EBRT: 10.0 |
CHT:40.0 |
| Ibarra et al. 201223 | Retrospective | 2001–2010 | 11 | 7.8 | Intra hepatic: 100; M1: 45.5 |
NR | Surgery: 50.0; RFA: 27.3; CHT: 45.5 |
NR |
| Jung et al. 201422 | Retrospective | 2005–2013 | 58 | 10 | Intra hepatic: 57.0; extra hepatic: 43.0; primary: 48.0; recurrence: 52.0 | NR | EBRT(40–63 Gy): 15.5; surgery: 51.7 | NR |
| Kopek et al. 201026 | Retrospective | 1999–2006 | 27 | 64 | Extra hepatic (Klatskin): 96.0; intra hepatic: 4.0 | 100 | NR | NR |
| Mahadevan et al. 201520 | Retrospective | 2006–2014 | 34 | 38 | Intra hepatic: 73.8; intra hepatic +extra hepatic: 21.4; extra hepatic: 4.8; primary: 85.3; positive margin: 5.9 | 38.2 | Chemoembolization: 2.9: surgery: 5.9 | CHT (Gemcitabine or Gemcitabine + cisplatin) |
| Polistina et al. 201125 | Retrospective | 2004–2009 | 10 | 35.5 | Extra hepatic:100; N+: 60.0 |
100 | CHT (Gemcitabine): 100 | CHT: 100 (Gemcitabine) |
| Sandler et al. 201619 | Retrospective | 2008–2015 | 31 | 11.5 | Intra hepatic: 19.0; extra hepatic: 81.0primary: 87.0;recurrence: 13.0;N+: 6.0 | NR | EBRT: 6.4; surgery: 3.2; surgery +CHT: 3.2 | Liver transplant: 16.0 |
| Shen et al. 201718 | Retrospective | 2009–2012 | 28 | 16 | Intra hepatic: 100 | No | Chemoembolization: 28.6 | NR |
| Tse et al. 200827 | Phase I | 2003–2006 | 10 | 17.6 | Intra hepatic: 100; N+ :60.0; M1: 40.0 |
NR | Surgery: 10.0 CHT: 40.0 |
NR |
| Welling et al. 201421 | Retrospective | NR | 12 | 14 | Extra hepatic: 100 | 100 | No | CHT (Capecitabine) liver transplant: 50.0 |
CHT, chemotherapy; EBRT, external beam radiotherapy; M, male; N+, positive lymph nodes; NR, not reported; RFA, radiofrequency ablation;SBRT, stereotactic body radiation therapy.
Table 2.
Technical components of treatment planning and dose delivery
| Study | Respiratory motion control/IGRT | Target definition (median tumor volume) | Dose prescription | Median dose (Gy)/fr |
BED10Gy
(median) |
TDT weeks |
| Barney et al. 201224 | 4D CT/yes | PTV: ITV + 5 (79.1 cc) | NR | 55/5 | 115.5 | 1 |
| Ibarra et al. 201223 | Yes/yes | PTV: GTV + 3–5 mm (80.2 cc) | To 70% isodose line | 30/3 | 60 | 2 |
| Jung et al. 201422 | Abdominal compression device/yes | PTV: ITV + 2–4 mm (40.0 cc) | To 70–80% isodose or 92–99% to cover at least 95% of the PTVs. | 45/3 | 112.5 | NR |
| Kopek et al. 201026 | Abdominal compression device/yes | PTV: CTV + 5 mm radial direction +10 mm CC direction (NR) | To the isocenter | 45/3 | 112.5 | 5–8 days |
| Mahadevan et al. 201520 | Tracking (two gold fiducials) /yes | NR (63.8 cc) | To 75% isodose line | 30/3 | 60 | 1 |
| Polistina et al. 201125 | Tracking/yes | PTV: GTV + 3 mm (NR) | To 80% isodose line | 30/3 | 60 | 3 days |
| Sandler et al. 201619 | 4D CT free breathing/yes | PTV: ITV + 5–8 mm (59.3 cc) | PTV Dmin ≥ 95% of the prescription dose | 40/5 | 72 | 1 |
| Shen et al. 201718 | Tracking/yes | PTV: GTV + 5 mm (267.4 cc) | PTV Dmin ≥ 95% of the prescription dose | 45/3 | 112.5 | 1 |
| Tse et al. 200827 | Exhale breath hold/yes | PTV: GTV + 8 mm (172 cc) | NR | 36/6 | 57.6 | 2 |
| Welling et al. 201421 | Active breathing control/yes | PTV: ITV + 5–8 mm (NR) | Isodose surface covering 99.5% of PTV | 50–60/3–5 | 100–180 | 2 |
BED, biologically effective dose;CTV, clinical target volume; GTV, gross tumor volume; IGRT, image-guided radiotherapy; ITV, internal target volume; NR, not reported; PTV, planning target volume; TDT, treatment delivery time; fr, fraction.
