Abstract
Background
Primary headache disorders (PHDs) are associated with sleep problems. It is suggested that headache and sleep disorder share anatomical and physiological characteristics. We hypothesised that patients with PHDs were exposed to a great risk for developing sleep apnoea (SA).
Methods
In this retrospective longitudinal study, the data obtained from the Longitudinal Health Insurance Database in Taiwan were analysed. The study included 1346 patients with PHDs who were initially diagnosed and 5348 patients who were randomly selected and age/sex matched with the study group as controls. PHDs, SA, comorbidities and other confounding factors were defined based on International Classification of Diseases, Ninth Revision, Clinical Modification. Cox proportional hazards regressions were employed to examine adjusted HRs after adjusting with confounding factors.
Results
Our data revealed that patients with PHDs had a higher risk (HR 2.17, 95% CI 1.259 to 3.739, p<0.05) to develop SA compared with matched cohorts, whereas patients with migraine exhibited a high risk (HR 2.553, 95% CI 1.460 to 4.395, p<0.01). The results showed that patients with PHDs aged 18–44 exhibited highest risk of developing SA. In addition, males with PHDs exhibited an HR 3.159 (95% CI 1.479 to 6.749, p<0.01) for developing SA, respectively. The impact of PHDs on SA risk was progressively increased by various follow-up time intervals.
Conclusion
Our results suggest that PHDs are linked to an increased risk for SA with sex-dependent and time-dependent characteristics.
Keywords: primary headache disorders, sleep apnea, migraine, population-based
Introduction
Primary headache disorders (PHDs) are considered as chronic disabling illness, which are characterised by repeated exacerbation and unidentifiable causes. They are of global health concern due to their high prevalence and impact on productivity and quality of life in the sufferers. The association between headache and sleep has been known to be bidirectional. It is observed in many primary headache including migraine, tension-type headache (TTH) and cluster headache.1–5 Sleep disturbances have been shown to be a trigger for migraine attacks. Recent research has reported that the sleep disturbance was positively associated with TTH.6 It has been reported that patients with cluster headache suffer from sleep-disordered breathing.7 8 Although the cause-and-effect relationship between primary headache and sleep disorder has been recognised for many decades, the understanding of actual mechanism underlying PHDs and sleep disorder is still sketchy.
Sleep apnoea (SA), a respiratory disturbance during sleep, has gained increasing attention for its increased global prevalence and consequent deteriorations.9 10 The disorders have been associated with various health problems including cardiovascular, metabolic and psychiatric disorder. SA has been associated with different degrees of headache.11 Obstructive SA is known to worsen primary headaches such as migraine and TTH.4 12 Cluster headache has been associated with SA syndrome during active cluster episode.13 SA has been shown to be associated with chronic pain.14 15 On the other hand, a recent study has reported that there is no clear relationship between migraine and obstructive SA in the general population.16 It has been shown that the presence and severity of SA have no influence on presence and attack frequency of TTH.17 However, the association between PHDs and SA remains controversial.
It is suggested that headache and sleep share a close relationship, showing a dense anatomical and physiological overlap in the central nervous system. The aim of the present study was to investigate the relationship between PHDs and SA in the general population. In addition, we hypothesised that patients with PHDs had increased risk for developing all type SA using data from the National Health Insurance Research Database (NHIRD).
Materials and methods
Database
This retrospective study analysed the data obtained from Longitudinal Health Insurance Database (LHID) released by the Taiwan National Health Research Institute. LHID contains all original claims data of 1 million beneficiaries, randomly sampled from the registry for Beneficiaries of NHIRD covering more than 99.5% of Taiwan population. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) was employed for coding diagnosis by the National Health Insurance programme. The data in the LHID were de-identified and therefore the signed informed consent of participants was waived.
