Abstract
Schizophrenia is associated with impaired and exaggerated Theory of Mind processes, pointing on alterations in generating a representation of another person’s mind. Despite recent work on healthy subjects suggesting that a coupling between the right temporoparietal junction (rTPJ) and the hippocampus is relevant for building representations of others’ intentions, the neural basis of related dysfunctions in patients with schizophrenia remains unclear. Therefore, we used structural and functional magnetic resonance imaging together with a modified prisoner’s dilemma game to test the hypotheses, that patients show dysfunctional social updating on behavioral level accompanied by altered rTPJ–hippocampus coupling on a functional and a structural level. During the task, 31 patients with schizophrenia and 20 healthy controls interacted with 3 playing partners, who behaved according to stable strategies competitively, cooperatively, or randomly. Our data show that patients adapted their social behavior less flexibly to the playing partners than healthy controls, indicating differences in forming mental representations of the counterparts’ intentions. Patients showed lower functional connectivity between the rTPJ and temporal lobe regions such as the hippocampus, the fusiform gyrus, and the middle temporal gyrus, indicating that in patients the rTPJ fails to integrate memory-informed processing streams during mental state inferences. Remarkably, the rTPJ–hippocampus coupling accounted for the participants’ adaptive social behavior in the task, suggesting that a neural pathway relevant for updating social knowledge and forming forward predictions in social interactions is altered in schizophrenia.
Keywords: Theory of Mind, mentalizing, social cognition, social learning, social decision making, fMRI, psychotic disorder
Introduction
Mental disorders are associated with deficits in social functioning1,2 with schizophrenia being particularly linked to marked impairments in social interactions.3,4 These social deficits have been associated with difficulties in representing other peoples’ minds,3,5,6 underlined by comprehensive empirical evidence indicating that patients show impairments in mental state inference processes.7–9 The clinical phenotype of schizophrenia, however, is characterized by irrational and biased reflections of other peoples’ intentions.10 More specifically, deluded patients have been shown to ascribe malevolent intentions toward other people, indicating aberrant inference processes during the formation of others’ intentions.3,11 Along this line, schizophrenia might be associated with deficits in constructing and updating a mental representation of another person, which we tested in this study with an interactive social decision-making game with counterparts holding different intention types for the first time.
Novel theories about brain regions underlying Theory of Mind (ToM) processes indicate that information about other peoples’ intentions are represented in mental models which are dynamically synchronized and updated according to their appropriateness in the current environment.12–14 Accordingly, adaptive social behavior can be conceptualized as a constructive process of retrieving, integrating, and reforming information from novel and previous episodes.12,15,16 Previous studies consistently found a neural network that shows enhanced activity during the momentary inference and representation of other peoples’ mental states consisting of the right temporoparietal junction (rTPJ), the medial prefrontal cortex (mPFC) and the posterior cingulate cortex (PCC).17 However, only elusive evidence exists how these brain regions are involved in the constructive mechanisms underlying the formation of social representations.18 Novel findings suggest that brain regions underlying ToM processes integrate short-term and long-term social information from brain regions relevant for memory processes during the inference of whether another person holds positive or negative interpersonal intentions12,18,19 or is extraverted or agreeable.15 Accordingly, brain regions relevant for declarative memory processes such as the hippocampus,12,20 the temporal poles,12,15 and the PCC15 have been implicated in the formation of a mental model of another person’s mental states.
In schizophrenia, functional alterations and disconnectivity in brain regions underlying memory formation and social cognition have been repeatedly shown.21 Exempli gratia, the rTPJ as one of the key regions in the ToM network17,22 has been found to be less activated during mental state inference processes in schizophrenia.23,24 Such reduced activation is often accompanied by mPFC25–27 and PCC dysfunctions,25 indicating that multiple brain regions are functionally altered in the ToM network. However, contradicting reports show higher activity in the TPJ during the presentation of ToM cartoon stories,25,28 supporting the notion that enhanced activity in the same brain regions could account for impaired and exaggerated mental state inferences.21,29
Whereas these studies contributed to our understanding of spontaneous inferences of another person’s mental states in schizophrenia, our paradigm that simulates real-life interactions allows to examine how patients acquire mental states of multiple others holding different interpersonal intentions. This could provide insight into whether a neural pathway that informs and updates social inferences by memory-information is impaired in schizophrenia as behavioral findings suggest.30–32 Recently, we reported that a coupling between the rTPJ and the hippocampus is associated with forming a representation of another person’s mind during iterative interactions,12 consistent with the idea that the hippocampus stores social information and flexibly recombines them to a coherent representation in novel situations, providing the basis for adaptive social behavior.18,33,34 Remarkably, the hippocampus is one of the most consistently altered neurobiological findings in schizophrenia,35,36 which has been linked to the social impairments occurring in the disorder.21 Hence, we speculate that aberrant ToM processes in schizophrenia3,6 arise from alterations in rTPJ–hippocampus connectivity.
In this study, we examine brain processes underlying the construction of mental states in patients with schizophrenia. During functional magnetic resonance imaging (fMRI), the participants interacted in a dynamic social decision environment with 3 playing partners, who systematically differed in their strategies, ie, interpersonal intentions. Participants can improve the outcome of their decisions by forming a mental model of the playing partners’ intentions based on their behavior, ie, to figure out who is holding selfish or cooperative interpersonal intentions and adapt their behavior adequately. Recent evidence suggests that healthy subjects update social representations during the experiment, indicating the emergence of a mental model of the interaction partners’ mind.12 Furthermore, the participants interacted against a control condition with no playing partner present, in which no ToM processes were relevant. We will test the hypotheses that (1) patients show dysfunctional social updating on behavioral level in ToM conditions, (2) reduced activation of the rTPJ and other brain regions in the ToM network, (3) reduced functional connectivity, and (4) structural covariance between the rTPJ and the left hippocampus.
Materials and Methods
Participants
A total of 31 patients (10 women) with a diagnosis of schizophrenia or schizoaffective disorder (International Classification of Diseases, Tenth Revision: F20 or F25) between 18 and 53 years of age (M ± STD = 31.29 ± 8.53) participated in the study. The control group contained 20 healthy participants (10 women) between 23 and 45 years of age (M ± STD = 29.55 ± 6.17) who had participated in a previous study.12 A structured clinical interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition37 was used to confirm the patients’ diagnosis and to exclude any psychiatric disorder in controls. The severity of patients’ symptoms during the last week was assessed with the Scale for the Assessment of Positive Symptoms (SAPS)38 and the Scale for the Assessment of Negative Symptoms (SANS).39 Participants were only included by sufficient fMRI data quality (details in supplementary material) and sufficient task engagement (not more than 25% misses of behavioral responses during the task). According to these criteria, 3 and 6 patients were not included in the analysis, from a total sample of 40 patients. Trends toward group differences existed in educational level (χ2 = 7.65, P = .054) and gender (χ2 = 2.57, P = .107), which were considered in the statistical analysis. All participants had normal or corrected-to-normal vision, reported no history of neurological disorders, and were right-handed.40 The study was approved by the local ethics committee at Philipps-University Marburg and all participants gave written informed consent before the experiment and were reimbursed for the time they spent at the study site.
