Abstract
Despite mixed findings, increasing evidence suggests that people with first-episode psychosis (FEP) show increased pro-inflammatory and pro-oxidative status. We used the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines to conduct a systematic literature search of cross-sectional studies comparing in vivo inflammatory and oxidative blood markers between FEP patients and healthy controls. We analyzed 61 independent samples from 59 publications, including 3002 patients with FEP (ie, patients with FEP, early psychosis, first-episode schizophrenia or early schizophrenia) and 2806 controls. After controlling for multiple comparisons, our meta-analysis showed that total antioxidant status and docosahexaenoic acid levels were significantly lower in FEP patients than in controls, whereas levels of homocysteine, interleukin-6 and tumor necrosis factor alpha were significantly higher in FEP patients than in controls. This suggests that FEP patients had reduced antioxidant status and a pro-inflammatory imbalance, and that these biological processes may be targets for managing FEP.
Keywords: schizophrenia, first-episode psychosis, biomarker, meta-analysis, inflammatory, oxidation, cytokine
Introduction
Psychotic disorders are a group of heterogeneous syndromes with indeterminate neurobiological mechanisms.1 The onset of a first-episode psychosis (FEP) is usually preceded for many years by several underlying biological processes at both the peripheral level and the central nervous system, including an increased systemic pro-inflammatory and pro-oxidative status.2–4
Oxidative stress and inflammation may play a major role in the onset and development of numerous disorders.5 The most parsimonious model of the link between inflammatory and oxidative activation and psychosis proposes that psychosis could be, at least in part, a consequence of a homeostatic signaling imbalance caused by oxidative stress and inflammation-associated factors, perhaps due to an immune dysregulation.6 Indeed, genome-wide association and postmortem studies suggest that immune dysregulation could be a core feature of the illness.7–10 This model suggests that oxidative and inflammation imbalances, such as the release of pro-inflammatory cytokines and the activation of microglia, would be convergent outcomes of multiple genetic and early environmental factors which, in turn, may promote the appearance of psychosis-related brain changes, such as deficits in neural connectivity and clinical symptoms.11–13
The last meta-analyses of inflammatory and oxidative markers in FEP patients found higher levels of some cytokines and oxidative stress markers in FEP patients than in controls, suggestive of an increased pro-inflammatory and pro-oxidative status.14–18 Since the publication of those meta-analyses more than 20 publications have compared inflammatory and/or oxidative markers in patients with psychosis and controls. An updated meta-analysis incorporating new findings on blood inflammatory and/or oxidative markers in FEP patients can add to the body of evidence about the involvement of inflammatory processes in the pathophysiology of psychosis.
Methods
Search Strategies
Using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines, we conducted a systematic 2-step literature search to identify appropriate studies.19 First, we searched PubMed from inception to October 2016 for studies comparing inflammatory and oxidative markers in patients with FEP and healthy controls. The initial search covered the combination of 2 concepts: “first episode psychosis” (OR “first-episode psychosis” OR “early schizophrenia” OR “first episode schizophrenia” OR “early psychosis”) AND “inflammation” (OR “inflamm*” OR “inflammatory” OR “anti-inflammatory” OR “cytokine” OR “interleukin” OR “interferon” OR “tumor necrosis factor” OR “oxidat*” OR “oxidation” OR “oxidative”). Second, we checked the reference lists of the selected articles for any additional relevant studies.
Selection Criteria
The initial literature search yielded 2468 results. After removing duplicates, we evaluated 601 potential studies.
We double-screened all papers in 3 phases and resolved discrepancies through discussion and consensus to identify original peer-reviewed articles published in English assessing in vivo blood inflammatory or oxidative markers of patients with FEP and healthy controls. See supplementary table 1 for additional details on phases 1 and 2. Of the 601 studies, 463 met phase 1 exclusion criteria, leaving 138 for inclusion in phase 2. Of those, 70 met the phase 2 exclusion criteria, leaving 68 for inclusion in phase 3.
Phase 3 screening involved identifying papers with overlapping samples to ensure that the final set of papers included independent samples. We excluded overlapping studies if they assessed the same markers. The hierarchical criteria we used were: (1) study with the largest FEP group sample; (2) study with the largest control group sample; (3) most recent publication. We performed meta-analyses when data from at least 3 studies were available for the outcome variable. Of the 68 studies, 59 contained at least 1 marker for which there was data available from 3 or more non-overlapping samples and thus qualified for the meta-analysis. Two studies reported separate information from 2 independent samples, so we included 61 independent samples from those 59 studies.
We considered a sample as FEP if it was described in the article as “first-episode,” using any one of the following terms: first-episode psychosis, FEP, early psychosis, first episode schizophrenia, first episode schizophrenia (FES), or early schizophrenia. If the study contained a mixture of first-episode and multi-episode participants, data on the FEP group had to be reported separately. We considered a specific measurement to be an inflammatory/anti-inflammatory or oxidative/anti-oxidative marker if its role in inflammation and/or oxidative/nitrosative stress processes had been previously described.20 We included only studies measuring in vivo inflammatory and oxidative markers in plasma, serum, peripheral blood mononuclear cells (PBMCs) and red blood cells (RBCs) because there were insufficient data for meta-analysis of parameters in other sources, such as cerebrospinal fluid, platelets or postmortem brain tissue. Based on previous findings reporting inverse results in oxidative markers from plasma/serum and RBCs for some markers,14 we conducted the meta-analyses by dividing the sample sources into 2 groups: serum/plasma and cells (PMBCs or RBCs). We categorized studies using whole blood as serum/plasma.
