Antimicrobial resistance is a global problem. The antimicrobial ceftiofur has been used worldwide for disease prevention in poultry production, resulting in a greatly increased resistance to this antimicrobial important in poultry and human medicine. Our study examined the impact of ceftiofur cessation and its replacement with the antimicrobial combination lincomycin-spectinomycin, a common practice in the industry. Our study demonstrated a decrease in ceftiofur resistance after the cessation of ceftiofur use, although the resistance genes remain ubiquitous in all phases of poultry production, showing that poultry remains a reservoir for ceftiofur resistance and requiring continued vigilance. We also observed a decrease in multidrug resistance involving different antimicrobial classes after cessation of ceftiofur but an increase following use of lincomycin-spectinomycin, indicating that this antimicrobial use should be questioned. Reduced resistance to ceftiofur in poultry may translate to better treatment efficacy, decreased morbidity/mortality, and enhanced food safety for humans.
KEYWORDS: antimicrobial resistance, blaCMY-2, blaCTX-M, ceftiofur, cessation, chicken, lincomycin, spectinomycin
ABSTRACT
Ceftiofur, a third-generation cephalosporin antimicrobial, was used in Canadian hatcheries for many years to prevent early mortality in chicks, leading to a high prevalence of cephalosporin resistance in Escherichia coli in chickens. Preventive use of ceftiofur in hatcheries ceased in 2014. We examined the effect of ceftiofur cessation (n = 40 flocks with ceftiofur and n = 28 flocks without antimicrobial at hatchery) and its replacement with an antimicrobial combination, lincomycin-spectinomycin (n = 32), at the hatchery on the proportion of samples with E. coli positive for extended-spectrum-β-lactamase (ESBL) and AmpC β-lactamase-related genes, and on the multidrug resistance profiles of ESBL/AmpC-positive E. coli in broilers and their associated breeders (n = 46 samples), at 1 year postcessation. For indicator E. coli from nonenriched media, a significant decrease postcessation in the proportion of samples harboring E. coli isolates positive for blaCMY-2 and/or blaCTX-M was observed. In contrast, following enrichment in medium containing ceftriaxone (1 mg/liter) to facilitate recovery of ESBL/AmpC β-lactamase-producing E. coli colonies, both pre- and postcessation, 99% of the samples harbored E. coli positive for blaCMY-2 or blaCTX-M. Among the 15 tested antimicrobial agents, flocks receiving lincomycin-spectinomycin after cessation of ceftiofur showed a significantly greater nonsusceptibility to aminoglycosides, folate inhibitors, phenicols, and tetracyclines and a greater proportion of possible extensively drug-resistant E. coli than those receiving ceftiofur or no antimicrobial at hatchery. This study clearly demonstrates an initial decrease in ESBL/AmpC-positive E. coli following the cessation of ceftiofur in the hatchery but an increase in antimicrobial non-β-lactam resistance of ESBL/AmpC-positive E. coli following replacement with lincomycin-spectinomycin.
IMPORTANCE Antimicrobial resistance is a global problem. The antimicrobial ceftiofur has been used worldwide for disease prevention in poultry production, resulting in a greatly increased resistance to this antimicrobial important in poultry and human medicine. Our study examined the impact of ceftiofur cessation and its replacement with the antimicrobial combination lincomycin-spectinomycin, a common practice in the industry. Our study demonstrated a decrease in ceftiofur resistance after the cessation of ceftiofur use, although the resistance genes remain ubiquitous in all phases of poultry production, showing that poultry remains a reservoir for ceftiofur resistance and requiring continued vigilance. We also observed a decrease in multidrug resistance involving different antimicrobial classes after cessation of ceftiofur but an increase following use of lincomycin-spectinomycin, indicating that this antimicrobial use should be questioned. Reduced resistance to ceftiofur in poultry may translate to better treatment efficacy, decreased morbidity/mortality, and enhanced food safety for humans.
INTRODUCTION
One of the most important causes of early mortality in broiler chicks is omphalitis, mostly caused by avian pathogenic Escherichia coli (APEC), a subgroup of extraintestinal pathogenic E. coli (ExPEC) (1, 2). Ceftiofur, a third-generation cephalosporin antimicrobial, has been administered for over 15 years either in ovo or by subcutaneous injection at the hatchery, in order to reduce early chick mortality in many countries (3). Consequently, an increased prevalence of extended-spectrum-β-lactamase (ESBL) and AmpC β-lactamase-producing Escherichia coli has been reported worldwide (4–6); this increased prevalence has resulted in an increased resistance to extended-spectrum cephalosporins in the broiler poultry production chain. This is a public health concern due to cross-resistance with other extended-spectrum cephalosporins, such as ceftriaxone and cephamycin, antimicrobials that are used widely in human medicine and classified by the World Health Organization as highest-priority critically important antimicrobials (7–10). ESBL/AmpC-associated resistance genes detected in chickens include blaCMY-2, blaSHV, blaCTX-M, blaOXA, and blaTEM (9, 11). Ceftiofur has been used in food-producing animals in North America since 1989, and the blaCMY-2 gene was first reported in 1998 from cattle (12).
In Canada in 2014, the poultry industry eliminated the preventive use of ceftiofur in hatcheries for the second time (3). The ceftiofur resistance issue was raised over a decade ago, and Canadian hatcheries had already voluntarily stopped ceftiofur use but used alternative preventive drugs such as gentamicin and lincomycin-spectinomycin. Following this first cessation in 2005, a decline in the prevalence of cephalosporin-resistant Salmonella enterica serovar Heidelberg isolates in chicken meat was observed, although the effect on prevalence of resistance in E. coli was not clear, as a decrease did not occur in all tested provinces (10). Ceftiofur was then reintroduced in 2007 and alternated with lincomycin-spectinomycin, both being off-label in ovo uses. Recent Canadian studies have shown a decrease in the proportion of clinical isolates possessing ESBL/AmpC-associated resistance genes after the second cessation in 2014 (13–15). In addition, the prevalence of resistant E. coli from healthy broilers on farms was decreased within a year after ceftiofur cessation at hatcheries in Japan, from 16.4% in 2010 and 16.8% in 2011 to 9.2% in 2012 and 4.6% in 2013 (4). A decrease in the prevalence of Salmonella harboring blaCMY-2 in chicken meat was also observed in Japan in the same years (16). In Canada, whereas some hatcheries completely stopped using antimicrobials following the cessation of ceftiofur in 2014 (17), other hatcheries replaced it with lincomycin-spectinomycin (3, 14). This use has recently been associated with coresistance to gentamicin in Canada (14).
To our knowledge, there has been no convenient sampling study of healthy flocks in Canada comparing the impact on resistant E. coli of ceftiofur use, use of no other antimicrobial in ovo, and replacement with lincomycin-spectinomycin at the hatchery. Based on the recent studies following the 2014 cessation of ceftiofur, we expected to see a decrease in the proportion of resistance to ceftiofur (and in the presence of blaCMY-2 and possibly blaCTX-M) in flocks without antimicrobial use in ovo, but an increase in phenotypic resistance to spectinomycin and gentamicin after replacement with use of lincomycin-spectinomycin in ovo. Hence, our objectives were to examine the effect of ceftiofur use, ceftiofur cessation, and replacement with lincomycin-spectinomycin at the hatchery on the proportion of samples with E. coli possessing the ESBL/AmpC genes blaCMY-2, blaSHV, blaOXA, blaCTX-M, and blaTEM and on the antimicrobial resistance profiles of these isolates in young chicks, broilers, and breeders of an integrated pyramid.