Clinical Characteristics
The patient group had a mean SAPS score of 12.35 ± 10.57 (M ± STD, range: 0–36) and a SANS score of 10.35 ± 11.74 (M ± STD, range: 0–39) leading to a global score of 22.70 ± 19.44 (M ± STD, range: 0–73). The duration of illness was 9.35 ± 8.40 years (M ± STD, range: 0–30 years). Twenty-four patients received stable doses of atypical antipsychotic medication (M ± STD = 387.89 ± 451.74 mg/d chlorpromazine equivalent41), whereas 7 patients did not receive any antipsychotic medication.
Experimental Task
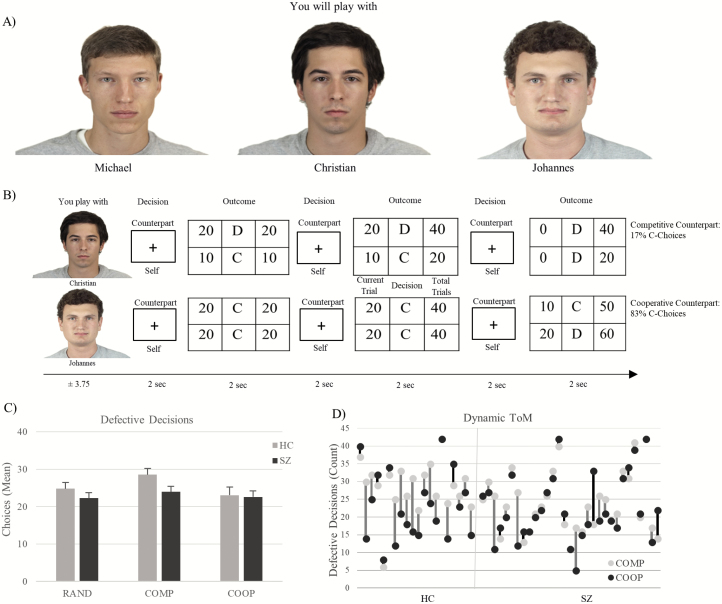
Participants were told that they would interact with 3 real playing partners in a social decision-making game in which the payoff depends on their own and the counterpart’s decisions (iterative modified prisoner’s dilemma game, for details see12). In fact, the participants interacted with fictive playing partners who pursued a stable strategy competitively (COMP; defecting in 83.3% of trials), cooperatively (COOP; cooperating in 83.3% of trials), or randomly (RAND; defecting/cooperating in 50% of trials, respectively). Each interaction block contained 6 decision phases (2 s) in which the participants had to decide to either defect or cooperate with the counterpart (figure 1). Afterwards the partners’ payoffs were presented (2 s). The decisions of the counterparts were pseudorandomized and the interaction sequences were counterbalanced. Participants interacted with each counterpart in alternate order in 7 blocks. A condition in which the picture of the playing partner was replaced by a red cross and the hint that due to technical reasons no playing partner is connected functioned as control condition. Participants had to alternately press the right and the left buttons and no outcomes were presented, which is why no ToM processes were necessary. The within-subjects design allowed the participants to accumulate evidence about the playing partners’ goals online during the experiment and to change their own strategy accordingly. Prior to the experiment, the participants were instructed carefully and practiced the game in a training session.
Fig. 1.
(A) Prior to the interactions, all playing partners were introduced to establish a social context. (B) Examples of the interaction sequences with the intentionally different counterparts. Each interaction block contained six decision phases in which the participants had to decide to either defect (D) or cooperate (C) with the counterpart. Afterwards the partners’ payoffs were presented. The COMP and COOP playing partners decided in 5 out of 6 decisions per block with either the D- or C-choice. (C) Mean D-Choices in the conditions. Error bars indicate SEM. (D) Distribution of the Dynamic ToM values, representing each participant’s D-choices in the COMP vs COOP condition. A positive value indicates higher D-choices in the COMP condition (gray line), a negative value vice versa (black line). ToM, Theory of Mind.
Behavioral Data Analysis and Dynamic ToM Value
Repeated-measures analysis of variances were conducted with SPSS20 (IBM Corp.) to examine the behavioral differences between conditions and groups. Furthermore, individual differences in the behavioral adaption to the playing partners with overt intentions (COMP and COOP) were examined to capture a participant’s adaptation toward multiple playing partners during the game (“Dynamic ToM”). The value represents the difference between the defective choices against the competitive and the cooperative playing partner. A positive value indicates a participant’s adaption to both partners’ strategies, i.e. higher competitive tendencies against the COMP but lower competitive tendencies against the COOP counterpart. Hence, the value indicates a participant’s understanding of the social environment, ie, the simultaneous consideration of multiple playing partners’ intentions.12
fMRI Data Analysis
Contrast images for each condition (COMP, COOP, RAND, and CONT) were calculated during a first-level analysis (details in supplementary material and Bitsch et al12) and implemented in a 2 × 4 second-level random effects analysis (flexible factorial design). The second-level design matrix contained the participants’ beta images and 2 covariates (educational level and gender). The contrasts of interest focused on commonalities and between-group differences in the mentalizing conditions compared to the control condition. The conjunction (null42) analysis was conducted according to HC∩SZ: (COMP > CONT)∩(COOP > CONT)∩(RAND > CONT). Between-group differences during ToM conditions were tested with the contrast HC vs SZ: (COMP & COOP & RAND > CONT). F-contrasts were conducted to examine between-group differences, followed by post hoc t-contrasts to examine the direction of effect. Brain–behavior associations were tested in the conditions with counterparts holding overt intentions (COMP & COOP > CONT). All whole-brain results were corrected for multiple comparisons on cluster level P < .05.43 For an individual voxel-type I error at P < .005, a corresponding cluster extend of 67 voxels had been calculated using the data-specific parameters and applied to all functional analyses. However, considering recent discussions about increased false-positive rates with low cluster-forming thresholds,44 we additionally highlight all results that also survive a cluster-forming threshold of P < .001 (k = 47).
