Abstract
Development of schizophrenia relates to both genetic and environmental factors. Functional deficits in many cognitive domains, including the ability to communicate in social interactions and impaired recognition of facial expressions, are common for patients with schizophrenia and might also be present in individuals at risk of developing schizophrenia. Here we explore whether an individual’s polygenic risk score (PRS) for schizophrenia is associated with the degree of interregional similarities in blood oxygen level–dependent (BOLD) signal and gray matter volume of the face-processing network and whether the exposure to early adversity moderates this association. A total of 90 individuals (mean age 22 years, both functional and structural data available) were used for discovery analyses, and 211 individuals (mean age 26 years, structural data available) were used for replication of the structural findings. Both samples were drawn from the Northern Finland Birth Cohort 1986. We found that the degree of interregional similarities in BOLD signal and gray matter volume vary as a function of PRS; lowest interregional correlation (both measures) was observed in individuals with high PRS. We also replicated the gray matter volume finding. We did not find evidence for an interaction between early adversity and PRS on the interregional correlation of BOLD signal and gray matter volume. We speculate that the observed group differences in PRS-related correlations in both modalities may result from differences in the concurrent functional engagement of the face-processing regions over time, eg, via differences in exposure to social interaction with other people.
Keywords: fMRI, the polygenic risk score for schizophrenia, cohort study, face-processing
Introduction
Schizophrenia has one of the highest heritability estimates in psychiatry. For example, in identical twins, the heritability estimate has been estimated as 80%.1 A large genome-wide association study (GWAS) identified 108 independent loci associated with schizophrenia.2
Both impaired face identification and poor performance in emotion processing from faces are common in patients with schizophrenia.3–5 Visual analysis of faces involves many cortical regions, including the fusiform face area (FFA), posterior superior temporal sulcus (STS), and occipital face area.6–12 These regions form the “core system” and provide face selectivity during visual exposure to faces.10 In a previous study, we found that regions within the core system exhibit a high number of connections with other face-processing regions but low population variance in connectivity.13 The core system connects with the “extended system” that provides further processing of faces (eg, emotion).10
Considering the central role of the core system in face processing, it is possible that impairments in face processing present in patients with schizophrenia4 are mediated in part via dysfunction in the core system and its neural connections to other face-processing regions. One possibility might be that genetic variants that predispose to schizophrenia affect neural interactions within the core system. A second possibility is that these genetic variants influence the interactions between the core and the extended system.
Robust epidemiological evidence implicates that exposure to different forms of early stress contributes to the development of schizophrenia,14,15 possibly in interaction with genetic vulnerability.16,17 Previous studies have shown that children exposed to early adversities have difficulties in recognizing different facial expressions.18–23 In our previous study, we found that early adversity correlates with brain response to faces and that this association varies—across the face-processing network—as a function of regional variation in glucocorticoid gene expression.24
Here we explore the possibility that interindividual differences in genetic predisposition to schizophrenia, as assessed with a polygenic risk score (PRS),2 are reflected in the variation of brain function and structure in the face-processing network. Second, given the importance of the interplay between genetic and environmental factors on the genesis of schizophrenia,25,26 we explore the possibility that early adversities interact with genetic predisposition to schizophrenia in functional and structural covariance within the face-processing network.
According to the disconnection hypothesis of schizophrenia,27 a failure of functional integration of the brain is central to the neural pathogenesis of schizophrenia. We hypothesized that the aggregation of schizophrenia-related genetic variants would reduce functional connectivity within the face-processing network. Also, we hypothesized that this reduced functional connectivity would—over time—result in a lower similarity in structural features of the critical nodes of the circuit.
We computed PRS in a sample from the Northern Finland Birth Cohort 1986 (NFBC 1986).28 Self-reported questionnaire data were used to assess the level of early adversity retrospectively. We used magnetic resonance imaging (MRI)–derived functional (blood oxygen level–dependent [BOLD] signal) and structural (gray matter volume) covariances as proxies of “connectivity” in the face-processing network. A total of 25 face-specific regions of interest (ROIs) were used to determine the face-processing network. Two independent samples drawn from the NFBC 1986 were used for the discovery (functional MRI [fMRI] and structural MRI available) and replication (structural MRI available) analyses.
Materials and Methods
Participants
Participants were born between July 1, 1985, and June 30, 1986, in the Northern Finland provinces.28 The local ethical committee approved the study. GWAS data in the NFBC 1986 originates from the visit conducted between August 2001 and June 2002 (N = 3743 in the final sample). Using this sample, we explored whether PRS relates to later-life schizophrenia diagnosis. The register-based information about the individual’s later-life schizophrenia (followed until 2016) was formed by using the following nation-wide registry: Care Register for Health Care, Finnish Centre for Pensions, and the registers of the National Social Insurance Institute. We used both “broad” (International Classification of Diseases, Tenth Revision [ICD-10]: F20, F22, F24, and F25) and “narrow” (ICD-10: F20) definition of schizophrenia.
