Abstract
Background
Measurement of glomerular filtration rate by iohexol disappearance (iGFR) has become a gold standard in the pediatric CKD population. The need for serial phlebotomy can be difficult and minimizing venipunctures would be beneficial. Furthermore, finger stick collection for dried blood spot (DBS) may be more tolerable in the pediatric population, and equivalence between these two methods may further simplify the process.
Methods:
This was a cross-sectional study in children and adolescents 1 to 21 years with Stage I-IV CKD. Iohexol was infused and blood drawn 10, 30, 120, and 300 minutes later. Blood spots on filter paper were collected by finger-stick after each of the latter two blood draws. The rate of iohexol plasma disappearance was used to calculate GFR. Pearson’s correlation coefficient and bias, Students t-test and Bland Altman graphical representations were used to compare methods.
Results:
Forty one patients were recruited. The mean creatinine was 1.13 mg/dL (SD 0.45), the mean 4-point iGFR was 73.2 ml/min/1.73m2 (SD 27.5) and the mean 2-point iGFR was 75.6 ml/min/1.73m2 (SD 27.3). Correlation between 2-point and 4-point venous GFR was r=0.97; p<0.001. The correlation between the DBS and the 2-point venous GFR was r=0.95; p<0.001, with no significant bias. Ninety four percent of the 2-point GFR’s were within 10% of the 4-point GFR’s and 80% of DBS-GFRs were within 10% of the 2-point GFR’s.
Conclusions:
The 2-point iGFR was highly correlated and agreed well with the 4-point iGFR. The same was true for the DBS method and the 2-point venous method. DBS sampling by finger stick sampling at 2 time points after iohexol infusion gave an acceptably accurate measurement of GFR.
Keywords: Pediatrics, Iohexol, Glomerular Filtration Rate, Chronic Kidney disease, Dried blood spot
Introduction
Accurate measurement of glomerular filtration rate (GFR) in children and adolescents is needed for monitoring chronic kidney disease (CKD) progression and in circumstances when precise medication dosing adjusted for renal function is crucial, such as chemotherapy for pediatric cancer patients. Creatinine clearance via 24-hour urine collection is difficult in children, often requiring placement of a bladder catheter in children who are not yet continent of urine in order to obtain a complete collection. Measurement of GFR by the plasma disappearance of iohexol (readily available as Omnipaque™) avoids this hurdle; and although accepted as a gold standard, it requires multiple blood draws, which can also be problematic in the pediatric population.[1–3]
Normally, iohexol GFR measurement involves a venipuncture for a baseline blood sample and infusion of 5mLs of iohexol, then blood samplings at 10 minutes, 30 minutes, 2 hours and 5 hours.[1] The subsequent timed blood samples are obtained through a small intravenous (IV) catheter; however the blood samplings are impossible to obtain if the IV clots or infiltrates, and there is frequently a need for repeated venipuncture. Recently the Chronic Kidney Disease in Children Study (CKiD) has modified the blood drawing schedule such that samples are taken at baseline and at 2 and 5 hours; thus decreasing the number of blood samples by omitting the early points.[4]
An alternative to the repeated blood samplings by venipuncture may be possible with a finger stick using a lancet and placement of the blood on filter paper (as commonly used for the newborn metabolic screen), the dried blood spot (DBS) method. This modification is especially important in children and adolescents who have limited vascular access where frequent venipuncture may not be feasible or desirable.
We designed a pilot study to assess whether modified technique for measuring GFR by iohexol disappearance would be accurate and reproducible in children and adolescents with chronic kidney disease (CKD), while minimizing the need for repeated venipuncture.
Materials and Methods
A cross-sectional multi-center pilot study was designed. Participating centers included the University of Rochester Medical Center, Johns Hopkins University and the University of New Mexico. The study was designed to address two specific aims; first, confirm that a limited blood sampling with 2 venipunctures determines GFR (iGFR2) and is comparable to the standard 4-point plasma disappearance of iohexol (iGFR4).[4] Secondly, determine if a DBS method for determination of the iohexol 2-point (iGFR-DBS) disappearance is as accurate as the iGFR2.
