Abstract
Purpose.
Extracellular protein toxins contribute to the pathogenesis of Staphylococcus aureus infections. The present study compared the effects of iclaprim and trimethoprim – two folic acid synthesis inhibitors – with nafcillin and vancomycin on production of Panton–Valentine leukocidin (PVL), alpha haemolysin (AH) and toxic-shock syndrome toxin I (TSST-1) in methicillin-resistant and vancomycin-intermediate S. aureus (MRSA and VISA, respectively).
Methodology.
Northern blotting and RT-PCR were used to assess gene transcription; toxin-specific bioassays were used to measure protein toxin production.
Results.
As shown previously, sub-inhibitory concentrations (sub-MIC) of nafcillin increased and prolonged MRSA toxin gene transcription and enhanced PVL, TSST-1 and AH production. Sub-inhibitory doses of iclaprim and trimethoprim delayed maximal AH gene (hla) transcription and suppressed AH production; both drugs delayed, but neither reduced, maximal TSST-1 production. Trimethoprim significantly increased lukF-PV expression and PVL production compared to both untreated and iclaprim-treated cultures. Higher concentrations of iclaprim and trimethoprim markedly suppressed MRSA growth, mRNA synthesis and toxin production. In VISA, iclaprim, vancomycin and nafcillin variably increased tst and hla expression, but only nafcillin increased toxin production. Despite its ability to increase hla expression, iclaprim was the most potent inhibitor of AH production.
Conclusions.
We conclude that, due to its ability to suppress toxin production, iclaprim should be effective against severe staphylococcal infections caused by toxin-producing MRSA and VISA strains, especially given its ability to concentrate at sites of infection such as skin and skin structures and the lung.
Keywords: Iclaprim, MRSA, VISA, Staphylococcus aureus, TSST-1, Panton–Valentine leukocidin, alpha haemolysin
Introduction
Folic acid plays a central role in anabolic metabolism by supplying single-carbon units at varied levels of oxidation for both nucleotide and amino acid biosynthesis. By blocking the enzyme dihydrofolate reductase (DHFR), folic acid inhibitor antibiotics, such as trimethoprim and iclaprim, deplete the available intracellular tetrahydrofolate (the active form of folic acid), thereby blocking the formation of thymidylate, purines, the amino acids methionine and glycine, and several other cell constituents [1, 2]. Lack of thymidylate, one of the precursors to DNA, disrupts DNA synthesis and cell growth ceases. DHFR inhibitor antibiotics have efficacy against Gram-positive and Gram-negative bacteria, both in vitro and in vivo and are classically considered bacteriostatic agents [1, 3]. However, halting the division of bacteria does not necessarily affect the duration of bacterial viability. This raises the possibility that such agents may have novel and clinically important effects on bacteria during antibiotic-induced stasis. Specifically, exotoxins that mediate the pathogenesis of Gram-positive infections are usually produced late in the bacterial growth cycle. It is possible that folic acid inhibitor antibiotics could arrest the growth cycle before the onset of this phase and therefore prevent production of these important virulence factors. Alternatively, these drugs could directly affect toxin production by interfering with the synthesis of mRNA. Thus, the efficacy of folic acid inhibitor antibiotics could be related to two factors: reduction of bacterial load by preventing bacterial replication and inhibition of toxin production by decreased intracellular folate availability.
In contrast, we have demonstrated that sub-inhibitory concentrations of beta-lactam antibiotics increase and prolong production of toxin-specific mRNA in methicillin-sensitive and -resistant S. aureus [4]. Such enhanced mRNA production translated directly into increased and sustained toxin production [4]. Toxins affected in this way included alpha haemolysin (AH), toxic shock syndrome toxin-1 (TSST-1) and Panton–Valentine leukocidin (PVL). We hypothesize that this phenomenon is, in part, related to a general stress response of the bacterium and that this response is directly related to systems controlling the growth cycle. We have further shown that protein synthesis inhibitors, such as clindamycin and linezolid, increased toxin gene transcription but blocked translation of this message at the ribosomal level [4].
In the current study, we compared the effects of two folic acid inhibitor antibiotics (iclaprim and trimethoprim) with the cell-wall-active agents, nafcillin and vancomycin on production of alpha toxin, TSST-1, and PVL in methicillin-resistant and vancomycin-intermediate S. aureus (MRSA and VISA, respectively).
Methods
Strains and culture conditions
Exotoxin profiles in S. aureus are strain-specific. Most strains produce alpha haemolysin (AH); however the ability to produce TSST-1 or PVL varies by strain [5], and co-production of both TSST-1 and PVL by a given S. aureus isolate is exceedingly rare [6]. Therefore, for the studies presented here, two clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) having distinct exotoxin profiles and one vancomycin-intermediate S. aureus (VISA) strain were used (Table 1). MRSA strain 04–014 was obtained from the Centers for Disease Control and Prevention (CDC 368–04). This strain was isolated from a case of community-acquired (CA)-MRSA infection and produces staphylococcal enterotoxin C, TSST-1 and alpha haemolysin but is negative for PVL. No multi-locus sequence typing or agr analysis was done on this strain. PFGE analysis was performed but did not yield a match (within 80%) to any known USA type. It was most closely related to strains of the USA 600 type (R. Carey, personal communication). Second, MRSA 1560 was provided by Dr Francoise Perdreau-Remington, University of California, San Francisco [7]. This CA-MRSA strain was isolated from a wound and belongs to ST1 (USA 400); its PFGE pattern is similar, but not identical to that of MW2; and it is alpha haemolysin- and PVL-positive and contains SCCmec IV and agr III (Table 1). Lastly, VISA strain Mu50 was obtained from the American Type Culture Collection, Manassas, VA (product 700699). Mu50 was originally isolated in 1997 from a 4-month-old male child with a MRSA wound infection who responded poorly to vancomycin therapy [8]. This strain was originally described as vancomycin-resistant with an MIC=8 µg ml−1 [8]; however strains with vancomycin MICs of 4–8 µg ml−1 were reclassified in 2006 as vancomycin-intermediate by the Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS) [9]. Mu50 carries the type II allelic form of the methicillin-resistance gene, mecA [10] and belongs to accessory gene regulator (agr) group II [11] (Table 1).
Table 1.