Functional Connectivity
Task-related connectivity changes were assessed with the generalized form of context-dependent psychophysiological interaction (gPPI45), given its higher sensitivity and specificity due to a better model fit, compared to the standard form of PPI.45 An a priori-defined region of interest (ROI) of the anterior rTPJ46 was used as seed region for the gPPI analysis. Whereas initial assumptions suggested that the anterior rTPJ has a higher relevance in attentional than in social cognitive processes,46 recent findings challenge this view and indicate that the anterior subregion is relevant for ToM and attention-reorienting processes,47–49 which is particularly relevant in strategic games, because a mental model of the counterparts’ intentions has to be formed and updated in the current context.12,50 Prior to the connectivity analysis, we ensured that the region is implicated in the mentalizing conditions (t = 5.02, P < .001, family-wise error (FWE) corrected for the small volume, main effect task). The entire regions eigenvariate was extracted and for each condition a PPI regressor was built and included in a first-level model with identical regressors as in the functional activity model plus the seed-regions time course. Contrast maps for TASK > CONT were calculated and submitted to a second-level random effects analysis (two sample t test) with covariates identical to the activity analysis. Because the correction on a cluster level biases findings toward larger brain regions and given our a priori hypothesis of rTPJ–hippocampus alterations in patients, we masked the corrected statistical parametric map (SPM) with an anatomically defined hippocampus mask (consisting of the CA1, CA2, CA3, DG, Subiculum, SPM Anatomy toolbox51) to limit findings between brain and behavior measurements to the hippocampus.
Structural Covariance
Structural covariance is an indirect measure of the anatomically coupling between a seed region and other brain regions52 allowing to identify anatomical networks that may underlie cognitive functions.53 Voxel-wise gray matter volume maps were derived from a voxel-based morphometry analysis with CAT12 (http://www.neuro.uni-jena.de/cat/) implemented in SPM12. The preprocessed volumes (preprocessing details in supplementary material) were implemented in a random effects analysis (2 sample t tests) with identical nuisance covariates as in the functional analysis and the estimated total intracranial volume to account for individual head size and volume. This general linear model (GLM) was used to extract each subject’s mean gray matter density of the rTPJ (identical ROI as in the functional connectivity analysis) with the REX toolbox (http://web.mit.edu/swg/software.htm). The participants’ rTPJ gray matter density was implicated as covariate of interest in the GLM described earlier. Identical second-level analyses were conducted for the structural covariance and functional connectivity analysis. The SPM was corrected for multiple comparisons again on a cluster level P < .05 (FWE, voxel threshold P = .001). A structural covariance ROI analysis with the suprathreshold hippocampus voxels found in the functional connectivity analysis was conducted via Marsbar (http://marsbar.sourceforge.net/).
Results
Behavioral Analysis
A main effect of condition shows that participants adapted their defective choices between the different playing partners, F(2,98) = 11.09, P < .001, ηp2 = .185. A group × condition interaction indicates that healthy controls adapted their decisions stronger between the playing partners than patients, F(2,98) = 3.55, P = .033, ηp2 = .068. The interaction was driven by more defective decisions in the COMP vs COOP interaction in controls compared to patients, F(1,49) = 5.53, P = .023, ηp2 = .101 (figure 2). No significant group differences existed between the COMP vs RAND conditions, F(1,49) = 2.21, P > .05.
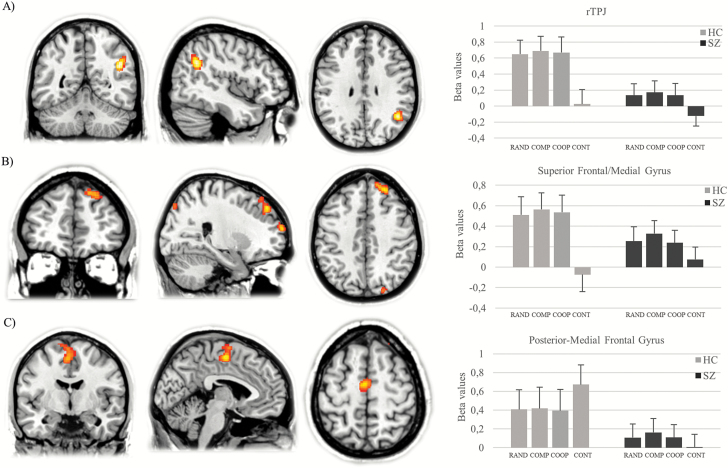
Fig. 2.
Between-group differences in brain activation between mentalizing and control condition indicate differences in the rTPJ (A), the right superior/medial frontal gyrus (B), and the left poster-medial frontal gyrus (C). rTPJ, right temporoparietal junction.
fMRI Analysis Common Functional Activity: Conjunction Analysis
The conjunction analysis of HC (COMP > CONT)∩HC(COOP > CONT)∩HC(RAND > CONT)∩SZ(COMP > CONT)∩SZ(COOP > CONT)∩SZ(RAND > CONT) revealed activation in large clusters comprising brain regions previously associated with ToM processes such as the right inferior parietal lobule, frontal lobe regions (right posterior-medial, bilateral superior, bilateral middle, and bilateral inferior frontal gyrus [IFG]), the right insula, and the mid-cingulate cortex [MCC] (table 1) indicating high activation commonalities in the ToM network across groups.
Table 1.