Study samples
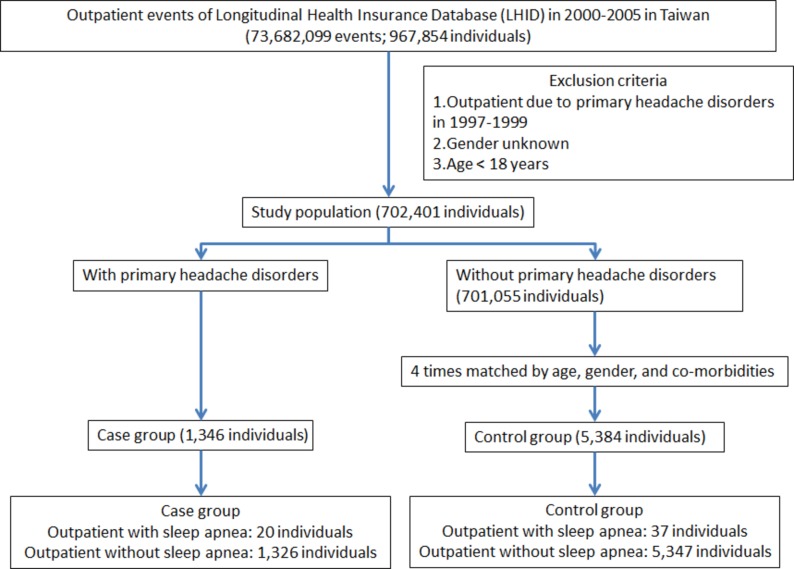
All patients in LHID who were diagnosed for PHDs (ICD-9-CM codes 346, 307.81, 339) for the first time from 2000 to 2005 were identified according to the International Classification of Headache Disorders, Second Edition criteria (N=1346). Patients diagnosed of PHDs before 2000 were excluded to increase the likelihood of identifying new cases. From the beginning of 2000 to the end of 2005 during which a patient was first diagnosed with PHDs was set as the index date. We randomly selected 5384 subjects (a sample size fourfold that of the PHDs group) from LHID, frequency matched with the study cohort in terms of age, sex, index date and comorbidities (chronic obstructive pulmonary disease [COPD], hypertension, diabetes, hyperlipidaemia, stroke, obesity and depression). Each patient was then followed up from the index date until the occurrence of SA. For those who did not have SA, the last day of follow-up was defined as the date of insurance withdrawal or the last day of the study period (31 December 2011) (figure 1). The diagnosis of SA was defined by ICD Classification (ICD-9-CM codes 327.2, 780.51, 780.53, 780.57). Comorbidities that are also SA risk factors were established before the index date based on outpatient data with the following ICD codes: (COPD; ICD-9-CM codes 490–496), hypertension (ICD-9-CM codes 401–405), diabetes mellitus (DM; ICD-9-CM code 250), hyperlipidaemia (ICD-9-CM code 272), stroke (ICD-9-CM codes 430–432, 433–437), obesity (ICD-9-CM codes 278, 649.1, 783.1) and depression (ICD-9-CM code 296).
Figure 1.
The flow chart of study sample selection from National Health Insurance Research Database in Taiwan. Primary headache disorders were including migraine (ICD-9-CM 346), tension-type headache (ICD-9-CM 307.81) and other headache syndromes (ICD-9-CM 339). Sleep apnoea was including organic sleep apnoea (ICD-9-CM 327.2), insomnia with sleep apnoea (ICD-9-CM 780.51), hypersomnia with sleep apnoea (ICD-9-CM 780.53) and unspecified sleep apnoea (ICD-9-CM 780.57). ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
Statistical analysis
Continuous variables were presented as mean SD and categorical variables as frequencies and percentages. Differences between study group and control cohort in the distribution of demographic characteristics (age and sex) and comorbidities (COPD, hypertension, DM, hyperlipidaemia, stroke, obesity and depression) were examined by X2/Fisher’s exact test. Cox proportional hazard regression analysis was performed to calculate adjusted HRs, with 95% CIs, for the impact of PHDs on developing SA. To investigate the interaction of covariates in relation to the association of PHDs and SA, we also calculated adjusted HR stratified by age (<45, 45–64, 65 years), sex and follow-up time. All statistical analyses were performed with SPSS software V.22.0. A two-tailed p<0.05 was considered statistically significant.
Results
In this retrospective study, a total of 1346 patients diagnosed with PHDs and 5384 sex-matched and age-matched controls for comparison were included in this study. Of the patients with PHDs, 71.25% were female and 60.92% were aged between 18 and 44 years old. There were no significant differences in distribution of age, sex and comorbidities between the PHDs group and the matched controls. The baseline and demographic characteristics of enrolled subjects were presented in table 1.
Table 1.
Baseline demographic status and comorbidities compared between comparison and PHDs group
| Variable | PHDs cohort N=1346 (%) |
Comparison cohort N=5384 (%) |
P value |
| Age, years (SD)* | 47.38 (14.56) | 46.74 (15.77) | 0.183 |
| <45 | 820 (60.92) | 3280 (60.92) | |
| 45–64 | 404 (30.01) | 1616 (30.01) | |
| ≥65 | 122 (9.07) | 488 (9.07) | |
| Sex | 0.999 | ||
| Female | 959 (71.25) | 3836 (71.25) | |
| Male | 387 (28.75) | 1548 (28.75) | |
| Comorbidities COPD |
2 (0.15) | 11 (0.20) | 0.070 |
| Hypertension | 22 (1.63) | 73 (1.36) | 0.255 |
| DM | 2 (0.15) | 12 (0.22) | 0.678 |
| Hyperlipidaemia | 4 (0.30) | 24 (0.45) | 0.160 |
| Stroke | 4 (0.30) | 10 (0.19) | 0.307 |
| Obesity | 0 (0) | 0 (0) | – |
| Depression | 6 (0.45) | 18 (0.33) | 0.154 |
*t-test.
COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; PHDs, primary headache disorders.
Of patients with PHDs, 20 patients (1.49%) developed SA with an overall rate of 3.12 cases per 1000 person-years, whereas 1.45% of control groups. Multivariate cox regression analysis revealed that patients with PHDs had a 2.17 times (95% CI 1.259 to 3.739) higher risk to develop SA in comparison with matched control cohort (table 2). We examined whether PHD itself is an age-dependent risk factor for SA. The patients were divided into three groups according to age, namely 18–44, 45–64 and ≥65 years. Our data showed that SA was prevalent in PHDs patients aged 18–44 with an HR risk of 2.509 (95% CI 1.148 to 5.481) compared with that of cohort controls, followed by patients aged 45–64 (HR 2.020; 95% CI 1.892 to 4.573) and ≥65 (HR 1.103; 95% CI 0.123 to 9.871), respectively (table 2). We next determined sex-dependent association of PHDs with SA. Our results revealed that male patients with PHDs had greater risk to develop SA (HR 3.159, 95% CI 1.479 to 6.749), whereas female patients with PHDs had an HR risk of 1.484 (95% CI 1.060 to 3.335). Unexpectedly, our data showed that there was no patient with PHD diagnosed with obesity.
Table 2.
Incidence of sleep apnoea and multivariate Cox proportional hazards regression analysis measured HR for study cohort
| Variable | PHDs cohort | Comparison cohort | Crude HR (95% CI) | Adjusted HR (95% CI) | ||||
| Event | PYs | Rate | Event | PYs | Rate | |||
| Total | 20 | 6406 | 3.12 | 37 | 25 443 | 1.45 | 2.216 (1.245 to 3.764)** | 2.170 (1.259 to 3.739)* |
| <45 years | 10 | 2.986 | 3.35 | 17 | 12 558 | 1.36 | 2.768 (1.650 to 3.986)** | 2.509 (1.148 to 5.481)* |
| 45–64 years | 9 | 2645 | 3.40 | 16 | 9461 | 1.69 | 2.122 (1.117 to 4.365)** | 2.020 (1.892 to 4.573)* |
| ≧65 years | 1 | 775 | 1.29 | 4 | 3437 | 1.16 | 1.954 (0.776 to 4.035) | 1.103 (0.123 to 9.871) |
| Male | 12 | 1814 | 6.62 | 15 | 7158 | 2.10 | 3.622 (1.855 to 7.914)** | 3.159 (1.479 to 6.749)** |
| Female | 8 | 4592 | 1.74 | 22 | 18 286 | 1.20 | 1.499 (1.016 to 2.107)** | 1.484 (1.060 to 3.335)* |
| Migraine | 19 | 6406 | 2.97 | 36 | 25 443 | 1.41 | 2.614 (1.350 to 4.442)*** | 2.553 (1.460 to 4.395)** |
| <45 years | 9 | 2.986 | 3.01 | 16 | 12 558 | 1.28 | 2.879 (1.115 to 5.997)** | 2.636 (1.184 to 5.872)* |
| 45–64 years | 9 | 2645 | 3.40 | 16 | 9461 | 1.69 | 2.794 (1.018 to 4.335)** | 2.618 (1.156 to 5.928)* |
| ≧65 years | 1 | 775 | 1.29 | 4 | 3437 | 1.16 | 1.533 (0.435 to 6.911) | 1.383 (0.155 to 0.735) |
| Male | 11 | 1814 | 6.07 | 15 | 7158 | 2.10 | 3.844 (1.124 to 7.680)*** | 3.693 (1.714 to 7.958)** |
| Female | 8 | 4592 | 1.74 | 21 | 18 286 | 1.15 | 1.975 (0.994 to 5.011) | 1.821 (0.810 to 4.093) |
| Tension-type headache | 1 | 6406 | 0.16 | 28 | 25 443 | 1.10 | 0.745 (0.116 to 4.252) | 0.500 (0.069 to 3.609) |
| <45 years | 1 | 2.986 | 0.33 | 12 | 12 558 | 0.96 | 1.133 (0.225 to 5.096) | 1.224 (0.166 to 9.019) |
| 45–64 years | 0 | 2645 | 0 | 12 | 9461 | 1.27 | 0 | 0 |
| ≧65 years | 0 | 775 | 0 | 3 | 3437 | 0.87 | 0 | 0 |
| Male | 1 | 1814 | 0.55 | 13 | 7158 | 1.82 | 0.901 (0.212 to 4.610) | 0.814 (0.110 to 5.999) |
| Female | 0 | 4592 | 0 | 15 | 18 286 | 0.82 | 0 | 0 |
Model adjusted for age, sex, COPD, hypertension, DM, hyperlipidaemia, stroke, obesity, depression. Rate: incidence rate, per 1000 PYs.