Next, two NFBC 1986 subsamples (referred to as neuroimaging subsamples hereinafter) were used for discovery and replication analyses. These subsamples are described in detail in the supplementary material. Briefly, the discovery sample (N = 90) was collected between 2007 and 2010 to investigate the risk of psychosis29 and the replication sample (N = 211) was obtained between 2011 and 2013 to examine associations between maternal smoking during pregnancy and the offspring’s brain and mental health.30
Polygenic Risk Score
Detailed information on the collection of the genetic samples and quality control of the genome-wide data are provided in the supplementary material. The results of the GWAS by the Schizophrenia Working Group of the Psychiatric Genomics Consortium were used for the calculation of PRS for schizophrenia in the NFBC 1986 sample (N = 3743). The score was calculated on single-nucleotide polymorphisms (SNPs) reaching genome-wide significance (P = 5 × 10−8). A total of 112 SNPs were found in the imputed NFBC 1986 GWAS dataset. For each participant (N = 3743), we calculated the sum of the risk SNPs multiplied by the effect size (logarithm of the odds ratio for schizophrenia).31 PRS was adjusted for 4 principal components to account for population stratification. In the neuroimaging subsamples, we used the median split of PRS to create “high” and “low” PRS groups.
Early Adversity
Early adversities in the neuroimaging subsamples were evaluated with the Trauma and Distress Scale (TADS).32,33 TADS self-report contains 43 statements, of which 25 statements are used to evaluate psychological traumas (emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect). All statements have 5 responding options scored on a scale from 0 to 4: Never, Rarely, Sometimes, Often, and Nearly always. This study used total TADS scores.
In a previous study, the internal consistency of TADS self-reports was 0.92 (Cronbach’s α), and intraclass coefficients for TADS were good to excellent when compared with the interviewed TADS as a gold standard.32 TADS has been shown to associate with depression and help-seeking for mental problems.32
In the discovery sample, TADS scores correlated with the prodromal syndrome as diagnosed with the SIPS interview.34 Furthermore, TADS scores were higher in individuals exposed (vs nonexposed) to maternal cigarette smoking during pregnancy (replication sample). For this reason, TADS scores were adjusted for the prodromal syndrome (discovery sample) and maternal smoking during pregnancy (replication sample) using linear regression. A median split of adjusted TADS scores was used to create “high” and “low” adversity groups in both samples.
Neuroimaging Data
Detailed descriptions relating to the neuroimaging parameters and preprocessing are provided in the supplementary material. Face-task fMRI data were available only in the discovery sample. T1-weighted MRI scans were available in both the discovery and the replication samples. Neuroimaging data were analyzed with FMRIB Software Library (FSL)35–40 and Analysis of Functional NeuroImages (AFNI)41 using the standard pipeline that included brain extraction, motion correction, spatial smoothing, prewhitening, high-pass filtering, and nonlinear normalization to the standard MNI-152 template (described in detail in the supplementary material).
Face-Task fMRI
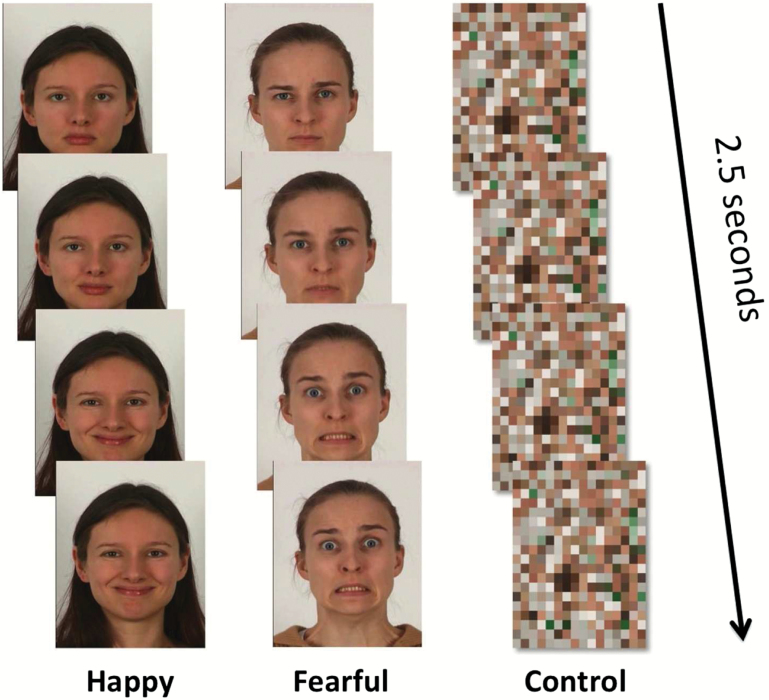
We conducted fMRI while participants viewed 4 blocks of video clips of happy and 4 blocks of video clips of fearful facial expressions presented in pseudorandom order separated by blocks of a dynamic mosaic baseline (control stimulus).42–43 The baseline stimulus was generated from the mosaic images of the facial expressions in the happy and fearful blocks. Blocks of happy and fearful facial expressions consisted of 12 × 2.5-second dynamic video clips showing a neutral facial expression changing into a happy/fearful expression (figure 1). The total duration of each block (facial expressions and dynamic mosaic) was 30 seconds. Six different people, each shown twice, performed the happy facial expressions. Fearful facial expression blocks consisted of facial expressions of 5 different people, each shown 2 or 3 times. The whole imaging session lasted 8 minutes 10 seconds.
Fig. 1.
The stimulus used in this study.