RESULTS
Decreased proportion of nonenriched samples with E. coli positive for blaCMY-2 or blaCTX-M following cessation of in ovo administration of ceftiofur in hatchery.
Before the cessation of ceftiofur, the proportion of samples with E. coli positive for blaCMY-2 was very high for the meconium (90%), decreasing to 60% in the feces of broilers at the end of fattening (P < 0.03) (Table 1). Similarly, the proportion of samples with E. coli positive for blaCTX-M was higher for the meconium (20%) than in broilers (5%). When no antimicrobial was used in ovo, a decrease in the proportion of samples with E. coli positive for blaCMY-2 was observed in the meconium and broiler feces, being significant (P = 0.002) only for the meconium (the same trend was observed for blaCTX-M but was not statistically different). The proportions of meconium and broiler samples in 2015 with E. coli positive for blaCMY-2 or blaCTX-M were not significantly different for flocks receiving lincomycin-spectinomycin and those that received no antimicrobial in ovo. Following the cessation of ceftiofur, as observed in flocks not receiving any antimicrobial in ovo, birds receiving lincomycin-spectinomycin showed a significant decrease in the proportion of samples harboring E. coli possessing the blaCMY-2 gene, from 90% to 50% (P < 0.01), for the meconium and a decrease, although not significant, in the proportion of samples harboring E. coli possessing blaCMY-2, from 60% to 44%, for the broiler feces. No significant differences were observed between groups for the proportion of samples with E. coli positive for the blaTEM gene. Overall, the proportion of samples with E. coli positive for the blaSHV gene was very low (see Table S3 in the supplemental material). No samples with E. coli positive for the blaOXA gene were detected.
TABLE 1.
Effect of cessation of in ovo administration of ceftiofur and replacement with lincomycin-spectinomycin on the proportion of non-enriched samples from newly hatched, broiler, and breeder birds with E. coli isolates positive for ESBL/AmpC resistance genes
| Sample origin | Before or after ceftiofur cessation in broiler | Antimicrobial(s) administered in ovo | No. of samples (no. of isolates)a | % of samples with one or more isolates positive for: |
||
|---|---|---|---|---|---|---|
| blaTEM | blaCMY-2b | blaCTX-M | ||||
| Meconium of newly hatched chicks | Before (2014) | Ceftiofur | 20 (96) | 45 | 90A | 20 |
| After (2015) | None | 14 (69) | 71 | 36B | 0 | |
| After (2015) | Lincomycin-spectinomycin | 16 (80) | 56 | 50B | 0 | |
| Pooled feces of broilers | Before (2014) | Ceftiofur | 20 (100) | 50 | 60 | 5 |
| After (2015) | None | 14 (70) | 43 | 50 | 0 | |
| After (2015) | Lincomycin-spectinomycin | 16 (79) | 69 | 44 | 6 | |
| Pooled feces of breeders | Before (2014) | Ceftiofur | 22 (109) | 64 | 0 | 0 |
| Before (2015) | Ceftiofur | 24 (119) | 60 | 16 | 0 | |
All isolates tested were uidA positive (likely E. coli).
Different letters in the same column of results for genes and same sample origin indicate significantly different results.
High proportion of E. coli positive for blaCMY-2 from all sources in potential ESBL/AmpC producer collection.
In contrast to the findings when examining the indicator E. coli collection, almost all ceftriaxone-enriched samples (145 of 146 tested) harbored cephalosporin-resistant E. coli (one negative meconium receiving ceftiofur) (Table 2). Almost all ceftriaxone-enriched samples demonstrating growth (n = 144) harbored E. coli positive for blaCMY-2, except one sample after the ceftiofur cessation that harbored only E. coli positive for blaCTX-M. Although the blaCTX-M gene was much less prevalent, it was present in the entire production chain of both years. Few differences between flocks before and after the cessation of in ovo administration of ceftiofur were observed with respect to the proportion of samples harboring E. coli isolates positive for blaCTX-M, blaTEM, or blaSHV. One exception was that the proportion of samples harboring E. coli positive for blaCTX-M (P < 0.05) or blaTEM (P < 0.01) was higher for pooled feces of broilers for lincomycin-spectinomycin-receiving flocks than for flocks with no in ovo antimicrobial administration. As observed for the nonenriched samples, the proportion of ceftriaxone-enriched samples harboring E. coli positive for blaSHV was very low (Table S3) and blaOXA was not detected in any sample.
TABLE 2.
Effect of cessation of in ovo administration of ceftiofur and the replacement with lincomycin-spectinomycin on the proportion of ceftriaxone-enriched samples from newly hatched, broiler, and breeder birds with E. coli isolates positive for ESBL/AmpC resistance genes
| Sample origin | Before or after ceftiofur cessation in broiler | Antimicrobial(s) administered in ovo | No. (%) of samples with growth after ceftriaxone enrichment | No. of isolatesa |
% of samples with one or more isolates positive forb
: |
||
|---|---|---|---|---|---|---|---|
| blaTEM | blaCMY-2 | blaCTX-M | |||||
| Meconium of newly hatched chicks | Before (2014) | Ceftiofur | 19 (95) | 57 | 26 | 100 | 16 |
| After (2015) | None | 14 (100) | 40 | 43 | 93 | 7 | |
| After (2015) | Lincomycin-spectinomycin | 16 (100) | 47 | 44 | 100 | 0 | |
| Pooled feces of broilers |
Before (2014) | Ceftiofur | 20 (100) | 60 | 40 | 100 | 20 |
| After (2015) | None | 14 (100) | 42 | 7A | 100 | 0A | |
| After (2015) | Lincomycin-spectinomycin | 16 (100) | 48 | 56B | 100 | 31B | |
| Pooled feces of breeders |
Before (2014) | Ceftiofur | 22 (100) | 66 | 45 | 100 | 14 |
| Before (2015) | Ceftiofur | 24 (100) | 72 | 67 | 100 | 8 | |
All isolates tested were uidA positive (likely E. coli). For each sample, 2 or 3 isolates were tested.
Different letters in the same column of results for genes and same sample origin indicate significantly different results.
Different antimicrobial nonsusceptibilities of blaCMY-2- and blaCTX-M-positive E. coli isolates in the ESBL/AmpC producer collection.
Thus, we observed in the indicator collection that replacement with lincomycin-spectinomycin did not appear to affect the proportion of samples harboring E. coli with resistance genes blaCMY-2 and blaCTX-M compared to that in flocks without in ovo administration of an antimicrobial. As enrichment with ceftriaxone showed that E. coli isolates with the ESBL/AmpC resistance gene blaCMY-2 are ubiquitous in most examined samples, we wished to determine the effect of lincomycin-spectinomycin administration on the nonsusceptibility of these isolates to antimicrobials of the different classes and on the level of multidrug resistance (MDR). In our previous studies, we saw an increase in the level of MDR of E. coli in the potential ESBL/AmpC producer collection compared to the indicator collection (18, 19); hence, we focused on the study of antimicrobial resistance only in this collection.
