Abstract
Main sources of manganese (Mn) in the general population are diet and drinking water. Mn is also found in ethylene bisdithiocarbamate (EBDC) fungicides used in agriculture or emitted into the air by ferromanganese plants and welding fumes, which can be additional environmental and occupational sources of exposure. High occupational Mn exposure has been linked with motor, behavioral, and cognitive impairment, but its effects on neural function remain poorly understood. We conducted a functional neuroimaging study in a sample of 48 farmworkers in Zarcero County, Costa Rica, an agricultural region where EBDC fungicides are sprayed. We measured Mn concentrations in farmworkers’ toenails (n = 40 farmworkers) and hair (n = 33 farmworkers), and recorded brain activity in the dorsolateral prefrontal cortex during a letter-retrieval working memory task using functional near-infrared spectroscopy (fNIRS). We estimated exposure-outcome associations using multivariable linear regression models adjusted for age and education level. Geometric mean (geometric standard deviation) toenail and hair Mn concentrations were 0.40 μg/g (3.52) and 0.24 μg/g (3.54), respectively. We did not find strong evidence that Mn concentrations were associated with working memory-related brain activity in this sample of farmworkers; we also found null associations between working memory task accuracy and brain activity. However, our small sample size may have limited our ability to detect small effect sizes with statistical precision. Our study demonstrates that fNIRS can be a useful and feasible tool in environmental epidemiology for examining the effects of toxicants, like Mn, on neural function. This may prove to be important for elucidating neuropathological pathways that underlie previously reported associations of elevated Mn exposure with neurotoxic effects.
Keywords: Manganese, Mancozeb, Pesticides, Farmworkers, Neuroimaging, Functional near-infrared spectroscopy, Working memory, Costa Rica
1. Introduction
Manganese (Mn) is a naturally-occurring element that is found ubiquitously in the environment. Though an essential nutrient for healthy cellular functioning, Mn can be neurotoxic at high levels (Agency for Toxic Substances and Disease Registry, 2012). Main sources of Mn for the general population are diet, such as nuts and tea, and drinking water (Agency for Toxic Substances and Disease Registry, 2012). Mn is also emitted into the air by ferromanganese plants (Bowler et al., 2015) and welding fumes (Chang et al., 2010), and is found in ethylene bisdithiocarbamate (EBDC) fungicides, such as mancozeb and maneb, which can contaminate drinking water (van Wendel de Joode et al., 2016). Previous occupational studies have found increased urinary levels of Mn (Canossa et al., 1993) and ethylenethiourea (ETU) (Runkle et al., 2013), the main metabolite of EBDCs, among farm-workers following mancozeb applications on agricultural fields. Another study found increased blood serum levels of Mn in farmworkers exposed to mancozeb compared to unexposed workers (Rocha et al., 2015). Environmental studies in communities living near agricultural fields have also found that closer residential proximity to agricultural use of EBDC fungicides was associated with increased Mn levels in pregnant women’s hair (Mora et al., 2014), prenatal dentin from children’s shed teeth (Gunier et al., 2013), and home dust samples (Gunier et al., 2014). Exposure to Mn from EBDC fungicides is therefore a particular concern among farmworkers and individuals living in proximity to agricultural fields.
Elevated Mn exposure has been linked with motor (Perl and Olanow, 2007), behavioral (Bouchard et al., 2007b; Donaldson, 1987), and cognitive impairment (Al-Lozi et al., 2017; Bowler et al., 2007), primarily in occupational studies of welders exposed to Mn from welding fumes. To our knowledge, there has been only one occupational study of Mn exposure in adult farmworkers, which reported higher prevalence of headache, fatigue, nervousness, memory complaints, and sleepiness in workers exposed to the EBDC fungicide maneb compared to unexposed workers (Ferraz et al., 1988). Much of the evidence for the impact of Mn exposure on cognitive abilities comes from studies of prenatal and early-life exposures (Coetzee et al., 2016). Though developmental exposure likely affects the brain differently than exposure during adulthood (Rice and Barone, 2000), these studies provide additional support that overexposure to Mn has an adverse impact on the brain.
Functional neuroimaging techniques, such as functional magnetic resonance imaging (fMRI) and functional near-infrared spectroscopy (fNIRS), measure localized changes in cerebral blood flow related to brain activity at the neural level and have the potential to elucidate the neuropathological pathway through which chemical exposures affect the brain (Boas et al., 2014; Horton et al., 2014). While fMRI is considered the gold standard in functional neuroimaging due to its high spatial resolution, fNIRS is emerging as a convenient and low-cost alternative, especially when working in remote regions (Baker et al., 2017). Although fNIRS has a lower signal-to-noise ratio than fMRI, the two methods have been found to correlate highly across a variety of cognitive tasks (Cui et al., 2011).
To date, few published studies have examined the association between occupational Mn exposure and brain activity using functional neuroimaging. A recent fNIRS case study of a 55-year-old man in Singapore, who was likely occupationally exposed to Mn at previous workplaces, had elevated urinary concentrations of Mn and decreased activation of the frontal lobe during a verbal fluency test compared to a healthy comparison subject (Ho et al., 2018). In addition, one fMRI study found increased brain activity of the prefrontal cortex during a verbal working memory task (Chang et al., 2010), while another found decreased brain activity in the same region during an executive function task (Seo et al., 2016) in Mn-exposed welders compared to un-exposed controls in Korea.
In the present study, we examined whether elevated Mn exposure was associated with brain activity in a sample of farmworkers in Zarcero Country, Costa Rica, a region where the Mn-containing fungicide mancozeb is the second most widely used agricultural pesticide (Ramírez et al., 2016). We hypothesized that higher Mn concentrations, as measured in hair and toenails, would be associated with altered brain activation patterns in the prefrontal cortex related to working memory.
2. Methods
2.1. Study participants and procedures
We conducted this cross-sectional study of 48 farmworkers from 14 smallholder horticultural farms [farms < 20 ha that operate as family businesses and use mainly family labor (FAO, 2012)] in Zarcero County, Costa Rica, between July and August of 2016. Zarcero is considered the “salad bowl” of Costa Rica because of the large amount and diversity of crops grown in the area. Notably, this is also the location where organic farming practices in the country originated (Rodríguez Miranda and Paniagua Guerrero, 1994).
We selected a convenience sample of participants from the Pesticide Use in Tropical Settings (PESTROP) study cohort, which is a study of 300 farmworkers aimed at assessing agricultural pesticide exposure and their potential health effects. Detailed methods for the PESTROP study have been described elsewhere (Fuhrimann et al., 2019). Sample size for the current study was limited by the availability of fNIRS equipment and technical staff. We included farmworkers from organic farms (n = 26) and conventional farms (n = 22) to ensure that there would be sufficient variability in pesticide exposure. We identified smallholder conventional farms using random Global Positioning System (GPS) points, and certified organic farms from a list provided by the organic farmworkers’ association. Eligible participants for this study were farmworkers on a conventional or organic farm within the study area and were ≥18 years of age. No participants self-reported a diagnosis of a psychiatric disorder or use of psychopharmacologic medications.
We assessed the 48 participants over the course of two study visits. At the first visit, trained research staff administered a structured questionnaire to gather information on sociodemographic characteristics, occupational history, current or past disease status, and computer literacy (i.e., whether they have ever used a computer or played video games). fNIRS data were also collected on the first visit. The second visit occurred about a month later [mean (SD), 29.7 (2.7) days]. We collected hair and toenail samples during the second visit in order to give us time to remind the participants to refrain from cutting their hair and clipping their toenails. Despite the gap in time between fNIRS and biological sample collection, hair and toenail Mn concentrations reflect Mn exposure over the past month and a seven-to 12-month period, respectively, thus capturing exposure at the time of and before outcome assessment (Gil et al., 2011; Laohaudomchok et al., 2011).
All study materials and procedures were approved by the Human Subjects Committee of the Universidad Nacional in Costa Rica (UNA-CECUNA-ACUE-04–2016) and the Ethical Board of the Ethikkommission Nordwest-und Zentralschweiz in Switzerland (EKNZ-UBE, 2016–00771). Written informed consent was obtained from all study participants at enrollment.