Subjects were recruited from our pediatric nephrology clinics during routine visits. All pediatric patients ages 1 to 21 with an estimated GFR between 15 and 150 ml/min/1.73 m2 (CKD Stage G1 to G4) were eligible to participate. Stable kidney transplant patients and those on dialysis were eligible. CKD subjects who were nephrotic, defined as having edema, nephrotic range proteinuria, and hypoalbuminemia were not eligible. Nephrotic range proteinuria was defined as a 24-hour urine protein of > 4 grams per day and/or a random urinary protein-to-creatinine ratio of > 2 mg/mg. Hypoalbuminemia was defined as serum albumin < 2g/dL. Pregnant females were also ineligible. Enrollment was voluntary, and subjects were compensated for their time. The study was approved by the Institutional Review Boards of each of the participating centers.
Single injection iohexol clearance to measure GFR:
Subjects were examined and current medications noted. A 22 gauge polyethylene catheter or butterfly was inserted for iohexol injection. An additional 22 gauge catheter was placed in the other arm for blood drawing. A zero time blood sample was collected for hematocrit, iohexol blank, and serum creatinine. Then, 5 ml of iohexol solution (Omnipaque 300™, corresponding to 647 mg iohexol per ml or 300 mg iodine per ml) was infused in each subject over 1–2 minutes followed by infusion of 10 ml of saline solution. This catheter was removed after the infusion. Blood (1 ml) was drawn through the contralateral intravenous catheter at 10, 30, 120, and 300 minutes, comprising the time points for iGFR4 (with iGFR2 using only the 120 and 300 minute points).[1] The time points for iGFR-DBS were also obtained after the venous draws at 120 and 300 minutes with 11.2 microliters of blood each placed as 2–4 large single droplets onto filter paper by finger stick (Schleicher and Schuell Grade 903). A 6.3 mm diameter punch of the DBS (containing 11.2 μl of blood) was treated with 170 μl 5% perchloric acid and samples were vortexed for 3 min, ultrasonicated for 15 min, incubated for 30 min on the bench at room temperature, and then spun at 14 000 g for 10 min. Iohexol was eluted from the DBS and analyzed as per venous samples by high pressure liquid chromatography (HPLC) at the University of Rochester according to the procedure of Nicolescu-Duvaz, incorporating the hematocrit, because iohexol is not distributed in red blood cells.[5] [6]
Statistical analysis
Calculation of iGFR4:
The plasma disappearance is resolved into two exponential curves, the fast curve being a distribution curve and the slow curve being a renal excretion curve.[7] iGFR4 was calculated from the dose and areas under the slopes of the two curves. The GFRs in mL/min were corrected to body surface area (BSA) according to the formula of Haycock et al (BSA (m2) = weight (kg)0.5378 x height (cm)0.3964 × 0.024265.8).[8]
Calculation of iGFR2 and iGFR-DBS:
Clearance of iohexol (C) was calculated according to the plasma disappearance of iohexol as derived by Ng, from the one-compartment clearance (C1) by the formula C = C1/[1+0.12(C1/100)].[4] In this calculation C1 = injected amount of iohexol divided by the slope of monoexponential line described by the two sample points of iohexol concentration in mg/mL back-extrapolated to time zero.[9]
We performed paired comparisons of iGFR4 versus iGFR2; and iGFR4 versus iGFR-DBS and iGFR2 versus iGFR-DBS. Means and standard deviations were calculated for the summary statistics; Pearson’s correlation coefficient was used to correlate the respective measurements and bias was calculated (defined as the mean difference between comparison groups). The above three comparisons were also graphically represented by scatter plots, and modified Bland-Altman and Students paired t-test analyses were used to further assess the agreement. Accuracy was also assessed by noting the percentage of results within 10% and 30% of the comparison study. Data were analyzed using STATA v12.1 (StataCorp, College Station, TX USA).