Characteristics of strains used in the present study
|
Strain |
Response to methicillin |
mecA type |
agr type |
MIC (μg ml− 1) |
Toxin profile (by gene probe) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Iclaprim |
Trimethoprim |
Nafcillin |
Vancomycin |
PVL |
TSST-1 |
AH |
||||
|
MRSA 04–014 |
Resistant |
IVc |
nd |
0.12 |
2.0 |
4.0 |
1.0 |
− |
+ |
+ |
|
MRSA 1560 |
Resistant |
IV |
III |
0.12 |
2.0 |
2.0 |
2.0 |
+ |
− |
+ |
|
VISA Mu50 |
Resistant |
II |
II |
0.25 |
4.0 |
64 |
8 |
− |
+ |
+ |
|
ATCC29213* |
Sensitive |
na |
nd |
0.12 |
2.0 |
0.25 |
2.0 |
nd |
nd |
nd |
*ATCC29213 is the CLSI control strain of S. aureus (methicillin-sensitive). The MICs given above were obtained in our laboratory and are within the defined ranges for this strain.
AH: Alpha heamolsin; na: Not applicable; nd: Not done; PVL: Panton–Valentine leukocidin; TSST-1: Toxic shock syndrome toxin-1.
All strains of staphylococcus were maintained in calcium- and magnesium-supplemented Mueller–Hinton broth (Oxoid, UK). Cultures were initiated from single bacterial colonies picked from sheep blood agar plates, and cultures were grown at 37 °C in 5% CO2 with shaking (200 r.p.m.). MICs of antibiotics were determined using a microdilution broth method following CLSI guidelines [12] using calcium- and magnesium-supplemented Mueller–Hinton broth. MICs for strains used in this study are given in Table 1.
Antibiotics
Antibiotics used, and their suppliers, were as follows: iclaprim, Motif BioSciences, New York, NY; nafcillin, trimethoprim and vancomycin, Sigma Chemical, St. Louis, MO.
Effects of antibiotics on toxin production
Two experimental strategies were used to study the effects of antibiotics on exotoxin production. The first utilized cultures that were initiated with high starting bacterial concentrations (~1×107 c.f.u. ml−1) as previously described [4]. This approach was employed to overcome the limited sensitivity of first-generation toxin assays and ensure detectable levels of toxin [4]. To compensate for the increased inoculum size, antibiotics were added such that the final concentrations were 0.1×, 1× or 5× the MIC [4]. Culture supernatant samples were collected over time, rendered bacteria-free by centrifugation and sterile filtration (0.45 µm), concentrated 50-fold by centrifugal concentrator (Amicon PVDF 10 000 mwt cut-off), then aliquoted and stored at −70 °C until used in toxin assays. In the second approach, toxin assay refinements permitted subsequent studies to be conducted using cultures initiated at 1×105 c.f.u. ml−1 and antibiotics at ≤1× MIC. These refinements principally included use of a higher affinity antibody to PVL, which obviated the need to concentrate the culture supernatant samples and therefore avoided any sample processing artifacts. In this approach, samples were rendered bacteria-free as described above, aliquoted and stored −70 °C for subsequent toxin assays.
In both methods, the number of viable bacteria (c.f.u. ml−1) in the samples was determined by plating duplicate 10 µl samples (or 10-fold dilutions thereof) onto sheep blood agar plates and this number was related to absorbance at OD600 nm. An OD600 nm of 1.0 was approximately equal to 1×109 c.f.u. ml−1.
Toxins assays
Alpha haemolysin (AH) activity was measured using rabbit erythrocytes as previously described [13–15]. Briefly, sterile filtered culture supernatant fluids were diluted twofold in DPBS in a microtitre plate and an equal volume of a washed, rabbit erythrocyte suspension (2% in DPBS) was added. Sterile deionized water served as the 100% haemolysis control. After 1 h at 37 °C, the plate was centrifuged, the supernatant removed to a fresh microtitre plate, and absorbance read at 550 nm. Activity, in haemolytic units (HU) ml−1, was defined as the inverse of the dilution causing 50% haemolysis multiplied by two.
The TSST-1 ELISA procedures have been previously described [16]. Briefly, a 96-well microplate was coated overnight at 4 °C with 5 µg ml−1 of rabbit polyclonal anti-TSST-1 (Toxin Technology, Sarasota, FL) in PBS, after which the wells were washed and blocked with 3% non-fat dry milk in PBS. Serially diluted bacterial culture supernatant fluids or recombinant TSST-1 (Toxin Technology) were added to duplicate wells and the plate incubated overnight at 4 °C. After washing in PBS-Tween-20 (PBST), rabbit anti-TSST-1 HRP conjugate (Toxin Technology, diluted 1:1000) was added for 2 h at room temperature. After washing, the colorimetric substrate TMB (Zymed, San Francisco, CA) was added. After 20 min, the reaction was stopped by addition of 1 N H2SO4 and absorbance was read at 450 nm. The concentration of TSST-1 in the culture supernatant was extrapolated from a standard curve prepared using recombinant TSST-1. The assay was linear over the range of 0.6–20 ng ml−1.
Similar methods were used for the PVL ELISA as previously described [5]. Antibodies used for improved PVL ELISA sensitivity included a mouse anti-LukS-PV monoclonal antibody (capture antibody; IBT Bioservices, Gaithersburg, MA; product 0221–001) and rabbit polyclonal anti-LukS-PV antibody (secondary antibody; also IBT Bioservices; product 04–0009).
Gene expression studies in MRSA
Northern blotting
Northern blotting followed methods described previously [4]. In brief, MRSA cultures were initiated at a concentration of ~1×107 c.f.u. ml−1 and treated with 0, 0.1, 1 or 5X MIC. At various times after treatment, ~1×109 MRSA organisms were collected by centrifugation and total RNA was purified by commercial kit (RiboPure-Bacteria kit, Ambion). The RNA yield and its quality were assessed by UV absorbance and by agarose-gel electrophoresis, respectively. Two micrograms of total RNA in a 4 µl volume was mixed with 10 µl of RNA loading buffer (catalog number R-4268; Sigma), and the sample was heated at 55 °C for 15 min. The sample was loaded onto a 1% denaturing agarose gel and was run under 100 V for 90 min. RNA was transferred, by capillary action, to a nylon membrane (Hybond-N; Amersham Life Science) by use of 20× standard saline citrate (SSC) buffer. The membrane was airdried, baked at 80 °C for 2 h, and prehybridized in Ultrahyb solution (Ambion) at 42 °C for 1 h, followed by hybridization with random labelled (Invitrogen) 32P probes (described below), at a concentration of 1×106 counts ml− 1, for 18 h at 42 °C. The membrane was washed twice, at 60 °C for 15 min, with 2× SSC and 1% SDS and twice, at 60 °C for 15 min, with 0.2× SSC and 0.5% SDS and then was exposed to Kodak BioMax light film (Sigma). Blots were imaged by Alpha Imager (Alpha Innotech).
