Abstract
Background
Auditory encoding abnormalities, gray-matter loss, and cognitive deficits are all candidate schizophrenia (SZ) endophenotypes. This study evaluated associations between and heritability of auditory network attributes (function and structure) and attention in healthy controls (HC), SZ patients, and unaffected relatives (UR).
Methods
Whole-brain maps of M100 auditory activity from magnetoencephalography recordings, cortical thickness (CT), and a measure of attention were obtained from 70 HC, 69 SZ patients, and 35 UR. Heritability estimates (h2r) were obtained for M100, CT at each group-difference region, and the attention measure.
Results
SZ patients had weaker bilateral superior temporal gyrus (STG) M100 responses than HC and a weaker right frontal M100 response than UR. Abnormally large M100 responses in left superior frontal gyrus were observed in UR and SZ patients. SZ patients showed smaller CT in bilateral STG and right frontal regions. Interrelatedness between 3 putative SZ endophenotypes was demonstrated, although in the left STG the M100 and CT function−structure associations observed in HC and UR were absent in SZ patients. Heritability analyses also showed that right frontal M100 and bilateral STG CT measures are significantly heritable.
Conclusions
Present findings indicated that the 3 SZ endophenotypes examined are not isolated markers of pathology but instead are connected. The pattern of auditory encoding group differences and the pattern of brain function−structure associations differ as a function of brain region, indicating the need for regional specificity when studying these endophenotypes, and with the presence of left STG function−structure associations in HC and UR but not in SZ perhaps reflecting disease-associated damage to gray matter that disrupts function−structure relationships in SZ.
Keywords: MEG, gray matter, auditory, M100, schizophrenia, attention, heritability
Introduction
Auditory encoding abnormalities, gray-matter loss, and cognitive deficits are increasingly considered schizophrenia (SZ) endophenotypes. In particular, recent electrophysiological studies have focused on 100 ms auditory encoding abnormalities,1–5 brain structure studies have focused on reduced gray matter,3,6–8 and neuropsychological studies have focused on attention deficits.2,3,9 These putative endophenotypes are usually examined separately and thus relationships infrequently discussed. There is evidence, however, that these endophenotypes are associated.3 The present study built upon these findings, now examining 100 ms auditory encoding activity throughout the brain in individuals with SZ and controls and, in regions where auditory encoding group differences were observed, measuring associations between brain structure and performance on a test of attention. To establish these measures as endophenotypes10 (as well as to better demonstrate hypothesized associations between measures), first-degree unaffected relatives (UR) of the SZ sample were also recruited.
Abnormally small 100 ms auditory response (N100 electroencephalography [EEG] or M100 magnetoencephalography [MEG]) is a robust electrophysiological finding in medicated and unmedicated SZ patients and their relatives,2,4,11–14 with studies indicating that 100 ms abnormalities are trait rather than state features. Some studies have reported normal N100 responses in first-degree relatives of SZ patients.15–21 However, reduced N100 response may be observed only in unaffected first-degree relatives with comorbid psychiatric or substance conditions.1 Studies have shown that N100 amplitudes are highly heritable.1,22,23 There is thus support for 100 ms auditory encoding measures as a viable endophenotype.
Gray-matter abnormalities are common in patients with SZ,24–26 with studies observing cortical thinning in SZ, particularly in temporal, frontal, and cingulate gyrus regions19–21 and with smaller superior temporal gyrus (STG) volume and cortical thickness (CT) being among the most reliably observed structural brain abnormality in SZ.27–30 Several studies indicate STG gray-matter pathology as a marker of conversion to SZ.31–33 Other studies examining first-episode schizophrenia (FES) show that STG gray-matter abnormalities are present at disease onset.34,35 Although STG pathology is often considered an endophenotype, some studies have not observed STG gray-matter abnormalities in either nonconverting at-risk individuals or UR, indicating that STG gray-matter abnormalities were specific to SZ.35,36 Although studies are inconsistent in whether STG pathology is observed in SZ relatives, heritability studies show that CT and brain volume are influenced by genetic factors.37
A small number of multimodal studies have examined associations between brain structure (STG gray matter) and brain function (auditory encoding processes).38,39 For example, using EEG, less left posterior STG and left planum temporale gray matter were associated with smaller left temporal auditory P300 in SZ40 and FES.41 Using MEG, Edgar et al3 showed that left STG (L-STG) CT was associated with L-STG M100 source strength, and Edgar et al42 showed that the low-frequency auditory transient response as well as 40 Hz auditory steady-state abnormalities in L-STG distinguish SZ patients and healthy controls (HC), with associations between 40 Hz steady-state activity and gray matter in controls but not SZ indicating a loss of structure−function associations in patients. Given the above studies, and given that Chen et al4 showed STG as well as frontal region auditory encoding abnormalities in SZ, studies relating gray matter and auditory encoding processes in multiple brain regions in SZ are needed.
Finally, with regard to cognitive measures, individuals with SZ experience cognitive problems particularly in attention and working memory.9,43–49 Studies from our laboratory have shown that STG CT and STG function (M100 activity) are both associated with performance on tests of attention in individuals with SZ.2,3 Associations between prefrontal gray matter and cognitive performance have also been reported in SZ50.
Study Goals and Hypotheses
Distributed source localization identified auditory encoding processes throughout the brain and evaluated relationships among group differences in brain function, brain structure, and attention performance. To determine whether these measures have a genetic component, heritability estimates were obtained for M100, gray matter, and attention. Given that most previous paired-click EEG and MEG studies have shown group differences for the first but not the second click,1,2 this study focused on first-click M100 activity with the following hypotheses: (1) Replicating previous findings,4 it was hypothesized that M100 group differences between HC, SZ patients, and UR would be observed in STG and frontal regions. (2) Individuals with SZ would show smaller STG CT, and gray-matter abnormalities in UR were expected to be small or absent. (3) CT would predict M100 source strength in HC and UR. Given gray-matter pathology in SZ, it was hypothesized that function−structure relationships would be missing in SZ. Finally, replicating Edgar et al,3 it was hypothesized that associations between M100 source strength, CT, and attention performance would be observed. (4) M100 source strength, CT, and attention performance would be heritable, with results from phenotypic correlation analyses demonstrating associations between the 3 putative endophenotypes.
