Abstract
During reproductive age, approximately one in seven couples are confronted with fertility problems. While the aetiology is diverse, including infections, metabolic diseases, hormonal imbalances and iatrogenic effects, it is becoming increasingly clear that genetic factors have a significant contribution. Due to the complex nature of infertility that often hints at a multifactorial cause, the search for potentially causal gene mutations in idiopathic infertile couples has remained difficult. Idiopathic infertility patients with a suspicion of an underlying genetic cause can be expected to have mutations in genes that do not readily affect general health but are only essential in certain processes connected to fertility. In this review, we specifically focus on genes involved in meiosis and maternal-effect processes, which are of critical importance for reproduction and initial embryonic development. We give an overview of genes that have already been linked to infertility in human, as well as good candidates which have been described in other organisms. Finally, we propose a phenotypic range in which we expect an optimal diagnostic yield of a meiotic/maternal-effect gene panel.
Keywords: clinical genetics, diagnostics, genetic screening/counselling, obstetrics and gynaecology
Background
It is estimated that 10%–15% of couples are affected by infertility during reproductive age, with equal distribution of subfertility between men and women.1 A significant proportion of couples are unsuccessful despite having healthy reproductive age, no detectable physical, endocrine or immune problems, apparently adequate quantity and quality of gametes and no apparent technical laboratory issues affecting the Artificial Reproduction Technologies (ART) procedures. For example, 50%–80% of cases diagnosed with primary ovarian insufficiency (POI) remain idiopathic2 3; likewise, in 80% of men with non-obstructive azoospermia, the cause remains unknown.4 For such individuals, there are currently limited options for intervention to optimise fertility. When confronted with idiopathic infertility patients, an important first test that is often used by fertility centres is karyotyping. In a cohort study of 1663 azoospermic men, 14% of the tested individuals had chromosomal abnormalities, stressing the importance of karyotyping as a first-tier test.5 Patients with a normal karyotype and with exclusion of other causes may however be warranted to undergo genetic analysis.
In a clinical setting, one of the routes that can be followed to accomplish this is diagnostic gene panel sequencing. In humans to date, only a limited number of genetic changes have been found, affecting fertility in small numbers of cases.4 6 These findings hint at a multifactorial genetic origin and/or environmental influences.7 In this scenario, the setup of genetic studies for infertility faces the risk of being underpowered because of an insufficient amount of samples and due to difficulties in clearly delineating the clinical pathophysiology. Therefore, to potentially increase the diagnostic yield of gene panels, both the patient phenotype and the disease spectrum of the investigated genes should be matched as well as possible. For example, when investigating the genetic causes of subfertility of individuals with no other overt health problems, and without other physical, environmental, endocrinological or structural problems, one of the potential causes could be found in the process of meiosis, an absolute prerequisite for both male and female gamete formation. In addition to this, defects in maternal-effect processes could be suspected as well.
We here suggest that during in-vitro fertilisation (IVF) treatment, errors in meiotic and maternal-effect genes can, in absence of an overt male factor, lead to a reduced fertilisation rate and an impaired early embryonic development. Meiotic defects have furthermore been described to be implicated in POI as well.8 9 However, the genetics of POI is broad, while in this review the emphasis is put on meiotic and maternal-effect genes with a potential clinical implication in infertility. Since genetic and functional evidence from humans is limited, our study will be mainly based on reports from animal models. Most particularly, research in mice has explored many reproductive processes and identified critical factors. nimal studies are cited when relevant, with the understanding that species differences limit the power of extrapolation to humans.
Meiosis
Meiosis is an essential process of gamete formation, and its genetic disruptions are likely to have a considerable impact on fertility. Expression of meiosis genes is implicated in considerations including ovarian reserve, ovarian response, and oocyte maturation and activation. Meiosis gene mutations may therefore lead to a number of clinical pathologies such as POI, insufficient oocyte maturation and low fertilisation rate.
Several distinct steps are necessary for meiotic completion, including the formation of double-strand breaks (DSBs), chromosome synapsis, homologous recombination (HR), separation of homologous chromosomes during first meiotic division (MI) and separation of sister chromatids during meiosis II (MII). Since the spatiotemporal regulation of meiosis is also dependent on somatic cells in humans, namely the granulosa cells in women and Sertoli cells in men, genes involved in the crosstalk between the somatic and the germline compartment are also relevant to meiotic success.
Below, we describe the molecular subprocesses of meiosis and as such define a collection of genes warranting inclusion in a diagnostic gene panel for idiopathic infertility. This will comprise both genes that have already been described in an idiopathic fertility setting, as well as unreported genes that have a high potential to lead to meiotic errors when disturbed (figure 1).
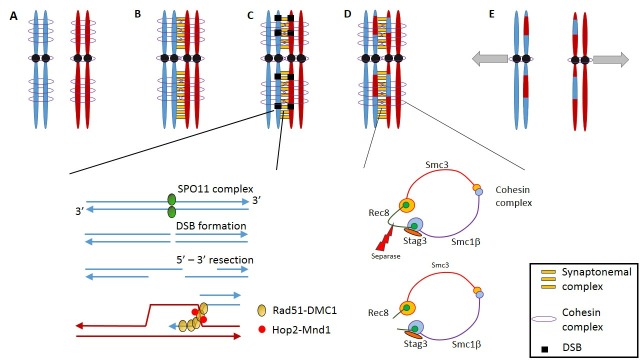
Figure 1.
Overview of critical processes during the MI stage. (A) After DNA replication, sister chromatids of both homologous chromosome pairs are held together by multiple units of the cohesin complex. (B) Alignment of the homologous chromosomes is facilitated by the synaptonemal complex. (C) The first step of homologous recombination occurs through the formation of double strand breaks (DBS). This process is Spo-11 dependent, and strand invasion is mediated by the Rad51-DMC1 complex, which is stabilised by Hop2-Mnd1. (D) After homologous recombination, the cohesin complex of the sister chromatids is cleaved by separase along the length of the sister chromatids. Cohesin at the centromeres is protected by shugosin, inhibiting the separase-mediated cleaving. (E) Sister kinetochores connect to microtubules emanating from the same spindle poles, as such separating the newly recombined homologues.
The synaptonemal complex (SC): basis for chromosome pairing, synapsis and recombination
An essential premise for meiosis to take place is the correct alignment of homologous chromosomes (pairing) during its initial stages. A crucial mediator for this process is the SC, a multiprotein structure that is assembled during meiotic prophase I and that is essential for synapsis, meiotic crossover10 and correct segregation of homologous chromosomes during anaphase in the first meiotic division.11 Given the pivotal role of the SC in meiosis, mutations in SC would be expected to give rise to fertility problems.
The SYCP3 protein is, together with SYCP2, one of the main components of the lateral elements of the SC and is essential for chromosome loading on the SC.12 Mutations in SYCP3 have been shown in men with non-obstructive azoospermia.13 Examination of testicular biopsies revealed that the most mature spermatogenic cells were early spermatocytes, indicating a meiotic arrest, whereas SYCP3 mutations in women do not seem to lead to a meiotic arrest but result in recurrent pregnancy loss, probably due to the presence of aneuploidies.14 This sexual dimorphism is speculated to arise from greater stringency of the pachytene checkpoint in men than in women.10 To date, no mutations have been found in SYCP2, but mouse Sycp2 mutants show a phenotype reminiscent of human SYCP3 mutations, including the sexual dimorphism.15 Females lacking the SYCP2-like gene product SYC2PL undergo accelerated reproductive ageing.16
Mutations in the SC component SYCE1 have been reported in cases of human infertility.17 SYCE1 is a component of the central element of the SC. Both male and female Syce1-mutant mice are infertile and are characterised by an arrest in prophase I.18 Reports of human SYCE1 variants identify azoospermia in affected men and women affected by premature ovarian insufficiency (POI).17 19 Additionally, in mice, the absence of Meiob and Spata22, two proteins associating with the SC and forming discrete foci on meiotic chromosomes causes failure of meiotic synapsis. Although Meiob ablation is associated with both male and female infertility in mice, in humans MEIOB mutation has been associated only with male azoospermia.20 21 Murine ablation of Spata22 is also associated with male and female infertility through failure of synapsis.22
Double strand break formation
Precise alignment of the homologous chromosomes allows the initiation of the next meiotic process, recombination or crossing over (figure 1). Crossover occurs at one or multiple sites along the length of each chromosomal arms, resulting in the formation of chiasmata, and these chiasmata are essential to maintain chromosome cohesion during meiosis. Reduced recombination or incorrect placement of chiasmata is associated with increased incidence of aneuploidy.23–25 Paucity of chiasmata is most likely to lead to aneuploidy in the smallest chromosomes, for example, chromosome 21, there is evidence that the genome-wide frequency of crossover may have some genetic basis. In families where one offspring has Trisomy 21, genome-wide analysis indicates that the frequency of crossovers is reduced in the individual affected by Trisomy 21 and in siblings26; and this crossover frequency may be partly accounted for by variation in the recombination factor PRDM9.27
Interestingly, the helicase-homologous protein HFM1, expressed in male and female germ tissues, appears to be required for formation or resolution of crossovers; in mice lacking this gene product, early steps in crossover are normal, but then most crossovers are eliminated and the majority of germ cells undergo apoptosis.28 Human HFM1 variants have been identified in women affected by POI.29 Furthermore, MCM8 and MCM9, two essential proteins required for HR drivenDNA repair, are more widely expressed in somatic tissues, and their ablation results in accumulation of DNA damage in response to replication stress, but nonetheless, the key phenotype of mice lacking these proteins is infertility, apparently due to errors in homologous recombination (HR).30 Variants in MCM8 have been identified in women affected by POI.31 32
Meiotic crossover requires the creation of DSBs in individual chromosomes and subsequent recombination between chromosome homologues. Meiotic DSB generation requires the highly conserved SPO11 topoisomerase-like protein (figure 1). In human, heterozygous SPO11 mutations have been shown in men with azoospermia.33 In mouse models entirely lacking Spo11, spermatogenesis arrested before the pachytene stage, while oocytes arrested in prophase I.34 35 SPO11−/− preleptotene spermatocytes lacked homologous pairing, independent of the SPO11 DSB catalytic activity.36 However, in a hypomorphic mouse model expressing 60% normal levels of Spo11, spermatocyte development was normal,37 and Spo11+/− male mice showed no reduction in fertility compared with wild-type animals.38
Genetic defects in the regulatory machinery of SPO11 could also contribute to a fertility phenotype. Studies in yeast have delineated distinct mechanisms for SPO11 regulation in meiosis, either through intrinsic control of SPO11 dimerisation and nuclear retention, or through regulation of its interaction with DNA recombination hotspots. For instance, Rec102, Rec104 and Ski8 are required for SPO11 dimerisation, DNA binding and nuclear retention in yeast.39–41 However, the SPO11 accessory proteins REC11, Mer2 and Mei4 form a complex that is essential for the DNA binding and guiding of SPO11 to DSB cleavage sites.42 Mei4−/− male mice are unable to initiate DSB formation in meiosis, resulting in synaptic defects and arrest of spermatogenesis.42 Mutations in homologous SPO11-associated genes have however not yet been described in humans.
In mice, an additional factor that has been shown to be necessary for DSB formation/maintenance is Hormad1. Knockout mice meiocytes show a strong reduction in single-stranded DSB ends, as is evidenced by the diminished presence of Dmc1/Rad51 foci.43 As both Hormad1 and its close paralogue Hormad2 associate with the axis of unsynapsed chromosomes and have been hypothesised as inhibitors of interstrand DNA repair, thus favourising interhomologous driven repair, chromosome synapsis is disrupted as well in the Hormad1/2−/− models.44 45 On synapsis, Hormad1/2 dissociate from the chromosomal axis, a process that is facilitated by Trip13. Trip13−/− mice oocytes show full chromosome synapsis but are unable to repair the Spo11-mediated DSBs, further supporting the role of Hormad1/2 in interhomologous repair.46 Failure of DSB repair leads to Chk2-dependent oocyte clearance. Interestingly, while testes of Hormad1−/− mice show progressive atrophy, ovarian development does not seem to be affected.47 However, embryos of Hormad1−/− females do not proceed further than the blastocyst stage.