Interregional Correlation of BOLD and Gray Matter Volume in the Face-Processing Network
We used ROIs that were defined in the IMAGEN subsample of 1110 adolescents (mean age = 14 years).6 A total of 25 ROIs relevant for face processing were identified using a probabilistic map as described in detail elsewhere.6 Our previous study showed that these ROIs highly overlap with BOLD response to faces in the NFBC 1986 sample.24
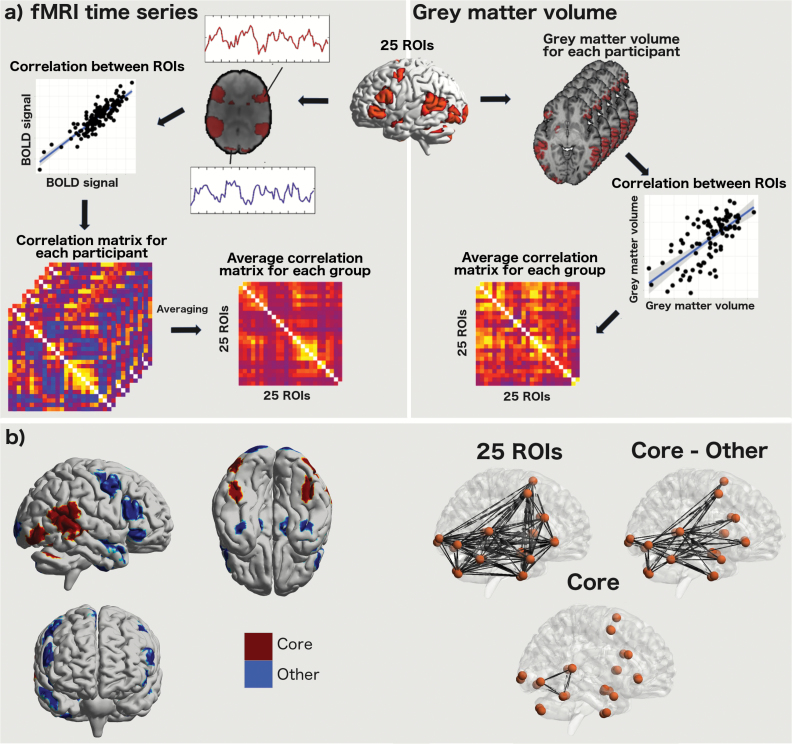
The fMRI data were analyzed as follows (figure 2). For each individual and each ROI, the mean BOLD signal time series was calculated by averaging the BOLD signal from all voxels constituting the ROI at every time point (150 in total). The BOLD time series for each face condition was realized by concatenating the BOLD signal from the corresponding blocks. Nuisance covariates including white matter signals, and cerebrospinal fluid signals, and 6 motion parameters were regressed out from the BOLD signals. For each group, we then created mean 25 × 25 correlation matrices for the BOLD signal by averaging Pearson correlation coefficients between each pair of ROIs. Group comparisons of fMRI data were conducted using these average correlation matrices.
Fig. 2.
(a) Workflow of processing of functional magnetic resonance imaging (fMRI) and structural data. (b) Regions used for the core and other face-processing regions, and “links” (ie, interregional correlation coefficients) of these systems. ROI, region of interest.
Structural data were analyzed as follows (figure 2). T1-weighted MRI scans were used to measure regional gray matter volume and were processed using FSL’s optimized voxel-based morphometry pipeline. From each of the 25 ROIs, we extracted the gray matter volume for each participant. For each group, we created 25 × 25 correlation matrices of gray matter data by correlating participants’ gray matter volume across the 25 ROIs. Group comparisons were conducted using these average correlation matrices of interregional gray matter volume.
Statistical Analyses
For statistical analyses, we used R (http://cran.r-project.org), version 3.4.0. Data were visually inspected to check for normality. If necessary, we used Box–Cox power transformation44 on the data to fulfill the normality criteria. ANOVA, linear regression, linear mixed model,45 logistic regression, Pearson correlation, and t tests were used as specified in “Results” section. Results are visualized with BrainNet Viewer,46 gplots,47 and the pirateplot.
The interregional correlation coefficients were estimated in the each groups’ average correlation matrices. Note, therefore, that we conducted the following group comparisons at the level of average correlation coefficients of each group. The group comparisons were carried out separately for BOLD signal (discovery sample) and gray matter volume data (discovery and replication sample). Correlation coefficients in each groups’ average correlation matrices were converted with Fisher r to z before statistical comparisons.
To analyze the PRS-related differences in interregional correlations, we used the following analytical strategy. First, we compared study groups for the mean of 300 pair-wise correlation coefficients (obtained from each groups’ average correlation matrices) using unpaired t test. Statistical significance was considered at P value of <.05 as we test here the average of all the interregional correlation coefficients within the face-processing network. Second, if this comparison was significant, we compared study groups’ average correlation matrices for the mean correlation coefficients: (1) between the core and the other face-processing regions (mean of 114 pair-wise correlations) and (2) within the core regions of face processing (mean of 15 pair-wise correlations). We defined the core system to include the lateral occipital cortex, posterior STS, and FFA (figure 2).
Results
PRS and Schizophrenia in the NFBC 1986
Using data from the Finnish healthcare registers, we confirmed that the calculated PRS predicts clinically defined “broad” (1 SD increase in PRS associated with OR = 1.8, 95% CI = 1.2–2.7) and “narrow” (1 SD increase in PRS associated with OR = 2.0, 95% CI = 1.3–3.1) schizophrenia in the NFBC 1986. Supplementary figure S1 presents the level of PRS in the NFBC 1986.
