Abstract
Schizophrenia is a complex, debilitating mental disorder characterized by wide-ranging symptoms including delusions, hallucinations (so-called positive symptoms), and impaired motor and speech/language production (so-called negative symptoms). Salience-monitoring theorists propose that abnormal functional communication between the salience network (SN) and default mode network (DMN) begets positive and negative symptoms of schizophrenia, yet prior studies have predominately reported links between disrupted SN/DMN functional communication and positive symptoms. It remains unclear whether disrupted SN/DMN functional communication explains (1) solely positive symptoms or (2) both positive and negative symptoms of schizophrenia. To address this question, we incorporate time-lag-shifted functional network connectivity (FNC) analyses that explored coherence of the resting-state functional magnetic resonance imaging signal of 3 networks (anterior DMN, posterior DMN, and SN) with fixed time lags introduced between network time series (1 TR = 2 s; 2 TR = 4 s). Multivariate linear regression analysis revealed that severity of disordered thought and attentional deficits were negatively associated with 2 TR-shifted FNC between anterior DMN and posterior DMN. Meanwhile, severity of flat affect and bizarre behavior were positively associated with 1 TR-shifted FNC between anterior DMN and SN. These results provide support favoring the hypothesis that lagged SN/DMN functional communication is associated with both positive and negative symptoms of schizophrenia.
Keywords: ICA, salience network, default mode network, positive symptoms, negative symptoms, resting-state fMRI
Introduction
The abnormal salience monitoring theory of schizophrenia (Sz) proposes that abnormal functional communication between the salience network (SN) and default mode network (DMN) begets wide-ranging symptoms including hallucinations, disorganized thought, and psychomotor poverty.1,2 When healthy subjects perform cognitive tasks requiring externally focused attention, the DMN deactivates and regions essential for executive functioning (eg, lateral prefrontal and parietal cortex) become active; DMN hubs include medial prefrontal cortex (MPFC)/anterior cingulate cortex (ACC; anterior midline), posterior cingulate/precuneus (posterior midline), and angular gyrus (posterior lateral).3,4 Both anterior and posterior midline hubs have strong structural connections to limbic regions involved in emotion and memory.5 But studies exploring DMN function during rest and across different tasks suggest that anterior DMN (aDMN) and posterior DMN (pDMN) hubs may play specialized functional roles. Tasks requiring explicit self-reference preferentially activate MPFC,6 whereas posterior midline hubs are thought to integrate self-referential judgments and play an important role in autobiographical memory.6–8 Finally, 2 studies exploring effective (directional) connectivity within the DMN reported that the anterior prefrontal cortex acts as a sink of propagated activity (eg, anterior prefrontal activity lags behind activity of pDMN hubs).9,10
The SN plays a critical role in monitoring the proximal salience of cues—from startling noises to changes in homeostatic state. The anterior insular hub receives convergent input from visual and auditory cortex,11–14 whereas the dorsal ACC hub projects to the spinal cord.15 These connections allow the SN to integrate incoming perceptual information and respond quickly when confronted with salient changes to internal states of the body and external states of the environment.15 Diminished white matter integrity of anterior insula–dorsal anterior cingulate tracts in individuals with traumatic brain injury disrupts normal patterns of DMN activation/deactivation,16,17 suggesting that the SN is required for regulating DMN activation.
Patients with Sz have an attenuated ability to deactivate DMN during task performance18–20 and elevated DMN resting-state functional connectivity (rs-FC).21–23 These abnormalities are associated with global assessments of positive symptoms (eg, delusions, hallucinations, and disorganized speech),18 working memory deficits,24 social deficits,25 and hallucinations.26,27 Depressed rs-FC with SN hubs in Sz is linked to hallucinations,26,28 general assessments of reality distortion (hallucinations + delusions),29 and defective error monitoring.30
An innovative study by Manoliu et al26 first examined resting-state functional magnetic resonance imaging (rs-fMRI) signal coherence of DMN and SN in Sz, and reported that the patients with Sz had seemingly normal rs-FC between the SN and the DMN relative to healthy controls. Next, a series of time-lag-shifted rs-FC analyses21 explored rs-fMRI signal coherence of SN and DMN, but introduced fixed time lags between network time series. When time lags of 1 TR (2 s) and 2 TRs (4 s) were introduced between network time series, Sz had abnormal rs-FC between DMN and SN relative to healthy controls. However, the researchers did not explore potential associations between symptom severity and time-lag-shifted rs-FC between DMN and SN.
Prior studies have predominately reported links between disrupted SN/DMN functional communication and positive symptoms of Sz.18,26–28 Yet, we know that the SN and the DMN play indispensable roles in monitoring internal and environmental states, and orienting attention. At present, it remains unclear whether altered SN/DMN functional communication explains exclusively positive symptoms, or, alternatively, both positive and negative symptoms. This study explores the relationship between positive and negative symptom expression in Sz and DMN/SN functional communication. Specifically, we explore the relationship between rs-FC (zero-lag) and time-lag-shifted (1 TR = 2 s; 2 TR = 4 s) rs-FC between resting-state networks (RSNs: aDMN, pDMN, and SN) and reported severity of 9 Sz symptom dimensions: hallucinations, delusions, bizarre behavior, positive formal thought disorder, affective flattening/blunting, alogia, avolition/apathy, anhedonia/asociality, and attention.
