Abstract
Bipolar disorder (BD) is a complex psychiatric disorder characterized by dominant symptom swings across different phases (manic, depressive, and euthymic). Different symptoms in BD such as abnormal episodic memory recall and psychomotor activity have been related to alterations in different regions, ie, hippocampus and motor cortex. How the abnormal regional distribution of neuronal activity relates to specific symptoms remains unclear, however. One possible neuronal mechanism of the relationship is the alteration of the global distribution of neuronal activity manifested in specific local regions; this can be measured as the correlation between the global signal (GS) and local regions. To understand the GS and its relationship to psychopathological symptoms, we here investigated the alteration of both GS variance and its regional topography in healthy controls and 3 phases of BD. We found that the variance of GS showed no significant difference between the 4 groups. In contrast, the GS topography was significantly altered in the different phases of BD, ie, the regions showing abnormally strong topographical GS contribution changed from hippocampus (and parahippocampus/fusiform gyrus) in depression to motor cortex in mania. Importantly, topographical GS changes in these regions correlated with psychopathological measures in both depression and mania. Taken together, our findings demonstrate the central importance of GS topography for psychopathological symptoms. This sheds lights on the neuronal mechanisms of specific psychopathological symptoms in BD, and its relevance in the relationship between global and local neuronal activities for behavior in general.
Keywords: bipolar disorder, hippocampus, psychomotor dysfunction, functional magnetic resonance imaging, global signal
Introduction
Bipolar Disorder and the Brain’s Spontaneous Activity
Bipolar disorder (BD) is an affective disorder that is characterized by extreme mood swings ranging from depression over euthymia to mania.1 However, psychopathological symptoms extend far beyond mood swings including cognitive changes and abnormal motor behavior. They, for instance, include strong rumination and extreme focus on recalling of the own autobiographical contents in depression2–4 whereas mania can be characterized by the abnormal dominance of motor behavior as in psychomotor excitation.1,5,6 The neural origin of such dominance of one specific behavioral function, ie, symptom, in the different phases of BD remains unclear though.
Several resting-state functional magnetic resonance imaging (fMRI) studies reported alterations in functional connectivity (FC) of a single resting-state network such as default-mode network (DMN),7–12 and, most recently, sensorimotor network9,13 in BD. However, as in other mental disorders such as depression14,15 and schizophrenia,16–21 altered resting-state activity is not limited to single networks in BD. Instead, the overall topographical pattern with the relationship between different networks as between DMN and somatomotor network seems to be altered in BD.9 Therefore, the exclusive focus on specific regions/networks may overlook the importance of topographical activity pattern across the whole brain, which, on the psychopathological level, may reflect the abnormal dominance of one particular behavioral function relative to others.
The Spontaneous Activity’s Global Signal: Psychopathological Relevance
The brain global activity can be measured by what is described as the global signal (GS) in fMRI (see later for methodological details). For data-cleaning purpose, GS must be eliminated and is therefore regressed out of the data.22–24 Studies combining electrocorticography/electrophysiology and fMRI, however, demonstrate a direct relationship between local electrophysiological measures and GS in fMRI.25,26 This suggests that GS is not merely nonneuronal physiological noise but also an important source of the neuronal activity itself. That has been proven to be relevant in psychiatric disorders such as schizophrenia where the variance of GS is abnormally elevated (while it showed normal levels in a group of euthymic BD), which, relying on computational modeling, was related to increased local and global functional connectivity (GFC).18
Moreover, the spatial topography of GS, ie, the representation of GS in specific regions, has also been examined in schizophrenia. Yang et al.19 observed abnormally elevated GS representation in association regions whereas sensory regions showed preferential GS-related reductions in schizophrenia. That leaves open the topographical pattern of GS in other psychiatric disorders such as BD including the relationship of GS topography to the different symptoms in depressed, euthymic, and manic phases. That is especially relevant as, for instance, psychomotor hyperactivity in mania and increased autobiographical memory recall in depression are related to abnormal activity in different regions, ie, motor cortex9,13 and hippocampus.27,28 Whether these abnormal regional activities are related to alterations in GS topographical distribution remains unclear.