Table 3.
Outcomes
| Study | LC % |
OS %
1 year |
Median OS (months) | Median PFS (months) | Toxicity scale | Acute toxicity G ≥ 3 % | Late toxicity G ≥ 3 % |
| Barney et al. 201224 | Crude: 100 | 73.0 | NR | NR | CTCAE vs 3.0 | 0.0 | Biliary stenosis: 8.3 Liver failure: 8.3 (G5) |
| Ibarra et al. 201223 | NR | 45.0 | 11 | 4.2 | CTCAE vs 3.0 | 7.0 | 0.0 |
| Jung et al. 201422 | 1y: 85.0, 2y: 72.0 |
45.0 2y: 20.0 |
10 | NR | CTCAE vs 4.0 | 0.0 | Cholangitis: 8.6 Biliary stenosis: 1.7 Gastric perforation: 1.7 Gastric ulcer :1.7 |
| Kopek et al. 201026 | 1y: 84.0 | NR | 10.6 | 6.7 | CTCAE vs 3.0/World Health Organization | Nausea: 3.7 Pain: 7.4 Liver enzyme: 55.5 |
Gastroduodenal ulceration: 22.2 Duodenal stenosis: 11.0 |
| Mahadevan et al. 201520 | 1y: 88.0 | 58.0, 2y: 31.0 |
17 | 10 | NR | 0.0 | Duodenal ulceration:5.9 Cholangitis:1.7 Liver abscess:1.7 |
| Polistina et al. 201125 | NR | 80.0 2y: 80.0 |
b 35.5 | 30.0 | CTCAE vs 3.0 | 0.0 | 0.0 |
| Sandler et al. 201619 | 1y: 78.0 2y :47.0 |
59.0 2y: 33 |
15.7 | 16.8 | CTCAE vs 4.0 | Duodenal stenosis: 3.2 | Duodenal stenosis: 6.4 Duodenal hemorrhage:10.3 Pain: 3.2 |
| Shen et al. 201718 | Crude: 89.3 | 57.1 2y:32.1 |
15 | 11 | CTCAE vs 4.0 | 0.0 | 0.0 |
| Tse et al. 200827 | NR | 58.0 | 15 | NR | CTCAE vs 3.0 | Liver Enzymes:20.0 Transient biliary obstruction: 20.0 |
Bowel obstruction: 10.0 |
| Welling et al. 201421 | NR | 83.0 (1) | NR | NR | SAEs | Cholangitis: 5.0 Dehydration: 7.0 Palmar-plantar erythrodysesthesia: 43.0 Diarrhea: 14.0 Wound infection post-surgery: 14.0 |
NR |
CTCAE, common terminology criteria for adverse events; G, grade; NR, not reported; OS, overall survival; PFS, progression free survival; SAEs, Serious Adverse Events.
(1): in six transplanted patients.
Median local progression free survival.
calculated from time of diagnosis.
Figure 1.
Flow chart study selection diagram.