Methods
Subjects
This study draws from the Functional Biomedical Informatics Research Network (FBIRN) Phase III study (see Hare et al,31 Ford et al,32 and Damaraju et al33). For a detailed description of the multiphase FBIRN project including subject characteristics and imaging/behavior assessments, see Keator et al.34 For this study, we analyzed rs-fMRI scans from a large, clinically diverse sample of 100 subjects with Sz (table 1).
Table 1.
Demographic Information
| Descriptive Statistics (For Continuous Variables, Means and SDs Are Reported) | Range | |
|---|---|---|
| Gender | 78 (male), 22 (female) | N/A |
| Handedness | 93 (right), 5 (left), 2 (both) | N/A |
| Smoking status | 43 (current smoker), 26 (ex-smoker), 31 (never) | N/A |
| Age in years | 39.3 (12.0) | 18–60 |
| Duration illness in years | 17.7 (11.4) | 1–41 |
| Chlorpromazine equivalents46 | 414.3 (407.9)a | 2–1800 |
| Scale for the Assessment of Positive Symptoms (SAPS) total score | 18.9 (15.0) | 0–63 |
| SAPS hallucinations subscale score (SAPS items 1–6 total score) | 3.7 (5.0) | 0–22 |
| SAPS delusions subscale score (SAPS items 8–19 total score) | 6.0 (6.2) | 0–33 |
| SAPS bizarre behavior subscale score (SAPS items 21–24 total score) | 1.0 (1.6) | 0–8 |
| SAPS thought disorder subscale score (SAPS Items 26–33 total score) | 3.1 (5.1) | 0–27 |
| Scale for the Assessment of Negative Symptoms (SANS) total score | 28.3 (17.0) | 0–80 |
| SANS affective flattening subscale score (SANS items 1–7 total score) | 5.3 (6.2) | 0–24 |
| SANS alogia subscale score (SANS items 9–12 total score) | 2.0 (2.4) | 0–13 |
| SANS avolition/apathy subscale score (SANS items 14–16 total score) | 4.6 (3.4) | 0–14 |
| SANS anhedonia/asociality subscale score (SANS items 18–21 total score) | 6.7 (5.3) | 0–19 |
| SANS attention subscale score (SANS items 23–24 total score) | 2.4 (2.2) | 0–8 |
aWe lacked data to derive chlorpromazine equivalents for 11/100 (11%) subjects. Mean and SD calculations are based on the sample of 89 subjects without missing data.
Raw imaging data were collected from 7 sites; written informed consent was obtained from all participants. The consent process was approved by institutional review boards of the University of California, Irvine; the University of California, Los Angeles; the University of California, San Francisco; Duke University/the University of North Carolina at Chapel Hill; the University of New Mexico; the University of Iowa; and the University of Minnesota.
All recruited study participants were between the ages of 18 and 62 years. All subjects in this study were diagnosed with Sz by experienced clinicians using the Structural Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) Axis I Disorders.35 Patients either were stable on antipsychotic medication or were not taking antipsychotic medication at the time of the study (only 4 unmedicated of 100 subjects with Sz). Exclusion criteria for all participants included history of major medical illness, insufficient eyesight to see with normal acuity with MRI compatible corrective lenses, contraindications for MRI, drug dependence in the last 5 years or a current substance abuse disorder, and an intelligence quotient less than 75.
Assessments of Symptoms
Symptom severity was assessed using the Scale for the Assessment of Positive Symptoms (SAPS)36 and the Scale for the Assessment of Negative Symptoms (SANS).37 Subscale scores for each symptom dimension were calculated by deriving the sum of individual items in each dimension: hallucinations (SAPS items 1–6); delusions (SAPS items 8–19); bizarre behavior (SAPS items 21–24); positive formal thought disorder (SAPS items 26–33); affective flattening/blunting (SANS items 1–7); alogia (SANS items 9–12); avolition/apathy (SANS items 14–16); anhedonia/asociality (SANS items 18–21); and attention (SANS items 23–24) (see table 1). Clinicians and research staff at each FBIRN site were designated to perform the symptom ratings. To successfully calibrate symptom ratings, they participated in mandatory training sessions, run by experienced clinicians.
Imaging
As part of the larger FBIRN Phase III study, data were acquired using six 3 T Siemens TIM Trio scanners and one 3 T GE MR750 scanner using an AC–PC aligned echo-planar imaging pulse sequence (TR/TE 2 s/30 ms, flip angle 77º, 32 slices collected sequentially from superior to inferior, 3.4 × 3.4 × 4 mm gap, 162 frames, 5:24 min) to obtain T2*-weighted images. Subjects were instructed to lie in the scanner with eyes closed.