The Spontaneous Activity’s GS: Methodological Considerations
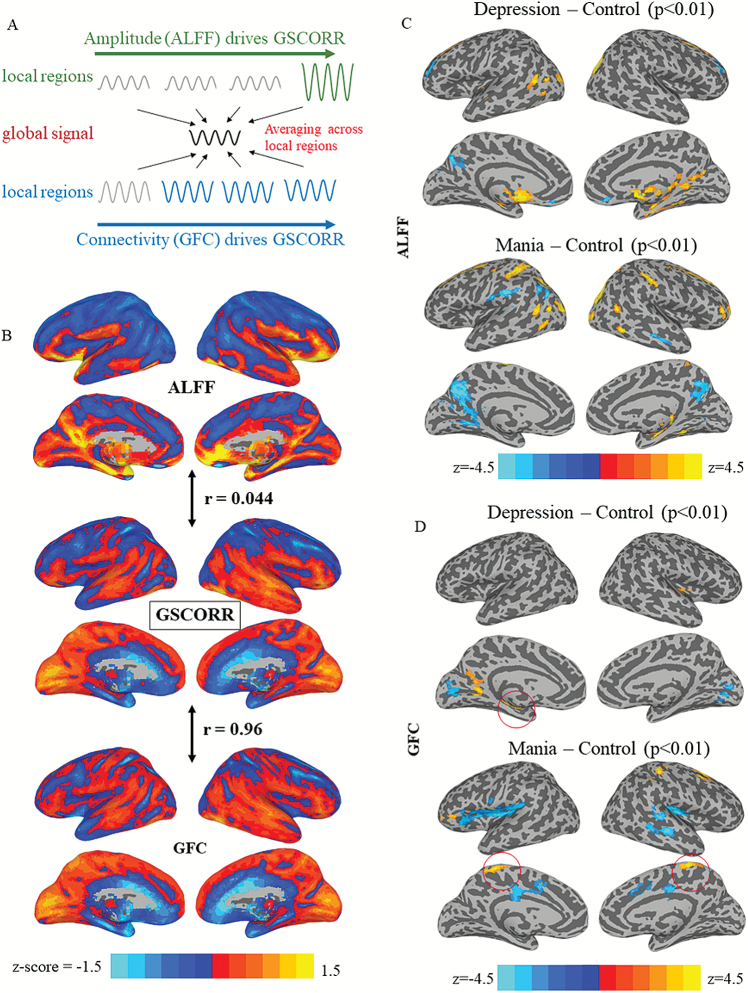
GS topography represents the relationship between local regions and GS. Two methods are widely used for investigating GS topography: (1) the correlation between GS and local voxels, ie, global signal correlation (GSCORR)26,29,30; and (2) the mean correlation between each voxel and the other voxels, ie, GFC.31,32 Mathematically, the 2 measurements can dissociate from each other. The GSCORR topography can be driven by connectivity or amplitude based on the definition of GS calculation. The GS is the averaging of time course in each voxel within gray matter; therefore, the regions with higher amplitude (figure 3A, the region in green color) or correlating more with other regions (figure 3A, the regions in blue color) can both increase their weights in GS. Although for GFC, it is only determined by the connectivity in local regions. The extent to GS topography is determined by amplitude or connectivity remains unclear, by which can be tested through the relationship between GSCORR, GFC, and amplitude of low-frequency fluctuations (ALFF). If GS topography is more amplitude driven, a higher correlation between GSCORR and ALFF will be observed, alternative, a higher correlation between GSCORR and GFC will be observed for connectivity driven (figure 3A).
Fig. 3.
(A) Schematic illustrations of the relationship between global signal correlation (GSCORR), global functional connectivity (GFC), and amplitude of low-frequency fluctuations (ALFF). GSCORR can be amplitude driven, ie, the region with higher amplitude correlates more with global signal (GS); or connectivity driven, ie, the regions with more connections to the others correlates more with GS. (B) A voxel-based correlation for GSCORR with ALFF and GFC across all subjects. (C) Voxel-wise group comparison for ALFF. (D) Voxel-wise group comparison for GFC.
In addition, previous studies show that the GS topography is nonuniform but stable across the brain.33,34 But it remains unclear whether such a topography relates to the distribution of neuronal or nonneuronal components in GS, which is crucial to verify the neuronal significance of GS topography.
Aims and Hypotheses
The main and overarching aim of this study was to investigate the relevance of GS for BD with its different phases in resting-state fMRI. Specifically, we first investigated whether the variance of GS was abnormal in BD across phases. Second, we investigated topography of GSCORR in 3 phases of BD and checked whether the abnormal GS topography is a phase or disease dependent. Third, we investigated the relationship between GSCORR and the severity of psychopathological symptoms using psychopathological scales (ie, Young Mania Rating Scale [YMRS] and Hamilton Depression Scale [HAMD]) to confirm whether the abnormal GS topography was related to symptoms. Finally, we checked whether the GSCORR is amplitude driven or phase driven and verified the influence of noise on GS topography (see supplementary methods and results).