Results of fMRI Activation Analysis
| Contrast | Anatomical region | Hemisphere | Voxel per cluster | X | Y | Z | F/t value |
|---|---|---|---|---|---|---|---|
| Conjunction | |||||||
| Cluster 1 | Inferior parietal lobule | Right | 30 278 | 32 | −50 | 44 | 9.24a,b |
| Supramarginal gyrus | Right | 50 | −44 | 42 | 9.06a,b | ||
| Superior parietal lobule | Right | 26 | −66 | 54 | 8.72a,b | ||
| Inferior parietal lobule | Right | 42 | −44 | 46 | 8.68a,b | ||
| Cerebellum | Left | −8 | −76 | −28 | 7.91a,b | ||
| Superior parietal lobule | Left | −24 | −66 | 52 | 7.41a,b | ||
| Inferior occipital gyrus | Left | −40 | −82 | −8 | 7.40a,b | ||
| Cluster 2 | Precentral gyrus | Right | 22 085 | 46 | 6 | 32 | 9.55a,b |
| Insula | Right | 32 | 22 | −2 | 8.84a,b | ||
| Inferior frontal gyrus | Right | 46 | 36 | 26 | 8.18a,b | ||
| Posterior-medial frontal gyrus | Right | 6 | 24 | 48 | 8.07a,b | ||
| Middle frontal gyrus | Right | 44 | 42 | 20 | 7.59a,b | ||
| Insula | Left | −34 | 18 | −2 | 6.67a,b | ||
| Superior frontal gyrus | Right | 26 | 16 | 62 | 6.18a,b | ||
| Cluster 3 | Precentral gyrus | Left | 3183 | −44 | 4 | 30 | 7.84a,b |
| Inferior frontal gyrus | Left | −44 | 30 | 28 | 5.59a,b | ||
| Middle frontal gyrus | Left | −44 | 22 | 42 | 4.10a,b | ||
| Superior frontal gyrus | Left | −22 | 16 | 62 | 2.76a,b | ||
| Cluster 4 | Middle frontal gyrus | Left | 871 | −38 | 46 | 2 | 4.48a,b |
| Cluster 5 | MCC | Right | 394 | 6 | −28 | 30 | 5.09a,b |
| Interaction effect of group × task | |||||||
| Cluster 1 | Posterior-medial frontal gyrus | Left | 457 | −4 | −8 | 54 | 15.29a,b |
| Cluster 2 | Temporoparietal junction | Right | 270 | 44 | −54 | 28 | 19.51a,b |
| Cluster 3 | Superior frontal gyrus | Right | 269 | 24 | 44 | 44 | 14.76a,b |
| Right superior medial gyrus | Right | 14 | 48 | 46 | 12.79a,b | ||
| Cluster 4 | Precentral gyrus | Left | 171 | −26 | −18 | 74 | 12.80a |
| Cluster 5 | Superior frontal gyrus | Right | 130 | 20 | 64 | 16 | 16.28a |
| Cluster 6 | Cerebellum | Left | 69 | −22 | −80 | −34 | 11.14a |
| Cluster 7 | Cuneus | Right | 67 | 22 | −84 | 46 | 11.43a |
| Post hoc t contrast | |||||||
| HC(TASK > CONT) > SZ(TASK > CONT) | |||||||
| Cluster 1 | Superior frontal gyrus | Right | 475 | 24 | 44 | 44 | 3.84a,b |
| Superior medial gyrus | Right | 14 | 48 | 46 | 3.58a,b | ||
| Cluster 2 | Temporoparietal junction | Right | 395 | 44 | −54 | 28 | 4.42a,b |
| Cluster 3 | Superior frontal gyrus | Right | 214 | 20 | 64 | 16 | 4.04a |
| Cluster 4 | Cuneus | Right | 133 | 22 | −84 | 46 | 3.38a |
| Cluster 5 | Cerebellum | Left | 133 | −22 | −80 | −34 | 3.34a |
| SZ(TASK > CONT) > HC(TASK > CONT) | |||||||
| Cluster 1 | Posterior-medial frontal gyrus | Left | 667 | −4 | −8 | 54 | 3.91a,b |
| Cluster 2 | Precentral gyrus | Left | 297 | −26 | −18 | 74 | 3.58a |
Note: All reported regions were corrected for multiple comparisons on a cluster threshold of P < .05.
aVoxel threshold: P = .005, k = 67 voxel.
bVoxel threshold: P = .001, k = 47 voxel.
Coordinates are reported in MNI space.
fMRI = functional magnetic resonance imaging.
Functional Activity Differences: HC (Task > CONT) vs SZ (Task > CONT)
Between-group differences existed in the rTPJ, the right superior frontal/medial frontal gyrus and the right posterior-medial frontal gyrus, left the precentral gyrus, the left cerebellum, and the right cuneus (table 1, figure 2). Post hoc t tests revealed that patients showed lower neural responses in the rTPJ, the superior/medial frontal gyrus, the cuneus, and the cerebellum during the task (table 1). The inverse contrast revealed that patients demonstrate the opposite activation pattern compared to the controls in the posterior-medial frontal gyrus (figure 2C).
rTPJ—Functional Connectivity
Within-Group Contrast: HC (Task > CONT).
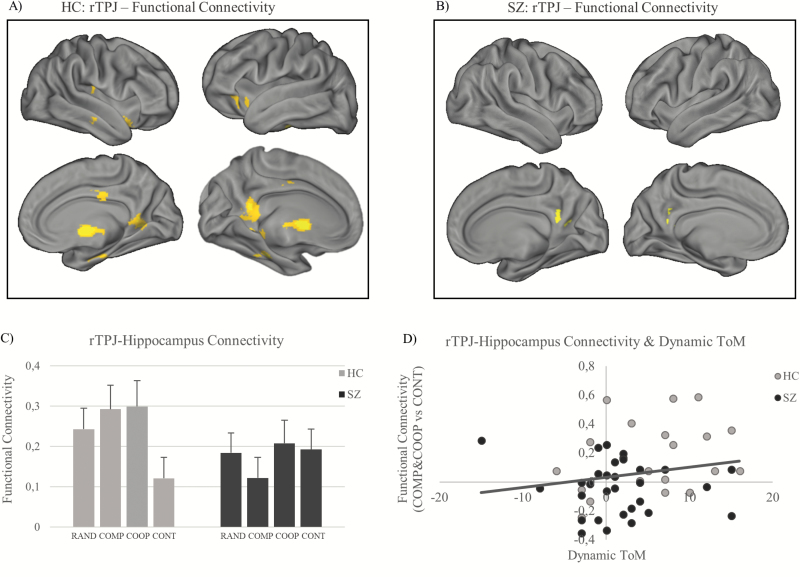
In controls, the rTPJ was functionally connected with multiple regions relevant for ToM and memory formation processes such as the left hippocampus, the left fusiform gyrus (FFG), the PCC, the precuneus, the left thalamus, the right parahippocampal gyrus, the right amygdala, the left IFG, the bilateral insula, and the MCC (table 2).
Table 2.
