Abstract
Background
The study aimed to (1) compare the risk of health care use, adverse health status, and work productivity loss of parents of patients with schizophrenia to parents of patients with multiple sclerosis (MS), rheumatoid arthritis (RA), epilepsy, and healthy controls; and (2) evaluate such outcome measures while considering disease severity of schizophrenia.
Methods
Based on linkage of Swedish registers, at least one parent was included (n = 18215) of patients with schizophrenia (information 2006–2013, n = 10883). Similarly, parental information was linked to patients with MS, RA, epilepsy, and matched healthy controls, comprising 11292, 15516, 34715, and 18408 parents, respectively. Disease severity of schizophrenia was analyzed. Different regression models yielding odds ratios (OR), hazard ratios (HR), or relative risks (RR) with 95% confidence intervals (CI) were run.
Results
Psychiatric health care use, mainly due to anxiety and affective disorders, showed a strongly increasing trend for parents of patients with schizophrenia throughout the observation period. During the follow-up, these parents had an up to 2.7 times higher risk of specialized psychiatric health care and receipt of social welfare benefits than other parents. Parents of the moderately severely ill patients with schizophrenia had higher risk estimates for psychiatric health care (RR: 1.12; 95% CI: 1.07–1.17) compared with parents of least severely ill patients.
Conclusions
Parents of patients with schizophrenia have a considerably higher risk of psychiatric health care and social welfare benefit receipt than other parents. Psychiatric health care use worsens over time and with increasing disease severity of the offspring.
Keywords: caregiver burden, schizophrenia, sickness absence, work productivity
Introduction
Schizophrenia is a severe psychiatric disorder with a chronic and relapsing course.1,2 The lifetime prevalence of schizophrenia is around 1%,2 with a geographical variation of up to 5 times.3 Schizophrenia is a disabling disease4 leading to approximately 80%–90% of the patients not being able to economically support themselves.5,6
Schizophrenia does not only have a strong influence on the affected patients, but also on their family members, particularly their parents.7–9 This negative influence in parents might arise from worries about the offspring’s future health and social situation, the stigma associated with schizophrenia, as well as from caring for a child with a chronic, severe disorders.10–12 This situation is exacerbated by the parents’ own morbidity. Schizophrenia is known to be a considerable degree heritable and therefore the parents themselves might suffer from the same disorder.13,14 The burden of parents of patients with schizophrenia seems also to be intensified by the patient’s symptom severity,7,15–20 which is considered to be one of the main determinants of subjective or objective caregiver burden.20,21 Moreover, low levels of functioning in patients with schizophrenia are associated with stress, anxiety, and depression in the patients’ parents.22,23
For the mentioned reasons, a worse health-related quality of life with more use of health care resources among parents of patients with schizophrenia compared with parents of patents with other diseases (such as bipolar and depressive disorders) or parents of healthy children has been reported.24,25 Moreover, the existing literature suggests that parents who are taking care of an offspring with schizophrenia have a greater loss of productivity compared with parents of healthy children.7,8,26 Research also suggests that having a child and particularly caring for a child with chronic conditions may negatively affect existing chronic conditions in the parents or even increase their risk of mortality.27–30 The full range of health and social consequences of having a child with schizophrenia is, however, still unclear, especially when it comes to consequences like loss of productivity. These negative effects on health and social outcome measures for parents of patients with schizophrenia should, however, be balanced with reports on positive effects of being a caregiver, such as satisfaction and meaning derived from caregiving, greater sensitivity to people with disabilities, a greater sense of inner strength and a greater sense of clarity regarding priorities in life.31,32
Part of the burden for parents of patients with schizophrenia may include the effect the disorder has on cognition and behavior.17,26,33 Additionally, stigma around schizophrenia can also contribute to higher burden.10,12,15,34 Significant burden has also been reported in case of somatic diseases, eg, multiple sclerosis (MS),35–37 rheumatoid arthritis (RA),38,39 and epilepsy.40,41 Studies, comparing health- and work-related factors between parents of patients with schizophrenia and parents of patients with chronic somatic diseases as well as with the general population, are, however, scarce.7,25 These previous studies investigated caregivers and reported a higher caregiver burden for caregivers of patients with schizophrenia compared with other somatic disorders (cancer, Alzheimer’s disease, and stroke) or depressive disorders. They were, however, based on small samples and self-reported survey data, with relatively short follow-up time and lack of analyses on newly diagnosed cases.42 To the best of our knowledge, this is the first study that is based on nationwide registers, with a long observation period (from 5 years’ prediagnosis to 7 years after diagnosis), covering a large number of parents of patients with schizophrenia, as well as parents of MS, RA, epilepsy, and healthy controls, and giving the possibility to take the disease severity of schizophrenia into account.
Aim
The study aimed to assess the risk of health care resource use, adverse health status, and work productivity loss in parents of patients with schizophrenia compared with parents of patients with MS, RA, epilepsy, and healthy controls. A further aim was to evaluate these outcome measures while taking the disease severity of schizophrenia into account.
Materials and Methods
Study Design and Population
This is a population-based cohort study based on the Insurance-Medicine-All-Sweden (IMAS) study with data derived from Swedish nationwide registers. Information regarding patients with schizophrenia and their parents was linked at individual level based on the personal id-number provided to all residents in Sweden. De-identified data on the patients were linked to data on their parents through the Multigeneration register. Additional information was gathered from the following nationwide registers:
1. Longitudinal integration database for health insurance and labour market studies (LISA) held by Statistics Sweden: gender, age, area of residence, family situation, annual disposable income, unemployment, and social welfare benefit.
2. National patient register (National Board of Health and Welfare, NBHW): diagnoses and dates for in- or specialized outpatient care.
3. Prescribed drug register (NBHW): type of medication, dispensing date, defined daily dose (DDD) and the Anatomical Therapeutic Chemical (ATC) Classification System code.
4. Cause of death register (NBHW): dates and causes of death.
5. Micro-data for analyses of social insurance (MiDAS) register, from the National Social Insurance Agency: date and diagnoses of sickness-absence (SA) and disability pension (DP).
Inclusion Criteria of Patients With Schizophrenia
Individuals living in Sweden, aged 16–45 years at cohort entry date (CED), diagnosed with schizophrenia from July 1, 2006 to December 31, 2013 (N = 10883, prevalent population), with at least one identifiable parent with information on gender and age, were included. Cases of schizophrenia were identified by using the International Classification of Diseases version 10 (ICD-10) codes F20 or F25 as a main diagnosis in the following instances: either discharged from psychiatric inpatient care, or visit at specialized psychiatric outpatient care, sickness absence (SA), or DP. The CED was set as the earliest of any of these events since July 1, 2006. To perform sensitivity analyses, an incident (newly diagnosed) population of patients with schizophrenia (n = 3379) was formed. This incident population was identified by excluding those patients from the prevalent population who had a main or side diagnosis of F20–F29 (ICD-10), or 295 (ICD-9) recorded in either psychiatric health care, SA, or DP, or use of any antipsychotics (ATC code N05A) before July 1, 2006.