Methods
Subjects
In this study, 70 HC (51 males; mean age 39.40 ± 11.67 years), 69 individuals with chronic SZ (57 males, mean age 41.08 ± 12.75 years), and 35 first-degree UR (14 parents, 4 children, and 17 siblings; 14 males; mean age 45.33 ± 13.24 years) were recruited. Selection criteria for SZ were: (1) diagnosis of SZ with no other Axis I diagnosis, determined by the Structured Clinical Interview for DSM-IV-Patient Edition (SCID-DSM-IV; American Psychiatric Association, 1994); (2) stable, continuous treatment with antipsychotic medication for at least 3 months; (3) no history of substance dependence (per SCID); (4) no history of alcohol or other substance abuse in the past 3 months (per SCID); (5) no history of head injury with loss of consciousness for more than 5 min; and (6) no psychiatric hospitalization in the last 3 months. Selection criteria for HC and UR are those provided in Chen et al.4 UR were ascertained by the participation of their SZ family members. See the online supplement for additional demographic information and, as shown in supplementary table 1, groups did not differ in age, education, or parental socioeconomic status (SES51). Patients’ SES was significantly lower than controls’ SES. In individuals with SZ, mean total scores of the Positive and Negative Syndrome Scale (PANSS)52 were 17.78 (SD = 5.64) for positive symptoms and 16.38 (SD = 4.44) for negative symptoms (N = 65; PANSS scores were not available in 4 subjects).
Attention Measures
For each participant, a measure of attention was obtained via the clinical index (confidence index score) from the Conners’ Continuous Performance Test II53 (CPT II). The CPT clinical index score is an estimate of the likelihood that the examinee’s response fits those given by individuals with attention deficits hyperactivity disorder. The CPT clinical index score is derived by an automated discriminant function analysis,53 and the higher the CPT clinical index score the more likely the individual has an attention deficit.
Paired-Click Paradigm
The paired-click paradigm followed the protocol of Adler et al,54 in which 3 ms binaural clicks were presented in pairs with a 500 ms interstimulus interval and with onset-to-onset intertrial interval jitter between 7 and 11 s, averaging 9 s. A total of 150 clicks were delivered through earphones placed in each ear canal. Prior to data acquisition, 500 Hz tones of 300 ms duration and 12.5 ms rise time were used to obtain auditory thresholds for each ear. Auditory thresholds were initially estimated via stepwise amplitude reduction until participants stopped verbally identifying the presence of the tone. For fine-tuning, tone loudness was then adjusted within ±10 dB of the preliminary threshold until a final threshold was confirmed (approximately 50% accuracy). For each ear, the peak intensity of the click was presented 35 dB above each subject’s hearing threshold. As previously noted, this study examined only first-click activity at 100 ms.
MEG and Magnetic Resonance Imaging Data Acquisition and Coregistration
MEG data were recorded in a magnetically shielded room (VACUUMSCHMELZE GmbH & Co. KG) using a 306-channel Vector-View MEG system (Elekta-Neuromag). After 0.1–330 Hz band-pass and 60 Hz notch filters, MEG signals were digitized at 1000 Hz. Electrooculogram and electrocardiogram were also obtained. The participant’s head position was monitored using 4 Head Position Indicator (HPI) coils attached to the scalp. Participants were asked to refrain from smoking for at least 1 h before the recording session. After the MEG session, T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE) structural MR images (sMRI) were collected on a Siemens 3T TIM Trio scanner at the Mind Research Network. Images were collected with a field of view of 256 × 256 mm, 192 sagittal slices, and 1 × 1 × 1 mm3 spatial resolution. This was a 5-echo sequence with echo times of 1.64, 3.5, 5.36, 7.22, and 9.08 ms, a repetition time of 2530 ms, a gray−white matter contrast enhancement inversion recovery time of 1200 ms, and 7° flip angle.
To coregister MEG and sMRI data, 3 fiducial landmarks (nasion, right, and left preauriculars) as well as an additional 250+ points on the scalp and face were digitized for each participant using the Probe Position Identification (PPI) System (Polhemus). A transformation matrix that involved rotation and translation between the MEG and sMRI coordinate systems was obtained by matching the PPI measurements to the sMRI.
Magnetic Source Analysis
MEG raw signals were first processed with Signal Space Separation (SSS55,56) using MaxFilter (Elekta-Neuromag; Elekta Oy). After SSS, first-click epochs 500 ms prestimulus to 500 ms poststimulus onset were averaged. Trials containing eyeblinks and large eye movements were excluded. On average, 108 trials were retained for HC, 110 trials for SZ patients, and 97 trials for UR (F(2,170) = 2.87, P > .05).
To calculate MEG forward solutions, a realistically shaped boundary element method (BEM) head model was created from each participant’s inner skull,57 with the BEM mesh obtained from tessellating the inner skull surface from the MRI into ~6000 triangular elements with ~5 mm size. VESTAL provided source images for each participant. VESTAL selects the source configuration that minimizes the absolute value of the source strength.
A 5−55 Hz band-pass filter was applied before VESTAL analyses. VESTAL analyses examined activity 80−130 ms poststimulus, producing a 4D activation map (3D volumes across time). The M100 VESTAL source image for each participant was obtained by summing source strength from VESTAL volumes between 80 and 130 ms. Before examining group difference in M100 activity throughout the brain, each participant’s M100 VESTAL source image was registered to MNI space (Montreal Neurological Institute, MNI-152 atlas) using an affine transformation (FLIRT-FMRIB’s Linear Image Registration Tool)58 in FSL (www.fmrib.ox.ac.uk/fsl/). A spatial smoothing of sigma 5 mm was applied. Group difference in M100 activity throughout the brain was examined using the 3dANOVA program in AFNI (https://afnilnimh.nig.gov/pub/dist/doc/program_help/3dANOVA.html). Analysis of variance (ANOVA) with diagnosis as between-subjects factor compared M100 VESTAL activity. For all whole-brain analyses described here and later, the cluster size needed to provide family-wise correction was determined using AFNI AlphaSim (https://afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html).
Cortical Thickness
To assess function−structure associations, a CT measure was obtained in regions where group M100 source strength differences were observed (figure 1). To this end, each subject’s CT measures from FreeSurfer surface space was first converted to voxel space. The CT volume from each subject was resampled to volume in MNI space. Regions of interest (ROIs) from VESTAL group difference maps served as masks, and mean CT for each region was obtained by averaging CT values across voxels within each ROI. ANOVA with diagnosis as a between-subjects factor assessed CT group differences at each ROI.