Characteristics of patients and SBRT technique
Patients’ median age ranged from 57 to 72 years.18–27 The studies were heterogeneous in terms of tumor features, treatment aim, treatment planning, delivery devices, and techniques. Patients underwent SBRT for unresectable or recurrent CC in nine studies18–27 except for two patients in the study of Mahadevan and colleagues who underwent post-operative SBRT for positive surgical margins.20 In two studies also patients with liver and/or distant metastases were included.23,27 Liver transplant after SBRT was performed in 16.0 and 50.0% of patients in two series.19,21 Two studies included only extra hepatic CC,21,25 three studies only intra hepatic CC,18,23,27 and five studies included both anatomical sites.19,20,22,24,26 Biliary stenting was performed in five series with percentage of patients ranging from 38.2 to 100%.19–21,25,26 Neoadjuvant CHT was administered in six studies19,20,22–25 and adjuvant CHT was prescribed after SBRT in two series.23,24
Respiratory motion management and image-guided RT were used in all studies18–27 with large variability among centers. This variability influenced the Planning Target Volume (PTV) definition which resulted heterogeneous. The PTV was not specified in one study.20 In four studies the PTV was defined as Gross Tumor Volume (GTV) plus 3–5 mm18,23,25,27 and as Internal Target Volume (ITV) plus 2–8 mm in four studies.19,21,22,24 The Clinical Target Volume (CTV) to PTV margin was 5 mm radially and 10 mm craniocaudally in one study.26 The median tumor volume reported in seven studies ranged between 40.0 and 267.4 cm3 (median: 79.1 cm3).18–20,22–24,27 Dose prescription methods and total dose/fraction were highly variable.18–27 Median prescribed SBRT dose ranged between 30 and 60 Gy in 3 to 5 fractions. Median computed BED ranged between 57.6 and 180.0 Gy. Different dose prescription modalities were reported in eight studies.18–23,25,26 In four studies, the dose was prescribed to ≥70% isodose.20,22,23,25 In one study, PTV dose was not less than 95% of the prescribed dose18 and in another series the dose was prescribed to the isodose covering at least 99.5% of the PTV.21 In one study, 95% of the PTV received the full prescribed dose19 and in another study the dose was prescribed to the isocenter.26 Table 2 reports in details the technical characteristics of treatment planning and delivery.
Outcomes
Overall survival
Median follow up was 15 months (range: 7.8–64.0 months).18–27 Median OS ranged from 10.0 to 35.5 months (median: 15 months). From nine studies,18–24,26,27 the pooled 1 year OS in 204 patients was 58.3% (95%Confidence Interval (CI), 50.2–66.1%) with very low heterogeneity between studies (Q2 test: p = 0.22; I2 = 24.8%) (Figure 2). The pooled 2 year OS reported in five studies18–20,22,24 (161 patients), was 35.5% (95%CI, 22.1–50.1%) with very high heterogeneity between studies (Q2 test: p = 0.0075; I2 = 71.3%) (Figure 3). According to the anatomical location of CC, 1 year OS was 57.1% (range: 45.0–58.0%), 81.5% (range: 80.0–83.0%), and 58.7% (range: 45.0–73.0%) in studies including intra hepatic CC, extra hepatic CC, and both sites, respectively.18–27
Figure 2.

Forest plot of the 1 year overall survival reported in the analyzed studies.
Figure 3.

Forest plot of the 2 year overall survival reported in the analyzed studies.
Local control
LC was reported in six studies.18–20,22,24,26 Data reported in four studies19,20,22,26 on 123 patients yielded a pooled rate for 1 year LC of 83.4% (95%CI, 76.5–89.4%) with low heterogeneity level (Q2 test: p = 0.5514; I2 = 0.00%) (Figure 4). The highest value of 100% was reported as crude rate with a median follow up of 14 months.24
Figure 4.
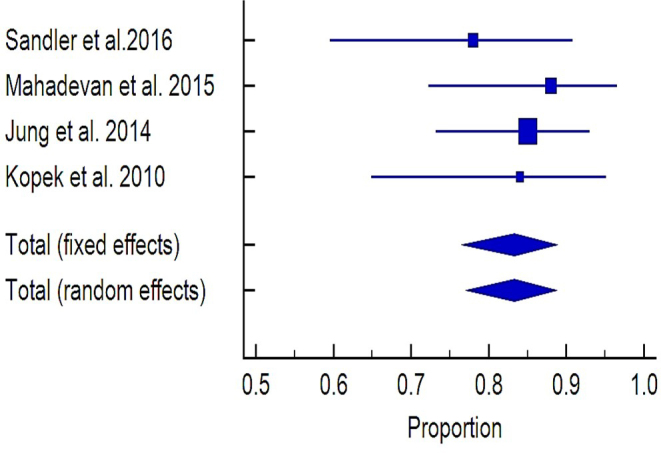
Forest plot of the 1 year local control reported in the analyzed studies.