2.2. Hair and toenail Mn measurement
We measured Mn concentrations in farmworkers’ hair and toenails. We collected hair samples from 33 (69%) participants (15 participants did not have enough hair to collect a sample). Using stainless steel scissors, we cut 20–30 strands of hair from the occipital region, approximately 2 mm from the scalp. We collected toenail samples from 40 (83%) participants (toenails of 8 participants were too short to safely acquire a sample). Prior to collection, participants were asked to wash their feet with soap and water and then clip their toenails with clean stainless-steel nail clippers. We stored hair and toenail samples in sterile plastic bags at room temperature until they were shipped to the Federal University of Bahia, Brazil for analysis.
To limit contamination, hair samples were cleaned using a technique detailed elsewhere (Eastman et al., 2013). Briefly, the nearest cm from the scalp of hair was sonicated for 20 min in 1% Triton, rinsed five times with ultra-pure water (Milli-Q, Millipore-Merck), sonicated for ten minutes in 1 N ultra-pure nitric acid, rinsed once with a 1 N ultra-pure nitric acid, and then rinsed five times with ultra-pure water. Approximately 10 mg of hair was digested with 2 mL of concentrated spectroscopic grade nitric acid in a microwave digester oven. The digested material was then diluted to 10 mL with ultra-pure water. Hair samples, certified reference material (Human hair IAEA-085), and reagent blanks were analyzed twice using graphite furnace atomic absorption spectrometry with Zeeman background correction. Toenail samples were analyzed for Mn using the same procedure described for hair samples.
The analytical limit of detection (LOD) of both hair and toenail Mn concentrations was 0.05 μg/g with precision ranging between 2.6% and 7.6%. For participants with two measurements above the LOD, we took the average. For participants with only one measurement above the LOD (n = 3 for hair), we used that single value. For participants whose measurements were both below the LOD (n = 3 for hair, n = 3 for toenail), we imputed their Mn concentration as (Lubin et al., 2004).
2.3. fNIRS data collection and preprocessing
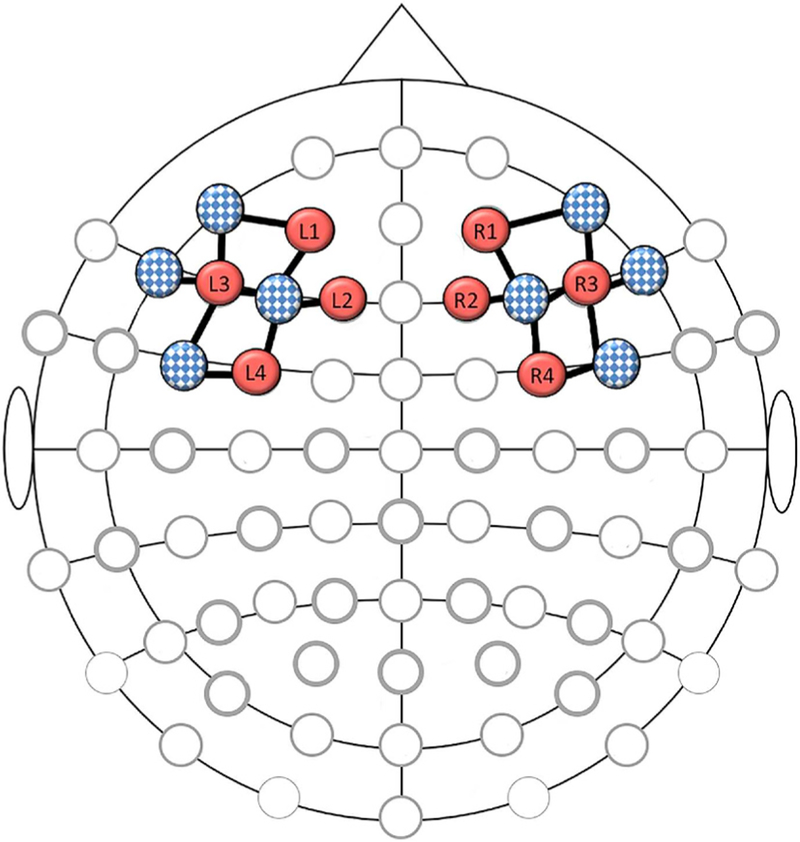
We measured brain activity during a letter-retrieval working memory task (Sternberg, 1969) with a portable fNIRS device (NIRSport, NIRx Medical Technologies, LLC). Details of fNIRS methods utilized in this study have been described elsewhere (Baker et al., 2017). Briefly, we projected near-infrared light with wavelengths of 760 nm and 850 nm into the cortex of each participant’s brain, and recorded samples of data at a rate of 7.81 Hz. We attached eight sources and eight detectors to a mesh cap using the international 10/20 system, resulting in 18 channels configured to record brain activity from the left and right dorsolateral prefrontal cortex. We grouped the 18 channels into eight functional regions of interest (ROIs), four in each brain hemisphere (Fig. 1). The dorsolateral prefrontal cortex is a region known to be involved in working memory based on previous fNIRS and fMRI studies of healthy adults (Rypma and D’Esposito, 1999; Sato et al., 2013) and it is also known to be affected by Mn exposure (Chang et al., 2010; Seo et al., 2016).
Fig. 1.
The functional near-infrared spectroscopy (fNIRS) optode configuration over the dorsolateral prefrontal cortex used in our study. Using the international 10/20 system, we configured eight sources (solid red circles) and eight detectors (checkered blue circles) to make 18 unique channels (bold black lines), creating eight regions of interest (ROIs)—four in the left hemisphere (L1–L4) and four in the right hemisphere (R1–R4). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
We selected an appropriately sized cap for each participant by measuring their head circumference; some participants (20%) also wore a dark overcap to cover the sources and detectors to decrease noise introduced by sunlight. Participants wore the fNIRS cap while seated in front of a laptop that presented the task stimuli (Fig. S1). The Sternberg letter-retrieval working memory task required participants to memorize a list of seven or eight letters that were displayed for 2 s (Encoding), hold them in their memory (Maintenance), and then determine whether a single letter was part of the previous list or not (Recall) (Sternberg, 1969). During Recall, participants pressed a button on the keyboard to indicate their response (yes or no), and reaction time and accuracy were recorded. The task consisted of 30 trials, with a jittered inter-trial interval with a mean of 4 s where participants passively viewed a fixation cross. Participants were asked to relax, remain still, and look at the fixation cross for 30 s at the beginning and end of the task (Rest).
We quality checked fNIRS data using software developed by Cui et al. (2010) and preprocessed data using a pipeline (Brigadoi et al., 2014) involving the Homer 2 package in Matlab (Brigadoi et al., 2014; Cui et al., 2010; Huppert et al., 2009). Briefly, we converted raw data to optical density prior to being corrected for motion-related artifacts using a wavelet-based correction procedure (Hosseini et al., 2017). Next, we band-pass filtered the data between 0.01 Hz and 0.5 Hz (Cui et al., 2010). Finally, we converted the preprocessed data into time series of oxygenated hemoglobin (HbO) and deoxygenated hemoglobin (HbR) concentrations using the modified Beers-Lambert law (Wyatt et al., 1986).
After preprocessing, we employed a general linear model (GLM) approach to assess task-related cortical responses related to each component of the task (i.e., Encoding, Maintenance, Recall, and Rest) within each recording channel (Schroeter et al., 2004). Due to a hypothesized difference in cognitive demands elicited by the Encoding and Recall phases of our task (Anderson et al., 2000), we made a contrast between the T-values estimated for these conditions within the GLM procedure. We then performed a functional localization procedure by selecting the channel with the greatest contrast value within each of the eight functional ROIs for use in group-level analyses (Fig. 1) (Hosseini et al., 2017). This procedure allows for individual participant variation in the task-responsive channels and underlying brain regions, and reduces the risk of committing Type II errors due to averaging across non-responsive channels. For consistency, we conducted the localization procedure first on the HbO data, then selected the same eight channels for the HbR data.