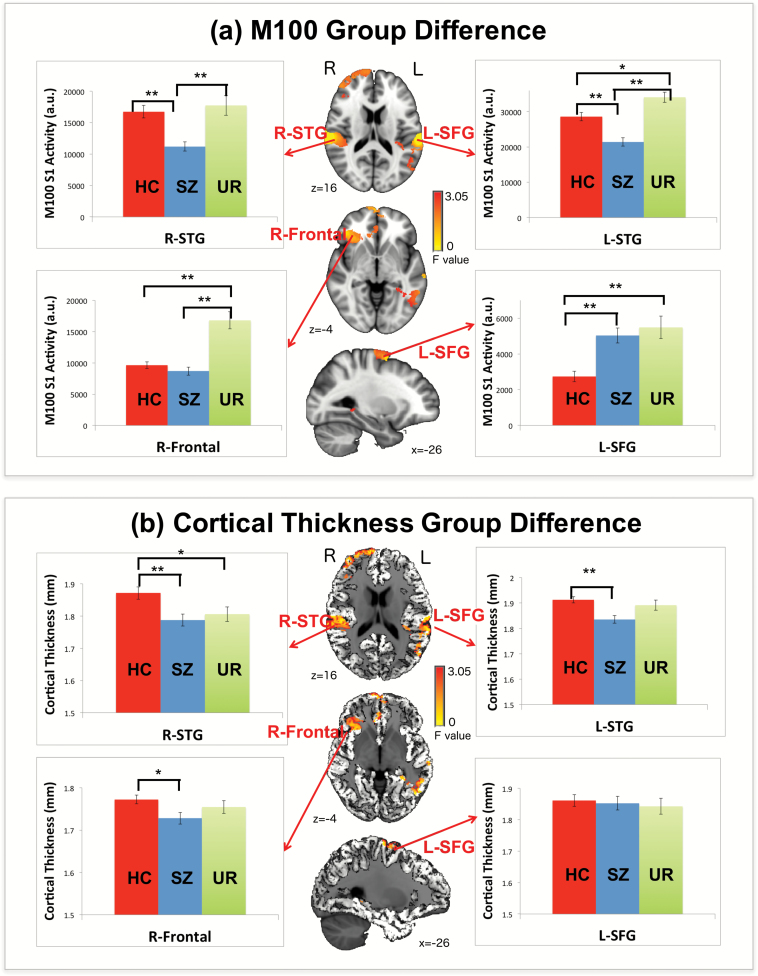
Fig. 1.
(a) M100 analysis of variance (ANOVA) group differences and associated bar charts showing results of simple-effects analyses at the 4 identified regions of interest (ROIs) (*P < .05, **P < .001). All shown points of M100 activity (axial and sagittal slices) are contained in 1 of the 4 ROIs. (b) Cortical thickness (CT) measures for each participant were derived from the 4 ROIs where M100 group differences were observed (shown for one participant). Associated bar charts show results of simple-effects analysis at each ROI (*P < .05, **P < .001).
Associations between M100 activity, CT, and Attention
Given the main hypothesis, associations between auditory encoding, gray matter, and attention were examined in each of the M100 group difference ROIs. In particular, for each ROI, hierarchical regressions were run entering M100 source strength or CT measures first, group second, and Group X M100 or CT interaction last, with the CPT attention score at the dependent measure. Except for the whole-brain VESTAL group difference analysis, all statistical analyses were performed using IBM SPSS Statistics 24 (IBM Corp)
Heritability Analyses
To help establish M100 auditory activity, CT, and the attention measure as candidate endophenotypes, estimates of additive genetic variance, heritability (h2r), were obtained for each dependent measure (M100 and CT at each group difference ROI, and the attention measure) using SOLAR-Eclipse software (http://www.solar-eclipse-genetics.org). Age and gender were used as covariates as both gray matter and electrophysiological response may show effects of age and gender.59,60 To avoid the covariates introducing interpretative confounds, these analyses were repeated with just one or neither covariate. Bivariate genetic correlation analysis was performed to calculate the proportion of common genetic and environmental variance that influences 2 traits that constitute the phenotypic correlation (ρP) between 2 traits. If the genetic correlation coefficient (ρG) is significantly different from 0, then the significant portion of the variability in 2 traits are considered to be influenced by shared genetic factors.61 The environmental correlation (ρE) is the component of the correlation due to shared environmental factors. All genetic analyses were performed after ensuring that each phenotype followed a normal distribution.
Results
M100 Group Differences
Figure 1a shows M100 ANOVA group differences. Based on the ANOVA results, ROIs were identified via thresholding the group difference F-stats map at P < .05 and with cluster-thresholded family-wise correction applied (>4000 voxels). Four group-difference ROIs were identified: L-STG, right STG (R-STG), right frontal regions (R-Frontal), and left superior frontal gyrus (L-SFG). Simple-effects analyses (all pairwise comparisons) were performed for each ROI, computing for each participant a single M100 measure at each ROI by summing across the voxels in each ROI. Simple effect analyses showed stronger M100 activity in HC than in SZ patients in L-STG (P < .05) and R-STG (P < .05), and stronger M100 activity in SZ patients than in HC in L-SFG (P < .001). Stronger M100 activity in UR than in HC and SZ patients was observed in L-STG and R-Frontal regions (Ps < .05). The only M100 abnormality common to SZ patients and UR was observed in L-SFG, with abnormally larger L-SFG M100 activity in UR than in HC (P < .001). Bar charts in figure 1 illustrate the aforementioned group-difference findings.
CT Group Differences
As shown in figure 1b, CT measures for each participant were obtained from the M100 group-difference ROIs (ie, L-STG, R-STG, R-Frontal, and L-SFG), computing for each participant a single CT measure at each ROI by averaging across the voxels in each ROI. Less CT in SZ patients than in HC was observed in the following regions: L-STG, R-STG, and R-Frontal. Less CT in UR than in HC was observed only for R-STG (P < .05).