Definition of Parents
In total, 18215 parents of patients with schizophrenia with a valid cohort entry date were selected. Parents of offspring with other chronic diseases comprised 11292 parents of patients with MS (ICD 10 code G35; N = 6462); 15516 parents of patients with RA (ICD 10 code M05, M06; N = 8900) and 34715 parents of patients with epilepsy (ICD 10 code G40; N = 19481). For parents of control individuals with other diseases, CED was defined as the earliest date of any of the 4 events of the offspring: discharged from inpatient care, visit at specialized outpatient care or SA, DP due the disease in question, from July 1, 2006 to December 31, 2013. The proportions of single parents in the groups of “parents of patients with schizophrenia” and “healthy controls” were similar due to matching (ie, 45%). This proportion was lower in the control groups of parents of offspring with MS, RA, and epilepsy (ie, 34%). The numbers of offspring in each group were as follows: 10883 with schizophrenia, 6462 with MS, 8900 with RA, 19481 with epilepsy, and 10963 healthy comparisons.
Healthy controls, referring to persons without schizophrenia, were matched 1:1 with patients with schizophrenia by gender, age (−3/+3 years) and inclusion year. In addition, parents of the patients with schizophrenia (N = 19065) were matched with parents of healthy controls (1:1, N = 19065) on gender, age (−5/+5 years), area of residence (3 categories), family situation (5 categories), and number of children (3 categories). For matched parents of healthy controls, CED was equal to the date of the corresponding matched parents of the patients with schizophrenia. Parents with multiple children matched in the process (multiple children with schizophrenia or multiple healthy children matched) were included only once, meaning that parents of children with specified chronic diseases were excluded if they were also parents of a s patient with schizophrenia (removal of duplicates of the same person, leaving 18597 parents of patients with schizophrenia and 19061 parents of healthy controls). Consequently, the cohort of parents of patients with schizophrenia was mutually exclusive with the cohorts of parents of offspring with RA, MS, or epilepsy. The parents of patients with RA, MS, or epilepsy were not mutually exclusive so, eg, a parent of a child with RA might have a child with MS. This overlap was marginal in most cases, however. In addition, matched parents not alive or resident in Sweden during the entire year of CED were excluded (leaving N = 18215 parents of the patients with schizophrenia and N = 18408 parents of healthy controls).
Outcome Measures
Health Care and Health Status
Health care use was assessed in terms of number of in- and specialized outpatient care visits due to psychiatric (ICD-10 codes: F00–F99) and somatic disorders (ie, diabetes mellitus type 2; diseases of the circulatory system; diseases of esophagus, stomach, and duodenum; liver disease; and dorsalgia: ICD-10 codes: E11–E14; I00–I99; K20–K31; K70–K77; and M54, respectively). Number of visits was used as a continuous outcome measure (Poisson regression), as mean annual number of visits and as a categorized variable (0, 1–2, 3–6, >6 visits; descriptive analyses). Outcomes variables related to the health status of parents comprised substance abuse (supplementary table 2), medication use for somatic and psychiatric disorders (supplementary table 3) and mortality.
Work Productivity
Long-term SA was defined as >90 annual gross days of SA (with benefits from the Social Insurance Agency). Information on granting of DP during follow-up was dichotomized. Annual income was based on yearly individualized disposable income derived from the family income (categorized based on quartiles as “no income” [yearly income is 0], “low” [first quartile], “medium low” [second quartile], “medium high” [third quartile], “high” [fouth quartile]). Long-term unemployment was measured as >180 annual days of unemployment. Social welfare benefits were calculated from the yearly individualized social welfare benefit derived from the family benefit (categorized as “yes” and “no”).
Disease Severity
The number of psychiatric inpatient care visits due to “schizophrenia, schizotypal, delusional, and other non-mood psychotic disorders” (ICD-10: F20–F29) was used as a proxy for disease severity of schizophrenia. The severity variable was categorized by calculating the number of visits per year during follow-up for each patient with schizophrenia at the end of follow-up. The patients in the first quartile were defined as “least severe,” those in the second–third quartiles as “moderately severe” and those in the fourth quartile as “most severe.”
Covariates
Sociodemographic characteristics, including information on gender, age, educational level, area of residence, number of children, and family situation were obtained for all parental groups (supplementary table 1). All factors measured at start of follow-up except area of residence and family situation which are measured at the calendar year of the outcome measure. Family situation included information on single or cohabiting parents. This covariate was included in the analyses as the proportion of single parents differed in the parental groups. If a study variable included missing values, a missing-value category was added to that variable. In the analyses of disease severity, prescribed antipsychotics (ATC codes N05A) and treatment persistence of the patient were also used as covariates. Treatment persistence was measured annually and categorized as “no persistence” if there was no on-going antipsychotic use, “low persistence” if the longest annual antipsychotic treatment period lasted at most 50% of the calendar days (excluding hospital days), “high persistence” if this treatment period was between 50% and 100%, and “total persistence” in case of 100% annual days of treatment period. Psychiatric morbidity at baseline was measured as diagnosis-specific psychiatric health care the year at cohort entry date. Four dichotomous diagnostic groups were formed: schizophrenia at baseline (ICD-10: F20–F29), mood disorders at baseline (ICD-10: F30–F39), anxiety disorders at baseline (F40–F49), and other psychiatric diagnoses at baseline (ICD-10: F00–F19, F50–F99).
Statistical Analyses
First, the mean number of diagnosis-specific specialized health care from 5 years before to 7 years after CED was plotted. Then, analyses in relation to comparison of parental groups, were performed for all outcome variables after CED during the entire 7-year follow-up period using pairwise comparisons: comparing parents of patients with schizophrenia to (1) parents of healthy controls (reference group); (2) parents of patients with MS, RA, epilepsy (reference groups). Yearly outcome variables were analyzed as dependent variables using logistic regression (“long-term sickness absence,” “disability pension,” “low or no income,” “long-term unemployment,” “social welfare benefit,” “medication use,” “substance abuse”), Cox regression (mortality), or Poisson regression (in- or specialized outpatient care due to psychiatric or somatic diagnoses). Related to these regression models, odds ratios (OR), hazard ratios (HR), and relative risks (RR) with 95% confidence intervals (CI) were calculated. Logistic regression analyses for longitudinal binary data yielding ORs were based on a model with repeated dichotomous measurements per individual. In these analyses, within-individual correlation between consecutive years was taken into account using generalized estimating equation modeling. Both unadjusted and adjusted analyses were conducted censoring for mortality. Analyses comparing parents of patients with schizophrenia and parents of healthy controls were controlled for gender, age, area of residence, family situation, number of children, and inclusion year by matching. Due to the small size of the population of parents of patients with other diseases (MS, RA, and epilepsy), matching was not possible. Instead, covariates (inclusion year, patient’s gender and age, parents’ gender and age, parents’ family situation and area of residence) were dealt as confounders in the multivariate analyses. In analyses on disease severity, parents of patients with least (reference), moderate and highest disease severity were compared. Here, additional adjustments were made by controlling for medication use of the parents and for annually prescribed antipsychotics and treatment persistence of the patients. Moreover, all analyses reported in tables 1–3 were adjusted for psychiatric morbidity at CED in the final models. Parents with on-going DP at CED were excluded from the analyses with SA and DP as outcome measures. Moreover, in all analyses with outcome measures related to work productivity, a general exclusion criterion of age being 65 years or over at CED was applied. Sensitivity analyses with regard to psychiatric or somatic specialized health care use were carried out for the incident cases to ascertain the comparability between parents of prevalent and incident patient populations with schizophrenia.