Results
Forty-one subjects were recruited. Not all patients had complete data to allow for all calculations to be performed on all subjects. There were 33 subjects with both iGFR2 and iGFR4 data and 29 with both iGFR2 and iGFR-DBS data (Table 1). Reasons for incomplete data included patient refusal of finger stick for DBS and IV infiltration with patient preference to not replace and continue.
Table 1.
Demographics by Comparison Group
| iGFR2 vs. iGFR4 N=33 Mean (SD) |
iGFR2 vs. iGFR-DBS N=29 Mean (SD) |
iGFR4 vs. iGFR-DBS N=24 Mean (SD) |
|
|---|---|---|---|
| Age (years) | 14.5 (3.6) | 14.6 (3.4) | 14.9 (3.4) |
| Male (%) | 64 | 76 | 71 |
| Height (cm) | 159.2 (18.8) | 160.4 (19.1) | 160.4 (19.0) |
| Weight (kg) | 58.5 (23.0) | 59.7 (24.3) | 59.2 (22.4) |
| BSA (m2) | 1.6 (0.41) | 1.6 (0.43) | 1.6 (0.40) |
| Creatinine (mg/dL) | 1.15 (0.5) | 1.09 (0.46) | 1.10 (0.5) |
| iGFR2 (ml/min/1.73m2) | 76.3 (28.9) | 79.1 (26.0) | N/A |
| iGFR4 (ml/min/1.73m2) | 73.2 (27.5) | N/A | 76.0 (27.1) |
| iGFR-DBS (ml/min/1.73m2) | N/A | 81.9 (28.4) | 82.4 (30.8) |