Data Processing
Preprocessing was performed using the Data Processing Assistant for rs-fMRI toolbox that runs with the REST software.38 The first 2 time frames were removed to allow for signal stabilization. Raw data underwent motion correction to the first image, slice-timing correction to the middle slice, normalization to standardized Montreal Neurological Institute (MNI) space, and spatial smoothing with an 8-mm full-width at half maximum (FWHM) Gaussian kernel. Framewise displacement (FD)—defined as the sum of the absolute values of the derivatives of the 6 realignment parameters (3 linear + 3 rotational converted from degrees to millimeters)39—was calculated for each image. The FD measurement differentiates head realignment parameters across frames and generates a 6-dimensional times series that represents instantaneous head motion.39 Mean FD was calculated for each subject by taking the average of the sum of the absolute values of the derivatives of the 6 realignment parameters (3 linear + 3 rotational). Although independent component analysis (ICA) has been shown to be resistant to motion artifacts,40 we also corrected for potentially confounding effects of head motion on the fMRI signal, by including mean FD as a subject-level covariate.
Group Spatial Independent Component Analysis
Group spatial ICA and functional network connectivity (FNC) correlation analyses were performed using GIFT software.41 As part of a prior network analysis of hallucinations in Sz, we performed group spatial ICA on a large sample of FBIRN subjects and analyzed FNC between 9 RSNs: 2 auditory networks, 2 visual networks, 2 subcortical networks, aDMN, pDMN, and SN.42 Back-reconstruction was performed using group information guided ICA, which takes the group maps and runs a spatially constrained ICA on individual subjects, producing individual subject component maps and time courses. This approach has been shown to be robust to artifacts as well as sensitive to individual and group differences.43,44 In this work, we performed a new analysis of spatial maps and time series of SN, aDMN, and pDMN (figure 1) to explore DMN/SN functional communication.
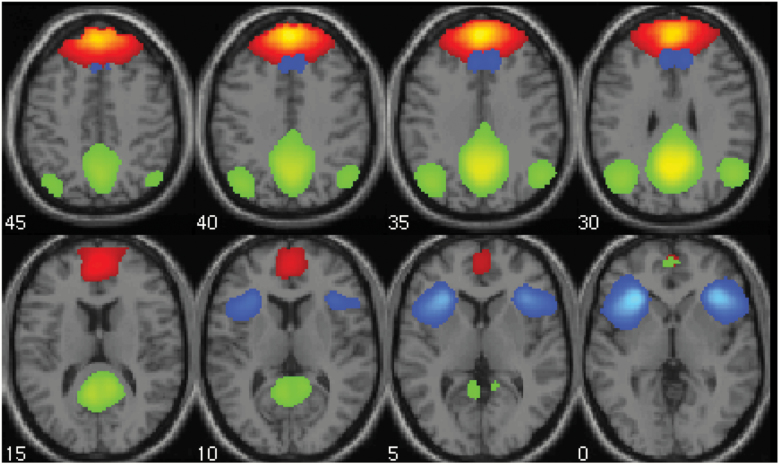
Fig. 1.
Anterior default mode, posterior default mode, and salience networks. The mean aggregate spatial maps of the 3 independent component networks analyzed in the functional network connectivity analysis are shown (threshold: Z > 2): anterior default mode network (red), posterior default mode network (green), and salience network (blue).
Subject time courses were detrended and despiked, then filtered with a high-frequency cutoff of 0.15 Hz before computing FNC correlations (zero-lag) and time-lag-shifted FNC correlations; FNC correlations (zero-lag) are defined as the pairwise correlations between network time courses, and time-lag-shifted FNC correlations are defined as pairwise correlations between one network’s time course and another network’s time course shifted by a specified lag. In a previous analysis, Manoliu et al26 performed time-lag-shifted FNC analyses with specified lags of 1 TR (2 s), 2 TR (4 s), and 3 TR (6 s), and found that Sz had abnormal 1-TR-shifted and 2-TR-shifted FNC between DMN and SN (but normal 3-TR-shifted FNC between DMN and SN) relative to healthy controls. Given these findings, we explored time-lag-shifted FNC between aDMN/pDMN and SN with specified time lags of 1 TR (2 s) and 2 TR (4 s). All FNC correlations (zero-lag and lagged) were transformed to z scores using Fisher’s transformation.
Statistical Analyses
We performed hierarchical linear regression analyses of FNC correlations (zero-lag and time-lag-shifted), controlling for confounding effects of nuisance variables in block 1 of the linear model (age, gender, scanning site, and mean FD). Symptom scores including the SANS37 subscale scores (affective flattening/blunting, alogia, avolition/apathy, anhedonia/asociality, attention) and the SAPS36 subscale scores (hallucinations, delusions, bizarre behavior, positive formal thought disorder) were entered in block 2 of the linear model. Subjects with residuals >3 SD from the mean were excluded (N ≥ 98 subjects for each regression analysis).
To ensure that observed associations between symptom severity and FNC were not driven by confounding effects of medication, we also performed regression analyses including total chlorpromazine equivalents45 as an additional covariate in block 1. We lacked information to derive chlorpromazine equivalents45 for 11 subjects with Sz, so we calculated the mean value of total chlorpromazine equivalents (based on the available data; n = 89 subjects) and interpolated the mean value for the 11 subjects with missing data. Results of these analyses are reported in supplementary table S2.