Toxicity
Acute toxicity was reported in all studies18–27 and late toxicity in nine series.18–20,22–27 One study used a non-validated toxicity scale,21 one study reported overall toxicity not specifying both type and grade23 while one study did not describe separately acute and late toxicity.18 Severe acute toxicity (≥G3) was recorded in four studies19,21,26,27 as cholangitis (50%),21 abnormal liver enzymes (range: 20.0–55.5%),26,27 duodenal obstruction (3.2%),19 pain (7.4%),26 and transient biliary obstruction (20%).27
Clinically relevant late toxicity (≥G2) was reported in six studies.19,20,22,24,26,27 The most frequent were duodenal complications (obstruction, ulceration, and hemorrhage ranging from 5.9 to 22.2%),19,20,25–27 cholangitis (1.7–8.6%,)20,22 and biliary stenosis (range: 1.7–8.3%).22,24 Other less frequent toxicities are reported in Table 3. Only one case of fatal liver failure was reported in one patient despite compliance with dose/volume constraints.24 According to the authors, this fatal event could have been related to subclinical liver damage due to previous CHT for breast cancer.24
Publication bias
The funnel plots were examined and none of them showed any asymmetry nor missing studies (figures not shown). The statistical analysis confirmed the absence of publication bias. However, caution regarding these results is warranted considering the small study numbers.
Discussion
To the best of our knowledge, this is the first systematic review analyzing the role of SBRT in CC. Our study is limited by obvious reasons that include: retrospective design of most studies, small number of enrolled patients, few number and quality of the studies, and the high heterogeneity in terms of tumor characteristics, treatment aim, and prescribed dose. Certainly, the usefulness of a systematic review on such a limited and heterogeneous body of evidence can be discussed. However, we felt that in the absence of evidence from large prospective studies, this modality could still be useful to contribute to the knowledge in this field.
Surgery with negative margins is considered to be the standard treatment in resectable CC. However, locally advanced/unresectable disease is the most common presentation of CC and Gemcitabine plus Cisplatin-based CHT is the standard treatment in these patients27 with a median OS of 11.7 months.28,29 Based on the ESMO guidelines,27 the role of chemoradiation remains unclear in the treatment of locally advanced non-metastatic CC.
However, if we compare the results of CHT with the ones of chemoradiation, they seem very similar. In fact, in the systematic review of Bisello and colleagues, in the series based on chemoradiation ± brachytherapy boost, median PFS and OS were 7.5 months (range: 6.8–10.5 months) and 13 months (range: 9.6–13.5 months), respectively.30
More recently, SBRT has been tested in the treatment of advanced CC as an alternative to chemoradiation.18–27 In fact, SBRT has several advantages like high biologically equivalent dose, short duration and therefore greater convenience for patients and departments, and easier integration with systemic therapies. Based on our analysis the results of SBRT in terms of survival are almost comparable with the ones of standard chemoradiation and CHT with 15.0 months median OS (range: 10.0–35.5 months). This result is particularly interesting considering that in the review of Bisello and colleagues,30 the series including metastatic patients were excluded unlike in our analysis.
Comparing the results of studies enrolling only patients with intra hepatic CC,18,23,27 we can observe that the highest 1 year OS rate (58%) was reported in the only prospective series included in this analysis.27 In that trial, the SBRT dose prescription was based on the volume of the irradiated liver and the risk of liver toxicity was estimated by the Lyman-Kutcher-Burman normal tissue complication model. The study with the lowest 1 year OS rate (45%) was a retrospective multicenter analysis on heavily pre-treated patients (surgery, radiofrequency ablation, CHT).23 This difference in terms of outcome might be related to the different study design, and to the different treatments performed before SBRT, the inclusion of metastatic patients, and the larger median PTV in the second study (172.0 cc vs 80.2 cc).