The mean age of the entire recruited cohort was 14.4 years (range 7 to 21 years) and 63% of subjects were male. Mean creatinine was approximately 1.1 mg/dL (range 0.47 to 2.75 mg/dL). Etiology of CKD was variable and included autoimmune vasculitis, glomerulonephritis, elevated serum creatinine, congenital anomalies of the kidneys and urinary tract, and nephrectomy due to malignancy.
The mean iohexol iGFR4 was 73.2 ml/min/1.73m2, with a standard deviation (SD) of 27.5 ml/min/1.73m2. Mean iGFR2 was 75.6 ml/min/1.73m2 (SD 27.3). Mean iohexol GFR-DBS was 81 ml/min/1.73m2 (SD 28.4). The paired t-test p-value for both iGFR2 vs. iGFR4 and iGFR2 vs. iGFR-DBS was 0.7, indicating no significant difference between the paired measurements. The p-value for iGFR-DBS vs iGFR4 was 0.45, also indicating no significant difference between the two paired measurements.
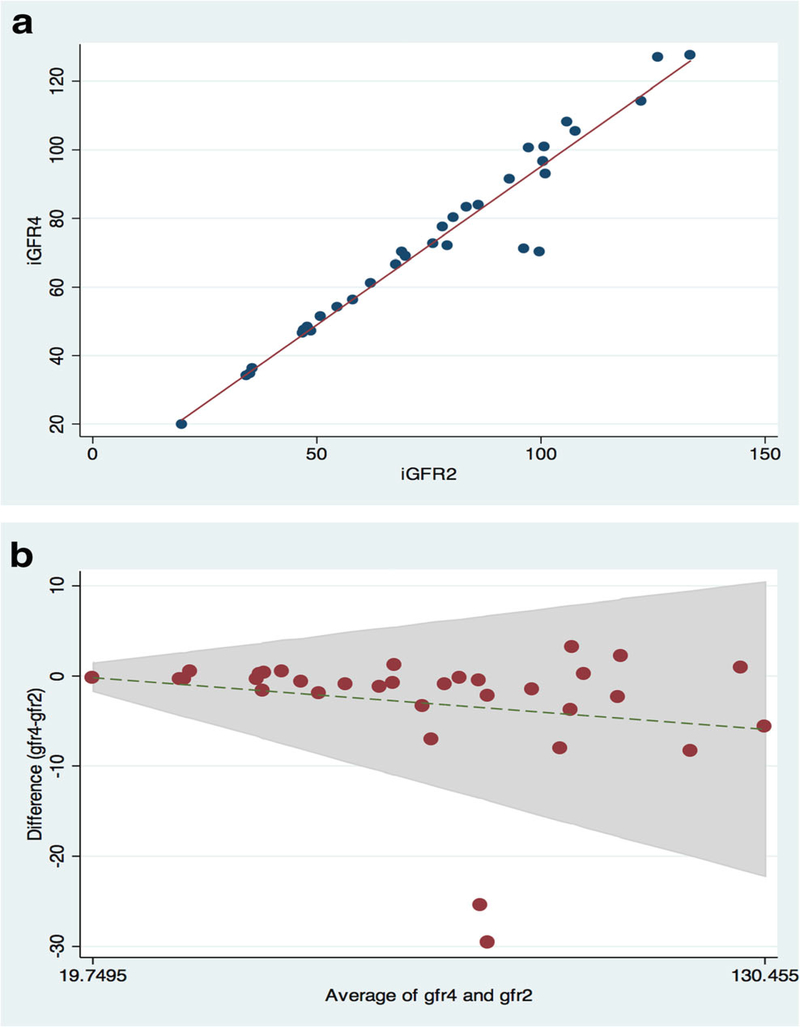
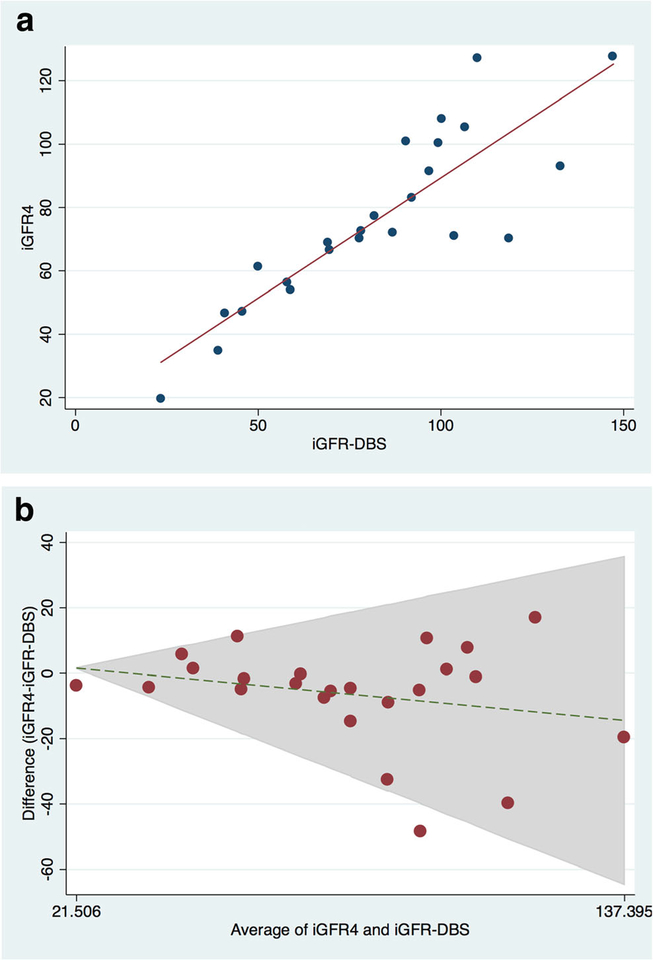
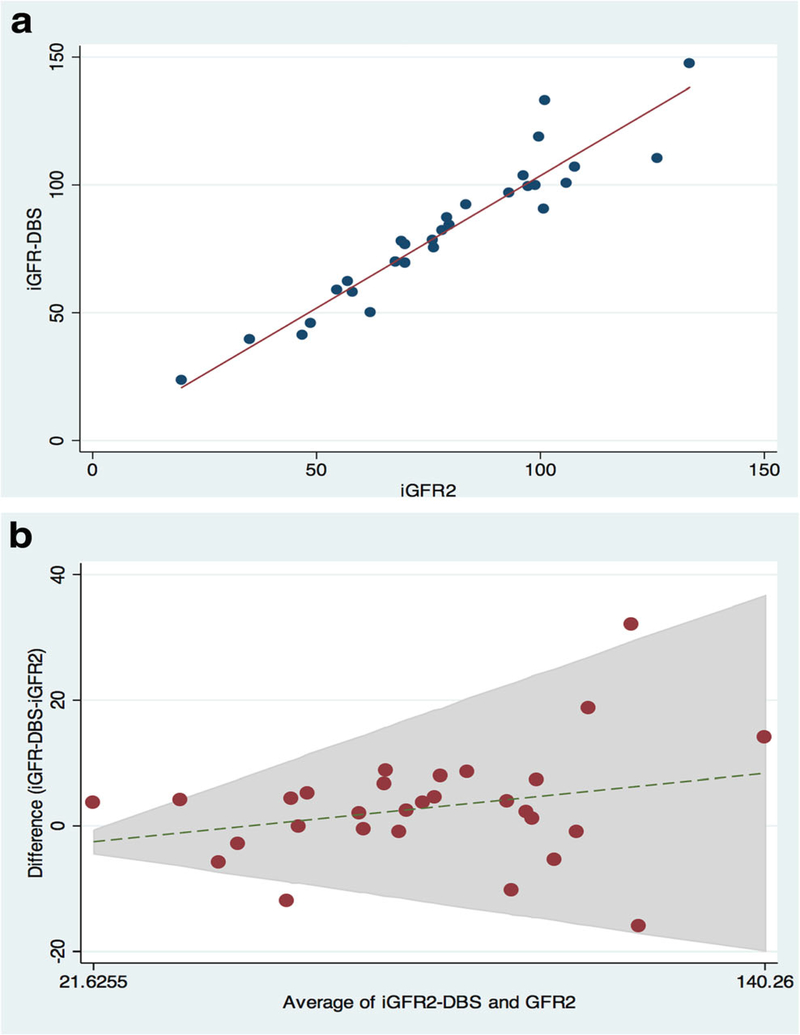
Pearson’s correlation coefficient (r) between iGFR4 and iGFR2 was 0.97 with an insignificant bias of 3.0 ml/min/1.73m2 (95% CI −10.5 – 16.5). Pearson’s correlation coefficient (r) between iGFR2 and iGFR-DBS was 0.95 with an insignificant bias of −2.9 