Abstract
Given the enormous increase in the risks of bone and cartilage defects with the rise in the aging population, the current treatments available are insufficient for handling this burden, and the supply of donor organs for transplantation is limited. Therefore, tissue engineering is a promising approach for treating such defects. Advances in materials research and high-tech optimized fabrication of scaffolds have increased the efficiency of tissue engineering. Electrospun nanofibrous scaffolds and hydrogel scaffolds mimic the native extracellular matrix of bone, providing a support for bone and cartilage tissue engineering by increasing cell viability, adhesion, propagation, and homing, and osteogenic isolation and differentiation, vascularization, host integration, and load bearing. The use of these scaffolds with advanced three- and four-dimensional printing technologies has enabled customized bone grafting. In this review, we discuss the different approaches used for cartilage and bone tissue engineering.
Keywords: tissue engineering, extracellular matrix, bioprinting, biomaterials
Introduction
Traffic accidents, work and sporting injuries, degenerative diseases, and wars present immense orthopedic trauma burdens, most of which are bone and cartilage fractures that require reconstruction as well as rehabilitation.1,2 Other than accidental fractures, aging-related osteoporosis and autoimmune diseases such as arthritis are causes of bone defects and increase the chances of bone fractures.3 Diseases such as osteomyelitis cause severe pain, fever, and redness in specific areas, and if not addressed, may lead to irreversible trauma (eg, amputation).4,5 In addition, cancers affect bone remodeling and induce fractures and anemia, and surgery at the cancer site can leave bone defects.6 Moreover, some hereditary diseases (eg, hereditary multiple exostoses and hereditary bone marrow failure syndromes) can induce bone injuries and require surgery or stem cell injections for restoration.7,8 The number of cosmetic surgery procedures is also increasing as societies develop.9 Worldwide, more than 900 million reconstructive surgery operations are performed annually in response to all these leading causes of bone fractures and defects.10 Despite the fact that various precautionary measures (eg, legislation and road safety improvements) have been implemented to decrease trauma-causing accidents, the number of orthopedic procedures carried out to repair large bone damage has still not shown a tangible decrease. In general, bone has the intrinsic property to repair itself, but there are many circumstances where full bone regeneration fails to occur and requires external stimulation.11 Therefore, millions of people with bone defects require a bone graft or substitute. Owing to the high demand for surgery, the market value for bone grafts and related materials reached 2.4 billion US dollars in 2016 and is expected to hit 11.5 billion US dollars by 2025.12 Other than the supply-and-demand phenomenon, grants for bone tissue engineering research, as well as the interest of research and development authorities in the progress of such technology, are key factors that boost the market value.13
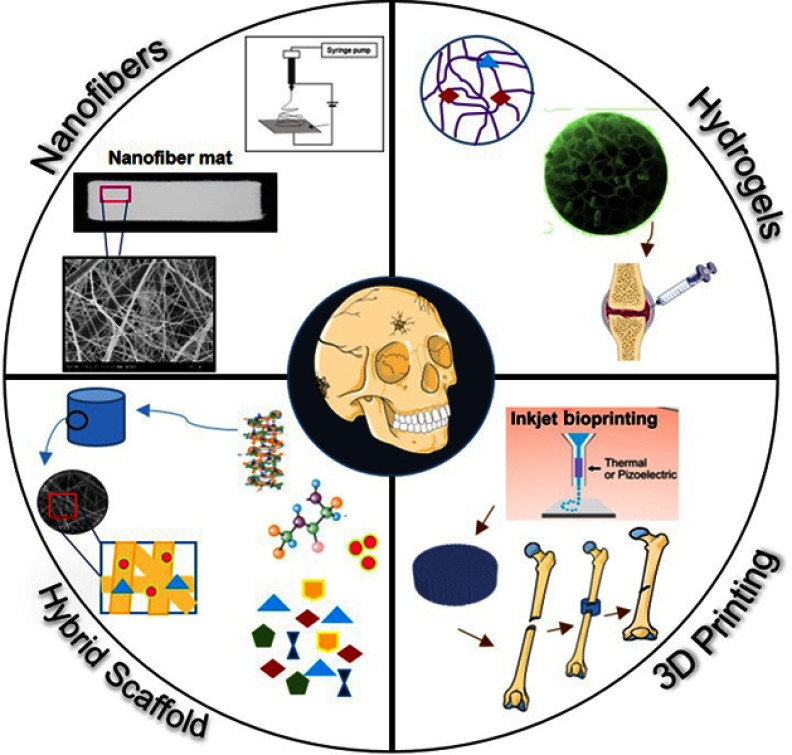
The solutions available to address bone defects and diseases include medical procedures, transplantation, and medication.11 Despite their success, however, they are still not able to fully meet the demands; therefore, tissue engineering has emerged as an alternative option for correcting bone damage and cartilage defects.13 Advances in the field of three-dimensional (3D) scaffold fabrication for tissue engineering, the use of 3D printers for in vitro implant construction for tissue repair, and the use of stem cells are expected to solve the problematic issues and help meet the future demands of cartilage and bone tissue repair. In this review, we discuss the current treatments and their limitations, advanced tissue engineering tools, 3D scaffolds (nanofibers, hydrogels), and 3D and four-dimensional (4D) printing applications for cartilage and bone tissue engineering (Figure 1).
Figure 1.
Different advanced strategies for scaffold fabrication used in bone and cartilage tissue engineering: nanofibers, hydrogels, and 3D printing.
Bone and cartilage biology, role of the extracellular matrix, and synchrotron imaging techniques
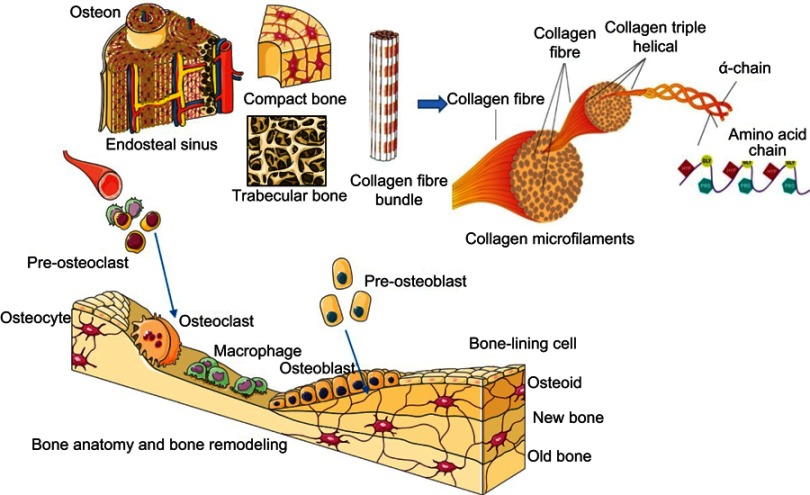
The engineering of soft and hard tissues for the repair of bone and cartilage tissues is an emerging trend in the field of medical sciences. However, although these two tissue types are important components of the human skeletal system, they differ widely in their composition and mechanics. Bone is hard and rigid, whereas cartilage is soft, flexible, and viscoelastic, and their physiologies vary according to body location.14,15 Thus, it is essential to know the basic anatomy of these tissues, as each one requires a special scaffold or material for its bioengineering. Bone is further composed of two types of osseous tissue: cancellous trabecular bone, which constitutes the inner bone and has a high porosity (50–90% vol); and cortical bone, which covers the outer bone surface and has a low porosity (10% vol) (Figure 2). The inner part of bone is metabolically active and helps in joint and limb movement, whereas the outer part is mechanically sound and provides support and protection. Mechanically, the compressive strength of the trabecular bone ranges from 2 to 12 MPa and its elastic modulus from 0.1 to 5 GPa, whereas the cortical bone is stronger, with a compressive strength of 170–193 MPa and an elastic modulus of 7–20 GPa.16 Furthermore, bone tissues are mineralized connective tissue and composed of three types of cells: osteocytes, osteoblasts (from pluripotent mesenchymal stem cells [MSCs]), and osteoclasts.17 These cells are arranged to work in synchronization, to constitute a unified bone organism.3 Bone is enriched with a vascular network and consists of 10% water, 30% matrix, and 60% minerals by weight.18
Figure 2.
(A) Anatomical hierarchy of bone and its types (compact bone, trabecular bone); (B) bone remodeling mechanism in which three types of bone cells (osteocyte, osteoclast, and osteoblast) participate.
The extracellular matrix (ECM) of bone exists as a biphasic system, with the major portion being composed of organic matter (mostly collagen type I fibers) and the rest consisting of inorganic matter and mineral bone salt (eg, hydroxyapatite [HA] and calcium phosphates).19 Collagen fibers are composed of three polypeptide chains that are twisted in a helical conformation and stabilized by intramolecular and intermolecular bonds, which increase the tensile strength of the fibers.20 Non-collagenous proteins, growth factors, and cytokines act as functional players, whereas proteoglycans and phospholipids have a regulatory effect in the calcification process.21,22 The highly mineralized ECM network of bone provides strength and support to the skeleton, which shapes the body and plays a role in the transmittance of muscular pull for movement. The mineralized ECM is able to provide protection to soft tissue in the pelvic bone, bone marrow, and thoracic and cranial cavities.19 Collagen fibers in ECMs reinforced with HA gave rise to a tough and flexible nanostructure that supported the adhesion, proliferation, and differentiation of bone cells.20 To some extent, bone tissues also have a self-renewal property, which they constantly undergo through a remodeling process to adapt to the body burden and to repair old micro-damaged bone with new cells to sustain the mechanical strength of bone (Figure 2).19,23 It was observed that 3% of cortical bone and 25% of trabecular bone are replaced every year with new bone,19 indicating that bones possess remarkable regeneration properties. Nonetheless, some bone diseases, tumor resections, and union fractures cause bone defects of a critical size that cannot be managed spontaneously and need external treatments and induction for regeneration.24,25 This process of bone formation, known as ossification, is regulated by osteoblasts along with the bone matrix. Two essential processes are involved in new bone formation: intramembranous ossification, in which the primitive connective tissue (mesenchyme) participates directly in bone formation (eg, in the mandible, skull, and clavicle); and endochondral ossification, where connective tissues (mesenchyme) first differentiate into cartilage and are then replaced with bone cells (eg, in the radius, femur, tibia, and humerus).21,22
Other than bone, cartilage is another important component of the body skeletal system, being composed of strong and elastic connective tissues that cover the bone surfaces at joints (articular cartilage), and are found in the ear, rib cage, nose, and other body components.15 There are three types of cartilage, based on ECM composition: elastic cartilage (ECM with elastic fibers), fibrocartilage (ECM enriched with collagenous fibers), and hyaline cartilage (ECM enriched with glycosaminoglycans [GAGs]).20 Hyaline cartilage, also known as articular cartilage, is present at the interfaces of gliding bones in the articular synovial joints. This cartilage reduces the friction in synovial joints and facilitates their free movement, and supports the high dynamic compression load. Being less mechanically strong then bone, cartilage has a shear modulus of 0.7 MPa, compressive modulus of 0.7–0.8 MPa, and tensile modulus of 0.3–10 MPa.26 Structurally, cartilage consists of a mineralized ECM that is produced by the chondrocytes embedded within it, and a hydrated ECM that is 80% water filled with hydrophilic proteoglycans composed of core proteins with covalently attached GAGs (mainly chondroitin sulfates) and collagen type II, which carry out the load-bearing function of the tissue.15 The ability of cartilage to withstand high compressive loads is due to the GAGs, and the high tensile strength and tolerance for high shear stress come from the collagen type II fibrils.15,20 Upon compression, the cartilage ECM is compacted with the efflux of water and the increasing load; subsequently, the water flow is decreased and the hydrostatic pressure is increased to hold the load. Because cartilage tissues are avascular in their anatomy, the supply of nutrients and infiltration of cells are poor, resulting in arrested wound healing during tissue injury or defect.15 Fibrocartilage that was developed to replace native cartilage tissue had inferior mechanical strength compared with the native cartilage. The treatment of bone and cartilage defects through tissue engineering requires the development of materials and scaffolds according to the native composition and physiological roles of these two tissues.