2.4. Statistical analysis
We assessed the relationship between toenail and hair Mn concentrations in farmworkers who provided both samples using a Spearman correlation test. To test for potential selection bias, we compared distributions of sociodemographic and occupational characteristics among all farmworkers included in our analyses (n = 48) with the subsets who provided hair (n = 33) and toenail samples (n = 40), using Wilcoxon rank-sum or χ2 tests. We assessed bivariate associations of Mn exposures with farmworker characteristics using Spearman correlation or Wilcoxon rank-sum tests.
We modeled brain activation (HbO and HbR) at all eight ROIs and accuracy of the working memory task (percentage of correct trials) as continuous outcomes. We used one-sample t-tests to determine whether there was significant brain activation in each ROI in response to the working memory task among the entire sample of 48 participants. We examined bivariate associations of brain activity with socio-demographic and occupational characteristics using two-sample t-tests, analyses of variance (ANOVA), or Pearson correlation tests. We also assessed the association between task accuracy and brain activity at each ROI using linear regression models.
We estimated associations of toenail and hair Mn concentrations with brain activity in each ROI using linear regression models. We also estimated associations of toenail and hair Mn concentrations with accuracy on the Sternberg task. Toenail and hair Mn concentrations were strongly right-skewed, with few farmworkers having higher concentrations, so we log2-transformed them to improve the linear fit of the model. Covariates associated with Mn exposure and cognition in previous studies [i.e., age at testing and education level (≤6th grade vs. 7–11th grade)] (Damalas and Koutroubas, 2017; de Azeredo Passos et al., 2015; Oulhote et al., 2014; Reuter-Lorenz and Cappell, 2008), including previous fMRI studies (Chang et al., 2010; Seo et al., 2016), were included in the models a priori.
We examined the impact of other potential confounders in sensitivity analyses using linear regression models. In separate analyses, we excluded females (n = 2), left-handed participants (n = 2), and those with self-reported neurological disorders (i.e., fibromyalgia and epilepsy) (n = 2), because these subgroups are known to have different patterns of brain activation (Cosgrove et al., 2007; Gur et al., 1982; Staud, 2011; Zhang et al., 2010). We also excluded farmworkers who performed the task poorly (accuracy < 50%) (n = 3) because we were unsure if their poor performance was due to inattention to the task. Given that people drinking from non-aqueduct sources might be exposed to elevated Mn levels due to external contamination of their water sources (e.g., from agricultural EBDC spraying) (van Wendel de Joode et al., 2016) or naturally-occurring Mn found in groundwater (Agency for Toxic Substances and Disease Registry, 2012), we excluded participants who did not drink water from an aqueduct at home (n = 2). We reran our models adjusting for household poverty level and computer literacy, separately, to determine if there was residual confounding after adjusting for education level. Missing household poverty data (n = 3) was imputed using predictions from a linear regression model of income per person per household per month on years worked in agriculture and years of education. Lastly, we included hair and toenail Mn concentrations in the same multivariable models to estimate their independent associations with brain activity, among the sub-sample that provided both hair and toenail samples (n = 32).
We performed all statistical analyses in RStudio version 1.1.419. We controlled for Type I error using the Benjamini-Hochberg False Discovery Rate (FDR) at p < 0.05, except for tests not involving fNIRS outcomes. We assessed for significant interaction effects at p < 0.20.
3. Results
3.1. Sociodemographic characteristics, Mn concentrations, and working memory
Participants included in the study were mostly male (96%), right-handed (94%), born in Costa Rica (71%), educated at or below the 6th grade level (65%), living above the poverty level (73%), and drank water from an aqueduct at home (96%) (Table 1). Notably, we did not find meaningful differences in sociodemographic characteristics between all study participants (n = 48) and those who provided hair samples (n = 33) or toenail samples (n = 40) (Table 1).
Table 1.
Characteristics of farmworkers with functional neuroimaging from the Pesticide Use in Tropical Settings (PESTROP) study, Zarcero County, Costa Rica, 2016.
| All participants (n = 48) | Participants with toenail samples (n = 40) | Participants with hair samples (n = 33) | |||
|---|---|---|---|---|---|
| Characteristics | n (%) | n (%) | p-valuea | n (%) | p-valuea |
| Ageb | 0.98 | 0.42 | |||
| 18–29 | 21 (43.8) | 17 (42.5) | 11 (33.3) | ||
| 30–49 | 13 (27.1) | 11 (27.5) | 10 (30.3) | ||
| ≥ 50 | 14 (29.1) | 12 (30.0) | 12 (36.4) | ||
| Sex | 1.00 | 1.00 | |||
| Female | 2 (4.2) | 2 (5.0) | 2 (6.1) | ||
| Male | 46 (95.8) | 38 (95.0) | 31 (93.9) | ||
| Education level | 1.00 | 0.90 | |||
| ≤ 6th grade | 31 (64.6) | 25 (62.5) | 20 (60.6) | ||
| 7–11th grade | 17 (35.4) | 15 (37.5) | 13 (39.4) | ||
| Country of birth | 0.84 | 0.23 | |||
| Costa Rica | 34 (70.8) | 30 (75.0) | 28 (84.8) | ||
| Nicaragua | 14 (29.2) | 10 (25.0) | 5 (15.2) | ||
| Poverty status | 1.00 | 0.98 | |||
| < Poverty line | 13 (27.1) | 11 (27.5) | 8 (24.2) | ||
| > Poverty line | 35 (72.9) | 29 (72.5) | 25 (75.8) | ||
| Handedness | 0.65 | 0.68 | |||
| Right | 45 (93.7) | 38 (95.0) | 32 (97.0) | ||
| Left | 2 (4.2) | 2 (5.0) | 1 (3.0) | ||
| Unknown | 1 (2.1) | 0 (0) | 0 (0) | ||
| Computer literacyc | 0.54 | 0.87 | |||
| Yes | 27 (56.2) | 26 (65.0) | 20 (60.6) | ||
| No | 21 (43.8) | 14 (35.0) | 11 (39.4) | ||
| Years worked in agricultureb | 0.91 | 0.90 | |||
| ≤14 | 19 (39.6) | 16 (40.0) | 11 (33.3) | ||
| 15–29 | 14 (29.2) | 12 (30.0) | 10 (30.3) | ||
| ≥30 | 15 (31.2) | 12 (30.0) | 12 (36.4) | ||
| Type of farm | 0.74 | 0.94 | |||
| Conventional | 22 (45.8) | 16 (40.0) | 14 (42.4) | ||
| Organic | 26 (54.2) | 24 (60.0) | 19 (57.6) | ||
| Drinking water source | 0.98 | 0.68 | |||
| Aqueduct | 46 (95.8) | 38 (95.0) | 32 (97.0) | ||
| Spring | 1 (2.1) | 1 (2.5) | 1 (3.0) | ||
| Other | 1 (2.1) | 1 (2.5) | 0 (0) | ||
Groups were compared using Wilcoxon rank-sum tests for continuous variables and Chi-squared tests for categorical variables.
Modeled as continuous variable when testing for group differences.
Defined as ever used a computer of played video games.
Geometric mean (GM) (geometric standard deviation, GSD) toenail and hair Mn concentrations were 0.40 μg/g (3.52) and 0.24 μg/g (3.54), respectively (Table 2). Toenail Mn concentrations ranged from 0.04 to7.45 μg/g, and hair Mn concentrations ranged from 0.04 to 9.45 μg/g (Table S1). We found that toenail and hair Mn concentrations trended positively among the 32 participants who provided both toenail and hair samples, but this correlation was not statistically significant [Spearman correlation coefficient (rs) = 0.21, p = 0.25]. Toenail Mn concentrations were negatively associated with age (rs = −0.36, p = 0.02) and years worked in agriculture (rs = −0.33, p = 0.04; data not shown). Farmworkers born in Nicaragua had higher toenail Mn concentrations (GM = 0.85, GSD = 2.87; p = 0.03) compared to those born in Costa Rica (GM = 0.31, GSD = 3.44; Table 2), which could be confounded by age since farmworkers born in Nicaragua were significantly younger [median = 22 years, interquartile range (IQR) = 4.8; p < 0.001] than those from Costa Rica (median = 43.7 years, IQR = 24.5). We also observed higher toenail Mn concentrations in workers from organic farms (GM = 0.56, GSD = 3.24; p = 0.04) than those from conventional farms (GM = 0.24, GSD = 3.46), but similar hair Mn concentrations in both groups (Table 2). Hair Mn concentrations were not associated with any other sociodemographic or occupational characteristics.
