Abstract
BACKGROUND
Pulmonary exacerbations (PEx) are associated with increased morbidity and mortality in individuals with CF. PEx management practices vary widely, and optimization through interventional trials could potentially improve outcomes. The object of this analysis was to evaluate current physician treatment practices and patient outcomes for PEx.
METHODS
The Standardized Treatment of Pulmonary Exacerbations (STOP) observational study enrolled 220 participants ≥12 years old admitted to the hospital for PEx at 11 U.S. CF centers. Spirometry and daily symptom scores were collected during the study. Physicians were surveyed on treatment goals and their management practices were observed. Treatment outcomes were compared to stated goals.
RESULTS
The mean (SD) duration of IV antibiotic treatment was 15.9 (6.0) days. Those individuals with more severe lung disease (<50% FEV1) were treated nearly two days longer than those with >50% FEV1. Physician-reported FEV1 improvement goals were 10% (95% CI: 5%, 14%) lower for patients with 6-month baseline FEV1 ≤50% predicted compared with those with 6-month baseline FEV1 >50% predicted. There were clinically and statistically significant improvements in symptoms from the start of IV antibiotic treatment to the end of IV antibiotic treatment and 28 days after the start of treatment. The mean absolute increase in FEV1 from admission was 9% predicted at end of IV antibiotic treatment, and 7% predicted at Day 28. Only 39% fully recovered lost lung function, and only 65% recovered at least 90% of lost lung function. Treatment was deemed successful by 84% of clinicians, although 6-month baseline FEV1 was only recovered in 39% of PEx.
CONCLUSIONS
In this prospective observational study of PEx, treatment regimens and durations showed substantial variation. A significant proportion of patients did not reach physician’s treatment goals, yet treatment was deemed successful.
Keywords: cystic fibrosis, pulmonary exacerbations, physician treatment practices, antibiotic therapy
INTRODUCTION
Pulmonary exacerbations (PEx) occur frequently in individuals with cystic fibrosis (CF), and are associated with loss of lung function (forced expiratory volume over one second [FEV1]), decreased survival, and worsened quality of life(1–9). A systematic review of PEx found insufficient evidence upon which to base recommendations on duration of antibiotic therapy, number of antibiotics to use, use of systemic corticosteroids, and site of treatment (home versus hospital)(10–12). Current practices for treatment of PEx vary widely for key treatment decisions such as these(9,10,13–16). In the US, the median duration of treatment with intravenous (IV) antibiotics for a PEx is 13.1 days for individuals < 18 years old and 14.0 days for those > 18 years old(11). However, there is a wide variation, with the median duration at individual centers varying from 4.0–21.0 days across pediatric programs, and 4.0–23.5 days across adult programs(11). An acute or sub-acute drop in lung function is a typical feature of a PEx and a significant proportion of patients do not fully recover lost lung function following treatment(1,9,17–19). A delayed or suboptimal treatment is one possible explanation for the lack of complete recovery. The wide variance in current treatment practices presents an opportunity to determine which practices are most efficacious.
The Standardized Treatment of Pulmonary Exacerbations in Patients with Cystic Fibrosis (STOP) study (clinicaltrials.gov: NCT02109822) was performed with the purpose of identifying clinical endpoints that could be used in future investigation of treatments for PEx. The objective of this analysis was to describe the treatment practices for patients with CF admitted to the hospital for a PEx, and the outcomes associated with this treatment. In addition, we identified the a priori goals of the admitting clinician(20) and compared outcomes at the end of treatment according to initial physician treatment goals in order to identify optimal treatment regimens.