Most (274/290) of the isolates from the ceftriaxone-enriched samples from all sources that were selected for antimicrobial susceptibility testing were positive for blaCMY-2, whereas 20/290 were positive for blaCTX-M. One isolate was negative for blaCMY-2 and blaCTX-M but positive for blaSHV and blaTEM. The isolates positive for blaCTX-M were nonsusceptible to a significantly lower number of different antimicrobials than those positive for blaCMY-2 (P < 0.01) or both blaCMY-2 and blaCTX-M (P < 0.0001). Among the 15 different antimicrobials examined, the maximum number of antimicrobials for which nonsusceptibility was observed in isolates positive for blaCTX-M alone (n = 15) was 8, whereas the maximum numbers of antimicrobials for which nonsusceptibility was observed in isolates positive for blaCMY-2 alone (n = 269) or for blaCMY-2 and blaCTX-M (n = 5) were 13 and 12, respectively. This could be explained by the significantly lower frequency of nonsusceptibility to penicillins with β-lactamase inhibitors, cephamycin, chloramphenicol, and gentamicin and a trend with spectinomycin in isolates with only blaCTX-M (Table S4). This statistical difference was expected only for penicillins with β-lactamase inhibitors and cephamycin, as blaCTX-M does not confer resistance to these two antimicrobial classes as observed for blaCMY-2. On the other hand, tetracycline and sulfisoxazole nonsusceptibility was statistically associated with isolates positive for blaCTX-M. Finally, isolates with both blaCMY-2 and blaCTX-M showed a higher proportion of nonsusceptibility for the antimicrobial classes of aminoglycosides, folate inhibitors, tetracyclines, and phenicols than did those with blaCMY-2 alone or blaCTX-M alone, being significant (P = 0.05) only for chloramphenicol.
Increased proportion of E. coli organisms nonsusceptible to antimicrobials of different classes in the ESBL/AmpC producer collection following substitution with lincomycin-spectinomycin.
As expected, with almost 100% of isolates positive for the blaCMY-2 gene, resistance to penicillins with and without β-lactamase inhibitors, third-generation cephalosporins, and cephamycin was almost 100%, irrespective of the antimicrobial used at the hatchery (Table 3). There was no difference in the proportions of samples harboring ESBL/AmpC-positive E. coli nonsusceptible to any of the six other antimicrobial classes in meconium or broiler fecal samples between flocks before and after cessation of in ovo administration of ceftiofur when there was no replacement with lincomycin-spectinomycin, except for the nonsusceptibility to trimethoprim-sulfamethoxazole in broiler feces, in which a significant decrease was observed following the ceftiofur cessation (P = 0.001) (Table 3). On the other hand, there was a significantly greater proportion of samples nonsusceptible to the aminoglycoside, folate inhibitor, phenicol, and tetracycline classes with lincomycin-spectinomycin than when no antimicrobial was used in ovo or with ceftiofur use (Table 3). Finally, the proportion of samples nonsusceptible to fluoroquinolones and macrolides was very low in all isolates of the potential ESBL/AmpC producer collection.
TABLE 3.
Effect of cessation of in ovo administration of ceftiofur and replacement with lincomycin-spectinomycin on the proportions of ceftriaxone-enriched samples from newly hatched, broiler, and breeder birds harboring isolates nonsusceptible to antimicrobials of different classes in the collection of ESBL/AmpC resistance gene-positive E. colia
| Sample origin | Before or after ceftiofur cessation in broiler |
Antimicrobial(s) administered in ovo |
No. of samples (isolates) |
Proportion (%) of nonsusceptible samples by category,b
by classc
of antimicrobial, and by antimicrobiald
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Critically important |
Highly important |
|||||||||||||||||
| Highest priority |
High priority |
|||||||||||||||||
| FLQ |
CPS |
MAC |
PEN |
PEN/I |
AMG |
CPM |
FOL |
PHE |
TET | |||||||||
| NA | CIP | TIO | CRO | AZM | AMP | AMC | GEN | SPT | STR | FOX | SXT | SSS | CHL | |||||
| Meconium | Before (2014) | Ceftiofur | 19 (38) | 5 | 0 | 100 | 100 | 5 | 100 | 100 | 47A | 37A | 63A | 100 | 5A | 68A | 16 | 68 |
| After (2015) | None | 14 (28) | 0 | 0 | 100 | 100 | 0 | 100 | 93 | 64 | 36A | 79 | 93 | 29 | 86 | 29 | 79 | |
| After (2015) | Lincomycin-spectinomycin | 16 (32) | 6 | 0 | 100 | 100 | 0 | 100 | 100 | 88B | 81B | 100B | 100 | 38B | 100B | 38 | 88 | |
|
Pooled feces of broilers |
Before (2014) | Ceftiofur | 20 (40) | 15 | 0 | 100 | 100 | 10 | 100 | 100 | 45 | 25A | 85 | 100 | 65A | 85 | 25 | 85 |
| After (2015) | None | 14 (28) | 7 | 0 | 100 | 100 | 0 | 100 | 100 | 71 | 50 | 71A | 100 | 7B | 86 | 7A | 50A | |
| After (2015) | Lincomycin-spectinomycin | 16 (32) | 13 | 0 | 100 | 100 | 0 | 100 | 100 | 69 | 69B | 100B | 94 | 75A | 100 | 44B | 94B | |
|
Pooled feces of breeders |
Before (2014) | Ceftiofur in 2014 | 22 (44) | 0 | 0 | 95 | 100 | 9 | 100 | 95 | 41 | 36 | 82 | 95 | 14 | 50 | 32 | 82 |
| Before (2015) | Ceftiofur in 2015 | 24 (48) | 4 | 4 | 100 | 100 | 4 | 100 | 96 | 50 | 38 | 75 | 96 | 17 | 58 | 25 | 71 | |
Tested by the Kirby-Bauer method. Different letters in the same column of results for antimicrobial and same sample origin indicate significantly different results.
Category of human antimicrobial importance according to the World Health Organization (WHO) (7).
Abbreviations for antimicrobial classes: FLQ, fluoroquinolones; CPS, cephalosporins; MAC, macrolides; PEN, penicillins; PEN/I, penicillins plus β-lactamase inhibitors; AMG, aminoglycosides; CPM, cephamycins; FOL, folate inhibitors; PHE, phenicols; TET, tetracyclines.
Abbreviations for antimicrobials: NA, nalidixic acid; CIP, ciprofloxacin; TIO, ceftiofur; CRO, ceftriaxone; AZM, azithromycin; AMP, ampicillin; AMC, amoxicillin-clavulanic acid; GEN, gentamicin; SPT, spectinomycin; STR, streptomycin; FOX, cefoxitin; SXT, trimethoprim-sulfamethoxazole; SSS, sulfisoxazole; CHL, chloramphenicol; TET, tetracycline.
Increased level of MDR in ESBL/AmpC resistance gene-positive E. coli isolates in ceftriaxone-enriched samples.