Because nicotine use is 2–3 times higher in patients with Sz than in the healthy population46 and has been shown to significantly impact brain functional connectivity,47 we examined Spearman correlations between FNC and smoking status (factor with 3 levels: “never smoker,” “ex-smoker,” “current smoker”). We found no significant correlations between smoking status and FNC measures, so smoking status was not included as a covariate.
Although we hypothesized that rs-FNC with DMN/SN would be linked predominately to positive symptoms,18,26–28 our FNC analyses were largely exploratory to test whether DMN/SN connectivity might also be linked to negative symptoms and to determine whether FNC-symptom associations depend on lag direction and/or magnitude between SN/DMN time courses. For clarity of reporting the results, lag magnitude is reported parenthetically, whereas lag direction is denoted with an arrow. For instance, “lagged (1 TR) aDMN→SN connectivity” refers to the correlation between aDMN and SN rs-fMRI signal when the time series of the SN lags behind the time series of the DMN by 1 TR (2 s). For each set of time-lag-shifted FNC analyses of a specified lag (1 TR, 2 TR), confidence was initially specified as P < .05, and then Bonferroni-corrected for six tests (SN→aDMN, aDMN→SN, SN→pDMN, pDMN→SN, aDMN→pDMN, pDMN→aDMN) (P < .0083).
Results
Below, we report significant associations between time-lag-shifted FNC and symptom dimension scores of the SAPS/SANS (table 2). Results of the zero-lag FNC analyses are reported in supplementary table S1a; nominally significant (non-Bonferroni-corrected, P < .05) results of the time-lag-shifted FNC analyses are reported in supplementary table S1b. To provide estimates of effect sizes, we parenthetically report standardized regression coefficients.
Table 2.
Associations Between Symptom Dimension Scores and Network Connectivity
| FNC | Lag Summary | Symptom Dimension | β | T-stat | P |
|---|---|---|---|---|---|
| aDMN→pDMN | pDMN time series lags aDMN time series by 2 TRs | Attention | −.31 | −3.0 | .003 |
| aDMN→pDMN | pDMN time series lags aDMN time series by 2 TRs | Thought disorder | −.31 | −2.9 | .005 |
| aDMN→SN | SN time series lags aDMN time series by 1 TR | Flat affect | .29 | 2.9 | .005 |
Note: FNC, functional network connectivity; aDMN, anterior default mode network; pDMN, posterior default mode network; SN, salience network.
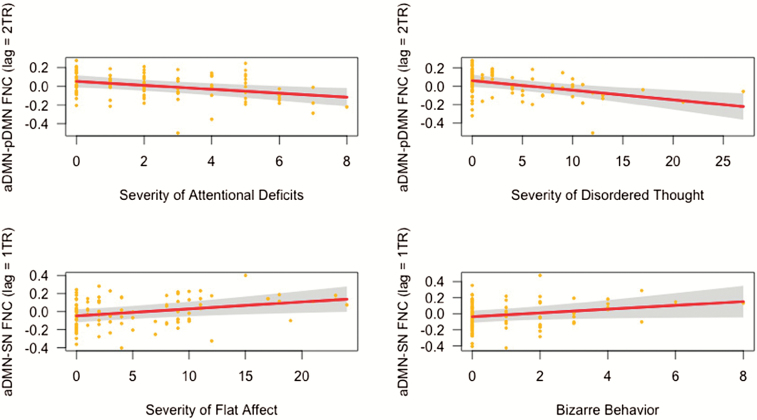
Associations between symptoms and FNC between aDMN and pDMN: Lagged (2 TR) aDMN→pDMN connectivity was negatively associated with severity of attentional deficits (β = −0.31, P = .003) and disordered thought (β = −.31, P = .005) (table 2, (figure 2).
Fig. 2.
Associations between symptom severity and time-lag-shifted functional network connectivity (FNC). Partial regression plots showing negative associations between lagged (2 TR) aDMN→pDMN connectivity and reported severity of attentional deficits (top left) and thought disorder (top right), in addition to positive associations between lagged (1 TR) aDMN→SN connectivity and severity of flat affect (bottom left) and bizarre behavior (bottom right). Covariates controlled for in the linear model included age, gender, scanning site, and mean framewise displacement. aDMN, anterior default mode network; pDMN, posterior default mode network; SN, salience network.
Associations between symptoms and FNC between aDMN and SN: Lagged (1 TR) aDMN→SN connectivity was positively associated with severity of flat affect (β = .29, P = .005) (table 2) and bizarre behavior (figure 2), although the latter association did not survive Bonferroni correction for multiple tests (β = .25, P = .014) (supplemental table S1b).
Discussion
The objective of this study was to ask whether functional communication between SN and DMN explains exclusively positive symptoms, or both positive and negative symptoms. Prior research suggests that traditional (zero-lag) FNC analyses may be ill-equipped to detect time-varying communication between different brain regions as well as group differences in communication between regions. We focused on functional communication between the SN and DMN and how this communication is affected by Sz.26 Specifically, we probed the roles of lag magnitude and direction to explore the relations between SN/DMN connectivity and targeted behavioral dimensions of Sz. We hypothesized that time-lag-shifted rs-FNC across 3 networks (aDMN, pDMN, and SN; see figure 1) would be linked predominately to positive symptoms.18,26–29 Instead, we found that specific patterns of time-lag-shifted rs-FNC were associated with negative symptoms (eg, attentional deficits and flat affect) as well as positive symptoms (eg disordered thought and bizarre behavior).