METHODS
STOP was an observational cohort study that enrolled 220 participants with CF from 11 centers that were admitted to the hospital for treatment of PEx from January 2014 to January 2015. A complete description of the study methods are described elsewhere(20). Patients were ≥ 12 years of age and currently hospitalized for treatment of a PEx with IV antibiotics. Complete inclusion/exclusion criteria and physician surveys are listed in the online supplement. Duration and choice of IV antibiotics were determined by the treating physician and observed. A survey was performed on day 1 that captured whether the treating physician’s primary goal was to recover lung function or to improve symptoms, and a target FEV1 that would constitute a treatment success was recorded. Spirometry was assessed at admission, day 7, end of IV treatment, and at 28 days after the start of IV treatment. FEV1% predicted was calculated using Global Lung Initiative equations(21). Symptoms were assessed daily using the Cystic Fibrosis Respiratory Symptom Diary and Chronic Respiratory Infection Symptom Score (CFRSD-CRISS), with total scores ranging from 0–100, where a higher score indicates greater symptom severity(22). A change of 11 units is considered clinically significant(23). At day 28, we asked clinicians if they considered the treatment a success (though we did not designate a definition of success) to evaluate the durability of treatment. This study design is unique in combining this prospective data with data from the Cystic Fibrosis Foundation Patient Registry (CFFPR), which was accessed to obtain retrospective data.
Descriptive statistics were used to summarize demographics, symptom duration and distribution, and spirometry at the time of enrollment. FEV1 % predicted was compared to historical values recorded in the CFFPR. Change from historical baseline (best in a 6 or 12-month period) and admission assessment were calculated at completion of IV therapy and day 28, and then compared to clinical characteristics, treatment practices, and treatment goals defined by the admitting clinician. T-tests were used to compare continuous variables by treatment goals and demographic values at baseline. Proportions were compared via Fisher’s exact test with corresponding 95% confidence intervals (CI) derived using the Newcombe-Wilson method. For sensitivity analyses, missing visit 3 data were imputed using last observation carried forward (LOCF) method to estimate effects of missing data on change from admission outcomes. Analyses were performed using SAS (version 9.4, SAS Institute Inc., Cary, NC, 2013), and R (versions 3.2.1, The R Foundation for Statistical Computing, Vienna, Austria, 2015). This study was approved by each of the participating center’s Institutional Review Board and all participants or guardians provided written informed consent and assent where required.
RESULTS
Baseline Demographics
Key demographic data, duration of symptoms, presenting PEx features, and historic lung function are described elsewhere(20). Briefly, 220 patients were enrolled (56% female), with a mean (SD) age 26.3 (9.5) years, 19% of which were adolescents (12–17 years old).
Treatment Practices
All patients were treated with IV antibiotics, and the mean (SD) duration of antibiotic treatment was 15.9 (6.0) days (range 2–51). Mean duration of antibiotic treatment for individuals <18 years was 14.5 (4.8) days and >18 years was 16.2 (6.2) days. This difference was not statistically significant (difference = 1.6%, 95% CI: −0.5, 3.8, p=0.14). Eleven percent of individuals were treated with antibiotics for ≤10 days (17% of pediatric patients, 10% of adult patients); 29% were treated between 11–14 days (28% of pediatric patients, 29% of adult patients); 60% were treated for >14 days (56% of pediatric patients, 61% of adult patients). Those individuals with more severe lung disease (best FEV1 in the 6 months’ prior <50% predicted) were treated nearly two days longer than those individuals with best FEV1 in the 6 months prior >50% predicted (16.7 vs. 14.8 days, difference = 1.9 days, 95% CI: 0.1, 3.7, p=0.04). There was no difference in duration of IV antibiotics in patients with Pseudomonas aeruginosa (Pa) or methicillin-resistant Staphylococcus aureus. The mean duration of hospital stay was 11.3 (5.6) days (range 3–36). Approximately half of patients completed antibiotic therapy in the hospital and the rest completed antibiotics at home.