Methods
Subjects and Clinical Assessment
The study included 64 healthy control subjects (39 females, age range 19–61 years), and bipolar patients with 30 in the manic phase, 35 in the depressive phase, and 34 in euthymic phase (62 females, age range 18–60 years for the whole BD group) after head motion checking (supplementary table S1). The details for clinical assessments, inclusion, and exclusion can be found in supplementary material methods.
fMRI Data Acquisition
Images were acquired using a 1.5 T GE scanner with a standard head coil. Functional images were collected using a gradient echo-planar imaging sequence, which is sensitive to BOLD contrast (repetition time/echo time = 2000/30 ms, flip angle = 90°, field of view = 24 cm). In addition, 3-dimensional T1-weighted anatomical images were acquired for all participants. For the details, see supplementary methods.
fMRI Data Preprocessing
Preprocessing steps were implemented in Analysis of Functional NeuroImages (AFNI) software.35 For the details, see supplementary methods.
Calculation of GS Variance
The GS was calculated by averaging time courses across all voxels in the gray matter after the bandpass filter (0.01–0.1 Hz). The variance of the GS was then calculated.
Calculation of Global Signal Correlation
The GSCORR was calculated as the Pearson correlation between the GS and the voxels in gray matter with Fisher z transformation.23 As various artifacts may uniformly increase GS representation in a local voxel, the GSCORR was spatially normalized as z score within gray matter for each subject.
Calculation of GFC
GFC,31,36 also called as global brain connectivity ,32 calculated the correlation (Pearson’s r) of the time series in each voxel with every other voxel in gray matter mask and storing the mean correlation after Fisher z transformation. The GFC was also normalized spatially as z score within gray matter for each subject.
Calculation of Amplitude of Low-frequency Fluctuations
The ALFF was calculated using REST software.37 The preprocessed time series was transformed to a frequency domain with a fast Fourier transform and the power spectrum were then calculated. The ALFF was calculated as the averaged square root across 0.01–0.10 Hz at each voxel. Similar to GSCORR, the ALFF was normalized spatially as z score within gray matter for each subject.
Relationship Between GSCORR, GFC, and ALFF
The relationships between ALFF and GSCORR as well as between GSCORR and GFC in the gray matter were tested by calculating voxel-based Pearson correlation on all subjects’ average map. In addition, to check whether the relationship was clinically relevant, the voxel-wise group comparison between depression, mania with healthy controls for GSCORR, ALFF, and GFC topography was calculated and the similarity of significant regions was checked (see “Methods” section).
Voxel-wise Group Comparison for GS Topography
The voxel-wise group comparison in this study was carried out by AFNI’s function “3dttest++” for the 2-sample t test. The results were thresholded at P < .01 after AlphaSim correction in AFNI with a minimum cluster size of 46 voxels (corrected P < .01).
Voxel-wise Correlations With Disorder Symptom Severity and Medication Load
To investigate the correlation between GSCORR and disorder symptoms, the total scores of HAMD and YMRS were used to correlate with GSCORR in whole-brain voxel way for patients with BD in depression, euthymia, and mania. The correlation maps were thresholded at r > ±3 (P = .0025) with cluster size larger than 24 voxels, which suggested the effect size of correlation reached medium and corrected P value in this analysis was P < .01.38
Results
Variance of GS in BD
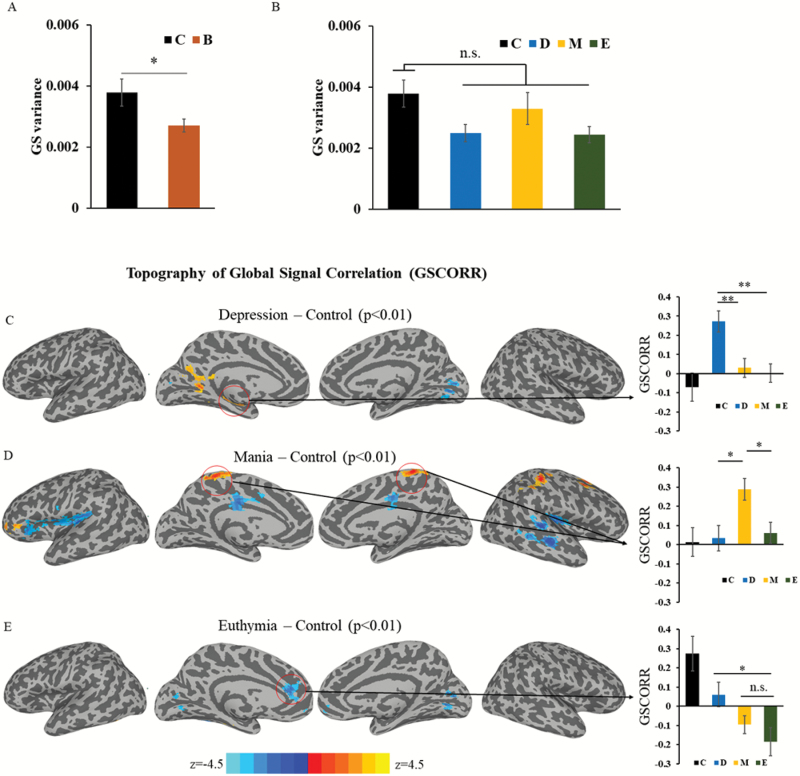
We first checked the group difference for GS variance between patients BD (n = 99, including depression, n = 35; mania, n = 30, euthymia, n = 34) and healthy controls (n = 64). A weak significance was observed when we compared BD group (as a whole) with healthy controls (t = 2.4, P = .017) (figure 1A). In addition, 1-way ANOVA between healthy controls and different phases in BD showed marginal significance (F = 2.56, P = .057) (figure 1B). This replicates the finding from the previous study that observed normal GS in a group of euthymic BD subjects.18
Fig. 1.