*P<0.05, **P<0.01, ***P<0.001.
COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; PHDs, primary headache disorders; PY, person-year.
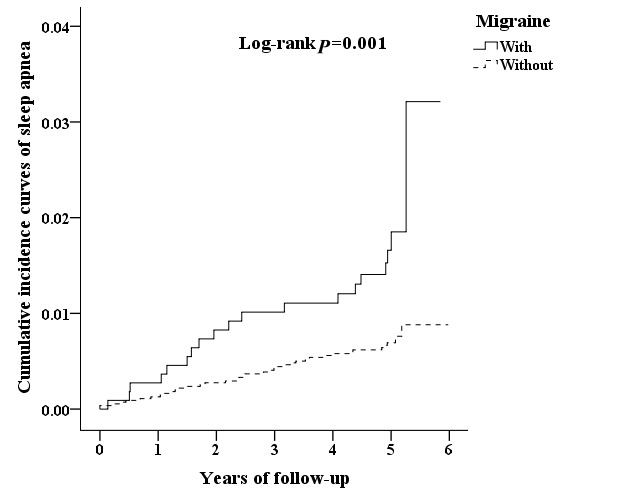
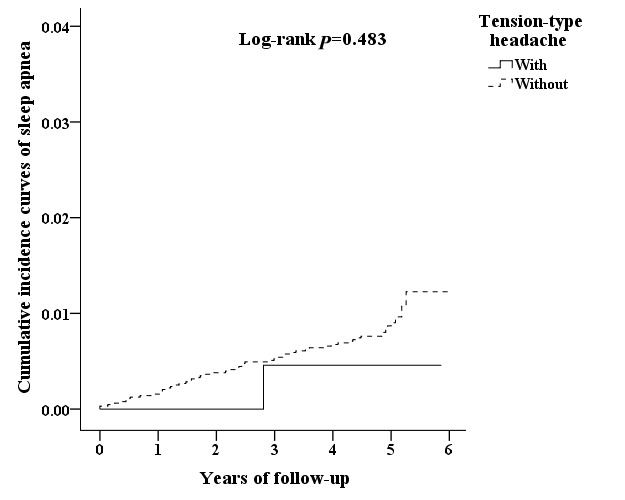
We next analysed the incidence of SA and the subtypes, showing that patients with migraine were more likely to develop SA compared with that of control cohorts (HR 2.553, 95% CI 1.460 to 4.395). We also assessed whether migraine is an age-dependent risk factor for SA by dividing patients into three groups, namely 18–44, 45–64 and ≥65 years. The results revealed that patients with migraine aged 18–44 had the highest risk of developing SA (HR 2.636, 95% CI 1.184 to 5.872) compared with that of cohort controls (table 2). In addition to age, the Cox regression analysis revealed that male patients with migraine had greater risk to develop SA (HR 3.693, 95% CI 1.714 to 7.958), whereas female patients with migraine had an HR risk of 1.821. There was no significant difference between TTH and control cohorts.
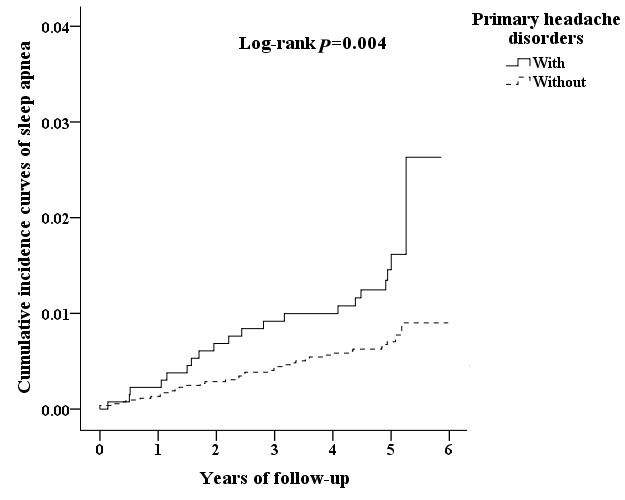
We analysed the incidence of SA in patients with PHDs using multivariate Cox proportional hazards regression analysis based on time intervals. The results showed that patients with PHDs were likely to develop SA within 2 years after diagnosis with time-dependent characteristic (table 3). Interestingly, patients with migraine exhibited SA relatively early in 1 year after diagnosis. Moreover, Kaplan-Meier analysis showed that, compared with the matched cohorts, patients with PHDs had significantly higher incidence of SA (log-rank test p=0.004) (figure 2). Patients with migraine exhibited higher cumulative incidence rate that that of controls cohorts (log-rank test p=0.001) (figure 3), whereas no significant difference between TTH and control cohorts (figure 4).