Gene-specific probes for LukF-PV, TSST-1 and alpha haemolysin were prepared by PCR amplification of S. aureus genomic DNA and random labelling of the oligonucleotides as previously described [4] and as listed in Table 2. The resulting PCR product was TA-TOPO cloned into the pCR2.1 vector and the sequence was confirmed by commercial sequencing. The purified plasmid was then digested with EcoRI and the ~0.9 kb fragment was gel purified and random labelled with 32P for use in Northern blotting. The lukF-PV probe was similarly created by PCR amplification using the primers given in Table 2.
Table 2.
Primers used for preparation of probes for Northern blotting
|
Gene |
Oligonucleotides |
|
|---|---|---|
|
Sense |
Antisense |
|
|
Tst |
GCTACAGATTTTACCCCTGTTCCCTTA |
TGATATGTGGATCCGTCATTCATTG |
|
Hla |
CCAATCGATTTTATATCTTTCTGAAGAACG |
ATTGCTAGGTTCCATATTAATGAATCCTGT |
|
lukF |
CTTCTCGAGGCTCAACATATCACACCTG |
ATTGGATCCCTAGCTCATAGGATTTTTTTCC |
qRT-PCR
Expression of hla, lukF and tst in MRSA was assessed by quantitative reverse transcriptase real-time PCR (qRT-PCR). RNA was isolated from washed bacteria using Qiagen RNeasy mini-kit following the manufacturer’s instructions with slight modification. Bacterial pellets (~1×109 c.f.u.) were dispersed in 200 µl of resuspension buffer (10 mM Tris-HCl, pH 7.5, 30 mM MgCl2, 30 mM NH4Cl plus 0.25 mg ml−1 lysostaphin; Sigma). After 30 min at 37 °C, 700 µl of RLT buffer with beta-mercaptoethanol (RLT/BME) was added, followed by 500 µl of 100% ethanol. The remaining steps followed the manufacturer’s protocol with on-column DNase treatment. RNA quantity and quality were assessed by Agilent TapeStation.
Primers for qRT-PCR are shown in Table 3. Altogether, 0.2 µg of total RNA in 20 µl total volume was converted to cDNA by First Strand cDNA Synthesis (NEB). qPCR reactions were performed with the LightCycler 480 SYBR green I Master mix (Roche) using cDNA from 5 ng total RNA and 0.5 µM primers per reaction. Primers directed toward S. aureus gyrB were used as an internal control. Reactions were run in duplicate using the following conditions: 1 cycle at 95 °C for 5 min and 45 cycles of 95 °C for 10 s, 60 °C for 10 s and 72 °C for 10 s. All data were analysed by using the LightCycler 480 software. Normalized expression was calculated as previously described by Livak and Schmittgen [17]. Briefly, the relative quantification of toxin gene expression in untreated cultures was determined by 2-ΔCt relative to the gyrB (housekeeping gene). Gene expression in antibiotic-treated cultures is given as 2-ΔΔCt and was made relative to the ‘no treatment’ control.
Table 3.
Primers used for qRT-PCR
|
Gene |
Oligonucleotides |
|
|---|---|---|
|
Sense |
Antisense |
|
|
Tst |
TCGCTACAGATTTTACCCCTG |
CGTTTGTAGATGCTTTTGCAG |
|
Hla |
GCTGCTTTCATAGAGCCATTTC |
TAGAGATTCTTGGAACCCGGTA |
|
lukF |
TGTATCTCCTGAGCCTTTTTCA |
CAGACAATGAATTACCCCCATT |
|
gyrB |
ATGTAGCAAGCCTTCCAGGTAA |
CAGAGTCCCCTTCGACTAAGAA |
Gene expression studies in VISA
A search of the NCBI database revealed that the VISA strain Mu50 carries the genes for alpha haemolysin and TSST-1, but not PVL (NCBI Reference Sequence: NC_002758.2) [18]. The effects of antibiotics on expression of hla and tst were assessed by qRT-PCR as described above for MRSA strains, except that bacterial cultures were initiated with low starting inocula (1×105 c.f.u. ml−1) and antibiotics were added such that the final concentration was ≤1× MIC. In these studies, the MRSA strain 04–014 was included as a comparator since its exotoxin gene profile is comparable to Mu50 (i.e. hla and tst positive; pvl negative) [4].
Results
Effects of antibiotics on growth and toxin production in MRSA under high-inoculum conditions
Effects of antibiotics on MRSA growth dynamics
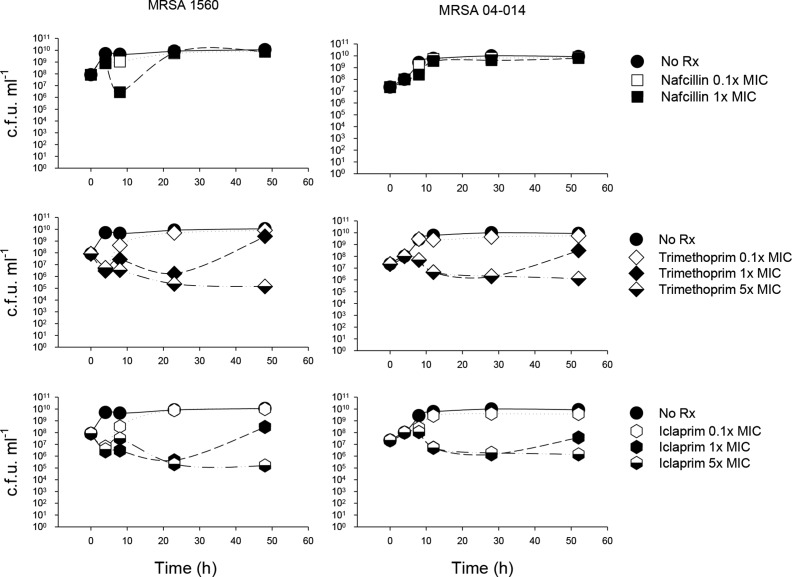
As mentioned above, initial studies of the effects of antibiotics on toxin production in MRSA required use of high starting inocula. Given that (a) the in vitro efficacies of antibiotics are variably sensitive to increasing inoculum size (i.e. inoculum effect [19]) and (b) that among some Gram-positive pathogens including S. aureus , reduced bacterial killing can sometimes be observed in vitro when antibiotic levels are above the MIC (i.e. paradoxical bactericidal effect [20]), we first examined the effects of 0.1×, 1× and 5× MIC of nafcillin, iclaprim and trimethoprim against higher bacterial concentrations.
Untreated MRSA organisms reached the post-exponential/early stationary phase of growth approximately 8–10 h after initiation of the bacterial culture, reaching a maximal bacterial concentration of ~1×1010 c.f.u. ml−1 (Fig. 1, closed circles). Addition of 0.1× MIC of nafcillin, iclaprim or trimethoprim did not markedly alter these overall growth dynamics (Fig. 1). In MRSA 1560, both iclaprim and trimethoprim at 0.1× MIC transiently (1–2 h) decreased viability at the time when untreated cultures transitioned from post-exponential to stationary phase growth (i.e. 8 h).