Homologous recombination (HR)
Creation of meiotic DSB is followed by HR, which is driven by cellular DNA repair machinery that is shared between germline and somatic cells (figure 1). DSB repair is initiated by the Mre11-Rad50-Nbs1 complex, which attracts both the ataxia telangiectasia mutated (ATM) and ataxia telangiectasia mutated and Rad3 related (ATR) kinases to the DSB sites and which in their turn phosphorylate histone H2AFX that acts as a beacon to attract novel repair associated proteins.48 In addition, ATM phosphorylates multiple DNA damage repair associated factors including CHK2, BRCA1/2 and P53, which subsequently orchestrate crucial cell cycle checkpoints and the potential decision to drive the cell towards apoptosis if DSB repair is unsuccessful.49 Repair by HR is mediated by DMC1 and Rad51 proteins, which form a nucleosome complex around the single strand overhangs of DSBs. Rad51 is an essential facilitator for DMC1-mediated interhomologous strand invasion.50 Interaction of the DMC1/Rad51 complex with the strand invasion structure is furthermore enhanced by the Hop2–Mnd complex.51 Spermatocytes or oocytes with unrepaired DSBs are expected to be eliminated due to apoptosis or undergo induced senescence. Dmc1−/− mice ovaries are devoid of follicles, while depletion of Chk2 can rescue the phenotype by preventing Chk2-dependent Trp53 (p53 in humans) activation and subsequent apoptosis.46 In humans, meiotic DSB repair is furthermore facilitated by the MSH4–MSH5 heterodimer, which specifically associates with Holliday junctions, thereby stabilising the DSB intermediates.52 MSH4/5 proteins are members of the MutS homologues which are mainly implicated in mismatch repair (MMR). While MSH2, 3 and 6 are implicated in mitotic MMR, MSH4/5 are specifically active during meiosis. Interestingly, MSH5 is also expressed in granulosa cells.53 Mutations in both MSH4 and MSH5 have been detected in POI families.53 54
DNA repair-deficient mice often result in early lethality, as has been demonstrated for Rad51, PalB2, Brca1 and Brca2 knockout models.55 Human mutations in DSB repair genes including ATM, ATR, CHEK2, RAD51 and BRCA1/2 are associated with morbid phenotypes including cancer predispositions, and to date, no clear link has been demonstrated between hypomorphic variants in these genes and an infertility phenotype. It is not clear at present whether they warrant inclusion in an infertility gene panel.
The specific case of BRCA1 and BRCA2 deserves further mention. Both proteins are involved in DSB repair and resolution of HR, and women carrying inactivating mutations are at elevated risk of cancer. A recent metastudy of carriers of BRCA1/2 variants did not reveal significant subfertility compared with a normal control population.56 However, BRCA2+/− mice show a significant reduction in germline cells.57 Spermatocytes do not progress further than early prophase I, while oocytes have been shown to progress through prophase, although with the presence of nuclear abnormalities. Likewise, BRCA1+/− mice are subfertile, characterised by an increase in oocyte apoptosis after hormonal stimulation and smaller litter sizes.58 Notwithstanding these observations, the association of BRCA variation with cancer susceptibility mandates caution in including these genes in a fertility gene panel.
Meiosis: cohesin is key
On completion of HR, MI is initiated. To keep the sister chromatids together until separation in MII, spatiotemporal regulation of the cohesin complex is necessary. While the cohesin complex is located along the whole length of the sister chromatids during synapsis and HR, cohesin is depleted from the arms of sister chromatids after MI but from centromeres only in MII59 (figure 1). Protection of centromeric cohesin prevents premature separation of the sister chromatids at MI, which could result from the mechanical pull of the kinetochores. Failure of maintaining centromeric cohesin integrity could potentially lead toaneuploidy. In humans, the cohesin complex consists of Smc1, Smc3, Rec8 and a STAG1-3/Scc3 subunit.60 After HR, phosphorylation of cohesin subunits (in particular Rec8) along the length of the sister chromatids permits separase cleavage of Rec8, breakdown of the cohesin complex and separation of chromatid arms.59 At the centromeres, cohesin association with the shugoshin–PP2A phosphatase complex blocks phosphorylation and prevents premature separase-induced cleavage. When cells enter MII, the shugosin–PP2A complex is antagonised by the SET protein, allowing Rec8 cleavage by separase and separation of the sister chromatids.61
Meiotic segregation errors (leading to aneuploidy) increase in frequency with age, because of the incremental depletion of cohesin and Sgo2.62 Both male and female mice lacking Sgo2 are infertile, but in humans, SGO2 mutation has been reported only once to date.63 In mice, Sgo2 is furthermore stabilised by Meikin and consequentially, oocytes of Meikin−/− females display a disrupted anaphase II due to premature separation of the sister chromatids.64 Furthermore, human homozygous mutations in STAG3 are associated with POI.65 This has been mimicked in Stag3−/− mice where further investigation showed a meiotic arrest at prophase I, leading to oocyte depletion. Moreover, mice that have a knockout for Rec8, the phosphoprotein acting as a switch for separase degradation, are born in a submendelian frequency and are sterile.66 However, since other cohesin subunits are essential for both mitosis and meiosis, mutations in these result in congenital morbidities rather than reprodcutive disorders; for example, SMC1A mutations cause Cornelia de Lange syndrome, an X-linked dominant disorder characterised by growth retardation, developmental delay and often microcephaly.67 68 It remains possible that hypomorphic variants in cohesin complex components and regulators may produce reproductive effects, warranting their inclusion in a diagnostic gene panel for fertility.
Failure of completing MI or MII: meiotic arrest
The impossibility of an oocyte to complete MI or MII will, in case the oocyte pool is not fully cleared in the ovaries, likely result in fertilisation failure even when intracytoplasmic sperm injection (ICSI) is applied, and this can, in theory, be caused by mutations in any of the genes described above. However, during recent years, multiple novel genes have been described as being essential for meiotic progression. Although most work has been performed in mice and Xenopus, it can be expected that similar effects can be seen in humans in the homologous genes. Mutations in PATL2 (shown in humans, mice and Xenopus), Lfng (shown in mice), Prkar2b (shown in mice), Cks1, Cks2, Mos (shown in Xenopus and mice) and Smc1b all have been shown to lead to failure to proceed through meiosis.64 69–73 The processes these genes are involved in are diverse. For instance, oocytes of Cks2 null mice fail to proceed after prophase I and while the same holds true for Cks1 null mice, the Cks2 null oocytes can be rescued by microinjection of Cks1 mRNA.69 74 Both Cks1 and Cks2 bind to Cdk1 and Cdk2 (cyclin dependent kinases 1 and 2, respectively) complexes thereby modulating the cell cycle.75 Interestingly, in Xenopus, it has been demonstrated that the CKS1 homologue strongly enhances phosphorylation of the downstream Cdk target Myt1, by which meiosis I entry is enabled.76 77 Furthermore, entry into MI in Xenopus requires Mos activation which, in turn, phosphorylates Myt1.78 Mos, which is an upstream activator of the mitogen-activating protein kinase (MAPK) pathway, is also implicated in maintaining the oocyte MII arrest (shown in mice and in Xenopus) by indirectly phosphorylating EMI2, an inhibitor of the anaphase promoting complex.79 A complementary mechanism by which MI is arrested prior to the oestrous cycle is through cyclic adenosine monophosphate (cAMP)-mediated phosphorylation of Pka(cAMP-dependent protein kinase), which activates the kinase Wee2 (or Wee1b) which, in turn, will phosphorylate Cdk1, allowing the maintenance of prophase arrest.80 Intriguingly, when the oocyte has progressed to MII, Wee2 is also necessary for final MII exit by phosphorylation of its target Cdc2. In mice oocytes, inhibition of Wee2 results in failure of pronucleus formation and consequently to the impossibility of fertilisation.81 Likewise, in humans, it has recently been shown that homozygous WEE2 mutations result in oocyte fertilisation failure. Injection of WEE2 mRNA could compensate for the mutations and effectively resulted in fertilisation.82
In contrast to cell cycle modulation, the Lfng protein is a regulator of Notch signalling by post-translationally modifying the N-acetylglucosamine content of the Notch receptor, resulting in alteration of its ligand binding capacity.72 While Lfng−/− mice are not born at Mendelian ratios, the surviving female mice are subfertile and are characterised by significantly reduced in vitro fertilisation rate as the consequence of failure to proceed to meiotic metaphase II.72 Interestingy, chemical inhibition of the Notch pathway in isolated mouse ovaries results in a marked downregulation of Lhx8, Figla, Sohlh2 and Nobox mRNA expression.83 In humans, mutations in LHX8, FIGLA, SOHLH2 and NOBOX have been demonstrated to lead to POI, thus providing a link between Notch signalling, meiotic arrest and POI.84 Furthermore, besides the Notch pathway, cAMP-dependent protein kinase A (PKA) signalling is crucial as well in meiotic progression. For instance, during oocyte maturation, the PKA regulator, PRKAR2b, is highly upregulated during metaphase I and RNAi-mediated PRKAR2b depletion results in failure of MI progression.85
The PATL2 gene has recently been demonstrated as another essential factor for MI progression.86 Biallelic PATL2 mutations in women resulting in complete loss of the protein display germinal vesicle arrest, while oocytes of compound heterozygous patients with less severe mutations effectively make it through MI. However, fertilisation rates are poor, and the small number of embryos that are obtained fail in early development.86 Relatively little is currently known about the function of PATL2. RNAseq experiments in PATL2−/− murine oocytes have revealed a crucial role in the transcriptional regulation of oocyte maturation genes both in the germinal vesicle and during MII. One of the transcripts that is markedly downregulated in Patl2 mutated mouse oocytes is Cdc25a, which has also been shown to be crucial for meiotic progression.87 88 In line with this finding, translational regulation during oocyte maturation has been shown to be under control by the CPEB1 and DAZL proteins, which are responsible for ribosome loading onto oocyte-specific mRNAs.89 Additional transcriptional control in oocyte development has been observed for the FIGLA gene. Female Figla null mice display overexpression of testes-specific genes in their ovaries.90 Correspondingly, FIGLA mutations have been described in women with POI.91 92 In mice, transcriptional modulation of oocyte-specific genes, including Dazl, Figla and Nobox, is under control by the master regulator Taf4b, which associates with their respective proximal promoter sequence.93 Consequently, mice deficient for Taf4b have oocytes displaying failure of prophase I progression going together with failure in synapsis. In conclusion, while the genes described in this section are necessary for meiotic progression, their molecular role is diverse, ranging from cell cycle control to transcriptionally initiating and fine-tuning oogenesis. This varied repertoire in functionality strongly suggests that still more genes are awaiting to be uncovered as essential for meiotic progression. A further possible implication could be that in certain patients, a multigenic origin can be causal for their phenotype.