Figure 1 shows the flowchart of the systematic literature search strategy.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram of the systematic literature search strategy. aTwo papers reported results from 2 independent samples each, yielding a final number of 61 independent samples from 59 records.
Quality Assessment
We assessed the quality of the 59 studies using a checklist constructed specifically for this review, based on a previous meta-analysis.14 We rated each study on a scale of 0 to 10, with higher values reflecting greater quality (supplementary table 2).
Data Extraction
Data extracted from each eligible study included: denomination of inflammatory and/or oxidative marker; first author; location and year of the study; study design; fasting status at blood extraction (as yes/no); number of FEP patients; number of controls; mean and SD for each inflammatory and/or oxidative marker (for both FEP patients and controls) and/or statistical comparison of each marker between FEP patients and controls. We also extracted putative moderator data from FEP patients, controls or both, as appropriate: mean age; sex (as percentage of male); diagnosis (as percentage of schizophrenia); mean body mass index (BMI); tobacco users (as percentage); cannabis use/abuse (as percentage); substance use/abuse (as percentage); exclusion of medical comorbidity (as yes/no); exclusion of patients treated with nonsteroidal anti-inflammatory drugs (NSAID)/anti-inflammatory drugs (as yes/no); mean duration of untreated psychosis (DUP); mean duration of illness; antipsychotic dose at blood extraction (as chlorpromazine equivalents); and type of antipsychotic (as first/second generation).
Statistical Analysis
We entered the data into an electronic database and analyzed it with a quantitative meta-analytical approach using Comprehensive Meta-Analysis (CMA) Software version 3 (Biostat, Inc.). We calculated effect sizes (ES) based on Cohen’s and their 95% CIs for every individual marker, considering Cohen’s d values from 0.1 to 0.5 small, values from 0.6 to 0.8 moderate, and greater than 0.8 large.21 Based on the known heterogeneity of studies, we used random-effects models by Der-Simonian and Laird.22 We checked the rigor of the principal findings by performing a jackknife sensitivity analysis, which consists of iteratively repeating the meta-analysis excluding one study at a time to establish whether the results are replicable.23
We assessed statistical heterogeneity through visual inspection of forest plots and using the Q statistic (a magnitude of heterogeneity) and the I2 statistic (a measure of the proportion of variance in the summary effect size attributable to heterogeneity).24 We considered I2 values greater than 50% to be significant heterogeneity not attributable to random error.25 To check for publication bias, we visually inspected funnel plots and used Orwin’s fail-safe N.26 This generated the number of missing or unpublished studies required to move ES to irrelevant values (Cohen’s d under 0.1). We used Egger’s linear regression method to quantify the bias captured by the funnel plot.27 When the funnel plot or test statistics suggested publication bias, we used the Duval and Tweedie trim-and-fill method to estimate an effect size corrected for publication bias.28
We performed meta-regressions using a random-effects model with unrestricted maximum likelihood to test effects of moderators on estimates with significant meta-analyses. The slope of meta-regression line – β coefficient: direct (+) or inverse (−) – indicates the strength of the relationship between moderator and outcome. We performed meta-regressions when at least 4 studies were available for the same moderator variable per each outcome.
In order to confirm the robustness of the main findings, we conducted subgroup sensitivity analyses including only studies with quality score equal to or higher than 5. We rated as robust results in which the direction and the magnitude of the relationship remained in these subgroup analyses.
Considering the heterogeneity of definitions of FEP in the included studies and the potential impact of previous antipsychotic exposure on the findings of oxidative and inflammatory parameters, we conducted an additional sensitivity subgroup analysis including only data from the antipsychotic-naïve samples.
As there is not a clear frontier between inflammatory and anti-inflammatory markers,3,20 we assessed each specific marker independently and did not conduct meta-analyses assessing pro-inflammatory and anti-inflammatory markers as a group.
The significance level was set at P = .05. To limit the risk of false positive (type I) errors in meta-analyses arising from multiple comparisons, we corrected P-values using the Bonferroni-Holm method.29
Results
Sixty-one independent samples from 59 publications qualified for meta-analysis, with an overall sample of 3002 FEP patients and 2806 healthy controls. The mean age of FEP patients and controls ranged from 14.4 to 35.7 and from 12.7 to 46.5 years, respectively. The percentage of male patients and controls ranged from 40% to 100% and from 24% to 100%, respectively. The percentage of FEP patients with a schizophrenia diagnosis ranged from 15.6% to 100%. Details of the included studies are in supplementary table 3.
