Abstract
Background
Evidence-based psychological interventions to support treatment decision-making capacity (capacity) in psychosis do not currently exist. This study sought to establish whether reducing the extent to which this group form conclusions based on limited evidence, also known as the “jumping-to-conclusions” (JTC) bias, could improve capacity.
Methods
In a randomized controlled open trial, 37 patients aged 16–65 years diagnosed with schizophrenia-spectrum disorders were randomly assigned (1:1) to receive a single-session intervention designed to reduce the JTC bias (MCT-JTC; adapted from Metacognitive Training [MCT]) or an attention control (AC) condition designed to control for therapist attention, duration, modality, and face validity. Primary outcomes were treatment decision-making capacity measured by the MacArthur Competency Assessment Tool for Treatment (MacCAT-T) and the jumping-to-conclusions reasoning bias measured by draws to decision on the beads task, each of which were administered by the psychologist delivering the intervention.
Results
Those receiving MCT-JTC had large improvements in overall capacity (d = 0.96, P < .05) and appreciation (d = 0.87, P < .05) compared to those receiving AC. Reduction in JTC mediated a large proportion of the effect of group allocation on understanding, appreciation, reasoning, and overall MacCAT-T scores.
Conclusion
This is the first experimental investigation of the effect of a psychological intervention on treatment decision-making capacity in psychosis. It provides early evidence that reducing the JTC bias is associated with large and rapid improvements in capacity. Due to limited resources, assessments were administered by the researchers delivering the intervention. Results should therefore be considered preliminary and a larger, definitive trial addressing methodological limitations is warranted.
Keywords: capacity, psychosis, RCT, jumping-to-conclusions, metacognitive therapy
Introduction
The concept of treatment decision-making capacity (capacity) is increasingly important in contemporary mental health care and has major implications for treatment, research, policy, and legislation.1 In jurisdictions that have adopted capacity-based mental health legislation, the extent to which a person is judged as having the capacity to make a decision about their psychiatric treatment has important implications not only for their freedom to consent to psychiatric treatment,2,3 but also for the responsibilities of clinicians who provide treatment. Estimates suggest that 50%–80% of inpatients with psychosis have impaired capacity,4 and clinicians are expected to support them to regain capacity, which may include referral for specialist support.2,3 However, very little robust research exists to inform practice in this area.5 Although a recent systematic review and meta-analysis found that shared decision-making improved subjective empowerment in psychosis, whether or not it improves capacity is unclear5,6 and psychiatrists may be reluctant to use this approach when their patients lack capacity.7 Clinicians may, on the other hand, be willing to provide “supported decision-making,” where the focus is instead on understanding and enhancing components of their patient’s decision-making ability.8 To aid this work, clinicians need theoretically driven psychological interventions that have been shown in randomised controlled trials RCTs to target and improve the mechanisms responsible for impaired capacity. Despite the concept of capacity being in existence for at least 30 years, such interventions are currently lacking for people with psychosis.5
The importance of respecting autonomy and minimizing compulsory, coercive, or paternalistic care means the development of these interventions is crucial, as is the development of an enhanced understanding of clinical and psychological mechanisms maintaining incapacity in psychosis. A recent meta-analytical review found that increased psychotic symptom severity, poorer verbal cognitive functioning, and fewer years of education were robustly associated with impaired capacity.5 However, aside from an established literature on the role of reduced insight in capacity, only 2 small studies have examined the potential contribution of other psychological factors to capacity.9,10 One found that metacognitive awareness of cognitive functioning had a stronger relationship with capacity than actual cognitive functioning.9 Another found that improvements in capacity in psychosis were correlated with the provision of Metacognitive Training (MCT).10 This was first developed as a group psychological intervention aiming to raise awareness of cognitive biases and decision-making styles known to be robustly and specifically associated with their psychotic symptoms.11 A later version, “MCT+,” adapted the package to target patients’ specific delusional ideas via an individualized approach.12
One bias that MCT aims to reduce is the “jumping-to-conclusions” (JTC) bias, which refers to a tendency to make decisions based on minimal evidence.13,14 Our meta-analysis of 55 studies found that individuals with psychosis were 4–6 times more likely to demonstrate the JTC bias than healthy individuals or people with nonpsychotic mental health problems.14 As predicted by the cognitive model of psychosis,15 there was evidence for specificity to delusions. Together with the finding of Naughton et al10 that an intervention targeting the JTC bias (and others) may improve capacity, the results suggest the possibility that the JTC bias may be involved in maintaining impaired capacity. As a consequence of forming conclusions too quickly, a person with the JTC bias may be limited in the degree to which they understand all the issues relevant to a particular decision and may struggle to properly weigh up and appreciate the relevant evidence. JTC may also contribute to impaired capacity via the formation of capacity-limiting delusional appraisals (eg, “my doctor is trying to kill me”). We tested this hypothesis recently, finding that the degree to which people with psychosis could understand treatment-relevant information was both directly and indirectly associated with the degree to which they gathered data before reaching a decision (A. Larkin, D. Turner, K. Whyte, and P. Hutton, unpublished data, 2017). It has been hypothesized that the JTC bias and similar data-gathering biases have origins in a “liberal acceptance” belief appraisal process among people with psychosis compared to nonpsychotic individuals, which suggests that a lowered-decision threshold leads to premature acceptance of hypotheses, subsequent bias in seeking hypothesis disconfirmation, and overconfidence in errors.16
If these preliminary observational findings reflect underlying causal relationships, then this has important implications for efforts to support decision-making in this group. If the JTC bias does contribute to impaired capacity, then simple interventions that successfully ameliorate this bias may improve capacity and support informed decision-making. A nonrandomized controlled trial has previously demonstrated a therapeutic effect of an adapted single-session MCT intervention targeting JTC and belief flexibility on severity and conviction of delusions, insight, and cognitive biases.17 The aim of this study was to determine whether a brief psychological intervention could improve capacity under experimental conditions.18 In the first study of its kind, people with psychosis were randomly assigned to receive either an intervention designed solely to reduce the JTC bias or a suitable control intervention, with the effect on their capacity assessed. We worked with the developer of MCT to identify its most effective components in reducing JTC and reasoned that if greater improvements in capacity occurred in those who received this “MCT-JTC” intervention, then this would be a strong evidence that the JTC bias is related to capacity in this group. We also reasoned that if changes in the amount of data people gathered before making a decision mediated the effect of group allocation, then this would be an even stronger evidence that data-gathering is an important mechanism underlying capacity in this group.