Demographic Data for the Neuroimaging Subsamples
We present demographic tables for the discovery and replication samples in the supplementary material. There were no PRS-related differences in age, sex, intelligence quotient, body mass index, reporting of TADS, alcohol dependency (Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders [Fourth Edition] Axis I disorders), smoking, prodromal syndrome, the parental risk of psychosis, and exposure to maternal cigarette smoking. Internal consistency of TADS was acceptable (Cronbach’s α > .7) in both the discovery and the replication samples: 0.76 and 0.79, respectively.
The Interregional Similarity in BOLD Signal (Discovery Sample)
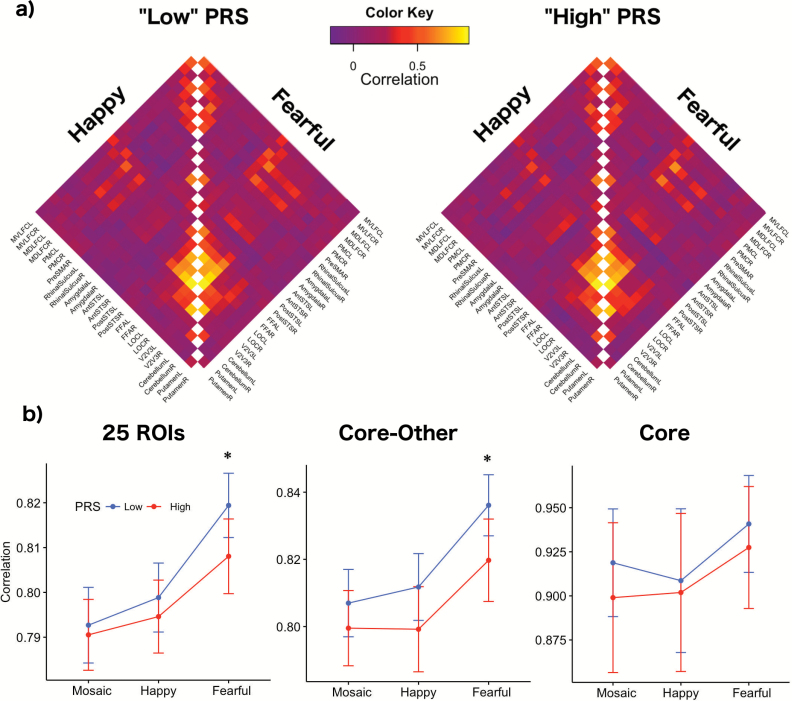
Figure 3 provides correlation matrices and interregional pair-wise correlations for “high” and “low” PRS. For happy facial expressions, we did not find any associations between PRS and mean interregional correlation coefficients in the BOLD signal (“high” vs “low” PRS, t test, P = .46, Cohen’s d = 0.06). For fearful faces, interregional similarities in the BOLD signal differed between “high” and “low” PRS groups (t test, P = .04, Cohen’s d = 0.17); the lowest interregional pair-wise correlation was observed in “high” PRS.
Fig. 3.
(a) Correlation matrices for fMRI data. Abbreviations: mid-ventrolateral frontal cortex (MVLFC), mid-dorsolateral frontal cortex (MDLFC), premotor cortex (PMC), pre-supplementary motor area (PreSMA), superior temporal sulcus (STS), fusiform face area (FFA), lateral occipital cortex (LOC), left (L), right (R), anterior (Ant), posterior (Post). (b) Interregional similarities in blood oxygen level–dependent (BOLD) fMRI signal during happy and fearful facial expressions and dynamic mosaic (control stimulus). Asterisk represents statistical significance (P < .05). PRS, polygenic risk score; ROI, region of interest.
The mean correlation in BOLD signal across the core and the other face-processing regions also varied as a function of the PRS; the lowest correlation was observed in the high PRS (“high” vs “low,” t test, P = .03, Cohen’s d = 0.28). There were no significant PRS-related correlation differences in BOLD signal within the core system in the discovery sample (“high” vs “low,” t test, P = .52, Cohen’s d = 0.24).
We also conducted linear mixed effects analyses of interregional correlations of the 25 ROIs to test for PRS (ie “high” vs “low”) × fMRI Stimulus interactions. PRS (“high” vs “low”) and Stimulus (dynamic mosaic control stimulus, and happy and fearful facial expressions) represented fixed effects. Random slope terms were included to take into account interstimuli variability of individual correlations (eg, between the amygdala and FFA) of the face-processing network. Linear mixed effect analysis showed PRS × fMRI Stimulus interaction on the average correlations of the 25 ROIs (ANOVA, F(2,1196) = 8.7, P = .0002) and the average correlations between the core and the other face-processing regions (ANOVA, F(2,1196) = 4.0, P = .02). No interactions were observed in the average correlations within the core network (ANOVA, F(2,1196) = 1.9, P = .16).