ml/min/1.73m2 (95% CI −20.9 – 15.1). Pearson’s correlation coefficient (r) between iGFR-DBS and iGFR4 was 0.84 with a bias of 6.4 ml/min/1.73m2 (95% CI −24.0–36.8). These bias calculations indicate no significant differences between the paired measurement techniques, although the agreement between iGFR-DBS and iGFR4 was slightly less robust than the other comparison groups. (Table 2).
Table 2.
Pearson’s Correlation, Bias and Accuracy by Comparison Group
| iGFR2 vs. iGFR4 |
iGFR2 vs. iGFR-DBS |
iGFR4 vs. iGFR-DBS |
|
|---|---|---|---|
| Pearson’s correlation coefficient (r) | 0.97 | 0.95 | 0.86 |
| Bias (ml/min/1.73m2) | 3.0 | −2.9 | 6.4 |
| Accuracy 10% | 94 | 80 | 67 |
| Accuracy 30% | 100 | 97 | 88 |
Accuracy (the number of measurements within both 10 and 30% of the comparison group) was assessed in all comparison groups. Ninety four percent (31/33) of the iGFR2 measurements were within 10% of the iGFR4 (100% were within 30%), 80% (23/29) of the iGFR-DBS measurements were within 10% of the iGFR2 (97% (28/29) were within 30%), while 67% (16/24) of the iGFR-DBS measurements were within 10% of the iGFR4 (88% (21/24) were within 30%) (Table 2).