Methods
Patients
People with psychosis were eligible to take part in the study if they were aged 16–65, were English-speaking, were in contact with National Health Service (NHS) mental health services, and had a diagnosis of schizophrenia, schizoaffective disorder, delusional disorder, brief psychotic disorder, or psychosis not otherwise specified (NOS). Capacity to consent to participation was assessed via clinical judgement by the patients’ respective NHS psychiatrist and confirmed by the researcher. Patients were unable to participate if their psychotic symptoms were the result of a general medical condition or substance misuse disorder, if they were under care of forensic mental health services or involved in ongoing legal proceedings, if they had a moderate or severe learning disability, or if there had been a deterioration in their condition, which suggested participation could be detrimental. Given the primary outcome was treatment decision-making capacity, no minimum or maximum symptom threshold or stage of illness was specified. The study was conducted across NHS Lanarkshire and NHS Dumfries and Galloway Health Boards (Scotland, UK), with the majority of participants (n = 36, 97%) being recruited from NHS Lanarkshire; hence, the research clinician based in NHS Lanarkshire assessed and treated the majority of participants. Recruitment was open between January 2016 and February 2017. Outpatients were recruited through contact with Community Mental Health Teams (CMHTs) and Psychological Therapies Teams (PTTs) whereas inpatients were recruited within both acute and rehabilitation inpatient psychiatric services. A presentation and Q&A session was delivered to CMHTs, PTTs, and psychiatrists introducing the study aims and referral procedure. The overall study aims were fully communicated to clinical staff while participants were informed that the study aimed to investigate how people with psychosis make decisions regarding their treatment.
Ethical approval was obtained from the South of Scotland Research Ethics Committee (REC no. 15/SS/0162) and the University of Edinburgh. All patients provided written informed consent.
Study Design
The study used a randomized controlled parallel experimental design. Participants were randomly assigned to receive a single-session of MCT-JTC or an attention control, specially designed to control for time and therapist attention. Outcome measures were administered at baseline and posttreatment. To minimize attrition bias from missing data, randomization was performed by the researcher only when participants arrived to attend the single intervention or control session. This was carried out in session using an online randomization service (sealedenvelope.com), which randomized participants according to a permuted random blocks randomization sequence concealed from the investigator, the participant, and clinical staff. Participants, but not clinical staff, were blind to the study hypothesis. Participants and clinical staff were blind to allocation; however, the same researcher carried out the assessments and delivered the interventions. Participants were fully debriefed about the study hypothesis at the end of the study.
Interventions
MCT-JTC: The intervention was derived from the MCT manual developed by Moritz et al.19 To provide an effective single-session version of MCT, the primary investigator (D.T.) and the developer of MCT (S.M.) amalgamated the JTC modules into an hour-long “best of” intervention. The intervention aimed to repeatedly engage the participant in an approach contrary to the JTC bias while reflecting on the pitfalls of JTC. Participants were encouraged to assign additional time in decision-making while assessing and interpreting all available evidence in given scenarios. The intervention was delivered individually via PowerPoint. Key components are provided in table 1. Due to the utilization of individual format, the method of delivery was closest to the “MCT+” variant of MCT.12 Participants were encouraged to engage and interact during the session while reflection on personal examples and misinterpretation of personally significant situations was encouraged.
Table 1.
Components of 1-Hour Metacognitive Training-Jumping to Conclusions (MCT-JTC) Intervention
| 1. An introduction to the jumping-to-conclusions bias in psychosis |
| 2. Inferences without 100% proof; examples from daily life (2 examples) |
| 3. Jumping-to-conclusions “in action”; examples from politics and medicine of the pitfalls of using jumping-to-conclusions in decision-making (4 examples) |
| 4. How jumping-to-conclusions promotes misinterpretation; discussion and examples including a worksheet for personal experiences and alternative interpretation |
| 5. Jumping-to-conclusion and its role in conspiracy theories; illustration via the moon landing conspiracy theory |
| 6. Worksheet exercise; providing evidence for and against personal delusional beliefs including conviction rating |
| 7. Picture-identification tasks (3 tasks); participants were required to identify all possible interpretations of images as progressive detail was revealed and state their confidence in their interpretation |
| 8. Face illusion tasks (3 tasks); participants were required to identify all details or alternative interpretations when presented with images, for example the old woman/young woman/old man face illusion |
| 9. Scene identification from cutout (4 tasks); 4 tasks in which a cutout image from a larger scene was provided from which participants were required to infer the correct wider context from 4 options using evidence in the picture and state confidence |
| 10. Misfits task (5 tasks): presentation of 5 classic paintings in which participants were required to identify the correct title from 4 options based on clues within the painting and state confidence |
| 11. Summary of jumping-to-conclusions session and suggested tactics |
Control condition: To control for therapist attention and time, while removing specific intervention factors addressing thinking biases, an individually delivered educational talk on the localization of brain function was provided. Participants were informed that the presentation was “about how different parts of the brain have different functions.” This condition was also delivered individually using PowerPoint and lasted 1 hour, ensuring that both conditions were closely matched for modality of presentation, time spent by the researcher with the participant, and nonspecific interaction factors. The content of the presentations was carefully chosen to ensure participants were masked to which was the active intervention.