(A) Comparison of global signal (GS) variance in healthy controls and bipolar disorder (BD) subjects. (B). One-way ANOVA for GS variance across groups. (C) Voxel-wise group comparison for global signal correlation (GSCORR) between depression and healthy controls. The region of interest (ROI) of hippocampus/parahippocampus is extracted from the significant clusters of the comparison of depression vs healthy controls. (D) Mania vs healthy controls. The ROI of the bilateral motor cortex is extracted from the comparison of mania vs healthy control. (E). Euthymia vs healthy controls. The ROI of pregenual anterior cingulate cortex is extracted from the comparison euthymia vs healthy control. The threshold of voxel-wise group comparison was set at P < .01 with cluster size larger than 46 voxels (corrected P < .01). Error bars represent standard error of the mean. Control (C); depression (D); mania (M); euthymia (E). *P < .05, **P < .01, ***P < .001.
To assess the contribution of potential motion artifact to GS variance, we tested the motion artifacts between the 4 groups. No differences in the degree of head motion, ie, variance, were observed between the 4 groups in both shifting (supplementary figure S1A) (F = 1.33, P = .27) and rotating (supplementary figure S1B) (F = 1.1, P = .35) movement in 1-way ANOVA.
Topographical Distribution of GS in BD Phases
To investigate specific alterations in GS topography in the different phases of BD, we first investigated GSCORR topographical distribution in depression by comparing it with healthy controls. The 2-sample t test yielded the strongest increase in the left hippocampus and parahippocampal gyrus in conjunction with fusiform gyrus (figure 1C). The region of interest (ROI) analysis of hippocampus/parahippocampus extracted from the significant clusters of the comparison further suggested the increase of GSCORR in this region is BD phase dependent (depression vs mania: t = 3.3, P = .0089; and depression vs euthymia: t = 3.7, P =.0011, Bonferroni corrected) (figure 1C).
Next, we conducted a 2-sample t test for manic vs healthy controls. This yielded strongest GSCORR increases in bilateral motor cortex (figure 1D). The ROI analysis of the bilateral motor cortex also suggested the increase of GSCORR in this region is phase dependent (mania vs depression: t = 2.9, P = .012; and mania vs euthymia: t = 2.8, P = .015, Bonferroni corrected) (figure 1D).
Finally, we conducted the comparison between euthymic and healthy subjects. This yielded the most prominent reduction of GSCORR in the pregenual anterior cingulate cortex (pACC). The ROI analysis of pACC, however, suggested that the decrease of GSCORR in pACC is phase independent, because it shows no difference between euthymia and mania (t = –1.0, P = .68, Bonferroni corrected) and remains just above significant level in the comparison between euthymia and depression (t = –2.5, P = .039, Bonferroni corrected) (figure 1E).
In addition, the robustness of GSCORR to physiological noise is checked by calculating the GSCORR in different ways: (1) with regression of head motion, white matter (WM) and cerebrospinal fluid (CSF) (as main results); (2) without regression of WM and CSF; and (3) without regression of head motion. After comparing the GSCORR topography before and after various regressors, we suggest that our results are unlikely altered by physiological (supplementary results).