To mimic the native composition of bone, visualization of its complex hierarchical structure is the first step toward understanding the nature of fracture and bone loss. Therefore, spatial temporal evolution with high spatial resolution at different lengths has been visualized to understand the regeneration process of bone tissues and to design scaffolds to support tissue regeneration.27 Micro-computed tomography (micro-CT), employing conventional X-ray sources, Raman spectroscopy, Fourier transform infrared spectroscopy, transmission electron microscopy, quantitative backscattered electron imaging, and X-ray scattering have been used to map the geometry and density distribution, imaging, quantification of the chemical contents, such as cross-links and mineralized crystallite, and the size, shape and density distribution of mineral particles within the bone, and to observe the size and shape of individual mineral crystals.28 But it has still not been possible to completely analyze bone structure, owing to the limitations of these techniques. Despite being gold-standard methods for 3D analysis of trabecular bone and structure of vascular (osteonal) porosity in cortical bone, conventional CT and micro-CT have limitations in imaging micro-damage.29 Synchrotron radiation (SR) coupled with micro-CT instead of standard X-ray beams has presented an alternative method to image the detailed microstructure and micro-damage of bone, and this technique has led to improved image quality and signal-to-noise ratio.30 SR applications made it possible to analyze bone microstructure and bone mineralization simultaneously. Various image processing software packages, such as VGStudio MAX (Heidelberg, Germany), Avizo (Hillsboro, OR, USA), and Mimics (Leuven, Belgium), are used to construct 3D images using synchrotron micro-CT slices.31 These 3D reconstructed models are used for quantitative assessment of micro-damage and printing of 3D scaffolds to regenerate the micro-damage.32 The advantages of using 3D reconstructions to measure the amount of micro-damage in bone are that the 3D model prevents overcounting or undercounting of micro-damage, which may occur when assessing micro-damage using two-dimensional (2D) slices.28 The use of SR-micro-CT provides better images in terms of quality and resolution, reduces the scan time, and facilitates the use of monochromatic (single-energy) X-rays. Synchrotron imaging techniques could be combined with in situ mechanical testing to visualize the structure and mechanical behavior of bone at these levels. Cooper et al used SR-micro-CT to visualize secondary osteons and porous structures in detail.29 This technique has also been applied to observe the bone regeneration process inside the scaffold; Campi et al used synchrotron high-resolution X-ray phase-contrast micro-tomography and synchrotron scanning micro-X-ray diffraction techniques to visualize the dynamics of collagen packing during ex vivo mineralization of ceramic porous HA implant scaffolds.33 The high-resolution images obtained from the synchrotron can play a vital role in 3D and 4D scaffold fabrication to repair bone and cartilage micro-damage.
Conventional treatments and their limitations
The causes of most bone injuries and defects are categorized into fractures, infections, cancer, old age, hereditary diseases, and cosmetic surgery,1 which can all cause damage to a certain extent. However, once the damage increases to a critical point or beyond the limit of the bone's self-renewal capacity, external stimuli are needed to initiate and boost bone regeneration and to restore normal bone volumes and functions. In general, bone defects are of two types: cavity defects, where the bone loss is limited, the bone biomechanics are not affected, and osteosynthesis is not interfered with; and segmental defects, where the normal bone biomechanics are compromised and the bone organ integrity is endangered.11 Cavity bone defects are easy to treat with the help of bone autografts, bone substitutes, morselized allografts, etc. If the cavity defects are small enough, they can be filled with implants. Segmental bone defects, which result from high-energy trauma in which the soft tissue is badly damaged, are the most problematic. Causes of segmental bone defects include bone tumor surgery; road accidents that injure the tibia, long bone (femur), and upper limbs; and wounds inflicted by weapons,34 many of which result in major functional disability and can lead to amputation.
Apart from autografts and surgery for infections and heredity bone defect diseases, the Osteoarthritis Research Society International and the American Academy of Orthopedic Surgeons also categorize physical measures and pharmacological therapy as treatment means.35–37 Physical measures help to manage the mechanical imbalance and reduce the risk of bone diseases, whereas pharmacological therapies use medications like non-steroidal anti-inflammatory drugs, paracetamol, and opioid analgesics to moderate pain. However, these medications are also associated with side effects, including gastrointestinal complications such as bleeding and perforated gastric ulcers, liver and kidney toxicity, nausea, dizziness, and constipation. Although the intra-articular injection of hyaluronic acid has also been used to treat cartilage lesions, its effect has been reported to be insignificant compared with that in a placebo group.37 Similarly, arthroscopic lavage and debridement were previously used as therapies, until the 2000s.38 Although all these treatments are effective in providing relief from symptoms, they cannot stop or prevent the progressive damage to the affected cartilage or bone. Severely injured patients will usually need surgical treatment or replacement implants. Currently, mosaicplasty, microfracture, and autologous chondrocyte implantation are being used to treat cartilage damage, but there are still doubts about their efficacy for complete recovery and the restoration of long-term function.39
Autologous bone grafts are still considered the gold standard for bone treatment.11,40 However, concerns about the size of bone to be harvested and problems associated with the donor site have not been addressed. Furthermore, donor-site morbidity effects and postoperative problems, including nerve injury, blood loss, hernia formation, and infection, are the leading limitations of this treatment method.41 In general, the techniques available for bone repair require invasive surgical procedures and a long healing time. As well as from the intense pain suffered by patients, surgery increases the risk of infection, and complete repair of the defects is not guaranteed. Moreover, the financial burden due to long-term hospitalization and psychological effects due to surgery further complicate the situation. Because of these limitations, surgeons continue to look for alternative methods to repair bone and cartilage defects. Another approach adopted to repair the damaged part is the reimplantation of an extruded bone segment. However, this is possible only for a limited time after the injury or accident and carries a high risk of infection, and guidelines or protocols for the sterilization and stabilization of reimplants are not clear.42 The few studies published in this regard have reported many concerns.43 Along with such bone transplant methods, the Masquelet procedure is another approach for repairing bone defects. In this procedure, an artificial chamber is created in situ using a temporary membrane. Once this membrane has been covered by the periosteum layer, it is removed and the gap is bridged with cancellous soft tissue.44 These treatments have also been used with combinations of growth factors, such as platelet-derived growth factor and bone morphogenetic proteins (BMPs), as well as cell injections, to focus on bone synthesis through matrix-forming cells.45 Another available option is the use of a demineralized bone matrix as a bone graft extender instead of the bone graft substitute.46 Ceramic bone graft substitutes have also been tested, but their very low mechanical stability and brittle nature limit their use in locations subject to high pressure. In this perspective, bone and cartilage tissue engineering has emerged as a promising alternative approach to effectively overcome the limitations of traditional implants and repair methods.
Tissue engineering
With the increasing demand for organs for transplantation and the complications associated with the conventional treatments of bone and cartilage defects, the importance of tissue engineering has increased.47 To overcome these issues, a potential solution, through the combination of cells with biodegradable and mechanically strong scaffolds, is required to repair the damaged tissue and restore its functions. The development of scaffolds with materials that exhibit strong mechanical support, biocompatibility, biodegradability, and osteoinductive properties is the basis for bone and cartilage tissue engineering. Materials that have been proposed and used to replace native bone and cartilage tissue at damage sites include ceramics, metals, and polymers.48 However, the poor degradability of ceramics, non-degradability of metallic compounds, and low mechanical strength of polymers are key challenges in bone and cartilage tissue engineering. Therefore, researchers have focused on the use of hybrid materials to develop scaffolds with optimum properties. In tissue engineering, biomaterials are used to produce scaffolds that act as a temporary matrix to establish a specific ECM for bone and cartilage regeneration. Natural polymers with high biodegradability and low immunogenicity are widely adopted for scaffold fabrication.49,50 By manipulating the polymer concentrations, scaffolds with optimized mechanical strengths, pore sizes, and surface charges can be obtained (Figure 3). To increase the bioactivity of scaffolds, their surface is functionalized with various bioactive materials, functional groups, proteins, and peptides.51 A scaffold is defined as an artificial support that provides a 3D environment for the development of the native tissue morphology.52 It can be used as an acellular system, as a delivery agent for cells or biological payloads (drugs, growth factors, etc), or as a cell-anchoring entity. If implanted as an acellular material on an injured site, the scaffold supports cell colonization, adhesion, and proliferation for tissue regeneration. Alternatively, if a scaffold is applied with cells and other biological molecules, it participates in bone formation in vivo by stimulating osteogenic differentiation. These cells can be grown and expanded ex vivo before delivery or implantation. A variety of cells can be manipulated for bone and cartilage tissue engineering.53 Herein, we discuss the fabrication of advanced scaffolds and their applications for bone and cartilage tissue regeneration.
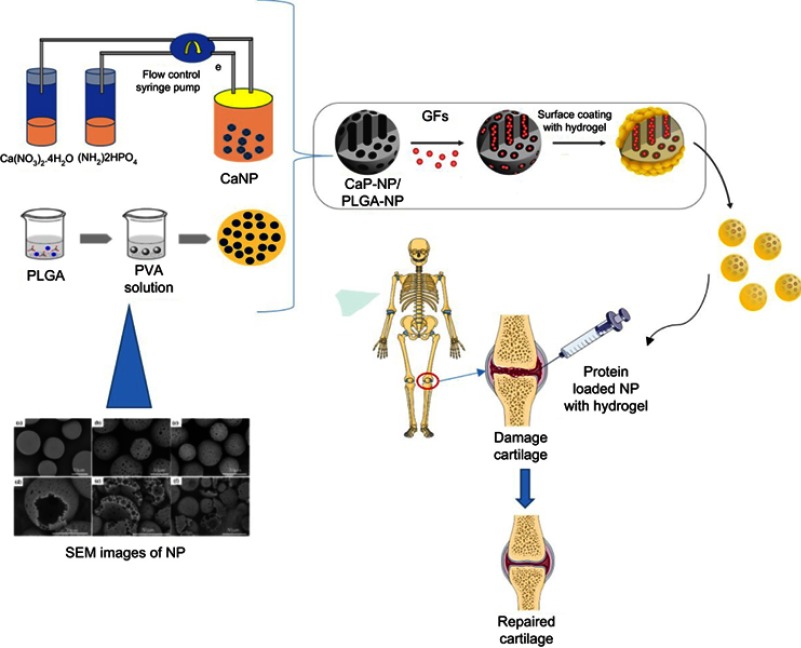
Figure 3.
Fabrication of hybrid scaffold for cartilage tissue engineering by using the Calcium nanoparticle or PLGA nanoparticles loaded with GFs and mixed with hydrogel to support cartilage regeneration.
Abbreviations: GF, growth factor; NP, nanoparticles; PLGA, poly(L-lactic-co-glycolic acid); PVA, poly(vinyl alcohol); SEM, scanning electron microscope.