Fig. 1.
Effects of antibiotics on MRSA growth at higher inoculum sizes. First-generation toxin assays required a high starting inoculum (>107 c.f.u. ml−1) to ensure detectable levels of toxin were achieved. Because standard MIC determinations are defined using 105 c.f.u. ml−1 and because in vitro efficacies of antibiotics are variably sensitive to increasing inoculum size (i.e. inoculum effect), these studies examined which doses of antibiotic were sub-inhibitory with higher bacterial concentrations. MRSA 1560 amd 04–014 were cultured as described in the text and adjusted to a starting concentration of 1−5×107 c.f.u. ml−1. Iclaprim, trimethoprim and nafcillin (at 0.1, 1.0 or 5× MIC) were added at mid-log phase and samples were taken at various times before, and up to 52 h after, antibiotic addition for bacterial quantitation. Nafcillin at 5× MIC was strongly bactericidal (not shown) and thus was not studied further. Data are from one representative experiment of two.
At 1× MIC, iclaprim and trimethoprim each caused a transient but marked ~1.5–2.0 log reduction in CFU in both MRSA strains over the first 24 hrs of culture; afterwards, organisms resumed growth such that the final bacterial concentrations were comparable to untreated controls. At 5× MIC, iclaprim and trimethoprim caused a sustained decrease in bacterial viability. Thus, only iclaprim and trimethoprim at 0.1× MIC were considered sub-inhibitory and neither drug displayed a paradoxical bactericidal effect in this system. Nafcillin at 5× MIC was strongly bactericidal (not shown) and was not studied further.
Effects of antibiotics on MRSA toxin gene transcription and translation
In untreated MRSA control cultures, Northern blotting revealed that transcription of hla, lukF-PV and tst peaked at the transition between exponential and stationary phase growth (~8 h) and declined thereafter (Fig. 2 upper panels); measurable increases in toxin production began at this time and continued over 12–52 h (Fig. 3, closed circles). These dynamics were confirmed by qRT-PCR (not shown).
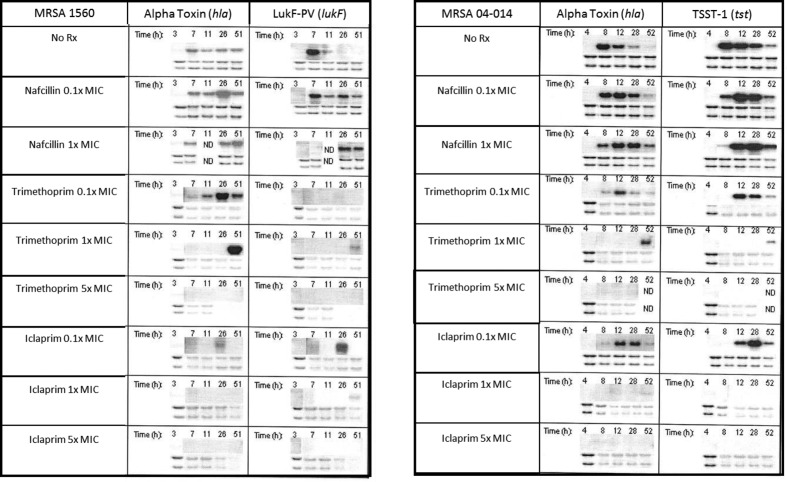
Fig. 2.
Effects of antibiotics on exotoxin gene expression in MRSA. MRSA were cultured using high initial inoculum conditions as described in Fig. 1. Before, and at various times after, antibiotic addition (0.1, 1.0 and 5× MIC), expression of hla, lukf and tst was analysed by Northern blotting. The lower two bands are total RNA loading controls.
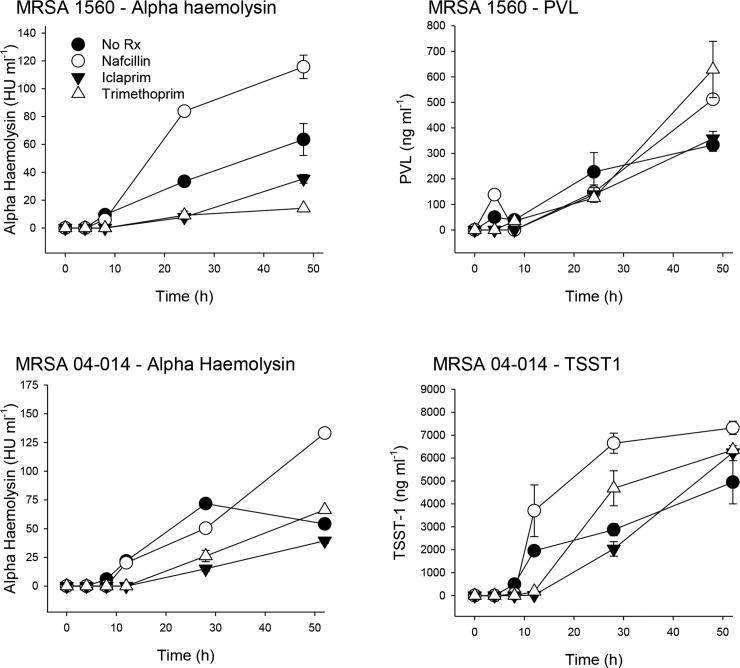
Fig. 3.
Effect of antibiotics on exotoxin protein production in MRSA. Methods used are as described in Fig. 1 except that MRSA were cultured in the presence of only 0.1× MIC (i.e. sub-inhibitory) doses of nafcillin, iclaprim or trimethoprim, or vehicle control. MRSA 1560 (top left, right) produces AH and PVL but not TSST-1; MRSA 04–014 (bottom left, right) produces AH and TSST-1 but not PVL. Samples were removed at various times after antibiotic addition, rendered bacteria-free by centrifugation and sterile filtration, concentrated by centrifugal 10 000 mwt filter, then aliquoted and frozen until assayed for toxins of interest as described in the text. Data are given as the means of duplicate measurements±standard deviation from one experiment.
Nafcillin
Nafcillin at 0.1× MIC did not alter the onset of hla, lukF or tst transcription but significantly increased and prolonged their expression (Fig. 2). This nafcillin-induced increase in gene expression resulted in significantly increased toxin production compared to untreated controls by 52 h (Fig. 3). These results with nafcillin are in agreement with our previously published findings [4]. Similar changes in the dynamics of toxin gene expression (Fig. 2) and protein production levels (not shown) were observed with nafcillin at 1× MIC.