Table 2.
Bivariate associations of manganese (Mn) concentrations (μg/g) with characteristics of farmworkers, Zarcero County, Costa Rica, 2016.
| Toenail Mn (n = 40)a | Hair Mn (n = 33)b | |||
|---|---|---|---|---|
| Characteristics | GM (GSD) | p-valuec | GM (GSD) | p-valuec |
| All | 0.40 (3.52) | 0.24 (3.54) | ||
| Aged | 0.02 | 0.64 | ||
| 18–29 | 0.76 (3.07) | 0.21 (4.32) | ||
| 30–49 | 0.25 (2.93) | 0.18 (3.08) | ||
| ≥50 | 0.24 (3.57) | 0.34 (3.35) | ||
| Sexe | ||||
| Female | 0.33 (1.73) | 0.19 (10.57) | ||
| Male | 0.40 (3.63) | 0.24 (3.42) | ||
| Education level | 0.62 | 0.13 | ||
| ≤ 6th grade | 0.36 (3.93) | 0.29 (2.92) | ||
| 7–11th grade | 0.48 (2.91) | 0.18 (4.51) | ||
| Country of birth | 0.03 | 0.06 | ||
| Costa Rica | 0.31 (3.44) | 0.21 (3.53) | ||
| Nicaragua | 0.85 (2.87) | 0.55 (2.78) | ||
| Poverty status | 0.35 | 0.60 | ||
| ≤ Poverty line | 0.25 (3.55) | 0.35 (6.14) | ||
| > Poverty line | 0.48 (3.43) | 0.21 (2.87) | ||
| Handednesse | ||||
| Right | 0.36 (3.41) | 0.24 (3.61) | ||
| Left | 2.20 (1.27) | 0.24 (NA) | ||
| Years worked in agricultured | 0.04 | 0.81 | ||
| ≤14 | 0.77 (3.17) | 0.32 (4.40) | ||
| 15–29 | 0.25 (3.30) | 0.15 (2.38) | ||
| ≥30 | 0.26 (3.17) | 0.29 (3.72) | ||
| Type of farm | 0.04 | 0.93 | ||
| Conventional | 0.24 (3.46) | 0.25 (3.50) | ||
| Organic | 0.56 (3.24) | 0.24 (3.69) | ||
| Drinking water sourcee | ||||
| Aqueduct | 0.40 (3.62) | |||
| Spring | 0.26 (NA) | 0.24 (3.61) | ||
| Other | 0.70 (NA) | 0.18 (NA) | ||
| Days between fNIRS and toenail/hair sample collectiond | 0.12 | 0.71 | ||
| < 30 | 0.61 (3.67) | 0.32 (5.40) | ||
| ≥30 | 0.26 (2.95) | 0.20 (2.45) |
Abbreviations: fNIRS, functional near-infrared spectroscopy; GM, geometric mean; GSD, geometric standard deviation; LOD, limit of detection; NA, not applicable.
37 farmworkers (92.5%) had toenail Mn concentrations above the LOD (0.05 μg/g).
30 farmworkers (90.1%) had hair Mn concentrations above the LOD (0.05 μg/g).
We estimated bivariate associations using Spearman correlation tests for continuous variables and Wilcoxon rank-sum tests for categorical variables.
Bivariate associations assessed using continuous variable.
Bivariate association not assessed due to small sample size.
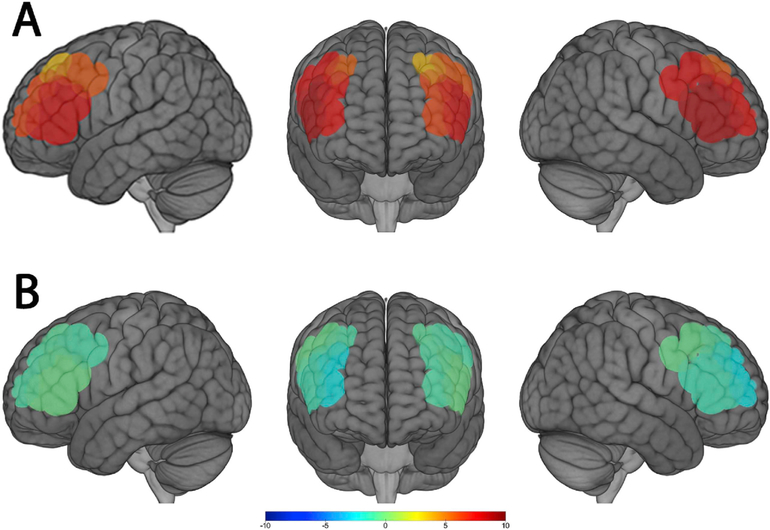
Among all study participants (n = 48), there was significant brain activation across all ROIs using HbO, but not using HbR (Fig. 2, Tables S2–S3). Additionally, there was significant brain deactivation in R2 using HbR. In bivariate analyses, age, years worked in agriculture, and type of farm were associated with HbO at some ROIs (Table S2); education level was associated with HbR at some ROIs (Table S3). These associations were null after correcting for multiple comparisons, except for the associations between type of farm and HbO at the most medial (L2, R2) and posterior (L4, R4) ROIs and the association between education level and HbR at R4.
Fig. 2.
Group mean estimates of brain activity across the eight regions of interest (ROIs) in response to the letter-retrieval working memory task (n = 48). Colors represent T-scores with warm colors indicating positive values and cool colors indicating negative values. (A) Brain activation using oxygenated hemoglobin concentrations (HbO). (B) Brain activation using deoxygenated hemoglobin concentrations (HbR).
3.2. Associations of working memory task performance with brain activity
Farmworkers performed the working memory task with a median accuracy of 67% (IQR = 11%) and a mean (SD) reaction time of 1.5 s (0.5 s). For each unit increase in HbO, there was a 0.3–0.5% increase in working memory task accuracy at all ROIs (Table S4). The positive associations between HbO and accuracy were strongest at the most lateral ROIs (L3, R3) and the right posterior ROI (R4), but they were not statistically significant after FDR correction. HbR was not associated with accuracy, neither before nor after FDR correction.
3.3. Associations of Mn concentrations with brain activity
We observed mostly null associations of toenail and hair Mn concentrations with brain activity (HbO and HbR) in our unadjusted (Table S3; Figs. S2–S5) and adjusted (Table 3) linear regression models. There were no statistically significant associations after adjusting for multiple comparisons with FDR correction. Nevertheless, associations between toenail Mn concentrations and HbO at all eight ROIs showed a negative trend, after controlling for age and education level (Table 3). More specifically, brain activity decreased with every two-fold increase in toenail Mn concentrations, with the strongest association observed at the most anterior ROI in the right hemisphere (R1) (β = −1.5, 95% CI: −3.4, 0.5). Associations between toenail Mn concentrations and HbR showed a positive trend, after controlling for age and education level (Table 3).
Table 3.