In total we performed 27 meta-analyses of cross-sectional comparisons of markers between FEP patients and controls: (1) in blood cells: catalase (CAT) and docosahexaenoic acid (DHA), and (2) in serum/plasma: C-reactive protein (CRP), dehydroepiandrosterone-sulfate (DHEA-S), glutathione (GSH), homocysteine (Hcy), interferon-γ (IFN-γ), interleukin-1α (IL-1α), interleukin-1β (IL-1β), interleukin-2 (IL-2), interleukin-3 (IL-3), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), interleukin-17 (IL-17), interleukin-18 (IL-18), lipid hydroperoxides (LOOH/Peroxides), total antioxidant status (TAS), thiobarbituric acid reactive substances (TBARS), tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF). Glutathione peroxidase (GPx) and superoxide dismutase (SOD) were measured both in blood cells and serum/plasma, so we conducted meta-analyses for each marker in each sample source separately.
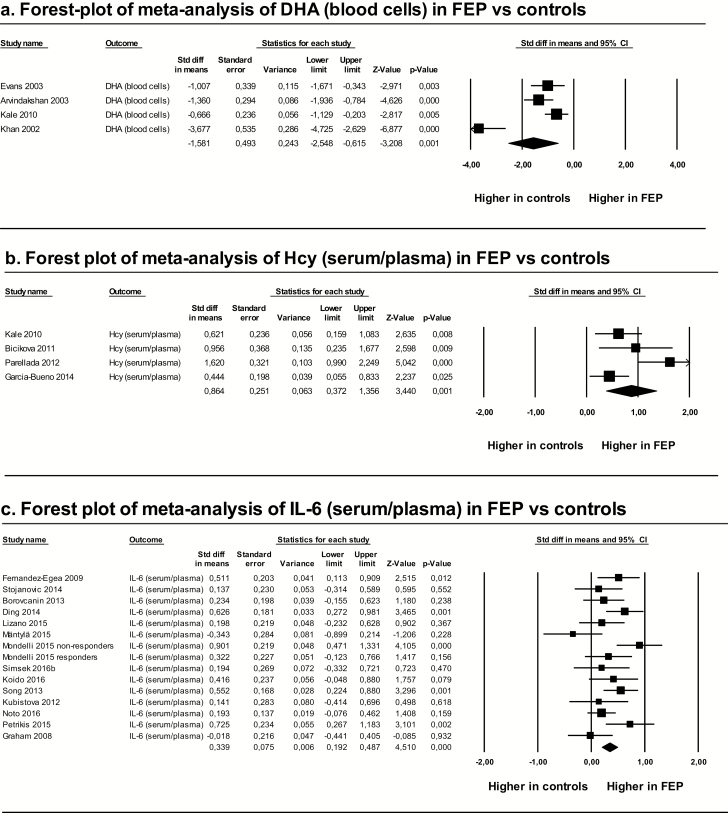
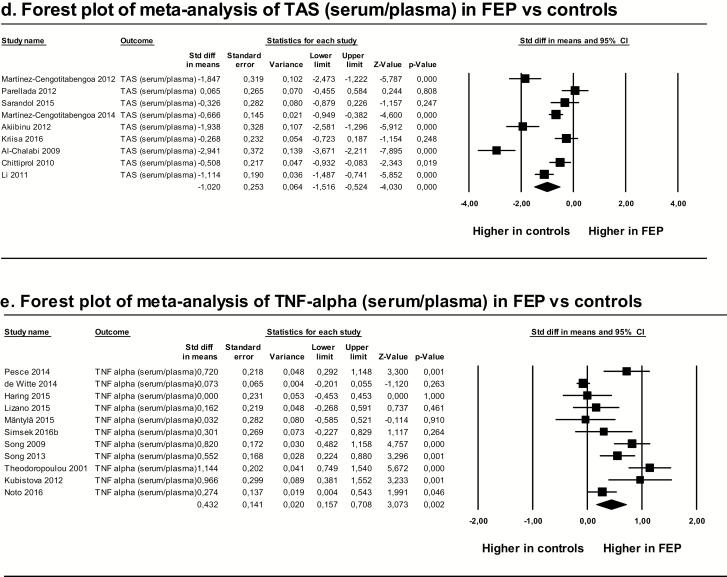
These meta-analyses showed that levels of DHA and TAS were significantly lower in FEP patients than in controls, whereas levels of Hcy, IL-6, and TNF-α were significantly higher in FEP patients than in controls. Heterogeneity among studies was high, with I2 values greater than 50%, except for IL-6, for which I2 was 46%. Risk of publication bias was low for DHA, Hcy, IL-6, and TAS, and high for TNF-α. Duval and Tweedie’s trim-and-fill method showed that the estimated effect sizes corrected for publication bias did not significantly differ from the observed ones (table 1 and figure 2).
Table 1.