Trimethoprim
Compared to untreated controls, trimethoprim at 0.1× MIC delayed the onset of hla expression in both MRSA strains from 8 to 12 h (Fig. 2) and shifted peak transcriptional activity from 8 to 28 h (Fig. 2). These effects were associated with a reduction in peak alpha haemolysin production in both strains (Fig. 3). Trimethoprim, but not iclaprim, significantly prolonged and increased hla expression but only in MRSA 1560. However, this was not associated with increased toxin production over the time frame studied.
In contrast to hla, sub-inhibitory concentrations of trimethoprim fully inhibited lukF expression in MRSA 1560, yet maximal PVL production was significantly greater in trimethoprim-treated cultures compared to controls (PVLcontrol vs trimethoprim:332.7±24.4 vs 628.9±110 ng ml−1; Fig. 3). As with hla, sub-inhibitory trimethoprim delayed the onset of tst expression in MRSA 04–014 and also reduced its peak expression level. These changes in tst expression were associated with an initial delay, but an overall increase, in TSST1 production (Fig. 3).
At 1× MIC, trimethoprim suppressed all toxin gene expression through 28 h; toxin gene transcription was only observed at 51–52 h when organisms had regrown to near untreated levels (Fig. 1); at 5× MIC, no toxin genes were expressed. Under these latter conditions, protein toxin production was virtually nil (not shown).
Iclaprim
Compared to untreated cultures, sub-inhibitory iclaprim (0.1× MIC) delayed the onset of hla, lukF and tst mRNA synthesis, and reduced the peak transcription level of hla (Fig. 2). Similarly, peak transcription of tst and lukF did not occur until 28 h compared to 8 h in control cultures. The iclaprim-induced temporal changes in hla gene transcription in MRSA 1560 were associated with a marked reduction in maximal alpha haemolysin production compared to that in untreated control cultures (alpha haemolysincontrol vs iclaprim=63.5 ± 11.4 vs 35.4±3.2 HU ml−1; (Fig. 3; top left). Similarly, peak alpha haemolysin production by MRSA 04–014 was markedly reduced by 0.1× iclaprim. Interestingly, maximal TSST-1 levels in iclaprim-treated cultures were not significantly different from untreated controls (TSST-1control vs iclaprim=4945 ± 947.5 vs 6240±92.8 ng ml−1).
In contrast to trimethoprim, iclaprim did not completely inhibit lukF expression, but rather shifted maximal gene expression from 7 to 26 h. Also compared to trimethoprim, iclaprim significantly reduced PVL production at 48 h (PVLiclaprim vs trimethoprim:357.7±28.6 vs 628.9±110 ng ml−1; Fig. 3 top right).
Effects of antibiotics on growth and toxin production in VISA under low-inoculum conditions
Effects of antibiotics on growth and toxin production in VISA
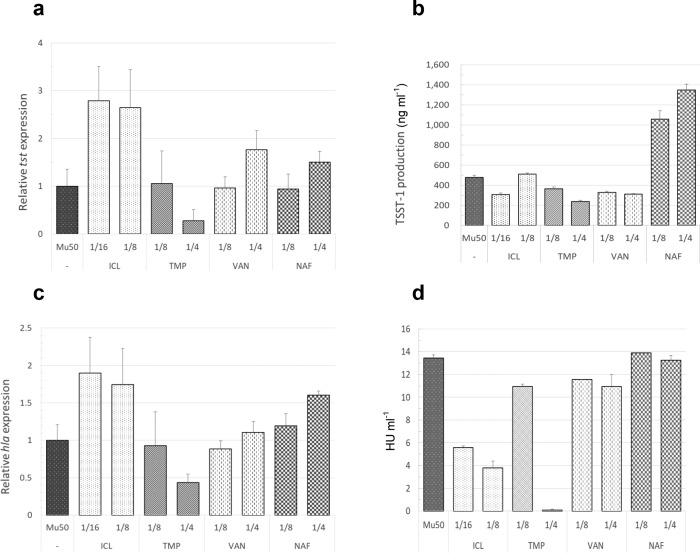
Vancomycin-intermediate S. aureus strain Mu50 carries the genes for TSST-1 and AH, but is negative for PVL. This toxin profile is comparable to MRSA strain 04–014 used above. Therefore, experiments examining toxin production in the Mu50 VISA strain included MRSA 04–014 as a comparator. Improved toxin assay sensitivity allowed studies with these strains to utilize lower starting inocula and sub-MIC antibiotics. Specifically, cultures were initiated at 1×105 c.f.u. ml−1 and treated at time zero (T 0) with iclaprim, trimethoprim, vancomycin or nafcillin at 1/4th, 1/8th or 1/16th× MIC (final concentrations). Optical density of the culture was followed at 600 nm. Samples for toxin gene expression and protein production were taken at 10 and 24 h. After removal of bacteria by centrifugation and sterile filtration, supernatant samples were used neat, or diluted in DPBS, for toxin assays.
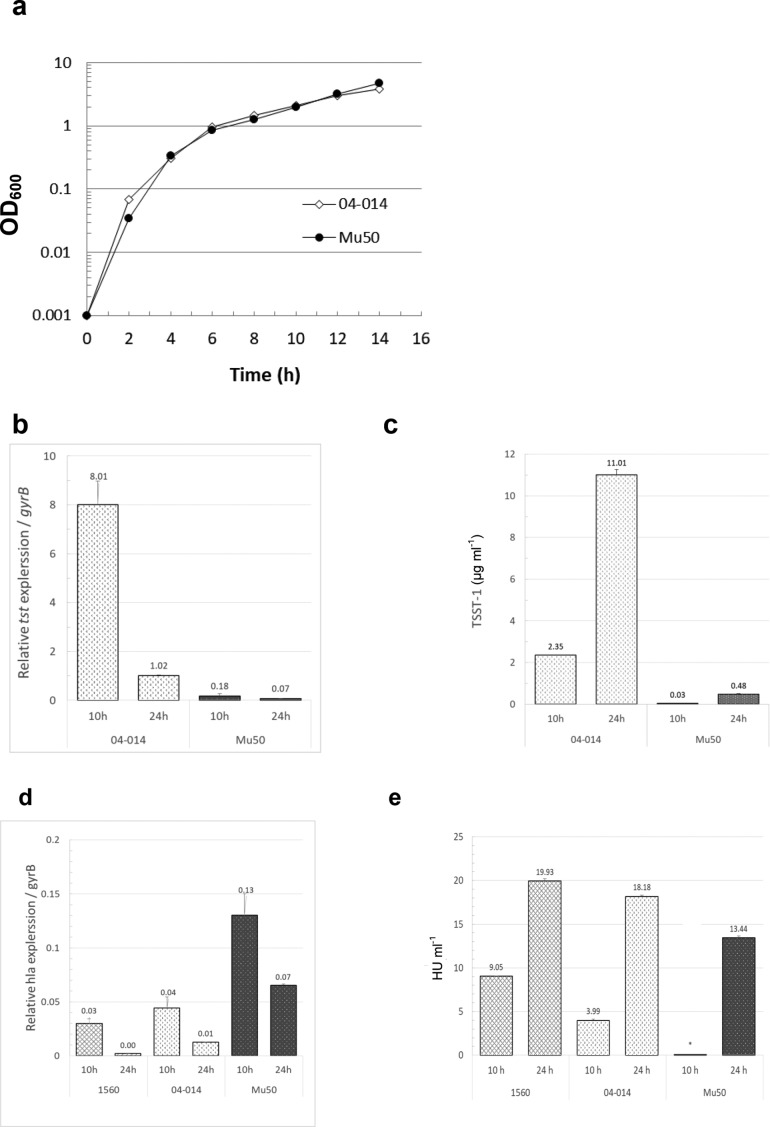
The growth dynamics of Mu50 were similar that of MRSA strain 04–014 (Fig. 4a) and, like the MRSA strain, expression of tst in Mu50 was maximal during its transition from late log phase to early stationary phase growth (8–10 h; Fig. 4b). However, the magnitude of tst expression at 10 h in the VISA Mu50 strain was nearly 50-fold lower than that in the MRSA 04–014 (Fig. 4b). This difference in gene expression paralleled large differences in toxin protein levels in that Mu50 produced 67- and 23-fold less TSST-1 at 10 and 24 h, respectively, compared to the MRSA strain (Fig. 4c). Interestingly, expression of hla at 10 h in VISA Mu50 was higher than MRSA strains 04–014 and 1560 (Fig. 4d). However, alpha haemolysin production by Mu50 was undetectable at 10 h and was 26 and 33% lower at 24 h compared to MRSA 04–014 and 1560, respectively (Fig. 4e).