Nanofibers
Electrospinning is a highly versatile and promising method for developing 3D nanofibrous scaffolds with a range of materials through the use of electrostatic force.49 In this technique, materials in solution are dispersed under electrostatic force into nano- and micro-sized continuous fibers. The elements required for electrospinning include a polymer source, a dispensing unit, a high-voltage supply, and a collector.54,55 The resulting scaffold mimics the natural ECM in terms of the fiber network, pore size, and surface-to-volume ratio. In addition, the nanofiber surface can be modified with various cross-linking and biological techniques according to the targeted tissues.56 Because of these properties, nanofibers have wide applications for skin, neural, heart, cartilage, and bone tissue engineering.57–60 For bone tissue engineering, nanofibrous scaffolds are considered close to ideal scaffolds owing to their similarity to collagen fibers in bone, and they mimic the native bone ECM.61–63 A wide variety of materials are used to fabricate nanofibrous scaffolds for bone and cartilage tissue engineering, including natural polymers (collagen, chitosan, silk, fibrogen, etc), synthetic polymers [poly(ε-caprolactone) (PCL), poly(L-lactic acid) (PLLA), polyglycolide, polyethylene glycol (PEG), etc], and hybrid materials (silver with PCL, etc) (Figure 3).49,64–66 Electrospun 3D nanofibrous scaffolds provide extra sites for cell attachment and high spatial interconnectivity. These nanofibers can also align in different patterns to support a cell arrangement similar to that of the native tissue. For example, Cai et al fabricated a practical 3D macroporous nanofibrous (MNF) scaffold for application in bone tissue regeneration.67 They seeded human embryonic stem cell-derived mesenchymal stem cells (hESC-MSCs) onto the MNF scaffold and observed the cell morphology with biochemical testing. The cells showed enhanced attachment and extended to spindle-like shapes. Those authors also implanted the MNF scaffold in vivo and evaluated the bone formation for 6 weeks. Histological and radiographic analyses showed the formation of 3D bony tissue after 6 weeks. Their study indicated that the MNF scaffold could be used for the culture of hESC-MSCs and their differentiation into bone cells, and revealed its potential for future clinical application in bone and cartilage tissue engineering.67 PLLA, a synthetic polymer approved by the US Food and Drug Administration (FDA), with good biodegradability, biocompatibility, and mechanical stability, has been applied extensively in bone repair.68 Laurencin used a PLLA-based biomimetic nanofibrous scaffold to deliver rat MSCs for ligament repair, resulting in enhanced mechanical strength and tissue regeneration.69 Similarly, Ito et al fabricated a nanocomposite scaffold comprising an atelocollagen sponge and a PLLA mesh to repair osteochondral damage in a rabbit model.70 PCL, another FDA-approved synthetic polymer, is used widely in tissue engineering both alone and in combination with other materials, such as collagen, chitosan, calcium, gelatin, silk, nano-hydroxyapatite (nHA), and beta-tricalcium phosphate (β-TCP), where it has shown improved mechanical properties when mixed with these materials.71,72 Lee et al used a PCL-gel–HA composite scaffold coated with the fibronectin 9–10 domain (FNIII9-10) and osteocalcin to evaluate the role of MSCs.73 The osteocalcin was conjugated with carboxyglutamic acid and aspartic acid, with calcium ions in the HA crystal. Similarly, Yoshimoto et al cultured mesenchymal stromal cells on PCL nanofibers and observed enhanced mineralization and collagen type I deposition.74 Observations of in vivo implantations of PCL-based nanofibrous scaffolds have also revealed the multilayered deposition of osteoblast-like cells, with the formation of bony tissue and a mineralized matrix enriched with osteocytes.75 In another study, Eap et al developed a 1-cm-thick PCL-based nanofibrous scaffold for culturing human osteoblasts and applied it for bone tissue engineering.76 Xu et al fabricated a PCL-based scaffold that was similar to the natural ECM, with high porosity (almost 97%), and observed better cell physiology for cartilage formation.77 The scaffold facilitated high cell viability in vitro, and the mouse bone marrow MSCs expressed BMP2-induced chondrogenic rather than osteogenic differentiation. The in vivo results were similar, proving the effectiveness of the nanofibrous scaffold as a supportive synthetic ECM for bone and cartilage regeneration via endochondral ossification.77
To mimic the natural ECM, nanofibrous scaffolds have been loaded with growth factors to repair complex and large-sized bone defects such as calvarial damage. Li et al fabricated an advanced form of nanofibrous scaffold, incorporating nanoparticles loaded with growth factors.78 They first encapsulated BMP2 into nanoparticles composed of bovine serum albumin, mixed them with a solution of PCL-co-PEG and dexamethasone (DEX), and then electrospun that mixture.78 The nanofibrous scaffold demonstrated a sustained release of DXE and BMP2 in vitro over 8 and 35 days, respectively. The authors also confirmed that the sustained release of these growth factors favored the propagation of osteoblasts in vitro. When they implanted this composite scaffold with drug-loaded nanoparticles to repair rat calvarial defects in vivo, they found that it cleared the defects more rapidly than the scaffold without drug-loaded nanoparticles did, owing to the combined effects of BMP2 and DEX.78 Similarly, PCL nanofibers loaded with magnetic particles facilitated enhanced MSC adhesion and proliferation and better osteogenic differentiation.79
Natural polymeric nanofibrous scaffolds for bone and cartilage tissue repair have also been reported. For example, Van Hong Thien et al fabricated nanofibers with HA and chitosan to increase the osteoconductivity of seeded cells, whereupon the scaffold showed increased cell proliferation and alkaline phosphate (ALP) activity.80 Similarly, the effects of micro-HA and nHA were compared by fabricating the nanofibrous scaffolds with chitosan, with results showing that nHA facilitated better MSC adhesion and propagation and estrogenic activity.81 Moreover, Runt-related transcription factor 2 (Runx2), ALP, BMP2, BMP4, and SMAD family member 1 were upregulated in the MSCs when seeded on these scaffolds. In a similar study, Mi et al used micro-HA and nHA with thermoplastic polyurethane (TPU) to fabricate nanofibrous scaffolds and evaluated the effect of the HA size on cell behavior.82 The TPU nanofibers with micro-HA showed a higher Young’s modulus and lower break strain then those with nHA. Overall, the tensile strength of the TPU nanofibers declined with the addition of either nHA or micro-HA. The nanofibers with TPU–nHA were soft owing to their shorter diameter, whereas those with micro-HA were hard. The soft nanofibers facilitated better osteoblast attachment, but cell migration and propagation were better on the surface of the hard scaffold. Furthermore, the soft scaffold with nHA enhanced the osteogenesis of human mesenchymal stem cells (hMSCs) compared with that without nHA. This study showed that the TPU–nHA scaffold could be applied for bone tissue engineering.82
Hybrid scaffolds with more than one type of material have shown synergetic effects for bone tissue engineering compared with scaffolds made from a single material alone. Therefore, the current trend is to study different combinations of materials to find ideal ones for hybrid scaffold fabrication. Vozzi et al fabricated a scaffold with collagen, gelatin, and genipin, seeded it with human primary osteoblasts, and evaluated it at regular intervals for 3 weeks for the expression of different bone-related genes.83 Increased cell adhesion, proliferation, and differentiation, with an increased trend for osteopontin, ALP, and osteocalcin expression over time, were observed.83 Similarly, Zhang et al fabricated gelatin−β-TCP nanofibers and observed the enhanced activity of bone-derived cells.84 Meanwhile, Wang et al developed electrospun poly-3-hydroxybutyrate-co-3-hydroxyhexanoate nanofibers to study the adipogenic and osteogenic abilities of osteoblasts and fibroblasts.85 Another synergetic nanofibrous scaffold, fabricated using silk and chitosan and cultured with bone marrow hMSCs, was used to evaluate osteogenic activity (using the discoloration of the biochemical stain alizarin red), ALP movement, and the expression of indicator genes of osteogenesis.86 The chitosan and silk mix increased the osteogenic separation and proliferation of hMSCs. Similarly, it was observed that 3D fibroin-based nanofibrous scaffolds possessed great porosity and showed bone regeneration potential.87
Although electrospun nanofibrous scaffolds have shown promising results for bone and cartilage tissue regeneration, their topography and morphology still present critical barriers to mimicking the natural ECM. These types of scaffolds lack macroscopic pores, making the diffusion of nutrients difficult. Therefore, a gap remains in the development of innovative methods for fabricating nanofibers with interconnected macropores to fully mimic the native ECM of bone. For large tissue defects in big bones, 3D nanofibrous mats have been recommended. Further studies are thus needed to enhance the morphology and architecture of nanofibers with nanopores and macropores, to support not only cell infiltration and nutrient supply but also vascular growth.
Hydrogels
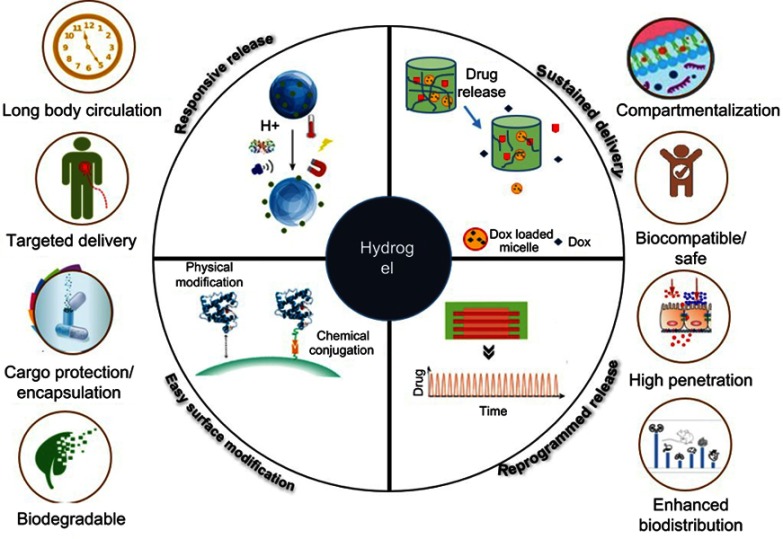
The majority of the previously discussed polymeric scaffold types need surgical and invasive support for implantation. Clinically, there is a demand for scaffolds that can be implanted with minimally invasive procedures. There is thus a demand for materials with a low viscosity to allow easy delivery by injection and that can be cross-linked in vivo to form a hard scaffold. A hydrogel is a physically and chemically cross-linked hydrophilic scaffold that can be used for the delivery of biological molecules and provides a 3D environment similar to that of the native ECM (Figure 4).88 Owing to their ECM-like structure, hydrogels can entrap proteins or cells in the mesh and control the release of the materials as required. Moreover, hydrogels are absorbable and demonstrate excellent integration with surrounding tissues, thereby avoiding the complexity of surgical removal and reducing the possibility of an inflammatory response.89 Owing to their high water-holding capacity (similar to that of soft tissues), hydrogels can support cell viability better than other 3D scaffolds.90 The low-viscosity hydrogels can be delivered via injection to the defect site, and they can incorporate growth factors, drugs, and cells for tissue engineering.91 Their properties can be modified according to the polymer used for their fabrication and the cross-linking agent.92 They have shown sustained, programmed, and thermoresponsive or pH-responsive payload delivery (Figure 4).93 Their properties protect the payload from chemical or physical degradation before reaching the target site and increase their circulation or presence time, thereby supporting the tissue regeneration process. Various 3D templates based on hydrogels have been developed that mimic the bioenvironments, depicting motifs inspired by the role of the ECM in regulating bone regeneration. Hydrogels fabricated with biocompatible, biodegradable, and responsive materials with fewer adverse reactions can be perfect for clinical applications.94,95
Figure 4.
Properties of hydrogel scaffolds used in cartilage and bone tissue engineering through delivery of growth factors and cells and different delivery mechanisms based on stimulus, target site, and material.