There were noted to be two outliers in the iGFR4 vs. iGFR2/iGFR-DBS comparisons. In both instances, the slope of the initial iohexol clearance was non-standard (one was higher than expected and the other lower). The accuracy of the two point calculation (iGFR2/iGFR-DBS) assumes a standard initial distribution, and without that the iGFR2 or iGFR-DBS may not agree well with the iGFR4. When the Pearson’s correlation coefficient (r) was recalculated between iGFR4 and iGFR2 with the two outliers removed r was 0.99 with a bias of 1.45 (95% CI −3.9 – 6.8) (p<0.001) and an accuracy of 100% within 10%. Additionally, when the Pearson’s correlation coefficient (r) was recalculated between iGFR-DBS and iGFR4 with the two outliers removed r improved to 0.93 with an insignificant bias of 3.35 (95% CI −19.6 – 26.2) and an accuracy of 73% (16/22) within 10% and 95% (21/22) within 30%.
Figures 1a through 3a are scatter plots demonstrating the strong correlation between venous iGFR2 and iGFR4 (Figure 1a), iGFR2 and iGFR-DBS (Figure 2a) and iGFR-DBS and iGFR4 (Figure 3a).Figures 1b through 3b are Bland-Altman representations of the means versus differences between venous iGFR2 and iGFR4 (Figure 1b), iGFR2 and iGFR-DBS (Figure 2b) and iGFR-DBS and iGFR4 (Figure 3b). The two outliers are evident in Figures 1a and 1b.Figures 2a and 2b show good general agreement iGFR2 and iGFR-DBS.Figures 3a and 3b also show good general agreement, with the two outliers again noted.
Figure 1 –
Comparison between 2 point GFR and 4 point GFR
a. Scatter Plot (r 0.97, p <0.001)
b. Bland Altman (bias 3.0 ml/min/1.73m2)
Figure 3 –
Comparison between GFR via dried blood spot and 4 point GFR
a. Scatter Plot (r 0.86, p<0.001)
b. Bland Altman (bias 6.4 ml/min/1.73m2)
Figure 2 –
Comparison between 2 point GFR and GFR via dried blood spot
a. Scatter Plot (r 0.95, p<0.001)
b. Bland Altman (bias −2.9 ml/min/1.73m2)
Discussion
The glomerular filtration rate is the universal measure for kidney health and used to assess individuals for CKD. Accurate measurement of GFR is important for measuring the rate of decline in kidney function, to appropriately adjust medications for decreased clearance, and to provide accurate anticipatory guidance for treatment of comorbid complications of decreased kidney function and renal replacement therapy. The determination of GFR in children and adolescents with autoimmune and oncologic disorders is critical to minimize the effect of nephrotoxic chemotherapeutic agents and thereby minimize renal complications. However, accurate measurement of GFR in children and adolescents can be cumbersome and difficult, due to unique differences between children and adults (variable bladder continence, difficult vascular access, poor cooperation etc.).
Schwartz et al. showed that a 10 point plasma iohexol disappearance accurately determined GFR and could be well approximated by 4 points selected at 10, 30, 120, and 300 minutes[1]. In our study, the 4-point plasma disappearance (iGFR4) curve was compared with a 2-point approximation using time points taken at 120 and 300 minutes (iGFR2); this has been shown to be an effective measurement in both children from the CKiD study as well as adult males from the Multicenter AIDS Cohort Study.[4] The goal of utilizing these 2 points rather than the standard 4 points was to develop a practical, simple but accurate alternative measure of GFR for monitoring kidney function in children and adolescents with CKD or those at risk for renal function decline. As an even less invasive option, we also compared the iohexol GFR using the 2 venous samples (iGFR2) to that determined from the dried blood spots (iGFR-DBS) obtained via finger stick. This latter procedure could ultimately replace the current iohexol GFR procedure and permit even less invasive blood collection.