Correlation of GS Topography With Symptoms and Medication
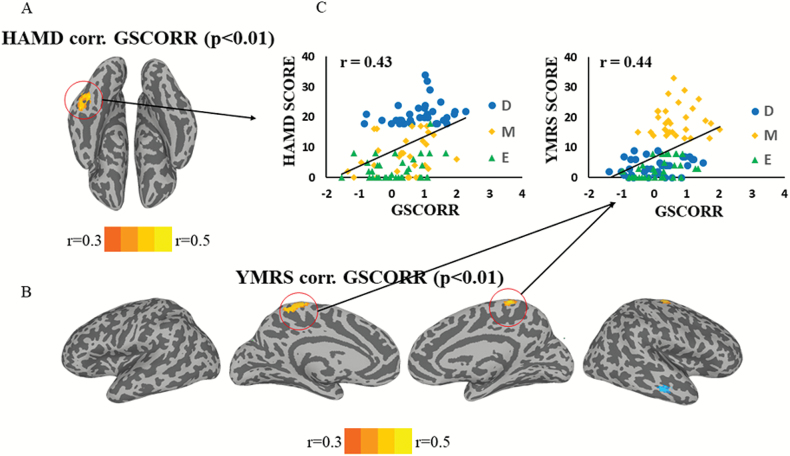
Next, we correlated GS topography with clinical symptoms. Conducting whole-brain voxel-wise correlation analyses, we observed the significant positive correlation between GSCORR and HAMD in the parahippocampus and fusiform gyrus (figure 2A). For correlation with YMRS, positive correlation with GSCORR was observed in the primary motor cortex (figure 2B). The correlation based on the ROIs extracted from significant voxels further suggested the effect size of correlations reached medium in both instances (r = .43, P < .001 for HAMD corr. GSCORR; r = .44, P < .001 for YMRS corr. GSCORR) (figure 2C).
Fig. 2.
Clinical correlation with psychopathological symptoms. Whole-brain voxel-wise correlation of global signal correlation (GSCORR) with Hamilton Depression Scale (HAMD) and Young Mania Rating Scale (YMRS) in the bipolar group, including depressive, manic, and euthymic groups. The threshold was set at r > ±.3 (P = .0025) with cluster size larger than 24 voxels (corrected P < .01). (A) The correlation for HAMD was found in parahippocampus and fusiform area for GSCORR. (B) The correlation for YMRS was found in the bilateral motor cortex for GSCORR. (C) Scatter plots for visualization of the correlation in significant regions.
Relationship Between GSCORR With GFC and ALFF
We next investigated to whether the GSCORR is driven by either amplitude (ie, measured by ALFF) or connectivity (ie, measured by GFC) (figure 3A). The topography of ALFF (averaging across all subjects) showed a different pattern when compared with the topography of GSCORR (voxel-based Pearson correlation: r = .044, P < .001) (figure 3B). In contrast, the spatial patterns of GSCORR and GFC were very similar (r = .96, P < .001) (figure 3B). In addition, the 2-sample t tests for ALFF and GFC further confirmed that the difference between depression, mania, and healthy controls showed more similarity of GFC with GSCORR when compared with GFC and ALFF (figure 3C, D). Taken together, our results confirm that GS topographical representation, measured as GSCORR, is more closely related to FC between the local voxel and GS (as measured by GFC) than to the amplitude (as measured by ALFF).
Discussion
We here investigated the GS in neuronal activity and its topographical distribution in BD including its different phases, ie, depressive, euthymic, and manic groups. Our main findings are as follows: (1) nonsignificance in the variance of GS across different phases; (2) abnormal topographical representation of GS, ie, GSCORR, in hippocampus (in association with parahippocampus and fusiform gyrus) in depression, bilateral motor cortex in mania, and pACC in euthymia; and (3) significantly positive correlation of abnormal GSCORR in parahippocampus/fusiform gyrus with depressive symptoms and GSCORR in motor cortex with manic symptoms. The influence of physiological noise on results was also checked, and we suggested that our results are unlikely generated by physiological noise (see supplementary discussion for details).