Paracrine regulation of female meiosis
In mammalian oocytes, meiosis is arrested at the diplotene stage until the time of ovulation. Only by an increase of preovulatory levels of luteinising hormone (LH) can meiotic resumption proceed. LH acts on the outer granulosa cells and initiates a signalling cascade that has to reach the oocyte, which is separated from the outer surface of the follicle by more than 10 cell layers.94 The LH signal transmission and subsequent control of meiotic progression is based on cGMP diffusion through these different layers. High levels of cGMP in the oocyte results in a meiotic arrest. However, the genes NPR2 and NPPC, which are responsible for cGMP production, are only expressed in the granulosa cells, and thus, diffusion is necessary in order to obtain high cGMP levels in the oocytes. In the oocyte, cGMP inhibits phosphodiesterase 3A activity, suppressing cAMP hydrolysis, leading to a subsequent activation of PKA, which modulates the cell cycle.95 96 The dependence of the meiotic arrest on the presence of cGMP has been demonstrated in Npr2 null mice, which are infertile due to premature meiotic resumption.97 The importance of diffusion has, however, been evidenced in connexin 37-deficient female mice, which are infertile due to an inhibition of meiotic completion.98
Connexin proteins assemble into gap junctions that are widely expressed in different cell types. In follicular tissue, connexin 37 is responsible for diffusion at the oocyte-granulosa boundary, while connexin 43-based gap junctions form the connection between the granulosa cells. Interestingly, tissue-specific overexpression of connexin 43 in connexin 37-deficient mice can restore oocyte maturation resulting in fertile female mice.99 Currently, two modes of action have been described that contribute to the control of the meiotic arrest under the influence of LH. Murine follicles exposed to LH show a significant decrease in estrogen receptor (ER) levels. Binding of ER to the NRP2 and NPPC promotor subsequently leads to their expression. Therefore, reduced ER levels results in lower cGMP levels, as such permitting meiotic progression.100 In a second study, it has been demonstrated that LH results in a significantly reduced permeability of the connexin 43 gap junctions in a MAPK-dependent way.96 As such, cGMP produced in the granulosa cells diffuses less efficiently to the oocyte, allowing the meiotic process to proceed.
While signalling from the granulosa cells towards the oocyte is crucial for follicular development, paracrine effects in the opposite directions play a key role as well. Oocyte expression of the Transforming Growth Factor beta (TGFβ) family member proteins GDF9 and BMP15 is essential for granulosa cell development.101 102 Binding of both proteins to the BMPRI and II receptor which are expressed on the granulosa and cumulus cells occurs from early follicilogenisis on and both proteins have been shown to interact with each other, forming the heterodimer cumulin, an activator of cumulus cells that is more potent than BMP15 or GDF9 alone.103 Gdf9-deficient mice are only able to form primary one-layer follicles.104 Interestingly, Gdf9 null oocytes grow faster and larger than controls despite incomplete follicle formation but nevertheless show abnormalities including the absence of cortical granules and aberrant clustering of organelles around the germinal vesicle.102 105 Regulation of GDF9 expression is under control of the transcription factor NOBOX, and mutations in both GDF9 and NOBOX have been shown to lead to POI in humans.106–110 Furthermore, NOBOX has been shown to interact with the FOXL2 transcription factor, in which mutations of the corresponding gene result in the blepharophimosis-ptosis-epicantus inversus syndrome, which is associated with POI as well.111 Additionally, mutations in BMP15 have been shown to lead to POI, suggesting that a disturbed BMP15–GDF9 interaction is contributive to the phenotype.107 Furthermore, regulation of BMP15 expression has recently been found to be influenced by basonuclein 1 (BNC1) expression.112 BNC1 mutations have been found in POI patients and resulted in reduced BMP15 expression in combination with meiotic defects in a mouse model.
Maternal-effect factors
The term ‘maternal-effect factor’ refers to maternally encoded gene products, typically expressed in her oocytes, defects that do not affect her health but compromise the development of her offspring. The majority of maternal-effect genes have been studied using mouse models, but similar mutations are now being detected in humans, in rare, clinically driven genomewide analyses. However, their prevalence and impact are not known in the wider landscape of clinical reproductive medicine.113 114
Some maternal-effect mutations directly affect the genome of the oocyte, and specifically the chromosome complement it delivers to the offspring. For example, a specific tubulin isoform, encoded by TUBB8, is required for the oocyte meiotic spindle, and maternal-effect mutations in TUBB8 can cause critical chromosomal defects affecting both oocytes and, remarkably, very early development of fertilised embryos.115 116
The majority of maternal-effect mutations affect proteins or mRNA deposited in the oocyte that are required for postfertilisation development. On fertilisation, the sperm genome enters the oocyte, and this triggers the completion of oocyte meiosis. The zygote then restructures and activates its genome through a coordinated sequence of functions, both epigenetically (changing the organisation of the zygote genome, and in particular the methylation of genomic DNA) and transcriptionally (potentiating expression of zygotic genes) (figure 2).
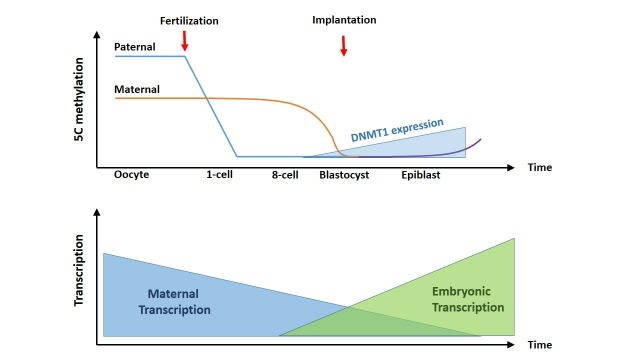
Figure 2.
Overview of the general methylation and transcriptional status of the oocyte, zygote and further developmental stages. From fertilisation on, the paternal DNA is actively demethylated. Demethylation of the maternal DNA occurs more passively, being not replaced during initial cell divisions. From the blastocyst stage on, expression of DNMT1 increases, which goes together with an increase of methylation of the embryonic DNA. Transcripts originating from the oocyte are very stable and constitute most of the mRNA during initial stages. However, from the 4–8 cell stage on, embryonic transcripts take over. SC, synaptonemal complex.
Epigenetic
The DNA methylation of oocyte and sperm are highly divergent, reflecting their highly differentiated state and gene expression patterns,117 118 but these patterns are essentially harmonised by the time of blastulation (figure 2).119 120 In the one-cell zygote, the paternal genome is rapidly and actively demethylated119–122 and appears to be predominantly passive, by reduction without replacement of DNA methylation over multiple cell cycles, possibly through restricted activity of the critical DNA methyltransferase DNMT1.123 124 By the blastula stage, the DNA methylation of the two genomes is broadly equivalent and low, with two exceptions. First, constitutive heterochromatin and repetitive DNA are highly methylated and transcriptionally repressed after a brief zygotic window of transcription.125 Second, a small number of sequences elude both DNA demethylation and remethylation, and thus retain the methylation state of their gamete of origin, in a phenomenon known as genomic imprinting.126
Transcriptional
The maturing oocyte accumulates significant stocks of RNA and proteins, but the mature oocyte silences transcription127 and remains transcriptionally dormant until full zygotic genome activation (ZGA) at two-cell stage (2C) in mice, and the eight-cell stage in humans.128 mRNA is very stable in the growing oocyte (with an average half-life of 10–14 days). On meiotic maturation, the average mRNA half-life returns to the normal level, of minutes or hours, and the mRNA content of the oocyte rapidly drops.129 The progressive destabilisation of maternal mRNA, by removal of 5′ caps and shortening of 3′ polyA tails, is believed to contribute to oocyte ‘ageing’, depriving zygotes of maternal mRNA necessary for early development and reducing their fitness.130–132
Maternal-effect genes and the zygote genome
During the remodelling of the embryonic genome, maternal-effect factors are essential, including epigenetic factors directly required for remodelling the genome and auxiliary factors that organise, stabilise and coordinate the use of maternally-provided RNA and protein until ZGA.
Epigenetic factors in the oocyte are also universally required in somatic cells, and thus highly penetrant mutations are incompatible with life; therefore, maternal-effect mutations are not readily found in human populations, and their effects have been explored in mouse models. For example, Trim28 forms a scaffold, linking DNA-binding zinc-finger proteins with DNA demethylases and chromatin modifiers. Ablation of oocyte Trim28 expression caused complete lethality: the majority of embryos failed around blastulation, and fetuses surviving beyond this time showed gross anatomical abnormalities. Interestingly, maternal null fetuses showed variably altered expression and DNA methylation of imprinted genes, suggesting that the lack of Trim28 in the first cell cycles exposed their differentially methylated regions to demethylation, which was not restored during later development.133 Remarkably, in both mice and humans, TRIM28 haploinsufficiency (in either maternal or paternal inheritance) predisposes to perturbed imprinted gene expression, particularly in adipose tissue, and resultant obesity.134 135 It remains to be determined whether more severe hypomorphism for TRIM28 is associated with reproductive compromise.
DNMT1, which methylates hemimethylated DNA, has both somatic and oocyte-specific isoforms. In mice, absence of maternally expressed Dnmt1 caused almost complete lethality of offspring, around midgestation, with a range of phenotypic abnormalities and DNA methylation defects, again including imprinted genes.123 124 136 Maternal haploinsufficiency for Dnmt1 has also been shown to compromise offspring outcomes and DNA methylation, though only in presence of another environmental challenge, assisted reproductive technology,137 but to date, human mutations have not been reported.
Another critical epigenetic factor is the zinc-finger DNA binding protein ZFP57. In mouse models, Zfp57 binds to a hexamer motif in hemimethylated DNA, which recruits Trim28 and thereby Dnmt1 to facilitate maintenance of DNA methylation.138 139 Combined maternal and zygotic knockout of Zfp57 in mouse results in loss of imprinted DNA methylation and midgestation lethality.140 Human ZFP57 is not a maternal-effect gene: it is expressed in the embryo, and somatic mutation carriers show imprinting disturbance and a congenital imprinting disorder, whereas maternal mutation carriers do not.141 Maternally provided Dppa3 (also known as Pgc7 or Stella) is essential for protection of methylation in the early murine embryo,142 but currently, its human homologue is not associated with any reproductive phenotype.
Maternal-effect genes and developmental competence
Imprinting disturbance is a recurring theme in the second class of maternal-effect mutations: those whose role may not be directly genomic but possibly epigenomic or organisational. The archetype of these is mutation in NLRP7.
Human NLRP7 has no murine homologue. It was identified as a maternal-effect gene through mutations in mothers causing a severe adverse reproductive outcome, complete hydatidiform mole. However, heterozygous maternal mutations have been identfied in the mothers of adverse reproductive outcomes, or offspring with altered DNA methylation.143–145 Molar pregnancies do not produce liveborn offspring but disorganised tissue resembling extraembryonic structures. The majority are sporadic, monospermic pregnancies with no maternal contribution, but women with homozygous NLRP7 inactivation, through mutation or gene deletion, show almost complete penetrance of molar pregnancy.146–148 NLRP7-associated moles have a normal biparental chromosome complement but complete loss of DNA methylation on maternally methylated imprints.149 Molar pregnancies also result from maternal-effect mutations of KHDC3L,150 whose protein product associates with NLRP7 in the oocyte.151
NLRP7 is one of a gene family, several of which are tandemly located and the products of recent duplication in mammalian evolution.152 Several NLRPs are involved in humoral immunity,153 while others are expressed almost exclusively and abundantly in the oocyte. Nlrp5 (also known as Mater) was one of the first maternal-effect genes identified.154 Along with four other factors, Padi6, Khdc3 (also known as Filia), Moep and Tle6, Nlrp5 is among the most highly expressed proteins in the oocyte.155 156 These proteins form a very high molecular weight complex, identified in some reports as the subcortical maternal complex140 and others as cytoplasmic lattices (CPLs).157 Maternal ablation of murine Nlrp5 causes arrest at the 2C stage.154 In these maternal-null zygotes, CPLs are not formed, and the majority of oocytes do not attain the ‘surrounded-nucleolus’ confirmation associated with early viability.157 Khdc3l and Moep both have RNA-binding domains and RNA-binding activity in vitro.158 Maternal-null Khdc3l mice have 50% fertility, with abnormalities of spindle assembly and chromosome alignment that cause delayed mitosis and gross aneuploidy.159 Maternal null Moep−/− embryos show delayed and asymmetric cell division resulting in arrest at 2C–4C. Padi6 interacts with the mitotic spindle and actin cytoskeleton of the oocyte, as well as with ribosomes; maternal ablation leads to disappearance of CPL, altered localisation of ribosomal components, reduced protein translation, reduced PolII transcription and developmental arrest at 2 C-4C.160 Tle6 is a phosphorylation target of PKA in oocyte maturation,161 but its function is not known. In humans, maternal-effect mutations have to date been identified in all these factors.162 Inactivating mutations of PADI6 and TLE6 were found in mothers undergoing IVF for infertility, whose embryos arrested at 2C163 164; KHCD3L mutations have been shown to cause familial hydatidiform mole. NLRP5 variants caused a range of developmental outcomes, including infertility, molar pregnancy, miscarriage and liveborn children affected by diverse imprinting disorders, and atypical imprinting disorders were also described in offspring of a mother with NLRP2 mutations.165 Other maternal-effect genes identified through murine studies but without currently identified human effects include Hsf1,166 Npm2 167 and Zfp36l2.168 Detailed characterisation of maternal-effect mutations in appropriate model systems is needed to reveal their mechanisms. It is plausible that complete or near-complete loss of function would cause zygote arrest before ZGA and apparent infertility. It is furthermore very likely that environmental, medical, genetic and epigenetic problems all contribute to infertility and reproductive wastage, but their relative contributions are unclear.