Naturally derived hydrogel-forming polymers have been extensively manipulated in bone and cartilage tissue engineering. For example, collagen is the most abundant protein of mammalian tissue ECMs and is used for the culture of osteoblasts.90,96 Similarly, hyaluronic acid-based hydrogels developed via copper(I)-catalyzed azide–alkene cycloaddition have been used as drug reservoirs and cell scaffolds.97 These natural polymers, with excellent biodegradability and low immunogenicity, and which facilitate high cell viability, adhesion, proliferation, and migration abilities, further add to the value of hydrogel scaffolds. Such scaffolds made of natural polymers, including fibrin, alginate, gelatin, and chitosan, have shown success in bone and cartilage tissue engineering.14,49,98,99 Chitosan, derived naturally from chitin, is a linear polysaccharide composed of N-acetylglucosamine.100 Owing to its similarity with cartilage glycosaminoglycan, it is considered one of the most favorable candidates for fabricating injectable hydrogels for cartilage repair.100 Naderi-Meshkin et al fabricated such an injectable hydrogel by mixing chitosan with glycerol phosphate and the cross-linking agent hydroxyethyl cellulose, and used it as a scaffold for cartilage repair.101 Similarly, the feasibility for MSC propagation and isolation for cartilage repair was evaluated using a chitosan-based injectable hydrogel.101 To constitute a hydrogel with responsive abilities, chitosan is mixed with stimulus-responsive polymers. For example, Sá-Lima et al demonstrated the thermoresponsiveness of an injectable hydrogel for cell delivery, which was fabricated with chitosan–glycerophosphate and different concentrations of starch.102 Moreover, Moreira et al produced an injectable hydrogel, composed of bioactive glass nanoparticles, collagen, and chitosan, with thermogelling properties.103 One of the limitations of chitosan is that it is insoluble in water and can only dissolve in acetic acid. Therefore, efforts have been made to develop a water-soluble form of chitosan so that its washing steps can be skipped during hydrogel fabrication. Expanding on the idea of a water-soluble, chitosan-based hydrogel, Kamoun developed an injectable hybrid hydrogel with non-toxic properties, made of N-succinyl chitosan–dialdehyde starch.104 The scaffold took less time to gel and exhibited low water uptake, high degradability, and a stiff surface, making it highly supportive for cartilage tissue engineering.104 To increase the cell viability and adhesion further, Santo et al added platelet-rich plasma (which, upon activation, is called platelet lysates) to chondroitin sulfate–chitosan hydrogels and showed the enhanced osteogenic differentiation of MSCs with high calcium deposition.105
Gelatin, another natural biopolymer that is extracted from collagen and has high biocompatibility and biodegradability, is also widely used in hydrogel fabrication.106 Oh et al fabricated a unified, dual-thermoresponsive, macroporous, gelatin-based injectable hydrogel using stable oil-in-water elevated inner-stage suspensions, with gelatin-graft-poly(N-isopropylacrylamide).107 Similarly, Geng et al fabricated a pH-responsive injectable hydrogel from amino gelatin, oxidized dextran, and 4-arm PEG acrylate in two steps and used it for pre-osteoblast seeding.108 The hydrogel showed high biocompatibility and facilitated cell adhesion and differentiation.108 Solorio et al fabricated microspheres (2–6 µm diameter) of gelatin hydrogels and loaded them with recombinant bone morphogenetic protein-2 (rhBMP2) to stimulate hMSCs for bone formation. These hydrogel microspheres showed a sustained released of rhBMP2, with their loading capacity increasing with a high degree of cross-linking, but with low cumulative release. The sustained released of rhBMP2 resulted in a three- to eight-fold increase in the expression of bone sialoprotein, confirming the use of the hydrogel for slow payload delivery and its impact on tissue regeneration.51
Fibrin is another natural polymer that is widely exploited in clinical studies for bone tissue engineering using stem cells.109 Seebach et al implanted fibrin glue hydrogels mixed with rat-derived MSCs into damage sites of rat femoral bone to test host-cell recruitment, immunomodulation, and tissue regeneration.98 Similarly, alginate has been used for bone and cartilage tissue engineering owing to its non-toxicity, non-immunogenicity, and scaffold-forming ability.99 Alginate is a polysaccharide derived from brown algae, and contains mannuronic and guluronic acids. Moshaverinia et al fabricated injectable hydrogels comprising alginate-based microbeads for use in enclosing dental-derived MSCs, including periodontal ligament stem cells and gingival MSCs.110 In their system, alginate was used to encapsulate a stem cell and calcium chloride (cross-linking agent) mixture for injection into a defect site to form a gel on the spot. Micro-CT analysis confirmed that the cells remained viable after gelation, and ectopic mineralization occurred both inside and around the microbeads owing to the efficient exchange of materials.110 Because hyaluronic acid is naturally responsible for chondrogenic differentiation, the matrix deposition of chondrocytes, the development of cartilage and limb buds, and the concentration of mesenchymal cells, it is considered a good candidate for bone and cartilage repair. Yu et al fabricated an injectable hyaluronic acid–PEG hydrogel with high mechanical stability for bone and cartilage tissue engineering, whereupon enhanced metabolic activity and cell propagation were observed inside the hydrogel.111 Similarly, Park et al fabricated an injectable hyaluronic acid-based hydrogel in which methacrylated glycol chitosan was incorporated to make the scaffold structurally similar to glycosaminoglycan.100 Chondrocytes mixed with the hydrogel showed increased propagation and extra deposition of cartilaginous ECM. On the basis of these results, it was concluded that this hydrogel system could be used for cartilage tissue repair.
Although hydrogel systems based on natural polymers have good biocompatibility and biodegradability, they do not have enough mechanical strength to support large-sized bone defects. Therefore, different metallic and non-organic nanoparticles have been used to increase the mechanical characteristics of hydrogel nanocomposites.106 These nanoparticles include graphene oxide, graphene, nHA, calcium phosphate, carbon nanotubes, and bioactive glass.104,112–114 The addition of these nanoparticles to scaffold supports has enhanced cell adhesion, proliferation, and differentiation. β-TCP, biphasic calcium phosphate, and synthetic hydroxyapatite (sHA) (a mixture of sHA and β-TCP) have mostly been used as ceramic granules.115,116 Similarly to sHA, bioactive glass showed a 10-fold increased bioactivity index, and expressed superior bone-bonding ability. The use of 2D nanosilicates for the osteogenic differentiation of human adipose stem cells117 and hMSCs118 has been reported without the addition of any growth factors such as BMP2. Similarly, Kerativitayanan et al fabricated porous nanocomposite scaffolds from poly(glycerol sebacate) and nanosilicates. The addition of the nanosilicates enhanced the physical reliability, mechanical strength, and stiffness of the scaffold without compromising its elastomericity. These physical characteristics of the hydrogel established a load-transducing environment for bone regeneration, which was enough to repair craniofacial defects.119
Similarly to natural polymers, synthetic polymers also offer various approaches for fabricating porous hydrogel scaffolds with controlled biodegradability and functionally active surfaces for bone and cartilage tissue engineering. Various synthetic polymers with a hydrophilic nature can auto-change to a hydrogel form through physical or chemical cross-linking; for example, poly(aminoamides), poly(N-isopropylacrylamide), poly(glycolic acid), poly(propylene fumarate), poly(hydroxyethyl methacrylate), poly(vinyl alcohol), and PEG.120–123 Nuttelman et al encapsulated hMSCs into a photocross-linkable injectable hydrogel composed of dimethacrylated PEG (MW 4.6 kDa).124 The PEG-based hydrogel system showed high cell viability and enhanced osteogenic differentiation values, which were confirmed by the upregulation of osteonectin and ALP gene expression. Furthermore, extensive mineralization of the ECM was confirmed by biochemical staining.124 A smart nanogel scaffold developed from poly(N-isopropylacrylamide-co-butyl methylacrylate) behaved like a gel at room temperature and changed to a hard scaffold at body temperature, making it suitable for bone tissue engineering.91,125 The thermoresponsive behavior of this nanogel scaffold was due to the low critical temperature of poly(N-isopropylacrylamide) being close to human body temperature (37°C).
Similarly, Cao et al used glycol chitosan and a multi-benzaldehyde-functionalized PEG analog [poly(ethylene oxide-co-glycidol)-CHO] to fabricate an injectable hydrogel for cartilage tissue repair.126 This hydrogel was cross-linked chemically in situ through a Schiff base reaction between the aldehyde groups of poly(ethylene oxide-co-glycidol)-CHO and the amino groups of glycol chitosan at room temperature.126 Furthermore, hydrogels formed by the Schiff base cross-linking of other biomaterials have been used for tissue engineering. Ma et al reported the fabrication of an injectable liposome–polymer-based hydrogel, using phosphatidylethanolamine liposomes and aldehyde-modified xanthan gum chemically cross-linked by a Schiff base reaction.127 This hydrogel, which was easily fabricated at room temperature and possessed excellent self-curing capability, expressed enzyme-based biodegradation and high cell viability. All these studies show that hydrogel scaffolds, fabricated using a range of natural, synthetic, and non-organic materials, have huge potential for use in bone repair and especially cartilage tissue regeneration. In the future, customized bone substitutes will be built using hydrogel scaffolds with advanced 3D and 4D technologies.
Three-dimensional printing scaffolds
Both bone and cartilage tissue engineering require scaffolds with high mechanical stability, biocompatibility, and tissue vascularization capability for implantation. An advanced manufacturing technology, named 3D printing, has been developed to overcome these challenges.128 The instrument for this technique is composed of a bioink reservoir with a controllable platform. This methodology has enough flexibility to build 3D structures with complex features. Using patient data from computed tomography/magnetic resonance imaging, advanced computer-aided design (CAD) technology, and rapid prototyping, a personalized scaffold for vasculature, with a bionic appearance and highly interconnected pores, can be 3D printed with precise structure (Figure 5).129 The mechanism of 3D bioprinting involves a layer-by-layer deposition of bioinks and biomaterials (ie, multiple cell types and biomaterials for tissue fabrication) in a process called additive manufacturing.130,131 This technology is further subdivided into fused deposition modeling, powder-based printing (3DP), stereolithography (SLA), selective laser sintering, and robocasting.132 In general, inkjets and microextrusion are used for the 3D printing of scaffolds, whereas extrusion-based 3D printing is used for bone.133,134
Figure 5.
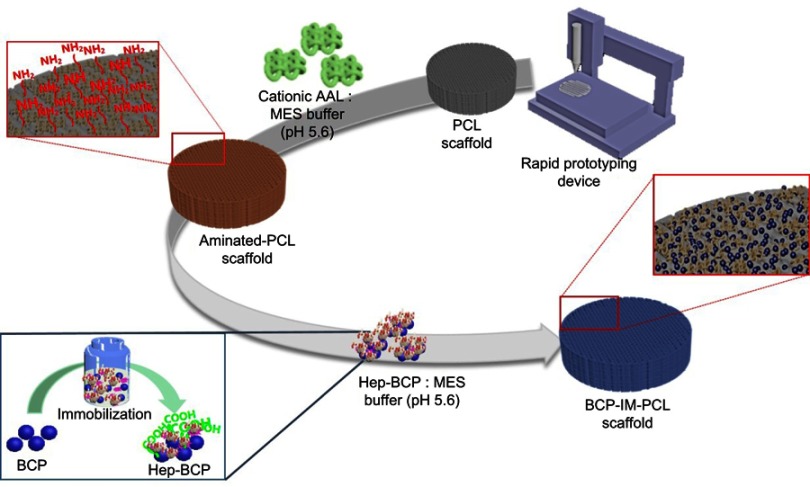
The 3D printing of a scaffold and its surface functionalization with active biological molecules to increase scaffold bioactivity: BCP conjugated with protein immobilized on a PCL 3D printed scaffold.
Abbreviations: AAL, L-Alanine; BCP, biphasic calcium phosphate; Hep, heparin; IM, immobilized; MES, 2-(N-morpholino)ethanesulfonic acid; PCL, poly (∂>+-caprolactone).
In the extrusion-based technique, a three-axis dispensing system is used to fabricate a 3D scaffold by printing the hydrogel (fiber)-laden material in layers according to CAD.95 The extrusion pressure is generated by compressed gas, and the printed scaffold is subjected to chemical or ultraviolet (UV) cross-linking according to the nature of the material, to maintain the fiber morphology.135 In the case of the inkjet technique, thermal or acoustic force is applied to eject the liquid drops.136 This is the best method for fine printing, with excellent resolution and controlled liquid volumes.137 Hydrogels are popular materials for bioprinting as they possess excellent biocompatibility and biodegradability and undergo phase transition under extreme thermal or chemical stress. Gao et al136 used the PEG–methacrylated gelatin (GelMA) hydrogel for inkjet printing, and showed an increase in the mechanical strength for bone constructs (by a modulus of 1–2 MPa) compared with that of the GelMA scaffold only. Similarly, poly(L-lactic-co-glycolic acid) (PLGA)–PEG–PLGA hydrogels have been used for the 3D printing of scaffolds.138 In another study, Gao et al139 used a PEG-based hydrogel mixed with hMSCs and peptides to bioprint the 3D bone and cartilage substitutes in a single step. The resultant matrix led to the development of bone and cartilage tissues with good deposition of mineralized ECM and cartilage. The addition of peptides to the 3D scaffold enhanced the cell differentiation over that of the hydrogel with polymer only.139 Similarly, Yin et al used GelMA–gelatin as bioinks for the controlled 3D bioprinting of microarchitectures, where the scaffold expressed a high cell viability rate (>90%).140 Furthermore, Daly et al used MSC-laden GelMA hydrogels to print scaffolds with interconnected microchannels for in vitro and in vivo bioanalyses of bone repair. They showed that the microchannel networks facilitated cell migration and increased the interaction between osteoclasts and immune cells.141 In advanced 3D printing methods, multiple printing heads are used to print functional large-sized bone constructs or full organs. Cui et al applied a dual head for the 3D bioprinting of large functional bone grafts enriched with microvascular structures.142
A variety of polymers have been applied for the 3D printing of bone and cartilage scaffolds, including PCL (Figure 6). Reichert et al143 used a PCL–TCP composite material for printing a highly porous 3D scaffold with high elasticity (22.2 MPa), which showed the formation of bone with high mechanical stability in vivo.143 Similarly, Wang et al144 used the SLA method to fabricate poly(propylene fumarate) scaffolds and demonstrated that the compressive modulus was comparable to that of the bone, with high mechanical stability and without any cytotoxicity while degrading.144 Kao et al145 used poly(lactic acid) for the fabrication of a 3D surface and modified it with poly(dopamine). This modification made the scaffold surface hydrophilic and increased the cell adhesion, migration, and differentiation over those on poly(lactic acid) alone. Similarly, Kuo et al146 used collagen type I to functionalize the surface of 3D-printed PLGA–chitosan scaffolds to increase MSC adhesion and proliferation.