Table 1.
Number of Events, Person Years During Follow-up (2006–2013), Adjusted and Unadjusted Risk Estimates for Comparison of Parents of Patients With Multiple Sclerosis (MS; n = 11292), Rheumatoid Arthritis (RA; n = 15516), Epilepsy (n = 34715), and Healthy Control (n = 18408) Against Parents of Patients With Schizophrenia (n = 18215)
| Outcome Measures | Events | Person Years | Unadjusted | P-Value | Adjusteda | P-Value |
|---|---|---|---|---|---|---|
| Psychiatric specialized health care usea (relative risk) | ||||||
| Parents of patients with schizophrenia | 14528 | 102366 | — | — | ||
| Compared with parents of MS | 2977 | 57458 | 2.74 (2.63–2.85) | <.0001 | 1.80 (1.73–1.88) | <.0001 |
| Compared with parents of RA | 3935 | 76102 | 2.74 (2.65–2.84) | <.0001 | 1.76 (1.70–1.83) | <.0001 |
| Compared with parents of epilepsy | 12097 | 182160 | 2.14 (2.08–2.19) | <.0001 | 1.71 (1.66–1.75) | <.0001 |
| Compared with parents of healthy controls | 6214 | 102659 | 2.34 (2.28–2.41) | <.0001 | 1.63 (1.58–1.68) | <.0001 |
| Somatic specialized health care usea (relative risk) | ||||||
| Parents of patients with schizophrenia | 19519 | 102366 | — | — | ||
| Compared with parents of MS | 11503 | 57458 | 0.95 (0.93–0.97) | <.0001 | 0.96 (0.94–0.98) | .00109 |
| Compared with parents of RA | 15962 | 76102 | 0.91 (0.89–0.93) | <.0001 | 0.93 (0.91–0.95) | <.0001 |
| Compared with parents of epilepsy | 31357 | 182160 | 1.11 (1.09–1.13) | <.0001 | 0.97 (0.96–0.99) | .00445 |
| Compared with parents of healthy controls | 19874 | 102659 | 0.98 (0.97–1.00) | .13231 | 1.00 (0.98–1.02) | .68587 |
| Medication usea (odds ratios) | ||||||
| Parents of patients with schizophrenia | 73891 | 102366 | — | — | ||
| Compared with parents of MS | 42402 | 57458 | 0.90 (0.78–1.03) | .13048 | 0.84 (0.73–0.97) | .01474 |
| Compared with parents of RA | 57260 | 76102 | 0.78 (0.68–0.88) | <.0001 | 0.66 (0.58–0.75) | <.0001 |
| Compared with parents of epilepsy | 125805 | 182160 | 1.51 (1.37–1.66) | <.0001 | 0.67 (0.61–0.73) | <.0001 |
| Compared with parents of healthy controls | 73291 | 102659 | 1.16 (1.03–1.31) | .01258 | 0.88 (0.79–0.98) | .0209 |
| Substance abusea (odds ratios) | ||||||
| Parents of patients with schizophrenia (%) | 990 (5.4) | 102366 | — | — | ||
| Compared with parents of MS (%) | 345 (3.1) | 57458 | 1.46 (0.91–2.34) | .11408 | 1.14 (0.30–4.29) | .84489 |
| Compared with parents of RA (%) | 541 (3.5) | 76102 | 1.18 (0.79–1.76) | .41499 | 0.95 (0.30–2.97) | .92865 |
| Compared with parents of epilepsy (%) | 1585 (4.6) | 182160 | 1.03 (0.76–1.41) | .83738 | 0.82 (0.43–1.58) | .55071 |
| Compared with parents of healthy controls (%) | 715 (3.9) | 102659 | 1.30 (0.90–1.87) | .16277 | 0.86 (0.37–2.01) | .73480 |
| Mortalitya (hazard ratios) | ||||||
| Parents of patients with schizophrenia (%) | 1591 (8.7) | 102366 | — | — | ||
| Compared with parents of MS (%) | 849 (7.5) | 57458 | 1.04 (0.96–1.13) | .35552 | 0.99 (0.91–1.08) | .80467 |
| Compared with parents of RA (%) | 1156 (7.5) | 76102 | 1.00 (0.93–1.08) | .90825 | 1.01 (0.91–1.08) | .91665 |
| Compared with parents of epilepsy (%) | 2128 (6.1) | 182160 | 1.32 (1.24–1.41) | <.0001 | 0.94 (0.88–1.00) | .05896 |
| Compared with parents of healthy controls (%) | 1534 (8.3) | 102659 | 1.04 (0.97–1.12) | .23965 | 1.05 (0.98–1.13) | .15779 |
aAdjusted for calendar year, patient’s gender, parent’s gender, patient’s age, parent’s age, number of children, parent’s family situation, parent’s area of residence, schizophrenia at baseline (F20–F29), mood disorders at baseline (ICD-10: F30–F39), anxiety disorders at baseline (ICD-10: F40–F49), and other psychiatric diagnoses at baseline (ICD-10: F00–F19, F50–F99).
Table 3.
Number of Events, Person Years (PY), Relative Risks (RR) of Health Care Use and Hazard Ratios (HR) of Mortality in Parents of Patients With Schizophrenia According to the Patients’ Disease Severity
| Outcome Measures | Events | PY | Unadjusted | P-Value | Model 1a | P-Value | Model 2b | P-Value |
|---|---|---|---|---|---|---|---|---|
| Psychiatric specialized health care use | ||||||||
| Least severe | 6167 | 49783 | 1 | 1 | 1 | |||
| Moderately severe | 3225 | 18786 | 1.39 (1.33–1.45) | <.0001 | 1.11 (1.06–1.16) | <.0001 | 1.12 (1.07–1.17) | <.0001 |
| Most severe | 1412 | 8362 | 1.36 (1.29–1.44) | <.0001 | 1.02 (0.96–1.09) | .43209 | 1.03 (0.97–1.10) | .27403 |
| Somatic specialized health care use | ||||||||
| Least severe | 9861 | 49783 | 1 | 1 | 1 | |||
| Moderately severe | 3789 | 18786 | 1.02 (0.98–1.06) | .34383 | 1.05 (1.01–1.09) | .00092 | 1.05 (1.01–1.09) | .01123 |
| Most severe | 1769 | 8362 | 1.07 (1.02–1.12) | .01087 | 1.12 (1.06–1.18) | <.0001 | 1.12 (1.06–1.18) | <.0001 |
| Mortality | ||||||||
| Least severe | 914 | 49783 | 1 | 1 | 1 | |||
| Moderately severe | 339 | 18786 | 0.98 (0.86–1.11) | .74079 | 1.07 (0.94–1.22) | .30738 | 1.06 (0.93–1.20) | .39184 |
| Most severe | 147 | 8362 | 0.95 (0.80–1.13) | .56745 | 1.05 (0.87–1.26) | .60804 | 1.03 (0.86–1.24) | .75205 |
aAdjusted for patient age and gender, parents’ age and gender, parent’s family situation, parent’s area of residence, medication use (parent), number of different antipsychotics used (patient) within a year before start of follow-up, treatment persistence (patient), schizophrenia at baseline (ICD-10: F20–F29), mood disorders at baseline (ICD-10: F30–F39), anxiety disorders at baseline (F40–F49), and other psychiatric diagnoses at baseline (ICD-10: F00–F19, F50–F99).