We have again demonstrated in this pilot study that the 2 point iGFR measurement is comparable to the 4 point iGFR measurement (exceptional agreement and minimal bias), as demonstrated by Ng et al.[4] Additionally the 2 point venous iGFR and 2 point DBS iGFR agree and correlate extremely well, as shown previously in adults by Nicolescu-Duval, suggesting that the 2 point iGFR-DBS is an acceptable surrogate for venous draws when measuring GFR with iohexol disappearance in children and adolescents.[5]
Whereas Salvador et. al. demonstrated good agreement between dried blood spot and venous samples in a younger population, their protocol required using seven venous and between two and four dried blood spot samples.[12] Additionally, they noted the dried blood spot GFR overestimated the venous GFR in subjects with a GFR >60 ml/min/1.73m2.[12] Although our study population was comparatively older, we demonstrate strong agreement and accuracy between both the 4 point iGFR and the 2 point iGFR when compared to the 2 point iGFR-DBS methods (both studies demonstrated accuracy greater than 95% within 30%). Additionally, two dried blood spot samples are likely to be better tolerated by children compared to three or four dried blood spot samples. While we did see a slight overestimation of GFR by dried blood spots in our 2 point comparison, the difference was clinically insignificant (bias −3.3 in those with a GFR >60 vs. −2.9 in all subjects).
Delanaye et. al. published their study comparing a single sample GFR method (the Jacobsson method) versus a multiple sample (a three or four point) method using iohexol or 51Cr-ethylenediaminetetraacetic acid (EDTA).[10] While they did show acceptable agreement with the single sample GFR to GFR by multiple samples, their study did find significantly lower agreement with the 120 minute sample than the 300 minute sample. Additionally, relying on a single measurement point increases the risk of error. Indeed, we saw in our small sample that one errant point (as seen with our two outliers) can change the measurement of GFR considerably. Additionally, the lower concordance at lower GFR’s may render it too unreliable for clinical management decisions, which become more significant as the GFR declines.
Whereas our study utilized non-volumetric samples for dried blood spot collection, Luis-Lima et. al. demonstrated improved accuracy when using volumetric sampling rather than non-volumetric sampling.[11] While they conclude that non-volumetric DBS sampling demonstrated insufficient agreement, they initially found that both collection strategies were insufficient in vitro, and added iopamidol as an internal standard, which improved their agreement to an acceptable level prior to proceeding with the in vivo study of adult patients. Our studies differed in methods. We used two separate collections (one venous and the other finger stick) while Luis-Lima et. al. collected the venous and DBS samples in one collection, then placing the blood onto the filter paper, which could increase the error.
Our study was not without limitations. It was a small pilot study with only 41 participants, and only 29 with complete iGFR2 and iGFR-DBS data. Small subject numbers may have failed to demonstrate a more significant difference between measurement methods. Future studies in a larger cohort of pediatric patients using the two point DBS method could further delineate the applicability, accuracy, and reproducibility of this method. Additionally, combining an accurate DBS method with a single point, such as with the Jacobsson method, might simplify the process further, which may deserve further study.
Conclusion
The iGFR2 is accurate and highly correlated with the iGFR4 as shown previously.[4] Additionally, the finger stick, dried blood spot method (iGFR-DBS) was highly correlated without bias, with the venous phlebotomy (iGFR2 and iGFR4) methods. This is the first study showing such an agreement between 2 and 4 point venous and 2 point dried blood spot GFRs in adolescents and older children. It appears that both venous and finger stick sampling at 2 time points after iohexol infusion provide an acceptable accuracy for GFR measurement.
Acknowledgements
We would like to acknowledge Ms. Paula Maier and Ms. Madeline Murray for their invaluable assistance with data management.
Funding
There were three sites that contributed subjects to the study. The University of New Mexico site was supported by an intramural grant awarded to AS by the UNM Department of Pediatrics and a CTSC grant (UL1TR001449). The University of Rochester site and all the biochemical assays were supported by funds for the Central Biochemistry Laboratory of the CKiD consortium (U01-DK82194). CW receives additional funding from the CKiD consortium (U01-DK066143).