Ten percent of participants were also prescribed inhaled antibiotics and 32% were prescribed one or more oral antibiotics. The addition of oral antibiotics to the IV antibiotic regimen was more common in the pediatric population (45% vs. 29% for adult patients, difference = 16%, 95% CI: 0%, 32%, p=0.065). For IV antibiotics, 54% were prescribed 2 antibiotics and 35% were given ≥3 antibiotics (Supplement Table 1). Thirteen percent of participants experienced a change in IV antibiotic regimen during treatment. Patients with Pa isolated in the 6 months prior to admission were more likely to be treated with IV tobramycin than those without Pa (71% vs. 40%, difference=31%, 95% CI: 17%, 44%). Additionally, 16% of Pa-positive patients were treated with IV colistimethate. Finally, 21% of patients were treated with corticosteroids.
Treatment Outcomes
The mean (SD) absolute increase in FEV1 from admission was 9% (10%) predicted at end of IV antibiotic treatment and 7% (11%) predicted at day 28. Figure 1 shows the mean absolute changes from the best FEV1 in the previous 6 months to admission, day 7, end of IV antibiotic treatment, and to day 28, categorized according to the primary goal of the treatment. There was greater lung function improvement in those patients for whom the treatment goal was lung function recovery compared to those whose goal was relief of symptoms (improvement of 10% vs 4% from admission to Day 28, difference = 6% predicted, 95% CI: 3%, 9%, p<0.001). However, these patients had a greater absolute drop in lung function at admission from their best measure in the 6 months prior (drop of 12% vs 7%, difference = 5% predicted, 95% CI: 2%, 9%, p=0.004). Patients with their best FEV1 in 6 months’ prior >50% predicted experienced a greater recovery of FEV1 from admission to Day 28 than those with 6-months best <50% predicted (10% vs 3%, diff=7%, 95% CI: 3%, 10%). Figure 2 shows the percentage of patients who recovered their lost lung function at Day 28 using different percentages of their best FEV1 in the previous 6 months. It is notable that a minority (39%) fully recovered lost lung function, and only 65% of patients recovered at least 90% of lost lung function.
Figure 1.
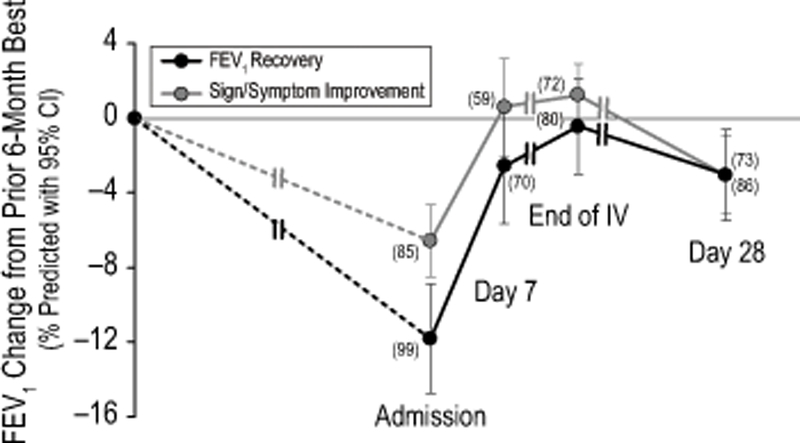
Absolute changes from the best FEV1 % predicted in the 6 months prior to admission compared to admission, day 7, end of IV antibiotic treatment, and day 28 FEV1 % predicted. Black lines represent the individuals whose treatment goal was lung function recovery, and gray line represents the individuals whose treatment goal was improvement in signs and symptoms. Time intervals between visits vary by subject (as noted by double hashes), as end of IV antibiotic treatment varied by subject.
Figure 2.
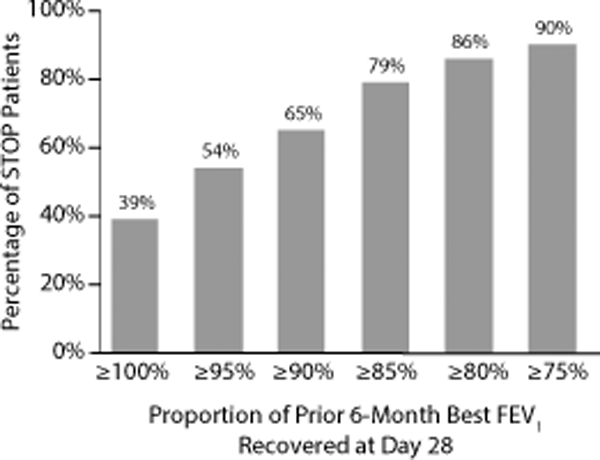
Percentage of STOP patients who recovered the specified proportion of their baseline lung function (defined as prior 6-month best FEV1) at day 28. For example, only 67% of individuals recovered 90% of their baseline lung function.