Adjusted coefficients (β) and (95% CI) in fNIRS oxygenated (HbO) and deoxygenated hemoglobin (HbR) per two-fold increase in toenail (n = 40) and hair (n = 33) Mn concentrations (μg/g) in farmworkers, Zarcero County, Costa Rica, 2016.
| Toenail Mn | Hair Mn | |||
|---|---|---|---|---|
| ROI | HbO | HbR | HbO | HbR |
| L1 | −1.0 (−2.9, 0.9) | 0.2 (−1.5, 1.9) | 0.4 (−1.9, 2.6) | 0.2 (−1.5, 2.0) |
| L2 | −0.6 (−2.2, 1.0) | 0 (−1.8, 1.8) | 0.3 (−1.6, 2.2) | −0.2 (−2.3, 1.9) |
| L3 | −1.0 (−2.8, 0.8) | 0.7 (−0.9, 2.2) | 0.9 (−1.2, 2.9) | −0.2 (−1.9, 1.5) |
| L4 | −0.7 (−2.5, 1.1) | 0.6 (−0.8, 1.9) | 0.7 (−1.4, 2.8) | 0.1 (−1.5, 1.7) |
| R1 | −1.5 (−3.4, 0.5) | 0.6 (−0.8, 1.9) | −0.5 (−2.9, 1.8) | −0.1 (−1.5, 1.3) |
| R2 | −0.3 (−2.0, 1.4) | −0.2 (−1.5, 1.1) | −0.3 (−2.2, 1.7) | −0.8 (−2.2, 0.6) |
| R3 | −1.0 (−2.8, 0.8) | 0.9 (−0.9, 2.6) | −0.1 (−2.2, 2.0) | −0.8 (−2.6, 0.9) |
| R4 | −0.7 (−2.7, 1.4) | 0.3 (−0.9, 1.5) | −0.1 (−2.4, 2.2) | 0.2 (−1.2, 1.5) |
Abbreviations: CI, confidence interval; fNIRS, functional near-infrared spectroscopy; ROI, region of interest; L, left hemisphere; Mn, manganese; R, right hemisphere; HbO, oxygenated hemoglobin; HbR, deoxygenated hemoglobin.
Models adjusted for age and education level.
Unadjusted p < 0.05.
FDR-corrected p < 0.05.
Associations between hair Mn concentrations and HbO in left ROIs also showed a positive trend; however, a negative trend was observed in right ROIs (Table 3). Associations between hair Mn concentrations and HbR were slightly positive in left anterior (L1) and bilateral posterior (L4, R4) ROIs and negative elsewhere.
We found null associations of toenail (β = −1.2, 95% CI: −4.1, 1.6) and hair Mn concentrations (β = 0.9, 95% CI: −2.3, 4.2) with working memory task accuracy, after adjusting for age and education level (data not shown).
3.4. Sensitivity analysis
Effect estimates for toenail (Table S6) and hair (Table S7) Mn concentrations after excluding females (n = 2), left-handed farm-workers (n = 2), poor performers (n = 3), participants with neurological diseases (n = 2, fibromyalgia, epilepsy), and farmworkers with water sources other than an aqueduct (n = 2) generally stayed the same. Similarly, when poverty level and computer literacy were added to the models, coefficients did not change meaningfully (Tables S6–S7). Lastly, when toenail and hair Mn concentrations were added simultaneously to the same multivariable models, effect estimates remain unchanged (Table S8).
4. Discussion
In a pilot study of smallholder farmworkers in Costa Rica, we did not find strong evidence that elevated Mn exposure, measured in toe-nails and hair, was associated with alterations in cortical brain activation during a working memory task. Overall, patterns of brain activation in relation to Mn were weak and inconsistent. We found that toenail Mn concentrations trended negatively with brain activity, concurrently with both HbO and HbR, suggesting that past Mn exposure may be weakly related to inefficient recruitment of the dorsolateral prefrontal cortex; however, our small sample size impeded our ability to measure subtle associations with precision. Higher hair Mn concentrations, on the other hand, were associated with a mixed pattern of brain activation across brain hemispheres.
Our study demonstrates the capacity to measure impacts of chemical exposures and metal toxicity on cognitive ability at the neural level using functional neuroimaging. These measures are potentially more sensitive than studies of neuropsychological test scores in detecting the neural consequences of environmental insults. For example, an elevated exposure to a neurotoxicant such as Mn could induce damage to the brain which results in neural compensation, where brain regions “work harder” and become overactivated in order to perform on tests within a normal range (Reuter-Lorenz and Cappell, 2008). Functional neuroimaging also provides an opportunity to study how elevated exposures to Mn affect neural pathways (de Water et al., 2017) and the function and structure of brain regions (Ho et al., 2018).
Our findings are not consistent with previous functional neuroimaging studies of welders that found associations of elevated Mn exposure with altered brain activation in the prefrontal cortex (Chang et al., 2010; Seo et al., 2016). A study in Korea found that Mn-exposed welders (n = 23) with similar accuracy in their performance on a verbal working memory task as the unexposed workers (n = 21) had significantly greater activity in the prefrontal cortex and a more widespread pattern of activity across the brain (Chang et al., 2010). In contrast, another study in Korea found that Mn-exposed welders (n = 53) performed worse on an executive function test and had less activation of the bilateral superior frontal cortex (part of the prefrontal cortex) compared to unexposed workers (n = 44) (Seo et al., 2016). In these studies, both increased and decreased activation of the prefrontal cortex are suggestive of neural dysfunction, and the distinction may depend on cognitive demand (Seo et al., 2016; Tomasi et al., 2007). Elevated Mn exposure may lead to inefficient engagement of neural resources (i.e., higher brain activation) in a task with lower cognitive demands [e.g., working memory task (Chang et al., 2010)], but this compensatory mechanism may no longer be effective in tasks with higher cognitive demands [e.g., executive function test (Seo et al., 2016) and verbal fluency test (Ho et al., 2018)], leading to equivalent or lower activation of brain regions.
Lower Mn exposure levels in our farmworker population compared with levels observed in two studies of welders (Chang et al., 2010; Seo et al., 2016), due to differences in exposure routes and background levels, could explain our null findings (United States Environmental Protection Agency, 2005). However, direct comparison of Mn exposure levels is not possible given that the studies of welders measured Mn in blood and we measured Mn in hair and toenails. Notably, hair and toenail Mn concentrations in our study were lower than those reported in previous occupational and environmental studies (Table S9) (Grashow et al., 2014; Hariri et al., 2018; Hassani et al., 2016; Mora et al., 2014; Ohgami et al., 2018; Reiss et al., 2016; Rodrigues et al., 2015; Rolle-McFarland et al., 2018; Ward et al., 2017), though it is unclear if these differences are real or due to variations in the cleaning procedures used to prepare the biological samples for analysis.
We used different tasks in our study, compared with studies of welders, which could explain our inconsistent findings. The Sternberg task that we used in our study assesses working memory using a letter-retrieval format. This is different than the tasks used in the welding studies, which included an N-back task that requires constant updating of memory (Chang et al., 2010) and the Wisconsin Card Sort Task that involves cognitive flexibility and executive control (Seo et al., 2016). Finally, our sample sizes differed slightly; one study had a similar sample size to ours (23 Mn-exposed welders and 21 controls) (Chang et al., 2010), but the other had a larger sample size (53 Mn-exposed welders and 44 controls) (Seo et al., 2016). Our small sample size may have limited our ability to detect subtle associations of elevated Mn exposure with brain activation.
In our pilot study, we did not find associations of hair and toenail Mn concentrations with working memory task accuracy. This could be due to our small sample size or to the fact that the working memory task that we used, while designed for optimal fNIRS data collection and analysis, was not a standardized neuropsychological test. Likewise, a previous study in Korea did not find differences in working memory task accuracy between Mn-exposed welders and the comparison group (Chang et al., 2010).
We observed higher toenail Mn concentrations in organic farm-workers and comparable hair Mn concentrations between organic and conventional farmworkers, which highlights the complexity of Mn sources. Mn is found in the EBDC fungicide mancozeb, which is the second most widely used pesticide in conventional farms in Zarcero County, our study area (Ramírez et al., 2016), but it is also found in fertilizers that are used in organic farms if deficiency of Mn in the soil is documented (Baker, 2009). Additionally, since the main sources of Mn for the general population are drinking water and food (Agency for Toxic Substances and Disease Registry, 2012), we do not know for certain how much agricultural sources contributed to Mn concentrations observed in our sample of farmworkers. Furthermore, this did not impede our ability to assess brain activity across a gradient of Mn exposure, as we had sufficient variability in the exposure based on the distributions of Mn concentrations in our sample. Future research is needed to determine how much agricultural sources, whether from EBDC fungicides or fertilizers, contribute to Mn exposure levels in farmworkers.