Phenotype selection
Errors in MI could lead to an outcome of POI: the impossibility to proceed to MI might trigger an apoptotic effect in the immature oocytes, whereas failure to stop the meiotic cycle after completion of MI has been shown to lead to a premature depletion of the oocyte pool. Mutations in genes implicated in the formation of DSBs, chromosome synapsis, HR and separation of homologous chromosomes, which are the main processes occurring during MI, could therefore potentially be involved in patients with a POI phenotype. Alterations in genes regulating maternal-effect processes are expected to result in embryos that halt further development at a certain (early) stage. Moreover, and especially in an IVF setting, aberrations in maternal effects might lead to an increase in low-quality embryos as well.
Additionally, errors in the mechanisms spanning the timeframe between ovulation and completion of MII postfertilisation could lead to a reduced fertilisation rate or failure of the embryo to further develop. It has to be noted however that diminished fertilisation can have other causes as well, ranging from paternal effects to defects in the acrosomal reaction, processes that are not included in this review.
In an ART/IVF clinical setting, defects in meiosis or maternal-effect genes are expected to give rise to a specific phenotype. We therefore propose to initiate gene panel testing in patients with the following characteristics in the IVF clinic: (1) oocyte maturation rate lower than 20% in the absence of endocrinological or technical issues in normal responders, (2) fertilisation rate lower than 10% in the absence of overt male factor and (3) embryo development rate lower than 10% in the absence of lab issues. However, prior to setting these criteria, severe parental phenotypes (including immune problems) and high levels of sperm damage should be excluded. Sperm parameters including concentration, motility and morphology have been associated with the success in clinical pregnancies after ICSI.169 We suggest to take into account the parameters proposed by the WHO as initial cut-off values.170 Contrastingly, the presence of high sperm DNA damage has not been unambiguously shown as a significant success parameter during ICSI.171 Furthermore, the couple should have been checked for karyotype errors. The presence of balanced translocations impacts heavily on meioisis leading to chromosomal imbalances in the gametes. Likewise, Robertsonian translocations, aneuploidies for the sex chromosomes and mosaic chromosomal abnormalities should be excluded as well. In summary, when these parameters are considered, we estimate that the contribution of meiotic or maternal-effect processes is likely.
Concluding remarks
The last decade has shown a significant increase in the genetic and molecular characterisation of fertility-related processes and has given us a more clear insight in the cellular machinery that drives meiosis and maternal-effect processes. This research has predominantly been done in yeast and mice and has revealed a myriad of novel proteins, both species specific and evolutionary conserved, adding further to the complex regulation of these processes. Given the molecular complexity of the meiotic process and its regulation, it is to be expected that multigenic alterations or polymorphisms could lead to gradation of an infertility phenotype resulting from a deregulated meiotic process. It is however unlikely that the use of a targeted gene panel will be able to identify these subtle effects. In order to accomplish this, one would need a much more detailed description of the phenotype as well as a large enough amount of samples with similar phenotype. However, by using a meiotic gene-specific panel in combination with a highly specific phenotype that is readily identifiable by fertility centres, one can hope to further uncover the contribution of single genes and as such identify the underlying cause of infertility of a proportion of idiopathic patients. Furthermore, this would greatly improve our understanding of the meiotic/maternal-effect process and bring into view the impact certain genes have on the severity of the phenotype. More importantly in terms of clinical practice, this would aid patients in their treatment regime as well as patient families in terms of counselling.
Meiosis heavily depends on the formation and repair of double stranded breaks and mutations in genes that are implicated in this process and have been associated with cases of familial cancer. When mutations are found in any of these particular genes, the consulting physician or the fertility centre should have implemented well-considered scenarios into their counselling practice. This is, however, complicated by the fact that while for some repair genes, for instance BRCA1 and BRCA2, the connection with familial breast and ovarian cancer is clear, while for other repair genes, this is much less clear or even unknown at the moment. One approach to avoid this ethical issue is to simply omit the repair genes in the panel. Whether the benefits of this approach outweigh the disadvantages should be decided by the individual fertility centres in close collaboration with ethicists.
In this review, we have described candidate genes involved in two cellular processes, namely meiosis and maternal effects, which are eligible for playing a role in specific cases of idiopathic infertility. By using this set of genes in a diagnostic grade panel in combination with a specifically selected phenotype may improve the diagnosis for idiopathic infertility patients who fall into the selected category. We realise that our gene set is not complete from a biological point of view. However, in terms of clinical applicability, the future implementation of a limited gene panel can bring a significant benefit to the follow-up, treatment and counselling of patients. An overview of the different genes described can be found in additional online supplementary table 1.
jmedgenet-2018-105513supp001.xlsx (10.9KB, xlsx)
Footnotes
Contributors: AG wrote the main text; DJGM and YC wrote the sections concerning maternal effect genes and critically read the manuscript; WV conceptualised the study and critically reviewed the manuscript.
Funding: This project was funded by a Willy Gepts Scholarship.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Esteves SC, Hamada A, Kondray V, Pitchika A, Agarwal A. What every gynecologist should know about male infertility: an update. Arch Gynecol Obstet 2012;286:217–29. 10.1007/s00404-012-2274-x [DOI] [PubMed] [Google Scholar]
- 2. Laissue P. Aetiological coding sequence variants in non-syndromic premature ovarian failure: From genetic linkage analysis to next generation sequencing. Mol Cell Endocrinol 2015;411:243–57. 10.1016/j.mce.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 3. De Vos M, Devroey P, Fauser BCJM. Primary ovarian insufficiency. The Lancet 2010;376:911–21. 10.1016/S0140-6736(10)60355-8 [DOI] [PubMed] [Google Scholar]
- 4. Lee JY, Dada R, Sabanegh E, Carpi A, Agarwal A. Role of genetics in azoospermia. Urology 2011;77:598–601. 10.1016/j.urology.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 5. Donker RB, Vloeberghs V, Groen H, Tournaye H, van Ravenswaaij-Arts CMA, Land JA. Chromosomal abnormalities in 1663 infertile men with azoospermia: the clinical consequences. Hum Reprod 2017;32:2574–80. 10.1093/humrep/dex307 [DOI] [PubMed] [Google Scholar]
- 6. Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D, Novara PV, Fadini R. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update 2015;21:427–54. 10.1093/humupd/dmv011 [DOI] [PubMed] [Google Scholar]
- 7. Venkatesh T, Suresh PS, Tsutsumi R. New insights into the genetic basis of infertility. Appl Clin Genet 2014;7:235–43. 10.2147/TACG.S40809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rossetti R, Ferrari I, Bonomi M, Persani L. Genetics of primary ovarian insufficiency. Clin Genet 2017;91:183–98. 10.1111/cge.12921 [DOI] [PubMed] [Google Scholar]
- 9. Harper JC, Aittomäki K, Borry P, Cornel MC, de Wert G, Dondorp W, Geraedts J, Gianaroli L, Ketterson K, Liebaers I, Lundin K, Mertes H, Morris M, Pennings G, Sermon K, Spits C, Soini S, van Montfoort APA, Veiga A, Vermeesch JR, Viville S, Macek M. on behalf of the European Society of Human Reproduction and Embryology and European Society of Human Genetics. Recent developments in genetics and medically-assisted reproduction: from research to clinical applications†‡. Hum Reprod Open 2017;2017 10.1093/hropen/hox015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geisinger A, Benavente R. Mutations in genes coding for synaptonemal complex proteins and their impact on human fertility. Cytogenet Genome Res 2016;150:77–85. 10.1159/000453344 [DOI] [PubMed] [Google Scholar]
- 11. Handel MA, Schimenti JC. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet 2010;11:124–36. 10.1038/nrg2723 [DOI] [PubMed] [Google Scholar]
- 12. Syrjänen JL, Pellegrini L, Davies OR. A molecular model for the role of SYCP3 in meiotic chromosome organisation. Elife 2014;3:02963 10.7554/eLife.02963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miyamoto T, Hasuike S, Yogev L, Maduro MR, Ishikawa M, Westphal H, Lamb DJ. Azoospermia in patients heterozygous for a mutation in SYCP3. Lancet 2003;362:1714–9. 10.1016/S0140-6736(03)14845-3 [DOI] [PubMed] [Google Scholar]
- 14. Bolor H, Mori T, Nishiyama S, Ito Y, Hosoba E, Inagaki H, Kogo H, Ohye T, Tsutsumi M, Kato T, Tong M, Nishizawa H, Pryor-Koishi K, Kitaoka E, Sawada T, Nishiyama Y, Udagawa Y, Kurahashi H. Mutations of the SYCP3 gene in women with recurrent pregnancy loss. Am J Hum Genet 2009;84:14–20. 10.1016/j.ajhg.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De La Fuente YD. J Cell Biol 2006;173:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou J, Stein P, Leu NA, Chmátal L, Xue J, Ma J, Huang X, Lampson MA, Schultz RM, Wang PJ. Accelerated reproductive aging in females lacking a novel centromere protein SYCP2L. Hum Mol Genet 2015;24:6505–14. 10.1093/hmg/ddv359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maor-Sagie E, Cinnamon Y, Yaacov B, Shaag A, Goldsmidt H, Zenvirt S, Laufer N, Richler C, Frumkin A. Deleterious mutation in SYCE1 is associated with non-obstructive azoospermia. J Assist Reprod Genet 2015;32:887–91. 10.1007/s10815-015-0445-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bolcun-Filas E, Hall E, Speed R, Taggart M, Grey C, de Massy B, Benavente R, Cooke HJ. Mutation of the mouse Syce1 gene disrupts synapsis and suggests a link between synaptonemal complex structural components and DNA repair. PLoS Genet 2009;5:e1000393 10.1371/journal.pgen.1000393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Vries L, Behar DM, Smirin-Yosef P, Lagovsky I, Tzur S, Basel-Vanagaite L. Exome sequencing reveals SYCE1 mutation associated with autosomal recessive primary ovarian insufficiency. J Clin Endocrinol Metab 2014;99:E2129–E2132. 10.1210/jc.2014-1268 [DOI] [PubMed] [Google Scholar]