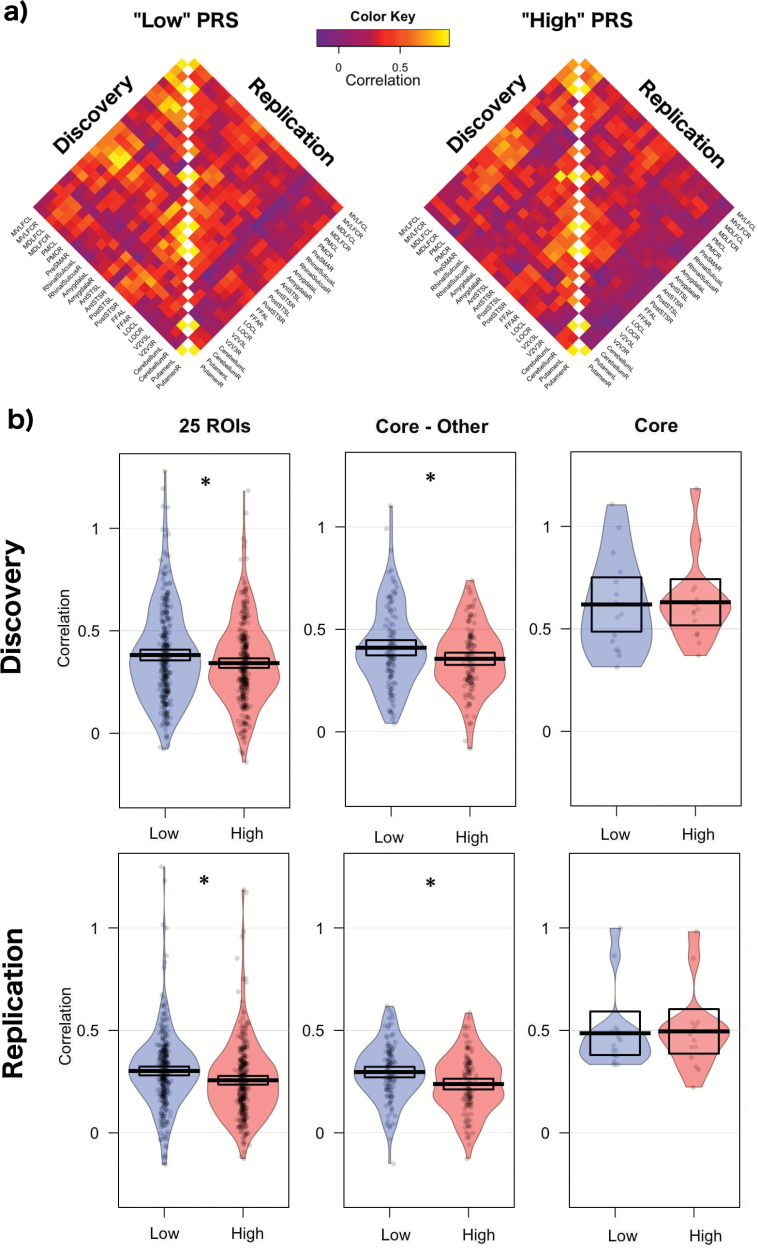
The Interregional Similarity in Gray Matter Volume (Discovery and Replication Samples)
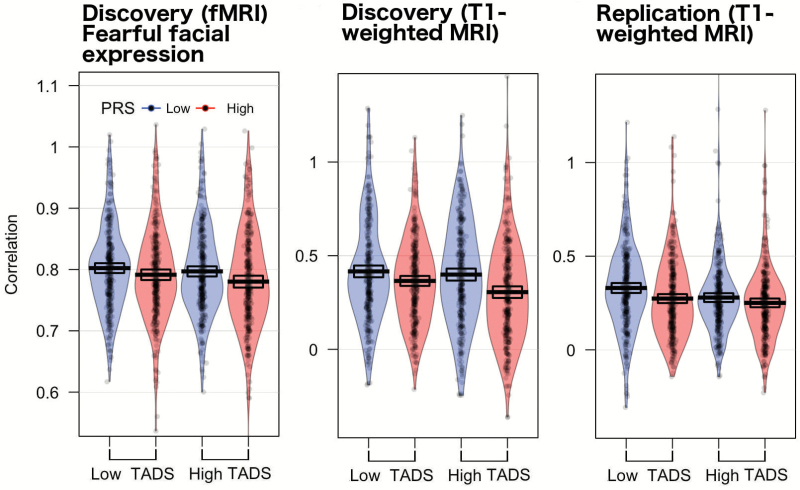
Figure 4 provides an overview of the degree of interregional similarities in gray matter volume across the 25 regions in each of the 2 groups. In the discovery sample, the mean correlation across the 25 regions varied as a function of the PRS; the mean interregional correlation coefficient was the lowest in the high PRS (t test, P = .03, Cohen’s d = 0.18). This result was similar in the replication sample: the mean interregional correlation coefficient was the lowest in the high PRS group (t test, P = .003, Cohen’s d = 0.24). The mean correlation in gray matter between the core and the other regions varied as a function of the PRS; the lowest correlation was observed in the high PRS in both the discovery (“high” vs “low”, t test, P = .03, Cohen’s d = 0.30) and the replication (“high” vs “low”, t test, P = .002, Cohen’s d = 0.42) samples. There were no significant PRS-related correlation differences in gray matter within the core system in the discovery sample (t test, P = .9, Cohen’s d = 0.05) nor in the replication sample (t test, P = .9, Cohen’s d = 0.05).
Fig. 4.
(a) Correlation matrices for gray matter volume data. (b) The degree of interregional similarities in gray matter volume. Asterisk represents statistical significance (P < 005). PRS, polygenic risk score; ROI, region of interest.
Interplay Between Early Adversity and PRS for Schizophrenia (Discovery and Replication Samples)
We also explored the interplay between early adversity and PRS for schizophrenia on the mean interregional pair-wise correlations (figure 5). We found no adversity by PRS interaction (ANOVA, F(1,1196) = 0.4, P = .5) on interregional similarity in BOLD signal. With structural data we did not detect adversity by PRS interactions in the discovery (ANOVA, F(1,1196) = 1.9, P = .16) nor in the replication (ANOVA, F(1,1196) = 1.3, P = .25) sample. Early adversity tended to show the main effect in fMRI data (ANOVA, F(1,1197) = 3.6, P = .06). With structural data, the main effect of early adversity was significant in both the discovery (ANOVA, F(1,1197) = 6.2, P = .01) and the replication (ANOVA, F(1,1197) = 9.0, P = .003) sample.