Topography of GS: Depressive BD
Depressed BD patients showed significantly increased GS representation in specifically the left hippocampus in association with parahippocampus and fusiform area. Several studies reported that hippocampus changes in BD are specific for depression, ie, phase specific.27,28 This is specified and extended by our finding of altered GS topography in the hippocampus in specifically the depressive phase whereas it was not observed in either manic or euthymic phases. That is also in line with the central role of the hippocampus in depression in general including both depressive BD and depression in major depressive disorder.14,39
Recent studies suggest that the hippocampus provides temporal organization of episodic memory,40,41 whereas the parahippocampus, known as “parahippocampus place area,”42 and the fusiform gyrus, whose major function is visual object recognition,43,44 may provide the contents of episodic memory.41 Therefore, presuming interplay between their timing and content-related functions, abnormal GS-based hippocampal and parahippocampal neuronal activities may be closely related to the frequently observed strong intrusions of contents from past events, ie, autobiographical memories, into the present inner mental life of depressed patients.45,46
Topography of GS: Manic BD
The central role of motor cortex in mania is well in line with recent studies.9,13 Moreover, we recently demonstrated abnormally increased neuronal variability in the somatomotor network in manic BD.9 That reflects well the clinical presentation as manic patients can be characterized by extreme psychomotor agitation and excitement.1,5,6 Most importantly, such psychomotor excitation links with an abnormally strong focus on external sensory contents, which dominates these patients’ outer behavior (and inner mental life).1,5,6,47
We, therefore, hypothesize the following relationship: The more strongly the GS is topographically represented in the motor cortex, the more its associated function, ie, psychomotor activity and motor execution, shifts into the forefront of the overall behavior in these patients (while shifting other behavioral functions into the background). Such interpretation is well supported by our finding of the direct correlation between increased GS representation in motor cortex and manic symptoms as characterized by externally directed behavior.
Topography of GS: Euthymic BD
Finally, we observed decreased GS topographical representation in pACC in euthymic state. This is most remarkable as pACC is strongly involved in both clinical and subclinical mood fluctuations.48 As mood fluctuations are also present in the euthymic state, we suppose that they may be traced to abnormally decreased GS representation in pACC; this, speculatively, renders pACC function unstable leading to mood instabilities even in the euthymic state.
Taken in this way, decreased GS representation in pACC may serve as a trait-specific marker for mood fluctuations in the euthymic state that predisposes mania and depression. In contrast, decreased GS in pACC does not reflect a state- or phase-specific marker as we observed similar findings in both depression and mania. Hence, unlike GS in motor cortex and hippocampus, reduced GS in pACC cannot be considered phase specific, ie, state marker, but a trait markers of BD in general.
Topography of GS and Clinical Symptoms: “Spatiotemporal Psychopathology”
Considering our correlation findings between GSCORR and psychopathological symptoms, we assume that increased global activity contribution to motor cortex and hippocampus is reflected in increased contributions of their respectively associated behavioral functions, ie, movement generation in mania and autobiographical memory recall in depression, to the subjects’ overall behavior. Clinically, the resulting overall behavior is consequently dominated by movements (in mania) and autobiographical recall (in depression). The psychopathological symptoms can thus be traced to an abnormal spatial structure in the topographical distribution of the brain’s global activity—this reflects the spatial part of what is described as “Spatiotemporal Psychopathology.”3,49,50
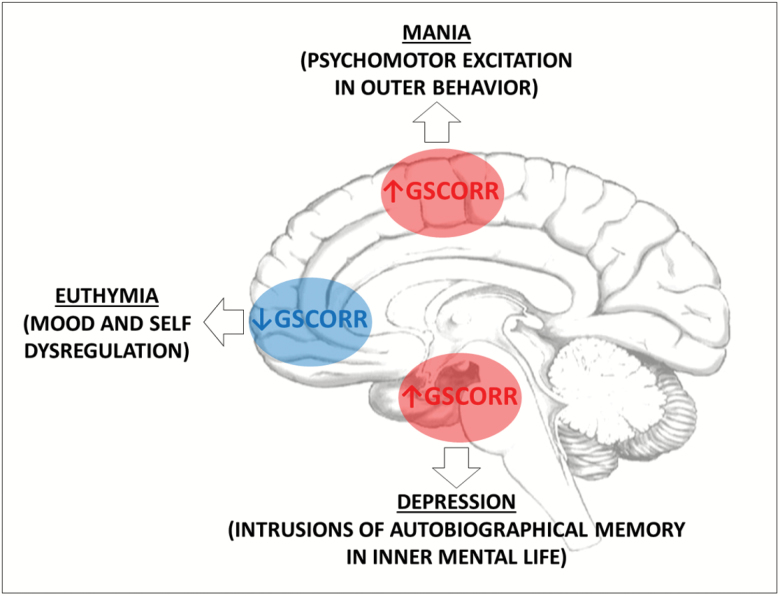
How about the temporal side? Our data clearly show that GSCORR is much stronger related to the phase coherence (as indexed by GFC) than the neuronal variability (as indexed by ALFF) (figure 3C and D). We thus assume that phase coherence is abnormally strong between (1) global and motor cortical activity in mania, and (2) global and hippocampal activity in depression. Abnormal spatial distribution of GS in mania and depression can thus be traced to temporal abnormalities, ie, phase coherence. The respectively associated symptoms such as psychomotor excitation and increased autobiographical memory recall may consequently show a truly spatiotemporal basis as suggested in Spatiotemporal Psychopathology3,49,50 (figure 4).