Fig. 4.
Comparison of growth dynamics and toxin production in MRSA and VISA under low inoculum conditions. MRSA strain 04–014 and VISA strain Mu50 have similar exotoxin profiles (i.e. hla and tst positive; pvl negative). (a) Using low initial inoculum conditions (cultures started with ~1×105 c.f.u. ml−1), growth in untreated cultures was followed over time by optical density at 600 nm. (b) Relative tst expression and (c) production of TSST-1 protein in untreated cultures were measured at 10 h and 24 h by qRT-PCR and ELISA, respectively. (d) Expression of hla and (e) production of alpha haemolysin were measured by qRT-PCR and rabbit erythrocyte lysis assay, respectively, in two untreated strains of MRSA and in the VISA Mu50 strain. *=below the level of detection. Data shown are the means of duplicate measurements±standard deviation from a single experiment.
The effects of antibiotics on toxin gene expression and protein production in Mu50 were antibiotic-specific (Fig. 5). In contrast to trimethoprim, sub-MIC doses of iclaprim increased tst gene expression in Mu50 (Fig. 5a). To a lesser extent, vancomycin and nafcillin at 1/4th MIC also stimulated tst gene transcription (Fig. 5a). Despite the ability of several antibiotics to upregulate gene expression in this strain, only nafcillin increased TSST-1 production (Fig. 5b). It is notable that despite significant differences in the magnitude of tst gene expression between MRSA 04–014 and VISA Mu50 (Fig. 4b), the fold-increase in TSST-1 production induced by nafcillin (~two–three fold) was similar in both strains (compare Figs 3 and 5b). Iclaprim, but not trimethoprim, potently increased expression of hla (Fig. 5c). However iclaprim was more effective at suppressing production of alpha haemolysin than the other antibiotics tested (Fig. 5d).
Fig. 5.
Effects of antibiotics on toxin gene expression and protein production in VISA. Cultures were initiated with ~1×105 c.f.u. ml−1 and antibiotics were added such that the final concentrations were 1/4th, 1/8th or 1/16th the MIC. The effects of antibiotics on expression of tst and hla at 10 h (a and c, respectively) or on protein production at 24 h (b and d, respectively) were measured as described above. ICL: iclaprim; TMP: trimethoprim; VAN: vancomycin; NAF: nafcillin. Data shown are the means of triplicate measurements on each samples obtained from a single experiment.
Summary
Strains of S. aureus vary in their exotoxin gene profiles and strains with similar genotypes can produce significantly different levels of toxins. As has been reported previously, nafcillin at sub-inhibitory doses consistently increased exotoxin gene expression and protein production for most strains and toxins studied. The present study sought to compare the effects of this beta-lactam antibiotic with that of trimethoprim and iclaprim – two folic acid synthesis inhibitors. In general, their effects on toxin gene expression and exotoxin production were strain- and toxin-dependent. Specifically, in both strains of MRSA, iclaprim and trimethoprim altered the dynamics of exotoxin gene transcription (hla, tst, lukF-PV), delaying the onset of mRNA production and shifting its peak production to later time points. This effect appeared independent of any effects on bacterial growth. Both iclaprim and trimethoprim suppressed, but did not eliminate, AH production and delayed, but did not reduce, maximal TSST-1 production in MRSA. Interestingly, in MRSA 1560, trimethoprim significantly increased maximal PVL production compared to either untreated or iclaprim-treated cultures. Whether this effect is consistent among all PVL-producing strains of MRSA remains to be determined.
Compared to the MRSA strains, toxin production was markedly lower in the VISA strain Mu50, yet as with MRSA 04–014, nafcillin significantly increased TSST-1 production in this strain. Although iclaprim increased both tst and hla gene expression in the VISA strain, this activity was not associated with an increase in either TSST-1 or alpha haemolysin protein production. In fact, iclaprim was the most effective antibiotic tested at suppressing toxin production in VISA Mu50.
Discussion
Antimicrobial efficacy in treating bacterial infection is dependent upon numerous parameters including resistance, achievable blood levels, percent protein binding and tissue penetration. The physiologic state of the microbe also influences efficacy and consequently, susceptibility testing is universally performed on log phase organisms. Here, beta-lactam antibiotics are rapidly bactericidal, largely because their targets – the penicillin binding proteins (PBPs) – are maximally expressed during the log phase of growth and decline thereafter [21]. In infections such as abscesses, osteomyelitis and deep-seated necrotizing soft tissue infections, the pathogen may not be in log phase and thus surgical debridement is a necessary component of effective treatment. In addition, microbes such as group A streptococcus, Staphylococcus aureus and Clostridium species cause disease by virtue of potent extracellular toxins, which are produced mainly in the late log/early stationary phase of growth where beta-lactam antibiotics begin losing antimicrobial killing capacity due to waning PBP expression. Furthermore, sub-inhibitory doses of beta-lactam antibiotics have been shown to increase protein toxin production in some Gram-positive pathogens, including S. aureus [4, 22, 23]. These factors largely explain why, in animal models and human cases of group A streptococcal or clostridial infection, beta-lactam antibiotics have demonstrated reduced clinical efficacy, whereas agents that inhibit bacterial protein synthesis are markedly superior (reviewed in [24]).