Study Evaluation
The first session consisted of administration of the complete baseline assessment battery. A follow-up appointment within 2 weeks was arranged to carry out the experimental procedures and postintervention assessment. The MacArthur Competency Assessment Tool for Treatment (MacCAT-T)20 was used to assess capacity at baseline and posttreatment. This semistructured interview schedule covers 4 domains: understanding information relevant to treatment (0–6); appreciation of diagnostic and treatment information (0–4); reasoning ability regarding treatment options (0–8); and expressing choice regarding treatment (0–2). Both raters (D.T. and A.L.) were trained in the administration of all clinical scales by the primary project supervisor (P.H.), who also supervised assessments and scoring while remaining blind to treatment allocation. Participants were first presented with an overview of their psychiatric diagnosis, 3 of its features and its expected course. Their capacity to accurately understand and retain information regarding the diagnostic information was then assessed. Participants were then introduced to 2 hypothetical treatment options relevant to their diagnosis (taking antipsychotics vs no medication) and the risks and benefits of each option. They were again assessed on their capacity to retain and understand this information. Participants were then asked to choose which treatment option seemed best for them based on the information provided. They were finally assessed on reasoning ability and ability to generate consequences of their choice. Although the scale does not provide a total score, in order to align with previous research an overall capacity score was calculated alongside the consideration of subscales.5 Each subscale has demonstrated inter-rater reliability of κ 0.80.4,21,22 Higher scores are indicative of greater capacity. MacCAT-T scale reliability (internal consistency) in the current sample was α = .80.
A computerized version of the “beads task” (described in detail elsewhere)23 was used to assess the JTC bias by recording how many pieces of information a participant required before making a decision regarding the jar of origin of the colored beads presented. We utilized the 60/40 version, which was chosen for consistency with other ongoing research within the project team. Participants were informed they could view as many beads as they wished until they were certain about their origin. The total number of beads a person requests before making their decision (“draws-to-decision” [DTD]) was taken as an index of data gathering and the JTC bias. The Cognitive Bias Questionnaire for Psychosis (CBQP) provided a secondary assessment of the JTC bias alongside related cognitive distortions.24 The scale reliability of this 30-item self-report measure was α = .89 in the current sample. The Positive and Negative Syndrome Scale (PANSS)25 was administered at baseline to characterize the clinical profile of the sample. Thirty clinician-rated items assess positive, negative, and general symptoms of psychosis alongside a total score. Higher scores indicate greater severity of symptoms. Scale reliability was α = .88 for the sample. The Hospital Anxiety and Depression Scale (HADS)26 provided a basic measure of depressive and anxiety symptoms. Scale reliability of this 14-tem measure was α = .83 for this sample.
Statistical Analyses
G*Power software27 was used to calculate the required sample size to detect a statistically significant effect, based on the assumption that a randomized study may need to able to detect smaller effects than the large effects (F = 1.29) reported on MacCAT-T total scores in the nonrandomized study by Naughton et al.10 It was estimated that 26 participants would be required in each group to detect an effect of F = 0.4 for the .05 α level at 80% power using an ANCOVA with one covariate.
All analyses followed intention-to-treat procedures.28 Multiple imputation was used to estimate missing scores at posttreatment by entering baseline PANSS scores and group assignment as predictors alongside baseline and posttreatment scores for the imputed outcome. All data were assessed for normality through visual examination of histograms and boxplots, alongside calculation of statistically significant skewedness or kurtosis. When significant skewedness or kurtosis suggested violation of the assumptions required for ANCOVA, post hoc nonparametric sensitivity analyses were performed using a Kruskal–Wallis H test in R. The main analyses of primary and secondary outcome measures were computed in SPSS using ANCOVAs by entering baseline scores as covariates to improve power and precision.29 Baseline scores correlate with magnitude of change, meaning they should be utilized as covariates when comparing groups on mean change and are important in guarding against floor and ceiling effects. To satisfy the assumption of independence of covariate and treatment effect,30 ANCOVA was only performed when group differences at baseline were not significant. When baseline differences were significant, a simple analysis of group differences in mean change in the variable was conducted instead. Each analysis was checked for consistency in both type I and type III ANCOVA models while lack of model fit was examined. Partial eta-squared effect sizes were converted to Cohen’s d.
To investigate whether changes in data gathering mediated the effect of group allocation on capacity, 2 methods of simple mediation analyses were performed. Mediation analyses based on the linear regression method described by Baron and Kenny31 were implemented using the RMediation package.32 Causal mediation analyses were also conducted following Preacher and Hayes method33 using the SPSS PROCESS-4 macro.34,35 Causal mediation analysis utilizes ordinary least squares path analysis and nonparametric bootstrapping, has higher precision than the Baron and Kenny approach, may accommodate data violating the assumption of normality, and improves inferences regarding causality.36
Preregistration
The study protocol was preregistered on the Open Science Framework before randomization and recruitment commenced (Open Science Framework registration; https://osf.io/kunc4/).