Fig. 4.
The hypothesis of the relationship between GS representation topography and dominant behaviors in bipolar disorder. The abnormal dominance of extreme psychomotor excitation in mania, the mood and self-dysregulation in euthymia, and the abnormal autobiographical memory recall in depression may be related to corresponding spatial, ie, topographical distribution of global neuronal activity. GSCORR = global signal correlation.
Conclusion
BD can be characterized by the abnormal predominance of specific behavioral functions in the different phases. We here investigated whether such abnormal behavioral predominance is related neuronally to the GS and its topographical distribution. Taken together, our results (1) illustrate the neuronal and behavioral relevance of the brain’s global neuronal activity (as measured by the GS) for behavior in general and its alterations in psychiatric disorders such as BD; (2) provide a novel mechanistic way of understanding psychopathological symptoms in terms of the brain’s spatiotemporal mechanisms entailing Spatiotemporal Psychopathology; and (3) show the potential clinical utility of the brain’s global topography in allowing for distinction between state- or -phase-specific (ie, motor cortex, hippocampus) and trait-specific (ie, pACC) markers of BD.
Funding
This work was supported by the grant from the Ministry of Science and Technology of China, National Key R&D Program of China (2016YFC1306700), the EJLB-Michael Smith Foundation, the Canada Institute of Health Research (CIHR), the Hope of Depression Foundation (HDRF), and the Start-up Research Grant in Hangzhou Normal University (to G.N.), the National Natural Science Foundation of China (No. 31271195 to Z.H.). The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
The authors thank Prof Gianluigi Mancardi for the access to the MRI Unit (Magnetic Resonance Research Center, Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genoa and IRCCS Ospedale Policlinico San Martino, Genoa, Italy). The authors also thank Bin-ke Yuan and Shi-jia Fan for suggestions on data analysis and Niall Duncan on data preparation and data transfer.
References
- 1. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. 5th ed (DSM-5). Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 2. Northoff G. Psychopathology and pathophysiology of the self in depression—neuropsychiatric hypothesis. J Affect Disord. 2007;104:1–14. [DOI] [PubMed] [Google Scholar]
- 3. Northoff G. Spatiotemporal psychopathology I: no rest for the brain’s resting state activity in depression? Spatiotemporal psychopathology of depressive symptoms. J Affect Disord. 2016;190:854–866. [DOI] [PubMed] [Google Scholar]
- 4. Northoff G, Wiebking C, Feinberg T, Panksepp J. The “resting-state hypothesis” of major depressive disorder-a translational subcortical–cortical framework for a system disorder. Neurosci Biobehav Rev. 2011;35:1929–1945. [DOI] [PubMed] [Google Scholar]
- 5. Kraepelin E. Clinical Psychiatry. London: Macmillan; 1902. [Google Scholar]
- 6. Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W. The quantitative assessment of motor activity in mania and schizophrenia. J Affect Disord. 2010;120:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ongür D, Lundy M, Greenhouse I, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martino M, Magioncalda P, Saiote C, et al. Abnormal functional–structural cingulum connectivity in mania: combined functional magnetic resonance imaging-diffusion tensor imaging investigation in different phases of bipolar disorder. Acta Psychiatr Scand. 2016;134:339–349. [DOI] [PubMed] [Google Scholar]
- 9. Martino M, Magioncalda P, Huang Z, et al. Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc Natl Acad Sci USA. 2016;113:4824–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magioncalda P, Martino M, Conio B, et al. Functional connectivity and neuronal variability of resting state activity in bipolar disorder–reduction and decoupling in anterior cortical midline structures. Hum Brain Mapp. 2015;36:666–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anticevic A, Brumbaugh MS, Winkler AM, et al. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 2013;73:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anticevic A, Savic A, Repovs G, et al. Ventral anterior cingulate connectivity distinguished nonpsychotic bipolar illness from psychotic bipolar disorder and schizophrenia. Schizophr Bull. 2015;41:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doucet GE, Bassett DS, Yao N, Glahn DC, Frangou S. The role of intrinsic brain functional connectivity in vulnerability and resilience to bipolar disorder. Am J Psychiatry. 2017;174:1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaiser RH, Whitfield-Gabrieli S, Dillon DG, et al. Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacology. 2016;41:1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carhart-Harris RL, Leech R, Erritzoe D, et al. Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull. 2013;39:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Northoff G, Duncan NW. How do abnormalities in the brain’s spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Prog Neurobiol. 2016;145–146:26–45. [DOI] [PubMed] [Google Scholar]