Figure 6.
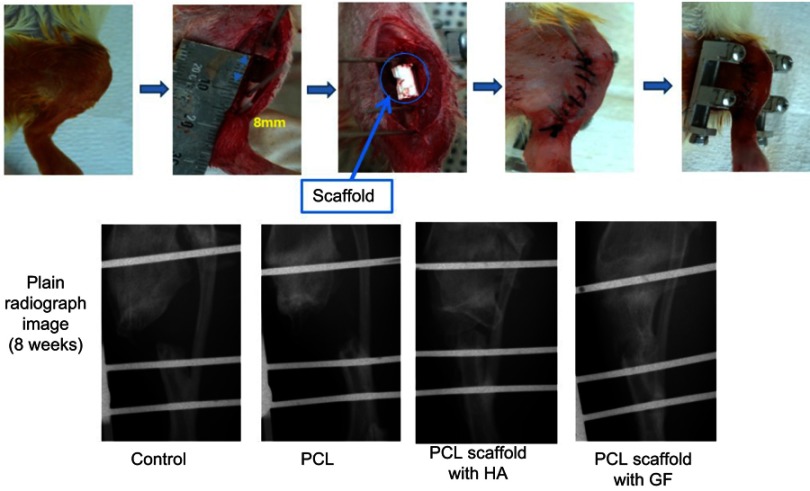
In vivo evaluation of a 3D-printed PCL scaffold for bone regeneration in a rat model through radiography with X-ray after 8 weeks.
Abbreviations: GF, growth factor; HA, hydroxyapatite; PCL, poly(ε-caprolactone).
Hybrid materials made of inorganic materials and polymers are also used in the 3D printing of scaffolds for cartilage and bone tissue engineering. The extrusion-based 3D printing of scaffolds with HA mixed with poly(propylene fumarate) has been used to mimic the natural mechanical strength of bone. The authors controlled the nHA distribution of the poly(propylene fumarate)-based scaffold and used it for in vivo implantation.135 Similarly, poly(lactic acid) and osteoconductive HA have been used to fabricate 3D-printed composite scaffolds for bone tissue engineering.147 Inorganic silicates alone, such as tricalcium silicate (Ca3SiO5, C3S) ceramics, are also used for the 3D printing of scaffolds to allow slow drug release. The 3D scaffold showed bone tissue formation in vivo when used with a nanocrystal morphology.148,149 Zhu et al combined the 3DP technique with a solid-state reaction method to fabricate pure-phased Sr5(PO4)2SiO4 ceramic scaffolds; these showed increased ALP activity and the upregulation of gene expression for osteogenesis (osteopontin, ALP, osteocalcin, and Runx2) in rabbit bone marrow-derived MSCs.150 Roohani-Esfahani et al fabricated 3D scaffolds with a triphasic microstructure using submicrometer gahnite (ZnAl2O4) mixed with strontium-doped hardystonite (Ca2ZnSi2O7) and a glass phase. They achieved a porous network of various pore sizes and geometries, with high compressive strengths.151 Similarly, Ti-6Al-4V was used with the inkjet method to develop 3D-printed scaffolds with different morphologies for use as substitutes for high-torsion bone defects.152 To improve bone and cartilage tissue engineering, the 3D printing technology should be combined with techniques such as nanosurface modification, digital medical imaging, finite element analysis, virtual surgical planning, and motion capture, so that they can be applied directly for clinical use after FDA (USA) approval. There is also a need to develop new 3D printing methods to meet the complex nature of biological materials, by using the right combination and quantity of seeded cells and evaluating the existing 3D printing methods for the use of hybrid materials as bioinks.
Four-dimensional implants
Four-dimensional printing technology has revolutionized the design of printed constructs.153 It is a printing method in which constructs being 3D printed are designed to modify over time according to stimuli from the environment. Therefore, this method provides many additional advantages and applications. As with 3D technology, 4D printing is dependent on new biomaterials, but these usually respond to stimuli (humidity, temperature, or chemicals).154 After the fabrication process, these stimuli change the form of the construct accordingly. Miao et al polymerized soybean oil by UV light to prepare epoxidized acrylate resin for 4D SLA of biomedical scaffolds with significant shape memory effects. The shape memory effect depends on temperature to affect the cross-linking of the molecules. Under low temperature, the cross-linker is frozen and the shape of the scaffold is fixed, whereas at elevated temperature, the scaffold retains its original shape. These scaffolds demonstrated biocompatibility and allowed stronger attachment of hMSCs and their higher proliferation, compared with PEG diacrylate-based scaffolds. In addition, there was no significant difference between the effects of poly(lactic acid) and PCL (clinically approved materials). Potentially, this study could use renewable resources for biomedical scaffold fabrication by SLA printing and it may advance to enable the development of renewable 4D scaffolds in bone engineering.153 Similarly, 4D scaffolds of polyurethane were fabricated using additive manufacturing technology, and seeded with cells to evaluate the polymer’s shape memory.155 After shape recovery of the polymer, the cells were significantly elongated, indicating that the mechanical stress induced by shape recovery could influence cell behavior.
To comply with traditional manufacturing methods, least possible variation has been employed for the structures, methodologies, and characteristics. Furthermore, in current bone implant designs, joint contact forces that occur during real-life activities are not taken into account.156 Therefore, a 4D design should consider the physiological condition of the defective cartilage and bone, including all the micro and macro aspects of the target tissue and consider them in the scaffold fabrication process.157 Thus, to increase the reliability of scaffolds for clinical application, all 3D-printed constructs must be subjected to multiscale finite element analysis (MFEA) and computational neuromusculoskeletal (NMS) evaluation, in addition to analyses of their mechanical and in vivo compatibility. Computational NMS models evaluate the load-holding capacity of scaffold and muscle forces according to real-life situations.158 In addition, they help researchers to simulate structural and functional relationships between the damage and the designed scaffolds, to understand the mechanism of injury. MFEA models optimize the scaffold before its fabrication by considering its micro- and nano-level aspects, predicting its output, and pointing out the weak areas in case of in vivo cyclic stress. Therefore, the future of bone and cartilage tissue engineering is associated with the 4D bioprinting of implants for full defect repair.
Conclusions and future prospects
Advances in the areas of biomaterials, nanotechnology, and methodologies of hydrogel- and nanofiber-based scaffold fabrications have presented significant success for cartilage and bone regeneration compared with conventional techniques. Nevertheless, significant barriers still exist in the development of fully functional bone constructs with enough mechanical strength to replace large bone defects. These barriers are associated with not only these technologies but also with problems in attaining appropriate vascularization, mechanical strength, and sustainability of the substitute. The introduction of medical imaging, computational modeling, precise personalized design, and 3D and 4D bioprinting to tissue engineering is a step toward facilitating the cost-effective construction of mechanically strong and functional bone and cartilage substitutes. This will also require the development of materials with biomimicry ability, responsiveness to stimuli, a highly porous nature, and mechanical stability for manipulation in 4D printing.
Acknowledgments
Although we are the authors of this review, we would never have been able to complete it without the great contribution of many people in the field of cardiac and vascular tissue engineering. We owe our gratitude to all those researchers who have made this review possible. We have cited as many references as permitted and apologize to the authors of those publications that we have not cited due to limitations of space. We apologize to other authors who have worked on these aspects but whom we have unintentionally overlooked. All figures were originally drawn/edited for this article. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (number NRF-2017R1A2B4008179). This work was also supported by grants from the National Research Foundation of Korea (NRF-2017R1C1B5017159) and the Ministry of Health & Welfare, Republic of Korea (HI18C0661).
Disclosure
Prof Dr Dong Sik Chae reports grants from National Research Foundation of Korea and the Ministry of Health & Welfare, Republic of Korea, during the conduct of study. The authors report no other conflicts of interest in this work.
References
- 1.Facts and Statistics | International Osteoporosis Foundation. Available from: https://www.iofbonehealth.org/facts-statistics. Accessed March 9, 2019.
- 2.Haagsma JA, Graetz N, Bolliger I, et al. The global burden of injury: incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj Prev. 2016;22:3–18. doi: 10.1136/injuryprev-2015-041944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrington JL. Aging bone and cartilage: cross-cutting issues. Biochem Biophys Res Commun. 2005;328:700–708. doi: 10.1016/j.bbrc.2004.12.193 [DOI] [PubMed] [Google Scholar]
- 4.Baroli B. From natural bone grafts to tissue engineering therapeutics: brainstorming on pharmaceutical formulative requirements and challenges. J Pharm Sci. 2009;98:1317–1375. doi: 10.1002/jps.21528 [DOI] [PubMed] [Google Scholar]
- 5.Wijewardena A, Vandervord E, Lajevardi SS, Vandervord J, Jackson CJ. Combination of activated Protein C and topical negative pressure rapidly regenerates granulation tissue over exposed bone to heal recalcitrant orthopedic wounds. Int J Low Extrem Wounds. 2011;10:146–151. doi: 10.1177/1534734611417342 [DOI] [PubMed] [Google Scholar]
- 6.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Ferng AS, Geffre CP, Mantyh PW. Bone cancer pain. Ann N Y Acad Sci. 2010;1198:173–181. doi: 10.1111/j.1749-6632.2009.05429.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan G, McCormick C, Tufaro F. The link between heparan sulfate and hereditary bone disease: finding a function for the EXT family of putative tumor suppressor proteins. J Clin Invest. 2001;108:511–516. doi: 10.1172/JCI13737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bizzetto R, Bonfim C, Rocha V, et al. Outcomes after related and unrelated umbilical cord blood transplantation for hereditary bone marrow failure syndromes other than Fanconi anemia. Haematologica. 2011;96:134–141. doi: 10.3324/haematol.2010.027839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidekrueger PI, Juran S, Ehrl D, Aung T, Tanna N, Broer PN. Global aesthetic surgery statistics: a closer look. J Plast Surg Hand Surg. 2017;51:270–274. doi: 10.1080/2000656X.2016.1248842 [DOI] [PubMed] [Google Scholar]
- 10.Grasman JM, Zayas MJ, Page RL, Pins GD. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater. 2015;25:2–15. doi: 10.1016/j.actbio.2015.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locker PH, Arthur J, Edmiston T, Puri R, Levine BR. Management of bone defects in orthopedic trauma. Bull Hosp Jt Dis. 2018;76:278–284. [Google Scholar]
- 12.Bone Grafts and Substitutes Market Size, Share & Trends Analysis Report by Material Type (Natural, Synthetic), by Application Type (Spinal Fusion, Craniomaxillofacial, Long Bone), by Region, and Segment Forecasts, 2018–2025. Grand View Research: San Francisco (CA); 2018. Available from: https://www.grandviewresearch.com/industry-analysis/bone-grafts-substitutes-market. Accessed May 23, 2019. [Google Scholar]
- 13.Zhang K, Wang S, Zhou C, et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018;6:31. doi: 10.1038/s41413-018-0032-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin CC, Ki CS, Shih H. Thiol-norbornene photoclick hydrogels for tissue engineering applications. J Appl Polym Sci. 2015;132:1–11. doi: 10.1002/app.41563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wojnar R. Bone and cartilage – its structure and physical properties. In: A Ochsner, W Ahmed, editors. Biomechanics of hard tissues: modeling, testing, and materials Weinheim: Wiley–VCH Verlag GmbH & Co.; 2010:1–75. [Google Scholar]