Results
Patients
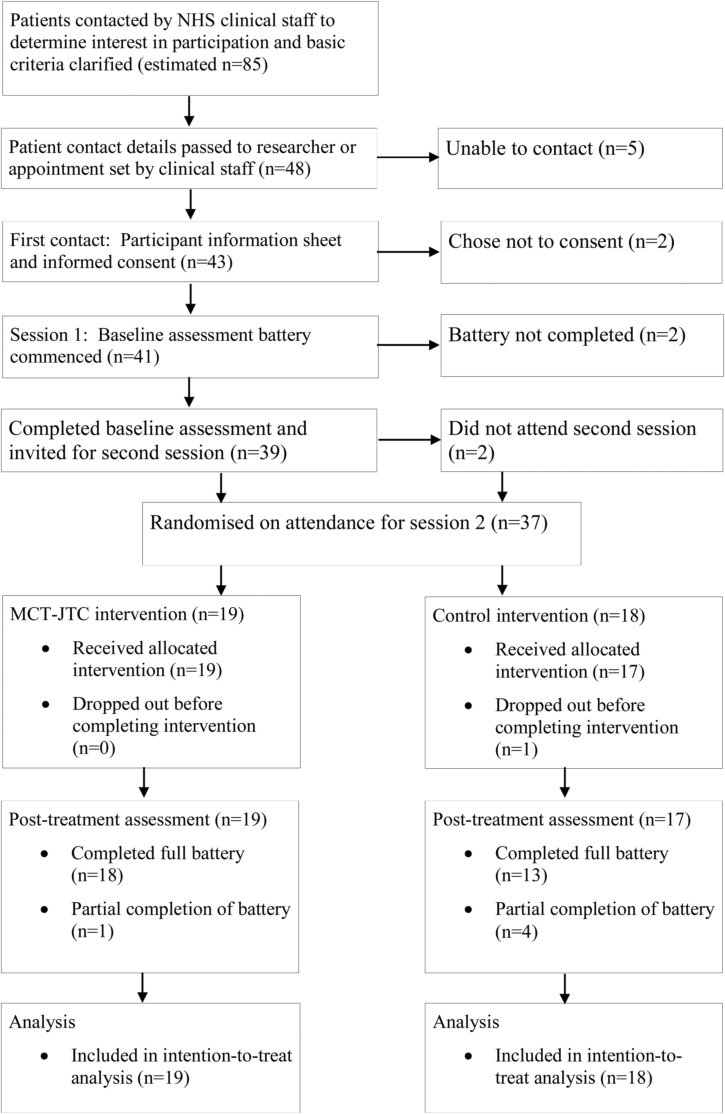
As shown in figure 1, 37 participants were randomized within the recruitment window. Resource and time constraints led to this smaller than planned sample size, but it was deemed sufficient to detect clinically meaningful effects. Nineteen participants were allocated to MCT-JTC and 18 were allocated to the control group. Missing data were minimal (3%) for the primary outcome, suggesting high acceptability and a very low risk of attrition bias.
Fig. 1.
Flow of participants through the study.
Table 2 provides information on demographic and clinical variables. The majority (n = 31, 84%) of participants were male and all patients were of self-reported white ethnicity. Thirty percent (n = 11) were inpatients and 70% (n = 26) were outpatients. The majority were diagnosed with schizophrenia (n = 26, 70%) whereas 14% (n = 5) were diagnosed with schizoaffective disorder and 16% (n = 6) as psychosis NOS. First diagnosis occurred over 10 years before baseline for the majority of participants (n = 27, 73%). On average, HADS scores fell within the moderate range.37 PANSS total scores indicated that participants were mildly to moderately ill on average.38 The majority of participants (81.1%) showed at worst mild impairment on the lack of judgement and insight item of the PANSS (see Supplementary Materials).
Table 2.
Demographic and Clinical Characteristics
| Overall (N = 37) | Experimental(n = 19) | Control(n = 17) | |
|---|---|---|---|
| Age, mean (SD) | 44.7 (12.8) | 45.3 (13.0) | 44 (12.9) |
| Gender (male:female) | 31:6 | 14:5 | 17:1 |
| Ethnicity (white:other) | 37:0 | 19:0 | 17:0 |
| Inpatient:outpatient | 11:26 | 5:14 | 6:12 |
| Diagnosis | |||
| Schizophrenia, n (%) | 26 (70) | 12 (63) | 14 (78) |
| Schizoaffective, n (%) | 5 (14) | 3 (16) | 2 (11) |
| Psychosis NOS, n (%) | 6 (16) | 4 (21) | 2 (11) |
| Duration | |||
| 0–1 years, n (%) | 3 (8) | 3 (16) | 0 (0) |
| 1–3 years, n (%) | 3 (8) | 2 (11) | 1 (6) |
| 3–5 years, n (%) | 2 (5) | 1 (5) | 1 (6) |
| 5–10 years, n (%) | 2 (5) | 1 (5) | 1 (6) |
| Over 10 years, n (%) | 27 (73) | 12 (63) | 15 (83) |
| PANSS positive, mean (SD) | 17.2 (7.1) | 17.2 (8.1) | 17.2 (6.1) |
| PANSS negative, mean (SD) | 15.1 (5.2) | 13.7 (4.4) | 16.6 (5.8) |
| PANSS general, mean (SD) | 36.2 (7.4) | 38.1 (7.2) | 34.3 (7.3) |
| PANSS total, mean (SD) | 68.8 (16.5) | 69.5 (16.8) | 68.1 (16.7) |
| HADS total, mean (SD) | 13.30 (7.2) | 15.4 (8.2) | 11.1 (5.4) |
| PANSS insight, mean (SD) | 2.51 (1.39) | 2.37 (1.38) | 2.67 (1.41) |
Note: PANSS, Positive and Negative Syndrome Scale; HADS, Hospital Anxiety and Depression Scale. Insight = Item 12 of PANSS General subscale. NB: data for Age, PANSS and HADS are reported as mean and standard deviation. There were no significant differences between groups using t test and chi-squared tests.