Symptoms improved from the start of IV antibiotic treatment with a mean (SD) CFRSD-CRISS 47.5 (11.2) to 21.5 (15.7) at the end of IV antibiotic therapy, difference = –26.1 (95% CI: –28.3, –23.8, p<.001) (Figure 1a,1b online supplement). The average improvement in symptoms regressed slightly by Day 28, but this difference was not clinically significant. Patients with their best FEV1 in 6 months’ prior >50% predicted experienced a greater reduction in symptoms, as measured by the CFRSD-CRISS (23.6 vs 15.4, diff=8.3, 95% CI: 2.8, 13.7). Eighty-three percent of patients achieved the clinically significant 11-point CRISS improvement(23) at end of IV antibiotic treatment, and 75% of all patients experienced this improvement at Day 28 (Figure 1a,1b, online supplement). Individuals with baseline FEV1 <50% predicted were less likely to achieve the 11-point improvement by day 28 than those with baseline FEV1 >50% predicted (68% versus 77%, difference= –8%, 95% CI: –23%, 6%, p=.33). There was no difference in symptom reduction over the course of 28 days by baseline FEV1 or specified treatment objectives. Treatments identified as ‘successes’ had a larger mean symptom reduction from admission to Day 28 than treatments identified as ‘not successes’ (22.6 vs. 12.3, 95% CI: 0.7, 19.9, p=0.036).
Importantly, patients with historical FEV1 data had admission FEV1 values greater than their best FEV1 value recorded in the 6 (19.6% of patients) and 12 (12.1%) months prior to admission. The 28 patients with admission FEV1 values exceeding their best prior 6-month FEV1 experienced no change in FEV1 and smaller average CFRSD-CRISS changes from admission to Day 28: 0.93% predicted [95% CI –1.18, 3.05] and –12.25 [–17.99, –6.51], respectively, Figure 3.
Figure 3.
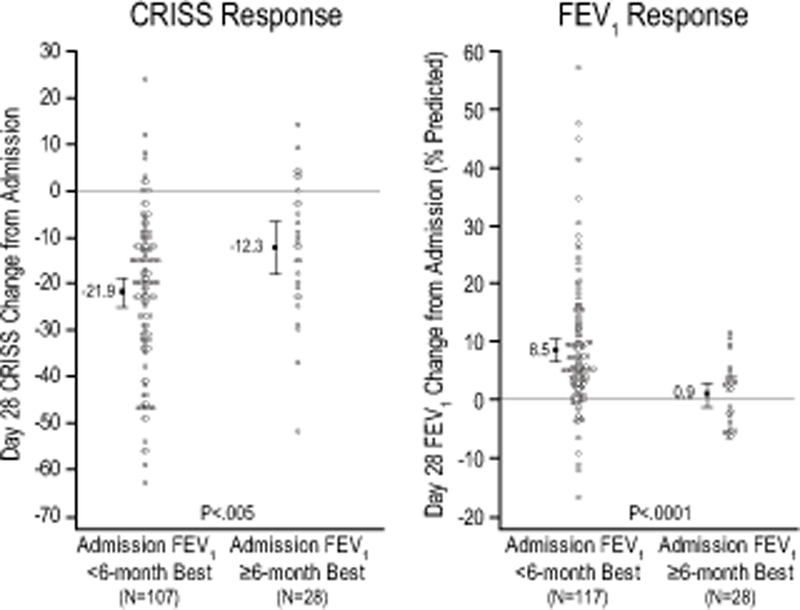
CRISS and FEV1 response from admission to day 28 in patients whose admission FEV1 was < 6 month best FEV1 compared to patients whose admission FEV1 was > 6 month best FEV1.