Table 3.
Incidence of sleep apnoea and multivariate Cox proportional hazards regression analysis measured HR for study cohort by various time intervals
| Variable | PHDs cohort | Comparison cohort | Crude HR (95% CI) | Adjusted HR (95% CI) | ||||
| Event | PYs | Rate | Event | PYs | Rate | |||
| Total | ||||||||
| Follow <1 year | 3 | 8 | 382.65 | 7 | 33 | 211.42 | 1.995 (0.579 to 5.678) | 1.974 (0.507 to 7.684) |
| Follow ≧1, <2 years | 6 | 31 | 193.99 | 8 | 106 | 75.14 | 2.712 (1.002 to 7.654)* | 2.602 (0.992 to 7.510) |
| Follow ≧2 years | 11 | 6367 | 1.73 | 22 | 25 304 | 0.87 | 2.255 (1.043 to 4.468)** | 2.203 (1.080 to 4.174)* |
| Migraine | ||||||||
| Follow <1 year | 3 | 8 | 382.65 | 7 | 33 | 211.42 | 4.556 (0.454 to 9.876) | 4.297 (0.968 to 7.288) |
| Follow ≥1, <2 years | 6 | 31 | 193.99 | 8 | 106 | 75.14 | 3.012 (1.021 to 5.998)* | 2.998 (1.037 to 8.662)* |
| Follow ≥2 years | 10 | 6367 | 1.57 | 21 | 25 304 | 0.83 | 2.511 (1.402 to 5.403)* | 2.225 (1.058 to 4.679)* |
| Tension-type headache | ||||||||
| Follow <1 year | 0 | 8 | 0 | 5 | 33 | 151.01 | 0 | 0 |
| Follow ≥1, <2 years | 0 | 31 | 0 | 7 | 106 | 65.75 | 0 | 0 |
| Follow ≥2 years | 1 | 6367 | 0.16 | 16 | 25 304 | 0.63 | 0.882 (0.005 to 7.416) | 0.871 (0.119 to 6.374) |
Model adjusted for age, sex, COPD, hypertension, DM, hyperlipidaemia, stroke, obesity, depression. Rate: incidence rate, per 1000 PYs.
*P<0.05, **P<0.01.
COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; PHDs, primary headache disorders; PYs, person years.
Figure 2.
The cumulative incidence curves of sleep apnoea for the individual with and without primary headache disorders.
Figure 3.
The cumulative incidence curves of sleep apnoea for the individual with and without migraine.
Figure 4.
The cumulative incidence curves of sleep apnoea for the individual with and without TTH.
Discussion
In this national population-based longitudinal study, we found that PHD itself was associated with an increased risk to develop SA in individuals without comorbid sleep problem. We showed that male patients with PHDs are more likely to develop SA. Our results also indicate that increasing age was not a risk factor for developing SA in patients with PHDs. Furthermore, the impact of PHDs on SA risk was progressively increased by various follow-up time intervals with time-dependent characteristic.