- 20. Gershoni M, Hauser R, Yogev L, Lehavi O, Azem F, Yavetz H, Pietrokovski S, Kleiman SE. A familial study of azoospermic men identifies three novel causative mutations in three new human azoospermia genes. Genet Med 2017;19:998–1006. 10.1038/gim.2016.225 [DOI] [PubMed] [Google Scholar]
- 21. Souquet B, Abby E, Hervé R, Finsterbusch F, Tourpin S, Le Bouffant R, Duquenne C, Messiaen S, Martini E, Bernardino-Sgherri J, Toth A, Habert R, Livera G. MEIOB targets single-strand DNA and is necessary for meiotic recombination. PLoS Genet 2013;9:e1003784 10.1371/journal.pgen.1003784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hays E, Majchrzak N, Daniel V, Ferguson Z, Brown S, Hathorne K, La Salle S. Spermatogenesis associated 22 is required for DNA repair and synapsis of homologous chromosomes in mouse germ cells. Andrology 2017;5:299–312. 10.1111/andr.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2001;2:280–91. 10.1038/35066065 [DOI] [PubMed] [Google Scholar]
- 24. Herbert M, Kalleas D, Cooney D, Lamb M, Lister L. Meiosis and maternal aging: insights from aneuploid oocytes and trisomy births. Cold Spring Harb Perspect Biol 2015;7:a017970 10.1101/cshperspect.a017970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. MacLennan M, Crichton JH, Playfoot CJ, Adams IR, development O. meiosis and aneuploidy. Semin Cell Dev Biol 2015;45:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oliver TR, Middlebrooks CD, Tinker SW, Allen EG, Bean LJ, Begum F, Feingold E, Chowdhury R, Cheung V, Sherman SL. An examination of the relationship between hotspots and recombination associated with chromosome 21 nondisjunction. PLoS One 2014;9:e99560 10.1371/journal.pone.0099560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oliver TR, Middlebrooks C, Harden A, Scott N, Johnson B, Jones J, Walker C, Wilkerson C, Saffold SH, Akinseye A, Smith T, Feingold E, Sherman SL. Variation in the zinc finger of prdm9 is associated with the absence of recombination along nondisjoined chromosomes 21 of maternal origin. J Down Syndr Chromosom Abnorm 2016;2:115 10.4172/2472-1115.1000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guiraldelli MF, Eyster C, Wilkerson JL, Dresser ME, Pezza RJ. Mouse HFM1/Mer3 is required for crossover formation and complete synapsis of homologous chromosomes during meiosis. PLoS Genet 2013;9:e1003383 10.1371/journal.pgen.1003383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang J, Zhang W, Jiang H, Wu BL. Primary Ovarian Insufficiency Collaboration. Mutations in HFM1 in recessive primary ovarian insufficiency. N Engl J Med 2014;370:972–4. 10.1056/NEJMc1310150 [DOI] [PubMed] [Google Scholar]
- 30. Lutzmann M, Grey C, Traver S, Ganier O, Maya-Mendoza A, Ranisavljevic N, Bernex F, Nishiyama A, Montel N, Gavois E, Forichon L, de Massy B, Méchali M. MCM8- and MCM9-deficient mice reveal gametogenesis defects and genome instability due to impaired homologous recombination. Mol Cell 2012;47:523–34. 10.1016/j.molcel.2012.05.048 [DOI] [PubMed] [Google Scholar]
- 31. Tenenbaum-Rakover Y, Weinberg-Shukron A, Renbaum P, Lobel O, Eideh H, Gulsuner S, Dahary D, Abu-Rayyan A, Kanaan M, Levy-Lahad E, Bercovich D, Zangen D. mutations result in primary gonadal failure. Minichromosome Maint Complex Compon 2015;8:391–9. [DOI] [PubMed] [Google Scholar]
- 32. AlAsiri S, Basit S, Wood-Trageser MA, Yatsenko SA, Jeffries EP, Surti U, Ketterer DM, Afzal S, Ramzan K, Faiyaz-Ul Haque M, Jiang H, Trakselis MA, Rajkovic A. Exome sequencing reveals MCM8 mutation underlies ovarian failure and chromosomal instability. J Clin Invest 2015;125:258–62. 10.1172/JCI78473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Christensen GL, Ivanov IP, Atkins JF, Mielnik A, Schlegel PN, Carrell DT. Screening the SPO11 and EIF5A2 genes in a population of infertile men. Fertil Steril 2005;84:758–60. 10.1016/j.fertnstert.2005.03.053 [DOI] [PubMed] [Google Scholar]
- 34. Baudat F, Manova K, Yuen JP, Jasin M, Keeney S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell 2000;6:989–98. 10.1016/S1097-2765(00)00098-8 [DOI] [PubMed] [Google Scholar]
- 35. Romanienko PJ, Camerini-Otero RD. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell 2000;6:975–87. 10.1016/S1097-2765(00)00097-6 [DOI] [PubMed] [Google Scholar]
- 36. Boateng KA, Bellani MA, Gregoretti IV, Pratto F, Camerini-Otero RD. Homologous pairing preceding SPO11-mediated double-strand breaks in mice. Dev Cell 2013;24:196–205. 10.1016/j.devcel.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Faieta M, Di Cecca S, de Rooij DG, Luchetti A, Murdocca M, Di Giacomo M, Di Siena S, Pellegrini M, Rossi P, Barchi M. A surge of late-occurring meiotic double-strand breaks rescues synapsis abnormalities in spermatocytes of mice with hypomorphic expression of SPO11 . Chromosoma 2016;125:189–203. 10.1007/s00412-015-0544-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bellani MA, Romanienko PJ, Cairatti DA, Camerini-Otero RD. SPO11 is required for sex-body formation, and Spo11 heterozygosity rescues the prophase arrest of Atm-/- spermatocytes. J Cell Sci 2005;118:3233–45. 10.1242/jcs.02466 [DOI] [PubMed] [Google Scholar]
- 39. Arora C, Kee K, Maleki S, Keeney S. Antiviral protein Ski8 is a direct partner of Spo11 in meiotic DNA break formation, independent of its cytoplasmic role in RNA metabolism. Mol Cell 2004;13:549–59. 10.1016/S1097-2765(04)00063-2 [DOI] [PubMed] [Google Scholar]
- 40. Sasanuma H, Murakami H, Fukuda T, Shibata T, Nicolas A, Ohta K. Meiotic association between Spo11 regulated by Rec102, Rec104 and Rec114. Nucleic Acids Res 2007;35:1119–33. 10.1093/nar/gkl1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prieler S, Penkner A, Borde V, Klein F. The control of Spo11’s interaction with meiotic recombination hotspots. Genes Dev 2005;19:255–69. 10.1101/gad.321105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumar R, Bourbon HM, de Massy B. Functional conservation of Mei4 for meiotic DNA double-strand break formation from yeasts to mice. Genes Dev 2010;24:1266–80. 10.1101/gad.571710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Daniel K, Lange J, Hached K, Fu J, Anastassiadis K, Roig I, Cooke HJ, Stewart AF, Wassmann K, Jasin M, Keeney S, Tóth A. Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1. Nat Cell Biol 2011;13:599–610. 10.1038/ncb2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wojtasz L, Daniel K, Roig I, Bolcun-Filas E, Xu H, Boonsanay V, Eckmann CR, Cooke HJ, Jasin M, Keeney S, McKay MJ, Toth A. Mouse HORMAD1 and HORMAD2, two conserved meiotic chromosomal proteins, are depleted from synapsed chromosome axes with the help of TRIP13 AAA-ATPase. PLoS Genet 2009;5:e1000702 10.1371/journal.pgen.1000702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rinaldi VD, Bolcun-Filas E, Kogo H, Kurahashi H, Schimenti JC. The DNA Damage checkpoint eliminates mouse oocytes with chromosome synapsis failure. Mol Cell 2017;67:1026–36. 10.1016/j.molcel.2017.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science 2014;343:533–6. 10.1126/science.1247671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shin YH, Choi Y, Erdin SU, Yatsenko SA, Kloc M, Yang F, Wang PJ, Meistrich ML, Rajkovic A. Hormad1 mutation disrupts synaptonemal complex formation, recombination, and chromosome segregation in mammalian meiosis. PLoS Genet 2010;6:e1001190 10.1371/journal.pgen.1001190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carson CT, Schwartz RA, Stracker TH, Lilley CE, Lee DV, Weitzman MD. The Mre11 complex is required for ATM activation and the G2/M checkpoint. Embo J 2003;22:6610–20. 10.1093/emboj/cdg630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marangos P, Carroll J. Oocytes progress beyond prophase in the presence of DNA damage. Curr Biol 2012;22:989–94. 10.1016/j.cub.2012.03.063 [DOI] [PubMed] [Google Scholar]
- 50. Lao JP, Cloud V, Huang CC, Grubb J, Thacker D, Lee CY, Dresser ME, Hunter N, Bishop DK. Meiotic crossover control by concerted action of Rad51-Dmc1 in homolog template bias and robust homeostatic regulation. PLoS Genet 2013;9:e1003978 10.1371/journal.pgen.1003978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bugreev DV, Huang F, Mazina OM, Pezza RJ, Voloshin ON, Camerini-Otero RD, Mazin AV. HOP2-MND1 modulates RAD51 binding to nucleotides and DNA. Nat Commun 2014;5:4198 10.1038/ncomms5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Snowden T, Acharya S, Butz C, Berardini M, Fishel R. hMSH4-hMSH5 recognizes Holliday Junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol Cell 2004;15:437–51. 10.1016/j.molcel.2004.06.040 [DOI] [PubMed] [Google Scholar]
- 53. Guo T, Zhao S, Zhao S, Chen M, Li G, Jiao X, Wang Z, Zhao Y, Qin Y, Gao F, Chen ZJ. Mutations in MSH5 in primary ovarian insufficiency. Hum Mol Genet 2017;26:1452–7. 10.1093/hmg/ddx044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carlosama C, Elzaiat M, Patiño LC, Mateus HE, Veitia RA, Laissue P. A homozygous donor splice-site mutation in the meiotic gene MSH4 causes primary ovarian insufficiency. Hum Mol Genet 2017;26:3161–6. 10.1093/hmg/ddx199 [DOI] [PubMed] [Google Scholar]
- 55. Prakash R, Zhang Y, Feng W, Jasin M. Homologous recombination and human health: the roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb Perspect Biol 2015;7:a016600 10.1101/cshperspect.a016600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith KR, Hanson HA, Hollingshaus MS. BRCA1 and BRCA2 mutations and female fertility. Curr Opin Obstet Gynecol 2013;25:207–13. 10.1097/GCO.0b013e32835f1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sharan SK. BRCA2 deficiency in mice leads to meiotic impairment and infertility. Development 2004;131:131–42. 10.1242/dev.00888 [DOI] [PubMed] [Google Scholar]
- 58. Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, Oktay K. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med 2013172:ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nasmyth K, Haering CH. Cohesin: its roles and mechanisms. Annu Rev Genet 2009;43:525–58. 10.1146/annurev-genet-102108-134233 [DOI] [PubMed] [Google Scholar]