Iclaprim is a second-generation dihydrofolate reductase (DHFR) inhibitor, similar in mechanism of action to trimethoprim. However, iclaprim exhibits a significantly stronger affinity for microbial DHFR and the mutated F98Y S. aureus enzyme compared with trimethoprim, which obviates the need for combination treatment with a sulfonamide [25]. The proposed indication for iclaprim is treatment of adults with acute bacterial skin and skin structure infections (ABSSSI) caused by susceptible strains of the following Gram-positive micro-organisms: S. aureus including methicillin-susceptible and -resistant strains, Streptococcus pyogenes , Enterococcus faecalis and S. agalactiae [26, 27].
Consistent with previous studies [4], nafcillin increased toxin gene and/or protein toxin production in MRSA. In contrast to nafcillin, both iclaprim and trimethoprim at sub-inhibitory concentrations significantly altered (delayed initiation and/or prolonged gene transcription) bacterial toxin gene expression dynamics in MRSA.
Similarly, in the VISA strain, nafcillin increased tst gene expression and protein toxin production. Although iclaprim greatly increased both tst and hla gene expression, this activity did not result in increased toxin production. In fact, iclaprim potently inhibited alpha haemolysin production in this strain.
Mechanisms responsible for antibiotic-induced toxin gene upregulation are not well understood. Even more perplexing is how an antibiotic can increase toxin gene expression without a concomitant increase in toxin protein production. In our previous studies, clindamycin and linezolid increased and/or prolonged S. aureus toxin gene expression [4]; yet protein toxin production was curtailed by the ability of these agents to block translation at the ribosomal level. Unlike clindamycin and linezolid, iclaprim is not a direct protein synthesis inhibitor antibiotic. Certainly, protein synthesis could be affected by inadequate intracellular folate levels. However, if this were the mechanism responsible, one would also expect a reduction in mRNA production and a diminution of growth rate. To the contrary, growth was unaffected by sub-inhibitory doses of iclaprim and toxin gene mRNA levels were increased in iclaprim-treated cultures. Together these results imply that intracellular folate levels were likely to be functionally unchanged such that general gene transcription and tRNA production could proceed unimpaired. This notion is supported by studies of trimethoprim-treated E. coli that demonstrated an excellent correlation between intracellular folate levels and growth rate [28].
Taken together, our findings suggest iclaprim may have a unique, as-yet undescribed, mechanism for inhibiting protein synthesis. One possibility is that iclaprim induces a reprogramming of the organism’s protein synthesis machinery. Such reprogramming is known to occur in response to various stresses and favours translation of those mRNAs whose protein products are required for appropriate countermeasures and survival responses [29]. In prokaryotes, mechanisms for such translational attenuation include conformational changes in the mRNA leader sequence [30] and ribosome stalling within an upstream ORF (reviewed in [31]). Whether iclaprim can uniquely stimulate events remains to be investigated.
In summary, these results demonstrate that folic acid antagonist antibiotics alter both mRNA synthesis dynamics and protein toxin production in MRSA and VISA at concentrations below those that inhibit bacterial growth. These results, plus the fact that iclaprim is at least 16-fold more active than trimethoprim (MRSA MICsiclaprim vs trimethoprim=0.12 vs 2.0 µg ml−1; VISA MICsiclaprim vs trimethoprim=.25 vs 4.0 µg ml−1) [32] and is active against MRSA isolates resistant or non-susceptible to vancomycin, linezolid and daptomycin [33], provide additional rationale for the use of iclaprim to treat complicated MRSA and VISA infections.
Funding information
Work was supported, in part, by the Department of Veterans Affairs, Biomedical Laboratory Research & Development program (AEB, DLS), by the National Institutes of General Medical Sciences (DLS; P20GM109007) and by Motif BioSciences.
Conflicts of interest
A.E.B., S.G., E.K. and D.L.S. have no conflict of interest to declare; D.B.H. is an employee of Motif BioSciences.
Footnotes
Abbreviations: agr, gene for accessory gene regulator; AH, alpha haemolysin; CFU, colony forming unit; DHFR, dihydrofolate reductase; DPBS, dulbecco’s phosphate buffered saline; hla, gene for alpha haemolysin; HRP, horseradish peroxidase; HU, hemolytic unit; lukF-PV, gene for Panton-Valentine leucocidin, F component; mecA, gene for PBP2a; confers resistance to methicillin; MIC, minimum inhibitory concentration; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus ; MSSA, methicillin-sensitive Staphylococcus aureus ; OD, optical density; ORF, open reading frame; PBP, penicillin-binding protein; PFGE, pulse field gel electrophoresis; PVL, Panton-Valentine leukocidin; SCCmec, Staphylococcal cassette chromosome mec; TSST-1, toxic shock syndrome toxin–1; tst, gene for toxic shock syndrome toxin-1; VISA, vancomycin-intermediate Staphylococcus aureus .