Results of the rTPJ Functional Connectivity and Structural Covariance Analysis
| Group/Contrast | Anatomical region | Hemisphere | Voxel per cluster | x | y | z | t value |
|---|---|---|---|---|---|---|---|
| Functional connectivity | |||||||
| HC:TASK > CONT | |||||||
| Cluster 1 | Precuneus | Left | 699 | −6 | −52 | 14 | 3.76a,b |
| Calcarine gyrus | Right | 20 | −50 | 8 | 3.76a | ||
| Cerebellar vermis | Right | 6 | −54 | 6 | 3.68a,b | ||
| Posterior cingulate cortex | Right | 14 | −42 | 10 | 3.61a | ||
| Lingual gyrus | Left | −6 | −46 | 2 | 2.90a | ||
| Cluster 2 | Cerebellum | Left | 671 | −6 | −44 | −46 | 3.40a,b |
| Cluster 3 | Thalamus (temporal) | Left | 300 | −2 | −6 | 0 | 4.68a,b |
| Thalamus (prefrontal) | Left | −2 | −20 | 0 | 3.30a,b | ||
| Cluster 4 | Fusiform gyrus | Left | 282 | −28 | −30 | −20 | 4.09a,b |
| Hippocampus | Left | −22 | −36 | −2 | 3.34a,b | ||
| Precuneus | Left | −16 | −42 | 0 | 2.95a | ||
| Cluster 5 | Inferior frontal gyrus | Left | 199 | −38 | 22 | −16 | 3.65a |
| Insula | Left | −34 | 14 | −12 | 3.32a | ||
| Cluster 6 | Parahippocampal gyrus (Subiculum) | Right | 157 | 22 | −20 | −22 | 3.29a |
| Parahippocampal gyrus (Amygdala) | Right | 20 | −8 | −22 | 3.25a | ||
| Cluster 7 | Middle temporal gyrus | Right | 124 | 56 | −14 | −16 | 3.55a |
| Cluster 8 | Insula | Right | 121 | 32 | 14 | −20 | 3.46a |
| Cluster 9 | MCC | Right | 104 | 6 | −20 | 30 | 3.29a |
| MCC | Left | −2 | −16 | 40 | 2.85a | ||
| Cluster 10 | Insula | Right | 74 | 34 | −20 | 16 | 3.15a |
| Area T 1.1 | Right | 42 | −22 | 12 | 3.15a | ||
| SZ:TASK > CONT | |||||||
| Cluster 1 | Posterior cingulate cortex | Left | 776 | −12 | −48 | 20 | 3.91a,b |
| Precuneus | Left | −12 | −46 | 14 | 3.83a,b | ||
| Cluster 2 | Precuneus | Right | 620 | 12 | −50 | 22 | 4.36a,b |
| Cluster 3 | Middle temporal gyrus | Right | 80 | 62 | −54 | 16 | 3.56a |
| Superior temporal gyrus | Right | 60 | −48 | 18 | 3.17a | ||
| Cluster 4 | Thalamus (prefrontal) | Right | 67 | 20 | −6 | 10 | 3.95a |
| HC(TASK > CONT) > SZ(TASK > CONT) | |||||||
| Cluster 1 | Cerebellum (IX) | Right | 369 | 10 | −44 | −40 | 4.19a |
| Cluster 2 | Middle temporal gyrus | Right | 177 | 60 | −12 | −16 | 3.59a |
| Superior temporal gyrus | Right | 54 | 2 | −12 | 2.77a | ||
| Cluster 3 | Fusiform gyrus | Left | 88 | −28 | −34 | −22 | 3.64a |
| Fusiform gyrus | Left | −30 | −30 | −20 | 3.51a | ||
| Hippocampus (DG, CA1, Subiculum) | Left | ||||||
| Cluster 4 | Precentral gyrus | Right | 82 | 16 | −18 | 80 | 3.67a |
| Cluster 5 | Superior temporal gyrus | Right | 77 | 52 | −28 | 10 | 3.53a |
| Heschls gyrus (Area OP1) | Right | 46 | −24 | 14 | 3.07a | ||
| SZ(TASK > CONT) > HC(TASK > CONT) | |||||||
| Cluster 1 | Superior parietal lobule | Right | 105 | 22 | −74 | 60 | 3.66a |
| Cluster 2 | Superior parietal lobule | Left | 84 | −38 | −48 | 60 | 2.99a |
| Inferior parietal lobule | Left | −48 | −44 | 60 | 2.72a | ||
| Cluster 3 | Temporoparietal junction | Right | 67 | ||||
| Superior temporal gyrus | Right | 60 | −46 | 18 | 3.39a | ||
| Middle temporal gyrus | Right | 64 | −54 | 16 | 3.21a | ||
| Supramarginal gyrus | Right | 62 | −44 | 26 | 3.16a | ||
| Structural covariance conjunction | |||||||
| Cluster 1 | Superior temporal gyrus | Right | 8280 | 60 | −41 | 15 | 16.42 |
| Supramarginal gyrus | Right | 59 | −36 | 27 | 6.98 | ||
| Area Ig1 | Right | 39 | −24 | −5 | 4.91 | ||
| Cluster 2 | Area Ig1/2 | Left | 2633 | −38 | −26 | −3 | 5.21 |
| Area Id1 | Left | −39 | −17 | −11 | 5.20 | ||
| Thalamus | Left | −30 | −27 | 3 | 4.74 | ||
| Middle temporal gyrus | Left | −45 | −48 | 6 | 4.28 | ||
| Area Ig1 | Left | −32 | −23 | 6 | 4.02 | ||
| Cluster 3 | Inferior parietal lobule | Left | 1020 | −45 | −53 | 36 | 4.58 |
| Cluster 4 | Inferior occipital gyrus | Left | 888 | −51 | −69 | −6 | 4.74 |
| Fusiform gyrus | Left | −39 | −65 | −14 | 4.54 | ||
| Inferior occipital gyrus | Left | −47 | −63 | −15 | 4.14 | ||
| Cluster 5 | Insula | Right | 654 | 42 | 10 | 7 | 3.96 |
| Inferior frontal gyrus | Right | 36 | 27 | 8 | 3.71 | ||
| Cluster 6 | Inferior frontal gyrus | Left | 393 | −36 | 30 | 8 | 4.12 |
| Insula | Left | −27 | 23 | 6 | 3.35 | ||
| Cluster 7 | Precuneus | Right | 267 | 6 | −66 | 24 | 3.74 |
| Cluster 8 | Inferior parietal lobe | Right | 260 | 39 | −38 | 50 | 5.00 |
Note: All reported regions were corrected for multiple comparisons on a cluster threshold of P < 0.05. aVoxel threshold: P = .005, k = 67 voxel.
bVoxel threshold: P = .001, k = 47 voxel.
Regions without coordinates represent activated areas within the cluster as indicated by probabilistic cytoarchitectonic mapping.51 The structural covariance analysis is corrected on a cluster threshold P < 0.05 (FWE, voxel threshold P = .001). Coordinates are reported in MNI space.
rTPJ = right temporoparietal junction.
Within-Group Contrast: SZ (Task > CONT).
In patients, the rTPJ was functionally connected with the PCC, the precuneus, the right middle temporal gyrus (MTG), the right superior temporal gyrus (STG), and the thalamus (table 2).
Common Functional Connectivity: HC (Task > CONT)∩SZ(Task > CONT).
A conjunction analysis showed no suprathreshold commonalities in functional connectivity between groups.
Functional Connectivity Differences: HC (Task > CONT) > SZ (Task > CONT).