- 16.Fyhrie DP. Osteoporosis in men. In: Orwoll ES, Bilezikian JP, Vanderschueren D, editors. The mechanical properties of bone. Amsterdam: Elsevier; 2010:51–67. [Google Scholar]
- 17.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743–765. doi: 10.1002/mabi.200300044 [DOI] [PubMed] [Google Scholar]
- 18.Oryan A, Monazzah S, Bigham-Sadegh A. Bone injury and fracture healing biology. Biomed Environ Sci. 2015;28:57–71. doi: 10.3967/bes2015.010 [DOI] [PubMed] [Google Scholar]
- 19.Alford AI, Kozloff KM, Hankenson KD. Extracellular matrix networks in bone remodeling. Int J Biochem Cell Biol. 2015;65:20–31. doi: 10.1016/j.biocel.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 20.Gentili C, Cancedda R. Cartilage and bone extracellular matrix. Curr Pharm Des. 2009;15:1334–1348. doi: 10.2174/138161209787846739 [DOI] [PubMed] [Google Scholar]
- 21.Reznikov N, Shahar R, Weiner S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014;10:3815–3826. doi: 10.1016/j.actbio.2014.05.024 [DOI] [PubMed] [Google Scholar]
- 22.Mackie EJ, Ahmed YA, Tatarczuch L, Chen K-S, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40:46–62. doi: 10.1016/j.biocel.2008.02.019 [DOI] [PubMed] [Google Scholar]
- 23.Ruimerman R. Modeling and Remodeling in Bone Tissue [PhD thesis]. Eindhoven: Eindhoven University of Technology; 2005. [Google Scholar]
- 24.Wiese A, Pape HC. Bone defects caused by high-energy injuries, bone loss, infected nonunions, and nonunions. Orthop Clin North Am. 2010;41:1–4. doi: 10.1016/j.ocl.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 25.Berner A, Reichert JC, Müller MB, et al. Treatment of long bone defects and non-unions: from research to clinical practice. Cell Tissue Res. 2012;347:501–519. doi: 10.1007/s00441-011-1184-8 [DOI] [PubMed] [Google Scholar]
- 26.Ode A, Duda GN, Geissler S, et al. Interaction of age and mechanical stability on bone defect healing: an early transcriptional analysis of fracture hematoma in rat. PLoS One. 2014;9:e106462. doi: 10.1371/journal.pone.0106462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastrogiacomo M, Campi G, Cancedda R, Cedola A. Synchrotron radiation techniques boost the research in bone tissue engineering. Acta Biomater. 2019;89:33–46. doi: 10.1016/j.actbio.2019.03.031 [DOI] [PubMed] [Google Scholar]
- 28.Ma S, Boughton O, Karunaratne A, et al. Synchrotron Imaging Assessment of Bone Quality. Clin Rev Bone Miner Metab. 2016;14:150–160. doi: 10.1007/s12018-016-9223-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper DML, Erickson B, Peele AG, Hannah K, Thomas CDL, Clement JG. Visualization of 3D osteon morphology by synchrotron radiation micro-CT. J Anat. 2011;219:481–489. doi: 10.1111/j.1469-7580.2011.01398.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labriet H, Nemoz C, Renier M, et al. Significant dose reduction using synchrotron radiation computed tomography: first clinical case and application to high resolution CT exams. Sci Rep. 2018;8:12491. doi: 10.1038/s41598-018-30902-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larrue A, Rattner A, Peter Z-A, et al. Synchrotron radiation micro-CT at the micrometer scale for the analysis of the three-dimensional morphology of microcracks in Human trabecular bone. PLoS One. 2011;6:e21297. doi: 10.1371/journal.pone.0021297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giuliani A, Mazzoni S, Mele L, Liccardo D, Tromba G, Langer M. Synchrotron phase tomography: an emerging imaging method for microvessel detection in engineered bone of craniofacial districts. Front Physiol. 2017;8:769. doi: 10.3389/fphys.2017.00769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campi G, Fratini M, Bukreeva I, et al. Imaging collagen packing dynamics during mineralization of engineered bone tissue. Acta Biomater. 2015;23:309–316. doi: 10.1016/j.actbio.2015.05.033 [DOI] [PubMed] [Google Scholar]
- 34.Giannoudis PV, Faour O, Goff T, Kanakaris N, Dimitriou R. Masquelet technique for the treatment of bone defects: tips-tricks and future directions. Injury. 2011;42:591–598. doi: 10.1016/j.injury.2011.03.036 [DOI] [PubMed] [Google Scholar]
- 35.Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21:571–576. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162. doi: 10.1016/j.joca.2008.02.013 [DOI] [PubMed] [Google Scholar]
- 37.Colen S, van Den Bekerom MPJ, Mulier M, Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis. BioDrugs. 2012;26:257–268. doi: 10.1007/BF03261884 [DOI] [PubMed] [Google Scholar]
- 38.Basad E, Wissing FR, Fehrenbach P, Rickert M, Steinmeyer J, Ishaque B. Matrix-induced autologous chondrocyte implantation (MACI) in the knee: clinical outcomes and challenges. Knee Surg Sport Traumatol Arthrosc. 2015;23:3729–3735. doi: 10.1007/s00167-014-3295-8 [DOI] [PubMed] [Google Scholar]
- 39.Petersen JP, Ruecker A, von Stechow D, et al. Present and Future therapies of articular cartilage defects. Eur J Trauma. 2003;29:1–10. doi: 10.1007/s00068-003-1215-6 [DOI] [Google Scholar]
- 40.Seebach C, Schultheiss J, Wilhelm K, Frank J, Henrich D. Comparison of six bone-graft substitutes regarding to cell seeding efficiency, metabolism and growth behaviour of human mesenchymal stem cells (MSC) in vitro. Injury. 2010;41:731–738. doi: 10.1016/S0020-1383(10)70016-4 [DOI] [PubMed] [Google Scholar]
- 41.Roberts TT, Rosenbaum AJ. Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8:114–124. doi: 10.4161/org.23306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afshar A. Reimplantation of a large extruded segment of bone in an open fracture. J Hand Surg Am. 2017;42:128–134. doi: 10.1016/j.jhsa.2016.11.024 [DOI] [PubMed] [Google Scholar]
- 43.Shanmuganathan R, Chandra Mohan AK, Agraharam D, Perumal R, Jayaramaraju D, Kulkarni S. Successful reimplantation of extruded long bone segments in open fractures of lower limb – A report of 3 cases. Injury. 2015;46:1389–1392. doi: 10.1016/j.injury.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 44.Masquelet AC, Fitoussi F, Begue T, Muller GP. [Reconstruction of the long bones by the induced membrane and spongy autograft]. Ann Chir Plast Esthet. 2000;45:346–353. [PubMed] [Google Scholar]
- 45.Guerado E, Fuerstenberg CH. What bone graft substitutes should we use in post-traumatic spinal fusion? Injury. 2011;42:S64–S71. doi: 10.1016/j.injury.2011.06.200 [DOI] [PubMed] [Google Scholar]
- 46.Drosos GI, Touzopoulos P, Ververidis A, Tilkeridis K, Kazakos K. Use of demineralized bone matrix in the extremities. World J Orthop. 2015;6:269–277. doi: 10.5312/wjo.v6.i2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White SL, Hirth R, Mahíllo B, et al. The global diffusion of organ transplantation: trends, drivers and policy implications. Bull World Health Organ. 2014;92:826–835. doi: 10.2471/BLT.13.126128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barradas AMC, Yuan H, van Blitterswijk CA, Habibovic P. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater. 2011;21:407–29; discussion 429. doi: 10.22203/eCM.v021a31 [DOI] [PubMed] [Google Scholar]
- 49.Balagangadharan K, Dhivya S, Selvamurugan N. Chitosan based nanofibers in bone tissue engineering. Int J Biol Macromol. 2017;104:1372–1382. doi: 10.1016/j.ijbiomac.2016.12.046 [DOI] [PubMed] [Google Scholar]
- 50.Hinderer S, Layland SL, Schenke-Layland K. ECM and ECM-like materials - Biomaterials for applications in regenerative medicine and cancer therapy. Adv Drug Deliv Rev. 2016;97:260–269. doi: 10.1016/j.addr.2015.11.019 [DOI] [PubMed] [Google Scholar]
- 51.Solorio L, Zwolinski C, Lund AW, Farrell MJ, Stegemann JP. Gelatin microspheres crosslinked with genipin for local delivery of growth factors. J Tissue Eng Regen Med. 2010;4:514–523. doi: 10.1002/term.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xavier JR, Thakur T, Desai P, et al. Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS Nano. 2015;9:3109–3118. doi: 10.1021/nn507282f [DOI] [PubMed] [Google Scholar]
- 53.Walmsley GG, Ransom RC, Zielins ER, et al. Stem cells in bone regeneration. Stem Cell Rev. 2016;12:524–529. doi: 10.1007/s12015-016-9665-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lv D, Zhu M, Jiang Z, et al. Green electrospun nanofibers and their application in air filtration. Macromol Mater Eng. 2018;303:1800336. doi: 10.1002/mame.v303.12 [DOI] [Google Scholar]
- 55.Lv D, Wang R, Tang G, et al. Ecofriendly electrospun membranes loaded with visible-light-responding nanoparticles for multifunctional usages: highly efficient air filtration, dye scavenging, and bactericidal activity. ACS Appl Mater Interfaces. 2019;11:12880–12889. doi: 10.1021/acsami.9b01508 [DOI] [PubMed] [Google Scholar]
- 56.Cui W, Li X, Xie C, Zhuang H, Zhou S, Weng J. Hydroxyapatite nucleation and growth mechanism on electrospun fibers functionalized with different chemical groups and their combinations. Biomaterials. 2010;31:4620–4629. doi: 10.1016/j.biomaterials.2010.01.042 [DOI] [PubMed] [Google Scholar]
- 57.Lee JY, Bashur CA, Goldstein AS, Schmidt CE. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials. 2009;30:4325–4335. doi: 10.1016/j.biomaterials.2009.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Y, Yang D, Chen X, Xu Q, Lu F, Nie J. Electrospun water-soluble Carboxyethyl Chitosan/Poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules. 2008;9:349–354. doi: 10.1021/bm7009015 [DOI] [PubMed] [Google Scholar]
- 59.Hsiao C-W, Bai M-Y, Chang Y, et al. Electrical coupling of isolated cardiomyocyte clusters grown on aligned conductive nanofibrous meshes for their synchronized beating. Biomaterials. 2013;34:1063–1072. doi: 10.1016/j.biomaterials.2012.10.065 [DOI] [PubMed] [Google Scholar]
- 60.Jang J-H, Castano O, Kim H-W. Electrospun materials as potential platforms for bone tissue engineering. Adv Drug Deliv Rev. 2009;61:1065–1083. doi: 10.1016/j.addr.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 61.Li C, Vepari C, Jin H-J, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–3124. doi: 10.1016/j.biomaterials.2006.01.022 [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Venugopal JR, El-Turki A, Ramakrishna S, Su B, Lim CT. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials. 2008;29:4314–4322. doi: 10.1016/j.biomaterials.2008.07.038 [DOI] [PubMed] [Google Scholar]
- 63.Li X, Xie J, Yuan X, Xia Y. Coating electrospun Poly(ε-caprolactone) fibers with gelatin and calcium phosphate and their use as biomimetic scaffolds for bone tissue engineering. Langmuir. 2008;24:14145–14150. doi: 10.1021/la802984a [DOI] [PubMed] [Google Scholar]
- 64.Bhattacharyya S, Kumbar SG, Khan YM, et al. Biodegradable polyphosphazene-nanohydroxyapatite composite nanofibers: scaffolds for bone tissue engineering. J Biomed Nanotechnol. 2009;5:69–75. doi: 10.1166/jbn.2009.032 [DOI] [PubMed] [Google Scholar]
- 65.Haisch A, Wanjura F, Radke C, et al. Immunomodulation of tissue-engineered transplants: in vivo bone generation from methylprednisolone-stimulated chondrocytes. Eur Arch Oto-Rhino-Laryngol. 2004;261:216–224. doi: 10.1007/s00405-003-0646-3 [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Cui W, Chou J, Wen S, Sun Y, Zhang H. Electrospun nanosilicates-based organic/inorganic nanofibers for potential bone tissue engineering. Colloids Surfaces B. 2018;172:90–97. doi: 10.1016/j.colsurfb.2018.08.024 [DOI] [PubMed] [Google Scholar]