While analyzing Mn concentrations in two biological matrices is a strength of our study, we cannot conclude which biomarker is more valid for characterizing long-term cumulative Mn exposure. Currently, there is no consensus on which biomarker of Mn most reliably reflects internal dose (Eastman et al., 2013; Skröder et al., 2017; Smith et al., 2007). Previous occupational studies have primarily used blood and urine, which are suitable for capturing short-term and recent exposures (hours to days) (Chang et al., 2010; Laohaudomchok et al., 2011; Smith et al., 2007). Given the mounting evidence that Mn accumulates in the brain over periods of exposure (Bowler et al., 2017; Guilarte, 2013; Zaiyang et al., 2014), biomarkers that reflect exposures over longer time intervals, such as hair and toenails, might be useful. While hair has been used in epidemiologic studies and is believed to reliably reflect Mn in the body from exposures over the past month (Bouchard et al., 2007a; Gil et al., 2011), toenails are a relatively new biomarker that reflect internal dose of Mn from seven to 12 months earlier (Laohaudomchok et al., 2011). More research is needed in order to determine which biomarker provides the best estimate of long-term cumulative exposure.
While our study has some notable strengths, including the novelty of conducting an epidemiologic field study with neuroimaging among a farmworker population, it also has some limitations. First, our small sample size limited our ability to estimate potentially subtle Mn-related associations with brain activity with precision. Second, we utilized a convenience sampling scheme to select farmworkers based on the availability of fNIRS equipment and technical staff, meaning our sample may not be representative of the entire farmworker population in our study area. Third, while farmworkers were reminded to refrain from cutting their toenails and hair two to four weeks prior to collection, some had forgotten and were unable to safely provide samples. We believe it is unlikely that selection bias strongly impacted our results as we did not find meaningful differences in farmworker characteristics between the subsets of participants who provided hair or toenail samples and all participants included in this study (n = 48). Future research could improve sample collection efforts by giving more reminders or allowing participants an allotted time frame within which they can provide a sample. Another limitation includes selection bias due to the healthy worker effect. For example, since we were unable to capture in our sampling scheme farmworkers who may have left their occupation due to illness related to elevated Mn exposure, we may have underestimated the effect that elevated Mn has on working memory-related brain activity. Lastly, in our study, we did not assess participants’ intelligence, which may be a potential confounder of the exposure-outcome associations of interest; however, we adjusted our main analyses for education level, which has been used as a proxy for intelligence (Deary and Johnson, 2010).
A limitation of most fNIRS recording devices is the inability to decouple neurovascular responses, used to infer functional brain activity, and hemodynamic activity from non-cortical sources (e.g., skin blood flow changes and global blood pressure changes) from fNIRS signal recordings (Caldwell et al., 2016; Scholkmann et al., 2014). An emerging solution to this problem is the inclusion of “short-channels” to record physiological signals that can be used as additional regressors in the GLM approach used to estimate brain activation (Tachtsidis and Scholkmann, 2016). Short-channels function the same as typical fNIRS recording channels, but have a much smaller distance between source and detector, which creates a shallower photon path and enables measurement of oxygen fluctuations from scalp blood. While our fNIRS device was not equipped with short-channels, the GLM approach that we used is robust and should not be highly influenced by extraneous interference when noise is spatially constant. Because our optode coverage was symmetrical and limited to the bilateral prefrontal cortex, noise from scalp blood flow was likely to be detected similarly across all fNIRS recording channels. We are therefore confident that any unexpected influences of scalp blood flow on our outcomes was minimal.
Lastly, we had a dominantly male sample with only two females, thus limiting the generalizability of our findings to women and ability to measure sex-dependent effects. Previous neuroimaging studies of Mn-exposed welders were also limited to men (Chang et al., 2010; Seo et al., 2016). Given the evidence of neurobiological differences between sexes (Cosgrove et al., 2007), higher Mn concentrations found in women (Oulhote et al., 2014), and sex-dependent effects of Mn concentrations on cognition in children (Bouchard et al., 2017), future studies should ensure that females are sufficiently represented in their sample using a stratified sampling scheme. While this may be difficult to accomplish in occupational populations that are predominantly male, efforts should be made in the study design to ensure the inclusion of females.
5. Conclusions
We did not find strong evidence that elevated Mn exposure was associated with working memory-related brain activity in this pilot study of smallholder farmworkers. We demonstrate that conducting a functional neuroimaging study using fNIRS in the field with a farm-worker population is feasible and may be a useful tool for future environmental and occupational epidemiologic studies with larger sample sizes. Measuring cognitive ability at the neural level with functional neuroimaging has potential for detecting adverse effects of chemical exposures that may not otherwise be detected with neuropsychological tests or behavior rating scales due to, for example, compensation of other neural resources. The ability to conveniently measure brain activity with fNIRS also has important public health implications for assessing the neurobehavioral effects of chemical exposures that may have subtle effects on the brain.
Supplementary Material
Acknowledgements
We gratefully acknowledge the study participants and staff. We thank Katherine Kogut, Maya Petersen, Arthur Reingold, and Alexandra Minnis for their helpful feedback of components of the manuscript. This work was supported by the Swiss Network for International Studies (SNIS), Swiss Federal Institute of Aquatic Science and Technology (EAWAG), Universidad Nacional in Costa Rica, and National Institutes of Health’ Environmental influences on Child Health Outcomes program (ECHO UG3OD023356). JMB’s effort was supported in part by an NIH Career Development Award (K99-HD092883), and the Stanford Maternal Child Health Research Institute. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funders.
Footnotes
Competing financial interest declaration
The authors declare they have no actual or potential competing financial interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2019.04.006.