Primary Outcomes
As shown in table 3, there was a large positive effect of MCT-JTC on the 2 capacity outcomes: the appreciation subscale (d = 0.87, P < .05) and the total score (d = 0.95, P < .01) for the MacCAT-T. There was a medium effect of MCT-JTC on the MacCAT-T reasoning subscale, which approached significance (d = 0.68, P = .055). MCT-JTC did not demonstrate superiority from control on the MacCAT-T understanding subscale. A significant degree of negative skew was present for the MacCAT-T appreciation at baseline and posttreatment across MCT-JTC and control groups, potentially violating the assumptions for ANCOVA. Data met the assumptions required for a post hoc Kruskal–Wallis H test; therefore, a sensitivity analysis was performed. The resulting chi-squared statistic was consistent with the main ANCOVA in showing a significant effect favoring MCT-JTC (x2 = 4.89, P < .05).
Table 3.
Main ANCOVA Results for All Outcome Measures by Treatment Group (Intention to Treat)
| Baseline | Posttreatment | F Test | Between-group effect size (d) | |||
|---|---|---|---|---|---|---|
| MCT-JTC | AC | MCT-JTC | AC | Group Effect (F) | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| MacCAT-T understanding | 3.62 (1.40) | 3.30 (1.46) | 4.30 (1.49) | 3.59 (1.57) | 2.06 | .49 |
| MacCAT-T appreciation | 3.21 (1.08) | 2.90 (1.28) | 3.86 (0.58) | 3.00 (1.35) | 6.45* | .87* |
| MacCAT-T reasoning | 6.2 (1.23) | 5.44 (1.89) | 6.89 (1.24) | 5.56 (2.00) | 3.95** | .68** |
| MacCAT-T expressing choice | 1.95 (0.23) | 1.89 (0.32) | 1.95 (0.23) | 1.93 (0.24) | b | b |
| MacCAT-T total | 14.93 (3.37) | 13.58 (4.17) | 16.8 (2.83) | 13.9 (4.37) | 7.78* | .96* |
| HADS anxiety | 9.21 (5.39) | 7.22 (4.13) | 9.16 (4.71) | 7.52 (4.55) | 2.21 | −.18 |
| HADS depression | 6.16 (4.14) | 3.39 (2.68) | 7.32 (3.60)a | 3.44 (2.39)a | b | .30 |
| HADS total | 15.38 (8.17) | 11.11 (5.40) | 16.26 (7.18) | 10.99 (5.87) | 2.21 | −.51 |
| CBQP JTC subscale | 11.21 (3.87) | 10.56 (2.20) | 10.67 (3.05) | 10.60 (1.57) | .33 | .20 |
| CBQP Total | 48.89 (11.10) | 45.44 (9.83) | 45.40 (9.42) | 43.54 (8.39) | .35 | .20 |
| Beads task | 3.84 (2.91) | 3.67 (2.68) | 6.16 (4.05) | 3.72 (3.36) | 7.35* | .93* |
Note: MCT, metacognitive training; AC, attention control; MacCAT-T, MacArthur Competency Assessment Tool for Treatment; HADS, Hospital Anxiety and Depression Scale; CBQP, Cognitive Biases Questionnaire for Psychosis: JTC, jumping-to-conclusions.
aMean change for MCT-JTC and control was 1.16 (SD 3.17) and 0.05 (SD 3.09), respectively.
bData violated necessary assumptions therefore ANCOVA was not possible. Between group difference for MacCAT-T Expressing Choice was nonsignificant.
*P < .05; **P < .1.
Secondary Outcomes
MCT-JTC did not have a significant effect on the CBQP JTC subscale or total. There was, however, a large positive effect of MCT-JTC on DTD, as assessed by the beads tasks (d = 0.93, P < .05). No significant group differences were found on the HADS anxiety subscale or total score. Although MCT-JTC participants had higher HADS depression subscale at postintervention, they also had significantly higher HADS depression scores at baseline (P = .022). It was not possible to adjust for these with ANCOVA, because doing so would violate the assumption of the independence of covariate and treatment effect.30 An analysis of mean change did not detect any significant group differences. The number of new cases of at least mild depression, defined as moving from a score of less than 8 on the HADS depression subscale at baseline to a score of 8 or more at posttreatment, was 3 and 2 for MCT-JTC and control, respectively. To examine whether participants became more depressed or anxious following the trial, post hoc within-group t tests and Wilcoxon signed ranks tests were conducted on HADS total and subscale scores for the sample as a whole and per group. No significant differences were detected (all comparisons P < .10). No other adverse effects were reported.