M100 and CT Associations
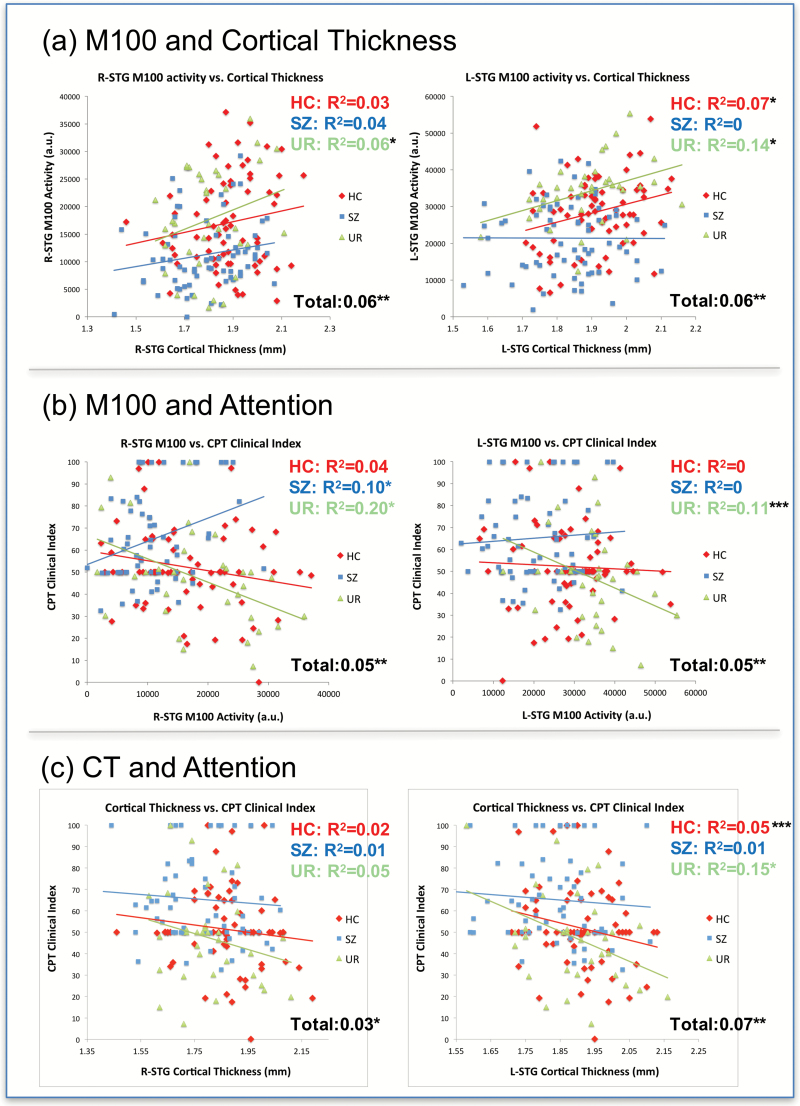
The 4 M100 group-difference ROIs were used for analyses evaluating structure−function associations. At each ROI, hierarchical regressions were run with CT entered first, group second (dummy-coded orthogonally as HC vs SZ patients, and UR vs others), and the CT X group interaction last, with M100 source strength as the dependent measure (table 1(a)). As hypothesized, in both left and right STG, larger CT predicted greater M100 source strength. Given previous findings showing a loss of L-STG structure−function associations in SZ42, the regression was restructured on a post hoc basis with group dummy coded as SZ vs others, which identified a marginally significant Group X L-STG CT interaction (P = .06), with a structure−function association in HC + UR but not in SZ patients (figure 2a). Figure 2a also shows significant associations between R-STG CT and M100 source strength in the full sample. No main effect of CT and no Group X CT interaction were observed in the 2 frontal regions.
Table 1.
Hierarchical Regressions With (a) CT Predicting M100 Source Strength; (b) M100 Predicting Attention (CPT Clinical Index); (c) CT Predicting Attention (CPT Clinical Index)
| (a) CT predicting M100 activity | ||||
|---|---|---|---|---|
| Regions | CT | Group | Group X CT | Total R2 |
| R 2 | R 2 Change | R 2 Change | ||
| L-STG | 0.06** | 0.17** | 0.02+ | 0.25*** |
| R-STG | 0.06** | 0.10*** | 0.00 | 0.16*** |
| R-Frontal | 0.01 | 0.22*** | 0.00 | 0.24*** |
| L-SFG | 0.00 | 0.13*** | 0.01 | 0.14*** |
| (b) M100 predicting attention | ||||
| M100 | Group | Group X M100 | Total R2 | |
| R 2 | R 2 Change | R 2 Change | ||
| L-STG | 0.05** | 0.07** | 0.02 | 0.14*** |
| R-STG | 0.05** | 0.08** | 0.08** | 0.20*** |
| R-Frontal | 0.00 | 0.12*** | 0.00 | 0.12** |
| L-SFG | 0.00 | 0.11*** | 0.01 | 0.12** |
| (c) CT predicting attention | ||||
| CT | Group | Group X CT | Total R2 | |
| R 2 | R 2 Change | R 2 Change | ||
| L-STG | 0.07** | 0.08** | 0.02 | 0.17*** |
| R-STG | 0.03* | 0.11*** | 0.00 | 0.14*** |
| R-Frontal | 0.11*** | 0.08*** | 0.01 | 0.21*** |
| L-SFG | 0.07** | 0.11*** | 0.00 | 0.18*** |
Note: CT, Cortical thickness; CPT, Continuous Performance Test; L-STG, left superior temporal gyrus; R-STG, right superior temporal gyrus; R-frontal, right frontal regions; L-SFG, left superior frontal gyrus.
*P < .05; **P < .01; ***P < .001; +P = .12.
Fig. 2.
(a) Association between M100 and cortical thickness (CT) in left superior temporal gyrus (L-STG) and right superior temporal gyrus (R-STG). (b) Associations between attention and L-STG and R-STG M100 source strength. (c) Associations between attention and L-STG and R-STG CT (*P < .05; **P < .01; ***P < .09).
Per recommendation of Miller and Chapman62 and Verona and Miller63 at all ROIs group M100 source strength differences remained after removing variance associated with CT (P’s < .01), indicating that M100 source strength group differences could not be explained as secondary to group differences in CT. Findings remained similar when hierarchical regressions were run with CT the dependent variable.