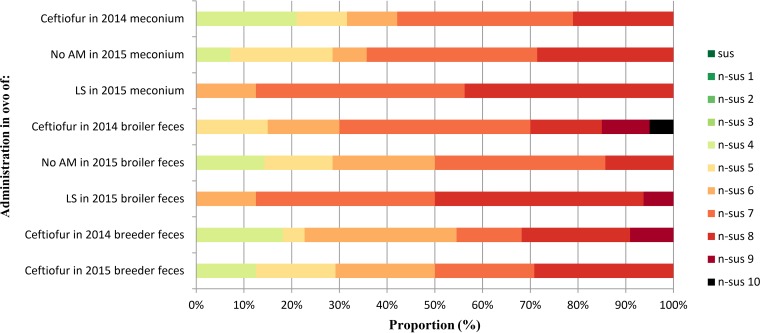
The proportion of ESBL/AmpC resistance gene-positive E. coli organisms from ceftriaxone-enriched samples nonsusceptible to 8 or more classes (referred to as possible extensively drug resistant [XDR] [20]) was greater for 2014 broiler and breeder feces than the meconium in 2014 before the cessation of the in ovo administration of ceftiofur (Fig. 1). The number of possible XDR isolates in broiler feces when no antimicrobial was administered in ovo was slightly lower than that observed in broiler feces before the cessation of ceftiofur. In contrast, flocks receiving lincomycin-spectinomycin demonstrated a level of possible XDR similar to that observed before ceftiofur cessation. Notably, all isolates in the lincomycin-spectinomycin-treated flocks demonstrated non-β-lactam nonsusceptibility in addition to β-lactam (penicillins with and without β-lactamase inhibitors, third-generation cephalosporins, and cephamycin) nonsusceptibility (nonsusceptibility being to 6 or more classes), whereas those in flocks prior to cessation of ceftiofur administration or following cessation of ceftiofur when no antimicrobial was administered in ovo often showed only β-lactam nonsusceptibility (nonsusceptibility being to 4 or more classes) (Table S5). Thus, samples from chicks having received lincomycin-spectinomycin in ovo showed nonsusceptibility to a higher number of antimicrobial classes than those of chicks having received ceftiofur or those who did not receive any antimicrobial in ovo. Finally, possible XDR bacteria in flocks that had received lincomycin-spectinomycin almost always demonstrated nonsusceptibility to spectinomycin and were mostly associated with nonsusceptibility to other non-β-lactams, such as gentamicin, streptomycin, sulfisoxazole, and tetracycline.
FIG 1.
Effect of cessation of in ovo administration of ceftiofur and replacement with lincomycin-spectinomycin on the proportion of multidrug resistance in ESBL/AmpC resistance gene-positive E. coli isolates in ceftriaxone-enriched samples from newly hatched, broiler, and breeder birds. LS, lincomycin-spectinomycin; No AM, no antimicrobial in ovo; sus, susceptible to all antimicrobial classes; n-sus 1 to n-sus 10, nonsusceptibility to 1 to 10 different antimicrobial classes. 2014 is before and 2015 is after the cessation of ceftiofur in Canada; no isolate was susceptible to fewer than 4 antimicrobial classes in the potential ESBL/AmpC-producing isolate collection.
DISCUSSION
Examination of the indicator E. coli in our study clearly demonstrated a decrease in prevalence of samples harboring isolates positive for the ESBL/AmpC resistance genes blaCMY-2 and blaCTX-M in the meconium of newly hatched birds after the cessation of in ovo administration of ceftiofur. This finding shows a beneficial effect of the cessation of the use of ceftiofur, which reinforces the findings of other studies in which a decrease in resistance to extended-spectrum cephalosporins was observed (4, 10, 13) and is consistent with the data from the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) (21). Nevertheless, we did not observe a statistically different effect in the broilers, as was the case in the previous studies (6). In addition, the replacement of ceftiofur with lincomycin-spectinomycin in the broiler hatchery did not have a significant impact on the presence of blaCMY-2 or blaCTX-M in this collection, reinforcing the idea that these genes were selected mainly due to the use of ceftiofur. When we used a more sensitive approach of enrichment with ceftriaxone, we found that almost all samples, irrespective of the production phase, harbored isolates positive for the ESBL/AmpC resistance genes, mostly blaCMY-2, before the cessation of the use of ceftiofur, and this was not affected by the cessation or replacement with lincomycin-spectinomycin, at least in the first year after it occurred. The enrichment with ceftriaxone provided an adequate condition to recover the E. coli isolates resistant to extended-spectrum cephalosporins, and this high proportion was expected in this collection, as already shown in other studies (6, 9, 11, 22, 23).
We observed E. coli positive for blaCTX-M across the entire poultry production chain, suggesting that this gene is more prevalent in Canadian flocks than previously reported (14). Our results following ceftriaxone enrichment demonstrated that cephalosporin resistance genes were still present among the population but that the prevalence was probably lower in the flocks following the cessation of ceftiofur. The lower prevalence of blaCTX-M than of blaCMY-2 in our study in contrast to the lower prevalence of blaCMY-2 reported in Europe (5) could be due to the presence of different plasmids containing blaCTX-M in Europe, such as IncN, IncI, IncL/M, and IncK (9). Nevertheless, a more recent study in Europe showed a higher proportion of blaCMY-2, with a prevalence of 91% of isolates in the ESBL/AmpC producer collection in 7-day-old chickens (24). The difference in relative predominance of the blaCTX-M and blaCMY-2 genes could also be explained in part by differences in geographical distribution of these genes (6). Further testing is needed to understand the difference in the multidrug resistance patterns between isolates with blaCMY-2 alone, blaCTX-M alone, or both. Our results suggest that the plasmids carrying blaCTX-M could harbor coresistance to fewer different classes than those carrying blaCMY-2 alone or with both genes. For these isolates, it is impossible at this point to differentiate if blaCTX-M and blaCMY-2 are on different plasmids. Ceftiofur resistance has largely been associated with blaCMY-2 plasmids in E. coli of poultry origin in Canada in the past, and the increased proportion of blaCTX-M needs further testing. Analyses such as plasmid sequencing will provide answers to these questions. The presence of E. coli isolates with a plasmid harboring blaCMY-2 or blaCTX-M and resistance to other non-β-lactam antimicrobial classes, a mechanism called coselection where antimicrobial resistance genes of different classes are in the same plasmid, has been reported in Quebec and elsewhere (14, 18, 19, 23, 25). In our studies, plasmids from Vietnam isolates carrying blaCTX-M or blaCMY-2 cotransferred resistance to gentamicin, chloramphenicol, sulfisoxazole, and tetracycline (18), whereas plasmids carrying blaCTX-M in Senegal cotransferred resistance to tetracycline and trimethoprim-sulfamethoxazole (19) and in isolates from Quebec, plasmids carrying blaCTX-M or blaCMY-2 cotransferred nonsusceptibility against tetracycline or sulfonamides (unpublished data). On the other hand, plasmids of several incompatibility groups, such as as IncIγ, IncB/O, IncFIB, IncFIC, and IncI1, have already been reported to contain blaCMY-2 genes alone, but not resistance to non-β-lactam antimicrobials (12, 26).