First, the (2 TR) aDMN→pDMN connectivity analysis revealed that patients with more severe thought disorder had less time-lag-shifted functional communication between DMN networks (specifically, aDMN activation preceding pDMN activation by 4 s). This lag might contribute to derailment and illogicality, symptoms of thought disorder.37 It is thought that the DMN supports internal mental processes (memories, thought, etc.),48,49 but it remains unclear how exactly the DMN supports these processes. Our findings suggest that functional communication between DMN hubs may be critical for organizing thoughts into coherent, meaningful utterances. Yet, this theory remains speculative until future research provides insight into how the DMN supports complex thought processes and addresses targeted associations between disrupted DMN function and wide-ranging formal thought disturbances in Sz—from derailment (eg, where the patient’s ideas slip off topic) to blocking (eg, where the patient’s train of thoughts is interrupted).
The same pattern of lagged aDMN→pDMN connectivity was also negatively associated with severity of attentional deficits. Put another way, patients with more severe attentional deficits had less temporally coherent (4 s-lagged) functional co-activation of aDMN and pDMN. In addition, we observed numerous nominally significant associations between attentional deficits and FNC between pDMN and SN (both pDMN→SN and SN→pDMN connectivity; see supplemental table S1b). A previous study found that elevated posterior cingulate activity was observed during lapses in attention when healthy research subjects performed a demanding perceptual task.50 In another study, increased activity in posterior midline regions predicted which words were forgotten on a memory task.51 Thus, our results are consistent with the theory that pDMN plays a critical role in regulating attention.5
Next, we observed flat affect was more pronounced in patients to the extent that the aDMN activation preceded SN activation by 2 s, as reflected in lagged aDMN→SN connectivity. The aDMN contains midline structures spanning the MPFC and ACC. Whitfield-Gabrieli et al52 reported that dorsal MPFC was preferentially engaged during performance of a task that required explicit self-reference, relative to DMN activation evoked by a rest condition. Meanwhile, ventral MPFC plays a critical role in the regulation of amygdala activity53; patients with ventral MPFC damage have marked reductions in autonomic arousal to emotionally charged stimuli.54 These findings suggest that anterior midline DMN hubs contain functional subdivisions essential for explicit self-reference (dorsal MPFC), and tracking the salience of emotional stimuli and regulating our responses to those stimuli (ventral MPFC). It is plausible that flat affect stems from elevated aDMN/SN functional communication that manifests as disturbances in emotional salience tracking/monitoring, and/or inability to disengage with self-reflective thought and engage with surroundings. Future studies should explore these functional MPFC subdivisions and their potential contributions to diminution of vocal inflection, and affective gestures, as well as inappropriately elevated displays of affect in Sz.
Finally, bizarre behavior was positively associated with the same FNC pattern (2-s-lagged aDMN→SN connectivity). However, this small effect (standardized β = .25) did not survive Bonferroni correction for multiple tests. Elevated functional communication between SN and DMN could result in awareness of mislabeled bursts of inner speech or thoughts. These experiences may, in turn, affect planning, social engagement, and engagement with the environment, resulting in bizarre behavior. Future studies in patients selected to have a broader range of bizarre behaviors may further examine this relationship.
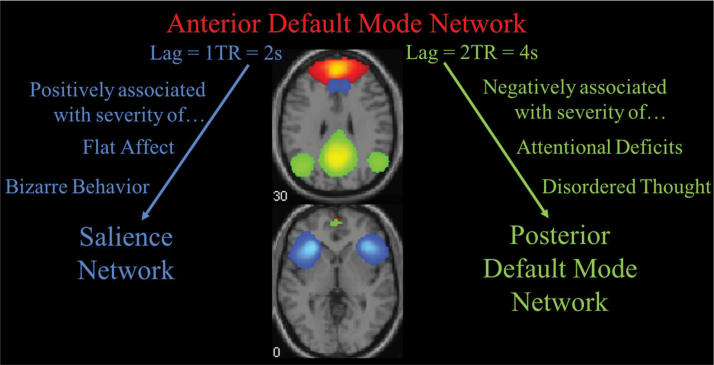
Our findings support the hypothesis that specific patterns of lagged DMN/SN functional communication are associated with both positive and negative symptoms. We observed 2 main trends: (2 TR) lagged aDMN→pDMN connectivity was negatively associated with symptom severity, whereas (1 TR) lagged aDMN→SN connectivity was positively associated with symptom severity (figure 3). On the one hand, to the extent that lagged functional communication between aDMN and pDMN hubs is reduced, patients had more severe cognitive disturbances (disordered thought and attentional deficits). On the other hand, patients had more pronounced flat affect and engaged in more bizarre behavior to the extent that aDMN activation consistently preceded SN activation (by 2 s).
Fig. 3.