Headache is a neurobiological disorder suggested to share an anatomical characteristics and pathophysiological mechanism with sleep disorders.18 Several PHDs have been associated with sleep disorders, including TTH, migraine and cluster headache. Migraine has been reported for its association with sleep disturbance.19 Patients with sleep-related breathing disorders have been shown to have a high risk of developing migraine.11 A cross-sectional population-based study reports a counterargument that migraine and obstructive SA are unrelated in the general population.16 TTH is suggested to be the most common primary headache, which is characterised by episodes of bilateral pressing pain. Increasing evidence has highlighted the relationship of TTH and SA. A cross-sectional population-based study conducted in Norway has revealed subjects with TTH is more likely to have comorbid obstructive SA.20 A recent study has reported that patients with obstructive SA have a higher risk for developing TTH as sleep-related headache.4 In contrast, Kristiansen et al conducted a population-based study showing that the association of SA with TTH was insignificant in the general population. In the present study, we found that PHDs are more prevalent in women than men, which is consistent with population-based studies.21–24 It is agreed that headache has female dominance attributed to the imbalance hormone particularly oestrogen. However, we were unable to distinguish the causes of PHD in our study population due to the nature of the database used. Our results revealed that patients initially diagnosed with PHDs exhibited a high risk for developing SA. Moreover, we found that patients with migraine were more likely to have SA than matched control cohort. There is a discrepancy in association of PHDs and SA between present study and the others. It may be explained by the nature of study designs, given that we included the patients initially diagnosed with PHDs and analysed the longitudinal data extracted from LHID. A recent study using LHID data has reported that obstructive SA is a risk factor for TTH.4 PHDs and SA are suggested to share both obesity and diabetes patients as possible comorbidities.25 26 In our study, PHDs showed low comorbidities with obesity and diabetes with an average age of 47.38. Given a female dominance in the study population, it is postulated that obesity and diabetes were yet to occur before diagnosis for PHD. Our data suggested that PHD itself represents a risk factor for SA in years to come. Given these findings, it is suggested that a vicious circle of PHDs and SA develops on first occurrence of symptoms and treatment for either disorder can have effects on the other. However, further prospective studies with control group are necessary to determine the interaction between PHDs and SA and the mechanism underlying the pathophysiology.
SA is a breathing abnormality specific to the sleeping state. Obstructive SA is caused by soft-tissue collapse in the upper airway such as pharynx. It is attributed to anatomic factor such as narrow airway or abnormal neuromuscular activity during sleep.27–30 Central SA is known to be attributed to a temporary cessation in respiratory rhythm involved in brainstem respiratory networks.31 It has been reported that the presence of a midbrain plaque is associated with an increased likelihood of headache, including migraine and TTH.32 A function imaging study using positron emission tomography has shown that lesion in rostral brainstem resulted in some clinical presentations such as TTH and migraine.33 This study provides biologically plausible evidence supporting that brainstem is pivotal in primary headache pathophysiology. In the present study, we found that PHD itself is associated with increased incidence of developing SA. Moreover, our results indicated that SA may occur in 2 years after initial diagnosis with PHDs. Our finding that there is a higher incidence of SA in patients with migraine is postulated to be attributed to the aberrant neurobiology in brain stem. This is supported by the evidence that brainstem lesion cause migraine-like characteristics.32 34 Although obstructive SA is prevalent in clinical practice, we were unable to separate obstructive SA from the other SA based on the database used. Chronic sleep disorders can exacerbate headache and lead to medication-overuse headache, which deteriorates the symptom of headache.35 36 However, further imaging studies are needed to elucidate the mechanism by which PHD itself is causative factor of SA.
There are several limitations to the present study. First, in this cohort study, there was an inability to validate diagnoses and objective measure of PHDs. There might be coding error occurring in the database. Second, as the nature of retrospective longitudinal study, the data of medication that might have effects on PHDs and/or SA was not taken into account for analysis. Medication for PHDs is suggested to lead to medication-overuse headache that might contribute to the symptoms of PHDs.37 Such medication record was not accessible from LHID. Third, we were unable to distinguish the causation of PHDs based on data extracted from LHID. We acknowledge that lack of information on some adaptable risk factors, such as sleep pattern, dietary habits and depression might lead to a misinterpretation of the findings.
In conclusion, we reported subjective evidence supporting the hypothesis that patients with PHDs are more likely to develop SA. The results suggest that PHD itself is a sex-dependent and time-dependent risk factor of SA. Further prospective studies with imaging are required to elucidate the underlying mechanism by which PHDs are pathophysiologically linked to SA.
What is already known on the subject.
The association between headaches and sleep problems is bidirectional.
Sleep apnea is a risk factor for the presence of neurologic diseases.
Main messages.
Patients initially diagnosed with primary headache disorders have a high risk for developing sleep apnoea.
Patients with migraine are likely to develop sleep apnoea in short period of time from first diagnosis of primary headache disorders.
A cause-and-effect relationship between primary headache and sleep apnoea is reported.
Current research questions.
Could chronic sleep disorders other than sleep apnoea contribute to primary headache disorders due to long-term medication for sleep problem?
What neuroimaging study for primary headache disorders could be performed on daily basis to distinguish the cause-and-effect relationship with sleep apnoea syndrome?
Would treatments for primary headache disorders reduce the risk for developing sleep apnoea including obstructive ones?