In controls, the rTPJ was stronger connected with the left FFG/hippocampus, the right MTG, the right STG, the right precentral gyrus, and the right cerebellum (table 2).
Functional Connectivity Differences: SZ (Task > CONT)> HC (Task > CONT).
In patients, the rTPJ was stronger connected with the bilateral superior parietal lobule, the inferior parietal lobe, and parts of the rTPJ (table 2).
rTPJ–Hippocampus Functional Connectivity—Dynamic ToM.
Across groups, the functional connectivity between the rTPJ and the hippocampus was significantly associated with the participants’ behavioral adaptation between the intentionally different counterparts (r = .274, P = .026, 1 tailed) (figure 3D).
Fig. 3.
Whole-brain rTPJ functional connectivity in controls (A) and patients (B). The SPM is corrected for multiple comparisons on cluster level P < .05 (voxel threshold: P = .005, cluster extent: k = 67). (C) Functional rTPJ–hippocampus connectivity in all conditions in both groups. (D) Across groups, rTPJ–hippocampus connectivity in COMP and COOP (vs CONT) is significantly associated with participants’ behavioral adaptation between counterparts (Dynamic-ToM). rTPJ, right temporoparietal junction; ToM, Theory of Mind.
rTPJ—Structural Covariance
Common Structural Covariance: HC (Task > CONT)∩SZ(Task > CONT).
The rTPJ gray matter density correlated with the gray matter density of other brain regions in the ToM network such as the bilateral STG, the precuneus, the bilateral insula, the bilateral IFG, the left MTG, and the left FFG in both groups to a similar extent (table 2).
Structural Covariance Differences Between Groups.
No suprathreshold differences in structural covariance existed between groups.
rTPJ–Hippocampus Structural Connectivity—ROI Analysis.
The hippocampus region that was functionally disconnected with the rTPJ in patients showed a trend toward alterations on a structural level as well as lower gray matter covariance in patients compared to healthy controls indicates (t = 1.43, P = .080).
Discussion
In this study, we tested whether neural mechanisms relevant for constructing and retrieving other peoples’ mental states are altered in schizophrenia using a social decision-making game. Our data show that patients adapted their social decisions less often between intentionally different counterparts, pointing on an impaired updating of mental representations of the playing partners’ different intentions. Activation differences point on reduced activation in core ToM brain regions such as the rTPJ and the mPFC in patients with schizophrenia. However, functional connectivity analysis suggests that differences in representing the playing partners’ intentions might arise due to a dysfunctional communication between the rTPJ and a temporal lobe network consisting of the hippocampus, the FFG, and the MTG. Remarkably, participants’ adaptive decisions between the counterparts were associated with a higher rTPJ–hippocampus coupling, indicating that in healthy subjects the drawing of social inferences is more enriched by previous experiences. On the basis of these results, we suggest that a dysfunctional integration of a memory-informed pathway into the rTPJ might underlie inaccurate associations of others’ intentions that can be observed in patients with impaired7,8 and exaggerated ToM processes.6,11
Schizophrenia has been associated with a diminished learning-dependent updating of beliefs, resulting in an aberrant sensory integration of the current environment.54–56 The rTPJ underlies such a cognitive process in which previous mental representations are recalibrated by novel information in the present context.47,57,58 Previous research showed that the rTPJ is a convergence zone of memory and attention-processing streams, which are recruited when the context requires these information49,59 in order to form the current social environment.46,60 These processes are essential during the formation of a coherent concept of other peoples’ traits, intentions, and feelings, which is the basis for adaptive social behavior.12,14,60 Our data indicate that in patients with schizophrenia the rTPJ fails to integrate such a memory-informed pathway from temporal lobe regions, such as the hippocampus, the FFG, and the MTG, indicating that rTPJ computations may be less enriched by prior information.
Meta-analytical evidence indicates that healthy persons show increased neural activity in the FFG and MTG during ToM processes,17 probably because the FFG61 and the MTG62 inform social cognition by semantic knowledge.63 In patients with first-episode schizophrenia as well as in chronic patients, structural alterations in the FFG64 and the MTG65 have been reported,66–68 pointing to distinct alterations in brain regions relevant for memory-informed ToM processes in schizophrenia.
Recently, however, it has been shown that the hippocampus is particularly relevant in social cognitive processes, because it enriches these with declarative knowledge from recent social experiences.12,16,18–20 Along this line, deficits in ToM processes have been found in patients with temporal lobe atrophy69 in similar brain regions we found in this study such as the hippocampus and the FFG, pointing on a relevance to link previous with current social information during mental state inferences. It can be assumed that the hippocampus relevance during ToM processes arises due to its function to flexibly recombine and adapt previous concepts in novel situations,33,34 which is relevant to create a consistent person schema18 and adaptive social behavior.12 Hippocampal alterations are one of the most consistent neurobiological alterations found in schizophrenia frequently located in the left hemisphere70–73 and present on a functional74 and an anatomical level.35,36 Previous studies in patients with schizophrenia indicate that a hippocampal discoupling is associated with impairments in memory-informed processes,75–77 echoing our results that impaired rTPJ–hippocampus connectivity is associated with deficits in forming representations of other peoples’ intentions.
Our neurobiological findings agree with theoretical assumptions30,31 and empirical evidence31,32 that ToM impairments in schizophrenia could arise due to a deficit in retrieving autobiographical memory information, which has been linked in healthy persons to an intact rTPJ and hippocampus functioning.19 Importantly, failures to reactivate prior social knowledge appropriately could be associated with previous findings of delayed ToM processes in schizophrenia78 and challenge assumptions that impairments in social cognitive processes are independent from general cognitive (ie, memory) processes.79
Further studies have yet to test how an insufficient access to short- or long-term information, that might be associated with a dysfunctional rTPJ updating and hence an incorrect model of the current environment,80 is related to alterated social cognitive processes in specific subpopulations in schizophrenia. Because hippocampal damage is associated with a more polarized evaluation of other peoples’ behavior, which might be associated with an impaired integration of contextual information during social inference processes,81 we expect that difficulties in forming an adequate representation of others’ mental states are relevant in deluded patients but also in patients with restricted mentalizing processes.
Remarkably, the rTPJ has been shown to connect with task-specific networks depending on the current cognitive process,59 pointing on the regions functional relevance to engage different brain regions.46,49 Functional connectivity might depend on the underlying anatomical network82,83 that has been linked to cognitive functioning.53 Our data show that the gray matter density between the rTPJ co-varied with regions implicated in ToM processes17 such as the STG, the insula, the precuneus, the IFG, the MTG, and the thalamus across groups. This finding indicates high similarities in anatomical network architecture. From a global perspective, the results raise the idea that alterations in patients’ mental state inferences are a matter of a rTPJ deficit to engage distant brain regions by intact structural underpinnings.