bAdjusted for patient’s age and gender, parent’s age and gender, parent’s family situation, parent’s area of residence, medication use (parent), amount of antipsychotics used (patient) within the previous calendar year, number of different antipsychotics used (patient) within a year before start of follow-up, schizophrenia at baseline (ICD-10: F20-29), mood disorders at baseline (ICD-10: F30–F39), anxiety disorders at baseline (ICD-10: F40–F49), and other psychiatric diagnoses at baseline (ICD-10: F00–F19, F50–F99).
Ethical Approval
The study was approved by the Regional Ethics Review Board of Stockholm, Sweden.
Results
Characteristics of the Schizophrenia Patients
The majority of the patients with schizophrenia were aged between 35 and 45 years (53%), of male gender (63%), with a medium level of education (50%), single and living alone (89%) and mostly living in big cities (39%) (data not shown). Almost two-thirds (64%) of the patients with schizophrenia had a disease with lowest clinical severity, whereas just above 11% were severely ill. Considerable differences in the sociodemographic characteristics compared with patients with schizophrenia were seen for gender: 71% and 77% were women among MS and RA patients, respectively (data not shown).
Characteristics of the Parents of Patients With Schizophrenia and Comparison Groups
Supplementary table 1 shows the distribution of sociodemographic characteristics for the different parental groups. The distribution of sociodemographic factors in the parents of the incident population with schizophrenia was similar to the prevalent population with one exception: individuals in the latter group were generally older (data not shown).
At start of follow-up, approximately twice as many parents of patients with schizophrenia consumed psychiatric specialized health care and 2–3 times as many received social welfare benefits compared with parents of other chronic diseases (MS, RA, and epilepsy) or parents of healthy controls (supplementary table 1). The proportion of parents of patients with schizophrenia with DP, low or no income, and long-term unemployment was also somewhat higher compared with the comparison groups, supplementary table 1. With regard to other measures of health care resource use, health status and work productivity, no major differences between the groups emerged.
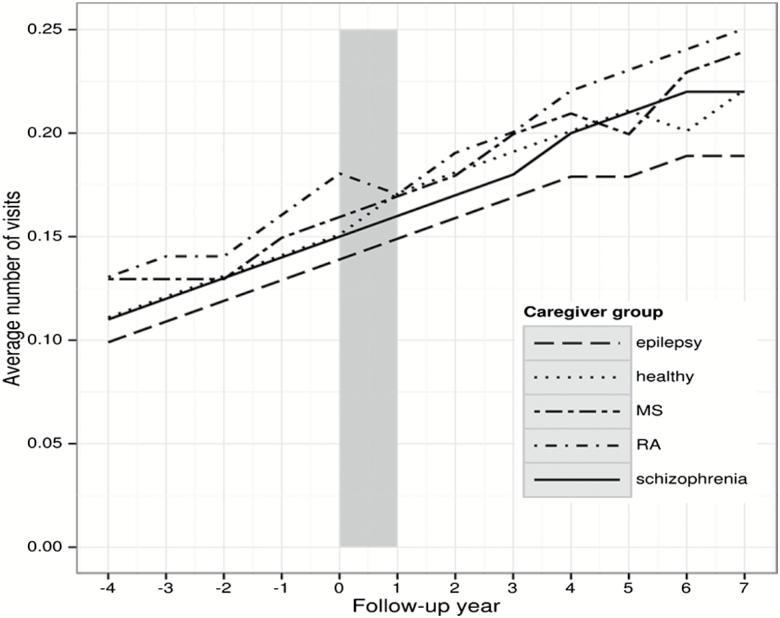
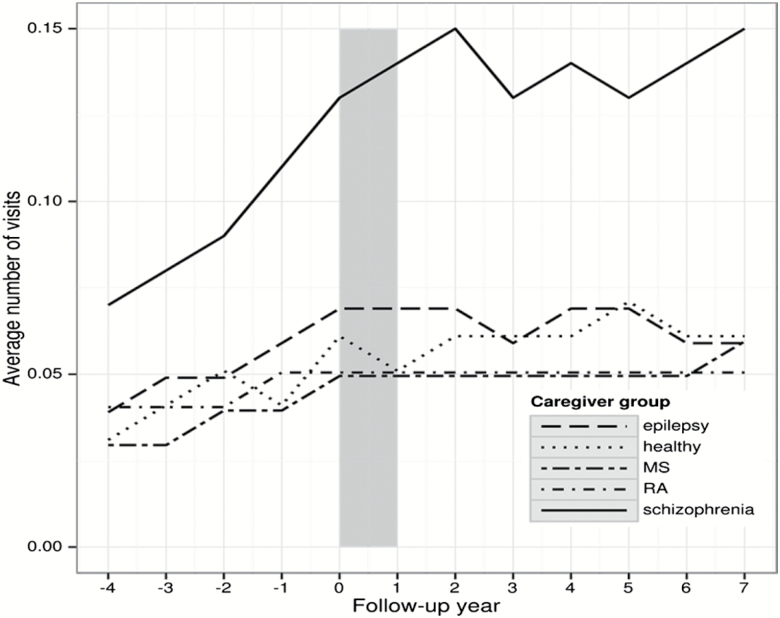
Use of specialized health care due to psychiatric or somatic diagnoses showed an increasing trend for all parents throughout the observation period, ie, before and after CED (figures 1 and 2). For somatic in- or specialized outpatient care, the frequencies were highest in the parents of patients with RA and lowest in the parents of patients with epilepsy (figure 1). Concerning psychiatric in- or specialized outpatient care among parents of patients with schizophrenia a sharp rise was observed from 4 years before CED, which stabilized—despite some fluctuations—following the year of CED (figure 2). Similar patterns were seen for the other parents groups, but on a much lower level. Sensitivity analyses revealed similar patterns of psychiatric and somatic specialized health care use for the parents of the incident population with schizophrenia. The most frequent diagnostic groups of psychiatric diagnoses among parents of patients with schizophrenia were affective and anxiety disorders as well as schizophrenia (supplementary table 4).