Another comparison can be done between two studies with similar characteristics in terms of CC type and site.22,24 In fact, both studies included patients with intra/extra hepatic and primary/recurrent CC. In these two series, published by Jung and colleagues22 and Barney and coworker,24 1 year OS was 45 and 73%, respectively. This difference could be related to the higher prescribed RT dose and to prescription of CHT in 40% of patients after SBRT in the study of Barney and colleagues.24
Surprisingly enough, the impact on survival of the inclusion of metastatic patients has been quite small. In fact, median 1 year survival was 51.1% (range: 45.0–58.0%) in series with M0-1 patients18–22,24,25 and 59.0 (range: 45.0–83.0%) in series with only M0 patients,23,27 respectively.
However, no clear impact of BED10Gy on OS and LC was recorded in our analysis. In fact, median 1 year OS was 57.1% in series with BED10Gy ≥100 Gy18,22,24 and 58.5% in studies with BED10Gy <100 Gy.19,20,23,25 Similarly, 1 year LC, in patients with BED10Gy ≥100 Gy and BED10Gy <100 Gy was 84.0–85.0%22,26 and 78.0–88.0%,19,20 respectively. On the contrary, patients receiving CHT after SBRT showed higher 1 year OS rates (median: 73.0%; range: 58.0–80.0%)20,24,25 compared to series without adjuvant CHT (median: 57.0%; range: 45.0–59.0%).18,19,22,23,27
Overall, treatment-related acute and late toxicities were acceptable even if with variable rates, and almost comparable with the ones reported after chemoradiation ± brachytherapy boost.30 Only one treatment-related death was reported.24 Unfortunately, it is impossible to correlate toxicity with dose and planning/delivery techniques due to the inhomogeneous and incomplete modalities of adverse events reporting.
This review demonstrates the minimal evidence available on this topic and highlights the need for high-quality studies in this area. Within this limit, the preliminary results in terms of OS seem not clearly different from the ones of standard chemoradiation. Moreover, SBRT seems reasonably effective in terms of LC with acceptable treatment-related toxicities. Again, considering the limitations of this analysis, its findings cannot justify changes in clinical practice or be considered as a recommendation. Therefore, SBRT can be considered as a therapeutic option at least in selected patients with CC, possibly combined with adjuvant CHT. Furthermore, from the excellent results recorded in patients undergoing neoadjuvant chemoradiation followed by orthotopic liver transplantation,31 this latter treatment should always be considered in patients in whom this combined modality therapy is feasible.
Further studies are warranted in this field to better define the role of this technique in the advanced CC setting. These studies could have the following objectives: (i) comparison between CHT and CHT + SBRT; (ii) comparison between chemoradiation and SBRT; (iii) evaluation of SBRT + CHT as neoadjuvant treatment aimed at tumor down-staging; (iv) combination of SBRT and CHT as bridge therapy in liver transplant candidates.
Contributor Information
Rezarta Frakulli, Email: rezarta.frakulli@gmail.com.
Milly Buwenge, Email: mbuwenge@gmail.com.
Gabriella Macchia, Email: macchiagabriella@gmail.com.
Silvia Cammelli, Email: silvia.cammelli2@unibo.it.
Francesco Deodato, Email: francesco.deodato@fgps.it.
Savino Cilla, Email: savinocilla@gmail.com.
Francesco Cellini, Email: francesco.cellini@policlinicogemelli.it.
Gian C. Mattiucci, Email: gcmattiucci@libero.it.
Silvia Bisello, Email: silvia.bisello11@gmail.com.
Giovanni Brandi, Email: giovanni.brandi@unibo.it.
Salvatore Parisi, Email: s.parisi@operapadrepio.it.
Alessio G. Morganti, Email: amorganti60@gmail.com.