End of treatment assessment
According to the treating physicians, the primary reason for stopping IV therapy was end of planned treatment duration for 31% of events, signs and symptoms resolved for 36%, FEV1 returned to established target for 24%, patient preference for 6%, and establishment of a new baseline for 2%. For participants with the primary goal listed as FEV1 recovery, Figure 4 compares Day 28 FEV1 to best FEV1 % predicted 12 months prior, 6 months prior, admission and target FEV1 by physician assessment of treatment outcome (success vs. non-success). The participants whose treatment was considered a ‘success’ by their physician had a greater improvement in Day 28 lung function as compared to admission than those whose treatment was considered a ‘non-success’ (p=0.002). The other differences were not significant. Success vs. non-success results are also compared between adolescent and adult patients (Table 2, online supplement). Among 35% of patients that failed to recover 90% of best FEV1 % predicted in 6 months’ prior, the treatment was reported by their physicians as stopped for the following reasons: 34% reached the end of planned treatment duration, 23% resolved signs and symptoms; 30% had FEV1 return to established target, 9% due to patient preference, and 4% established a new baseline.
Figure 4.

Among participants with lung function recovery as the primary goal listed, this figure compares absolute difference in FEV1 % predicted from Day 28 spirometry measurement to the best measurement in the 12 months prior and 6 months prior, admission, and target FEV1. The groups are separated by treatment outcome (success vs non-success).
Among the 116 patients whose physicians had reported a target FEV1, the mean (SD) FEV1 at the end of therapy was 5.0 (10.0) predicted below that target. Only 61% of individuals reached ≥90% of their target FEV1 at the end of IV therapy, yet 87% of physicians felt their patients had an improvement in lung function from admission at the end of therapy, and 84% deemed PEx treatment a success at Day 28. Among subjects whose treatment was deemed a success, mean Day 28 FEV1 % predicted was 5% below the target, compared to 11% for those whose treatment was not successful, (difference = 6%, 95% CI: −2%, 13%: p=0.135). Among patients with baseline FEV1 <50% predicted, treatment was less likely to have been deemed a success compared to those with baseline FEV1 >50% predicted (72% versus 90%, respectively, 95% CI: – 31%, –7%, p=0.003).
Sensitivity analyses were completed to account for missing data, to assess whether this affected outcomes, using the LOCF method. The number of evaluable FEV1 measures increased from 160 (72.7%) to 203 (92.3%) and evaluable CFRSD-CRISS measures increased from 158 (71.8%) to 209 (95.0%). Missing data were observed to be fairly evenly distributed across the treatment population as a function of age, lung function, or sex, CF-related diabetes status, or microbiology data (Table 3, online supplement), and imputation of missing day 28 data by LOCF method produced little effect on change from admission FEV1 and CRISS statistics (Figure 5).
Figure 5.

Change in CRISS and FEV1 from admission to day 28 in patients with all data available compared to available and imputed data combined. Missing data was computed using the Last Observation Carried Forward (LOCF) method.
DISCUSSION
This study corroborates previous reports of the wide variety of treatment practices for PEx, specifically with respect to the wide variation in duration of IV antibiotic therapy (median duration at individual centers varies from 4.0–23.5 days(11)). There was no standardization on the site of completion of IV antibiotics, with half of patients completing their therapy in the hospital. Furthermore, there was a broad range in choice of antibiotic therapies, with multiple routes of administration (inhaled, oral, and IV), and number of antibiotics prescribed. This study adds to previous reports by identifying clinician goals and the rationale for treatment decisions in comparison to PEx outcomes. Both lung function and symptoms improved during PEx treatment, but the improvement in lung function often fell short of previous baseline values and clinician goals. Nonetheless, PEx treatments were generally described as successful. We required a study visit at the end of IV therapy as well as at day 28 in order to compare potential endpoints for a future study, but also to determine the durability of treatment response. In clinical practice, success or failure of treatment is usually assessed at the end of IV antibiotic therapy. However, very little is known what happens in the day to weeks after treatment has ended, and whether the response to IV antibiotics is sustained.