Disrupted anterior default mode network functional communication linked to positive and negative symptoms. Time-lag-shifted functional network connectivity (FNC) between anterior default mode network (red) and salience network (blue) is positively associated with bizarre behavior and severity of flat affect. Meanwhile, time-lag-shifted FNC between anterior default mode network and posterior default mode (green) was negatively associated with attentional deficits and severity of disordered thought. Arrows denote direction of lag and do not imply causal relationships.
Given Manoliu et al’s report of a significant negative correlation between strength of functional connectivity within the right anterior insula and hallucination severity in the patients with Sz,26 we predicted that SN functional communication would be linked to hallucination severity. Yet, we observed no associations between hallucination severity and SN functional communication, and only a nominally significant negative association between hallucination severity and (zero-lag) aDMN–pDMN connectivity (supplemental table S1a). Notably, our analysis of 100 patients with Sz drew from a larger sample than in Manoliu et al26 (n = 18 patients), and we modeled effects of symptom severity on FNC, controlling for extraneous effects of motion, age, gender, and scanning site (vs performing bivariate correlation analyses). Thus, our null findings might be treated as evidence favoring rejection of the hypothesis that abnormal SN function underlies hallucinations in Sz. However, a targeted analysis of FNC between SN and sensory networks by our group42 revealed that elevated FNC between SN and an auditory network was positively associated with severity of auditory hallucinations. Future analyses should continue to explore and test targeted hypotheses of hallucinations by exploring potential associations between hallucination severity and SN functional communication.
Observed associations between symptom severity and FNC were dependent on lag direction. In resting-state analyses of healthy subjects, the anterior midline DMN hub acts as a sink of propagated activity (eg, anterior midline activity lags behind posterior midline activity during rest).9,10 In this study, we observed that symptom severity was associated with atypical aDMN→pDMN connectivity and aDMN→SN connectivity. Converging evidence from rs-FC analyses,18–20 along with a dynamic rs-FNC analysis55 demonstrating that Sz shows reduced dynamic switching of network states, suggests that patients may be stuck in DMN states associated with self-referential processing. As such, it makes sense that DMN activity might precede activity in networks such as the SN. Although it remains unclear why lagged FNC with aDMN (aDMN→pDMN, aDMN→SN) was associated with reported symptom severity, this is an interesting result that requires further investigation with other modalities such as electroencephalography (EEG) and/or magnetoencephalography (MEG), which provide more precise timing information.
Associations between symptom severity and FNC were also dependent on lag magnitude. In healthy subjects, brief delays are observed between network sources of propagated activity and subsequent activation of network sinks such as the anterior frontal cortex (typically <0.5 s).10 We observed that symptom severity was associated with lagged FNC with longer, atypical delays of 2 and 4 s. However, our methodological approach in this study limits us in making the strong claim that symptoms are caused by these lags. Future investigations must explore precise timing of activation of functional network hubs, and how this relates to behavioral task performance and Sz symptomology.
Given that the physiological basis of the blood-oxygenation-level-dependent (BOLD) fMRI signal remains controversial, this entails some speculation will be required when considering the significance of multisecond lags between BOLD hemodynamic responses of RSN hub regions. Lags in BOLD fMRI signaling may be caused by vascular effects, changes in neural signaling, or a combination of factors. Prior findings suggest that changes in neural signaling contribute to observed BOLD hemodynamic lags10 and that vascular effects alone cannot account for BOLD signal lag structure.56 Prior research also suggests that infra-slow neuronal oscillations (0.01–0.1 Hz) play a key role in generating the BOLD-fMRI response.57,58 Although, direct (causal) links between BOLD fluctuations and infra-slow neuronal oscillations in humans remain unestablished, it is widely acknowledged that proper functional network communication depends on dynamic phase coupling of fast neural rhythms (eg, γ; >30 Hz) to slower rhythms (eg, δ, θ; <8 Hz).59,60 It is plausible that coherent BOLD signal fluctuations in RSN hubs of healthy subjects may reflect frequency-dependent coupling of network hub activation. In Sz, cross-frequency coupling of activity across DMN hubs is disrupted.60,61 We propose that these disruptions may manifest as measurable lags between hemodynamic responses of RSNs. At the same time, we acknowledge that additional physiological factors/interactions are associated with BOLD signal fluctuations and that exact (causal) relationships between oscillatory coupling disturbances and measurable changes in FNC using BOLD fMRI remain unknown.
Although our study was the first to examine targeted relationships between time-lagged FNC between SN and DMN and wide-ranging Sz symptoms, we must acknowledge several limitations. Although we were able to probe potential links between rs-FNC and a relatively broad set of 9 symptom dimensions, the SAPS/SANS clinical assessments limited our ability to explore links with an even broader array of symptoms and targeted behavioral outcomes such as working memory deficits. Next, the cross-sectional nature of this analysis limited our ability to explore how neural function changed in patients over time; it remains unclear whether observed FNC effects reflect chronic dispositions. Third, all but 4 of the 100 subjects with Sz were taking antipsychotic medication at the time of the FBIRN study, introducing potentially confounding effects on brain FNC. We controlled for these potentially confounding effects by introducing total chlorpromazine equivalents45 as an additional covariate in our regression analyses of FNC. Including chlorpromazine equivalents as covariate had no significant impact on the results (see supplemental table S2). Finally, our FNC analyses explore correlations between the rs-fMRI signals of DMN and SN. In our discussion of results, we use arrows to denote direction of lag. This effort to enhance clarification should not be taken to imply causation (eg, that one network’s activity exerts causal influence over another network’s activity).