The study findings must be interpreted in light of some limitations. First, the majority of our patients received stable antipsychotic treatment, which has been found to influence functional connectivity84 and brain tissue.85 Therefore, further studies have to test to what extent intake duration and dosage influence the cognitive mechanisms reported here. Second, we did not collect data about participants’ real-life social functioning, which might provide further insight how alterations in the identified biomarkers impact social life. In addition, it should be considered that a functional connectivity analysis and the resulting conclusions are limited to a predefined seed region. Further studies might examine functional integration between brain regions relevant for ToM within a network approach, thereby characterizing the communication between the network nodes. Given the relevance of functional integration for the pathophysiology of schizophrenia,86,87 this will hopefully further progress the understanding whether neural pathways underlying ToM are different or less effective in patients.
In sum, our data indicate that schizophrenia is associated with deficits in memory-informed updating of mental representations during the construction of other peoples’ mental states. The interaction between the rTPJ and the left hippocampus seems to have a specific relevance during this process and warrants future exploration in specific patient groups.
Supplementary Material
References
- 1. Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16:559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15:663–668. [DOI] [PubMed] [Google Scholar]
- 3. Brüne M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31:21–42. [DOI] [PubMed] [Google Scholar]
- 4. Burns J. The social brain hypothesis of schizophrenia. World Psychiatry. 2006;5:77–81. [PMC free article] [PubMed] [Google Scholar]
- 5. Frith CD. The Cognitive Neuropsychology of Schizophrenia. New York: Psychology press; 1992. [Google Scholar]
- 6. Frith CD. Schizophrenia and theory of mind. Psychol Med. 2004;34:385–389. [DOI] [PubMed] [Google Scholar]
- 7. Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109:1–9. [DOI] [PubMed] [Google Scholar]
- 8. Sprong M, Schothorst P, Vos E, Hox J, van Engeland H. Theory of mind in schizophrenia: meta-analysis. Br J Psychiatry. 2007;191:5–13. [DOI] [PubMed] [Google Scholar]
- 9. Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. [DOI] [PubMed] [Google Scholar]
- 10. American Psychological Association. Diagnostic and statistical manual of mental disorders (DSM-5®). Arlington, VA: American Psychiatric Pub; 2013. [Google Scholar]
- 11. Abu-Akel A, Bailey AL. The possibility of different forms of theory of mind impairment in psychiatric and developmental disorders. Psychol Med. 2000;30:735–738. [DOI] [PubMed] [Google Scholar]
- 12. Bitsch F, Berger P, Nagels A, Falkenberg I, Straube B. The role of the right temporo-parietal junction in social decision-making. Hum Brain Mapp. 2018;39:3072–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown EC, Brüne M. Evolution of social predictive brains?Front Psychol. 2012;3:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koster-Hale J, Saxe R. Theory of mind: a neural prediction problem. Neuron. 2013;79:836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hassabis D, Spreng RN, Rusu AA, Robbins CA, Mar RA, Schacter DL. Imagine all the people: how the brain creates and uses personality models to predict behavior. Cereb Cortex. 2014;24:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Montagrin A, Saiote C, Schiller D. The social hippocampus. In: Hippocampus. In press. [DOI] [PubMed] [Google Scholar]
- 17. Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev. 2014;42:9–34. [DOI] [PubMed] [Google Scholar]
- 18. Laurita AC, Spreng RN. The hippocampus and social cognition. In: Hannula DE, Duff MC, eds. The Hippocampus from Cells to Systems. Cham: Springer International Publishing; 2017:537–558. [Google Scholar]
- 19. Spreng RN, Mar RA. I remember you: a role for memory in social cognition and the functional neuroanatomy of their interaction. Brain Res. 2012;1428:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tavares RM, Mendelsohn A, Grossman Y, et al. A map for social navigation in the human brain. Neuron. 2015;87:231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wible CG. Hippocampal temporal-parietal junction interaction in the production of psychotic symptoms: a framework for understanding the schizophrenic syndrome. Front Hum Neurosci. 2012;6:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. [DOI] [PubMed] [Google Scholar]
- 23. Lee J, Quintana J, Nori P, Green MF. Theory of mind in schizophrenia: exploring neural mechanisms of belief attribution. Soc Neurosci. 2011;6:569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Das P, Lagopoulos J, Coulston CM, Henderson AF, Malhi GS. Mentalizing impairment in schizophrenia: a functional MRI study. Schizophr Res. 2012;134:158–164. [DOI] [PubMed] [Google Scholar]
- 25. Brüne M, Ozgürdal S, Ansorge N, et al. An fMRI study of “theory of mind” in at-risk states of psychosis: comparison with manifest schizophrenia and healthy controls. Neuroimage. 2011;55:329–337. [DOI] [PubMed] [Google Scholar]
- 26. Dodell-Feder D, Tully LM, Lincoln SH, Hooker CI. The neural basis of theory of mind and its relationship to social functioning and social anhedonia in individuals with schizophrenia. Neuroimage Clin. 2014;4:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLoS One. 2011;6:e25322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brüne M, Lissek S, Fuchs N, et al. An fMRI study of theory of mind in schizophrenic patients with “passivity” symptoms. Neuropsychologia. 2008;46:1992–2001. [DOI] [PubMed] [Google Scholar]
- 29. Crespi B, Badcock C. Psychosis and autism as diametrical disorders of the social brain. Behav Brain Sci. 2008;31:241–61; discussion 261. [DOI] [PubMed] [Google Scholar]
- 30. Corcoran R. Theory of Mind and Schizophrenia. Washington, DC: American Psychological Association; 2001. [Google Scholar]
- 31. Corcoran R, Frith CD. Autobiographical memory and theory of mind: evidence of a relationship in schizophrenia. Psychol Med. 2003;33:897–905. [DOI] [PubMed] [Google Scholar]
- 32. Mehl S, Rief W, Mink K, Lüllmann E, Lincoln TM. Social performance is more closely associated with theory of mind and autobiographical memory than with psychopathological symptoms in clinically stable patients with schizophrenia-spectrum disorders. Psychiatry Res. 2010;178:276–283. [DOI] [PubMed] [Google Scholar]
- 33. Buckner RL. The role of the hippocampus in prediction and imagination. Annu Rev Psychol. 2010;61:27–48, C1. [DOI] [PubMed] [Google Scholar]
- 34. Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: remembering, imagining, and the brain. Neuron. 2012;76:677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. [DOI] [PubMed] [Google Scholar]
- 36. Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. [DOI] [PubMed] [Google Scholar]