- 60. Higgins JM, Herbert M. Nucleosome assembly proteins get SET to defeat the guardian of chromosome cohesion. PLoS Genet 2013;9:e1003829 10.1371/journal.pgen.1003829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chambon JP, Touati SA, Berneau S, Cladière D, Hebras C, Groeme R, McDougall A, Wassmann K. The PP2A inhibitor I2PP2A is essential for sister chromatid segregation in oocyte meiosis II. Curr Biol 2013;23:485–90. 10.1016/j.cub.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 62. Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, Jessberger R, Kirkwood TB, Höög C, Herbert M. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol 2010;20:1511–21. 10.1016/j.cub.2010.08.023 [DOI] [PubMed] [Google Scholar]
- 63. Faridi R, Rehman AU, Morell RJ, Friedman PL, Demain L, Zahra S, Khan AA, Tohlob D, Assir MZ, Beaman G, Khan SN, Newman WG, Riazuddin S, Friedman TB. Mutations of SGO2 and CLDN14 collectively cause coincidental perrault syndrome. Clin Genet 2017;91:328–32. 10.1111/cge.12867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim J, Ishiguro K, Nambu A, Akiyoshi B, Yokobayashi S, Kagami A, Ishiguro T, Pendas AM, Takeda N, Sakakibara Y, Kitajima TS, Tanno Y, Sakuno T, Watanabe Y. Meikin is a conserved regulator of meiosis-I-specific kinetochore function. Nature 2015;517:466–71. 10.1038/nature14097 [DOI] [PubMed] [Google Scholar]
- 65. Caburet S, Arboleda VA, Llano E, Overbeek PA, Barbero JL, Oka K, Harrison W, Vaiman D, Ben-Neriah Z, García-Tuñón I, Fellous M, Pendás AM, Veitia RA, Vilain E. Mutant cohesin in premature ovarian failure. N Engl J Med 2014;370:943–9. 10.1056/NEJMoa1309635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu H, Beasley MD, Warren WD, van der Horst GTJ, McKay MJ. Absence of mouse rec8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell 2005;8:949–61. 10.1016/j.devcel.2005.03.018 [DOI] [PubMed] [Google Scholar]
- 67. Limongelli G, Russo S, Digilio MC, Masciadri M, Pacileo G, Fratta F, Martone F, Maddaloni V, D’Alessandro R, Calabro P, Russo MG, Calabro R, Larizza L. Hypertrophic cardiomyopathy in a girl with Cornelia de Lange syndrome due to mutation in SMC1A. Am J Med Genet A 2010;152A:2127–9. 10.1002/ajmg.a.33486 [DOI] [PubMed] [Google Scholar]
- 68. Hoppman-Chaney N, Jang JS, Jen J, Babovic-Vuksanovic D, Hodge JC. In-frame multi-exon deletion of SMC1A in a severely affected female with Cornelia de Lange Syndrome. Am J Med Genet A 2012;158A:193–8. 10.1002/ajmg.a.34360 [DOI] [PubMed] [Google Scholar]
- 69. Spruck CH, de Miguel MP, Smith AP, Ryan A, Stein P, Schultz RM, Lincoln AJ, Donovan PJ, Reed SI. Requirement of Cks2 for the first metaphase/anaphase transition of mammalian meiosis. Science 2003;300:647–50. 10.1126/science.1084149 [DOI] [PubMed] [Google Scholar]
- 70. Libby BJ, De La Fuente R, O’Brien MJ, Wigglesworth K, Cobb J, Inselman A, Eaker S, Handel MA, Eppig JJ, Schimenti JC. The mouse meiotic mutation mei1 disrupts chromosome synapsis with sexually dimorphic consequences for meiotic progression. Dev Biol 2002;242:174–87. 10.1006/dbio.2001.0535 [DOI] [PubMed] [Google Scholar]
- 71. Araki K, Naito K, Haraguchi S, Suzuki R, Yokoyama M, Inoue M, Aizawa S, Toyoda Y, Sato E. Meiotic abnormalities of c-mos knockout mouse oocytes: activation after first meiosis or entrance into third meiotic metaphase. Biol Reprod 1996;55:1315–24. 10.1095/biolreprod55.6.1315 [DOI] [PubMed] [Google Scholar]
- 72. Hahn KL, Johnson J, Beres BJ, Howard S, Wilson-Rawls J. Lunatic fringe null female mice are infertile due to defects in meiotic maturation. Development 2005;132:817–28. 10.1242/dev.01601 [DOI] [PubMed] [Google Scholar]
- 73. Edelmann W, Cohen PE, Kane M, Lau K, Morrow B, Bennett S, Umar A, Kunkel T, Cattoretti G, Chaganti R, Pollard JW, Kolodner RD, Kucherlapati R. Meiotic pachytene arrest in MLH1-deficient mice. Cell 1996;85:1125–34. 10.1016/S0092-8674(00)81312-4 [DOI] [PubMed] [Google Scholar]
- 74. Martinsson-Ahlzén HS, Liberal V, Grünenfelder B, Chaves SR, Spruck CH, Reed SI. Cyclin-dependent kinase-associated proteins Cks1 and Cks2 are essential during early embryogenesis and for cell cycle progression in somatic cells. Mol Cell Biol 2008;28:5698–709. 10.1128/MCB.01833-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Egan EA, Solomon MJ. Cyclin-stimulated binding of Cks proteins to cyclin-dependent kinases. Mol Cell Biol 1998;18:3659–67. 10.1128/MCB.18.7.3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Patra D, Wang SX, Kumagai A, Dunphy WG. The xenopus Suc1/Cks protein promotes the phosphorylation of G2/M regulators. J Biol Chem 1999;274:36839–42. 10.1074/jbc.274.52.36839 [DOI] [PubMed] [Google Scholar]
- 77. Ruiz EJ, Vilar M, Nebreda AR. A two-step inactivation mechanism of Myt1 ensures CDK1/cyclin B activation and meiosis I entry. Curr Biol 2010;20:717–23. 10.1016/j.cub.2010.02.050 [DOI] [PubMed] [Google Scholar]
- 78. Tang W, Wu JQ, Guo Y, Hansen DV, Perry JA, Freel CD, Nutt L, Jackson PK, Kornbluth S. Cdc2 and Mos regulate Emi2 stability to promote the meiosis I-meiosis II transition. Mol Biol Cell 2008;19:3536–43. 10.1091/mbc.e08-04-0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Miyagaki Y, Kanemori Y, Baba T. Possible involvement of mitogen- and stress-activated protein kinase 1, MSK1, in metaphase-II arrest through phosphorylation of EMI2 in mouse oocytes. Dev Biol 2011;359:73–81. 10.1016/j.ydbio.2011.08.021 [DOI] [PubMed] [Google Scholar]
- 80. Carroll J, Marangos P. The DNA damage response in mammalian oocytes. Front Genet 2013;4 10.3389/fgene.2013.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Oh JS, Susor A, Conti M. Protein tyrosine kinase Wee1B is essential for metaphase II exit in mouse oocytes. Science 2011;332:462–5. 10.1126/science.1199211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sang Q, Li B, Kuang Y, Wang X, Zhang Z, Chen B, Wu L, Lyu Q, Fu Y, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Wang L. Homozygous mutations in WEE2 cause fertilization failure and female infertility. Am J Hum Genet 2018;102:649–57. 10.1016/j.ajhg.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen CL, Fu XF, Wang LQ, Wang JJ, Ma HG, Cheng SF, Hou ZM, Ma JM, Quan GB, Shen W, Li L. Primordial follicle assembly was regulated by Notch signaling pathway in the mice. Mol Biol Rep 2014;41:1891–9. 10.1007/s11033-014-3038-4 [DOI] [PubMed] [Google Scholar]
- 84. Bouilly J, Beau I, Barraud S, Bernard V, Azibi K, Fagart J, Fèvre A, Todeschini AL, Veitia RA, Beldjord C, Delemer B, Dodé C, Young J, Binart N. Identification of multiple gene mutations accounts for a new genetic architecture of primary ovarian insufficiency. J Clin Endocrinol Metab 2016;101:4541–50. 10.1210/jc.2016-2152 [DOI] [PubMed] [Google Scholar]
- 85. Yoon H, Jang H, Kim EY, Moon S, Lee S, Cho M, Cho HJ, Ko JJ, Chang EM, Lee KA, Choi Y. Knockdown of PRKAR2B results in the failure of oocyte maturation. Cell Physiol Biochem 2018;45:2009–20. 10.1159/000487978 [DOI] [PubMed] [Google Scholar]
- 86. Chen B, Zhang Z, Sun X, Kuang Y, Mao X, Wang X, Yan Z, Li B, Xu Y, Yu M, Fu J, Mu J, Zhou Z, Li Q, Jin L, He L, Sang Q, Wang L. Biallelic mutations in PATL2 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet 2017;101:609–15. 10.1016/j.ajhg.2017.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Christou-Kent M, Kherraf ZE, Amiri-Yekta A, Le Blévec E, Karaouzène T, Conne B, Escoffier J, Assou S, Guttin A, Lambert E, Martinez G, Boguenet M, Fourati Ben Mustapha S, Cedrin Durnerin I, Halouani L, Marrakchi O, Makni M, Latrous H, Kharouf M, Coutton C, Thierry-Mieg N, Nef S, Bottari SP, Zouari R, Issartel JP, Ray PF, Arnoult C. PATL2 is a key actor of oocyte maturation whose invalidation causes infertility in women and mice. EMBO Mol Med 2018;10:e8515 10.15252/emmm.201708515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Solc P, Saskova A, Baran V, Kubelka M, Schultz RM, Motlik J. CDC25A phosphatase controls meiosis I progression in mouse oocytes. Dev Biol 2008;317:260–9. 10.1016/j.ydbio.2008.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sousa Martins JP, Liu X, Oke A, Arora R, Franciosi F, Viville S, Laird DJ, Fung JC, Conti M. DAZL and CPEB1 regulate mRNA translation synergistically during oocyte maturation. J Cell Sci 2016;129:1271–82. 10.1242/jcs.179218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hu W, Gauthier L, Baibakov B, Jimenez-Movilla M, Dean J. FIGLA, a basic helix-loop-helix transcription factor, balances sexually dimorphic gene expression in postnatal oocytes. Mol Cell Biol 2010;30:3661–71. 10.1128/MCB.00201-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chen B, Li L, Wang J, Li T, Pan H, Liu B, Zhou Y, Cao Y, Wang B. Consanguineous familial study revealed biallelic FIGLA mutation associated with premature ovarian insufficiency. J Ovarian Res 2018;11:48 10.1186/s13048-018-0413-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tosh D, Rani HS, Murty US, Deenadayal A, Grover P. Mutational analysis of the FIGLA gene in women with idiopathic premature ovarian failure. Menopause 2015;22:520–6. 10.1097/GME.0000000000000340 [DOI] [PubMed] [Google Scholar]
- 93. Grive KJ, Gustafson EA, Seymour KA, Baddoo M, Schorl C, Golnoski K, Rajkovic A, Brodsky AS, Freiman RN. TAF4b Regulates Oocyte-Specific Genes Essential for Meiosis. PLoS Genet 2016;12:e1006128 10.1371/journal.pgen.1006128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Clift D, Schuh M. Restarting life: fertilization and the transition from meiosis to mitosis. Nat Rev Mol Cell Biol 2013;14:549–62. 10.1038/nrm3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol 2003;258:385–96. 10.1016/S0012-1606(03)00134-9 [DOI] [PubMed] [Google Scholar]
- 96. Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 2009;136:1869–78. 10.1242/dev.035238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Geister KA, Brinkmeier ML, Hsieh M, Faust SM, Karolyi IJ, Perosky JE, Kozloff KM, Conti M, Camper SA. A novel loss-of-function mutation in Npr2 clarifies primary role in female reproduction and reveals a potential therapy for acromesomelic dysplasia, Maroteaux type. Hum Mol Genet 2013;22:345–57. 10.1093/hmg/dds432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature 1997;385:525–9. 10.1038/385525a0 [DOI] [PubMed] [Google Scholar]
- 99. Li TY, Colley D, Barr KJ, Yee SP, Kidder GM. Rescue of oogenesis in Cx37-null mutant mice by oocyte-specific replacement with Cx43. J Cell Sci 2007;120:4117–25. 10.1242/jcs.03488 [DOI] [PubMed] [Google Scholar]