Meta-analyses of Cross-Sectional Comparisons of Oxidative/Inflammatory Markers Between FEP Patients and Controls
| Marker (Source) | # Studies | N FEP | N Control | Effect Size (FEP vs Controls) | ||
|---|---|---|---|---|---|---|
| Cohen’s d (95% CI) | Z value | P value* | ||||
| CAT (blood cells) | 5 | 191 | 202 | −0.308 (−0.890 to 0.274) | −1.037 | >.995 |
| CRP (serum/plasma) | 5 | 276 | 189 | 0.996 (−0.179 to 2.720) | 1.661 | >.995 |
| DHA (blood cells) | 4 | 89 | 134 | −1.581 (−2.548 to −0.615) | −3.208 | .032 a |
| DHEA-S (serum/plasma) | 3 | 89 | 74 | 0.449 (−1.014 to 1.912) | 0.601 | >.995 |
| GPx (blood cells) | 6 | 185 | 209 | 1.294 (−0.121 to 2.709) | 1.792 | >.995 |
| GPx (serum/plasma) | 3 | 88 | 87 | −0.521 (−1.170 to 0.128) | −1.574 | >.995 |
| GSH (serum/plasma) | 4 | 185 | 194 | −0.274 (−0.819 to 0.270) | −0.988 | >.995 |
| Hcy (serum/plasma) | 4 | 138 | 140 | 0.864 (0.372 to 1.356) | 3.440 | .015 b |
| IFN-γ (serum/plasma) | 10 | 565 | 696 | 0.262 (0.056 to 0.468) | 2.498 | .237 |
| IL-1α (serum/plasma) | 3 | 241 | 393 | 0.767 (−0.659 to 2.193) | 1.054 | >.995 |
| IL-1β (serum/plasma) | 9 | 472 | 434 | 0.396 (0.062 to 0.729) | 2.326 | .340 |
| IL-2 (serum/plasma) | 11 | 390 | 411 | −0.191 (−0.581 to 0.199) | −0.961 | >.995 |
| IL-3 (serum/plasma) | 3 | 116 | 88 | −0.906 (−1.527 to −0.284) | −2.857 | .094 |
| IL-4 (serum/plasma) | 7 | 314 | 289 | 0.247 (−0.027 to 0.520) | 1.768 | >.995 |
| IL-6 (serum/plasma) | 15 | 879 | 648 | 0.339 (0.192 to 0.487) | 4.510 | <.001 c |
| IL-8 (serum/plasma) | 5 | 175 | 197 | 0.460 (0.093 to 0.826) | 2.459 | .251 |
| IL-10 (serum/plasma) | 11 | 764 | 821 | 0.203 (−0.051 to 0.457) | 1.569 | >.995 |
| IL-17 (serum/plasma) | 5 | 288 | 211 | −0.039 (−0.373 to 0.295) | −0.229 | >.995 |
| IL-18 (serum/plasma) | 3 | 272 | 441 | 0.047 (−0.163 to 0.256) | 0.439 | >.995 |
| LOOH/Peroxides (serum/plasma) | 4 | 193 | 205 | 2.567 (0.585 to 4.550) | 2.538 | .223 |
| SOD (blood cells) | 5 | 189 | 199 | −0.089 (−1.139 to 0.961) | −0.167 | .868 |
| SOD (serum/plasma) | 4 | 151 | 181 | −0.118 (−0.666 to 0.431) | −0.420 | >.995 |
| TAS (serum/plasma) | 9 | 387 | 386 | −1.020 (−1.516 to −0.524) | −4.030 | .001 d |
| TBARS (serum/plasma) | 6 | 213 | 218 | 0.958 (0.240 to 1.676) | 2.616 | .187 |
| TNF-α (serum/plasma) | 11 | 815 | 798 | 0.432 (0.157 to 0.708) | 3.073 | .049 e |
| Uric acid (serum/plasma) | 5 | 128 | 169 | −0.174 (−0.679 to 0.331) | −0.676 | >.995 |
| VEGF (serum/plasma) | 3 | 107 | 100 | −0.546 (−1.737 to 0.645) | −0.898 | >.995 |
Note: CAT, catalase; CRP, C-reactive protein; DHA, docosahexaenoic acid; DHEA-S, dehydroepiandrosterone-sulfate; FEP, first-episode psychosis; FSN, fail-safe number (number of missing studies needed to bring Hedges’s g under 0.1); GPx, glutathione peroxidase; GSH, glutathione; Hcy, homocysteine; IFN-γ, interferon-γ; IL-1α, interleukin-1α; IL-1β, interleukin-1β; IL-2, interleukin-2; IL-3, interleukin-3; IL-4, interleukin-4; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; IL-17, interleukin-17; IL-18, interleukin-18; LOOH/Peroxides, lipid hydroperoxides; N/A, not applicable; SOD, superoxide dismutase; TAS, total antioxidant status; TBARS, thiobarbituric acid reactive substances; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
aHeterogeneity markers: Q-value = 27.174, P-value < .001, I2 (%) = 88.960; Publication bias markers: Orwin’s FSN = 44, Egger’s regression test, P (2-tailed) = .068.
bHeterogeneity markers: Q-value = 10.325, P-value = .016, I2 (%) = 70.943; Publication bias markers: Orwin’s FSN = 26, Egger’s regression test, P (2-tailed) = .201.
cHeterogeneity markers: Q-value = 26.101, P-value = .025, I2 (%) = 46.363; Publication bias markers: Orwin’s FSN = 37, Egger’s regression test, P (2-tailed) = .487.
dHeterogeneity markers: Q-value = 79.857, P-value < .001, I2 (%) = 89.982; Publication bias markers: Orwin’s FSN = 66, Egger’s regression test, P (2-tailed) = .191.
eHeterogeneity markers: Q-value = 70.766, P-value < .001, I2 (%) = 85.869; Publication bias markers: Orwin’s FSN = 14, Egger’s regression test, P (2-tailed) = .029, Duval and Tweedie trim-and-fill method: Point estimate (95% CI): 0.432 (0.157 to 0.708).