Fig. 1.
Number of specialized somatic health care visits during the observation period in parents of patients with schizophrenia, multiple sclerosis (MS), rheumatoid arthritis (RA), epilepsy, and healthy controls. Note: observation period from −4 to +7 years after diagnosis of the offspring/cohort entry date, t0.
Fig. 2.
Number of specialized psychiatric health care visits during the observation period in parents of patients with schizophrenia, multiple sclerosis (MS), rheumatoid arthritis (RA), epilepsy, and healthy controls. Note: observation period from −4 to +7 years after diagnosis of the offspring/cohort entry date, t0.
Parents of patients with schizophrenia had a higher risk of psychiatric specialized health care use compared with the other parental groups (range of RRs: 1.63–1.80) during follow-up, however rather similar risk estimates were observed for specialized somatic health care use in similar comparisons (range of RRs: 0.93–1.00) (table 1). Parents of patients with schizophrenia had a lower risk of medication use compared with the parents of healthy controls and the other comparison groups. A lower risk of substance abuse among parents of patients with schizophrenia than parents of patients with RA was observed (table 1).
While the subsequent risk of long-term SA was somewhat higher in parents of patients with schizophrenia than that in parents of patients with RA and healthy controls, no differences in risk of DP, long-term unemployment or income loss were observed between the most of the groups during follow-up (table 2). Moreover, parents of patients with schizophrenia were considerably more likely to receive social welfare benefits than their peers of other parental groups (range of ORs 1.20 to 2.74). Finally, a slightly higher mortality risk of parents of patients with schizophrenia was seen only when compared with the parents of healthy controls (HR 1.05; 95% CI: 0.98–1.13).
Table 2.
Number of Events, Person Years During Follow-up (2006–2013), Adjusted and Unadjusted Risk Estimates for Comparison of Parents of Patients With Multiple Sclerosis (MS; n = 11292), Rheumatoid Arthritis (RA; n = 15516), Epilepsy (n = 34715) and Healthy Control (n = 18408) Against Parents of Patients With Schizophrenia (n = 18215)
| Outcome Measures | Events | Person Years | Unadjusted | P-Value | Adjusted | P-Value |
|---|---|---|---|---|---|---|
| Sickness absence (>90 days)a (odds ratios) | ||||||
| Parents of patients with schizophrenia (%) | 2158 (11.8) | 82943 | — | — | ||
| Compared with parents of MS (%) | 1346 (11.9) | 49160 | 0.93 (0.74–1.17) | .52855 | 0.57 (0.39–0.82) | .00301 |
| Compared with parents of RA (%) | 1504 (9.7) | 64518 | 1.04 (0.83–1.29) | .75060 | 1.15 (1.15–1.16) | <.0001 |
| Compared with parents of epilepsy (%) | 5244 (15.1) | 155622 | 0.74 (0.62–0.87) | .00026 | 0.68 (0.67–0.68) | <.0001 |
| Compared with parents of healthy controls (%) | 1974 (10.7) | 89389 | 1.13 (0.93–1.38) | .21768 | 1.62 (1.16–2.26) | .00441 |
| Disability pensiona (odds ratios) | ||||||
| Parents of patients with schizophrenia (%) | 1298 (7.1) | 82943 | — | — | ||
| Compared with parents of MS (%) | 691 (6.1) | 49160 | 1.09 (0.59–1.99) | .78773 | 1.13 (0.32–3.99) | .84954 |
| Compared with parents of RA (%) | 722 (4.7) | 64518 | 1.28 (1.28–1.28) | <.0001 | 0.71 (0.12–4.33) | .70877 |
| Compared with parents of epilepsy (%) | 2425 (7.0) | 155622 | 0.97 (0.62–1.53) | .90945 | 1.30 (0.37–4.56) | .68443 |
| Compared with parents of healthy controls (%) | 950 (5.2) | 89389 | 1.43 (0.84–2.45) | .19115 | 0.98 (0.41–2.30) | .95452 |
| Low or no incomea (odds ratios) | ||||||
| Parents of patients with schizophrenia (%) | 11942 (65.6) | 45147 | — | — | ||
| Compared with parents of MS (%) | 5206 (46.1) | 24310 | 1.36 (1.12–1.66) | .00237 | 0.97 (0.69–1.37) | .85987 |
| Compared with parents of RA (%) | 6733 (43.4) | 31574 | 1.36 (1.13–1.64) | .00104 | 0.85 (0.68–1.05) | .13731 |
| Compared with parents of epilepsy (%) | 25232 (72.7) | 106405 | 1.26 (1.09–1.46) | .00179 | 1.03 (0.83–1.27) | .80195 |
| Compared with parents of healthy controls (%) | 9319 (50.6) | 42745 | 1.40 (1.18–1.67) | .00010 | 0.84 (0.61–1.15) | .27785 |
| Unemployment (>180 days)a (odds ratios) | ||||||
| Parents of patients with schizophrenia (%) | 1150 (6.3) | 102366 | — | — | ||
| Compared with parents of MS (%) | 514 (4.6) | 57458 | 1.18 (0.86–1.61) | .30891 | 1.03 (0.54–1.97) | .93449 |
| Compared with parents of RA (%) | 593 (3.8) | 76102 | 1.30 (0.96–1.75) | .08985 | 1.02 (0.52–2.02) | .95495 |
| Compared with parents of epilepsy (%) | 2208 (6.4) | 182160 | 0.90 (0.72–1.12) | .34179 | 1.59 (0.99–2.55) | .05413 |
| Compared with parents of healthy controls (%) | 943 (5.1) | 102659 | 1.16 (0.89–1.50) | .26534 | 1.77 (1.06–2.96) | .02781 |
| Social welfare benefita (odds ratios) | ||||||
| Parents of patients with schizophrenia (%) | 6231 (34.2) | 102366 | — | — | ||
| Compared with parents of MS (%) | 849 (7.5) | 57458 | 3.86 (2.82–5.29) | <.0001 | 2.69 (1.20–6.02) | .01599 |
| Compared with parents of RA (%) | 1254 (8.1) | 76102 | 3.48 (2.67–4.53) | <.0001 | 2.74 (1.30–5.74) | .00783 |
| Compared with parents of epilepsy (%) | 6373 (18.4) | 182160 | 1.72 (1.45–2.04) | <.0001 | 1.20 (0.82–1.76) | .33786 |
| Compared with parents of healthy controls (%) | 2610 (14.2) | 102659 | 2.48 (2.00–3.07) | <.0001 | 2.30 (1.31–4.05) | .00379 |
aAdjusted for calendar year, patient’s gender, parent’s gender, patient’s age, parent’s age, number of children, parent’s family situation, parent’s area of residence, schizophrenia at baseline (ICD-10: F20–F29), mood disorders at baseline (ICD-10: F30–F39), anxiety disorders at baseline (ICD-10: F40–49), and other psychiatric diagnoses at baseline (ICD-10: F00–F19, F50–F99).