The finding that patient symptoms improve during treatment of an exacerbation is not surprising, and unfortunately symptoms begin to worsen on average within 2 weeks after completing therapy. We do not know whether a patient’s symptoms returned to baseline, as the CFRSD-CRISS is not routinely measured. However, when we use an 11-point minimal clinically important difference (MCID) change in CFRSD-CRISS(22,23), we can see that there is a subset of patients that do not achieve this goal at end of treatment (17%), or at day 28 (25%). Patients are also known to have lost FEV1 at the time of PEx diagnosis when compared to their highest values recorded in the past 12 months. Our data demonstrate this loss to be substantial, approximately 14% predicted on average. When clinicians defined recovery of lost lung function as the primary goal of therapy, their established targets of FEV1 were comparable to the best lung function measured in the past year, suggesting they aspire to complete recovery. Similar to other reports, most patients do not fully recover their lost lung function(1,17,18,24). It is likely that some patients only present to clinic when they are sick, making it likely that the peak FEV1 recorded in the previous 6–12 months is not representative of their true best FEV1. Therefore, the extent of failure of treatment to reach baseline is likely underestimated by these data. Furthermore, what is worrisome is that despite patients falling short of a target lung function goal, clinicians generally characterized treatments as a success. Moreover, clinicians did not acknowledge that their patients had not achieved the prospectively declared target lung function or that they were accepting a new baseline lung function. It is interesting to note that ~20% of individuals had an admission FEV1 that was higher than their best FEV1 recorded in the previous 6 months. This group had an overall lower FEV1 response, which might have been expected, but also a lower CRISS response, than those patients whose admission FEV1 was lower than the best FEV1 in the previous 6 months.
The accelerated loss of lung function associated with a PEx(1,17,18,24) suggests either that we need to prevent such events or improve our approaches to treatment. It is encouraging that physicians in this study expressed a general willingness to enroll patients in different interventional studies of PEx, as indicated in the accompanying manuscript by Sanders et al(20). Additionally, this study allowed evaluation of several possible clinical endpoints that could be used in a large study, and the importance of carefully selected meaningful efficacy measures is further discussed by the accompanying manuscript by VanDevanter at al(25).
There are some limitations to the generalizability of this study. It will be necessary to survey other clinicians as to their approaches to care and their willingness to enroll patients in a study of treatment of PEx. Although we assessed clinicians’ willingness to enroll their patients in an intervention study, we did not query those same patients with respect to their interest in participating in such trials. This would be an important next step. Another limitation is that any additional oral and/or inhaled antibiotics that might have been given after the end of IV antibiotics treatment was not recorded, which may have impacted the 28-day outcomes. Lastly, while there were some missing data observed, that was little evidence that missing day 28 data from STOP substantially biased outcome statistics based on LOCF imputation.