*P-values were corrected using the Bonferroni-Holm method. Significant corrected P-values are in bold.
Fig. 2.
Forest plots of significant meta-analyses after Bonferroni-Holm correction. DHA, docosahexaenoic acid; FEP, first-episode psychosis; Hcy, homocysteine; IL-6, interleukin-6; TAS, total antioxidant status; TNF-α, tumor necrosis factor-α.
Meta-regression analyses showed that the effect sizes for the difference in DHA, TAS, Hcy, IL-6, and TNF-α between FEP patients and controls were not significantly associated with moderators (year of publication, quality, mean age, sex, diagnosis, BMI, tobacco use, cannabis use/abuse, substance use/abuse, exclusion of medical comorbidity and exclusion of NSAID/anti-inflammatory drugs), except for Hcy and quality, where higher quality was associated with lower effect size differences in Hcy between FEP and controls. We could not perform meta-regressions for mean DUP, mean duration of illness, type of antipsychotic, antipsychotic dose and antipsychotic monotherapy because there were fewer than 4 studies with data available for each of these variables for any marker (supplementary table 4).
Sensitivity subgroup meta-analyses including only studies with a quality score equal to or greater than 5 (yielding a total of 48 independent samples from 47 studies), confirmed the direction, magnitude and significance of the associations for TAS, Hcy, and IL-6. For TNF-α and DHA, there was little change in the direction and magnitude of the effect sizes for the differences between FEP patients and controls, but they lost significance (P = .090, and P = .147 after Bonferroni-Holm correction, respectively). Details of these subgroup meta-analyses are shown in supplementary table 5.
Sensitivity subgroup meta-analyses including only studies with antipsychotic-naïve FEP samples (yielding a total of 38 independent samples from 37 studies) confirmed the direction, magnitude, and significance of the associations for TAS, DHA, TAS, IL-6, and TNF-α. We could not perform meta-analyses for Hcy because data were not available from at least 3 independent studies. Further, we found that serum/plasma levels of uric acid were significantly lower in antipsychotic-naïve FEP patients than in controls (Cohen’s d = −0.602 (−0.917 to −0.287), P = .003) and that levels of IL-1β in serum/plasma were significantly greater in antipsychotic-naïve FEP patients than in controls (Cohen’s d = 0.726 (0.376 to 1.076), P = .001). Details of these subgroup meta-analyses are shown in supplementary table 6.
Discussion
This meta-analysis, based on cross-sectional comparisons between FEP patients and controls, included 61 independent samples from 59 studies with an overall sample of 3002 FEP patients and 2806 healthy controls. To our knowledge, this is the largest meta-analysis to date exploring differences in oxidative and inflammatory parameters between FEP patients and controls. We found that, relative to healthy controls, patients with FEP have lower antioxidant capacity (as measured by means of TAS), lower levels of DHA, and higher levels of Hcy, IL-6, and TNF-α, 3 markers usually considered pro-inflammatory. All the effect sizes for these differences were large, except for IL-6, which was only moderate. Sensitivity subgroup analyses based on quality scores suggested that these findings were robust.
Our results are consistent with previous findings of an increased pro-inflammatory and reduced antioxidant status in people with FEP,14–18 and provide further support for their involvement in the physiopathology of psychotic disorders. Recent data from studies conducted in earlier stages of the disease, such as the psychosis high-risk state, provide further evidence for that hypothesis.30–33 Our findings provide a partial confirmation of previous meta-analyses of first-episode schizophrenia,14,15,17 which also reported lower TAS and higher levels of TNF-α and IL-6 in FEP patients relative to controls. The lack of replication of some of the markers identified in these meta-analyses (eg, TGF-β, IL-12, IFN-γ, sIL-2R, catalase, nitrites, and SOD) could be due to different methodological approaches: we used a more restrictive P-value for identifying significant differences, and included studies conducted on different FEP populations and not FES-only populations. Differences in the degree of control for potentially confounding factors between earlier and later studies might also contribute to these differences. Meta-regression of Hcy studies suggested that the quality of the included studies may influence effect sizes of the differences between FEP and controls, since the higher the quality of the studies, the lower the differences found between FEP and controls. Indeed, among the studies included in our meta-analysis, quality was positively and significantly associated with the year of publication (r = .255, P = .047), so that more recent studies tended to show higher quality scores, indicative of better control for potentially confounding variables such as smoking, BMI and concomitant treatment with anti-inflammatory or antioxidant agents. Thus, our inclusion of more recent better-designed studies, could have led to smaller effect size estimates for some markers.