References
- Agency for Toxic Substances, Disease Registry, 2012. Toxicological Profile for Manganese. Agency for Toxic Substances and Disease Registry, Atlanta, GA. [PubMed] [Google Scholar]
- Al-Lozi A, Nielsen SS, Hershey T, Birke A, Checkoway H, Criswell SR, Racette BA, 2017. Cognitive control dysfunction in workers exposed to manganese-containing welding fume. Am. J. Ind. Med 60, 181–188. 10.1002/ajim.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ND, Iidaka T, Cabeza R, Kapur S, McIntosh AR, Craik FI, 2000. The effects of divided attention on encoding- and retrieval-related brain activity: a PET study of younger and older adults. J. Cogn. Neurosci 12, 775–792. [DOI] [PubMed] [Google Scholar]
- Baker B, 2009. Can I Use This Fertilizer on My Organic Farm? [WWW Document]. Org. Farms Resour. Conserv. Nat. Resour. Conserv. Serv. U. S. Dep. Agric URL, .21.18. https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs144p2_045863.pdf. [Google Scholar]
- Baker JM, Rojas-Valverde D, Gutiérrez D, Winkler M, Fuhrimann S, Eskenazi B, Reiss AL, Mora AM, 2017. Portable functional neuroimaging as an environmental epidemiology tool: a how-to guide for the use of fNIRS in field studies. Environ. Health Perspect. 125 10.1289/EHP2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas DA, Elwell CE, Ferrari M, Taga G, 2014. Twenty years of functional near-infrared spectroscopy: introduction for the special issue. Neuroimage 85, 1–5. Celebrating 20 Years of Functional Near Infrared Spectroscopy (fNIRS). 10.1016/j.neuroimage.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D, 2007a. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ. Health Perspect 115, 122–127. 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M, Mergler D, Baldwin M, Panisset M, Roels H, 2007b. Neuropsychiatric symptoms and past manganese exposure in a ferro-alloy plant. Neurotoxicology 28, 290–297. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Surette C, Cormier P, Foucher D, 2017. Low level exposure to manganese from drinking water and cognition in school-age children. Neurotoxicology. 10.1016/j.neuro.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Kornblith ES, Gocheva VV, Colledge MA, Bollweg G, Kim Y, Beseler CL, Wright CW, Adams SW, Lobdell DT, 2015. Environmental exposure to manganese in air: associations with cognitive functions. Neurotoxicology 49, 139–148. 10.1016/j.neuro.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, Koller W, Bowler RP, Mergler D, Bouchard M, Smith D, Gwiazda R, Doty RL, 2007. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup. Environ. Med 64, 167–177. 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler RM, Yeh C-L, Adams SW, Ward EJ, Ma RE, Dharmadhikari S, Snyder SA, Zauber SE, Wright CW, Dydak U, 2017. Association of MRI T1 relaxation time with neuropsychological test performance in manganese-exposed welders. Neurotoxicology. 10.1016/j.neuro.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigadoi S, Ceccherini L, Cutini S, Scarpa F, Scatturin P, Selb J, Gagnon L, Boas DA, Cooper RJ, 2014. Motion artifacts in functional near-infrared spectroscopy: a comparison of motion correction techniques applied to real cognitive data. Neuroimage 85, 181–191. Celebrating 20 Years of Functional Near Infrared Spectroscopy (fNIRS). 10.1016/j.neuroimage.2013.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell M, Scholkmann F, Wolf U, Wolf M, Elwell C, Tachtsidis I, 2016. Modelling confounding effects from extracerebral contamination and systemic factors on functional near-infrared spectroscopy. Neuroimage 143, 91–105. 10.1016/j.neuroimage.2016.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canossa E, Angiuli G, Garasto G, Buzzoni A, De Rosa E, 1993. Dosage indicators in farm workers exposed to mancozeb. Med. Lav 84, 42–50. [PubMed] [Google Scholar]
- Chang Y, Lee J-J, Seo J-H, Song H-J, Kim J-H, Bae S-J, Ahn J-H, Park S-J, Jeong KS, Kwon YJ, Kim SH, Kim Y, 2010. Altered working memory process in the manganese-exposed brain. Neuroimage 53, 1279–1285. 10.1016/j.neuroimage.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Coetzee DJ, McGovern PM, Rao R, Harnack LJ, Georgieff MK, Stepanov I, 2016. Measuring the impact of manganese exposure on children’s neurodevelopment: advances and research gaps in biomarker-based approaches. Environ. Health Global Access Sci. Source 15, 91 10.1186/s12940-016-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK, 2007. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry 62, 847–855. 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Bray S, Bryant DM, Glover GH, Reiss AL, 2011. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage 54, 2808–2821. 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Bray S, Reiss AL, 2010. Functional Near Infrared Spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. Neuroimage 49, 3039 10.1016/j.neuroimage.2009.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damalas CA, Koutroubas SD, 2017. Farmers’ training on pesticide use is associated with elevated safety behavior. Toxics 5. 10.3390/toxics5030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azeredo Passos VM, Giatti L, Bensenor I, Tiemeier H, Ikram MA, de Figueiredo RC, Chor D, Schmidt MI, Barreto SM, 2015. Education plays a greater role than age in cognitive test performance among participants of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). BMC Neurol. 15 10.1186/s12883-015-0454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Water E, Proal E, Wang V, Medina SM, Schnaas L, Téllez-Rojo MM, Wright RO, Tang CY, Horton MK, 2017. Prenatal manganese exposure and intrinsic functional connectivity of emotional brain areas in children. Neurotoxicology. 10.1016/j.neuro.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Johnson W, 2010. Intelligence and education: causal perceptions drive analytic processes and therefore conclusions. Int. J. Epidemiol 39, 1362–1369. 10.1093/ije/dyq072. [DOI] [PubMed] [Google Scholar]
- Donaldson J, 1987. The physiopathologic significance of manganese in brain: its relation to schizophrenia and neurodegenerative disorders. Neurotoxicology 8, 451–462. [PubMed] [Google Scholar]
- Eastman RR, Jursa TP, Benedetti C, Lucchini RG, Smith DR, 2013. Hair as a biomarker of environmental manganese exposure. Environ. Sci. Technol 47, 1629–1637. 10.1021/es3035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO, 2012. Smallholders and Family Farmers. [WWW Document]. URL, .3.19. http://www.fao.org/fileadmin/templates/nr/sustainability_pathways/docs/Factsheet_SMALLHOLDERS.pdf.
- Ferraz HB, Bertolucci PH, Pereira JS, Lima JG, Andrade LA, 1988. Chronic exposure to the fungicide maneb may produce symptoms and signs of CNS manganese intoxication. Neurology 38, 550–553. [DOI] [PubMed] [Google Scholar]
- Fuhrimann S, Winkler MS, Staudacher P, Weiss FT, Stamm C, Eggen RI, Lindh CH, Menezes-Filho JA, Baker JM, Ramírez-Muñoz F, Gutiérrez-Vargas R, Mora AM, 2019. Exposure to pesticides and health effects on farm owners and workers from conventional and organic agricultural farms in Costa Rica: protocol for a cross-sectional study. JMIR Res. Protoc 8, e10914 10.2196/10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil F, Hernández AF, Márquez C, Femia P, Olmedo P, López-Guarnido O, Pla A, 2011. Biomonitorization of cadmium, chromium, manganese, nickel and lead in whole blood, urine, axillary hair and saliva in an occupationally exposed population. Sci. Total Environ 409, 1172–1180. 10.1016/j.scitotenv.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Grashow R, Zhang J, Fang SC, Weisskopf MG, Christiani DC, Cavallari JM, 2014. Toenail metal concentration as a biomarker of occupational welding fume exposure. J. Occup. Environ. Hyg 11, 397–405. 10.1080/15459624.2013.875182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, 2013. Manganese neurotoxicity: new perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Front. Aging Neurosci. 5 10.3389/fnagi.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB, Bradman A, Jerrett M, Smith DR, Harley KG, Austin C, Vedar M, Arora M, Eskenazi B, 2013. Determinants of manganese in prenatal dentin of shed teeth from CHAMACOS children living in an agricultural community. Environ. Sci. Technol 47, 11249–11257. 10.1021/es4018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB, Jerrett M, Smith DR, Jursa T, Yousefi P, Camacho J, Hubbard A, Eskenazi B, Bradman A, 2014. Determinants of manganese levels in house dust samples from the CHAMACOS cohort. Sci. Total Environ 497–498, 360–368. 10.1016/j.scitotenv.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Younkin D, Rosen AD, Skolnick BE, Reivich M, 1982. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science 217, 659–661. 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- Hariri A, Mohamad Noor N, Paiman NA, Ahmad Zaidi AM, Zainal Bakri SF, 2018. Heavy metals found in the breathing zone, toenails and lung function of welders working in an air-conditioned welding workplace. Int. J. Occup. Saf. Ergon. JOSE 24, 646–651. 10.1080/10803548.2017.1368950. [DOI] [PubMed] [Google Scholar]