REFERENCES
- 1. Khan SA , Thomas HC , Davidson BR , Taylor-Robinson SD . Cholangiocarcinoma . The Lancet 2005. ; 366 : 1303 – 14 . doi: 10.1016/S0140-6736(05)67530-7 [DOI] [PubMed] [Google Scholar]
- 2. Forsmo HM , Horn A , Viste A , Hoem D , Øvrebø K . Survival and an overview of decision-making in patients with cholangiocarcinoma . Hepatobiliary Pancreat Dis Int 2008. ; 7 : 412 – 7 . [PubMed] [Google Scholar]
- 3. Ghafoori AP , Nelson JW , Willett CG , Chino J , Tyler DS , Hurwitz HI , et al. . Radiotherapy in the treatment of patients with unresectable extrahepatic cholangiocarcinoma . Int J Radiat Oncol Biol Phys 2011. ; 81 : 654 – 9 . doi: 10.1016/j.ijrobp.2010.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vern-Gross TZ , Shivnani AT , Chen K , Lee CM , Tward JD , MacDonald OK , et al. . Survival outcomes in resected extrahepatic cholangiocarcinoma: effect of adjuvant radiotherapy in a surveillance, epidemiology, and end results analysis . Int J Radiat Oncol Biol Phys 2011. ; 81 : 189 – 98 . doi: 10.1016/j.ijrobp.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 5. Nakeeb A , Pitt HA . Radiation therapy, chemotherapy and chemoradiation in hilar cholangiocarcinoma . HPB 2005. ; 7 : 278 – 82 . doi: 10.1080/13651820500373028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morganti AG , Trodella L , Valentini V , Montemaggi P , Costamagna G , Smaniotto D , et al. . Combined modality treatment in unresectable extrahepatic biliary carcinoma . Int J Radiat Oncol Biol Phys 2000. ; 46 : 913 – 9 . doi: 10.1016/S0360-3016(99)00487-3 [DOI] [PubMed] [Google Scholar]
- 7. Alden ME , Mohiuddin M . The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer . Int J Radiat Oncol Biol Phys 1994. ; 28 : 945 – 51 . doi: 10.1016/0360-3016(94)90115-5 [DOI] [PubMed] [Google Scholar]
- 8. Deodato F , Clemente G , Mattiucci GC , Macchia G , Costamagna G , Giuliante F , et al. . Chemoradiation and brachytherapy in biliary tract carcinoma: long-term results . Int J Radiat Oncol Biol Phys 2006. ; 64 : 483 – 8 . doi: 10.1016/j.ijrobp.2005.07.977 [DOI] [PubMed] [Google Scholar]
- 9. Mahadevan A , Miksad R , Goldstein M , Sullivan R , Bullock A , Buchbinder E , et al. . Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer . Int J Radiat Oncol Biol Phys 2011. ; 81 : e615 – 22 . doi: 10.1016/j.ijrobp.2011.04.045 [DOI] [PubMed] [Google Scholar]
- 10. Chang DT , Schellenberg D , Shen J , Kim J , Goodman KA , Fisher GA , et al. . Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas . Cancer 2009. ; 115 : 665 – 72 . doi: 10.1002/cncr.24059 [DOI] [PubMed] [Google Scholar]
- 11. Rusthoven KE , Kavanagh BD , Cardenes H , Stieber VW , Burri SH , Feigenberg SJ , et al. . Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases . J Clin Oncol 2009. ; 27 : 1572 – 8 . doi: 10.1200/JCO.2008.19.6329 [DOI] [PubMed] [Google Scholar]
- 12. Macchia G , Morganti AG , Cilla S , Ippolito E , Massaccesi M , Picardi V , et al. . Quality of life and toxicity of stereotactic radiotherapy in pancreatic tumors: a case series . Cancer Invest 2012. ; 30 : 149 – 55 . doi: 10.3109/07357907.2011.640649 [DOI] [PubMed] [Google Scholar]
- 13. Shamseer L , Moher D , Clarke M . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement . Systematic Reviews 2015. ; 349 : g7647 . doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hutton B , Salanti G , Caldwell DM , Chaimani A , Schmid CH , Cameron C , et al. . The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations . Ann Intern Med 2015. ; 162 : 777 – 84 . doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 15. Fowler JF . The linear-quadratic formula and progress in fractionated radiotherapy . Br J Radiol 1989. ; 62 : 679 – 94 . doi: 10.1259/0007-1285-62-740-679 [DOI] [PubMed] [Google Scholar]