Footnotes
Contributors: J-HY and J-TL were involved in the study concept and design, the data collection, the analysis and interpretation of data and the drafting of manuscript. S-YC, C-CL, Y-FS, C-HCho, C-HChu, W-CC, F-CY, C-KT, C-LT and G-YL were involved in the study design, the analysis of data and interpretation of data. All of the authors critically reviewed the manuscript and gave final approval of the version to be published.
Funding: This study was supported in part by grants from the Tri-Service General Hospital (TSGH-C104-084; TSGH-C105-084; TSGH-C100-101; TSGH-C101-080; TSGH-C103-085; TSGH-C104-083; TSGH-C105-085), Ministry of Science and Technology (MOST104-2314-B-016–017-MY3; MOST 105–2314-B-016–004-), Teh-Tzer Study Group for Human Medical Research Foundation (A1031031).
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: This study protocol was reviewed and approved by Institutional Review Board of Tri-Service General Hospital (TSGHIRB No.: 1-104-05-112).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Gupta R, Bhatia MS, Dahiya D, et al. Impact of primary headaches on subjective sleep parameters among adolescents. Ann Indian Acad Neurol 2008;11:164–9. 10.4103/0972-2327.42936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache 2005;45:904–10. 10.1111/j.1526-4610.2005.05159.x [DOI] [PubMed] [Google Scholar]
- 3. Wang Y, Xie J, Yang F, et al. Comorbidity of poor sleep and primary headaches among nursing staff in North China. J Headache Pain 2015;16:88 10.1186/s10194-015-0571-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chiu YC, Hu HY, Lee FP, et al. Tension-type headache associated with obstructive sleep apnea: a nationwide population-based study. J Headache Pain 2015;16:34 10.1186/s10194-015-0517-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Tommaso M, Delussi M, Vecchio E, et al. Sleep features and central sensitization symptoms in primary headache patients. J Headache Pain 2014;15:64 10.1186/1129-2377-15-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang J, Huang Q, Li N, et al. Triggers of migraine and tension-type headache in China: a clinic-based survey. Eur J Neurol 2013;20:689–96. 10.1111/ene.12039 [DOI] [PubMed] [Google Scholar]
- 7. Chervin RD, Zallek SN, Lin X, et al. Timing patterns of cluster headaches and association with symptoms of obstructive sleep apnea. Sleep Res Online 2000;3:107–12. [PubMed] [Google Scholar]
- 8. Chervin RD, Zallek SN, Lin X, et al. Sleep disordered breathing in patients with cluster headache. Neurology 2000;54:2302–6. 10.1212/WNL.54.12.2302 [DOI] [PubMed] [Google Scholar]
- 9. Young T. Sleep-disordered breathing in older adults: is it a condition distinct from that in middle-aged adults? Sleep 1996;19:529–30. 10.1093/sleep/19.7.529 [DOI] [PubMed] [Google Scholar]
- 10. Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–5. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 11. Harnod T, Wang YC, Kao CH. Association of migraine and sleep-related breathing disorder: a population-based cohort study. Medicine 2015;94:e1506 10.1097/MD.0000000000001506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson KG, Ziemba AM, Garb JL. Improvement in headaches with continuous positive airway pressure for obstructive sleep apnea: a retrospective analysis. Headache 2013;53:333–43. 10.1111/j.1526-4610.2012.02251.x [DOI] [PubMed] [Google Scholar]
- 13. Evers S, Barth B, Frese A, et al. Sleep apnea in patients with cluster headache: a case-control study. Cephalalgia 2014;34:828–32. 10.1177/0333102414544038 [DOI] [PubMed] [Google Scholar]
- 14. Pampati S, Manchikanti L. What is the prevalence of symptomatic obstructive sleep apnea syndrome in chronic spinal pain patients? An assessment of the correlation of OSAS with chronic opioid therapy, obesity, and smoking. Pain Physician 2016;19:E569–79. [PubMed] [Google Scholar]
- 15. Shapiro CM, Chung SA, Wylie PE, et al. Home-use servo-ventilation therapy in chronic pain patients with central sleep apnea: initial and 3-month follow-up. Sleep Breath 2015;19:1285–92. 10.1007/s11325-015-1161-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kristiansen HA, Kværner KJ, Akre H, et al. Migraine and sleep apnea in the general population. J Headache Pain 2011;12:55–61. 10.1007/s10194-010-0268-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kristiansen HA, Kværner KJ, Akre H, et al. Tension-type headache and sleep apnea in the general population. J Headache Pain 2011;12:63–9. 10.1007/s10194-010-0265-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holland PR. Headache and sleep: shared pathophysiological mechanisms. Cephalalgia 2014;34:725–44. 10.1177/0333102414541687 [DOI] [PubMed] [Google Scholar]