Fig. 5.
The interaction between early adversity (total Trauma and Distress Scale [TADS] scores) and polygenic risk score (PRS) on the degree of interregional similarities of blood oxygen level–dependent (BOLD) fMRI and gray matter volume.
Discussion
PRS offers an exciting extension to previous studies using the familial risk of schizophrenia data to investigate schizophrenia-related variation in heritability across healthy individuals. We demonstrated that aggregation of these genetic variants associate with clinically defined schizophrenia in the NFBC 1986. In this light, PRS can be considered as a proxy for genetic vulnerability for schizophrenia with clinical implications in the NFBC 1986.
Young adults with “high” genetic vulnerability to schizophrenia (vs “low”) appear to differ, albeit with a small effect-size, in the degree of similarity in BOLD signal and gray matter volume across the face-processing network. Specifically, our findings demonstrated that the degree of interregional similarities in BOLD signal and gray matter volume varies as a function of PRS group; lower covariance was observed in individuals with high vs low PRS. We were able to replicate the above structural-covariance finding. With fMRI, the association between PRS and interregional correlation appeared to be stimulus-specific (ie, during visual exposure to fearful facial expressions).
We explored further whether the lower interregional similarities in BOLD signal and gray matter volume are partly driven by (1) the interconnection correlations of the core and other face-processing regions or (2) the intraconnection differences within the core system. We showed consistently with both modalities that the interregional similarities between the core and the other face processing vary as a function of PRS for schizophrenia; the lowest correlation is observed in individuals with high PRS for schizophrenia. No such differences, however, were found in the associations within the core system. We speculate that this finding indicates that the aggregation of PRS may not affect the neural network responsible of recognition of faces (vs other objects) but may lead to perturbations in the extraction of information relating to the significance of faces (eg, identity or different emotions).
To the best of our knowledge, this is the first study to explore the association between PRS and interregional “connectivity” of the face-processing network. Previous fMRI studies comparing individuals with vs without familial risk of psychosis have found lower functional connectivity between different face-processing regions during visual exposure to faces (eg, between the amygdala and prefrontal cortex).42,48,49 These results combined with our findings might suggest that the heritability of schizophrenia plays a role in face processing in the brain, possibly by affecting the links of the face-processing network.
Similarities in gray matter volume across the brain have been linked to structural maturation (ie, the degree of interregional loss in the gray matter over time).50,51 Therefore, we cannot entirely rule out the possibility that the observed structural MRI findings arise in part from different global gray matter development patterns in the brain that may encompass other networks in addition to the face-processing network. BOLD fMRI results in our study, however, were in line with the structural findings, which supports the conclusion that the structural results are reflected in part by the functional engagements of the face-processing network. This is not implausible as one previous study demonstrated that the properties of functional connectivity in fMRI BOLD signal in different brain regions across the brain are shared in part with the degree of morphological similarities in the gray matter of the same brain regions.51 In this light, we speculate that the observed PRS-related correlation differences may result from differences in the concurrent functional engagement of the same areas over time, eg, via differences in exposure to social interaction with other people.
As environmental factors interplay with genetic vulnerability to schizophrenia according to the different etiological models of schizophrenia25,26 and given the substantial epidemiological evidence supporting a link between early adversity and schizophrenia,14,15,52,53 we further investigated the interplay between early adversities and PRS for schizophrenia. We did not find early adversity by PRS interaction on interregional correlation in the face-processing network with BOLD fMRI or structural data. Nonetheless, we demonstrated a main effect of adversity on the correlations of the 25 ROIs.
There were several strengths in the present study. Using both functional and structural imaging, we were able to explore the functional and structural properties of interregional similarities in the face-processing network. For both the discovery and the replication analyses, we used a unique dataset in NFBC 1986; participants of our study were born in the same geographical region, had a similar ethnic and cultural background, and were of the same age at the time of the study. Furthermore, participants in our study were in their 20s, which is the peak age for developing schizophrenia.54,55 In this light, the timing of our study can be considered as optimal.
This study also has limitations that should be taken into account in future studies. First, we used a median split of PRS to allow for calculation of correlation matrices in structural MRI data. Our study had a relatively modest sample size for genetic analyses. Future studies with larger samples should expand our work by using more granular PRS-based stratification (eg, deciles). Our analyses were conducted at the group level as one cannot calculate structural covariance at the level of individual participant. Thus, one should also interpret our results at the group level as conclusions on individual participants cannot be drawn here. Also, a limitation is the lack of BOLD fMRI in the replication sample.
Also, questionnaires that measure early adversity may be affected by recall bias, although the TADS has been shown to be a valid and reliable instrument for assessing retrospectively reported childhood traumas.32,33 Information from families, local international adoption consultation services, or social services, however, could have been used as additional information for the degree of experienced early adversity. Future studies with larger sample size should also address the interaction PRS and different adversity types on the face-processing network.