Mediation Analysis
Results of both methods of simple mediation analysis are provided in table 4. The prespecified Baron and Kenny31 analysis found that postintervention draws-to-decision significantly mediated the effect of group allocation on the MacCAT-T total score at posttreatment (d = 0.64, P < .05), accounting for 39% of treatment effects. However, this comparison did not meet the assumptions described by Baron and Kenny because the second step in the 3-part model was not significant (P ≤ .06). No significant mediating effects of postintervention draws-to-decision were observed on the MacCAT-T subscales. A post hoc Preacher and Hayes33 analysis of mediation was also performed because this approach does not require data to be normally distributed and incorporates bootstrapping, meaning it has increased power to detect effects in smaller samples.39 The magnitude of the mediation effects in this analysis were the same as those observed with the Baron and Kenny approach; however, using the Preacher and Hayes approach all were significant. Postintervention draws-to-decision accounted for 63%, 36%, 29%, and 39% of the effect of group allocation on understanding (d = 0.45, P < .05), appreciation (d = 0.55, P < .05), reasoning (d = 0.59, P < .05), and overall MacCAT-T scores (d = 0.64, P < .05), respectively.
Table 4.
Data Gathering as a Mediator of Group Allocation on Capacity
| Total Effect (SE), P | Direct Effect (SE), P | Mediated Effect (SE), 95% CI | Proportion Mediated, % | d | |
|---|---|---|---|---|---|
| Baron and Kenny method | |||||
| MacCAT-T understanding | −0.71 (0.50), .17 | −0.26 (0.48), .59 | −0.45 (0.28), −1.10, 0.00 | 63 | 0.45 |
| MacCAT-T appreciation | −0.71 (0.34), .04* | −0.45 (0.34), .19 | −0.25 (0.17), −0.66, 0.01 | 35.7 | 0.55 |
| MacCAT-T reasoning | −1.34 (0.55), .02* | −0.95 (0.55), .09 | −0.39 (0.27), −1.03, 0.02 | 28.8 | 0.59 |
| MacCAT-T total | −2.89 (1.21), .02* | −1.77 (1.14), .13 | −1.12 (0.69), −2.69, −.00* | 38.7* | 0.64 |
| Preacher and Hayes method | |||||
| MacCAT-T understanding | −0.71 (0.50), .17 | 0.26 (0.48), .59 | −0.45 (0.28), −1.19, −0.05* | 63* | 0.45 |
| MacCAT-T appreciation | −0.71 (0.34), .04* | −0.46 (0.34), .19 | −0.25 (0.15), −0.66, −0.04* | 35.7* | 0.55 |
| MacCAT-T reasoning | 1.34 (0.55), .02* | −0.95 (0.55), .09 | −0.39 (0.25), −1.08, −0.03* | 28.8* | 0.59 |
| MacCAT-T total | −2.89 (1.21), .02* | −1.77 (1.14), .13 | −1.12 (0.65), −2.86, −0.14* | 38.7* | 0.64 |
Note: MacCAT-T, MacArthur Competency Assessment Tool for Treatment. SE, standard error. CI, confidence interval; P, probability level. Mediation model; x = group, y = MacCAT-T, M = draws to decision at posttreatment. *P < .05.
Discussion
This study represents the first randomized controlled investigation of the effect of a psychological intervention on capacity in psychosis and provides evidence that data gathering is associated with capacity in this group. Hypotheses, methodology, outcomes, and analytical plan were preregistered in the public domain, reducing the risk of selective reporting bias. The duration of the intervention, use of late randomization, and use of intention-to-treat analysis also minimized the risk of attrition bias. Late randomization, together with the careful design of the control intervention and the brief nature of the intervention, helped to ensure participants and clinicians were masked to allocation, therefore minimizing performance bias. The close matching of the control and experimental interventions in relation to duration, modality, and researcher–participant interaction also greatly reduced the risk of group differences being attributable to nonspecific therapeutic factors. Nevertheless, firmer conclusions await larger trials that could address the limitations of this study, most notably lack of rater blinding.
The findings were broadly consistent with previous observational research.10 The relative magnitude and significance of the effect on the appreciation subscale over the reasoning subscale contrasts with the findings of a previous nonrandomized trial, although the medium magnitude of the nonsignificant effect on reasoning, alongside small samples sizes in each trial, suggests the possibility of type II error. It should also be noted that because outcome measures were administered directly following the treatment, the current RCT does not demonstrate a persistent effect of MCT-JTC but rather a snapshot of capacity at postintervention. Naughton et al10 were able to demonstrate a dose-response relationship between changes in MacCAT-T reasoning and the number of sessions completed. These changes were also associated with changes in general functioning. Consequently, the opportunity remains to investigate such effects via a randomized trial integrating multiple sessions. Although we expected differences on the MacCAT-T total, we did not make specific a priori predictions of differences across subscales. Nevertheless, we hypothesize that a positive impact on the JTC bias, as demonstrated by the effect on the beads task, may be influential in increasing metacognitive awareness of the pitfalls of one’s own biased decision-making process. This in turn may lead to greater (and less biased) appreciation regarding one’s own disorder and treatment options. A larger trial administering multiple sessions with session-by-session outcome measures may have the potential to shed further light on this possibility alongside the sustainability of such effects.
The prediction that change in data gathering would mediate the effect of group assignment on capacity was confirmed, therefore suggesting that MCT-JTC successfully changed this mechanism, and that this change accounted for the observed improvements. Importantly, the noninvasive and brief nature of the MCT-JTC made it highly acceptable to participants. By operating at the metacognitive level, MCT-JTC avoided protracted discussions of the evidence for and against particular beliefs. By focusing on decision-making, emotive discussion of the merits or otherwise of psychiatric treatments was also avoided. The focus of MCT-JTC on only one mechanism—the JTC bias—makes it unlikely that changes in other psychological variables accounted for the changes in capacity.