Attention Performance Group Differences
The mean CPT clinical index scores for each group were 52.12 (SD = 19.65) for HC, 65.19 (SD = 20.43) for SZ patients, and 47.28 (SD = 21.99) for UR. ANOVAs showed a group main effect (F(2,157) = 10.42, P < .001), with the expected better attention performance in HC and UR than in SZ patients (P < .001).
M100, CT, and Attention Associations
Hierarchical regressions with attention as the dependent variable were run with M100 or CT entered first, group second (dummy coded as HC vs SZ patients, and UR vs others), and the M100 or CT X Group interaction last, with M100 source strength or CT from each ROI analyzed separately (table 1(b) and (c)). As detailed later, M100 and CT analyses provided support for brain function and brain structure associations with attention. Of note, for all analyses, after removing variance associated with M100 or CT, attention group differences remained significant (P’s < .001).
As shown in table 1(b) and (c), brain function (M100) and brain structure (CT) predicted variance in attention. Figure 2b and c shows relationships for L-STG and R-STG M100 measures and attention as well as L-STG and R-STG CT measures and attention for each group. Specifically, M100 in L-STG and R-STG predicted attention (figure 2b), although with a significant Group X R-STG M100 interaction indicating R-STG M100 source strength and attention associations only in UR (figure 2b). CT at all ROIs predicted attention, with no significant Group X CT interactions.
Heritability
Heritability estimates for all 3 phenotypes are presented in table 2. As in most heritability studies, age and gender were entered as covariates. The only M100 measure showing significant heritability was R-Frontal M100. CT measures showing significant heritability were L-STG and R-STG. To better understand the effect of the covariates, analyses were rerun with only age or gender entered as a covariate, as well as with no covariate.62,63 The heritability results with only age as a covariate were similar to the results with both age and gender as covariates. With only gender as a covariate as well as no covariate, only R-Frontal M100 was found to be heritable. The pattern of results thus suggests a possible influence of gender on the CT heritability findings.
Table 2.
Heritability Estimates (h2r) for Attention, M100 Source Strength, and CT– Overall Sample N = 170–174
| Covariate P values | |||||
|---|---|---|---|---|---|
| Measures | h 2 r | SE | P value | Age | Gender |
| CPT clinical index | 0 | N/A | .5 | .02 | .02 |
| L-STG M100 | 0.02 | 0.23 | .46 | .29 | .42 |
| R-STG M100 | 0.02 | 0.24 | .46 | .70 | .60 |
| R-Frontal M100* | 0.87 | 0.32 | .05 | .59 | .24 |
| L-SFG M100 | 0 | N/A | .5 | .19 | .43 |
| L-STG CT* | 0.88 | 0.34 | .04 | <.001 | .06 |
| R-STG CT* | 0.93 | 0.32 | .01 | <.001 | .14 |
| R-Frontal CT | 0.72 | 0.37 | .10 | <.001 | .05 |
| L-SFG CT | 0 | N/A | .5 | .001 | .32 |
Note: Abbreviations are explained in the footnote to Table 1.
*Significant heritability values (P < .05).
Phenotypic Correlations
Table 3 presents the phenotypic correlation results for M100, CT, and attention for the full sample. ρP is the estimate of the strength of the observed correlation attributed to either environmental or genetic factors. Significant phenotypic correlations were found between (1) attention and M100 activity in L-STG and R-STG; (2) attention and CT in L-STG, R-Frontal, and L-SFG; and (3) M100 and CT in L-STG, R-STG, and R-Frontal. Marginally significant phenotypic correlations were found between attention and CT in R-STG (P = .06) and between attention and M100 in L-SFG (P = .07).
Table 3.
Bivariate Correlations Between M100, CT, and Attention (CPT Clinical Index)
| Trait 1 | Trait 2 | ρP | P value |
|---|---|---|---|
| M100 and attention | |||
| L-STG M100 | CPT clinical index | −0.22 | .005** |
| R-STG M100 | CPT clinical index | −0.18 | .02* |
| R-Frontal M100 | CPT clinical index | −0.05 | .53 |
| L-SFG M100 | CPT clinical index | 0.15 | .07 |
| CT and attention | |||
| L-STG CT | CPT clinical index | −0.19 | .02* |
| R-STG CT | CPT clinical index | −0.15 | .06 |
| R-Frontal CT | CPT clinical index | −0.26 | <.001*** |
| L-SFG CT | CPT clinical index | −0.29 | <.001*** |
| M100 and CT | |||
| L-STG M100 | L-STG CT | 0.28 | <0.001*** |
| R-STG M100 | R-STG CT | 0.22 | <0.001*** |
| R-Frontal M100 | R-Frontal CT | 0.20 | <0.001*** |
| L-SFG M100 | L-SFG CT | −0.01 | 0.91 |
Note: Abbreviations are explained in the footnote to Table 1. *P < .05; **P < .01; ***P < .001.
Discussion
Auditory encoding, CT, and attention abnormalities were observed in SZ patients, with results demonstrating that these measures are not isolated markers of pathology but instead are mechanistically connected, with the analyses including UR providing evidence for treating each as an endophenotype and also their coincidence as an endophenotype. Here, we review the primary findings with respect to the 4 study hypotheses and within the context of previous findings. A likely clinically informative pattern of function−structure associations in HC and UR but not in SZ patients in left STG is also discussed.