Interestingly, we were only able to replicate the finding of increased IL-1β from previous meta-analyses in the antipsychotic-naïve sample and not in the full sample. This suggests that antipsychotic treatment, and possibly clinical status, could affect the levels of some cytokines. Studies on oxidative and inflammatory imbalances in individuals with psychosis are still inconsistent regarding which factors are state-specific (specific to acute phases, first episode or early stages) and which are more durable and may be a trait of the psychotic condition. Miller’s and Goldsmith’s meta-analyses suggested that IL-6 levels might be state markers.15,17 Increases of IL-6 levels occur not only in acute states of schizophrenia or psychosis but also in acute states of bipolar disorder and major depressive disorder, suggesting that there may be a common stress-related phenomenon in acutely ill patients across different conditions.17,34 Conversely, TNF-α levels, which have consistently been reported to be elevated in people with FEP in comparison with controls,15,35 remain elevated after the symptoms of the acute exacerbations are controlled, so some researchers consider TNF-α a trait marker of psychosis.18,35 Our methodological approach did not allow us to assess which markers could be considered state or trait markers but we found that both IL-6 and TNF-α were elevated both in the general and the antipsychotic-naïve samples, thus supporting the idea that increases in these pro-inflammatory markers are present in FEP before onset of pharmacological treatment.
The replication of the finding of reduced TAS (both in the general and the antipsychotic-naïve samples) supports the hypothesis of the presence of reduced antioxidants and higher oxidative status in people with psychosis. Insufficient antioxidant levels leads to higher levels of reactive oxygen species, causing oxidation of macromolecules such as DNA, proteins or fatty acids, and thereby altering cell behavior and underlying some of the brain abnormalities found in patients with psychosis.6,36,37 We have previously linked low baseline levels of antioxidants, such as GSH, in early-onset psychosis (ie, onset of psychotic symptoms before age 18) to longitudinal decreases in gray matter volume and cognitive deficits 2 years after a FEP.38,39 In the present meta-analysis, we also found increased levels of Hcy, a non-protein amino acid that interacts with NMDA receptors and usually induces pro-inflammatory status, oxidative stress and mitochondrial dysfunction.40 Others have reported that Hcy levels have a positive correlation with early cognitive deficits in schizophrenia, regardless of the use of medication and the duration of the disease.41 Our confirmation of that report suggests that oxidative stress and mitochondrial dysfunction in people with psychosis may be an early signal of the disease.
Our findings support that some patients with FEP may have an imbalance of their antioxidant and anti-inflammatory biochemical pathways, thus suggesting that anti-inflammatory and/or antioxidant strategies may have clinical value for treating people with these disorders.42,43 However, the efficacy of such treatments remains unknown.44 Stratification of patients based on these baseline parameters, in accordance with personalized or precision medicine,45,46 could be a method for increasing the effectiveness of interventions, as suggested by recent trials of anti-inflammatory agents for treatment-resistant depression47 and psychotic symptoms.48 Since inflammation and oxidation are 2-faced responses whose mechanisms are based on a complex interplay of factors integrated in a finely regulated system, the restoration of physiological inflammatory/anti-inflammatory and oxidative/antioxidant balances could lead to better outcomes.3,49
This work has several limitations. First, there was great heterogeneity among the included studies. The definition of FEP varied across studies and separate information based on diagnostic subgroups was not available for all the studies included in the meta-analyses. However, we decided to include a wide spectrum of psychotic disorders, considering that there is increasing evidence supporting the presence of shared etiological and physiopathological mechanisms,50 and current clinical diagnostic classifications do not seem to be designed to appropriately facilitate biological differentiation.51 Our meta-regression analyses did not find a significant effect of the percentage of patients with schizophrenia on the effect sizes for TNF-α, IL-6, and TAS, thus suggesting that these biological alterations could be shared among different types of FEP. An additional source of heterogeneity could be developmental stage. The effects of development on inflammatory and oxidative status are still understudied in healthy individuals. A recent meta-analysis on oxidative stress and inflammatory markers in early-onset FEP found no significant differences between patients and controls,52 which could be due to the paucity of available studies, but also could have been influenced by age. Further, considering the numbers of available studies, we assessed data from plasma/serum and RBC/PMBCs in the same meta-analyses. Although findings were consistent overall, this could have introduced an additional source of heterogeneity. Second, although individuals with psychosis show increased cytokine levels in both peripheral blood and CSF according to one study,15 our meta-analysis only assessed peripheral biomarkers, which do not necessarily mirror the inflammatory and oxidative state of the brain. However, recent studies have found that CSF and plasma levels of markers such as IL-6 are significantly correlated in patients with recent-onset schizophrenia,53 suggesting that peripheral and central inflammatory systems probably operate in a parallel, synchronized way54 and supporting the idea that blood-based biomarkers could be valid measures for investigating psychosis.55 Further, an increase of some cytokines, such as IL-1β, has been consistently reported in people with schizophrenia relative to controls in peripheral blood, CSF, and postmortem brain tissue.10 Third, we did not perform specific meta-analyses assessing the groups of pro-inflammatory and anti-inflammatory markers separately. Cytokines often show overlapping activity regulating inflammatory and anti-inflammatory mechanisms, depending on the type and developmental state of the cells involved. Cytokines such as IL-6 are elevated in most inflammatory states. However, IL-6 exerts a pleiotropic role, showing both pro- and anti-inflammatory properties.56 Since inflammatory and anti-inflammatory markers are closely interrelated in the context of complex homeostatic regulatory processes,3,20 we decided not to perform separate meta-analyses assessing the groups of pro-inflammatory and anti-inflammatory markers. Finally, we could not perform meta-regressions for some potential moderators because the included studies lacked the necessary data.