- 16. Sterne JA , Hernán MA , Reeves BC , Savović J , Berkman ND , Viswanathan M , et al. . ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions . BMJ 2016. ; 355 : i4919 . doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DerSimonian R , Laird N . Meta-analysis in clinical trials . Control Clin Trials 1986. ; 7 : 177 – 88 . doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 18. Shen ZT , Zhou H , Li AM , Li B , Shen JS , Zhu XX . Clinical outcomes and prognostic factors of stereotactic body radiation therapy for intrahepatic cholangiocarcinoma . Oncotarget 2017. ; 8 : 93541 – 50 . doi: 10.18632/oncotarget.19972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sandler KA , Veruttipong D , Agopian VG , Finn RS , Hong JC , Kaldas FM , et al. . Stereotactic body radiotherapy (SBRT) for locally advanced extrahepatic and intrahepatic cholangiocarcinoma . Adv Radiat Oncol 2016. ; 1 : 237 – 43 . doi: 10.1016/j.adro.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mahadevan A , Dagoglu N , Mancias J , Raven K , Khwaja K , Tseng JF , et al. . Stereotactic body radiotherapy (SBRT) for intrahepatic and hilar cholangiocarcinoma . J Cancer 2015. ; 6 : 1099 – 104 . doi: 10.7150/jca.13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Welling TH , Feng M , Wan S , Hwang SY , Volk ML , Lawrence TS , et al. . Neoadjuvant stereotactic body radiation therapy, capecitabine, and liver transplantation for unresectable hilar cholangiocarcinoma . Liver Transpl 2014. ; 20 : 81 – 8 . doi: 10.1002/lt.23757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jung DH , Kim MS , Cho CK , Yoo HJ , Jang WI , Seo YS , et al. . Outcomes of stereotactic body radiotherapy for unresectable primary or recurrent cholangiocarcinoma . Radiat Oncol J 2014. ; 32 : 163 – 9 . doi: 10.3857/roj.2014.32.3.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ibarra RA , Rojas D , Snyder L , Yao M , Fabien J , Milano M , et al. . Multicenter results of stereotactic body radiotherapy (SBRT) for non-resectable primary liver tumors . Acta Oncol 2012. ; 51 : 575 – 83 . doi: 10.3109/0284186X.2011.652736 [DOI] [PubMed] [Google Scholar]
- 24. Barney BM , Olivier KR , Miller RC , Haddock MG . Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma . Radiat Oncol 2012. ; 7 : 67 – 73 . doi: 10.1186/1748-717X-7-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Polistina FA , Guglielmi R , Baiocchi C , Francescon P , Scalchi P , Febbraro A , et al. . Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience . Radiother Oncol 2011. ; 99 : 120 – 3 . doi: 10.1016/j.radonc.2011.05.016 [DOI] [PubMed] [Google Scholar]
- 26. Kopek N , Holt MI , Hansen AT , Høyer M . Stereotactic body radiotherapy for unresectable cholangiocarcinoma . Radiother Oncol 2010. ; 94 : 47 – 52 . doi: 10.1016/j.radonc.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 27. Tse RV , Hawkins M , Lockwood G , Kim JJ , Cummings B , Knox J , et al. . Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma . JCO 2008. ; 26 : 657 – 64 . doi: 10.1200/JCO.2007.14.3529 [DOI] [PubMed] [Google Scholar]
- 28. Valle JW , Borbath I , Khan SA , Huguet F , Gruenberger T , Arnold D , et al. . Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up . Ann Oncol 2016. ; 27 ( suppl_5 ): v28 – 37 . doi: 10.1093/annonc/mdw324 [DOI] [PubMed] [Google Scholar]
- 29. Valle J , Wasan H , Palmer DH , Cunningham D , Anthoney A , Maraveyas A , et al. . Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer . N Engl J Med 2010. ; 362 : 1273 – 81 . doi: 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 30. Bisello S , Buwenge M , Zamagni A , Deodato F , Macchia G , Arcelli A . Chemoradiation in unresectable biliary tract cancer: a systematic review . J Gastrointest Oncol 2018. ;. [Google Scholar]
- 31. Rana A , Hong JC . Orthotopic liver transplantation in combination with neoadjuvant therapy: a new paradigm in the treatment of unresectable intrahepatic cholangiocarcinoma . Curr Opin Gastroenterol 2012. ; 28 : 258 – 65 . doi: 10.1097/MOG.0b013e32835168db [DOI] [PubMed] [Google Scholar]