- 100. Liu W, Xin Q, Wang X, Wang S, Wang H, Zhang W, Yang Y, Zhang Y, Zhang Z, Wang C, Xu Y, Duan E, Xia G. Estrogen receptors in granulosa cells govern meiotic resumption of pre-ovulatory oocytes in mammals. Cell Death Dis 2017;8:e2662 10.1038/cddis.2017.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. McNatty KP, Juengel JL, Reader KL, Lun S, Myllymaa S, Lawrence SB, Western A, Meerasahib MF, Mottershead DG, Groome NP, Ritvos O, Laitinen MP. Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function in ruminants. Reproduction 2005;129:481–7. 10.1530/rep.1.00517 [DOI] [PubMed] [Google Scholar]
- 102. Otsuka F, McTavish KJ, Shimasaki S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol Reprod Dev 2011;78:9–21. 10.1002/mrd.21265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mottershead DG, Sugimura S, Al-Musawi SL, Li JJ, Richani D, White MA, Martin GA, Trotta AP, Ritter LJ, Shi J, Mueller TD, Harrison CA, Gilchrist RB. Cumulin, an Oocyte-secreted Heterodimer of the Transforming Growth Factor-β Family, Is a Potent Activator of Granulosa Cells and Improves Oocyte Quality. J Biol Chem 2015;290:24007–20. 10.1074/jbc.M115.671487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 1996;383:531–5. 10.1038/383531a0 [DOI] [PubMed] [Google Scholar]
- 105. Carabatsos MJ, Elvin J, Matzuk MM, Albertini DF. Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice. Dev Biol 1998;204:373–84. 10.1006/dbio.1998.9087 [DOI] [PubMed] [Google Scholar]
- 106. Li L, Wang B, Zhang W, Chen B, Luo M, Wang J, Wang X, Cao Y. Kee K. A homozygous NOBOX truncating variant causes defective transcriptional activation and leads to primary ovarian insufficiency. Hum Reprod Oxf Engl 2017;32:248–55. [DOI] [PubMed] [Google Scholar]
- 107. Patiño LC, Walton KL, Mueller TD, Johnson KE, Stocker W, Richani D, Agapiou D, Gilchrist RB, Laissue P, Harrison CA. BMP15 Mutations associated with primary ovarian insufficiency reduce expression, activity, or synergy with GDF9. J Clin Endocrinol Metab 2017;102:1009–19. 10.1210/jc.2016-3503 [DOI] [PubMed] [Google Scholar]
- 108. Bouilly J, Roucher-Boulez F, Gompel A, Bry-Gauillard H, Azibi K, Beldjord C, Dodé C, Bouligand J, Mantel AG, Hécart AC, Delemer B, Young J, Binart N. New NOBOX mutations identified in a large cohort of women with primary ovarian insufficiency decrease KIT-L expression. J Clin Endocrinol Metab 2015;100:994–1001. 10.1210/jc.2014-2761 [DOI] [PubMed] [Google Scholar]
- 109. Bouilly J, Bachelot A, Broutin I, Touraine P, Binart N. Novel NOBOX loss-of-function mutations account for 6.2% of cases in a large primary ovarian insufficiency cohort. Hum Mutat 2011;32:1108–13. 10.1002/humu.21543 [DOI] [PubMed] [Google Scholar]
- 110. França MM, Funari MFA, Nishi MY, Narcizo AM, Domenice S, Costa EMF, Lerario AM, Mendonca BB. Identification of the first homozygous 1-bp deletion in GDF9 gene leading to primary ovarian insufficiency by using targeted massively parallel sequencing. Clin Genet 2018;93:408–11. 10.1111/cge.13156 [DOI] [PubMed] [Google Scholar]
- 111. Bouilly J, Veitia RA, Binart N. NOBOX is a key FOXL2 partner involved in ovarian folliculogenesis. J Mol Cell Biol 2014;6:175–7. 10.1093/jmcb/mju006 [DOI] [PubMed] [Google Scholar]
- 112. Zhang D, Liu Y, Zhang Z, Lv P, Liu Y, Li J, Wu Y, Zhang R, Huang Y, Xu G, Qian Y, Qian Y, Chen S, Xu C, Shen J, Zhu L, Chen K, Zhu B, Ye X, Mao Y, Bo X, Zhou C, Wang T, Chen D, Yang W, Tan Y, Song Y, Zhou D, Sheng J, Gao H, Zhu Y, Li M, Wu L, He L, Huang H. Basonuclin 1 deficiency is a cause of primary ovarian insufficiency. Hum Mol Genet 2018;27:3787–800. 10.1093/hmg/ddy261 [DOI] [PubMed] [Google Scholar]
- 113. Rai R, Regan L. Recurrent miscarriage. The Lancet 2006;368:601–11. 10.1016/S0140-6736(06)69204-0 [DOI] [PubMed] [Google Scholar]
- 114. van den Berg MM, van Maarle MC, van Wely M, Goddijn M. Genetics of early miscarriage. Biochim Biophys Acta 2012;1822:1951–9. 10.1016/j.bbadis.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 115. Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, Shi J, Tian G, Luchniak A, Fukuda Y, Li B, Yu M, Chen J, Xu Y, Guo L, Qu R, Wang X, Sun Z, Liu M, Shi H, Wang H, Feng Y, Shao R, Chai R, Li Q, Xing Q, Zhang R, Nogales E, Jin L, He L, Gupta ML, Cowan NJ, Wang L. Mutations in TUBB8 and Human Oocyte Meiotic Arrest. N Engl J Med 2016;374:223–32. 10.1056/NEJMoa1510791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Feng R, Yan Z, Li B, Yu M, Sang Q, Tian G, Xu Y, Chen B, Qu R, Sun Z, Sun X, Jin L, He L, Kuang Y, Cowan NJ, Wang L. Mutations in TUBB8 cause a multiplicity of phenotypes in human oocytes and early embryos. J Med Genet 2016;53:662–71. 10.1136/jmedgenet-2016-103891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Stewart KR, Veselovska L, Kelsey G. Establishment and functions of DNA methylation in the germline. Epigenomics 2016;8:1399–413. 10.2217/epi-2016-0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Stewart KR, Veselovska L, Kim J, Huang J, Saadeh H, Tomizawa S, Smallwood SA, Chen T, Kelsey G. Dynamic changes in histone modifications precede de novo DNA methylation in oocytes. Genes Dev 2015;29:2449–62. 10.1101/gad.271353.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Smallwood SA, Tomizawa S, Krueger F, Ruf N, Carli N, Segonds-Pichon A, Sato S, Hata K, Andrews SR, Kelsey G. Dynamic CpG island methylation landscape in oocytes and preimplantation embryos. Nat Genet 2011;43:811–4. 10.1038/ng.864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Smith ZD, Chan MM, Mikkelsen TS, Gu H, Gnirke A, Regev A, Meissner A. A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature 2012;484:339–44. 10.1038/nature10960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Guo F, Li X, Liang D, Li T, Zhu P, Guo H, Wu X, Wen L, Gu TP, Hu B, Walsh CP, Li J, Tang F, Xu GL. Active and passive demethylation of male and female pronuclear DNA in the mammalian zygote. Cell Stem Cell 2014;15:447–59. 10.1016/j.stem.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 122. Shen L, Inoue A, He J, Liu Y, Lu F, Zhang Y. Tet3 and DNA replication mediate demethylation of both the maternal and paternal genomes in mouse zygotes. Cell Stem Cell 2014;15:459–71. 10.1016/j.stem.2014.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Howell CY, Bestor TH, Ding F, Latham KE, Mertineit C, Trasler JM, Chaillet JR. Genomic imprinting disrupted by a maternal effect mutation in the Dnmt1 gene. Cell 2001;104:829–38. 10.1016/S0092-8674(01)00280-X [DOI] [PubMed] [Google Scholar]
- 124. Hirasawa R, Chiba H, Kaneda M, Tajima S, Li E, Jaenisch R, Sasaki H. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev 2008;22:1607–16. 10.1101/gad.1667008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. De Iaco A, Planet E, Coluccio A, Verp S, Duc J, Trono D. DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat Genet 2017;49:941–5. 10.1038/ng.3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hanna CW, Kelsey G. The specification of imprints in mammals. Heredity (Edinb). Nat Rev Genet 2014;113:176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bouniol-Baly C, Nguyen E, Besombes D, Debey P. Dynamic organization of DNA replication in one-cell mouse embryos: relationship to transcriptional activation. Exp Cell Res 1997;236:201–11. 10.1006/excr.1997.3708 [DOI] [PubMed] [Google Scholar]
- 128. Lee MT, Bonneau AR, Giraldez AJ. Zygotic genome activation during the maternal-to-zygotic transition. Annu Rev Cell Dev Biol 2014;30:581–613. 10.1146/annurev-cellbio-100913-013027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Paynton BV, Rempel R, Bachvarova R. Changes in state of adenylation and time course of degradation of maternal mRNAs during oocyte maturation and early embryonic development in the mouse. Dev Biol 1988;129:304–14. 10.1016/0012-1606(88)90377-6 [DOI] [PubMed] [Google Scholar]
- 130. Ma J, Flemr M, Strnad H, Svoboda P, Schultz RM. Maternally recruited DCP1A and DCP2 contribute to messenger RNA degradation during oocyte maturation and genome activation in mouse. Biol Reprod 2013;88:11 10.1095/biolreprod.112.105312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dankert D, Demond H, Trapphoff T, Heiligentag M, Rademacher K, Eichenlaub-Ritter U, Horsthemke B, Grümmer R. Pre- and postovulatory aging of murine oocytes affect the transcript level and poly(A) tail length of maternal effect genes. PLoS One 2014;9:e108907 10.1371/journal.pone.0108907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Eichenlaub-Ritter U. Oocyte ageing and its cellular basis. Int J Dev Biol 2012;56:841–52. 10.1387/ijdb.120141ue [DOI] [PubMed] [Google Scholar]
- 133. Messerschmidt DM, de Vries W, Ito M, Solter D, Ferguson-Smith A, Knowles BB. Trim28 is required for epigenetic stability during mouse oocyte to embryo transition. Science: In Germline mutation in NLRP2 (NALP2) in a familial imprinting disorder (Beckwith-Wiedemann, 2012:1499–502. [DOI] [PubMed] [Google Scholar]
- 134. Whitelaw NC, Chong S, Morgan DK, Nestor C, Bruxner TJ, Ashe A, Lambley E, Meehan R, Whitelaw E. Reduced levels of two modifiers of epigenetic gene silencing, Dnmt3a and Trim28, cause increased phenotypic noise. Genome Biol 2010;11:R111 10.1186/gb-2010-11-11-r111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Dalgaard K, Landgraf K, Heyne S, Lempradl A, Longinotto J, Gossens K, Ruf M, Orthofer M, Strogantsev R, Selvaraj M, Lu TT, Casas E, Teperino R, Surani MA, Zvetkova I, Rimmington D, Tung YC, Lam B, Larder R, Yeo GS, O’Rahilly S, Vavouri T, Whitelaw E, Penninger JM, Jenuwein T, Cheung CL, Ferguson-Smith AC, Coll AP, Körner A, Pospisilik JA. Trim28 Haploinsufficiency Triggers Bi-stable Epigenetic Obesity. Cell 2016;164:353–64. 10.1016/j.cell.2015.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ratnam S, Mertineit C, Ding F, Howell CY, Clarke HJ, Bestor TH, Chaillet TJR. J.M. Dynamics of Dnmt1 methyltransferase expression and intracellular localization during oogenesis and preimplantation development. Dev Biol 2002;15:304–14. [DOI] [PubMed] [Google Scholar]
- 137. Whidden L, Martel J, Rahimi S, Chaillet JR, Chan D, Trasler JM. Compromised oocyte quality and assisted reproduction contribute to sex-specific effects on offspring outcomes and epigenetic patterning. Hum Mol Genet 2016;25:ddw293–60. 10.1093/hmg/ddw293 [DOI] [PubMed] [Google Scholar]