Conclusions
PRS may relate to differences in the interregional correlations between the core and other face-processing regions. Also, we found that both the exposure to early adversity and PRS have an additive effect on the interregional degree of similarity of BOLD signal and gray matter volume. Further research with larger sample sizes and different PRS cutoffs is needed to replicate our findings. We suggest that our results indicate that “high” (vs “low”) PRS may not affect the neural network responsible of recognition of faces but may lead to perturbations in the extraction of information relating to the significance of faces.
Funding
Finnish Medical Association (to JL); Yrjö Jahnsson’s Foundation (to JL); Psykiatrian tutkimussäätiö (Finnish Foundation for Psychiatric Research) (to JL); Jalmari and Rauha Ahokas Foundation (to JL); Academy of Finland (124257, 212818, 214273 to JV); Sigrid Juselius Foundation (to JV); Signe and Ane Gyllenberg Foundation, Finland (to JV); Alfred Kordelin Foundation (to JL); Orion Foundation (to JL).
Supplementary Material
Acknowledgments
The facial stimuli used in this study were contributed by Professor Mikko Sams, Aalto University, Finland. Dr Kiviniemi, Dr Barnett, Dr Murray, Professor Jones, Professor Paus and Professor Veijola planned the NFBC 1986 substudy data collections. Dr Kiviniemi conducted the brain imaging in the NFBC 1986 sample. Professor Veijola performed the data collection of NFBC 1986. Dr Nordström and MD Lieslehto analyzed the genetic data. MD Lieslehto analyzed the fMRI data and conducted statistical analyses. MD Lieslehto wrote the first draft of the manuscript and all authors contributed to its subsequent revisions. All authors have accepted the final version of the manuscript. All the authors declared no conflicts of interest.
References
- 1. Cardno AG, Gottesman II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet. 2000;97:12–17. [PubMed] [Google Scholar]
- 2. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan RC, Li H, Cheung EF, Gong QY. Impaired facial emotion perception in schizophrenia: a meta-analysis. Psychiatry Res. 2010;178:381–390. [DOI] [PubMed] [Google Scholar]
- 4. Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kohler CG, Bilker W, Hagendoorn M, Gur RE, Gur RC. Emotion recognition deficit in schizophrenia: association with symptomatology and cognition. Biol Psychiatry 2000;48:127–136. [DOI] [PubMed] [Google Scholar]
- 6. Tahmasebi AM, Artiges E, Banaschewski T, et al. ; IMAGEN Consortium Creating probabilistic maps of the face network in the adolescent brain: a multicentre functional MRI study. Hum Brain Mapp. 2012;33:938–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perrett DI, Smith PA, Potter DD, et al. . Neurones responsive to faces in the temporal cortex: studies of functional organization, sensitivity to identity and relation to perception. Hum Neurobiol. 1984;3:197–208. [PubMed] [Google Scholar]
- 8. Perrett DI, Smith PA, Potter DD, et al. . Visual cells in the temporal cortex sensitive to face view and gaze direction. Proc R Soc Lond B Biol Sci. 1985;223:293–317. [DOI] [PubMed] [Google Scholar]
- 9. Hasselmo ME, Rolls ET, Baylis GC. The role of expression and identity in the face-selective responses of neurons in the temporal visual cortex of the monkey. Behav Brain Res. 1989;32:203–218. [DOI] [PubMed] [Google Scholar]
- 10. Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. [DOI] [PubMed] [Google Scholar]
- 11. Clark VP, Keil K, Maisog JM, Courtney S, Ungerleider LG, Haxby JV. Functional magnetic resonance imaging of human visual cortex during face matching: a comparison with positron emission tomography. Neuroimage 1996;4:1–15. [DOI] [PubMed] [Google Scholar]
- 12. Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994;14:6336–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dickie EW, Tahmasebi A, French L, et al. ; IMAGEN consortium; IMAGEN consortium; IMAGEN consortium Global genetic variations predict brain response to faces. PLoS Genet. 2014;10:e1004523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varese F, Smeets F, Drukker M, et al. . Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matheson SL, Shepherd AM, Pinchbeck RM, Laurens KR, Carr VJ. Childhood adversity in schizophrenia: a systematic meta-analysis. Psychol Med. 2013;43:225–238. [DOI] [PubMed] [Google Scholar]
- 16. Tienari P. Interaction between genetic vulnerability and family environment: the Finnish Adoptive Family Study of Schizophrenia. Acta Psychiatr Scand. 1991;84:460–465. [DOI] [PubMed] [Google Scholar]
- 17. Wahlberg KE, Wynne LC, Oja H, et al. . Gene-environment interaction in vulnerability to schizophrenia: findings from the Finnish Adoptive Family Study of Schizophrenia. Am J Psychiatry. 1997;154:355–362. [DOI] [PubMed] [Google Scholar]
- 18. Camras LA, Grow JG, Ribordy SC. Recognition of emotional expression by abused children. J Clin Child Psychol. 1983;12:325–328. [Google Scholar]
- 19. Camras LA, Ribordy S, Hill J, Martino S, Spaccarelli S, Stefani R. Recognition and posing of emotional expressions by abused children and their mothers. Dev Psychol. 1988;24:776–781. [Google Scholar]
- 20. Camras LA, Ribordy S, Hill J, et al. . Maternal facial behavior and the recognition and production of emotional expression by maltreated and nonmaltreated children. Dev Psychol. 1990;26:304–312. [Google Scholar]
- 21. During SM, McMahon RJ. Recognition of emotional facial expressions by abusive mothers and their children. J Clin Child Adolesc Psychol. 1991;20:132–139. [Google Scholar]