- 18. Yang GJ, Murray JD, Repovs G, et al. Altered global brain signal in schizophrenia. Proc Natl Acad Sci USA. 2014;111:7438–7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang GJ, Murray JD, Wang XJ, et al. Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc Natl Acad Sci USA. 2016;113:E219–E228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krystal JH, Anticevic A, Yang GJ, et al. Impaired tuning of neural ensembles and the pathophysiology of schizophrenia: a translational and computational neuroscience perspective. Biol Psychiatry. 2017;81:874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carhart-Harris RL, Muthukumaraswamy S, Roseman L, et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci USA. 2016;113:4853–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Power JD, Plitt M, Laumann TO, Martin A. Sources and implications of whole-brain fMRI signals in humans. Neuroimage. 2017;146:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saad ZS, Gotts SJ, Murphy K, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schölvinck ML, Maier A, Frank QY, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci USA. 2010;107:10238–10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lv Y, Margulies DS, Cameron Craddock R, et al. Identifying the perfusion deficit in acute stroke with resting-state functional magnetic resonance imaging. Ann Neurol. 2013;73:136–140. [DOI] [PubMed] [Google Scholar]
- 27. Blumberg HP, Kaufman J, Martin A, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. [DOI] [PubMed] [Google Scholar]
- 28. Frey BN, Andreazza AC, Nery FG, et al. The role of hippocampus in the pathophysiology of bipolar disorder. Behav Pharmacol. 2007;18:419–430. [DOI] [PubMed] [Google Scholar]
- 29. Murphy K, Fox MD. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fox MD, Zhang D, Snyder AZ, Raichle ME. The sglobal signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, Martin A. The perils of global signal regression for group comparisons: a case study of Autism Spectrum Disorders. Front Hum Neurosci. 2013;7:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. Neuroimage. 2010;49:3132–3148. [DOI] [PubMed] [Google Scholar]
- 33. Liu X, de Zwart JA, Schölvinck ML, et al. Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nat Commun. 2018;9:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang GJ, Murray JD, Glasser M, et al. Altered global signal topography in schizophrenia. Cereb Cortex. 2017;27:5156–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 36. Huang Z, Wang Z, Zhang J, et al. Altered temporal variance and neural synchronization of spontaneous brain activity in anesthesia. Hum Brain Mapp. 2014;35:5368–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cohen J. Statistical power analysis. Curr Dir Psychol Sci. 1992;1:98–101. [Google Scholar]
- 39. Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G. Is subcortical-cortical midline activity in depression mediated by glutamate and GABA? A cross-species translational approach. Neurosci Biobehav Rev. 2010;34:592–605. [DOI] [PubMed] [Google Scholar]
- 40. Nielson DM, Smith TA, Sreekumar V, Dennis S, Sederberg PB. Human hippocampus represents space and time during retrieval of real-world memories. Proc Natl Acad Sci USA. 2015;112:11078–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eichenbaum H. Time cells in the hippocampus: a new dimension for mapping memories. Nat Rev Neurosci. 2014;15:732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. [DOI] [PubMed] [Google Scholar]
- 43. Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bar M, Tootell RB, Schacter DL, et al. Cortical mechanisms specific to explicit visual object recognition. Neuron. 2001;29:529–535. [DOI] [PubMed] [Google Scholar]
- 45. Patel T, Brewin CR, Wheatley J, Wells A, Fisher P, Myers S. Intrusive images and memories in major depression. Behav Res Ther. 2007;45:2573–2580. [DOI] [PubMed] [Google Scholar]
- 46. Brewin CR, Reynolds M, Tata P. Autobiographical memory processes and the course of depression. J Abnorm Psychol. 1999;108:511–517. [DOI] [PubMed] [Google Scholar]
- 47. Raymond Lake C. Disorders of thought are severe mood disorders: the selective attention defect in mania challenges the Kraepelinian dichotomy a review. Schizophr Bull. 2008;34:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. [DOI] [PubMed] [Google Scholar]
- 49. Northoff G. The brain’s spontaneous activity and its psychopathological symptoms—“Spatiotemporal binding and integration”. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:81–90. [DOI] [PubMed] [Google Scholar]
- 50. Northoff G. Spatiotemporal psychopathology II: how does a psychopathology of the brain’s resting state look like? Spatiotemporal approach and the history of psychopathology. J Affect Disord. 2016;190:867–879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.