In conclusion, the results of the STOP study showed that patients generally have a substantial amount of lung function to recover with PEx treatment, and while clinicians aim to fully recover that loss, most patients will not achieve that goal, yet their treatments will be deemed successful. Even when observed, physicians made decisions that were inconsistent with their original plans, often failing to achieve treatment goals. There is considerable variation in physician treatment practices in regards to duration, choice, and location of antibiotic therapy, which may play a role in the lack of full recovery of lung function. Identifying best practices and standardizing PEx treatment in a future interventional trial has the potential to improve outcomes. The results from this study will be used to design a large interventional trial in treatment of pulmonary exacerbations, with the goal to establish concrete evidence-based guidelines that can improve outcomes in CF.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the patients and their families who participated in the clinical study, as well as the participating sites (investigator, lead research coordinator): Arizona Health Services (Cori Daines, Osmara Molina de Rodriguez); Case Western Reserve University (Elliott Dasenbrook, David Weaver and Bobbi Ksenich); Children’s Hospital of Pittsburgh (Jonathan Spahr, Elizabeth Hartigan); Johns Hopkins University (Natalie West, Abigail Thaxton); Medical University of South Carolina (Patrick Flume, Elizabeth Poindexter); Seattle Children’s Hospital (Ron Gibson, Sharon McNamara); University of Alabama at Birmingham (George M. Solomon, Heather Hathorne, Katie Brand); National Jewish Health, Denver (Jerry Nick, Katie Poch); University of Texas Southwestern (Raksha Jain, Ashley Keller); University of Washington (Christopher Goss, Ellen Wilhelm); University of Wisconsin, Madison (Don Sanders, Linda Makholm).
We thank the Cystic Fibrosis Foundation for the coordinated efforts to collect study data through the CF Foundation Patient Registry (Alex Elbert, Christopher Dowd, and Bruce Marshall). We also recognize the anonymous peer reviewers from the CF Therapeutics Development Network Publication Committee for their contributions in strengthening this manuscript.
FUNDING
This study was supported by grants from the Cystic Fibrosis Foundation Therapeutics (SANDERS14A0, HELTSH13A1, GOSS13A0, FLUME13A1, CLANCY09Y0, SORSCH15RO, ORENST14Y0, NICKR0, DAINES14Y0), the National Institutes of Health (KL2 TR000428, P30 DK089507), and the University of Wisconsin-Madison ICTR (NIH UL1 TR000427). This project was also supported by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina through National Institutes of Health grant UL1TR001450.The study sponsors had no role in study design, analysis, the writing of this manuscript, or the decision to submit for publication.
Abbreviations:
- CF
Cystic fibrosis
- CFFPR
Cystic Fibrosis Foundation Patient Registry
- CFRSD-CRISS
Cystic Fibrosis Respiratory Symptom Diary - Chronic Respiratory Infection Symptom Score Questionnaire
- CI
Confidence Interval
- FEV1
Forced expiratory volume at 1 second
- IV
Intravenous
- LOCF
Last observation carried forward
- MCID
Minimal clinically important difference
- Pa
Pseudomonas aeruginosa
- PEx
Pulmonary exacerbation
- SD
Standard deviation
- STOP
Standardized Treatment of Pulmonary exacerbations
Footnotes
Conflicts of interest: none
REFERENCES
- 1.Sanders DB, Hoffman LR, Emerson J, Gibson RL, Rosenfeld M, Redding GJ, et al. Return of FEV1 after pulmonary exacerbation in children with cystic fibrosis. Pediatr Pulmonol. 2010. February;45(2):127–34. [DOI] [PubMed] [Google Scholar]
- 2.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001. February 15;153(4):345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002. December 15;166(12 Pt 1):1550–5. [DOI] [PubMed] [Google Scholar]
- 4.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002. August;34(2):91–100. [DOI] [PubMed] [Google Scholar]
- 5.Ellaffi M, Vinsonneau C, Coste J, Hubert D, Burgel PR, Dhainaut JF, et al. One-year outcome after severe pulmonary exacerbation in adults with cystic fibrosis. Am J Respir Crit Care Med. 2005. January 15;171(2):158–64. [DOI] [PubMed] [Google Scholar]
- 6.de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax. 2011. August;66(8):680–5. [DOI] [PubMed] [Google Scholar]