- Hassani H, Golbabaei F, Shirkhanloo H, Tehrani-Doust M, 2016. Relations of biomarkers of manganese exposure and neuropsychological effects among welders and ferroalloy smelters. Ind. Health 54, 79–86. 10.2486/indhealth.2014-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CSH, Ho RCM, Quek AML, 2018. Chronic manganese toxicity associated with voltage-gated potassium channel complex antibodies in a relapsing neuropsychiatric disorder. Int. J. Environ. Res. Public Health 15 10.3390/ijerph15040783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MK, Margolis AE, Tang C, Wright R, 2014. Neuroimaging is a novel tool to understand the impact of environmental chemicals on neurodevelopment. Curr. Opin. Pediatr 26, 230–236. 10.1097/MOP.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SMH, Bruno JL, Baker JM, Gundran A, Harbott LK, Gerdes JC, Reiss AL, 2017. Neural, physiological, and behavioral correlates of visuomotor cognitive load. Sci. Rep 7, 8866 10.1038/s41598-017-07897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Diamond SG, Franceschini MA, Boas DA, 2009. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt 48, D280–D298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Christiani DC, Weisskopf MG, 2011. Toenail, blood and urine as biomarkers of manganese exposure. J. Occup. Environ. Med. Am. Coll. Occup. Environ. Med 53, 506–510. 10.1097/JOM.0b013e31821854da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P, 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ. Health Perspect 112, 1691–1696. 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, van Wendel de Joode B, Mergler D, Córdoba L, Cano C, Quesada R, Smith DR, Menezes-Filho JA, Lundh T, Lindh CH, Bradman A, Eskenazi B, 2014. Blood and hair manganese concentrations in pregnant women from the infants’ environmental health study (ISA) in Costa Rica. Environ. Sci. Technol 48, 3467–3476. 10.1021/es404279r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami N, Li X, Yajima I, Oshino R, Ohgami K, Kato Y, Ahsan N, Akhand AA, Kato M, 2018. Manganese in toenails is associated with hearing loss at high frequencies in humans. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 23, 533–539. 10.1080/1354750X.2018.1458153. [DOI] [PubMed] [Google Scholar]
- Oulhote Y, Mergler D, Bouchard MF, 2014. Sex- and age-differences in blood manganese levels in the U.S. general population: national health and nutrition examination survey 2011–2012. Environ. Health Global Access Sci. Source 13, 87 10.1186/1476-069X-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl DP, Olanow CW, 2007. The neuropathology of manganese-induced Parkinsonism. J. Neuropathol. Exp. Neurol 66, 675–682. 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Ramírez F, Chaverri F, Fournier M, de la Cruz E, Bravo V, Echeverría S, 2016. Las buenas prácticas agrícolas en el uso y manejo de agroquímicos en la zona hortícola de Zarcero, Alajuela: informe de avance de resultados primer año. Instituto Regional de Estudios en Sustancias Tóxicas (IRET), Heredia, Costa Rica. [Google Scholar]
- Reiss B, Simpson CD, Baker MG, Stover B, Sheppard L, Seixas NS, 2016. Hair manganese as an exposure biomarker among welders. Ann. Occup. Hyg 60, 139–149. 10.1093/annhyg/mev064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA, 2008. Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci 17, 177–182. 10.1111/j.1467-8721.2008.00570.x. [DOI] [Google Scholar]
- Rice D, Barone S, 2000. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 108 (Suppl. 3), 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha GHO, Lini RS, Barbosa F, Batista BL, de Oliveira Souza VC, Nerilo SB, Bando E, Mossini SAG, Nishiyama P, 2015. Exposure to heavy metals due to pesticide use by vineyard farmers. Int. Arch. Occup. Environ. Health 88, 875–880. 10.1007/s00420-014-1010-1. [DOI] [PubMed] [Google Scholar]
- Rodrigues EG, Kile M, Dobson C, Amarasiriwardena C, Quamruzzaman Q, Rahman M, Golam M, Christiani DC, 2015. Maternal-infant biomarkers of prenatal exposure to arsenic and manganese. J. Expo. Sci. Environ. Epidemiol 25, 639–648. 10.1038/jes.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez Miranda G, Paniagua Guerrero JJ, 1994. Horticultura Orgánica 2.
- Rolle-McFarland D, Liu Y, Zhou J, Mostafaei F, Zhou Y, Li Y, Fan Q, Zheng W, Nie LH, Wells EM, 2018. Development of a cumulative exposure index (CEI) for manganese and comparison with bone manganese and other biomarkers of manganese exposure. Int. J. Environ. Res. Public Health 15 10.3390/ijerph15071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runkle JD, Tovar-Aguilar JA, Economos E, Flocks J, Williams B, Muniz JF, Semple M, McCauley L, 2013. Pesticide risk perception and biomarkers of exposure in Florida female farmworkers. J. Occup. Environ. Med 55, 1286–1292. 10.1097/JOM.0b013e3182973396. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M, 1999. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc. Natl. Acad. Sci. U. S. A 96, 6558–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Yahata N, Funane T, Takizawa R, Katura T, Atsumori H, Nishimura Y, Kinoshita A, Kiguchi M, Koizumi H, Fukuda M, Kasai K, 2013. A NIRS-fMRI investigation of prefrontal cortex activity during a working memory task. Neuroimage 83, 158–173. 10.1016/j.neuroimage.2013.06.043. [DOI] [PubMed] [Google Scholar]
- Scholkmann F, Kleiser S, Metz AJ, Zimmermann R, Mata Pavia J, Wolf U, Wolf M, 2014. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 85, 6–27. Celebrating 20 Years of Functional Near Infrared Spectroscopy (fNIRS). 10.1016/j.neuroimage.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Bücheler MM, Müller K, Uludağ K, Obrig H, Lohmann G, Tittgemeyer M, Villringer A, von Cramon DY, 2004. Towards a standard analysis for functional near-infrared imaging. Neuroimage 21, 283–290. 10.1016/j.neuroimage.2003.09.054. [DOI] [PubMed] [Google Scholar]
- Seo J, Chang Y, Jang KE, Park JW, Kim Y-T, Park S-J, Jeong KS, Kim A, Kim SH, Kim Y, 2016. Altered executive function in the welders: a functional magnetic resonance imaging study. Neurotoxicol. Teratol 56, 26–34. 10.1016/j.ntt.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Skröder H, Kippler M, Nermell B, Tofail F, Levi M, Rahman SM, Raqib R, Vahter M, 2017. Major limitations in using element concentrations in hair as biomarkers of exposure to toxic and essential trace elements in children. Environ. Health Perspect. 125, 067021 10.1289/EHP1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Gwiazda R, Bowler R, Roels H, Park R, Taicher C, Lucchini R, 2007. Biomarkers of Mn exposure in humans. Am. J. Ind. Med 50, 801–811. 10.1002/ajim.20506. [DOI] [PubMed] [Google Scholar]
- Staud R, 2011. Brain imaging in fibromyalgia syndrome. Clin. Exp. Rheumatol 29, S109–S117. [PubMed] [Google Scholar]
- Sternberg S, 1969. Memory-scanning: mental processes revealed by reaction-time experiments. Am. Sci 57, 421–457. [PubMed] [Google Scholar]
- Tachtsidis I, Scholkmann F, 2016. False positives and false negatives in functional near-infrared spectroscopy: issues, challenges, and the way forward. Neurophotonics 3, 031405 10.1117/1.NPh.3.3.031405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli EC, Ernst T, 2007. Different activation patterns for working memory load and visual attention load. Brain Res. 1132, 158–165. 10.1016/j.brainres.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency, 2005. Reregistration Eligibility Decision for Mancozeb.
- van Wendel de Joode B, Barbeau B, Bouchard MF, Mora AM, Skytt Å, Córdoba L, Quesada R, Lundh T, Lindh CH, Mergler D, 2016. Manganese concentrations in drinking water from villages near banana plantations with aerial mancozeb spraying in Costa Rica: results from the Infants’ Environmental Health Study (ISA). Environ. Pollut. Barking Essex 1987 (215), 247–257. 10.1016/j.envpol.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Ward EJ, Edmondson DA, Nour MM, Snyder S, Rosenthal FS, Dydak U, 2017. Toenail manganese: a sensitive and specific biomarker of exposure to manganese in career welders. Ann. Work expo. Health 62, 101–111. 10.1093/annweh/wxx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt JS, Delpy DT, Cope M, Wray S, Reynolds EOR, 1986. Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet 8515 328, 1063–1066. Originally published as Volume 2, Issue. 10.1016/S0140-6736(86)90467-8. [DOI] [PubMed] [Google Scholar]
- Zaiyang L, Yue-Ming J, Xiang-Rong L, William F, Jun X, Chien-Lin Y, Li-Ling L, Hai-Lan L, Jaroslaw H, James BM, Wei Z, Ulrike D, 2014. Vulnerability of welders to manganese exposure – a neuroimaging study. Neurotoxicology 0, 285–292. 10.1016/j.neuro.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Liao W, Zhengge Wang, Zhongqiu Wang, Li K, Chen H, Liu Y, 2010. Altered spontaneous neuronal activity of the default-mode network in mesial temporal lobe epilepsy. Brain Res. 1323, 152–160. 10.1016/j.brainres.2010.01.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.