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest. Preliminary results were presented in abstract form at the 16th Congress of the International Pediatric Nephrology Association, September 2013, in Shanghai, China.
Contributor Information
Amy Staples, University of New Mexico, MSC10-5590, 1 University of New Mexico, Albuquerque, NM, 87131.
Craig Wong, University of New Mexico, MSC10-5590, 1 University of New Mexico, Albuquerque, NM, 87131.
George J. Schwartz, University of Rochester, 601 Elmwood Ave, Rochester NY, 14642.
References
- 1.Schwartz GJ, Furth S, Cole SR, Warady B, Munoz A (2006) Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int 69 (11):2070–2077. doi: 10.1038/sj.ki.5000385 [DOI] [PubMed] [Google Scholar]
- 2.Back SE, Krutzen E, Nilsson-Ehle P (1988) Contrast media as markers for glomerular filtration: a pharmacokinetic comparison of four agents. Scand J Clin Lab Invest 48 (3):247–253 [DOI] [PubMed] [Google Scholar]
- 3.Olsson B, Aulie A, Sveen K, Andrew E (1983) Human pharmacokinetics of iohexol. A new nonionic contrast medium. Invest Radiol 18 (2):177–182 [DOI] [PubMed] [Google Scholar]
- 4.Ng DK, Schwartz GJ, Jacobson LP, Palella FJ, Margolick JB, Warady BA, Furth SL, Munoz A (2011) Universal GFR determination based on two time points during plasma iohexol disappearance. Kidney Int 80 (4):423–430. doi: 10.1038/ki.2011.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niculescu-Duvaz I, D’Mello L, Maan Z, Barron JL, Newman DJ, Dockrell ME, Kwan JT (2006) Development of an outpatient finger-prick glomerular filtration rate procedure suitable for epidemiological studies. Kidney Int 69 (7):1272–1275. doi: 10.1038/sj.ki.5000240 [DOI] [PubMed] [Google Scholar]
- 6.Krutzen E, Back SE, Nilsson-Ehle P (1990) Determination of glomerular filtration rate using iohexol clearance and capillary sampling. Scand J Clin Lab Invest 50 (3):279–283 [DOI] [PubMed] [Google Scholar]
- 7.Silkalns GI, Jeck D, Earon J, Edelmann CM Jr, Chervu LR, Blaufox MD, Spitzer A (1973) Simultaneous measurement of glomerular filtration rate and renal plasma flow using plasma disappearance curves. J Pediatr 83 (5):749–757 [DOI] [PubMed] [Google Scholar]
- 8.Haycock GB, Schwartz GJ, Wisotsky DH (1978) Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 93 (1):62–66 [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20 (3):629–637. doi: 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delanaye P, Flamant M, Dubourg L, Vidal-Petiot E, Lemoine S, Cavalier E, Schaeffner E, Ebert N, Pottel H (2018) Single versus multiple-sample method to measure glomerular filtration rate. Nephrol Dial Transplant. doi: 10.1093/ndt/gfx345 [DOI] [PubMed] [Google Scholar]
- 11.Luis-Lima S, Gaspari F, Negrin-Mena N, Carrara F, Diaz-Martin L, Jimenez-Sosa A, Gonzalez-Rinne F, Torres A, Porrini E (2017) Iohexol plasma clearance simplified by dried blood spot testing. Nephrol Dial Transplant. doi: 10.1093/ndt/gfx323 [DOI] [PubMed] [Google Scholar]
- 12.Salvador CL, Tondel C, Morkrid L, Bjerre A, Brun A, Bolann B, Brackman D, Bergan S (2015) Glomerular filtration rate measured by iohexol clearance: A comparison of venous samples and capillary blood spots. Scand J Clin Lab Invest 75 (8):710–716. doi: 10.3109/00365513.2015.1091091 [DOI] [PubMed] [Google Scholar]