Despite these limitations, this meta-analysis provides further evidence for the role of pro-/anti-inflammatory and oxidative/nitrosative dysregulations in the pathophysiology of FEP. These alterations might facilitate the development of personalized or precision clinical approaches to psychosis, by helping stratify patients and implement biologically-tailored interventions using pharmacological strategies aimed at restoring physiological anti-inflammatory and antioxidant pathways.
Funding
Supported by the Spanish Ministry of Economy, Industry and Competitiveness, Instituto de Salud Carlos III (PI12/01303, PI14/00397, PI17/00481), co-financed by ERDF Funds from the European Commission, “A way of making Europe,” CIBERSAM, Madrid Regional Government (B2017/BMD-3740 AGES-CM-2), EU Structural Funds, EU Seventh Framework Program (FP7-HEALTH-2009-2.2.1-2-241909 [Project EU-GEI], FP7-HEALTH-2009-2.2.1-3-242114 [Project OPTiMISE], FP7- HEALTH-2013-2.2.1-2-603196 [Project PSYSCAN], and FP7-HEALTH-2013-2.2.1-2-602478 [Project METSY]); and EU H2020 Program under the Innovative Medicines Initiative 2 Joint Undertaking (grant agreement 115916; Project PRISM), Fundación Familia Alonso, Fundación Alicia Koplowitz, and Fundación Mutua Madrileña.
Supplementary Material
Acknowledgments
D.F. has been a consultant and/or has received fees from Angelini, Eisai, IE4Lab, Janssen, Lundbeck, and Otsuka. He has also received grant support from Instituto de Salud Carlos III (Spanish Ministry of Economy and Competitiveness) and from Fundación Alicia Koplowitz. C.M.D-C. has previously held grants from Instituto de Salud Carlos III (Spanish Ministry of Economy and Competitiveness) and from Fundación Alicia Koplowitz. M.A., S.R., and F.H-Á. have no conflicts of interest. A.R-Q has previously held a “Río Hortega” grant from Instituto de Salud Carlos III (Spanish Ministry of Economy and Competitiveness). J.C.L. has received grant support from Instituto de Salud Carlos III (Spanish Ministry of Economy, Industry, and Competitiveness), CIBERSAM, Fundación Alicia Koplowitz and Fundación Mutua Madrileña. C.A. has been a consultant to or has received honoraria or grants from Acadia, Abbot, AMGEN, AstraZeneca, Bristol-Myers Squibb, Caja Navarra, CIBERSAM, Dainippon Sumitomo Pharma, Fundación Alicia Koplowitz, Forum, Instituto de Salud Carlos III, Gedeon Richter, Janssen Cilag, Lundbeck, Merck, Ministerio de Ciencia e Innovación, Ministerio de Sanidad, Ministerio de Economía y Competitividad, Mutua Madrileña, Otsuka, Pfizer, Roche, Servier, Shire, Schering Plough, Sunovio, and Takeda.
References
- 1. Kahn RS, Sommer IE, Murray RM, et al. . Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. [DOI] [PubMed] [Google Scholar]
- 2. Kahn RS, Sommer IE. The neurobiology and treatment of first-episode schizophrenia. Mol Psychiatry. 2015;20:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leza JC, García-Bueno B, Bioque M, et al. . Inflammation in schizophrenia: a question of balance. Neurosci Biobehav Rev. 2015;55:612–626. [DOI] [PubMed] [Google Scholar]
- 4. Meyer U, Schwarz MJ, Müller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther. 2011;132:96–110. [DOI] [PubMed] [Google Scholar]
- 5. Camps J, García-Heredia A. Introduction: oxidation and inflammation, a molecular link between non-communicable diseases. Adv Exp Med Biol. 2014;824:1–4. [DOI] [PubMed] [Google Scholar]
- 6. Landek-Salgado MA, Faust TE, Sawa A. Molecular substrates of schizophrenia: homeostatic signaling to connectivity. Mol Psychiatry. 2016;21:10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pouget JG, Gonçalves VF, Spain SL, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium Genome-wide association studies suggest limited immune gene enrichment in schizophrenia compared to 5 autoimmune diseases. Schizophr Bull. 2016;42:1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016;21:1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Kesteren CF, Gremmels H, de Witte LD, et al. . Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry. 2017;7:e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stojanovic A, Martorell L, Montalvo I, et al. . Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology. 2014;41:23–32. [DOI] [PubMed] [Google Scholar]
- 12. Mäntylä T, Mantere O, Raij TT, et al. . Altered activation of innate immunity associates with white matter volume and diffusion in first-episode psychosis. PLoS One. 2015;10:e0125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. García Bueno B, Caso JR, Madrigal JL, Leza JC. Innate immune receptor Toll-like receptor 4 signalling in neuropsychiatric diseases. Neurosci Biobehav Rev. 2016;64:134–147. [DOI] [PubMed] [Google Scholar]
- 14. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Kemp WJ, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. A meta-analysis of the polyunsaturated fatty acid composition of erythrocyte membranes in schizophrenia. Schizophr Res. 2012;141:153–161. [DOI] [PubMed] [Google Scholar]
- 17. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Capuzzi E, Bartoli F, Crocamo C, Clerici M, Carrà G. Acute variations of cytokine levels after antipsychotic treatment in drug-naïve subjects with a first-episode psychosis: a meta-analysis. Neurosci Biobehav Rev. 2017;77:122–128. [DOI] [PubMed] [Google Scholar]