Finally, parents of the moderately severely ill patients with schizophrenia had higher risk estimates for psychiatric specialized health care (RR: 1.12; 95% CI: 1.07–1.17) compared with the parents of least severely ill patients with schizophrenia (table 3). Higher risk estimates for somatic specialized health care use was also observed among the parents of most severely ill patients (RR: 1.12; 95% CI: 1.06–1.18) (table 3).
Discussion
Strengths and Limitations
The prospective cohort design, the population-based and very large cohort of parents with annual and detailed data for all individuals and practically no drop-out rates are the main strengths of this study. Another strength is the high quality of data in the used administrative registers. Moreover, the wide range of different variables and the outcome measures were recorded independently from each other.
Limitations of the study include that knowledge about the validity of the SA or DP diagnoses is limited, although one study published on this reported an acceptable validity.43 The register data contains only proxy information on disease severity, ie, number of inpatient care stays. This study could not elucidate the role of inherited frailty, ie, genetic factors or the environment during upbringing. Therefore, the current analyses point toward correlation and not causal mechanism. Nevertheless, this article primarily intended to assess the current burden of parents of patients with schizophrenia by assessing different associations rather than looking at causal inferences. Parents of patients with schizophrenia were matched with parents of healthy comparisons on several sociodemographic factors, among them family situation and the number of children. While the number of matching factors available is a clear advantage, matching on particularly family situation and number of children might have led to an underestimation of the expected differences between these groups (due to potential overrepresentation of over-burdened healthy control). Finally, given the nature of this study, we could not determine to which extent parents were actually providing care.
Health Care Use in Parents
Higher frequencies of psychiatric health care use were observed in parents of patients with schizophrenia compared with parents of the different control groups. Such psychiatric health care rose sharply from 4 years before up to the year of inclusion, stabilizing thereafter. These findings suggest that mental health deteriorates strongly in parents of patients with schizophrenia up to the time point when the offspring gets the diagnosis and stays at a high level thereafter. It is possible that parents of patients with schizophrenia are strongly affected during the period when the offspring suffers from symptoms but gets no or nonspecifically treatment. Previous literature suggests that parents experience greater burden due to common mental disorders before a formal diagnosis of schizophrenia is made.44 Such burden can be reflected in increasing health care use.
Additionally, adequate treatment is likely to be initiated following a newly diagnosed case, resulting in better compliance of the patients and thereby reducing the burden for the parents,15,20,45 which ultimately may lead to stabilization of psychiatric health care use following diagnosis. There is also a possibility of a ceiling effect of psychiatric health care use for the parents following a diagnosis of schizophrenia of the offspring as the psychiatric health care use was already at a high level. Different psychosocial interventions aiming at parents of patients with schizophrenia have been reported to be helpful to reduce stress,15,46,47 which may also play a role in stabilizing mental health of such parents. Unfortunately, no data were available for this study regarding the proportion of parents attending such intervention. Another important issue in this aspect is heredity of the considered disorders.48–51 Specifically for parents of offspring with schizophrenia, it is possible that the parent also has a psychiatric diagnosis,48 which affects the use of health care and additionally may worsen with additional burden from caregiving.24,52 This is also supported by the finding that specialized psychiatric health care use was already high among the parents of patients with schizophrenia at baseline. It is important to mention that affective and anxiety disorders were the most frequent diagnostic groups of specialized health care among the parents of patients with schizophrenia and that schizophrenia was much more common among these parents than in the comparison groups. Still, the proportions of parents with schizophrenia were very low: The annual proportion of parents having in- or specialized outpatient health care visit due to schizophrenia during the observation period was around 0.1%–1% among parents of patients with schizophrenia and 0%–0.1% among the other parent groups. Differences in the psychiatric health care use between different groups of parents can also be influenced by the disease-specific clinical differences and advancements of disease-specific management, eg, by newer and better drugs, etc.53–56
Health Status in Parents
About 70% of the parents of patients with schizophrenia used medication for psychiatric or somatic diseases at CED. The proportions of parents of patients with MS, RA, epilepsy, or healthy controls who used medication at CED were similar. Literature suggests that taking care of patients with chronic disease is associated with psychiatric disorders, like depression, anxiety, or stress in the parents and also may worsen pre-existing psychiatric or somatic morbidity in parents,27,30,57,58 which may lead to increased medication use. Considering the higher risk for specialized psychiatric health care use of parents of patients with schizophrenia compared with parents of patients with other diseases, it is notable that their mortality risk was not increased. We found only a slightly higher (nonsignificant) risk for mortality among the parents of patients with schizophrenia compared with parents of healthy controls. This is in line with a previous study.28
Productivity Loss in Parents
The risk of DP, low or no income or unemployment did not differ between most of the comparison groups. Income loss, financial burden,8,12,15,26,59 and labor market exit60 have been reported for parents of patients with schizophrenia, partly because of spending a considerable amount of time taking care and thereby reducing productive working hours.22 It is possible that the Swedish social insurance system can compensate for a potential income loss. Here, it should be noted that nearly one in every 5 parents of patients with schizophrenia was already on DP at baseline.
We found that parents of patients with schizophrenia were up to 2.7 times more likely to receive social welfare benefits during follow-up compared with other groups of parents. A possible explanation for this finding can be the specific social welfare system, maybe in a Swedish context, parents of patients with schizophrenia are more likely to fall through other social safety nets like unemployment and SA benefits. Future studies are required to investigate risk factors for social benefit dependence among parents of patients with schizophrenia.
Conclusions
Parents of patients with schizophrenia have considerably higher rates of psychiatric health care, mainly due to anxiety and affective disorders, and social welfare dependence than parents of patients with RA, MS, epilepsy, or healthy controls. The burden measured as psychiatric health care use worsens with increasing severity of the disease of the offspring with schizophrenia and over time. Such health care use increased continuously from 4 years before diagnosis of the offspring up to 7 years after diagnosis.
Funding
The work has been supported by Janssen Cilag (R092670SCH4045). Drs E.J., J.S., A.L. and D.E., co-authors employed by Janssen Cilag, were involved in study content and design and critical revision of the manuscript for important intellectual content. Their authorship roles adhere to ICMJE criteria.
Supplementary Material
Acknowledgments
J.T. reports serving as a consultant to the Finnish Medicines Agency Fimea, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, F. Hoffman-La Roche, Janssen-Cilag, Lundbeck, and Organon; receiving fees for giving expert testimonies to AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, Lundbeck, Otsuka, and Pfizer; receiving lecture fees from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, Lundbeck, Novartis, Otsuka, Pfizer; receiving grants from Stanley Foundation and the Sigrid Jusélius Foundation; serving as a member of the advisory boards for AstraZeneca, Eli Lilly, Janssen-Cilag, and Otsuka, and participating in research projects funded by Janssen-Cilag and Eli Lilly with grants paid to Karolinska Institutet. F.H., M.M., and J.M. reported being employed by EPID Research, which is a contract research organization that performs commissioned pharmacoepidemiological studies, and thus its employees have been and currently are working in collaboration with several pharmaceutical companies. A.T. and H.T. reported participating in research projects funded by Janssen-Cilag and Eli Lilly with grants paid to the Karolinska Institutet. A.T. reported serving as a member of advisory board for Janssen-Cilag. E.J., D.E., A.L., and J.S. are employed by Janssen Cilag Pharmaceuticals. E.M.-R. and S.R. report no conflict of interest.