The objective of this study was to address whether functional communication between SN and DMN explains exclusively positive symptoms, or both positive and negative symptoms. To achieve this aim, we explored associations between time-lag-shifted FNC between SN and DMN and heterogeneous behavioral outcomes in Sz. We found strong associations between time-lag-shifted FNC with aDMN (specifically aDMN→SN and aDMN→pDMN) and both positive and negative symptoms of Sz (figure 3); all other reported FNC-symptom associations did not survive Bonferroni correction for multiple tests. Our results suggest that altered aDMN functional communication may play a crucial role in the pathophysiology of Sz and etiology of both positive and negative symptoms. Future studies should build upon these findings and explore time-lag-shifted FNC between SN/DMN hubs and sensory networks, motor networks, and attention networks to gain a more complete, nuanced understanding of the neural mechanisms underlying specific symptoms.
Supplementary Material
Acknowledgments
Functional Biomedical Informatics Research Network was supported by a grant from National Center for Research Resources to Potkin (1U24 RR021992). This work was supported in part through a Provost Dissertation Fellowship to Hare, and by a grant from National Institute of Mental Health to Turner and Calhoun (1R01MH094524). Dr Mathalon has served as a consultant for Boehringer Ingelheim, Alkermes, Takeda, and Upsher-Smith. All other authors have declared no conflicts of interest.
References
- 1. Menon V. Salience network. In: Toga AW, ed. Brain Mapping: An Encyclopedic Reference. Vol 2 Elsevier Inc; 2015:597–611. http://linkinghub.elsevier.com/retrieve/pii/B978012397025100052X [Google Scholar]
- 2. Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. [DOI] [PubMed] [Google Scholar]
- 4. Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:413–427. [DOI] [PubMed] [Google Scholar]
- 5. Washington SD, VanMeter JW. Anterior-posterior connectivity within the default mode network increases during maturation. Int J Med Biol Front. 2015;21:207–218. [PMC free article] [PubMed] [Google Scholar]
- 6. Whitfield-Gabrieli S, Moran JM, Nieto-Castañón A, Triantafyllou C, Saxe R, Gabrieli JD. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55:225–232. [DOI] [PubMed] [Google Scholar]
- 7. Wang H, Zeng LL, Chen Y, Yin H, Tan Q, Hu D. Evidence of a dissociation pattern in default mode subnetwork functional connectivity in schizophrenia. Sci Rep. 2015;5:14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qin P, Liu Y, Shi J, et al. Dissociation between anterior and posterior cortical regions during self-specificity and familiarity: a combined fMRI-meta-analytic study. Hum Brain Mapp. 2012;33:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deshpande G, Santhanam P, Hu X. Instantaneous and causal connectivity in resting state brain networks derived from functional MRI data. Neuroimage. 2011;54:1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mitra A, Snyder AZ, Hacker CD, Raichle ME. Lag structure in resting-state fMRI. J Neurophysiol. 2014;111:2374–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. [DOI] [PubMed] [Google Scholar]
- 12. Bamiou DE, Musiek FE, Luxon LM. The insula (Island of Reil) and its role in auditory processing. Literature review. Brain Res Brain Res Rev. 2003;42:143–154. [DOI] [PubMed] [Google Scholar]
- 13. Butti C, Hof PR. The insular cortex: a comparative perspective. Brain Struct Funct. 2010;214:477–493. [DOI] [PubMed] [Google Scholar]
- 14. Mesulam MM, Mufson EJ. Insula of the old world monkey. III: efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. [DOI] [PubMed] [Google Scholar]
- 15. Fries W. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol. 1984;230:55–76. [DOI] [PubMed] [Google Scholar]
- 16. Bonnelle V, Ham T, Leech R, et al. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109:4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jilka SR, Scott G, Ham T, et al. Damage to the salience network and interactions with the default mode network. J Neurosci. 2014;34:10798–10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. [DOI] [PubMed] [Google Scholar]
- 20. Pomarol-Clotet E, Salvador R, Sarró S, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network?Psychol Med. 2008;38:1185–1193. [DOI] [PubMed] [Google Scholar]
- 21. Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu H, Kaneko Y, Ouyang X, et al. Schizophrenic patients and their unaffected siblings share increased resting-state connectivity in the task-negative network but not its anticorrelated task-positive network. Schizophr Bull. 2012;38:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou Y, Liang M, Jiang T, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007;417:297–302. [DOI] [PubMed] [Google Scholar]
- 24. Zhou L, Pu W, Wang J, et al. Inefficient DMN suppression in schizophrenia patients with impaired cognitive function but not patients with preserved cognitive function. Sci Rep. 2016;6:21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holt DJ, Cassidy BS, Andrews-Hanna JR, et al. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol Psychiatry. 2011;69:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manoliu A, Riedl V, Zherdin A, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. 2014;40:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alonso-Solís A, Vives-Gilabert Y, Grasa E, et al. Resting-state functional connectivity alterations in the default network of schizophrenia patients with persistent auditory verbal hallucinations. Schizophr Res. 2015;161:261–268. [DOI] [PubMed] [Google Scholar]