M100 Source Strength and CT as Univariate Endophenotypes
Consistent with previous literature,4,64,65 present findings showed that M100 group differences were observed in STG and frontal regions, again confirming that auditory encoding abnormalities are not specific to STG, thus indicating that individuals with SZ show abnormalities in multiple nodes of a concurrently activated auditory network. In Chen et al,4 we hypothesized that the low STG and inferior frontal M100 activity and the high superior frontal M100 activity in individuals with SZ indicate that HC activate the ventral “what” auditory pathway more strongly than individuals with SZ when passively encoding auditory stimuli, and with individuals with SZ perhaps compensating for insufficient ventral auditory pathway activation (STG to inferior frontal connections) by over-activating the dorsal “where” auditory pathway (STG to superior frontal connections). Replicating Chen et al,4 present findings showed again showed 2 abnormalities in frontal activity in SZ—weaker inferior frontal activity and stronger superior frontal activity. In addition to M100 abnormalities, the present CT findings replicate studies showing reduced STG CT (bilateral) and reduced frontal CT (right frontal) in SZ.29,30,66
UR findings provided support for the consideration of M100 auditory encoding and CT measures as “univariate” endophenotypes, although with present and previous findings suggesting that these endophenotypes have regional specificity. For example, the M100 encoding abnormalities in unaffected family members were not identical to those observed in SZ; rather than reduced L-STG and R-STG M100 activity, abnormally high R-Frontal activity was observed in UR. Similar to SZ patients, however, UR showed abnormally high L-SFG activity. These results perhaps shed light on findings from Turetsky et al,1 who showed a nonsignificant larger N100 Cz response (P = .18) in UR without a comorbid condition (similar to the UR sample in this study) than HC. The present findings of greater L-STG, L-SFG, and R-Frontal M100 activity in UR than HC mirror those of Turetsky et al,1 although with HC and UR findings in this study significant, with greater statistical sensitivity in this study perhaps due to an examination of brain activity in source vs sensor space. As previously noted, based on the findings in Chen et al,4 individuals with SZ might be compensating insufficient ventral auditory pathway by overactivating dorsal auditory pathway (abnormally high L-SFG activity). With greater functionality, UR might be overactivating both ventral and dorsal auditory pathways (high activity in STG, R-Frontal, and L-SFG in UR than HC). The aforementioned findings thus suggest that the identification of brain markers as possible endophenotypes depends on the brain regions and family members examined, suggesting that in diagnostically “clean” UR only L-SFG M100 auditory encoding processes should be considered a potential endophenotype as this was the only auditory encoding abnormality observed in both SZ patients and UR.
Similar regional specificity was observed for CT, with present CT findings replicating studies showing reduced STG CT in SZ patients,29,30,66 but with findings for reduced CT in UR observed only in right STG. This finding of reduced right STG CT in UR is somewhat surprising given previous findings suggesting left > right STG abnormalities in SZ.2,40–42 However, given brain structure−function findings indicating greater damage to L-STG in SZ in this study (see later) as well as in Edgar et al,42 R-STG CT may better predict risk for conversion to SZ. Finally, the lack of SFG CT group differences between SZ patients and HC suggests that, compared with other frontal regions, SFG is more structurally intact in SZ and, therefore, can serve as compensatory or secondary frontal region during auditory encoding processes that, as detailed above, normally involve inferior frontal and STG regions in controls.
More generally, the aforementioned findings suggest the need to consider the possibility of region-specific brain findings in research domain criteria (RDoC) studies that involve SZ. For example, although SZ and autism spectrum disorder (ASD) are both neurodevelopmental disorders, and both SZ and ASD show M100 auditory encoding abnormalities,2,4,67,68 similar to the SZ and UR findings in this study, SZ and ASD may be similar in just one node of the auditory encoding network. Indeed, given that STG CT abnormalities are characteristic of SZ but not ASD, even in the presence of a shared functional regional abnormality the casual mechanisms may differ, with previous and present findings demonstrating the advantage of multimodal imaging studies to help interpret functional findings.42,69–71
Associations Between M100 Source Strength, CT, and Attention
Function−structure associations were observed in left and right STG, with larger STG CT predicting a stronger M100 STG response. Associations between STG measures (M100 and CT) and attention were also observed (table 1(b) and (c)). For example, at all ROIs CT predicted attention performance. One possibility is that findings implicate low CT as a factor contributing to the M100 and attention problems. A casual association in the other direction, however, is also possible, with functional abnormalities resulting in gray-matter pathology.
An interesting finding was the lack of L-STG M100 and CT associations in SZ (figure 2a). Our finding is consistent with previous studies, with Edgar et al3 showing that left STG CT was associated with left STG M100 source strength, and Edgar et al42 showing that low- and high-frequency auditory 40 Hz steady-state abnormalities in left STG distinguish SZ patients and HC, with associations between 40 Hz steady-state activity and STG CT in controls but not SZ indicating a loss of structure−function associations in many individuals with SZ. In particular, it was hypothesized that disease-associated damage to STG gray matter in SZ disrupts STG gamma-band function−structure relationships observed in HC. Examination of the left hemisphere associations in figure 2c suggests a similar loss of function−structure associations in SZ for left CT and attention, with larger N studies needed to replicate and extend present findings.
Heritability of M100, CT, and Attention Measures
Univariate heritability analyses showed that R-Frontal M100 activity, L-STG CT, and R-STG CT were heritable. The present STG CT heritability findings are consistent with studies showing that familial history explains a large proportion (40%–80%) of the variance in CT.8,37,59,72 A caveat for the CT heritability findings, however, is that L-STG CT and R-STG CT were not significantly heritable if gender was not included as a covariate, suggesting a possible influence of gender on the CT heritability findings.
Bivariate phenotypic correlations supported the hypothesized interrelatedness between M100, CT, and attention. Although findings were especially significant regarding the phenotypic correlations between L-STG and R-STG M100 activity, CT, and attention, phenotypic correlations were also observed between L-SFG and R-frontal structure and attention, and a trending association between L-SFG M100 and attention. Given the relatively small samples and the phenotypic correlation analysis thus potentially underpowered, the observation of such associations suggests fairly strong relationships. These findings thus suggest simultaneous genetic influence on these 3 measures.
Given the present sample size and study design, these phenotypic associations can only be attributed to shared and/or genetic factors, with the present sample size not allowing differentiation of the unique influences of genes vs environment. Nevertheless, the bivariate phenotypic correlation (table 3) among STG function and structure as well as attention indicate that the patterns observed at the individual level are also manifest at the family level. These findings indicate that these measures cluster and are heritable, evidence for treating each as an endophenotype and also their coincidence as an endophenotype. As the bivariate phenotypic correlation analyses showed commonality between the 3 measures, especially for L-STG and R-STG, and as individuals with SZ showed function and structure abnormalities in L-STG and R-STG, present findings suggest further joint study of these STG brain measures in SZ and individuals at-risk for SZ to better understand the pathway(s) for developing SZ.
Similar to the previously discussed function−structure associations, a model that accounts for the mechanisms relating the 3 endophenotypes is unfortunately difficult to derive empirically. Future studies with larger samples will perhaps allow evaluation of causality (eg, via structural equation model) to help determine the directionality of the observed associations.