- 19. Stark CD, Stark RJ. Sleep and chronic daily headache. Curr Pain Headache Rep 2015;19:468 10.1007/s11916-014-0468-6 [DOI] [PubMed] [Google Scholar]
- 20. Ødegård SS, Engstrøm M, Sand T, et al. Associations between sleep disturbance and primary headaches: the third Nord-Trøndelag health study. J Headache Pain 2010;11:197–206. 10.1007/s10194-010-0201-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manandhar K, Risal A, Steiner TJ, et al. The prevalence of primary headache disorders in Nepal: a nationwide population-based study. J Headache Pain 2015;16:95 10.1186/s10194-015-0580-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. El-Sherbiny NA, Masoud M, Shalaby NM, et al. Prevalence of primary headache disorders in Fayoum Governorate, Egypt. J Headache Pain 2015;16:85 10.1186/s10194-015-0569-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oshinaike O, Ojo O, Okubadejo N, et al. Primary headache disorders at a tertiary health facility in Lagos, Nigeria: prevalence and consultation patterns. Biomed Res Int 2014;2014:1–5. 10.1155/2014/782915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ayzenberg I, Katsarava Z, Sborowski A, et al. The prevalence of primary headache disorders in Russia: a countrywide survey. Cephalalgia 2012;32:373–81. 10.1177/0333102412438977 [DOI] [PubMed] [Google Scholar]
- 25. Ho ML, Brass SD. Obstructive sleep apnea. Neurol Int 2011;3:e15 10.4081/ni.2011.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sugerman HJ, Felton WL, Salvant JB, et al. Effects of surgically induced weight loss on idiopathic intracranial hypertension in morbid obesity. Neurology 1995;45:1655–9. 10.1212/WNL.45.9.1655 [DOI] [PubMed] [Google Scholar]
- 27. McGinley BM, Schwartz AR, Schneider H, et al. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol 2008;105:197–205. 10.1152/japplphysiol.01214.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horner RL. Contributions of passive mechanical loads and active neuromuscular compensation to upper airway collapsibility during sleep. J Appl Physiol 2007;102:510–2. 10.1152/japplphysiol.01213.2006 [DOI] [PubMed] [Google Scholar]
- 29. He J, Kastin AJ, Wang Y, et al. Sleep fragmentation has differential effects on obese and lean mice. J Mol Neurosci 2015;55:644–52. 10.1007/s12031-014-0403-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chung S-D, Lin C-C, Liu S-P, et al. Obstructive sleep apnea increases the risk of bladder pain syndrome/interstitial cystitis: a population-based matched-cohort study. Neurourol Urodyn 2014;33:278–82. 10.1002/nau.22401 [DOI] [PubMed] [Google Scholar]
- 31. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol 2013;3:141–63. 10.1002/cphy.c110057 [DOI] [PubMed] [Google Scholar]
- 32. Gee JR, Chang J, Dublin AB, et al. The association of brainstem lesions with migraine-like headache: an imaging study of multiple sclerosis. Headache 2005;45:670–7. 10.1111/j.1526-4610.2005.05136.x [DOI] [PubMed] [Google Scholar]
- 33. Goadsby PJ. Neurovascular headache and a midbrain vascular malformation: evidence for a role of the brainstem in chronic migraine. Cephalalgia 2002;22:107–11. 10.1046/j.1468-2982.2002.00323.x [DOI] [PubMed] [Google Scholar]
- 34. Duning T, Deppe M, Brand E, et al. Brainstem involvement as a cause of central sleep apnea: pattern of microstructural cerebral damage in patients with cerebral microangiopathy. PLoS One 2013;8:e60304 10.1371/journal.pone.0060304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manni R, Ghiotto N. Hypnic headache. Handb Clin Neurol 2010;97:469–72. 10.1016/S0072-9752(10)97041-3 [DOI] [PubMed] [Google Scholar]
- 36. Dodick DW, Eross EJ, Parish JM. Clinical, anatomical, and physiologic relationship between sleep and headache. Headache 2003;43:282–92. 10.1046/j.1526-4610.2003.03055.x [DOI] [PubMed] [Google Scholar]
- 37. Kristoffersen ES, Lundqvist C. Medication-overuse headache: epidemiology, diagnosis and treatment. Ther Adv Drug Saf 2014;5:87–99. 10.1177/2042098614522683 [DOI] [PMC free article] [PubMed] [Google Scholar]