Author Contributions
Ms M.M. and Dr J.M. had full access to all the data in the study and take responsible for the integrity of the data and the accuracy of the data analysis. J.T., E.M.-R., F.H., E.J., D.E., A.L., J.S., and A.T. were involved in study concept and design. E.M.-R., S.R., M.M., J.M., F.H., A.T., and H.T. were involved in acquisition, analysis, or interpretation of data. Drafting of the manuscript was done by E.M.-R. and S.R. Critical revision of the manuscript for important intellectual content was done by E.M.-R., S.R., J.T., M.M., J.M., F.H., E.J., D.E., A.L., J.S., A.T., and H.T. Statistical analysis has been carried out by M.M., J.M., F.H., A.T., and H.T.
References
- 1. WHO. Schizophrenia. World Health Organization; 2016. http://www.who.int/mediacentre/factsheets/fs397/en/. Accessed March 22, 2017. [Google Scholar]
- 2. Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. [DOI] [PubMed] [Google Scholar]
- 3. McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. [DOI] [PubMed] [Google Scholar]
- 4. Barbato A. Psychiatry in transition: outcomes of mental health policy shift in Italy. Aust N Z J Psychiatry. 1998;32:673–679. [DOI] [PubMed] [Google Scholar]
- 5. Kooyman I, Dean K, Harvey S, Walsh E. Outcomes of public concern in schizophrenia. Br J Psychiatry Suppl. 2007;50:s29–s36. [DOI] [PubMed] [Google Scholar]
- 6. Marwaha S, Johnson S. Schizophrenia and employment—a review. Soc Psychiatry Psychiatr Epidemiol. 2004;39:337–349. [DOI] [PubMed] [Google Scholar]
- 7. Gupta S, Isherwood G, Jones K, Van Impe K. Assessing health status in informal schizophrenia caregivers compared with health status in non-caregivers and caregivers of other conditions. BMC Psychiatry. 2015;15:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta S, Isherwood G, Jones K, Van Impe K. Productivity loss and resource utilization, and associated indirect and direct costs in individuals providing care for adults with schizophrenia in the EU5. Clinicoecon Outcomes Res. 2015;7:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caqueo-Urízar A, Rus-Calafell M, Craig TK, et al. Schizophrenia: impact on family dynamics. Curr Psychiatry Rep. 2017;19:2. [DOI] [PubMed] [Google Scholar]
- 11. Ekim A. OC29—caregiver burden in childhood asthma. Nurs Child Young People. 2016;28:75. [DOI] [PubMed] [Google Scholar]
- 12. von Kardorff E, Soltaninejad A, Kamali M, Eslami Shahrbabaki M. Family caregiver burden in mental illnesses: the case of affective disorders and schizophrenia—a qualitative exploratory study. Nord J Psychiatry. 2016;70:248–254. [DOI] [PubMed] [Google Scholar]
- 13. Sakamoto K, Crowley JJ. A comprehensive review of the genetic and biological evidence supports a role for microRNA-137 in the etiology of schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2018;177:242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shorter KR, Miller BH. Epigenetic mechanisms in schizophrenia. Prog Biophys Mol Biol. 2015;118:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Awad AG, Voruganti LN. The burden of schizophrenia on caregivers: a review. Pharmacoeconomics. 2008;26:149–162. [DOI] [PubMed] [Google Scholar]
- 16. Hjärthag F, Helldin L, Karilampi U, Norlander T. Illness-related components for the family burden of relatives to patients with psychotic illness. Soc Psychiatry Psychiatr Epidemiol. 2010;45:275–283. [DOI] [PubMed] [Google Scholar]
- 17. Wolthaus JE, Dingemans PM, Schene AH, et al. Caregiver burden in recent-onset schizophrenia and spectrum disorders: the influence of symptoms and personality traits. J Nerv Ment Dis. 2002;190:241–247. [DOI] [PubMed] [Google Scholar]
- 18. Parabiaghi A, Lasalvia A, Bonetto C, et al. Predictors of changes in caregiving burden in people with schizophrenia: a 3-year follow-up study in a community mental health service. Acta Psychiatr Scand Suppl 2007;(437):66–76. [DOI] [PubMed] [Google Scholar]
- 19. Grandón P, Jenaro C, Lemos S. Primary caregivers of schizophrenia outpatients: burden and predictor variables. Psychiatry Res. 2008;158:335–343. [DOI] [PubMed] [Google Scholar]
- 20. Lasebikan VO, Ayinde OO. Effects of psychopathology, functioning and anti-psychotic medication adherence on caregivers’ burden in schizophrenia. Indian J Psychol Med. 2013;35:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flyckt L, Fatouros-Bergman H, Koernig T. Determinants of subjective and objective burden of informal caregiving of patients with psychotic disorders. Int J Soc Psychiatry. 2015;61:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flyckt L, Löthman A, Jörgensen L, Rylander A, Koernig T. Burden of informal care giving to patients with psychoses: a descriptive and methodological study. Int J Soc Psychiatry. 2013;59:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gater A, Rofail D, Tolley C, et al. “Sometimes it’s difficult to have a normal life”: results from a qualitative study exploring caregiver burden in schizophrenia. Schizophr Res Treatment. 2014;2014:368215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Popovic D, Goldberg S, Fenchel D, et al. Risk of hospitalization for psychiatric disorders among siblings and parents of probands with psychotic or affective disorders: a population-based study. Eur Neuropsychopharmacol. 2018;28:436–443. [DOI] [PubMed] [Google Scholar]
- 25. Koujalgi SR, Patil SR. Family burden in patient with schizophrenia and depressive disorder: a comparative study. Indian J Psychol Med. 2013;35:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caqueo-Urízar A, Gutiérrez-Maldonado J, Miranda-Castillo C. Quality of life in caregivers of patients with schizophrenia: a literature review. Health Qual Life Outcomes. 2009;7:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim Y, Carver CS, Cannady RS, Shaffer KM. Self-reported medical morbidity among informal caregivers of chronic illness: the case of cancer. Qual Life Res. 2013;22:1265–1272. [DOI] [PubMed] [Google Scholar]
- 28. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. [DOI] [PubMed] [Google Scholar]
- 29. Shaffer KM, Kim Y, Carver CS, Cannady RS. Effects of caregiving status and changes in depressive symptoms on development of physical morbidity among long-term cancer caregivers. Health Psychol. 2017;36:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull. 2003;129:946–972. [DOI] [PubMed] [Google Scholar]