Taken together, these findings lend encouragement to researchers, clinicians, and legal experts who are interested in developing effective strategies to support treatment decision-making in psychosis. The complexity of psychosis means it is very unlikely that the JTC bias alone accounts for impaired capacity; therefore, studies investigating the potential role of other psychological variables including attributional biases, self-esteem, and metacognitive awareness of cognitive impairment are also required. The results of this study demonstrate the advantage of considering how the processes underlying a particular condition might also adversely affect decision-making and autonomy. As the concepts of capacity and supported decision-making become increasingly important in mental health legislation, so too will the need for empirical evidence. Application of an interventionist–causal framework18 to generating this evidence may help to accelerate the development of effective decision-support interventions.5
Study Limitations
Our study had several further limitations. It had limited power; therefore, the estimates of effect were imprecise. Achieving adequate precision must await studies with larger sample sizes.40 As indicated, limited resources precluded researcher blinding; therefore, there was a high risk of detection bias.41 The use of masked raters is associated with a reduction in effect sizes of 9%–17% across medicine42; therefore, we emphasize that the large effects we observed here, while promising, should be treated as preliminary until replicated independently by larger rater-blind studies. Further questions for future research include whether our findings can be generalized to populations with early psychosis, those presenting with more severe symptoms, or samples in which capacity is more severely impaired at baseline. We also note minor imbalances at baseline on secondary outcome measures, namely the HADS and PANSS subscales, which a larger trial may help resolve.
These limitations notwithstanding, the study provides evidence that a widely studied psychological mechanism associated with psychotic symptoms, the JTC bias,13,14 may influence treatment decision-making capacity in this group. In addition, the study has also demonstrated that capacity in psychosis is modifiable by intervention, which is consistent with previous observational research,43 and that it is feasible to perform RCTs with capacity as a primary outcome.
Funding
There was no funding for this work.
Supplementary Material
Acknowledgments
Dr Turner conceived of and designed the study, secured ethical approval, recruited participants, gathered and analyzed the data, and wrote the manuscript. Dr MacBeth helped with recruitment of participants, analysis of data, and contributed to the manuscript. Dr Larkin helped with recruitment of participants and gathering of data and contributed to the manuscript. Dr Moritz helped to design the intervention and contributed to the manuscript. Dr Livingstone helped to design the study, helped with recruitment of participants, and contributed to the manuscript. Dr Campbell helped with recruitment of participants and contributed to the manuscript. Dr Hutton conceived of and helped to design the study, helped secure ethical approval, helped with recruitment of participants, helped to analyze the data, and helped to write the final manuscript. Dr Turner, Dr MacBeth, Dr Larkin, Dr Livingstone, and Dr Campbell report no competing or conflicting interests. Dr Moritz is the originator of Metacognitive Therapy for psychosis and has conducted a number of studies and trials to examine its efficacy. He reports no author competing or conflicting interests. Dr Hutton has been a coinvestigator on research grants from the National Institute of Health Research to evaluate the efficacy of cognitive therapy for people with psychosis who are not taking antipsychotic medication and is a member of a committee developing National Institute for Clinical and Social Care Excellence (NICE) guidelines on supporting decision-making for people who may lack mental capacity.
References
- 1. Owen SG, Freyenhagen F, Richardson G, Hotopf M. Mental capacity and decisional autonomy: an interdisciplinary challenge. Inquiry. 2009;52(1):79– 107. doi: 10.1080/00201740802661502. [Google Scholar]
- 2. Department of Health. Mental Capacity Act. London, UK: The Stationery Office; 2005. [Google Scholar]
- 3. Scottish Executive. Adults with Incapacity (Scotland) Act 2000. Edinburgh, UK: The Stationery Office; 2000. [Google Scholar]
- 4. Cairns R, Maddock C, Buchanan A, et al. Prevalence and predictors of mental incapacity in psychiatric in-patients. Br J Psychiatry. 2005;187(4):379–385 [DOI] [PubMed] [Google Scholar]
- 5. Larkin A, Hutton P. Systematic review and meta-analysis of factors that help or hinder treatment decision-making capacity in psychosis. Br J Psychiatry. 2017;211:205–215. [DOI] [PubMed] [Google Scholar]
- 6. Stovell D, Morrison AP, Panayiotou M, Hutton P. Shared treatment decision-making and empowerment-related outcomes in psychosis: systematic review and meta-analysis. Br J Psychiatry. 2016;209(1):23–28. doi: 10.1192/bjp.bp.114.158931. [DOI] [PubMed] [Google Scholar]
- 7. Hamann J, Mendel R, Cohen R, et al. Psychiatrists’ use of shared decision making in the treatment of schizophrenia: patient characteristics and decision topics. Psychiatr Serv. 2009;60(8):1107–1112. doi: 10.1176/appi.ps.60.8.1107. [DOI] [PubMed] [Google Scholar]
- 8. Drake RE, Deegan PE, Rapp C. The promise of shared decision making in mental health. Psychiatr Rehabil J. 2010;34:7–13. [DOI] [PubMed] [Google Scholar]
- 9. Koren D, Poyurovsky M, Seidman LJ, Goldsmith M, Wenger S, Klein EM. The neuropsychological basis of competence to consent in first-episode schizophrenia: a pilot metacognitive study. Biol Psychiatry. 2005;57:609–616. [DOI] [PubMed] [Google Scholar]