References
- 1.Gleckman R, Blagg N, Joubert DW. Trimethoprim: mechanisms of action, antimicrobial activity, bacterial resistance, pharmacokinetics, adverse reactions, and therapeutic indications. Pharmacotherapy. 1981;1:14–19. doi: 10.1002/j.1875-9114.1981.tb03548.x. [DOI] [PubMed] [Google Scholar]
- 2.Hartman PG. Molecular aspects and mechanism of action of dihydrofolate reductase inhibitors. J Chemother. 1993;5:369–376. [PubMed] [Google Scholar]
- 3.Entenza JM, Haldimann A, Giddey M, Lociuro S, Hawser S, et al. Efficacy of iclaprim against wild-type and thymidine kinase-deficient methicillin-resistant Staphylococcus aureus isolates in an in vitro fibrin clot model. Antimicrob Agents Chemother. 2009;53:3635–3641. doi: 10.1128/AAC.00325-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, et al. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus . J Infect Dis. 2007;195:202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton SM, Bryant AE, Carroll KC, Lockary V, Ma Y, et al. In vitro production of Panton-Valentine Leukocidin (PVL) among strains of methicillin-resistant Staphylococcus aureus causing diverse infections. Clin Infect Dis. 2007;45:1550–1558. doi: 10.1086/523581. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Stevens DL, Hamilton SM, Parimon T, Ma Y, et al. Fatal S. aureus hemorrhagic pneumonia: genetic analysis of a unique clinical isolate producing both PVL and TSST-1. PLoS One. 2011;6:e27246. doi: 10.1371/journal.pone.0027246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diep BA, Sensabaugh GF, Somboona NS, Carleton HA, Perdreau-Remington F. Widespread skin and soft-tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J Clin Microbiol. 2004;42:2080–2084. doi: 10.1128/JCM.42.5.2080-2084.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing. sixteenth informational supplement. Wayne, PA: 2006. p. M100–S16. [Google Scholar]
- 10.Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–1827. doi: 10.1016/S0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 11.Sakoulas G, Eliopoulos GM, Moellering RC, Wennersten C, Venkataraman L, et al. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 2002;46:1492–1502. doi: 10.1128/AAC.46.5.1492-1502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards . Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS Approved standard. 2003. p. M7–06. [Google Scholar]
- 13.Gemmell CG, Peterson PK, Schmeling DJ, Quie PG. Effect of staphylococcal alpha-toxin on phagocytosis of staphylococci by human polymorphonuclear leukocytes. Infect Immun. 1982;38:975–980. doi: 10.1128/iai.38.3.975-980.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kernodle DS, McGraw PA, Barg NL, Menzies BE, Voladri RK, et al. Growth of Staphylococcus aureus with nafcillin in vitro induces alpha-toxin production and increases the lethal activity of sterile broth filtrates in a murine model. J Infect Dis. 1995;172:410–419. doi: 10.1093/infdis/172.2.410. [DOI] [PubMed] [Google Scholar]
- 15.Lominski I, Arbuthnott JP. Some characteristics of Staphylococcus alpha hæmolysin. J Pathol. 1962;83:515–520. doi: 10.1002/path.1700830225. [DOI] [PubMed] [Google Scholar]
- 16.van Langevelde P, van Dissel JT, Meurs CJ, Renz J, Groeneveld PH. Combination of flucloxacillin and gentamicin inhibits toxic shock syndrome toxin 1 production by Staphylococcus aureus in both logarithmic and stationary phases of growth. Antimicrob Agents Chemother. 1997;41:1682–1685. doi: 10.1128/AAC.41.8.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Ohta T, Hirakawa H, Morikawa K, Maruyama A, Inose Y. Nucleotide substitutions in Staphylococcus aureus strains, Mu50, Mu3, and N315. DNA Res. 2004;11:51–56. doi: 10.1093/dnares/11.1.51. [DOI] [PubMed] [Google Scholar]
- 19.Brook I. Inoculum effect. Rev Infect Dis. 1989;11:361–368. doi: 10.1093/clinids/11.3.361. [DOI] [PubMed] [Google Scholar]
- 20.Holm SE, Tornqvist IO, Cars O. Paradoxical effects of antibiotics. Scand J Infect Dis Suppl. 1990;74:113–117. [PubMed] [Google Scholar]
- 21.Stevens DL, Van S, Bryant AE. Penicillin-binding protein expression at different growth stages determines penicillin efficacy i n vitro and i n vivo: an explanation for the inoculum effect. J Infect Dis. 1993;167:1401–1405. doi: 10.1093/infdis/167.6.1401. [DOI] [PubMed] [Google Scholar]
- 22.Aldape MJ, Packham AE, Nute DW, Bryant AE, Stevens DL. Effects of ciprofloxacin on the expression and production of exotoxins by Clostridium difficile. J Med Microbiol. 2013;62:741–747. doi: 10.1099/jmm.0.056218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens DL, Maier KA, Mitten JE. Effect of antibiotics on toxin production and viability of Clostridium perfringens . Antimicrob Agents Chemother. 1987;31:213–218. doi: 10.1128/AAC.31.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases Society of America. Clin Infect Dis. 2014;59:e10–e52. doi: 10.1093/cid/ciu444. [DOI] [PubMed] [Google Scholar]
- 25.Oefner C, Bandera M, Haldimann A, Laue H, Schulz H, et al. Increased hydrophobic interactions of iclaprim with Staphylococcus aureus dihydrofolate reductase are responsible for the increase in affinity and antibacterial activity. J Antimicrob Chemother. 2009;63:687–698. doi: 10.1093/jac/dkp024. [DOI] [PubMed] [Google Scholar]
- 26.Holland TL, O'Riordan W, McManus A, Shin E, Borghei A, et al. A phase 3, randomized, double-blind, multicenter study to evaluate the safety and efficacy of intravenous Iclaprim versus vancomycin for treatment of acute bacterial skin and skin structure infections suspected or confirmed to be due to gram-positive pathogens (REVIVE-2 study) Antimicrob Agents Chemother. 2018;62:e02580–17. doi: 10.1128/AAC.02580-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang DB, O'Riordan W, Overcash JS, Heller B, Amin F, et al. A phase 3, randomized, double-blind, multicenter study to evaluate the safety and efficacy of intravenous iclaprim vs vancomycin for the treatment of acute bacterial skin and skin structure infections suspected or confirmed to be due to gram-positive pathogens: REVIVE-1. Clin Infect Dis. 2018;66:1222–1229. doi: 10.1093/cid/cix987. [DOI] [PubMed] [Google Scholar]
- 28.Rohlman CE, Matthews RG. Role of purine biosynthetic intermediates in response to folate stress in Escherichia coli . J Bacteriol. 1990;172:7200–7210. doi: 10.1128/jb.172.12.7200-7210.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spriggs KA, Bushell M, Willis AE. Translational regulation of gene expression during conditions of cell stress. Mol Cell. 2010;40:228–237. doi: 10.1016/j.molcel.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Babitzke P, Gollnick P. Posttranscription initiation control of tryptophan metabolism in Bacillus subtilis by the trp RNA-binding attenuation protein (TRAP), anti-TRAP, and RNA structure. J Bacteriol. 2001;183:5795–5802. doi: 10.1128/JB.183.20.5795-5802.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez J, Yaman I, Huang C, Liu H, Lopez AB, et al. Ribosome stalling regulates IRES-mediated translation in eukaryotes, a parallel to prokaryotic attenuation. Mol Cell. 2005;17:405–416. doi: 10.1016/j.molcel.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Huang DB, File TM, Dryden M, Corey GR, Torres A, et al. Surveillance of iclaprim activity: in vitro susceptibility of gram-positive pathogens collected from 2012 to 2014 from the United States, Asia Pacific, Latin American and Europe. Diagn Microbiol Infect Dis. 2018;90:329–334. doi: 10.1016/j.diagmicrobio.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Huang DB, Hawser S, Gemmell CG, Sahm DF. In vitro activity of Iclaprim against methicillin-resistant Staphylococcus aureus nonsusceptible to daptomycin, linezolid, or vancomycin: a pilot study. Can J Infect Dis Med Microbiol. 2017:epub 2017 Dec 17. doi: 10.1155/2017/3948626. [DOI] [PMC free article] [PubMed] [Google Scholar]