- 19. Shamseer L, Moher D, Clarke M, et al. ; PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 20. Schiavone S, Trabace L. Inflammation, Stress response, and redox dysregulation biomarkers: clinical outcomes and pharmacological implications for psychosis. Front Psychiatry. 2017;8:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen J. Statistical power analysis for the behavioral sciences, 2nd ed Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 22. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 23. Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. [DOI] [PubMed] [Google Scholar]
- 24. Lipsey M, Wilson D.. Practical meta-analysis. Thousand Oaks, CA: Sage Publications; 2000. [Google Scholar]
- 25. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 26. Orwin RG. A fail-safe N for effect size in meta-analysis. J Edu Stat. 1983;8:157–159. [Google Scholar]
- 27. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 29. Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86:726–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cannon TD. Brain biomarkers of vulnerability and progression to psychosis. Schizophr Bull. 2016;42(Suppl 1):S127–S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheutlin AB, Jeffries CD, Perkins DO, et al. . The role of microRNA expression in cortical development during conversion to psychosis. Neuropsychopharmacology. 2017;42:2188–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yee JY, Lee TS, Lee J. Levels of serum brain-derived neurotropic factor in individuals at ultra-high risk for psychosis - Findings from the Longitudinal Youth at Risk Study (LYRIKS). Int J Neuropsychopharmacol. 2018. doi: 10.1093/ijnp/pyy036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perry BI, Upthegrove R, Thompson A, et al. . Dysglycaemia, inflammation and psychosis: findings from the UK ALSPAC birth cohort. Schizophr Bull. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manu P, Correll CU, Wampers M, et al. . Markers of inflammation in schizophrenia: association vs. causation. World Psychiatry. 2014;13:189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barron H, Hafizi S, Andreazza AC, Mizrahi R. Neuroinflammation and oxidative stress in psychosis and psychosis risk. Int J Mol Sci. 2017;18:pii: E651. doi: 10.3390/ijms18030651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prabakaran S, Swatton JE, Ryan MM, et al. . Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–697, 643. [DOI] [PubMed] [Google Scholar]
- 38. Fraguas D, Gonzalez-Pinto A, Micó JA, et al. . Decreased glutathione levels predict loss of brain volume in children and adolescents with first-episode psychosis in a two-year longitudinal study. Schizophr Res. 2012;137:58–65. [DOI] [PubMed] [Google Scholar]
- 39. Martínez-Cengotitabengoa M, Micó JA, Arango C, et al. . Basal low antioxidant capacity correlates with cognitive deficits in early onset psychosis. A 2-year follow-up study. Schizophr Res. 2014;156:23–29. [DOI] [PubMed] [Google Scholar]
- 40. Perna AF, Ingrosso D, De Santo NG. Homocysteine and oxidative stress. Amino Acids. 2003;25:409–417. [DOI] [PubMed] [Google Scholar]
- 41. Moustafa AA, Hewedi DH, Eissa AM, Frydecka D, Misiak B. Homocysteine levels in schizophrenia and affective disorders-focus on cognition. Front Behav Neurosci. 2014;8:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. García-Bueno B, Bioque M, Mac-Dowell KS, et al. . Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull. 2014;40:376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garcia-Bueno B, Bioque M, MacDowell KS, et al. . Pro-/antiinflammatory dysregulation in early psychosis: results from a 1-year follow-up study. Int J Neuropsychopharmacol. 2014;18:pii: pyu037. doi: 10.1093/ijnp/pyu037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fraguas D, Díaz-Caneja CM, State MW, O’Donovan MC, Gur RE, Arango C. Mental disorders of known aetiology and precision medicine in psychiatry: a promising but neglected alliance. Psychol Med. 2017;47:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martinez-Cengotitabengoa M, MacDowell KS, Alberich S, et al. ; FLAMM-PEPs BDNF and NGF signalling in early phases of psychosis: relationship with inflammation and response to antipsychotics after 1 year. Schizophr Bull. 2016;42:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raison CL, Rutherford RE, Woolwine BJ, et al. . A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Conus P, Seidman LJ, Fournier M, et al. . N-acetylcysteine in a double-blind randomized placebo-controlled trial: toward biomarker-guided treatment in early psychosis. Schizophr Bull. 2018;44:317–327. doi: 10.1093/schbul/sbx093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. García-Bueno B, Caso JR, Leza JC. Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev. 2008;32:1136–1151. [DOI] [PubMed] [Google Scholar]
- 50. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. [DOI] [PubMed] [Google Scholar]
- 51. Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it?Mol Psychiatry. 2012;17:1174–1179. [DOI] [PubMed] [Google Scholar]
- 52. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, Arango C. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Coughlin JM, Wang Y, Ambinder EB, et al. . In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry. 2016;6:e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fernandes BS, Steiner J, Bernstein HG, et al. . C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry. 2016;21:554–564. [DOI] [PubMed] [Google Scholar]
- 55. Harris LW, Pietsch S, Cheng TM, Schwarz E, Guest PC, Bahn S. Comparison of peripheral and central schizophrenia biomarker profiles. PLoS One. 2012;7:e46368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.