Although the in ovo administration of ceftiofur ceased in Canada in 2014, this practice was only stopped in parent flocks in the United States in 2015, after the present study had been completed; hence, vertical transmission of resistance genes may have occurred (6, 27, 28). In fact, the results of our study are similar to those obtained in Denmark, where the parent flocks that did not receive ceftiofur but for which the grandparent flocks received cephalosporins (23) demonstrated a 93% prevalence of cephalosporin-resistant E. coli (most of the samples being positive for blaCMY-2). In addition, Nilsson et al. demonstrated the vertical transmission of E. coli carrying blaCMY-2 from grandparent flocks that had been exposed to ceftiofur to parent and broiler flocks that had never received ceftiofur (27). Zurfluh et al. demonstrated similar results: E. coli organisms carrying blaCTX-M were vertically transmitted from parent flocks to hatchery and broiler flocks (28). Thus, the administration of ceftiofur in ovo to U.S. breeders could have resulted in an increase in the proportion of resistance to third-generation cephalosporins in broiler E. coli even though the administration of this antimicrobial had ceased in Canadian hatcheries. In the Danish study, a decrease in prevalence of samples positive for cephalosporin-resistant E. coli to 27% in broiler flocks originating from breeders that did not receive ceftiofur was observed (23). Finally, horizontal transmission has also been reported for ESBL/AmpC-associated resistance genes that are found on horizontally transferable plasmids (29). Maintenance of the high proportion of positive samples in the Canadian broilers at 1 year after the cessation of administration of ceftiofur could be explained by environmental contamination which may have persisted after birds ceased to excrete E. coli positive for ESBL/AmpC resistance genes (24). Antimicrobial use causing coselection to other antimicrobial classes, such as penicillin and amoxicillin in the breeder, could also be an important factor (30). For example, Danish broiler flocks which did not receive ceftiofur in ovo (whereas their breeders received it) had significantly higher occurrences of blaCMY-2 when aminopenicillins were used up to 6 months before sampling (23). Unfortunately, we do not know the antimicrobial use for the broilers in our study after the hatchery. Vertical transmission of blaCMY-2 has already been reported (23); thus, further testing is needed to evaluate if vertical transmission of blaCMY-2 and blaCTX-M occurs between the breeders and broilers of our study.
Our finding of an increased prevalence of nonsusceptibility of ESBL/AmpC-positive E. coli to the aminoglycosides, phenicols, tetracyclines, and folate inhibitors in 2015 for birds receiving lincomycin-spectinomycin clearly showed that the use of this combination resulted in an increase in MDR among the cephalosporin-resistant E. coli organisms. These results suggested that there may be a coselection phenomenon when using lincomycin-spectinomycin, which would explain the high MDR for this group. The association of nonsusceptibility to spectinomycin with nonsusceptibility to other non-β-lactams, such as gentamicin, streptomycin, trimethoprim-sulfamethoxazole, sulfisoxazole, chloramphenicol, and tetracyclines, could be due to the presence of a plasmid or transferable elements (integrons, transposons, and insertion sequences) that carry blaCMY-2 and other resistance genes in addition to those encoding resistance to spectinomycin. In a study of E. coli isolates from clinical cases of colibacillosis in chickens in Quebec, Canada, Chalmers et al. (14) demonstrated that the use of spectinomycin could increase coselection. Indeed, resistances to spectinomycin [aac(3)-VI gene] and gentamicin (aadA), the latter being an antimicrobial that is no longer routinely used in Canadian poultry, were strongly associated statistically and the genes were located on a modified class 1 integron. These authors also demonstrated that the use of spectinomycin did not seem to select cephalosporin-resistant E. coli. The aac(3)-VI and blaCMY-2 genes were negatively associated, but when present together, they were generally located on the same plasmid (14). This result may explain the increased resistance to gentamicin in Canada between 2013 and 2016 demonstrated by CIPARS (21). This study could also explain why resistance to gentamicin was higher in flocks receiving lincomycin-spectinomycin than in those receiving ceftiofur in the present study, which was also carried out in Quebec, where no gentamicin was used (21). An increase in the prevalence of isolates with coselection of antimicrobial resistance and greater MDR would thus be expected with the use of lincomycin-spectinomycin and could allow the emergence of a bacterial flora that contains more MDR and, possibly, XDR bacteria.
In a study of MDR E. coli harboring blaCMY-2 or blaCTX-M from sick pigs in Canada, Jahanbakhsh et al. (12) demonstrated the presence of an IncA/C plasmid positively associated with resistance to gentamicin, kanamycin, streptomycin, trimethoprim-sulfamethoxazole, sulfisoxazole, chloramphenicol, and tetracycline in E. coli with resistance to at least one antimicrobial of eight different classes and the presence of blaTEM and class 1 integron. Furthermore, other plasmids, such as IncFIB, had a positive association with IncA/C, whereas IncI1 plasmid had a negative association with these plasmids (12). The presence of such plasmids in our study may explain why multidrug-resistant bacteria were selected when using lincomycin-spectinomycin at the hatchery. Studies in Japan and the Netherlands also found IncA/C plasmids harboring blaCMY-2 and resistance to non-β-lactam antimicrobials (24, 26). As discussed above, the plasmids selected in our study may have contained blaCMY-2 genes and other resistance genes or certain clonal lineages may harbor one plasmid with the blaCMY-2 genes concomitantly with a second plasmid conferring resistance to non-β-lactam antimicrobials.
We observed a significant decrease in nonsusceptibility to trimethoprim-sulfamethoxazole in the broiler feces for the flocks that did not receive any antimicrobial in ovo following the cessation of ceftiofur, thus demonstrating the potential for a positive cessation effect for this class of antimicrobials. Furthermore, resistance to phenicols and tetracyclines was lower, although not significantly, for the same flocks, showing a beneficial effect with respect to the prevalence of MDR. This decrease could be explained by the effect of a selective disadvantage of blaCMY-2 on certain plasmids at the animal level when ceftiofur is stopped and no antimicrobial is used subsequently (24). Nevertheless, no significant differences were seen between flocks receiving ceftiofur and flocks in which no antimicrobial was used with respect to nonsusceptibility to gentamicin, spectinomycin, sulfisoxazole, tetracyclines, and chloramphenicol, which suggests that resistance to these antimicrobials is mainly due to the use of lincomycin-spectinomycin. If broiler flocks not receiving antimicrobials in ovo have a lower level of resistance to certain antimicrobials (such as trimethoprim-sulfamethoxazole), stopping antimicrobials at the hatchery could lead to clinically more effective antimicrobial treatments during fattening.
We observed very little nonsusceptibility to the fluoroquinolones (nalidixic acid and ciprofloxacin) and macrolides (azithromycin) in E. coli isolates obtained following ceftriaxone enrichment. This result was expected, as these classes of antimicrobial agent were not approved for use in poultry in Canada during the years of our study, during which time ceftiofur, bacitracin, virginiamycin, avilamycin, and lincomycin-spectinomycin were the most commonly used antimicrobials (3). In addition, ceftriaxone enrichment is not a selective method for detection of quinolone or macrolide resistance. Finally, resistance to quinolones is mainly due to the acquisition of chromosomal mutations rather than to resistance genes present on plasmids as is the case for ESBL/AmpC production (31).
Overall, our results demonstrate a decrease in the proportion of cephalosporin-resistant E. coli harboring blaCMY-2 and blaCTX-M after the ceftiofur cessation and replacement with lincomycin-spectinomycin. No differences were seen in the proportions of blaSHV, blaOXA, and blaTEM. On the other hand, on examination of the potential ESBL/AmpC producer collection, 99% of samples were shown to harbor cephalosporin-resistant E. coli, indicating that at the sample level, there had not yet been a reduction in the prevalence of E. coli carrying the ESBL/AmpC resistance genes 1 year after the ceftiofur cessation. The 2015 ceftiofur cessation in U.S. breeders could possibly decrease this proportion in the future. Finally, we observed a high proportion of antimicrobial resistance of ESBL/AmpC E. coli in young chicks, broilers, and breeders, with an increase in the proportion of E. coli possibly extensively resistant in flocks receiving lincomycin-spectinomycin, which is a concern for public health.