- 37. Wittchen HU, Zaudig M, Fydrich T.. Structured Clinical Interview for DSM-IV Axis I and II—SCID. Göttingen: Hogrefe; 1997. [Google Scholar]
- 38. Andreasen NC. Scale for Assessment of Positive Symptoms (SAPS). Iowa City: University of Iowa; 1984. [Google Scholar]
- 39. Andreasen NC. Scale for the assessment of negative symptoms (SANS): conceptual and theoretical foundations. J Neurosci. 1989;(25):8303–8310. [PubMed] [Google Scholar]
- 40. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 41. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. [DOI] [PubMed] [Google Scholar]
- 43. Slotnick SD, Moo LR, Segal JB, Hart J Jr. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res. 2003;17:75–82. [DOI] [PubMed] [Google Scholar]
- 44. Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bzdok D, Langner R, Schilbach L, et al. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage. 2013;81:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Krall SC, Rottschy C, Oberwelland E, et al. The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Struct Funct. 2015;220:587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krall SC, Volz LJ, Oberwelland E, Grefkes C, Fink GR, Konrad K. The right temporoparietal junction in attention and social interaction: a transcranial magnetic stimulation study. Hum Brain Mapp. 2016;37:796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schuwerk T, Schurz M, Müller F, Rupprecht R, Sommer M. The rTPJ’s overarching cognitive function in networks for attention and theory of mind. Soc Cogn Affect Neurosci. 2017;12:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schurz M, Perner J. An evaluation of neurocognitive models of theory of mind. Front Psychol. 2015;6:1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. [DOI] [PubMed] [Google Scholar]
- 52. Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. J Neurosci. 2005;25:8303–8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. Proc Natl Acad Sci U S A. 2010;107(42):18191–18196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. The computational anatomy of psychosis. Front Psychiatry. 2013;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10:48–58. [DOI] [PubMed] [Google Scholar]
- 56. Frith CD, Friston KJ. False perceptions and false beliefs: understanding schizophrenia. Neurosci Hum Pers New Perspect Hum Act. 2013;121:1–15. [Google Scholar]
- 57. Geng JJ, Vossel S. Re-evaluating the role of TPJ in attentional control: contextual updating?Neurosci Biobehav Rev. 2013;37:2608–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee SM, McCarthy G. Functional heterogeneity and convergence in the right temporoparietal junction. Cereb Cortex. 2016;26:1108–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn Sci. 2012;16:338–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carter RM, Huettel SA. A nexus model of the temporal-parietal junction. Trends Cogn Sci. 2013;17:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci U S A. 2002;99:15238–15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci U S A. 2007;104(15):6430–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee CU, Shenton ME, Salisbury DF, et al. Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59:775–781. [DOI] [PubMed] [Google Scholar]
- 65. Kuroki N, Shenton ME, Salisbury DF, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in first-episode schizophrenia: an MRI study. Am J Psychiatry. 2006;163:2103–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Onitsuka T, Shenton ME, Kasai K, et al. Fusiform gyrus volume reduction and facial recognition in chronic schizophrenia. Arch Gen Psychiatry. 2003;60:349–355. [DOI] [PubMed] [Google Scholar]
- 67. Nestor PG, Onitsuka T, Gurrera RJ, et al. Dissociable contributions of MRI volume reductions of superior temporal and fusiform gyri to symptoms and neuropsychology in schizophrenia. Schizophr Res. 2007;91:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Onitsuka T, Shenton ME, Salisbury DF, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am J Psychiatry. 2004;161:1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Duval C, Bejanin A, Piolino P, et al. Theory of mind impairments in patients with semantic dementia. Brain. 2012;135:228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Crow TJ. Temporal lobe asymmetries as the key to the etiology of schizophrenia. Schizophr Bull. 1990;16:433–443. [DOI] [PubMed] [Google Scholar]
- 71. Niemann K, Hammers A, Coenen VA, Thron A, Klosterkötter J. Evidence of a smaller left hippocampus and left temporal horn in both patients with first episode schizophrenia and normal control subjects. Psychiatry Res. 2000;99:93–110. [DOI] [PubMed] [Google Scholar]
- 72. Shenton ME, Kikinis R, Jolesz FA, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327:604–612. [DOI] [PubMed] [Google Scholar]
- 73. Zierhut KC, Graßmann R, Kaufmann J, Steiner J, Bogerts B, Schiltz K. Hippocampal CA1 deformity is related to symptom severity and antipsychotic dosage in schizophrenia. Brain. 2013;136:804–814. [DOI] [PubMed] [Google Scholar]
- 74. Zierhut K, Bogerts B, Schott B, et al. The role of hippocampus dysfunction in deficient memory encoding and positive symptoms in schizophrenia. Psychiatry Res. 2010;183:187–194. [DOI] [PubMed] [Google Scholar]
- 75. Bähner F, Meyer-Lindenberg A. Hippocampal-prefrontal connectivity as a translational phenotype for schizophrenia. Eur Neuropsychopharmacol. 2017;27:93–106. [DOI] [PubMed] [Google Scholar]
- 76. Genzel L, Dresler M, Cornu M, et al. Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biol Psychiatry. 2015;77:177–186. [DOI] [PubMed] [Google Scholar]
- 77. Meyer-Lindenberg AS, Olsen RK, Kohn PD, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. [DOI] [PubMed] [Google Scholar]
- 78. Pedersen A, Koelkebeck K, Brandt M, et al. Theory of mind in patients with schizophrenia: is mentalizing delayed?Schizophr Res. 2012;137:224–229. [DOI] [PubMed] [Google Scholar]
- 79. Montag C, Dziobek I, Richter IS, et al. Different aspects of theory of mind in paranoid schizophrenia: evidence from a video-based assessment. Psychiatry Res. 2011;186:203–209. [DOI] [PubMed] [Google Scholar]
- 80. Mengotti P, Dombert PL, Fink GR, Vossel S. Disruption of the right temporoparietal junction impairs probabilistic belief updating. J Neurosci. 2017;37:5419–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Croft KE, Duff MC, Kovach CK, Anderson SW, Adolphs R, Tranel D. Detestable or marvelous? Neuroanatomical correlates of character judgments. Neuropsychologia. 2010;48:1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1: 13–36. [DOI] [PubMed] [Google Scholar]
- 83. Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342:1238411. [DOI] [PubMed] [Google Scholar]
- 84. Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. [DOI] [PubMed] [Google Scholar]
- 85. Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30(2):115–125 [DOI] [PubMed] [Google Scholar]
- 87. Friston KJ, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2–3):83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.