- 7.Konstan MW, Wagener JS, VanDevanter DR, Pasta DJ, Yegin A, Rasouliyan L, et al. Risk factors for rate of decline in FEV1 in adults with cystic fibrosis. J Cyst Fibros. 2012. September;11(5):405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of Recent Pulmonary Exacerbations on Quality of Life in Patients With Cystic Fibrosis. Chest. 2002. January;121(1):64–72. [DOI] [PubMed] [Google Scholar]
- 9.Wagener JS, Rasouliyan L, VanDevanter DR, Pasta DJ, Regelmann WE, Morgan WJ, et al. Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2013. July;48(7):666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flume PA, Mogayzel PJ, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, et al. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009. November 1;180(9):802–8. [DOI] [PubMed] [Google Scholar]
- 11.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry, 2014 Annual Data Report. Bethesda, Maryland: ©2015. Cystic Fibrosis Foundation. [Google Scholar]
- 12.Collaco JM, Green DM, Cutting GR, Naughton KM, Mogayzel PJ. Location and duration of treatment of cystic fibrosis respiratory exacerbations do not affect outcomes. Am J Respir Crit Care Med. 2010. November 1;182(9):1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraynack NC, Gothard MD, Falletta LM, McBride JT. Approach to treating cystic fibrosis pulmonary exacerbations varies widely across US CF care centers. Pediatr Pulmonol. 2011. September;46(9):870–81. [DOI] [PubMed] [Google Scholar]
- 14.VanDevanter DR, O’Riordan MA, Blumer JL, Konstan MW. Assessing time to pulmonary function benefit following antibiotic treatment of acute cystic fibrosis exacerbations. Respir Res. 2010. October 6;11:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.VanDevanter DR, Flume PA, Morris N, Konstan MW. Probability of IV antibiotic retreatment within thirty days is associated with duration and location of IV antibiotic treatment for pulmonary exacerbation in cystic fibrosis. J Cyst Fibros. 2016. November;15(6):783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schechter MS, Regelmann WE, Sawicki GS, Rasouliyan L, VanDevanter DR, Rosenfeld M, et al. Antibiotic treatment of signs and symptoms of pulmonary exacerbations: A comparison by care site. Pediatr Pulmonol. 2015;50(5):431–40. [DOI] [PubMed] [Google Scholar]
- 17.Sanders DB, Bittner RC, Rosenfeld M, Redding GJ, Goss CH. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol. 2011. April;46(4):393–400. [DOI] [PubMed] [Google Scholar]
- 18.Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010. September 1;182(5):627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanojevic S, McDonald A, Waters V, MacDonald S, Horton E, Tullis E, et al. Effect of pulmonary exacerbations treated with oral antibiotics on clinical outcomes in cystic fibrosis. Thorax [Internet]. 2016. September 9; Available from: http://thorax.bmj.com/content/early/2016/09/09/thoraxjnl-2016-208450.abstract [DOI] [PubMed] [Google Scholar]
- 20.Sanders DB, Solomon GM, Beckett VV, Daines CL, Heltshe SL, VanDevanter DR, et al. Standardized treatment of pulmonary exacerbations (STOP) study: observations at the initiation of intravenous antibiotics for cystic fibrosis pulmonary exacerbations. J Cyst Fibros Submitt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012. December;40(6):1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goss CH, Edwards TC, Ramsey BW, Aitken ML, Patrick DL. Patient-reported respiratory symptoms in cystic fibrosis. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2009. July;8(4):245–52. [DOI] [PubMed] [Google Scholar]
- 23.Goss CH, Caldwell E, Gries K, Leidy N, Edwards T, Flume PA, et al. Validation of a novel patient-reported respiratory symptoms instrument in cystic fibrosis: CFRSD-CRISS. In Pediatr Pulmonol; 2013. p. A251. [Google Scholar]
- 24.Heltshe SL, Goss CH, Thompson V, Sagel SD, Sanders DB, Marshall BC, et al. Short-term and long-term response to pulmonary exacerbation treatment in cystic fibrosis. Thorax. 2016. March;71(3):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanDevanter DR, Heltshe SL, Spahr JE, Beckett VV, Daines CL, Dasenbrook EC, et al. Rationalizing Endpoints For Prospective Studies Of Pulmonary Exacerbation Treatment Response In Cystic Fibrosis. J Cyst Fibros. Submitted; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