To conclude, present findings suggest the interrelatedness of 3 putative SZ endophenotypes: M100 source strength, CT, and attention performance, with regional precision needed when discussing SZ endophenotypes. There is currently a great focus on developing cognitive and pharmacological treatments that normalize neural network abnormalities and thus hopefully patient symptoms.73 Present findings indicate the need for whole-brain analyses to identify regionally specific abnormalities, and with UR findings indicating differences between treatment targets based on brain abnormalities specific to SZ vs treatment targets based on brain abnormalities observed in SZ patients as well as at-risk UR. Future brain imaging studies should thus focus on whole-brain imaging measures, with multimodal studies identifying regionally specific brain function and structure measures that best predict treatment response and thus intervention targets.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institute of Mental Health (R01 MH65304 to Dr Cañive, K01 MH108822 to Dr Chen, and K08 MH085100 to Dr Edgar), a VA Merit grant (VA Merit CSR&D: IIR-04-212-3 to Dr Cañive), and University of California at San Diego Merit Review Grant from the Department of Veterans Affairs to Dr Huang. The authors would like to thank the subjects who enrolled in this study; Megan Schendel, Kim Paulson, Cassandra Wootton, and Emerson Epstein, who helped with data collection; and Lawrence Calais, Gloria Fuldauer, and Nickolas Lemke for their help with subject recruitment and administrative support related to this project. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Turetsky BI, Greenwood TA, Olincy A, et al. Abnormal auditory N100 amplitude: a heritable endophenotype in first-degree relatives of schizophrenia probands. Biol Psychiatry. 2008;64:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith AK, Edgar JC, Huang M, et al. Cognitive abilities and 50- and 100-msec paired-click processes in schizophrenia. Am J Psychiatry. 2010;167:1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edgar JC, Hunter MA, Huang M, et al. Temporal and frontal cortical thickness associations with M100 auditory activity and attention in healthy controls and individuals with schizophrenia. Schizophr Res. 2012;140:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen YH, Edgar JC, Huang M, et al. Frontal and superior temporal auditory processing abnormalities in schizophrenia. Neuroimage Clin. 2013;2:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edgar JC, Fisk CL IV, Chen YH, et al. Identifying auditory cortex encoding abnormalities in schizophrenia: the utility of low-frequency versus 40 Hz steady-state measures [published online ahead of print March 23, 2018]. Psychophysiology. doi: 10.1111/psyp.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moran ME, Hulshoff Pol H, Gogtay N. A family affair: brain abnormalities in siblings of patients with schizophrenia. Brain. 2013;136:3215–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prasad KM, Keshavan MS. Structural cerebral variations as useful endophenotypes in schizophrenia: do they help construct “extended endophenotypes”?Schizophr Bull. 2008;34:774–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turner JA, Calhoun VD, Michael A, et al. Heritability of multivariate gray matter measures in schizophrenia. Twin Res Hum Genet. 2012;15:324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schulze-Rauschenbach S, Lennertz L, Ruhrmann S, et al. Neurocognitive functioning in parents of schizophrenia patients: attentional and executive performance vary with genetic loading. Psychiatry Res. 2015;230:885–891. [DOI] [PubMed] [Google Scholar]
- 10. Miller GA, Rockstroh B. Endophenotypes in psychopathology research: where do we stand?Annu Rev Clin Psychol. 2013;9:177–213. [DOI] [PubMed] [Google Scholar]
- 11. Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008;64:376–384. [DOI] [PubMed] [Google Scholar]
- 12. Salisbury DF, Collins KC, McCarley RW. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophr Bull. 2010;36:991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ethridge LE, Hamm JP, Pearlson GD, et al. Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder. Biol Psychiatry. 2015;77:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosburg T, Boutros NN, Ford JM. Reduced auditory evoked potential component N100 in schizophrenia–a critical review. Psychiatry Res. 2008;161:259–274. [DOI] [PubMed] [Google Scholar]
- 15. Frangou S, Sharma T, Alarcon G, et al. The Maudsley Family Study, II: endogenous event-related potentials in familial schizophrenia. Schizophr Res. 1997;23:45–53. [DOI] [PubMed] [Google Scholar]
- 16. Karoumi B, Laurent A, Rosenfeld F, et al. Alteration of event related potentials in siblings discordant for schizophrenia. Schizophr Res. 2000;41:325–334. [DOI] [PubMed] [Google Scholar]
- 17. Winterer G, Egan MF, Rädler T, Coppola R, Weinberger DR. Event-related potentials and genetic risk for schizophrenia. Biol Psychiatry. 2001;50:407–417. [DOI] [PubMed] [Google Scholar]
- 18. Waldo MC, Adler LE, Freedman R. Defects in auditory sensory gating and their apparent compensation in relatives of schizophrenics. Schizophr Res. 1988;1:19–24. [DOI] [PubMed] [Google Scholar]
- 19. Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. [DOI] [PubMed] [Google Scholar]
- 20. Narr KL, Bilder RM, Toga AW, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–719. [DOI] [PubMed] [Google Scholar]
- 21. White T, Andreasen NC, Nopoulos P, Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biol Psychiatry. 2003;54:418–426. [DOI] [PubMed] [Google Scholar]
- 22. Anokhin AP, Vedeniapin AB, Heath AC, Korzyukov O, Boutros NN. Genetic and environmental influences on sensory gating of mid-latency auditory evoked responses: a twin study. Schizophr Res. 2007;89:312–319. [DOI] [PubMed] [Google Scholar]
- 23. Renvall H, Salmela E, Vihla M, et al. Genome-wide linkage analysis of human auditory cortical activation suggests distinct loci on chromosomes 2, 3, and 8. J Neurosci. 2012;32:14511–14518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–1356. [DOI] [PubMed] [Google Scholar]
- 25. Gur RE, Turetsky BI, Bilker WB, Gur RC. Reduced gray matter volume in schizophrenia. Arch Gen Psychiatry. 1999;56:905–911. [DOI] [PubMed] [Google Scholar]
- 26. Takayanagi Y, Takahashi T, Orikabe L, et al. Volume reduction and altered sulco-gyral pattern of the orbitofrontal cortex in first-episode schizophrenia. Schizophr Res. 2010;121:55–65. [DOI] [PubMed] [Google Scholar]
- 27. Ehrlich S, Brauns S, Yendiki A, et al. Associations of cortical thickness and cognition in patients with schizophrenia and healthy controls. Schizophr Bull. 2012;38:1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mitelman SA, Brickman AM, Shihabuddin L, et al. A comprehensive assessment of gray and white matter volumes and their relationship to outcome and severity in schizophrenia. Neuroimage. 2007;37:449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smiley JF, Rosoklija G, Mancevski B, Mann JJ, Dwork AJ, Javitt DC. Altered volume and hemispheric asymmetry of the superficial cortical layers in the schizophrenia planum temporale. Eur J Neurosci. 2009;30:449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borgwardt SJ, Riecher-Rössler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–1156. [DOI] [PubMed] [Google Scholar]