- 28. Pu W, Li L, Zhang H, et al. Morphological and functional abnormalities of salience network in the early-stage of paranoid schizophrenia. Schizophr Res. 2012;141:15–21. [DOI] [PubMed] [Google Scholar]
- 29. Gradin VB, Waiter G, O’Connor A, et al. Salience network-midbrain dysconnectivity and blunted reward signals in schizophrenia. Psychiatry Res. 2013;211:104–111. [DOI] [PubMed] [Google Scholar]
- 30. Polli FE, Barton JJ, Thakkar KN, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131:971–986. [DOI] [PubMed] [Google Scholar]
- 31. Hare SM, Ford JM, Ahmadi A, et al. ; Functional Imaging Biomedical Informatics Research Network Modality-dependent impact of hallucinations on low-frequency fluctuations in schizophrenia. Schizophr Bull. 2017;43:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ford JM, Palzes VA, Roach BJ, et al. ; Functional Imaging Biomedical Informatics Research Network Visual hallucinations are associated with hyperconnectivity between the amygdala and visual cortex in people with a diagnosis of schizophrenia. Schizophr Bull. 2015;41:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Damaraju E, Allen EA, Belger A, et al. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin. 2014;5:298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keator DB, van Erp TG, Turner JA, et al. ; FBIRN The function biomedical informatics research network data repository. Neuroimage. 2016;124:1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. First MB, Spitzer RL, Gibbon MG, Williams JBW.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Patient Edition (SCID-I/P, 11/2002 revision). New York: Biometric Research Department, New York State of Psychiatric Institute; 2002. [Google Scholar]
- 36. Andreasen N. Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, IA: University of Iowa College of Medicine; 1984. [Google Scholar]
- 37. Andreasen N. Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa College of Medicine; 1984. [Google Scholar]
- 38. Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robinson SD, Schöpf V, Cardoso P, et al. Applying independent component analysis to clinical fMRI at 7 T. Front Hum Neurosci. 2013;7:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hare SM, Law A, Ford JM, et al. Disrupted network cross talk, hippocampal dysfunction and hallucinations in schizophrenia. Schizophr Res. March 2018. doi: 10.1016/j.schres.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Du Y, Allen EA, He H, Sui J, Wu L, Calhoun VD. Artifact removal in the context of group ICA: a comparison of single-subject and group approaches. Hum Brain Mapp. 2016;37:1005–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salman Y, Du E, Damaraju E, Calhoun V.. Group Information Guided ICA Shows More Sensitivity to Group Differences Than Dual-Regression. In: IEEE International Symposium on Biomedical Imaging; Melbourne, Australia, 2017. [Google Scholar]
- 45. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 46. de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–157. [DOI] [PubMed] [Google Scholar]
- 47. Jasinska AJ, Zorick T, Brody AL, Stein EA. Dual role of nicotine in addiction and cognition: a review of neuroimaging studies in humans. Neuropharmacology. 2014;84:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 49. Buckner RL. The brain’s default network: origins and implications for the study of psychosis. Dialogues Clin Neurosci. 2013;15:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. [DOI] [PubMed] [Google Scholar]
- 51. Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Curr Biol. 2001;11:1528–1530. [DOI] [PubMed] [Google Scholar]
- 52. Whitfield-Gabrieli S, Moran JM, Nieto-Castañón A, Triantafyllou C, Saxe R, Gabrieli JD. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55:225–232. [DOI] [PubMed] [Google Scholar]
- 53. Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry. 2015;77:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41:81–94. [DOI] [PubMed] [Google Scholar]
- 55. Miller RL, Yaesoubi M, Turner JA, et al. Higher dimensional meta-state analysis reveals reduced resting fMRI connectivity dynamism in schizophrenia patients. PloS One. 2016;11:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mitra A, Snyder AZ, Blazey T, Raichle ME. Lag threads organize the brain’s intrinsic activity. Proc Natl Acad Sci U S A. 2015;112:E2235–E2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Palva JM, Palva S. Infra-slow fluctuations in electrophysiological recordings, blood-oxygenation-level-dependent signals, and psychophysical time series. Neuroimage. 2012;62:2201–2211. [DOI] [PubMed] [Google Scholar]
- 58. Hiltunen T, Kantola J, Abou Elseoud A, et al. Infra-slow EEG fluctuations are correlated with resting-state network dynamics in fMRI. J Neurosci. 2014;34:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lisman J, Buzsáki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull. 2008;34:974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hunt MJ, Kopell NJ, Traub RD, Whittington MA. Aberrant network activity in Schizophrenia. Trends Neurosci. 2017;40:371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baenninger A, Palzes VA, Roach BJ, Mathalon DH, Ford JM, Koenig T. Abnormal coupling between default mode network and delta and beta band brain electric activity in psychotic patients. Brain Connect. 2017;7:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.