- 22. Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: developmental effects of child abuse and neglect. Dev Psychol. 2000;36:679–688. [DOI] [PubMed] [Google Scholar]
- 23. Koizumi M, Takagishi H. The relationship between child maltreatment and emotion recognition. PLoS One. 2014;9:e86093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lieslehto J, Kiviniemi V, Mäki P, et al. ; IMAGEN. Early adversity and brain response to faces in young adulthood. Hum Brain Mapp. 2017;38:4470–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104:667–685. [DOI] [PubMed] [Google Scholar]
- 26. Jones SR, Fernyhough C. A new look at the neural diathesis–stress model of schizophrenia: the primacy of social-evaluative and uncontrollable situations. Schizophr Bull. 2007;33:1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome?Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 28. Järvelin MR, Hartikainen-Sorri AL, Rantakallio P. Labour induction policy in hospitals of different levels of specialisation. Br J Obstet Gynaecol. 1993;100:310–315. [DOI] [PubMed] [Google Scholar]
- 29. Veijola J, Mäki P, Jääskeläinen E, et al. . Young people at risk for psychosis: case finding and sample characteristics of the Oulu Brain and Mind Study. Early Interv Psychiatry. 2013;7:146–154. [DOI] [PubMed] [Google Scholar]
- 30. Ramsay H, Barnett JH, Murray GK, et al. . Smoking in pregnancy, adolescent mental health and cognitive performance in young adult offspring: results from a matched sample within a Finnish cohort. BMC Psychiatry. 2016;16:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kendler KS. The schizophrenia polygenic risk score: to what does it predispose in adolescence?JAMA Psychiatry. 2016;73:193–194. [DOI] [PubMed] [Google Scholar]
- 32. Salokangas RK, Schultze-Lutter F, Patterson P, et al. . Psychometric properties of the trauma and distress scale, TADS, in an adult community sample in Finland. Eur J Psychotraumatol. 2016;7:30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patterson P, Skeate A, Birchwood M.. TADS-EPOS 1.2. Birmingham: University of Birmingham; 2002. [Google Scholar]
- 34. McGlashan TH, Miller TJ, Woods SW, Rosen JL, Hoffman RE, Davidson L.. SIPS, Structured Interview for Prodromal Syndromes - Version for present Prodromal syndromes. Version 3.0. New haven, CT: PRIME Research Clinic, Yale School of Medicine; 2001. [Google Scholar]
- 35. Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. [DOI] [PubMed] [Google Scholar]
- 36. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 37. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. [DOI] [PubMed] [Google Scholar]
- 38. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. [DOI] [PubMed] [Google Scholar]
- 40. Worsley KJ. Statistical analysis of activation images. ch 14. In: Jezzard P, Matthews PM, Smith SM, eds. Functional MRI: An Introduction to Methods. Oxford, UK: Oxford University Press; 2001. [Google Scholar]
- 41. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 42. Pulkkinen J, Nikkinen J, Kiviniemi V, et al. . Functional mapping of dynamic happy and fearful facial expressions in young adults with familial risk for psychosis-Oulu Brain and Mind Study. Schizophr Res. 2015;164:242–249. [DOI] [PubMed] [Google Scholar]
- 43. Rahko JS, Paakki JJ, Starck TH, et al. . Valence scaling of dynamic facial expressions is altered in high-functioning subjects with autism spectrum disorders: an fMRI study. J Autism Dev Disord. 2012;42:1011–1024. [DOI] [PubMed] [Google Scholar]
- 44. Fox J, Weisberg S.. An {R} Companion to Applied Regression. 2nd ed Thousand Oaks, CA: Sage; 2011. Http://socserv.socsci.mcmaster.ca/jfox/Books/Companion [Google Scholar]
- 45. Kuznetsova A, Brockhoff P, Christensen R. lmerTest package: tests in linear mixed effects models. Journal of Statistical Software. 2017;82:1–26. [Google Scholar]
- 46. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Warnes GR, Bolker B, Bonebakker L, et al. . Gplots: various R programming tools for plotting data. R package 3.0.1.
- 48. Diwadkar VA, Wadehra S, Pruitt P, et al. . Disordered corticolimbic interactions during affective processing in children and adolescents at risk for schizophrenia revealed by functional magnetic resonance imaging and dynamic causal modeling. Arch Gen Psychiatry. 2012;69:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cao H, Bertolino A, Walter H, et al. . Altered functional subnetwork during emotional face processing: a potential intermediate phenotype for schizophrenia. JAMA Psychiatry. 2016;73:598–605. [DOI] [PubMed] [Google Scholar]
- 50. Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14:322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alexander-Bloch A, Raznahan A, Bullmore E, Giedd J. The convergence of maturational change and structural covariance in human cortical networks. J Neurosci. 2013;33:2889–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morgan C, Gayer-Anderson C. Childhood adversities and psychosis: evidence, challenges, implications. World Psychiatry. 2016;15:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bentall RP, Wickham S, Shevlin M, Varese F. Do specific early-life adversities lead to specific symptoms of psychosis? A study from the 2007 the adult psychiatric morbidity survey. Schizophr Bull. 2012;38:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Häfner H, an der Heiden W, Behrens S, et al. . Causes and consequences of the gender difference in age at onset of schizophrenia. Schizophr Bull. 1998;24:99–113. [DOI] [PubMed] [Google Scholar]
- 55. Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence?Nat Rev Neurosci. 2008;9:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.