- 67.Cai YZ, Zhang GR, Wang LL, Jiang YZ, Ouyang HW, Zou XH. Novel biodegradable three-dimensional macroporous scaffold using aligned electrospun nanofibrous yarns for bone tissue engineering. J Biomed Mater Res Part A. 2012;100 A:1187–1194. doi: 10.1002/jbm.a.34063 [DOI] [PubMed] [Google Scholar]
- 68.Narayanan G, Vernekar VN, Kuyinu EL, Laurencin CT. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv Drug Deliv Rev. 2016;107:247–276. doi: 10.1016/j.addr.2016.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peach MS, Ramos DM, James R, et al. Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering. PLoS One. 2017;12:e0174789. doi: 10.1371/journal.pone.0174789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ito Y, Ochi M, Adachi N, et al. Repair of osteochondral defect with tissue-engineered chondral plug in a rabbit model. Arthrosc J Arthrosc Relat Surg. 2005;21:1155–1163. doi: 10.1016/j.arthro.2005.06.016 [DOI] [PubMed] [Google Scholar]
- 71.Binulal NS, Natarajan A, Menon D, Bhaskaran VK, Mony U, Nair SV. PCL–gelatin composite nanofibers electrospun using diluted acetic acid–ethyl acetate solvent system for stem cell-based bone tissue engineering. J Biomater Sci Polym Ed. 2014;25:325–340. doi: 10.1080/09205063.2013.859872 [DOI] [PubMed] [Google Scholar]
- 72.Hwang PTJ, Murdock K, Alexander GC, et al. Poly(ɛ-caprolactone)/gelatin composite electrospun scaffolds with porous crater-like structures for tissue engineering. J Biomed Mater Res Part A. 2016;104:1017–1029. doi: 10.1002/jbm.a.35614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee JH, Park J-H, El-Fiqi A, et al. Biointerface control of electrospun fiber scaffolds for bone regeneration: engineered protein link to mineralized surface. Acta Biomater. 2014;10:2750–2761. doi: 10.1016/j.actbio.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 74.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/S0142-9612(02)00635-X [DOI] [PubMed] [Google Scholar]
- 75.Fang R, Zhang E, Xu L, Wei S. Electrospun PCL/PLA/HA based nanofibers as scaffold for osteoblast-like cells. J Nanosci Nanotechnol. 2010;10:7747–7751. doi: 10.1166/jnn.2010.2831 [DOI] [PubMed] [Google Scholar]
- 76.Eap S, Ferrand A, Palomares CM, et al. Electrospun nanofibrous 3D scaffold for bone tissue engineering. Biomed Mater Eng. 2012;22:137–141. [DOI] [PubMed] [Google Scholar]
- 77.Xu T, Miszuk JM, Zhao Y, Sun H, Electrospun Polycaprolactone FH. 3D nanofibrous scaffold with interconnected and hierarchically structured pores for bone tissue engineering. Adv Healthc Mater. 2015;4:2238–2246. doi: 10.1002/adhm.201500345 [DOI] [PubMed] [Google Scholar]
- 78.Li L, Zhou G, Wang Y, Yang G, Ding S, Zhou S. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect. Biomaterials. 2015;37:218–229. doi: 10.1016/j.biomaterials.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 79.Daňková J, Buzgo M, Vejpravová J, et al. Highly efficient mesenchymal stem cell proliferation on poly-ε-caprolactone nanofibers with embedded magnetic nanoparticles. Int J Nanomed. 2015;10:7307–7317. doi: 10.2147/IJN.S93670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Hong Thien D, Hsiao SW, Ho MH, Li CH, Shih JL. Electrospun chitosan/hydroxyapatite nanofibers for bone tissue engineering. J Mater Sci. 2013;48:1640–1645. doi: 10.1007/s10853-012-6921-1 [DOI] [Google Scholar]
- 81.Frohbergh ME, Katsman A, Mondrinos MJ, et al. Osseointegrative properties of electrospun hydroxyapatite-containing nanofibrous chitosan scaffolds. Tissue Eng. Part A. 2015;21:970–981. doi: 10.1089/ten.tea.2013.0789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mi H-Y, Palumbo S, Jing X, Turng L-S, Li W-J, Peng X-F. Thermoplastic polyurethane/hydroxyapatite electrospun scaffolds for bone tissue engineering: effects of polymer properties and particle size. J Biomed Mater Res B Appl Biomater. 2014;102:1434–1444. doi: 10.1002/jbm.b.33122 [DOI] [PubMed] [Google Scholar]
- 83.Vozzi G, Corallo C, Carta S, et al. Collagen-gelatin-genipin-hydroxyapatite composite scaffolds colonized by human primary osteoblasts are suitable for bone tissue engineering applications: in vitro evidences. J Biomed Mater Res Part A. 2014;102:1415–1421. doi: 10.1002/jbm.a.34823 [DOI] [PubMed] [Google Scholar]
- 84.Zhang X, Meng S, Huang Y, et al. Electrospun Gelatin/β-TCP composite nanofibers enhance osteogenic differentiation of BMSCs and in vivo bone formation by activating Ca2+ sensing receptor signaling. Stem Cells Int. 2015;2015:1–13. doi: 10.1155/2015/328957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y-W, Yang F, Wu Q, et al. Effect of composition of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) on growth of fibroblast and osteoblast. Biomaterials. 2005;26:755–761. doi: 10.1016/j.biomaterials.2004.03.023 [DOI] [PubMed] [Google Scholar]
- 86.Chen J-P, Chen S-H, Lai G-J. Preparation and characterization of biomimetic silk fibroin/chitosan composite nanofibers by electrospinning for osteoblasts culture. Nanoscale Res Lett. 2012;7:170. doi: 10.1186/1556-276X-7-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu J, Zheng A, Liu Y, et al. Enhanced bone regeneration of the silk fibroin electrospun scaffolds through the modification of the graphene oxide functionalized by BMP-2 peptide. Int J Nanomed. 2019;14:733–751. doi: 10.2147/IJN.S187664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu B, Li Y, Deng B, Liu X, Wang L, Zhu QL. Chitosan hydrogel improves mesenchymal stem cell transplant survival and cardiac function following myocardial infarction in rats. Exp Ther Med. 2017;13:588–594. doi: 10.3892/etm.2017.4026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ding Q, Xu X, Yue Y, et al. Nanocellulose-mediated electroconductive self-healing hydrogels with high strength, plasticity, viscoelasticity, stretchability, and biocompatibility toward multifunctional applications. ACS Appl Mater Interfaces. 2018;10:27987–28002. doi: 10.1021/acsami.8b09656 [DOI] [PubMed] [Google Scholar]
- 90.Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hayashi C, Hasegawa U, Saita Y, et al. Osteoblastic bone formation is induced by using nanogel-crosslinking hydrogel as novel scaffold for bone growth factor. J Cell Physiol. 2009;220:1–7. doi: 10.1002/jcp.v220:1 [DOI] [PubMed] [Google Scholar]
- 92.Qasim M, Baipaywad P, Udomluck N, Na D, Park H. Enhanced therapeutic efficacy of lipophilic amphotericin B against Candida albicans with amphiphilic poly(N-isopropylacrylamide) nanogels. Macromol Res. 2014;22:1125–1131. doi: 10.1007/s13233-014-2162-2 [DOI] [Google Scholar]
- 93.Wang J, Hurren C, Sutti A, Lin T, Wang X. Thermo-responsive PNIPAM nanofibres crosslinked by OpePOSS. Proc SPIE. 2013;8793:879318. [Google Scholar]
- 94.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater. 2009;21:3307–3329. doi: 10.1002/adma.v21:32/33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076 [DOI] [PubMed] [Google Scholar]
- 96.Celikkin N, Mastrogiacomo S, Jaroszewicz J, Walboomers XF, Swieszkowski W. Gelatin methacrylate scaffold for bone tissue engineering: the influence of polymer concentration. J Biomed Mater Res Part A. 2018;106:201–209. doi: 10.1002/jbm.a.36226 [DOI] [PubMed] [Google Scholar]
- 97.Crescenzi V, Cornelio L, Di Meo C, Nardecchia S, Lamanna R. Novel hydrogels via click chemistry: synthesis and potential biomedical applications. Biomacromolecules. 2007;8:1844–1850. doi: 10.1021/bm700879t [DOI] [PubMed] [Google Scholar]
- 98.Seebach E, Freischmidt H, Holschbach J, Fellenberg J, Richter W. Mesenchymal stroma cells trigger early attraction of M1 macrophages and endothelial cells into fibrin hydrogels, stimulating long bone healing without long-term engraftment. Acta Biomater. 2014;10:4730–4741. doi: 10.1016/j.actbio.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 99.Chang SC-N, Tai C-L, Chung H-Y, Lin T-M, Jeng L-B. Bone marrow mesenchymal stem cells form ectopic woven bone in vivo through endochondral bone formation. Artif Organs. 2009;33:301–308. doi: 10.1111/j.1525-1594.2009.00728.x [DOI] [PubMed] [Google Scholar]
- 100.Park H, Choi B, Hu J, Lee M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater. 2013;9:4779–4786. doi: 10.1016/j.actbio.2012.10.038 [DOI] [PubMed] [Google Scholar]
- 101.Naderi-Meshkin H, Andreas K, Matin MM, et al. Chitosan-based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol Int. 2014;38:72–84. doi: 10.1002/cbin.10181 [DOI] [PubMed] [Google Scholar]
- 102.Sá-Lima H, Tuzlakoglu K, Mano JF, Reis RL. Thermoresponsive poly(N-isopropylacrylamide)-g-methylcellulose hydrogel as a three-dimensional extracellular matrix for cartilage-engineered applications. J Biomed Mater Res Part A. 2011;98A:596–603. doi: 10.1002/jbm.a.33140 [DOI] [PubMed] [Google Scholar]
- 103.Moreira CDF, Carvalho SM, Mansur HS, Pereira MM. Thermogelling chitosan–collagen–bioactive glass nanoparticle hybrids as potential injectable systems for tissue engineering. Mater Sci Eng C. 2016;58:1207–1216. doi: 10.1016/j.msec.2015.09.075 [DOI] [PubMed] [Google Scholar]
- 104.Kamoun EA. N-succinyl chitosan–dialdehyde starch hybrid hydrogels for biomedical applications. J Adv Res. 2016;7:69–77. doi: 10.1016/j.jare.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Santo VE, Gomes ME, Mano JF, Reis RL. Chitosan-chondroitin sulphate nanoparticles for controlled delivery of platelet lysates in bone regenerative medicine. J Tissue Eng Regen Med. 2012;6:s47–s59. doi: 10.1002/term.v6.S3 [DOI] [PubMed] [Google Scholar]
- 106.Heo DN, Ko W-K, Bae MS, et al. Enhanced bone regeneration with a gold nanoparticle–hydrogel complex. J Mater Chem B. 2014;2:1584–1593. doi: 10.1039/C3TB21246G [DOI] [PubMed] [Google Scholar]
- 107.Oh BHL, Bismarck A, Chan-Park MB. Injectable, interconnected, high-porosity macroporous biocompatible gelatin scaffolds made by surfactant-free emulsion templating. Macromol Rapid Commun. 2015;36:364–372. doi: 10.1002/marc.201400524 [DOI] [PubMed] [Google Scholar]
- 108.Geng X, Mo X, Fan L, Yin A, Fang J. Hierarchically designed injectable hydrogel from oxidized dextran, amino gelatin and 4-arm poly(ethylene glycol)-acrylate for tissue engineering application. J Mater Chem. 2012;22:25130. doi: 10.1039/c2jm34737g [DOI] [Google Scholar]
- 109.Steck E, Fischer J, Lorenz H, Gotterbarm T, Jung M, Richter W. Mesenchymal stem cell differentiation in an experimental cartilage defect: restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 2009;18:969–978. doi: 10.1089/scd.2008.0213 [DOI] [PubMed] [Google Scholar]
- 110.Moshaverinia A, Chen C, Akiyama K, et al. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J Biomed Mater Res Part A. 2013. doi: 10.1002/jbm.a.34546 [DOI] [PubMed] [Google Scholar]
- 111.Yu F, Cao X, Li Y, Zeng L, Yuan B, Chen X. An injectable hyaluronic acid/PEG hydrogel for cartilage tissue engineering formed by integrating enzymatic crosslinking and Diels–Alder “click chemistry.”. Polym Chem. 2014;5:1082–1090. doi: 10.1039/C3PY00869J [DOI] [Google Scholar]
- 112.Lyu C-Q, Lu J-Y, Cao C-H, et al. Induction of osteogenic differentiation of human adipose-derived stem cells by a novel self-supporting graphene hydrogel film and the possible underlying mechanism. ACS Appl Mater Interfaces. 2015;7:20245–20254. doi: 10.1021/acsami.5b05802 [DOI] [PubMed] [Google Scholar]