MATERIALS AND METHODS
Sampling.
Two vertical samplings of meat chickens of an integrated pyramid in the province of Quebec in Canada were made. The first sampling period was between March and May 2014, when chicks (n = 24 broiler flocks) were routinely receiving 0.08 to 0.2 mg of ceftiofur per egg as an in ovo injection (32, 33). The second sampling was done between June and October 2015, 1 year after preventive administration of ceftiofur at the hatchery had ceased. Chicks of 16 of the 30 broiler flocks in the 2015 sampling period received lincomycin and spectinomycin (2.5 mg of lincomycin and 5 mg of spectinomycin per chick [33]) in ovo, whereas chicks on the remaining farms (n = 14) did not receive any preventive antimicrobial at the hatchery.
Breeding sampling.
Eight broiler breeder flocks belonging to the same hatchery were conveniently selected for both sampling periods. In both periods, birds of all breeder flocks were born in the United States and received ceftiofur subcutaneously in the U.S. hatchery before delivery in their first week of age to Canada. During production, some breeder flocks received penicillin or amoxicillin in the water line. For both sampling years, three successive samplings within 5 weeks were made in the 31- to 57-week-old breeder flocks. For fecal sampling, the floor of each breeder house was divided into quarters and approximately 10 fresh fecal droppings were collected from each quarter, put on ice, and delivered to the E. coli laboratory (EcL) of the Faculty of Veterinary Medicine at Saint-Hyacinthe, where they were kept overnight at 4°C. The next day, feces of each quarter were mixed manually and 10 g of feces was mixed in 45 ml of peptone water (Oxoid Canada, Nepean, Ontario, Canada). After standing for 30 min, 8.5 ml of the peptone water suspension was collected and frozen with 1.5 ml of glycerol at −80°C.
Broiler sampling.
In the month following the beginning of the sampling of the eight broiler breeders, chicks from corresponding sampled breeder flocks were selected in the province of Quebec (Canada) for sampling at hatch and at the end of the growing period. A list of farms was obtained from the hatchery company. The first farmers who agreed to participate were recruited. Study farms housed 5,000 to 30,000 chickens. All eggs of the broiler flocks were incubated at the same hatchery. The flocks receiving no antimicrobial or lincomycin-spectinomycin in ovo were from the same breeder in 2015. No flock received gentamicin at the hatchery or on the farm in the province (21), but information on other antimicrobial use on broiler farms was not available. Shortly after hatch and placement into the delivery box, the paper under the chicks was collected to sample meconium as previously described (6). Papers were delivered to the EcL laboratory, where they were kept overnight at 4°C. The next day, 8 to 10 pieces of 3-cm by 3-cm cardboard containing meconium were cut out and put in 30 ml of peptone water. The mixture was then incubated at 37°C overnight. On the next day, 8.5 ml of the peptone water solution was mixed with 1.5 ml of glycerol and frozen at −80°C. Upon chick delivery at the farm, care was taken to place the sampled chicks in a single and well-identified pen to ensure traceability. Between 18 and 29 days of age, fecal sampling was done for each flock using the protocol previously described for the broiler breeder fecal sampling.
In 2014, during ceftiofur use, a total of 22 composite fecal samples from the 8 breeder flocks (1 flock was sampled only once before going to slaughter), 20 composite meconium samples (4 meconium samples each covered the number of chicks needed for the production of two different broiler flocks, giving a total of 20 meconium samples that covered 24 broiler flocks) and 20 composite fecal samples of broiler flocks from the 24 participating farms (traceability to the breeders was lost for 4 lots) were selected. In 2015, a total of 24 samples from 8 breeder flocks, with corresponding meconium and composite fecal samples from 14 broiler chicken flocks for which no in ovo antimicrobials were administered and 16 flocks for which lincomycin-spectinomycin was administered in ovo, were taken.
Colony isolation of Escherichia coli.
From the frozen samples, two different isolation protocols were used to obtain an indicator E. coli collection and a potential ESBL/AmpC producer collection.
Indicator E. coli collection.
Samples were streaked on MacConkey agar (Becton, Dickinson and Company) to obtain individual colonies. After overnight incubation at 37°C, five well-isolated lactose-positive colonies, when possible, were reinoculated on MacConkey agar and incubated at 37°C overnight. Five well-isolated colonies were then selected and incubated in Luria-Bertani (LB) (Becton, Dickinson and Company) broth overnight at 37°C. Finally, 750 μl of the bacterial suspension for each isolate was mixed with 750 μl of 30% glycerol and frozen at −80°C.
Potential ESBL/AmpC producer collection.
We used the protocol described previously by Agerso and colleagues (22, 23), with some modifications. A volume of 50 μl of each thawed sample was inoculated in 5 ml of peptone water containing 1 mg/ml of ceftriaxone (34). After incubation at 37°C overnight, cultures were streaked on MacConkey agar plates with 1 mg/ml of ceftriaxone. After another overnight incubation at 37°C, 5 colonies were selected (preferably lactose positive) and inoculated overnight at 37°C on MacConkey agar containing 1 mg/ml of ceftriaxone. Five well-isolated colonies on agar were placed in LB culture medium and incubated overnight at 37°C. Finally, 750 μl of each bacterial suspension was mixed with 750 μl of 30% glycerol and frozen at −80°C. Selective enrichment with the cephalosporin ceftriaxone permitted the detection of third-generation-cephalosporin-resistant E. coli organisms that are not usually found by standard monitoring (indicator collection), and we chose ceftriaxone among the various cephalosporins because it has some ability in separating ESBL and AmpC isolates (22, 34).
DNA extraction and uidA PCR.
For each isolate, DNA was extracted from the overnight culture in LB medium at 37°C as previously used for colony isolation. DNA templates were prepared from the samples by boiled cell lysis for examination by PCR, as described previously by Maluta et al. (35). All lactose-positive isolates were confirmed as E. coli by PCR for detection of housekeeping gene uidA, which encodes beta-glucuronidase, with control strain ECL7805 (36) (list of primers in Table S1). PCR conditions used to detect the uidA gene included initial denaturation (95°C for 2 min), 24 cycles of denaturation (94°C for 30 s), annealing (65°C for 30 s), extension (72°C for 30 s), and a hold at 4°C.
Detection of antimicrobial resistance genes.
Escherichia coli isolates positive for uidA were analyzed for the presence of bla genes by multiplex PCR (list of primers in Table S1). Five β-lactamase resistance genes (blaSHV, blaTEM, blaCMY-2, blaOXA, and blaCTX-M) were tested in a subset of E. coli isolates from the potential ESBL/AmpC producer collection (3 of 5 isolates maximum per sample for a total of 432 tested) and for all the E. coli isolates in the indicator collection (5 colonies maximum per sample for a total of 722 tested). The protocol was provided by the National Microbiology Laboratory of the Public Health Agency of Canada and used with some minor adjustments. The control strains ECL3482, PMON38, and CTX-M15 were used (37).
Phenotypic antimicrobial susceptibility testing in the potential ESBL/AmpC producer collection.