- 32. Fusar-Poli P, Broome MR, Woolley JB, et al. Altered brain function directly related to structural abnormalities in people at ultra high risk of psychosis: longitudinal VBM-fMRI study. J Psychiatr Res. 2011;45:190–198. [DOI] [PubMed] [Google Scholar]
- 33. Cannon TD, Chung Y, He G, et al. ; North American Prodrome Longitudinal Study Consortium Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Onitsuka T, Shenton ME, Salisbury DF, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am J Psychiatry. 2004;161:1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu M, Li J, Eyler L, et al. Decreased left middle temporal gyrus volume in antipsychotic drug-naive, first-episode schizophrenia patients and their healthy unaffected siblings. Schizophr Res. 2013;144:37–42. [DOI] [PubMed] [Google Scholar]
- 36. Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Winkler AM, Kochunov P, Blangero J, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rasser PE, Schall U, Todd J, et al. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr Bull. 2011;37:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCarley RW, Shenton ME, O’Donnell BF, et al. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry. 1993;50:190–197. [DOI] [PubMed] [Google Scholar]
- 41. McCarley RW, Salisbury DF, Hirayasu Y, et al. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:321–331. [DOI] [PubMed] [Google Scholar]
- 42. Edgar JC, Chen YH, Lanza M, et al. Cortical thickness as a contributor to abnormal oscillations in schizophrenia?Neuroimage Clin. 2014;4:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Unschuld PG, Buchholz AS, Varvaris M, et al. Prefrontal brain network connectivity indicates degree of both schizophrenia risk and cognitive dysfunction. Schizophr Bull. 2014;40:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. [DOI] [PubMed] [Google Scholar]
- 45. Heaton R, Paulsen JS, McAdams LA, et al. Neuropsychological deficits in schizophrenics. Relationship to age, chronicity, and dementia. Arch Gen Psychiatry. 1994;51:469–476. [DOI] [PubMed] [Google Scholar]
- 46. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. [DOI] [PubMed] [Google Scholar]
- 47. Barch DM. What can research on schizophrenia tell us about the cognitive neuroscience of working memory?Neuroscience. 2006;139:73–84. [DOI] [PubMed] [Google Scholar]
- 48. Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39:889–905. [DOI] [PubMed] [Google Scholar]
- 49. Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. [DOI] [PubMed] [Google Scholar]
- 50. Gur RE, Cowell PE, Latshaw A, et al. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000;57:761–768. [DOI] [PubMed] [Google Scholar]
- 51. Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Soc Sci Med. 2003;56:769–784. [DOI] [PubMed] [Google Scholar]
- 52. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 53. Conners CK. Conner’s Continuous Performance Test II (CPT II). Toronto, Canada: Multi-Health Systems; 2002. [Google Scholar]
- 54. Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. [DOI] [PubMed] [Google Scholar]
- 55. Song T, Cui L, Gaa K, et al. Signal space separation algorithm and its application on suppressing artifacts caused by vagus nerve stimulation for magnetoencephalography recordings. J Clin Neurophysiol. 2009;26:392–400. [DOI] [PubMed] [Google Scholar]
- 56. Taulu S, Kajola M, Simola J. Suppression of interference and artifacts by the signal space separation method. Brain Topogr. 2004;16:269–275. [DOI] [PubMed] [Google Scholar]
- 57. Hämäläinen MS, Sarvas J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng. 1989;36:165–171. [DOI] [PubMed] [Google Scholar]
- 58. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. [DOI] [PubMed] [Google Scholar]
- 59. Kochunov P, Charlesworth J, Winkler A, et al. Transcriptomics of cortical gray matter thickness decline during normal aging. Neuroimage. 2013;82:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kochunov P, Glahn DC, Nichols TE, et al. Genetic analysis of cortical thickness and fractional anisotropy of water diffusion in the brain. Front Neurosci. 2011;5:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Almasy L, Dyer TD, Blangero J. Bivariate quantitative trait linkage analysis: pleiotropy versus co-incident linkages. Genet Epidemiol. 1997;14:953–958. [DOI] [PubMed] [Google Scholar]
- 62. Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. [DOI] [PubMed] [Google Scholar]
- 63. Verona E, Miller GA. Analysis of covariance. In: Cautin RL, Lilienfeld SO eds. The Encyclopedia of Clinical Psychology. New York: Wiley; 2015:136–142. [Google Scholar]
- 64. Korzyukov O, Pflieger ME, Wagner M, et al. Generators of the intracranial P50 response in auditory sensory gating. Neuroimage. 2007;35:814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boutros NN, Gjini K, Urbach H, Pflieger ME. Mapping repetition suppression of the N100 evoked response to the human cerebral cortex. Biol Psychiatry. 2011;69:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mitelman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int Rev Psychiatry. 2007;19:345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Edgar JC, Fisk Iv CL, Berman JI, et al. Auditory encoding abnormalities in children with autism spectrum disorder suggest delayed development of auditory cortex. Mol Autism. 2015;6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Edgar JC, Khan SY, Blaskey L, et al. Neuromagnetic oscillations predict evoked-response latency delays and core language deficits in autism spectrum disorders. J Autism Dev Disord. 2015;45:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Calhoun VD, Sui J. Multimodal fusion of brain imaging data: a key to finding the missing link(s) in complex mental illness. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roberts TP, Lanza MR, Dell J, et al. Maturational differences in thalamocortical white matter microstructure and auditory evoked response latencies in autism spectrum disorders. Brain Res. 2013;1537:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Berman JI, Edgar JC, Blaskey L, et al. Multimodal diffusion-MRI and MEG assessment of auditory and language system development in autism spectrum disorder. Front Neuroanat. 2016;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chiang MC, McMahon KL, de Zubicaray GI, et al. Genetics of white matter development: a DTI study of 705 twins and their siblings aged 12 to 29. Neuroimage. 2011;54:2308–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2012;62:1504–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.