- 138. Quenneville S, Verde G, Corsinotti A, Kapopoulou A, Jakobsson J, Offner S, Baglivo I, Pedone PV, Grimaldi G, Riccio A, Trono D. In embryonic stem cells, ZFP57/KAP1 recognize a methylated hexanucleotide to affect chromatin and DNA methylation of imprinting control regions. Mol Cell 2011;44:361–72. 10.1016/j.molcel.2011.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Strogantsev R, Krueger F, Yamazawa K, Shi H, Gould P, Goldman-Roberts M, McEwen K, Sun B, Pedersen R, Ferguson-Smith AC. Allele-specific binding of ZFP57 in the epigenetic regulation of imprinted and non-imprinted monoallelic expression. Genome Biol 2015;16:112 10.1186/s13059-015-0672-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Li X, Ito M, Zhou F, Youngson N, Zuo X, Leder P, Ferguson-Smith AC. A maternal-zygotic effect gene, Zfp57, maintains both maternal and paternal imprints. Dev Cell 2008;15:547–57. 10.1016/j.devcel.2008.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Mackay DJ, Callaway JL, Marks SM, White HE, Acerini CL, Boonen SE, Dayanikli P, Firth HV, Goodship JA, Haemers AP, Hahnemann JM, Kordonouri O, Masoud AF, Oestergaard E, Storr J, Ellard S, Hattersley AT, Robinson DO, Temple IK. Hypomethylation of multiple imprinted loci in individuals with transient neonatal diabetes is associated with mutations in ZFP57. Nat Genet 2008;40:949–51. 10.1038/ng.187 [DOI] [PubMed] [Google Scholar]
- 142. Nakamura T, Arai Y, Umehara H, Masuhara M, Kimura T, Taniguchi H, Sekimoto T, Ikawa M, Yoneda Y, Okabe M, Tanaka S, Shiota K, Nakano T. PGC7/Stella protects against DNA demethylation in early embryogenesis. Nat Cell Biol 2007;9:64–71. 10.1038/ncb1519 [DOI] [PubMed] [Google Scholar]
- 143. Messaed C, Chebaro W, Di Roberto RB, Rittore C, Cheung A, Arseneau J, Schneider A, Chen MF, Bernishke K, Surti U, Hoffner L, Sauthier P, Buckett W, Qian J, Lau NM, Bagga R, Engert JC, Coullin P, Touitou I, Slim R. H M Collaborative Group. NLRP7 in the spectrum of reproductive wastage: rare non-synonymous variants confer genetic susceptibility to recurrent reproductive wastage. J Med Genet 2011;48:540–8. 10.1136/jmg.2011.089144 [DOI] [PubMed] [Google Scholar]
- 144. Caliebe A, Richter J, Ammerpohl O, Kanber D, Beygo J, Bens S, Haake A, Jüttner E, Korn B, Mackay DJ, Martin-Subero JI, Nagel I, Sebire NJ, Seidmann L, Vater I, von Kaisenberg CS, Temple IK, Horsthemke B, Buiting K, Siebert R. A familial disorder of altered DNA-methylation. J Med Genet 2014;51:407–12. 10.1136/jmedgenet-2013-102149 [DOI] [PubMed] [Google Scholar]
- 145. Soellner L, Begemann M, Degenhardt F, Geipel A, Eggermann T, Mangold E. Maternal heterozygous NLRP7 variant results in recurrent reproductive failure and imprinting disturbances in the offspring. Eur J Hum Genet 2017;25:924–9. 10.1038/ejhg.2017.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wang CM, Dixon PH, Decordova S, Hodges MD, Sebire NJ, Ozalp S, Fallahian M, Sensi A, Ashrafi F, Repiska V, Zhao J, Xiang Y, Savage PM, Seckl MJ, Fisher RA. Identification of 13 novel NLRP7 mutations in 20 families with recurrent hydatidiform mole; missense mutations cluster in the leucine-rich region. J Med Genet 2009;46:569–75. 10.1136/jmg.2008.064196 [DOI] [PubMed] [Google Scholar]
- 147. Akoury E, Gupta N, Bagga R, Brown S, Déry C, Kabra M, Srinivasan R, Slim R. Live births in women with recurrent hydatidiform mole and two NLRP7 mutations. Reprod Biomed Online 2015;31:120–4. 10.1016/j.rbmo.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 148. Reddy R, Nguyen NM, Sarrabay G, Rezaei M, Rivas MC, Kavasoglu A, Berkil H, Elshafey A, Abdalla E, Nunez KP, Dreyfus H, Philippe M, Hadipour Z, Durmaz A, Eaton EE, Schubert B, Ulker V, Hadipour F, Ahmadpour F, Touitou I, Fardaei M, Slim R. The genomic architecture of NLRP7 is Alu rich and predisposes to disease-associated large deletions. Eur J Hum Genet 2016;24:1445–52. 10.1038/ejhg.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sanchez-Delgado M, Martin-Trujillo A, Tayama C, Vidal E, Esteller M, Iglesias-Platas I, Deo N, Barney O, Maclean K, Hata K, Nakabayashi K, Fisher R, Monk D. Absence of maternal methylation in biparental hydatidiform moles from women with nlrp7 maternal-effect mutations reveals widespread placenta-specific imprinting. PLoS Genet 2015;11:e1005644 10.1371/journal.pgen.1005644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Parry DA, Logan CV, Hayward BE, Shires M, Landolsi H, Diggle C, Carr I, Rittore C, Touitou I, Philibert L, Fisher RA, Fallahian M, Huntriss JD, Picton HM, Malik S, Taylor GR, Johnson CA, Bonthron DT, Sheridan EG. Mutations causing familial biparental hydatidiform mole implicate c6orf221 as a possible regulator of genomic imprinting in the human oocyte. Am J Hum Genet 2011;89:451–8. 10.1016/j.ajhg.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Akoury E, Zhang L, Ao A, Slim R. NLRP7 and KHDC3L, the two maternal-effect proteins responsible for recurrent hydatidiform moles, co-localize to the oocyte cytoskeleton. Hum Reprod 2015;30:159–69. 10.1093/humrep/deu291 [DOI] [PubMed] [Google Scholar]
- 152. Tian X, Pascal G, Monget P. Evolution and functional divergence of NLRP genes in mammalian reproductive systems. BMC Evol Biol 2009;9:202 10.1186/1471-2148-9-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Radian AD, de Almeida L, Dorfleutner A, Stehlik C. NLRP7 and related inflammasome activating pattern recognition receptors and their function in host defense and disease. Microbes Infect 2013;15:630–9. 10.1016/j.micinf.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, Dean J, Nelson LM. Mater, a maternal effect gene required for early embryonic development in mice. Nat Genet 2000;26:267–8. 10.1038/81547 [DOI] [PubMed] [Google Scholar]
- 155. Wang S, Kou Z, Jing Z, Zhang Y, Guo X, Dong M, Wilmut I, Gao S. Proteome of mouse oocytes at different developmental stages: In Proc Natl Acad Sci. 107, 2010:17639–44. 10.1073/pnas.1013185107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Virant-Klun I, Leicht S, Hughes C, Krijgsveld J. Identification of maturation-specific proteins by single-cell proteomics of human oocytes. Mol Cell Proteomics 2016;15:2616–27. 10.1074/mcp.M115.056887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Monti M, Zanoni M, Calligaro A, Ko MS, Mauri P, Redi CA. Developmental arrest and mouse antral not-surrounded nucleolus oocytes. Biol Reprod 2013;88:2 10.1095/biolreprod.112.103887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Herr JC, Chertihin O, Digilio L, Jha KN, Vemuganti S, Flickinger CJ. Distribution of RNA binding protein MOEP19 in the oocyte cortex and early embryo indicates pre-patterning related to blastomere polarity and trophectoderm specification. Dev Biol 2008;314:300–16. 10.1016/j.ydbio.2007.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zheng P, Dean J. Role of Filia, a maternal effect gene, in maintaining euploidy during cleavage-stage mouse embryogenesis. Proc Natl Acad Sci U S A 2009;106:7473–8. 10.1073/pnas.0900519106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yurttas P, Vitale AM, Fitzhenry RJ, Cohen-Gould L, Wu W, Gossen JA, Coonrod SA. Role for PADI6 and the cytoplasmic lattices in ribosomal storage in oocytes and translational control in the early mouse embryo. Development 2008;135:2627–36. 10.1242/dev.016329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Duncan FE, Padilla-Banks E, Bernhardt ML, Ord TS, Jefferson WN, Moss SB, Williams CJ. Transducin-like enhancer of split-6 (TLE6) is a substrate of protein kinase A activity during mouse oocyte maturation. Biol Reprod 2014;90:63 10.1095/biolreprod.113.112565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Begemann M, Rezwan FI, Beygo J, Docherty LE, Kolarova J, Schroeder C, Buiting K, Chokkalingam K, Degenhardt F, Wakeling EL, Kleinle S, González Fassrainer D, Oehl-Jaschkowitz B, Turner CLS, Patalan M, Gizewska M, Binder G, Bich Ngoc CT, Chi Dung V, Mehta SG, Baynam G, Hamilton-Shield JP, Aljareh S, Lokulo-Sodipe O, Horton R, Siebert R, Elbracht M, Temple IK, Eggermann T, Mackay DJG. Maternal variants in NLRP and other maternal effect proteins are associated with multilocus imprinting disturbance in offspring. J Med Genet 2018;55:497–504. 10.1136/jmedgenet-2017-105190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Alazami AM, Awad SM, Coskun S, Al-Hassan S, Hijazi H, Abdulwahab FM, Poizat C, Alkuraya FS. TLE6 mutation causes the earliest known human embryonic lethality. Genome Biol 2015;16:240 10.1186/s13059-015-0792-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Xu Y, Shi Y, Fu J, Yu M, Feng R, Sang Q, Liang B, Chen B, Qu R, Li B, Yan Z, Mao X, Kuang Y, Jin L, He L, Sun X, Wang L. Mutations in padi6 cause female infertility characterized by early embryonic arrest. Am J Hum Genet 2016;99:744–52. 10.1016/j.ajhg.2016.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Meyer E, Lim D, Pasha S, Tee LJ, Rahman F, Yates JR, Woods CG, Reik W, Maher ER. Germline mutation in NLRP2 (NALP2) in a familial imprinting disorder (Beckwith-Wiedemann Syndrome). PLoS Genet 2009;5:e1000423 10.1371/journal.pgen.1000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Christians E, Davis AA, Thomas SD, Benjamin IJ. Maternal effect of Hsf1 on reproductive success. Nature 2000;407:693–4. 10.1038/35037669 [DOI] [PubMed] [Google Scholar]
- 167. Burns KH, Viveiros MM, Ren Y, Wang P, DeMayo FJ, Frail DE, Eppig JJ, Matzuk MM. Roles of NPM2 in chromatin and nucleolar organization in oocytes and embryos. Science 2003;300:633–6. 10.1126/science.1081813 [DOI] [PubMed] [Google Scholar]
- 168. Ramos SB, Stumpo DJ, Kennington EA, Phillips RS, Bock CB, Ribeiro-Neto F, Blackshear PJ. The CCCH tandem zinc-finger protein Zfp36l2 is crucial for female fertility and early embryonic development. Development 2004;131:4883–93. 10.1242/dev.01336 [DOI] [PubMed] [Google Scholar]
- 169. Zhang J, Xue H, Qiu F, Zhong J, Su J. Testicular spermatozoon is superior to ejaculated spermatozoon for intracytoplasmic sperm injection to achieve pregnancy in infertile males with high sperm DNA damage. Andrologia 2018:e13175 10.1111/and.13175 [DOI] [PubMed] [Google Scholar]
- 170. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231–45. 10.1093/humupd/dmp048 [DOI] [PubMed] [Google Scholar]
- 171. Cissen M, Wely MV, Scholten I, Mansell S, Bruin JP, Mol BW, Braat D, Repping S, Hamer G. Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: a systematic review and meta-analysis. PLoS One 2016;11:e0165125 10.1371/journal.pone.0165125 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jmedgenet-2018-105513supp001.xlsx (10.9KB, xlsx)