For each sample from the potential ESBL/AmpC producer collection, the first 2 of 3 isolates tested by PCR were selected as described by Agerso et al. (23) for examination by the disk diffusion (Kirby-Bauer) assay (total of 290 isolates), as previously described by the Clinical and Laboratory Standards Institute (CLSI) (38, 40). Susceptibility of isolates was tested for 14 antimicrobials belonging to 10 classes as used in the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) for food-producing animals and agents of interest in human and veterinary medicine (39), with addition of spectinomycin (total of 15 antimicrobials and 10 classes) (list of antimicrobial disks in Table S2). Breakpoints were those recommended by the CLSI in 2015 (M100-S25) for Enterobacteriaceae for most of the antimicrobials (40). There were three exceptions for which recommendations in the CLSI in 2015 for animals (VET01S) were used: tetracyclines and ceftiofur, for which the breakpoints selected were for Enterobacteriaceae, and spectinomycin, for which the zone diameter interpretative standards used were for Pasteurella multocida (38). Intermediate and resistant isolates together were classified as nonsusceptible as proposed by an international committee (20). Escherichia coli strain ATCC 25922 was used as a quality control for susceptibility testing.
Multidrug resistance (MDR) was defined as nonsusceptibility to at least one agent in three different antimicrobial classes and possible extreme drug resistance (XDR) as nonsusceptibility to at least one agent in all but two or fewer antimicrobial classes tested, as suggested by Magiorakos et al. (20). In order to more precisely compare the levels of MDR between groups, the level of MDR for each sample was classified from 0 to 10, representing the number of antimicrobial classes to which the sample was nonsusceptible.
Statistical analysis.
The unit of interest for statistical analysis was the flock, one pooled sample representing one flock. A sample was considered nonsusceptible to an antimicrobial when at least one of its isolates demonstrated nonsusceptibility. A sample was considered positive to a resistance gene when at least one of its isolates was positive. The associations between the various groups (in ovo administration of ceftiofur, no antimicrobial, or lincomycin-spectinomycin) were tested using exact chi-square with SAS v.9.3 (SAS, Cary, NC). The alpha value was set at 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Agriculture and Agri-Food Canada and by Éleveurs de volailles du Québec, Agri-Innovation Program grant AIP P270.
We thank Ghyslaine Vanier, Gabriel Desmarais, and Jocelyn Bernier-Lachance for their technical assistance, Benoît Lanthier for assistance in the field, and Les Fonds du Centenaire of the faculty of veterinary medicine of Université de Montréal for their contribution.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00037-19.
REFERENCES
- 1.Kemmett K, Williams NJ, Chaloner G, Humphrey S, Wigley P, Humphrey T. 2014. The contribution of systemic Escherichia coli infection to the early mortalities of commercial broiler chickens. Avian Pathol 43:37–42. doi: 10.1080/03079457.2013.866213. [DOI] [PubMed] [Google Scholar]
- 2.Giovanardi D, Campagnari E, Ruffoni LS, Pesente P, Ortali G, Furlattini V. 2005. Avian pathogenic Escherichia coli transmission from broiler breeders to their progeny in an integrated poultry production chain. Avian Pathol 34:313–318. doi: 10.1080/03079450500179046. [DOI] [PubMed] [Google Scholar]
- 3.Agunos A, Leger DF, Carson CA, Gow SP, Bosman A, Irwin RJ, Reid-Smith RJ. 2017. Antimicrobial use surveillance in broiler chicken flocks in Canada, 2013–2015. PLoS One 12:e0179384. doi: 10.1371/journal.pone.0179384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiki M, Kawanishi M, Abo H, Kojima A, Koike R, Hamamoto S, Asai T. 2015. Decreased resistance to broad-spectrum cephalosporin in Escherichia coli from healthy broilers at farms in Japan after voluntary withdrawal of ceftiofur. Foodborne Pathog Dis 12:639–643. doi: 10.1089/fpd.2015.1960. [DOI] [PubMed] [Google Scholar]
- 5.Dierikx C, van Essen-Zandbergen A, Veldman K, Smith H, Mevius D. 2010. Increased detection of extended spectrum beta-lactamase producing Salmonella enterica and Escherichia coli isolates from poultry. Vet Microbiol 145:273–278. doi: 10.1016/j.vetmic.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Baron S, Jouy E, Larvor E, Eono F, Bougeard S, Kempf I. 2014. Impact of third-generation-cephalosporin administration in hatcheries on fecal Escherichia coli antimicrobial resistance in broilers and layers. Antimicrob Agents Chemother 58:5428–5434. doi: 10.1128/AAC.03106-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. 2017. Critically important antimicrobials for human medicine, 5th revision 2016. Ranking of medically important antimicrobials for riskmanagement of antimicrobial resistancedue to non-human use. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 8.Gouvernement du Canada. 2009. Categorization of antimicrobial drugs based on importance in human medicine. https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importance-human-medicine.html. Accessed 17 April 2019.
- 9.Liebana E, Carattoli A, Coque TM, Hasman H, Magiorakos AP, Mevius D, Peixe L, Poirel L, Schuepbach-Regula G, Torneke K, Torren-Edo J, Torres C, Threlfall J. 2013. Public health risks of enterobacterial isolates producing extended-spectrum beta-lactamases or AmpC beta-lactamases in food and food-producing animals: an EU perspective of epidemiology, analytical methods, risk factors, and control options. Clin Infect Dis 56:1030–1037. doi: 10.1093/cid/cis1043. [DOI] [PubMed] [Google Scholar]
- 10.Dutil L, Irwin R, Finley R, Ng LK, Avery B, Boerlin P, Bourgault AM, Cole L, Daignault D, Desruisseau A, Demczuk W, Hoang L, Horsman GB, Ismail J, Jamieson F, Maki A, Pacagnella A, Pillai DR. 2010. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerg Infect Dis 16:48–54. doi: 10.3201/eid1601.090729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dierikx CM, van der Goot JA, Smith HE, Kant A, Mevius DJ. 2013. Presence of ESBL/AmpC-producing Escherichia coli in the broiler production pyramid: a descriptive study. PLoS One 8:e79005. doi: 10.1371/journal.pone.0079005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahanbakhsh S, Smith MG, Kohan-Ghadr HR, Letellier A, Abraham S, Trott DJ, Fairbrother JM. 2016. Dynamics of extended-spectrum cephalosporin resistance in pathogenic Escherichia coli isolated from diseased pigs in Quebec, Canada. Int J Antimicrob Agents 48:194–202. doi: 10.1016/j.ijantimicag.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Caffrey N, Nekouei O, Gow S, Agunos A, Checkley S. 2017. Risk factors associated with the A2C resistance pattern among E. coli isolates from broiler flocks in Canada. Prev Vet Med 148:115–120. doi: 10.1016/j.prevetmed.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Chalmers G, Cormier AC, Nadeau M, Cote G, Reid-Smith RJ, Boerlin P. 2017. Determinants of virulence and of resistance to ceftiofur, gentamicin, and spectinomycin in clinical Escherichia coli from broiler chickens in Quebec, Canada. Vet Microbiol 203:149–157. doi: 10.1016/j.vetmic.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Gouverment du Canada. 2016. Programme Intégré Canadien de Surveillance de la Résistance aux Antimicrobiens (PICRA). https://www.canada.ca/fr/sante-publique/services/publications/medicaments-et-produits-sante/programme-integre-canadien-surveillance-resistance-antimicrobiens-bulletin.html?%02undefined&wbdisable%02true Accessed 13 November 2018.