- 31. Zarit SH. Positive aspects of caregiving: more than looking on the bright side. Aging Ment Health. 2012;16:673–674. [DOI] [PubMed] [Google Scholar]
- 32. Kulhara P, Kate N, Grover S, Nehra R. Positive aspects of caregiving in schizophrenia: A review. World J Psychiatry. 2012;2:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mantovani LM, Ferretjans R, Marçal IM, Oliveira AM, Guimarães FC, Salgado JV. Family burden in schizophrenia: the influence of age of onset and negative symptoms. Trends Psychiatry Psychother. 2016;38:96–99. [DOI] [PubMed] [Google Scholar]
- 34. Caqueo-Urízar A, Miranda-Castillo C, Lemos Giráldez S, Lee Maturana SL, Ramírez Pérez M, Mascayano Tapia F. An updated review on burden on caregivers of schizophrenia patients. Psicothema. 2014;26:235–243. [DOI] [PubMed] [Google Scholar]
- 35. Hillman L. Caregiving in multiple sclerosis. Phys Med Rehabil Clin N Am. 2013;24:619–627. [DOI] [PubMed] [Google Scholar]
- 36. Kuipers E, Yesufu-Udechuku A, Taylor C, Kendall T. Management of psychosis and schizophrenia in adults: summary of updated NICE guidance. BMJ (Clinical research ed) 2014;348:g1173. [DOI] [PubMed] [Google Scholar]
- 37. Topcu G, Buchanan H, Aubeeluck A, Garip G. Caregiving in multiple sclerosis and quality of life: a meta-synthesis of qualitative research. Psychol Health. 2016;31:693–710. [DOI] [PubMed] [Google Scholar]
- 38. Boonen A, Severens JL. The burden of illness of rheumatoid arthritis. Clin Rheumatol. 2011;30(suppl 1):S3–S8. [DOI] [PubMed] [Google Scholar]
- 39. Brouwer WB, van Exel NJ, van de Berg B, Dinant HJ, Koopmanschap MA, van den Bos GA. Burden of caregiving: evidence of objective burden, subjective burden, and quality of life impacts on informal caregivers of patients with rheumatoid arthritis. Arthritis Rheum. 2004;51:570–577. [DOI] [PubMed] [Google Scholar]
- 40. Karakis I, Cole AJ, Montouris GD, San Luciano M, Meador KJ, Piperidou C. Caregiver burden in epilepsy: determinants and impact. Epilepsy Res Treat. 2014;2014:808421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riechmann J, Strzelczyk A, Reese JP, et al. ; EpiPaed Study Group Costs of epilepsy and cost-driving factors in children, adolescents, and their caregivers in Germany. Epilepsia. 2015;56:1388–1397. [DOI] [PubMed] [Google Scholar]
- 42. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017;74:686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ljungdahl LO, Bjurulf P. The accordance of diagnoses in a computerized sick-leave register with doctor’s certificates and medical records. Scand J Soc Med. 1991;19:148–153. [DOI] [PubMed] [Google Scholar]
- 44. Outram S, Harris G, Kelly B, et al. ‘We didn’t have a clue’: family caregivers’ experiences of the communication of a diagnosis of schizophrenia. Int J Soc Psychiatry. 2015;61:10–16. [DOI] [PubMed] [Google Scholar]
- 45. Cooper D, Moisan J, Grégoire JP. Adherence to atypical antipsychotic treatment among newly treated patients: a population-based study in schizophrenia. J Clin Psychiatry. 2007;68:818–825. [DOI] [PubMed] [Google Scholar]
- 46. Okpokoro U, Adams CE, Sampson S. Family intervention (brief) for schizophrenia. The Cochrane Database Syst Rev. 2014;(3):Cd009802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caqueo-Urízar A, Rus-Calafell M, Urzúa A, Escudero J, Gutiérrez-Maldonado J. The role of family therapy in the management of schizophrenia: challenges and solutions. Neuropsychiatr Dis Treat. 2015;11:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boshes RA, Manschreck TC, Konigsberg W. Genetics of the schizophrenias: a model accounting for their persistence and myriad phenotypes. Harv Rev Psychiatry. 2012;20:119–129. [DOI] [PubMed] [Google Scholar]
- 49. Frisell T, Holmqvist M, Källberg H, Klareskog L, Alfredsson L, Askling J. Familial risks and heritability of rheumatoid arthritis: role of rheumatoid factor/anti-citrullinated protein antibody status, number and type of affected relatives, sex, and age. Arthritis Rheum. 2013;65:2773–2782. [DOI] [PubMed] [Google Scholar]
- 50. Speed D, O’Brien TJ, Palotie A, et al. Describing the genetic architecture of epilepsy through heritability analysis. Brain. 2014;137:2680–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Westerlind H, Ramanujam R, Uvehag D, et al. Modest familial risks for multiple sclerosis: a registry-based study of the population of Sweden. Brain. 2014;137:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aschbrenner KA, Greenberg JS, Seltzer MM. Parenting an adult child with bipolar disorder in later life. J Nerv Ment Dis. 2009;197:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Needham E, Zandi MS. Recent advances in the neuroimmunology of cell-surface CNS autoantibody syndromes, Alzheimer’s disease, traumatic brain injury and schizophrenia. J Neurol. 2014;261:2037–2042. [DOI] [PubMed] [Google Scholar]
- 54. Ransohoff RM, Hafler DA, Lucchinetti CF. Multiple sclerosis—a quiet revolution. Nat Rev Neurol. 2015;11:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang H, Zhou H, Wang N. Recent advances in epilepsy management. Cell Biochem Biophys. 2015;73:7–10. [DOI] [PubMed] [Google Scholar]
- 56. Wang Q, Sun X. Recent advances in nanomedicines for the treatment of rheumatoid arthritis. Biomater Sci. 2017;5:1407–1420. [DOI] [PubMed] [Google Scholar]
- 57. Allen AP, Curran EA, Duggan Á, et al. A systematic review of the psychobiological burden of informal caregiving for patients with dementia: focus on cognitive and biological markers of chronic stress. Neurosci Biobehav Rev. 2017;73:123–164. [DOI] [PubMed] [Google Scholar]
- 58. Loh AZ, Tan JS, Zhang MW, Ho RC. The global prevalence of anxiety and depressive symptoms among caregivers of stroke survivors. J Am Med Dir Assoc. 2017;18:111–116. [DOI] [PubMed] [Google Scholar]
- 59. Madianos M, Economou M, Dafni O, Koukia E, Palli A, Rogakou E. Family disruption, economic hardship and psychological distress in schizophrenia: can they be measured?Eur Psychiatry. 2004;19:408–414. [DOI] [PubMed] [Google Scholar]
- 60. Schneider U, Trukeschitz B, Mühlmann R, Ponocny I. “Do I stay or do I go?”—job change and labor market exit intentions of employees providing informal care to older adults. Health Econ. 2013;22:1230–1249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.