- 10. Naughton M, Nulty A, Abidin Z, Davoren M, O’Dwyer S, Kennedy HG. Effects of group metacognitive training (MCT) on mental capacity and functioning in patients with psychosis in a secure forensic psychiatric hospital: a prospective-cohort waiting list controlled study. BMC Res Notes. 2012;5(1):302. doi: 10.1186/1756-0500-5-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moritz S, Andreou C, Schneider BC, et al. Sowing the seeds of doubt: a narrative review on metacognitive training in schizophrenia. Clin Psychol Rev. 2014;34(4):358–366. doi: 10.1016/j.cpr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 12. Vitzthum FB, Veckenstedt R, Moritz S. Individualized Metacognitive Therapy Program for patients with psychosis (MCT+): introduction of a novel approach for psychotic symptoms. Behav Cogn Psychother. 2013;42(1):105–110. doi: 10.1017/S1352465813000246. [DOI] [PubMed] [Google Scholar]
- 13. Huq SF, Garety PA, Hemsley DR. Probabilistic judgements in deluded and non-deluded subjects. Q J Exp Psychol Sect A. 1988;40(4):801–812. doi: 10.1080/14640748808402300. [DOI] [PubMed] [Google Scholar]
- 14. Dudley R, Taylor P, Wickham S, Hutton P. Psychosis, delusions and the “Jumping to Conclusions” reasoning bias: a systematic review and meta-analysis. Schizophr Bull. 2016;42(3):652–665. doi: 10.1093/schbul/sbv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol Med. 2001;31(2):189–195. doi: 10.1017/S0033291701003312. [DOI] [PubMed] [Google Scholar]
- 16. Moritz S, Balzan RP, Kulagin SC, Andreou C. A new paradigm to measure probabilistic reasoning and a possible answer to the question why psychosis-prone individuals jump to conclusions. J Abnorm Psychol. 2017;126(4):406. [DOI] [PubMed] [Google Scholar]
- 17. Balzan RP, Delfabbro PH, Galletly CA, Woodward TS. Metacognitive training for patients with schizophrenia: preliminary evidence for a targeted, single-module programme. Aust N Z J Psychiatry. 2014;48(12):1126–1136. doi: 10.1177/0004867413508451. [DOI] [PubMed] [Google Scholar]
- 18. Kendler KS, Campbell J. Interventionist causal models in psychiatry: repositioning the mind–body problem. Psychol Med. 2009;39(06):881. doi: 10.1017/S0033291708004467. [DOI] [PubMed] [Google Scholar]
- 19. Moritz S, Woodward TS, Burlon M.. Metacognitive Training for Patients with Schizophrenia (MCT) Manual. Hamburg, Germany: VanHam Campus Verlag; 2007. [Google Scholar]
- 20. Grisso T, Appelbaum PS.. MacArthur Competence Assessment Tool for Treatment (MacCAT-T). Sarasota, FL: Professional Resource Press/Professional Resource Exchange; 1998. [Google Scholar]
- 21. Raffard S, Fond G, Brittner M, et al. Cognitive insight as an indicator of competence to consent to treatment in schizophrenia. Schizophr Res. 2013;144(1–3):118–121. doi: 10.1016/j.schres.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 22. Cairns R, Maddock C, Buchanan A, et al. Reliability of mental capacity assessments in psychiatric in-patients. Br J Psychiatry. 2005;187:372–378. doi: 10.1192/bjp.187.4.372. [DOI] [PubMed] [Google Scholar]
- 23. Moritz S, Woodward TS. Jumping to conclusions in delusional and non-delusional schizophrenic patients. Br J Clin Psychol. 2005;44(2):193–207. doi: 10.1348/014466505X35678. [DOI] [PubMed] [Google Scholar]
- 24. Peters ER, Moritz S, Schwannauer M, et al. Cognitive Biases Questionnaire for Psychosis. Schizophr Bull. 2014;40(2):300–313. doi: 10.1093/schbul/sbs199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 26. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 27. Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 28. White IR, Carpenter J, Horton NJ. Including all individuals is not enough: lessons for intention-to-treat analysis. Clin Trials. 2012;9(4):396–407. doi: 10.1177/1740774512450098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borm GF, Fransen J, Lemmens WAJG. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60(12):1234–1238. doi: 10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 30. Field A. Discovering Statistics Using IBM SPSS. 4th ed London, UK: Sage; 2013. [Google Scholar]
- 31. Baron RM, Kenny D. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 32. Tofighi D, MacKinnon DP.RMediation: an R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43(3):692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- 34. Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. doi: 10.3758/BF03206553. [DOI] [PubMed] [Google Scholar]
- 35. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression Based Approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- 36. MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Breeman S, Cotton S, Fielding S, Jones GT. Normative data for the Hospital Anxiety and Depression Scale. Qual life Res. 2015;24(2):391–398. doi: 10.1007/s11136-014-0763-z. [DOI] [PubMed] [Google Scholar]
- 38. Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?Schizophr Res. 2005;79(2–3): 231–238. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 39. Fritz MS, MacKinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18(3):233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence - Imprecision. J Clin Epidemiol. 2011;64(12):1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 41. Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. 2011;343:889–893. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Higgins JPT, Green S (eds.). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011. http://www.handbook.cochrane.org. Accessed September 17, 2018. [Google Scholar]
- 43. Fernandez C, Kennedy HG, Kennedy M. The recovery of factors associated with decision-making capacity in individuals with psychosis. BJPsych Open. 2017:113–119. doi: 10.1192/bjpo.bp.116.004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.