- 113.Mohan YM, Premkumar T, Lee K, Geckeler KE. Fabrication of silver nanoparticles in hydrogel networks. Macromol Rapid Commun. 2006;27:1346–1354. doi: 10.1002/(ISSN)1521-3927 [DOI] [Google Scholar]
- 114.Xu G, Wang X, Deng C, et al. Injectable biodegradable hybrid hydrogels based on thiolated collagen and oligo(acryloyl carbonate)–poly(ethylene glycol)–oligo(acryloyl carbonate) copolymer for functional cardiac regeneration. Acta Biomater. 2015;15:55–64. doi: 10.1016/j.actbio.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 115.Xidaki D, Agrafioti P, Diomatari D, et al. Synthesis of hydroxyapatite, β-tricalcium phosphate and biphasic calcium phosphate particles to act as local delivery carriers of curcumin: loading, release and in vitro studies. Materials (Basel). 2018;11:595. doi: 10.3390/ma11081451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garrido CA, Lobo SE, Turíbio FM, Legeros RZ. Biphasic calcium phosphate bioceramics for orthopaedic reconstructions: clinical outcomes. Int J Biomater. 2011;2011:129727. doi: 10.1155/2011/129727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mihaila SM, Gaharwar AK, Reis RL, Khademhosseini A, Marques AP, Gomes ME. The osteogenic differentiation of SSEA-4 sub-population of human adipose derived stem cells using silicate nanoplatelets. Biomaterials. 2014;35:9087–9099. doi: 10.1016/j.biomaterials.2014.01.026 [DOI] [PubMed] [Google Scholar]
- 118.Carrow JK, Cross LM, Reese RW, et al. Widespread changes in transcriptome profile of human mesenchymal stem cells induced by two-dimensional nanosilicates. Proc Natl Acad Sci. 2018;115:E3905–E3913. doi: 10.1073/pnas.1716164115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kerativitayanan P, Tatullo M, Khariton M, Joshi P, Perniconi B, Gaharwar AK. Nanoengineered osteoinductive and elastomeric scaffolds for bone tissue engineering. ACS Biomater Sci Eng. 2017;3:590–600. doi: 10.1021/acsbiomaterials.7b00029 [DOI] [PubMed] [Google Scholar]
- 120.Zhong M, Liu YT, Liu XY, et al. Dually cross-linked single network poly(acrylic acid) hydrogels with superior mechanical properties and water absorbency. Soft Matter. 2016;12:5420–5428. doi: 10.1039/c5sm02053k [DOI] [PubMed] [Google Scholar]
- 121.Qasim M, Udomluck N, Chang J, Park H, Kim K. Antimicrobial activity of silver nanoparticles encapsulated in poly-N-isopropylacrylamide-based polymeric nanoparticles. Int J Nanomed. 2018;13:235–249. doi: 10.2147/IJN.S177627 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122.Howk D, Chu T-MG. Design variables for mechanical properties of bone tissue scaffolds. Biomed Sci Instrum. 2006;42:278–283. [PubMed] [Google Scholar]
- 123.Bai X, Gao M, Syed S, Zhuang J, Xu X, Zhang X-Q. Bioactive hydrogels for bone regeneration. Bioact Mater. 2018;3:401–417. doi: 10.1016/j.bioactmat.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nuttelman CR, Tripodi MC, Anseth KS. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J Biomed Mater Res. 2004;68A:773–782. doi: 10.1002/(ISSN)1097-4636 [DOI] [PubMed] [Google Scholar]
- 125.Ovsianikov A, Deiwick A, Van Vlierberghe S, et al. Laser fabrication of three-dimensional CAD scaffolds from photosensitive gelatin for applications in tissue engineering. Biomacromolecules. 2011;12:851–858. doi: 10.1021/bm1015305 [DOI] [PubMed] [Google Scholar]
- 126.Cao L, Cao B, Lu C, Wang G, Yu L, Ding J. An injectable hydrogel formed by in situ cross-linking of glycol chitosan and multi-benzaldehyde functionalized PEG analogues for cartilage tissue engineering. J Mater Chem B. 2015;3:1268–1280. doi: 10.1039/C4TB02051K [DOI] [PubMed] [Google Scholar]
- 127.Ma Y-H, Yang J, Li B, Jiang Y-W, Lu X, Chen Z. Biodegradable and injectable polymer–liposome hydrogel: a promising cell carrier. Polym Chem. 2016;7:2037–2044. doi: 10.1039/C5PY01773D [DOI] [Google Scholar]
- 128.Qasim M, Haq F, Kang MH, Kim JH. 3D printing approaches for cardiac tissue engineering and role of immune modulation in tissue regeneration. Int J Nanomed. 2019;14:1311–1333. doi: 10.2147/IJN.S189587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Mori A, Peña Fernández M, Blunn G, et al. 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers (Basel). 2018;10:285. doi: 10.3390/polym10030285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.An J, Teoh JEM, Suntornnond R, Chua CK. Design and 3D printing of scaffolds and tissues. Engineering. 2015;1:261–268. doi: 10.15302/J-ENG-2015061 [DOI] [Google Scholar]
- 131.Zhang L, Yang G, Johnson BN, Jia X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84:16–33. doi: 10.1016/j.actbio.2018.11.039 [DOI] [PubMed] [Google Scholar]
- 132.Bracaglia LG, Smith BT, Watson E, Arumugasaamy N, Mikos AG, Fisher JP. 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater. 2017;56:3–13. doi: 10.1016/j.actbio.2017.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Turnbull G, Clarke J, Picard F, et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018;3:278–314. doi: 10.1016/j.bioactmat.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nandi SK, Fielding G, Banerjee D, Bandyopadhyay A, Bose S. 3D-printed β-TCP bone tissue engineering scaffolds: effects of chemistry on in vivo biological properties in a rabbit tibia model. J Mater Res. 2018;33:1939–1947. doi: 10.1557/jmr.2018.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Trachtenberg JE, Placone JK, Smith BT, Fisher JP, Mikos AG. Extrusion-based 3D printing of poly(propylene fumarate) scaffolds with hydroxyapatite gradients. J Biomater Sci Polym Ed. 2017;28:532–554. doi: 10.1080/09205063.2017.1286184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao G, Schilling AF, Hubbell K, et al. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol Lett. 2015;37:2349–2355. doi: 10.1007/s10529-015-1921-2 [DOI] [PubMed] [Google Scholar]
- 137.Yoon S, Park JA, Lee HR, Yoon WH, Hwang DS, Jung S. Inkjet–spray hybrid printing for 3D freeform fabrication of multilayered hydrogel structures. Adv Healthc Mater. 2018;7:1–10. [DOI] [PubMed] [Google Scholar]
- 138.Sawkins MJ, Mistry P, Brown BN, Shakesheff KM, Bonassar LJ, Yang J. Cell and protein compatible 3D bioprinting of mechanically strong constructs for bone repair. Biofabrication. 2015;7:035004. doi: 10.1088/1758-5090/7/3/035004 [DOI] [PubMed] [Google Scholar]
- 139.Gao G, Yonezawa T, Hubbell K, Dai G, Cui X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol J. 2015;10:1568–1577. doi: 10.1002/biot.201400635 [DOI] [PubMed] [Google Scholar]
- 140.Yin J, Yan M, Wang Y, Fu J, Suo H. 3D bioprinting of low-concentration cell-laden gelatin methacrylate (GelMA) bioinks with a two-step cross-linking strategy. ACS Appl Mater Interfaces. 2018;10:6849–6857. doi: 10.1021/acsami.7b16059 [DOI] [PubMed] [Google Scholar]
- 141.Daly AC, Pitacco P, Nulty J, Cunniffe GM, Kelly DJ. 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials. 2018;162:34–46. doi: 10.1016/j.biomaterials.2018.01.057 [DOI] [PubMed] [Google Scholar]
- 142.Cui H, Zhu W, Nowicki M, Zhou X, Khademhosseini A, Zhang LG. Hierarchical fabrication of engineered vascularized bone biphasic constructs via dual 3D bioprinting: integrating regional bioactive factors into architectural design. Adv Healthc Mater. 2016;5:2174–2181. doi: 10.1002/adhm.201600505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reichert JC, Wullschleger ME, Cipitria A, et al. Custom-made composite scaffolds for segmental defect repair in long bones. Int Orthop. 2011;35:1229–1236. doi: 10.1007/s00264-010-1146-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang MO, Piard CM, Melchiorri A, Dreher ML, Fisher JP. Evaluating changes in structure and cytotoxicity during in vitro degradation of three-dimensional printed scaffolds. Tissue Eng. Part A. 2015;21:1642–1653. doi: 10.1089/ten.tea.2014.0495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kao C-T, Lin -C-C, Chen Y-W, Yeh C-H, Fang H-Y, Shie M-Y. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Mater Sci Eng C. 2015;56:165–173. doi: 10.1016/j.msec.2015.06.028 [DOI] [PubMed] [Google Scholar]
- 146.Kuo Y-C, Yeh C-F. Effect of surface-modified collagen on the adhesion, biocompatibility and differentiation of bone marrow stromal cells in poly(lactide-co-glycolide)/chitosan scaffolds. Colloids Surfaces B. 2011;82:624–631. doi: 10.1016/j.colsurfb.2010.10.032 [DOI] [PubMed] [Google Scholar]
- 147.Zhang H, Mao X, Du Z, et al. Three dimensional printed macroporous polylactic acid/hydroxyapatite composite scaffolds for promoting bone formation in a critical-size rat calvarial defect model. Sci Technol Adv Mater. 2016;17:136–148. doi: 10.1080/14686996.2016.1145532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao W, Wang J, Zhai W, Wang Z, Chang J. The self-setting properties and in vitro bioactivity of tricalcium silicate. Biomaterials. 2005;26:6113–6121. doi: 10.1016/j.biomaterials.2005.04.025 [DOI] [PubMed] [Google Scholar]
- 149.Yang C, Wang X, Ma B, et al. 3D-printed bioactive ca3sio5 bone cement scaffolds with nano surface structure for bone regeneration. ACS Appl Mater Interfaces. 2017;9:5757–5767. doi: 10.1021/acsami.6b14297 [DOI] [PubMed] [Google Scholar]
- 150.Zhu H, Zhai D, Lin C, et al. 3D plotting of highly uniform Sr5(PO4)2SiO4 bioceramic scaffolds for bone tissue engineering. J Mater Chem B. 2016;4:6200–6212. doi: 10.1039/C6TB01692H [DOI] [PubMed] [Google Scholar]
- 151.Roohani-Esfahani S-I, Newman P, Zreiqat H. Design and fabrication of 3D printed scaffolds with a mechanical strength comparable to cortical bone to repair large bone defects. Sci Rep. 2016;6:19468. doi: 10.1038/srep19468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barui S, Chatterjee S, Mandal S, Kumar A, Basu B. Microstructure and compression properties of 3D powder printed Ti-6Al-4V scaffolds with designed porosity: experimental and computational analysis. Mater Sci Eng C. 2017;70:812–823. doi: 10.1016/j.msec.2016.09.040 [DOI] [PubMed] [Google Scholar]
- 153.Miao S, Zhu W, Castro NJ, et al. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci Rep. 2016;6:27226. doi: 10.1038/srep27226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Su J-W, Tao X, Deng H, et al. 4D printing of a self-morphing polymer driven by a swellable guest medium. Soft Matter. 2018;14:765–772. doi: 10.1039/c8sm00535d [DOI] [PubMed] [Google Scholar]
- 155.Hendrikson WJ, Rouwkema J, Clementi F, van Blitterswijk CA, Farè S, Moroni L. Towards 4D printed scaffolds for tissue engineering: exploiting 3D shape memory polymers to deliver time-controlled stimulus on cultured cells. Biofabrication. 2017;9:031001. doi: 10.1088/1758-5090/aa8114 [DOI] [PubMed] [Google Scholar]
- 156.Wang MO, Vorwald CE, Dreher ML, et al. Evaluating 3D-printed biomaterials as scaffolds for vascularized bone tissue engineering. Adv Mater. 2015;27:138–144. doi: 10.1002/adma.201403943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miao S, Castro N, Nowicki M, et al. 4D printing of polymeric materials for tissue and organ regeneration. Mater Today. 2017;20:577–591. doi: 10.1016/j.mattod.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Geris L. In Vivo, in Vitro, in Silico: Computational Tools for Product and Process Design in Tissue Engineering. 2012:1–15. [Google Scholar]