- 16.Kataoka Y, Murakami K, Torii Y, Kimura H, Maeda-Mitani E, Shigemura H, Fujimoto S, Murakami S. 2017. Reduction in the prevalence of AmpC beta-lactamase CMY-2 in Salmonella from chicken meat following cessation of the use of ceftiofur in Japan. J Glob Antimicrob Resist 10:10–11. doi: 10.1016/j.jgar.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Chicken Farmers of Canada. 2018. The antimicrobial use reduction strategy. https://www.chickenfarmers.ca/the-antimicrobial-use-reduction-strategy/. Accessed 1 August 2018.
- 18.Vounba P, Arsenault J, Bada-Alambédji R, Fairbrother JM. 2019. Pathogenic potential and the role of clones and plasmids in beta-lactamase-producing E. coli from chicken faeces in Vietnam. BMC Vet Res 15:106. doi: 10.1186/s12917-019-1849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vounba P, Arsenault J, Bada-Alambédji R, Fairbrother JM. 2019. Prevalence of antimicrobial resistance and potential pathogenicity, and possible spread of third generation cephalosporin resistance, in Escherichia coli isolated from healthy chicken farms in the region of Dakar, Senegal. PLoS One 14:e0214304. doi: 10.1371/journal.pone.0214304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 21.CIPARS. 2016. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS), 2016 annual report. Public Health Agency of Canada, Ottawa, Canada. [Google Scholar]
- 22.Agerso Y, Aarestrup FM, Pedersen K, Seyfarth AM, Struve T, Hasman H. 2012. Prevalence of extended-spectrum cephalosporinase (ESC)-producing Escherichia coli in Danish slaughter pigs and retail meat identified by selective enrichment and association with cephalosporin usage. J Antimicrob Chemother 67:582–588. doi: 10.1093/jac/dkr507. [DOI] [PubMed] [Google Scholar]
- 23.Agerso Y, Jensen JD, Hasman H, Pedersen K. 2014. Spread of extended spectrum cephalosporinase-producing Escherichia coli clones and plasmids from parent animals to broilers and to broiler meat in a production without use of cephalosporins. Foodborne Pathog Dis 11:740–746. doi: 10.1089/fpd.2014.1742. [DOI] [PubMed] [Google Scholar]
- 24.Dame–Korevaar A, Fischer EAJ, Stegeman A, Mevius D, van Essen-Zandbergen A, Velkers F, van der Goot J. 2017. Dynamics of CMY-2 producing E. coli in a broiler parent flock. Vet Microbiol 203:211–214. doi: 10.1016/j.vetmic.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Abraham S, Kirkwood RN, Laird T, Saputra S, Mitchell T, Singh M, Linn B, Abraham RJ, Pang S, Gordon DM, Trott DJ, O’Dea M. 2018. Dissemination and persistence of extended-spectrum cephalosporin-resistance encoding IncI1-blaCTXM-1 plasmid among Escherichia coli in pigs. ISME J 12:2352–2362. doi: 10.1038/s41396-018-0200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiki M, Usui M, Kojima A, Ozawa M, Ishii Y, Asai T. 2013. Diversity of plasmid replicons encoding the bla(CMY-2) gene in broad-spectrum cephalosporin-resistant Escherichia coli from livestock animals in Japan. Foodborne Pathog Dis 10:243–249. doi: 10.1089/fpd.2012.1306. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson O, Borjesson S, Landen A, Bengtsson B. 2014. Vertical transmission of Escherichia coli carrying plasmid-mediated AmpC (pAmpC) through the broiler production pyramid. J Antimicrob Chemother 69:1497–1500. doi: 10.1093/jac/dku030. [DOI] [PubMed] [Google Scholar]
- 28.Zurfluh K, Wang J, Klumpp J, Nuesch-Inderbinen M, Fanning S, Stephan R. 2014. Vertical transmission of highly similar bla CTX-M-1-harboring IncI1 plasmids in Escherichia coli with different MLST types in the poultry production pyramid. Front Microbiol 5:519. doi: 10.3389/fmicb.2014.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daehre K, Projahn M, Semmler T, Roesler U, Friese A. 2018. Extended-spectrum beta-lactamase-/AmpC beta-lactamase-producing Enterobacteriaceae in broiler farms: transmission dynamics at farm level. Microb Drug Resist 24:511–518. doi: 10.1089/mdr.2017.0150. [DOI] [PubMed] [Google Scholar]
- 30.Birkegård AC, Halasa T, Græsbøll K, Clasen J, Folkesson A, Toft N. 2017. Association between selected antimicrobial resistance genes and antimicrobial exposure in Danish pig farms. Sci Rep 7:9683. doi: 10.1038/s41598-017-10092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hricova K, Roderova M, Pudova V, Hanulik V, Halova D, Julinkova P, Dolejska M, Papousek I, Bardon J. 2017. Quinolone-resistant Escherichia coli in poultry farming. Cent Eur J Public Health 25:163–167. doi: 10.21101/cejph.a4328. [DOI] [PubMed] [Google Scholar]
- 32.Janzen D, Davies M, Greydanus J, Clarke P, Evans C, Weiss R. 2013. Preventive use of category I antibiotics in the poultry and egg sectors. http://bcchicken.ca/wp-content/uploads/2012/11/Antibiotic-FAQ-2013.pdf. Accessed March 2018.
- 33.Agunos A, Leger D, Carson C. 2012. Review of antimicrobial therapy of selected bacterial diseases in broiler chickens in Canada. Can Vet J 53:1289–1300. [PMC free article] [PubMed] [Google Scholar]
- 34.Aarestrup FM, Hasman H, Veldman K, Mevius D. 2010. Evaluation of eight different cephalosporins for detection of cephalosporin resistance in Salmonella enterica and Escherichia coli. Microb Drug Resist 16:253–261. doi: 10.1089/mdr.2010.0036. [DOI] [PubMed] [Google Scholar]
- 35.Maluta RP, Fairbrother JM, Stella AE, Rigobelo EC, Martinez R, de Ávila FA. 2014. Potentially pathogenic Escherichia coli in healthy, pasture-raised sheep on farms and at the abattoir in Brazil. Vet Microbiol 169:89–95. doi: 10.1016/j.vetmic.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Walk ST, Alm EW, Gordon DM, Ram JL, Toranzos GA, Tiedje JM, Whittam TS. 2009. Cryptic lineages of the genus Escherichia. Appl Environ Microbiol 75:6534–6544. doi: 10.1128/AEM.01262-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mataseje LF, Bryce E, Roscoe D, Boyd DA, Embree J, Gravel D, Katz K, Kibsey P, Kuhn M, Mounchili A, Simor A, Taylor G, Thomas E, Turgeon N, Mulvey MR. 2012. Carbapenem-resistant Gram-negative bacilli in Canada 2009–10: results from the Canadian Nosocomial Infection Surveillance Program (CNISP). J Antimicrob Chemother 67:1359–1367. doi: 10.1093/jac/dks046. [DOI] [PubMed] [Google Scholar]
- 38.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals, CLSI supplement VET01S, p 128 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 39.CIPARS. 2015. Canadian Integrated Program for Antimicrobial Resistance Surveillance, 2015 annual report. Public Health Agency of Canada, Ottawa, Canada. [Google Scholar]
- 40.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement, M100-S25, p 240 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.